Abstract
Osteoporosis is a skeletal pathology characterized by compromised bone strength leading to increased risk of fracture, mainly the spine and hip fractures. Osteoporosis affects more than 200 million people worldwide and because of the skeletal fractures it causes, represents a major cause of morbidity, disability and mortality in older people. Recently, the new discoveries of osteoimmunology have clarified many of the pathogenetic mechanisms of osteoporosis, helping to identify new immunological targets for its treatment opening the way for new and effective therapies with biological drugs. Currently, there are basically two monoclonal antibodies for osteoporosis therapy: denosumab and romosozumab. Here, we focus on the modern approach to the osteoporosis management and in particular, on current and developing biologic drugs targeted to new immunological checkpoints, in the landscape of osteoimmunology.
Keywords: Osteoporosis, osteoimmunology, bone remodeling, cytokines, immunological checkpoints, biological therapies
1. INTRODUCTION
Osteoporosis is a systemic pathology of the skeleton, which affects more than 200 million people worldwide, resulting in bone fragility and increased risk of fractures [1]. The definition of osteoporosis based on World Health Organization (WHO) criteria is a reduction in Bone Mineral Density (BMD), assessed by Dual-energy x-ray Absorptiometry (DXA), of 2.5 standard deviations or more, below that of the mean peak BMD of young adults. The result is expressed as T-score: values between -1 and -2.5 identify a condition of osteopenia, while very low T score values and the presence of fractures identify severe osteoporosis conditions [2, 3]. It is an insidious disease which proceeds silently until the appearance of skeletal fractures, especially of the spine and the femur, involving high morbidity, mortality and reduction of quality of life, with significant socio-sanitary and economic impact [4].
Osteoporosis is a predominantly female pathology, whose prevalence increases drastically with age and is currently growing due to the constant aging of the population [5]. It can be divided into primary osteoporosis, which includes postmenopausal osteoporosis (Type I) and senile osteoporosis (type II), and secondary osteoporosis, which recognizes clearly definable etiologic mechanisms and is determined by a large number of pathologies and drugs [6-8]. Most of the pathologies capable of inducing osteoporosis are predominantly characterized by a chronic inflammatory substratum, the same partly shared by menopause and inflammaging [9]. Osteoporosis is actually a multi-factorial disease: the etiopathogenetic mechanisms involved are variously combined and in each patient, the clinical picture is peculiar, depending on the influence of different factors. In addition, the skeletal homeostasis is functionally connected to different organs and systems [10, 11]. The major clinical challenges are to identify patients at high risk for osteoporotic fractures, search for new effective and safe drugs against specific cell targets, design combined or sequential treatments and personalize diagnostic and therapeutic protocols [3].
Currently available osteoporosis drugs are either antiresorptive (inhibiting the osteoclasts) or bone-forming (stimulating the osteoblasts) [12, 13]. All these categories of drugs have the goal to make the bone stronger, acting on density, quality and bone turnover that is on bone remodeling. The most utilized antiresorptive treatments are bisphosphonates [14], widely used for over 20 years, and Selective Estrogen Receptor Modulators (SERMs) that either cause osteoclast apoptosis through farnesyl pyrophosphate synthase inhibition (bisphosphonates) or inhibit osteoclast recruitment through mimicking estrogen activity on a bone [15]. Although bisphosphonates and SERMs reduce the risk of osteoporotic-related fractures, they are associated respectively with serious skeletal side effects and increased risk of venous thromboembolism and stroke. Calcitonin, a peptide produced by the parafollicular thyroid cells, binds to osteoclasts and inhibits bone resorption. It is currently available in the USA for osteoporosis treatment, but it is not a very powerful antiresorptive drug and is associated with increased risk of cancer in long-term treatments [12]. Teriparatide (recombinant human parathyroid hormone analogue - rPTH) is an osteoanabolic drug inducing bone formation [16]. However, the use of teriparatide is limited to two years because of side effects and potential neoplastic risk. The osteoanabolic and antiresorptive strontium ranelate, used in the past to treat osteoporosis, has now been withdrawn from the market due to serious cardiovascular, cutaneous and hepatic side effects. Biological and immunological drugs have also been developed for osteoporosis, such as Receptor Activator of Nuclear factor-κB Ligand (RANKL) Monoclonal Antibody (MoAb), placed on the market about 10 years ago. It is the first biological antiresorptive drug against an osteoclastogenic cytokine, which inhibits osteoclast recruitment [17]. Other biological drugs are under study, which target key molecules of bone turnover [13].
Most reviews on osteoporosis therapy list the main categories of drugs currently used in clinical practice, based on updated guidelines, or alternatively focus on the biochemical structure and mechanism of action of specific drugs. Recent advances in the field of osteoimmunology have improved our understanding of the mechanisms of action of drugs already used in the clinic and have allowed the identification of other potential therapeutic targets. The purpose of this review is to provide an updated view of both the MoAbs used in osteoporosis therapy and the new potential biological targets with clinical-translational implications.
2. IMMUNE REGULATION OF BONE REMODELING
Bone is a metabolically active and plastic tissue formed by a matrix of proteins and mineral salts in which the bone cells (osteoblasts, osteocytes and osteoclasts) are embedded. Bone is remodeled throughout life by a process of resorption of old bone, mediated by osteoclasts (multinuclear giant cells of the myeloid lineage, containing lysosomal enzymes, such as acid tartrate-resistant phosphatase and cathepsins) followed by new bone formation, mediated by osteoblasts (derived from a mesenchymal stem cell that can also differentiate into bone marrow stromal cells and adipocytes) [18]. Osteoblasts are the precursors of osteocytes, the structural cells of the bone. Bone remodeling is finely tuned by cytokines and growth factors, which are also central for immunological functions, and factors involved in inflammation are linked with those critical for bone remodeling process [11].
Central in this process is the osteoclast, regulated by various signal pathways, the most important of which is undoubtedly the system of RANKL, produced by osteoblasts but also by other cell types, mainly activated immune cells. It binds to its receptor RANK on the membrane of the osteoclast precursors and mature osteoclasts stimulating osteoclastogenesis and bone resorption [19]. A natural inhibitor of RANKL, Osteoprotegerin (OPG), functions as a decoy receptor, hindering osteoclastogenesis. In addition, inflammatory cytokines, such as Tumour Necrosis Factor-α (TNF-α), Interleukin (IL)-1 and IL-6, also induce osteoblasts to further express RANK-L, leading to an imbalance between bone formation and resorption, with consequent osteoporosis [20].
Another complex signaling pathway, centrally involved in regulating bone mass, is the Wingless/ Integrated (Wnt) system, which exerts, unlike the RANK/ RANKL pathway, a prevailing osteoanabolic function, mainly activating osteoblasts and blocking their apoptosis. In response to mechanical loading, it promotes differentiation, proliferation, survival and mineralizing activity of osteoblasts, and only indirectly blocks osteoclastogenesis and adipocyte differentiation [21].
Wnt pathway encompasses various proteins, both inhibitory and stimulating, that bind to complex transmembrane receptors formed by the association of Frizzled Proteins (FRZ) and low-density Lipoprotein Receptor proteins 5 and 6 (LPR 5/6) which promote β-catenin translocation in the nucleus of osteoblasts to activate the transcription of Wnt signal target genes. Osteocytes express several negative regulators of this signal pathway [22]. Among them, the Dickkopf-related protein 1 (DKK1), a high-affinity ligand of Kremen proteins, also involved in embryonic development, plays a central role. It inhibits the Wnt/β-catenin signalling pathway by binding to and antagonizing LRP5/6 co-receptor, therefore preventing the activation of the receptor signal [23]. Other inhibitors of the Wnt pathway are Secreted-Frizzled Related Proteins (SFRPs), members of the DKK family, and sclerostin. Sclerostin is a glycoprotein also produced by osteocytes and encoded by the SOST gene on the long arm of chromosome 17 that both inhibits bone formation by blocking the Wnt pathway and increases bone resorption through the induction of RANKL production by osteocytes. Sclerostin binding to LPR 5/6 and FRZ receptor proteins leads to the phosphorylation and cytoplasmic degradation of β-catenin blocking osteoblast stimulation [24]. Glucocortocoids and TNF-β increase the expression of sclerostin and DKK1, thus suppressing bone formation [25, 26]. Anti-TNF therapy, therefore, exerts positive effects on bone through both its anti-inflammatory action and its osteoanabolic effect mediated by the enhancement of the Wnt signal.
A complex network of cytokines is involved in the regulation of bone remodeling. The Macrophage-Colony Stimulating growth Factor (M-CSF) enhances the osteoclastic differentiation signal mediated by RANK. IL-6, IL-17, IL-31, TNF-α and interferon-γ (IFN-γ) are osteoclastogenic cytokines and inhibit osteoblasts, while other cytokines, such as IL-4, IL-10, IL-12 and IL-33, suppress osteoclasts and promote osteogenetic function [11]. For the most part, the cytokines of the Th1 profile, unlike Th2, are osteoclastogenic [20, 27]. This is evident in clinical practice: for example in rheumatoid arthritis, periarticular erosions caused by bone resorption are blocked by therapy with anti-TNF-α monoclonal antibodies, which have long been used to treat this disease [28]. In patients with multiple myeloma, the osteolytic skeletal pathology is induced by a complex of osteoclastogenic factors, including, in addition to DKK1, also IL-6, produced by the neoplastic plasma cells, against which targeted therapies have been proposed [29]. IL-31, although belonging to the Th2 profile, induces the production of various inflammatory cytokines and matrix metalloproteinases, which respectively stimulate osteoclast production and induce bone resorption [30], suggesting the possibility of future anti-osteoporotic therapies against it. On the contrary, the multifunctional Th2 cytokine IL-33 exhibits anti-osteoclastic effects in postmenopausal women [31]. Low IL-33 serum levels in postmenopausal osteoporotic women have been recently demonstrated [32], suggesting the possibility of using recombinant IL-33 in osteoporosis therapy. Therefore, osteoporosis can no longer be considered only as a Th1 profile disease, since also a Th2 profile, classically considered osteoprotective, especially in the elderly, could variously contribute to skeletal damage [33, 34].
Immune regulation of bone remodeling also intervenes in the mechanisms driving bone metastases, realizing positive feedback between osteoporosis and neoplastic progression [35]. The normal bone remodeling is deeply altered by neoplastic cells in the bone: exacerbated osteoclast activity is a common finding in bone metastases and bone resorption and tumour growth potentiate each other. The identification of new shared targets for both osteoporosis and cancer represents an interesting research field [36]. Several antiresorptive molecules, frequently successfully used for the treatment of postmenopausal osteoporosis (bisphosphonates, denosumab) also have specific indications in cancer therapy and are routinely used to treat bone metastases. While breast cancer cells stimulate osteoclastogenesis thus inducing bone resorption and osteolytic metastases [37], prostate cancer cells act by stimulating bone formation and inducing osteosclerotic metastases through the secretion of Bone Morphogenetic Proteins (BMPs) which increase osteoblast activity and consequently bone mineralization. Multiple myeloma cells secrete DKK1 inhibiting osteoblast maturation [38]. RUNX2, a transcription factor in mature osteoblasts, exerts pleiotropic roles in the metastatic process, promoting both breast and prostate bone metastasis [39].
3. BIOLOGICAL TARGETS IN OSTEOPOROSIS THERAPY
3.1. RANKL
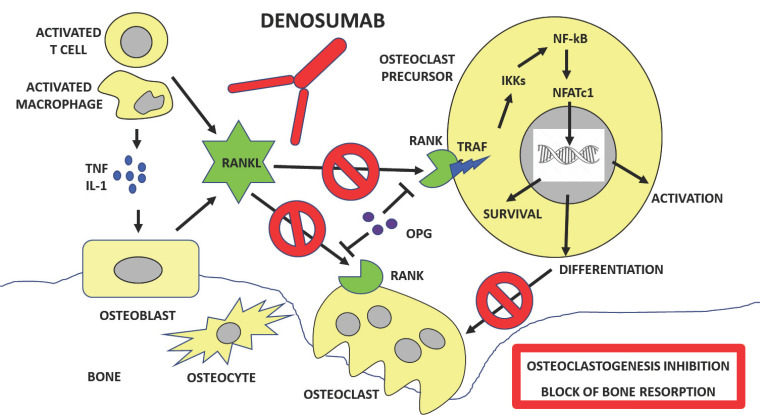
The first MoAb recognizing a biological target for osteoporosis therapy had been denosumab (hMoAb AMG162), specifically produced against RANKL, the essential mediator of bone resorption. Denosumab is a humanized MoAb which binds RANKL with high specificity, thus inhibiting osteoclastogenesis and the excessive bone resorption which accompanies osteoporosis. It is, therefore, a powerful anti-resorbent drug, able to inhibit osteoclast formation, function and survival [40].
Successfully used in clinical practice by about 10 years, denosumab is approved for the treatment of osteoporosis in postmenopausal women at increased risk of fracture, and is given as a subcutaneous injection of 60 mg once every 6 months. It is also utilised in the setting of breast cancer bone metastasis at a higher dosage (120 mg monthly). Subcutaneous administration is followed by a reduction in osteoclastic bone resorption and, subsequently, a reduction in neoformative activity. Like bisphosphonates, it is, therefore, an antiresorptive drug, however, the mechanism of action is quite different: bisphosphonates concentrate in the bone tissue where they block the enzyme farnesyl bisphosphate synthase in the osteoblasts that is essential for their activity, whereas denosumab binds RANKL thus inhibiting both osteoclast formation and recruitment [41]. Figure 1 shows the mechanisms through which denosumab inhibits bone resorption.
The anti-fracture efficacy of denosumab at 36 months and at 6 years has been demonstrated by the Freedom and Freedom extension studies respectively, a phase 3 Double-blind Placebo-controlled (DBPC) clinical trial on postmenopausal women with previous vertebral fractures, with primary endpoint reducing the risk of new fractures. Both at 3 and 6 years, patients treated with denosumab showed a reduction of more than 65% of the risk of new vertebral fractures compared to placebo [42]. The anti-fracture efficacy of denosumab was also demonstrated for non-vertebral fractures, in particular, femoral fractures, with a 20% risk reduction, showing that denosumab also acts, although with less power, on the cortical bone [43]. Denosumab significantly improved bone mineralization and consequently, BMD increased at the lumbar spine and total hip through 6 years of treatment [44]. However, although it represents a turning point in the treatment of osteoporosis, denosumab therapy also has some limitations. At the end of the therapy the osteoclasts, initially blocked by denosumab, resume their destructive activity so that multiple fractures may appear and BMD may decrease even below the starting value [45]. Recently, with the aim of strengthening, consolidating and maintaining over time the anti-fracture efficacy of denosumab, possibly reducing its side effects, further studies have been performed. Denosumab and Teriparatide Administration (DATA) study evaluated the efficacy of the combination therapy with denosumab, as an antiresorptive drug, and teriparatide, the only osteoanabolising drug currently available in Europe, administered both in sequence and simultaneously, in severe and advanced osteoporosis [46]. The results of the 1 and 2-year DATA study on BMD at the level of the whole column and hip neck showed that denosumab and teriparatide administered together are more effective than in monotherapy. Furthermore, the antiresorptive therapy with denosumab after teriparatide or the combination consolidates the recovery of bone mass. Interestingly, the behavior of the bone remodeling markers Osteocalcin (OC) and C-terminal Telopeptide (CTX), respectively linked to osteoblast and osteoclast activity, confirmed the block of resorption with the maintenance of osteoformation. In the most severe forms of osteoporosis, an additional BMD gain has been documented when denosumab is combined with teriparatide in the sequence teriparatide-denosumab but not vice versa [47]. This is in contrast to the common practice of first administering an antiresorptive, for example, bisphosphonates, and then, in the most severe and progressive forms, the osteoanabolising agent. Antiresorptive therapy with denosumab administered after an anabolic agent consolidates the anabolic bone density gain. Efficacy of combination and sequential therapy may be related to denosumab ability to fully block teriparatide pro-resorptive effects maintaining however its osteoanabolizing function [48]. In general, the more effective the antiresorptive drug is, the longer the therapy, the greater the risk of adverse events, especially in some types of patients, such as neoplastic and debilitated patients. On the other hand, since osteoporosis is a chronic disease, prolonged treatments are very frequent. For example, denosumab therapy is associated with the risk of some adverse reactions such as infections of the urinary tract, cellulitis, hypocalcaemia, musculoskeletal pain, Osteonecrosis of the Jaw (ONJ) and Atypical Femoral Fractures (AFF), among others. In long treated patients, the most frightening, although rare, are ONJ and AFF, that however may also occur as a result of bisphosphonate therapy [49, 50]. Teriparatide, although effective, has limited effect to no more than 2 years and could be associated with the risk of osteosarcoma, at least in the experimental animal [51]. So the need to look for new drugs and treatment options.
3.2. Sclerostin
Among the many molecules, potential therapeutic targets, which regulate bone remodeling, sclerostin, which is part of the canonical Wnt-β-catenin signaling pathway, seems particularly promising. It is a natural inhibitor of Wnt signal in bone [52, 53].
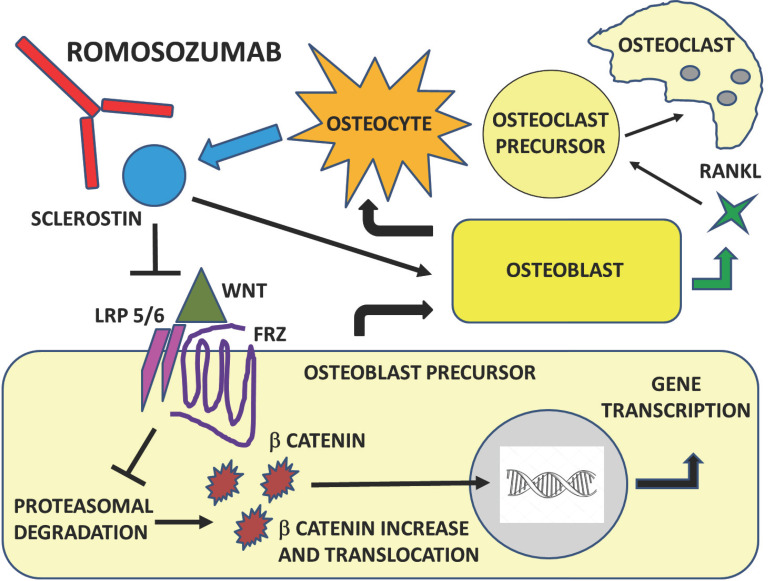
Figure 2 shows the role of WNT pathway and sclerostin in bone remodeling and the effect of sclerostin inhibition. The Wnt pathway is activated by the interaction between LRP5/6, Wnt and Frizzled proteins. As a consequence, β-catenin is released, enters the nucleus and activates transcription from Wnt target genes. Sclerostin inactivates the Wnt pathway by binding to LRP5/6 and as a consequence β-catenin is phosphorylated and degraded.
The observation of two rare recessive genetic diseases, sclerosteosis and van Buchem's disease, helped to clarify its function. These disease entities are clinically characterized by the presence of high BMD and a low risk of fractures. In particular, sclerosteosis is determined by loss of function mutations in the SOST gene, whereas in van Buchem's disease there is a mutation in the regulatory region of SOST [54]. Less than 100 cases of sclerosteosis worldwide have been described, characterized by robust bone growth evident by mid-childhood, with strongly fracture-resistant bones, normal, but dense bone architecture and clinical symptoms due to bone overgrowth, such as deafness and neurological abnormalities, mainly intracranial hypertension and cranial nerve paralysis due to the entrapment in their pathologically restricted foramina. Heterozygous gene carriers are clinically asymptomatic and exhibit higher BMD only, therefore suggesting that sclerostin function may be modulated. Buchem's syndrome exhibits less severe clinical manifestations [55]. The discovery of the effects of sclerostin has suggested the development of specific inhibitors for the treatment of osteoporosis, leading to the synthesis of a new humanized MoAb against this glycoprotein, romosozumab. The sclerostin block by romosozumab prevents both its inhibitory function on osteoblasts and therefore on bone formation and the induction of RANKL production by osteoblasts and therefore osteoclastogenesis [56].
Romosozumab (AMG 785) is a MoAb against sclerostin. The antibody is humanized, that is non-human, but the amino-acid sequence is modified to increase similarity with a human antibody. Romosozumab is administered subcutaneously with the absorption of 50-70% and a half-life of 6-7 days. It represents a promising approach for the prevention of fractures. Its clinical potential for fracture prevention in postmenopausal osteoporotic women has been investigated in two recent phase 3 clinical trials, FRAME (Fracture Study in Postmenopausal Women with Osteoporosis) [57] and ARCH (Active-Controlled Fracture Study in Postmenopausal Women with Osteoporosis at High Risk) [58].
FRAME is a randomized trial on osteoporotic women who were given monthly subcutaneous injections of romosozumab 210 mg or placebo one year, followed by denosumab for 12 months in both groups. After one year of therapy, romosozumab has been shown to reduce the risk of vertebral fractures by 75% with an increase of 13% of BMD compared to placebo, showing osteoanabolic activity but also antiresorptive activity. The marked increase in BMD was attributed to increased bone formation and decreased bone resorption. The lower risk of vertebral fracture than placebo was also observed at 24 months after the transition to denosumab in the treated group [59].
The ARCH study is a head-to-head comparative study between romosozumab and alendronate mono-therapy for one year and therefore sequential therapy with alendronate in both groups, with fractures as the primary endpoint. The incidence of new vertebral fractures at one year with romosozumab was significantly lower than bisphosphonate, and at two years the romosozumab and alendronate group had a fracture risk reduction of 48% and 38% respectively at the column and femur compared to the group that had previously hired alendronate. In conclusion, this study definitively demonstrated that in postmenopausal women with osteoporosis at high risk of fracture, treatment with romosozumab for 12 months followed by alendronate significantly reduced the risk of both vertebral and femoral fractures compared to alendronate alone [60, 61]. This is probably due to the alendronate-mediated strengthening of the indirect antiresorbing action of romosozumab, as suggested by the suppression of bone turnover markers by alendronate [57].
Another clinical trial, the STudy evaluating the effect of RomosozUmab Compared with Teriparatide in postmenopaUsal women with osteoporosis at high risk for fracture pReviously treated with bisphosphonatE therapy (STRUCTURE), compared the effects of romosozumab and teriparatide on BMD and bone strength in postmenopausal women previously treated with bisphosphonates. It is, therefore, a head-to-head study to compare the anabolic effect of romosozumab with that of teriparatide in patients already treated with bisphosphonates, representing the most common situation in real life, where the antiosteoporotic drugs administered as a first therapeutic option are more often bisphosphonates. Romosozumab increased vertebral and femoral neck BMD and strength more than teriparatide. In contrast to intermittent PTH, romosozumab increases bone formation but also decreases bone resorption markers [62]. Therefore, the osteoanabolic effect of teriparatide is limited by the concomitant increase in bone resorption; moreover, teriparatide can only be used once for up to 24 months due to safety concerns, in particular, the risk of the onset of osteosarcoma. Abaloparatide, a parathyroid hormone-related protein analog drug used to treat osteoporosis, approved by the Food and Drug Administration (FDA) in the USA but not available in Europe yet, seems to exert a more potent anabolic action than teriparatide, but is accompanied by equally important risks [2, 44].
However, in the ARCH study, a significantly higher number of serious cardiovascular adverse events in the romosozumab group, compared to alendronate, unexpectedly emerged during the first year of treatment. On the other hand, sclerostin is expressed not only at the bone level but also in other tissues, including vascular smooth muscle [61, 62]. After a careful revision of the results from the ARCH study, romosozumab has been finally approved by FDA for the treatment of osteoporosis in postmenopausal women with a high risk of fracture, and it is currently still under review for final approval by the European Medicines Agency (EMA). In any case, since it has been confirmed that the drug can increase the risk of heart attack, stroke and cardiovascular death, it is important to carefully select patients for this therapy, avoiding use in those who have had a heart attack or stroke within the previous year.
4. CYTOKINES, TRANSCRIPTION FACTORS AND STEM CELLS
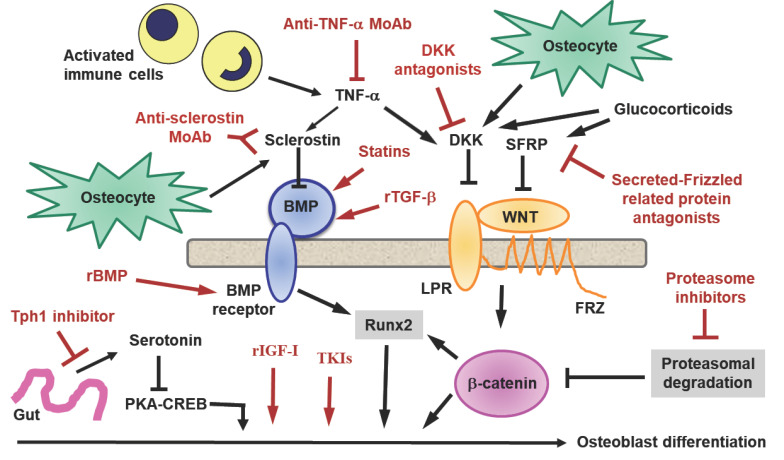
Other potential targets for osteoporosis therapy include growth factors, such as BMPs, Transforming Growth Factor-beta (TGF-β), Growth Hormone (GH), and Insulin-like Growth factor-1 (IGF-1), and against some of them have been produced monoclonal antibodies or recombinant analogues which are currently being tested (Fig. 3). TGF-β effects on bone are based on autocrine and paracrine signalling and extracellular matrix production and ossification, resulting in bone healing. Moreover, through its chemotactic ability, TGF-β recruits osteoprogenitor cells, fibroblasts, and immune cells and at the same time inhibits activation, proliferation, and differentiation of osteoclasts and induces their apoptosis and additionally promotes the development of callus and prevents its premature resorption [63]. GH and its downstream mediator IGF-1 exert their effects on osteogenic cells via binding to their cognate receptor, leading to activation of an array of genes that mediate cellular differentiation and function. Moreover, they both interact with other skeletal regulator hormones, such as sex steroids, thyroid hormone, and parathyroid hormone, to facilitate skeletal growth and metabolism. IGF-1, released from bone matrix, stimulates osteoblastic differentiation of MSCs by activation of mTOR during bone remodeling [64].
Fig. (3).
Other potential targets: cytokines and transcription factors. Other potential targets for osteoporosis therapy have been identified and against some of them have been produced monoclonal antibodies or recombinant analogues. In addition to anti-sclerostin monoclonal antibody (Anti-sclerostin MoAb) and Anti-tumor necrosis factor-α monoclonal antibody (Anti-TNF-α MoAb), other relevant checkpoints of the Wingless/Integrated (Wnt) pathway in osteoblasts seem to be promising targets: Dickkopf-related protein (DKK) antagonists, Proteasome inhibitors, Tyrosynkinase inhibitors (TKIs), Recombinant Bone morphogenetic protein (rBMP), Tryptophan hydroxylase 1 (Tph1), Recombinant transforming growth factor-β (rTGF-β), Recombinant insulin-like growth factor-1 (rIGF-1), Secreted frizzled-related protein 1 (SFRP1) antagonists.
Abbreviations: Tumor Necrosis Factor (TNF), Interleukin-1 (IL-1), Receptor Activator of Nuclear-factor k-B (RANK), Receptor Activator of Nuclear-factor k-B Ligand (RANKL), Osteoprotegerin (OPG), Tumor Necrosis Factor receptor-associated Factor 6 (TRAF6), Inhibitor of nuclear factor Kappa-B Kinases (IKKs), Nuclear factor-kB (NF-kB), Nuclear factor of activated T-cells, cytoplasmic 1 (NFATc1). (A higher resolution / colour version of this figure is available in the electronic copy of the article).
Molecules belonging to the complex Wnt pattern, other than sclerostin, are also promising targets for osteoporosis therapy. Among them, Bone Morphogenetic Protein-2 (BMP-2) plays an important role in the development of bone and cartilage [65]. Like other components of the BMP family, it is able to stimulate osteoblast differentiation from a variety of cell types. Therefore, BMP-2 fundamentally stimulates the production of bone. Recombinant human BMP-2 (rhBMP-2) is currently available and is administered using a variety of delivery system and biomaterial carriers in orthopedic and dentistry procedures [66]. The statins, employed to treat hypercholesterolemia, also exhibit osteoanabolic functions. Statin-like molecules induce BMP-2 and Vascular Endothelial Growth Factor (VEGF) gene expression in osteoblasts and stimulate fracture healing in mice [67]. DKK1 antagonizes the Wnt/β-catenin pathway by reducing β-catenin expression. An anti-DKK1 MoAb (RH2-18) has been developed and its subcutaneous administration increases trabecular BMD in ovariectomized mice and attenuates erosions in rheumatoid arthritis [68]. Conversely, enhancement of DKK1 function may inhibit new bone formation and prevent joint fusion in ankylosing spondylitis [69]. Deregulated Wnt–β-catenin signaling is implicated in the development of osteosarcoma and exerts a crucial role in the pathophysiology of multiple myeloma progression. Increased production of DKK1 by cancer cells is associated with the development of osteolytic lesions in human metastatic bone disease. MoAbs against DKK1 and DKK agonist molecules, as well as proteasomal inhibitors utilised in the therapy of multiple myeloma, such as bortezumab, are able to counteract neoplastic bone resorption [70]. Similarly, tyrosynkinase inhibitors used in the therapy of chronic myeloid leukemia and other neoplasms, such as dasatinib, exert anabolic and anti-resorbent functions [71].
Secreted Frizzled-related Protein 1 (SFRP1) is a member of the SFRP family which acts as a soluble modulator of Wnt signaling. SFRP glycoproteins contain cysteine-rich domains homologous to the Wnt-binding site of Frizzled proteins. SFRP1 is a biphasic modulator of Wnt signaling, counteracting the effects of Wnt at high concentrations, and favoring them at lower concentrations. The gene that encodes SFRP1 is located in a region of chromosome 8 frequently deleted in a variety of cancer, mainly breast cancer suggesting that it may also play a role as a tumor suppressor protein. Small-molecule antagonists to SFRP1 stimulate bone growth in organ culture [72].
The serotonin system has also recently been successfully targeted in mouse models of bone loss through development of a small-molecule Tryptophan Hydroxylase 1 (Tph1) inhibitor. Tph1, regulated by LRP5 in the gut, synthesizes peripheral serotonin that inhibits osteoblast function through the stimulation of a membrane serotoninergic receptor which suppresses the intracytoplasmic transcription factor cAMP Response Element-binding Protein (CREB), thus inhibiting osteoblastic proliferation and bone formation, with consequent osteoporosis. This explains why the antidepressants that selectively inhibit the reuptake of the neurotransmitter serotonin, induce osteoporosis [73]. A synthesized small molecule (LP533401) that does not cross the blood-brain barrier, specifically inhibits intestinal Tph1. Administered orally, it is already being tested to evaluate its effectiveness and safety in humans [74].
Cathepsin K is a lysosomal cysteine protease responsible for the degradation of the organic bone matrix by osteoclasts. Its rare genetic deficiency (pycnodysostosis), is characterized by high BMD, in addition to several skeletal deformities. Several inhibitors of catepsin k have been developed in the last decade and variously tested in experimental settings. Early cathepsin K inhibitors, such as balacatib, demonstrated undesirable off-target effects (skin hardening), that stopped their clinical development. Odanacatib, an orally administered selective inhibitor of cathepsin K, has been successfully tested in several clinical studies, demonstrating a high anti-absorptive action on bone. It reduces the reabsorption mediated by osteoclasts, while the formation mediated by osteoblasts remains unchanged. However, a deeper analysis of a phase III study in post-menopausal women suffering from osteoporosis discovered an increased risk of stroke and its use has not yet been approved [75].
The use of stem cells for bone regeneration is becoming an attractive alternative or additional osteoporosis treatment. Mesenchymal Stem Cells (MSC), from which osteoblasts arise, are multipotent adult stem cells with immunosuppressive properties that are deemed safe for clinical use by the FDA. Bone formation and fracture repair are dependent on the appropriate number and function of resident MSCs. Senile osteoporosis is characterized by both a reduction and a compromised function of resident bone marrow MSCs. Exogenous introduction of MSCs, usually derived from bone marrow, adipose and umbilical cord blood tissues, or treatments with drugs or small compounds able to recruit endogenous stem cells to osteoporotic sites, improves BMD and reduces the susceptibility of fractures, by either increasing the numbers or restoring the function of resident MSCs that can proliferate and differentiate into bone-forming cells [76]. Cell transplantation can be done either locally or systemically. MSCs directly delivered at the site of bone injury in animal models revealed a good survival and retention, leading to bone regeneration. Scaffold carriers, such as biocompatible, biodegradable and non-immunogenic gelatin-based hydrogel polymers, could also be used to support cell viability and function. Human tonsil-derived MSC, embedded in gelatin hydrogel and delivered subcutaneously in ovariectomized mouse models of systemic osteoporosis, have proved useful in inducing trabecular bone structure recovery and improvement of serum markers of bone turnover [77].
5. SMALL MOLECULES, NANOPARTICLES AND NATURAL PRODUCTS
Small non-coding nucleotide Ribonucleic Acids (RNAs) are important regulators of bone turnover. Among them, microRNAs (miRNAs) and short interfering RNAs (siRNAs) have shown potential therapeutic value for osteoporosis. They exert important epigenetic modification, by mediating post-transcriptional regulation of target genes, cell differentiation and apoptosis. Several miRNAs have been discovered which are involved in the regulation of osteoblast and osteoclast differentiation, by targeting to bone-related genes and different signaling pathways. RANKL is directly regulated by miR-338-3p. In experimental settings, miRNAs 20a-5p and mmu-miR-17-5p suppress glucocorticoid-induced osteoclast differentiation and inhibit RANKL in osteoblasts. Similarly, mmumiR-29a-3p protects rats from glucocorticoid-induced bone loss by regulating Wnt and its inhibitor Dkk-1 and inhibiting the histone deacetylase-4, thus restoring the acetylation state of b-catenin and Runx2, and improving osteoblastic mineralization. Other miRNAs, hsamiR-133a-3p and hsa-miR-194-5p, negatively regulate osteoclast related gene expression and represent possible biomarkers in postmenopausal osteoporosis. Interestingly, hsa-miR-382-3p and hsa-miR-550a-5p seem to be the most representative circulating miRNAs in diabetic bone disease, whereas hsa-miR-382-3p and hsa-miR-188-3p are the most important circulating miRNAs in postmenopausal osteoporosis, suggesting the possibility of exploiting differentiated and personalized target therapies in the management of patients suffering from specific forms of osteoporosis. The expression of mmu-miR-705 is significantly enhanced in ovariectomized mice through the TNF-α activated NF-kB pathway. Since complete inhibition of NF-kB pathway, which represents a major target for osteoporosis treatment, may be harmful in clinics because of its great importance for immune system functions, the modulation of its downstream signaling through specific miRNAs could be an alternative promising therapeutic strategy for osteoporosis [78]. Resveratrol, a polyphenolic phytoestrogen with osteogenic properties, prevents osteoporosis in ovariectomized rats by suppressing the expression of miR-338-3p and increasing the expression of Runx2. Curcumin improves bone microarchitecture by activating miRNA-365 targeting matrix metalloproteinase-9 in a glucocorticoid-induced osteoporosis mice model. Since miRNA may cause off-target effects, such as unintended gene knockdown and inflammation-related cell toxicity, the full understanding of the function of miRNAs involved in various bone physiological and pathological activities must represent the first step for the development of specific and accurate gene-silencing therapeutics [79]. Moreover, since miRNAs and siRNAs are prone to nuclease degradation, it is necessary to facilitate their cellular uptake to the target sites by idoneous delivery systems which protect them from premature nuclease degradation. To improve the stability of miRNAs and siRNAs as therapeutic agents, specific chemical modifications have been proposed. For example, PEGylated nanoparticles incorporating targeting ligands could be effective for avoiding reticuloendothelial system clearance, thus prolonging the circulation time of miRNA and siRNA delivery systems. Lipid-based and polymer-based systems are currently the major categories of drug delivery systems used for silencing gene expression at post-transcriptional levels by siRNA in osteoporosis. An
Legend to table/Abreviations: SERMs (selective estrogen receptor modulators); rhPTH 1-34 (recombinant human parathyroid hormone 1-34); rhPTHrP (recombinant human parathyroid hormone-related peptide); Anti-TNFα MoAbs (anti-tumor necrosis factor-alpha monoclonal antibodies); TGF-β (transforming growth factor-beta); GH (growth hormone); IGF-1 (insulin-like growth factor-1); rhBMP-2 (recombinant human bone morphogenetic protein-2); Anti-DKK1 MoAb (anti-Dickkopf-related protein 1 monoclonal antibody); Tph-1 (tryptophan hydroxylase-1); SFRP1 (secreted frizzled-related protein 1); MSCs (mesenchimal stem cells); sncRNAs (small non-coding ribonucleic acids); OCP (osteoclast progenitors); OBP (oteoblast progenitors); RANKL (receptor activator of nuclear factor kappa-Β ligand); OB (osteoblast); OC (osteoclast); OPG (osteoprotegerin); VEGF (vascular endothelial growth factor); HMG-CoA (3-hydroxy-3-glutaryl-coenzyme A); cAMP-PKA (cyclic AMP-dependent protein kinase A); RUNX2 (Runt-related transcription factor 2); Wnt (Wingless/Integrated); mTOR (mammalian target of rapamycin); LRP6 (Low-density lipoprotein receptor-related protein); CREB (cAMP response element-binding protein); NF-𝜅B (nuclear factor-kB); ROS (reactive oxygen species) osteogenic siRNA targeting casein kinase-2 interacting protein-1, encompassed into liposomes, promotes a significant increase of bone formation in both healthy and osteoporotic rats. The same siRNA has been encapsulated into more efficient osteoblast-specific aptamer-functionalized lipid nanoparticles to promote its selective uptake and induce bone formation [80]. A site-specific bone-targeting system based on an octa-aspartic acid sequence and bisphosphonates, strongly binds to hydroxyapatite in the bone. The epigenetic regulation of osteoblastic cell function by synthetic hydroxyapatite nanoparticles is significantly different in osteoblastic cells derived from osteoporotic rat bone compared to the healthy ones, with significant implication on defining design parameters for potential therapeutic use of nanomaterials [81]. Farnesyl pyrophosphate synthase, the enzyme of the mevalonate pathway targeted by nitrogen-containing bisphosphonates, such as alendronate, could be selectively inhibited by specific siRNAs, leading to osteoclast-mediated bone resorption inhibition and bone mass maintenance promotion without negative effects on the proliferation of preosteoblasts. Both the efficacy and duration of silencing effect after miRNA or siRNA administration depend on several factors (rate of RNA release from the delivery system, stability of the RNA molecules, type of target tissue and turnover rate of the target protein). Incorporation of miRNA or siRNA inside injectable biodegradable matrices might facilitate their localization to the sites of bone defects. Orthopaedic applications for therapeutics RNAs have the advantage that RNAs can be administered topically into the surgical site for repair in combination with a device. The addition of targeting moieties also improves the efficacy of RNA delivery. The most commonly used moieties for osteoporosis therapy are bisphosphonates, bone-specific oligopeptides and aptamers, all characterized by high bone affinity [82]. Water-dispersible magnetic nanoparticles with radiofrequency-induced thermogenic properties, such as bisphosphonate-conjugated iron oxide nanoparticles, suitable for incorporation into osteoclasts, have been developed with the potential of limiting the aggravation of osteoporosis by reducing the activity of osteoclasts through thermolysis [83].
Several natural products also exert bone protective effects without exhibiting significant toxicity or deleterious side effects. Inflammation-modifying foods, alone or associated with other therapies, could be useful to prevent osteoporosis. The bone protecting effects of vitamin D are well known, but also other compounds, such as vitamin C, B12 and folates seem to exert beneficial anti-inflammatory and metabolic effects on bone health. Both L-arginine and vitamin E coadministration reduces TNF-α and IL-6 levels induced by zinc oxide nanoparticle administration in rats, counteract its deleterious effects on bone turn over [84]. Tart cherry (Prunus cerasus), a fruit rich in polyphenols, such as flavonols, hydroxycinnamic acids, proanthocyanidins and anthocyanidins, has been reported to display antioxidant and anti-inflammatory properties and could be used as a supplement to prevent inflammation-mediated bone loss in TNF-overexpressing transgenic mice [85]. Traditional Chinese medicines comprising herbal formulas are an interesting alternative to prevent osteoporosis because of their effective bone protecting properties and the lower reported side effects compared with synthetic drugs. Zhuanggu Guanjie pill is a traditional Chinese medicine formula consisting of 12 herbs including rhizoma drynariae, radix dipsaci, rhizoma cibotii and fructus psoraleae, which have beneficial effects in osteoporosis patients [86]. BHH10, a traditional Korean herbal medicine including three herbs (A. membranaceus, C. cassia, and P. amurense), has also proved effective and safe [87]. However, overall, the evidence for the use of herbal medicine in patients with osteoporosis is still inconsistent.
Table 1 summarizes the relevant osteometabolic mechanisms of action of drugs, both approved and experimental, for osteoporosis therapy.
Table I.
Summary of characteristics of several approved and experimental agents for treating osteoporosis.
| Therapeutic Agents | Mechanism of Action | |
|---|---|---|
| Antiresorptive | Osteoanabolic | |
| Estrogens [88] | ↓ osteoclastogenic cytokines ↓ RANKL ↑ OPG |
↑ OB proliferation ↓ OB apoptosis ↑ IGF and TGFβ |
| Bisphosphonates [14] | ↓ farnesyl pyrophosphate synthase ↓ OC function and life span |
- |
| SERMs [15] | ↓ OC recruitment | ↑ osteoblastogenesis ↑ TGF-β |
| Calcitonin [12] | ↓ OC proteolytic enzyme secretion | - |
| Statins [67] | ↓ HMG-CoA reductase in OCs ↓ mevalonate pathway in OCs |
↑ BMP-2 and VEGF in OBs |
| Strontium ranelate [88] | ↓ osteoclastogenesis ↑ OPG |
↑ calcium-sensing receptor stimulation ↑ osteoblastogenesis |
| Teriparatide (rhPTH 1-34) Abaloparatide (rhPTHrP) [16] | - | ↑ PTH1R signaling in OBs ↑ cAMP-PKA dependent intracellular Ca2+ IGF-1, ↑ IGF-2, TGF-β, Wnt, RUNX2 |
| Anti-TNFα MoAbs [28] | ↓ inflammatory osteoclastogenesis | ↓ sclerostin and DKK1 ↑ Wnt signaling |
| Denosumab [41] | ↓ RANKL signaling in OCs | - |
| Romosozumab [56] | ↓ RANKL production in OBs | ↓ sclerostin ↑ Wnt signaling in OBs |
| TGF-β [63] | ↓ OC function and survival | ↑ OBP recruitment ↑ extracellular matrix and ossification |
| GH [64] | - | ↑ OB differentiation and function |
| IGF-1 [64] | - | ↑ mTOR activation ↑ OB differentiation |
| rhBMP-2 [66] | - | ↑ osteoblastogenesis |
| Anti-DKK1 MoAb [68] | - | ↑ LRP6 coreceptor signaling ↑ Wnt/β-catenin pathway |
| Src tyrosynkinase inhibitors [71] | ↓ Src kinase activity ↓ OC survival and function |
↑ OB differentiation ↓ Src kinase expression |
| Tph-1 inhibitor LP533401 [74] | - | ↓ peripheral serotonin biosynthesis ↑ CREB in OBs ↑ bone formation |
| Proteasomal inhibitors [70] | ↓ NF-𝜅B signaling ↓ RANKL induced osteoclastogenesis |
- |
| SFRP1 antagonists [72] | - | ↓ SFRP1 activity ↑ Wnt signaling |
| Odanacatib [75] | ↓ cathepsin K activity in OCs | - |
| MSCs [76] | - | ↑ bone forming cell number and function |
| sncRNAs [78, 79] | - | - |
| miRNAs 20a-5p mmu-miR-17-5p |
↓ RANKL ↓ osteoclastogenesis |
- |
| mmumiR-29a-3p | - | ↓ Dkk-1 and histone deacetylase-4 ↑ β-catenin and Runx2 acetylation ↑ OB mineralization |
| hsa-miR-133a-3p hsa-miR-194-5p |
↓ OC related gene expression ↓ NF-kB pathway signaling |
- |
| Vitamins and natural products [85, 88] | ↓ ROS ↓ inflammatory bone resorption |
- |
conclusion
In conclusion, despite the current availability of antiresorptive and anabolic drugs of proven efficacy for osteoporosis [88], not all patients respond or tolerate them and the search for new drugs is ongoing. Most of their biological targets physiologically intervene in many immunological functions, such as defense against infections and tumors or the modulation of allergic and autoimmune reactions [89, 90]. The aim is therefore to tailor treatments for osteoporosis that are both effective and safe.
Fig. (1).
Mechanism of action of denosumab. Denosumab is a humanized monoclonal antibody capable of neutralizing RANKL, a cytokine produced by osteoclasts and immune cells, that interacts with the RANK receptor on the membrane of osteoclast precursors and mature osteoclasts, where it blocks the activation cascade of intracellular transcription factors thus affecting osteoclast recruitment, maturation, and survival. Denosumab, therefore, suppresses bone resorption by inhibiting both osteoclast formation and function.
Abbreviations: Wingless/Integrated (Wnt), Receptor activator of nuclear-factor k-B ligand (RANKL), Frizzled proteins (FRZ), Low-density lipoprotein receptor proteins 5 and 6 (LPR 5/6). (A higher resolution / colour version of this figure is available in the electronic copy of the article).
Fig. (2).
Mechanism of action of romosozumab. The Wnt pathway is activated by the interaction between LRP5/6, Wnt and Frizzled. As a consequence, b-catenin is released, enters the nucleus and activates transcription from Wnt target genes. Sclerostin binding to LRP5/6 and Frizzled coreceptors on osteoblasts inhibits Wnt canonical pathway and b-catenin is consequently phosphorylated and degraded. Sclerostin inhibits bone formation by inhibiting osteoblasts and increases bone resorption by increasing RANKL production by osteoblasts. Romosozumab inhibits sclerostin allowing for increased Wnt signaling. Sclerostin inactivates the Wnt pathway by binding to LRP5/6 and b-catenin is consequently phosphorylated and degraded.
Abbreviations: Low-density lipoprotein receptor proteins (LRP), Runt-related transcription factor 2 (Runx2), Protein kinase A (PKA), cAMP response element-binding protein (CREB). (A higher resolution / colour version of this figure is available in the electronic copy of the article).
Acknowledgements
Declared none.
LIST OF ABBREVIATIONS
- AFF
Atypical Femoral Fractures
- BMD
Bone Mineral Density
- BMPs
Bone Morphogenetic Proteins
- cAMP-PKA
Cyclic AMP-dependent Protein Kinase A
- CREB
cAMP Response Element-binding Protein
- CTX
C-terminal Telopeptide
- DBPC
Double Blind Placebo Controlled
- DKK1
Dickkopf-related Protein 1
- DXA
Dual-energy x-ray Absorptiometry
- EMA
European Medicines Agency
- FDA
Food and Drug Administration
- FZR
Frizzled Proteins
- GH
Growth Hormone
- HMG-CoA
3-hydroxy-3-glutaryl-coenzyme A
- IFN-γ
Interferon-γ
- IGF-1
Insulin-like Growth Factor-1
- IKKs
Inhibitor of Nuclear Factor Kappa-B Kinases
- IL
Interleukin
- LPR 5/6
Low Density Lipoprotein Receptor Proteins 5 and 6
- MCS
Mesenchimal Stem Cell
- M-CSF
Macrophage-colony Stimulating Growth Factor
- miRNAs
microRNAs
- MoAb
Monoclonal Antibody
- mTOR
Mammalian Target of Rapamycin
- NFATc1
Nuclear Factor of Activated T-cells, Cytoplasmic 1
- NF-kB
Nuclear Factor-kB
- OBP
Oteoblast Progenitors
- OC
Osteocalcin
- OCP
Osteoclast Progenitors
- ONJ
Osteonecrosis of the Jaw
- OPG
Osteoprotegerin
- PKA
Protein Kinase A
- RANKL
Receptor Activator of Nuclear Factor-κB Ligand
- rhBMP-2
Recombinant Human BMP-2
- rhPTHrP
Recombinant Human Parathyroid Hormone-related Peptide
- rIGF
Recombinant Insulin-like Growth Factor-1
- RNAs
Ribonucleic Acids
- ROS
Reactive Oxygen Species
- rPTH
Recombinant Human Parathyroid Hormone Analogue
- rTGFβ
Recombinant Transforming Growth Factor-β
- RUNX2
Runt-related Transcription Factor 2
- SERMs
Selective Estrogen Receptor Modulators
- SFRPs
Secreted-Frizzled Related Proteins
- siRNAs
Short Interfering RNAs
- sncRNAs
Small Non-coding Ribonucleic Acids
- SOST
Sclerostin
- TGF-β
Transforming Growth Factor- β
- TKI
Tyrosynkinase Inhibitors
- TNF-α
Tumour Necrosis Factor-α
- Tph1
Tryptophan Hydroxylase 1
- TRAF6
Tumor Necrosis Factor Receptor Associated Factor 6
- VEGF
Vascular Endothelial Growth Factor
- Wnt
Wingless/Integrated
Consent for Publication
Not applicable.
Funding
None.
Conflict of Interest
The authors declare no conflict of interest, financial or otherwise.
REFERENCES
- 1.Ensrud K.E., Crandall C. J. Osteoporosis. Ann. Intern. Med. 2017;167(3):17–32. doi: 10.7326/AITC201708010. [DOI] [PubMed] [Google Scholar]
- 2.Akkawi I., Zmerly H. Osteoporosis: Current Concepts. Joints. 2018;6(2):122–127. doi: 10.1055/s-0038-1660790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nuti R., Brandi M.L., Checchia G., Di Munno O., Dominguez L., Falaschi P., Fiore C.E., Iolascon G., Maggi S., Michieli R., Migliaccio S., Minisola S., Rossini M., Sessa G., Tarantino U., Toselli A., Isaia G.C. Guidelines for the management of osteoporosis and fragility fractures. Intern. Emerg. Med. 2019;14(1):85–102. doi: 10.1007/s11739-018-1874-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hernlund E., Svedbom A., Ivergård M., Compston J., Cooper C., Stenmark J., McCloskey E.V., Jönsson B., Kanis J.A. Osteoporosis in the European Union: medical management, epidemiology and economic burden. A report prepared in collaboration with the International Osteoporosis Foundation (IOF) and the European Federation of Pharmaceutical Industry Associations (EFPIA). Arch. Osteoporos. 2013;8:136. doi: 10.1007/s11657-013-0136-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.De Martinis M., Di Benedetto M.C., Mengoli L.P., Ginaldi L. Senile osteoporosis: is it an immune-mediated disease? Inflamm. Res. 2006;55(10):399–404. doi: 10.1007/s00011-006-6034-x. [DOI] [PubMed] [Google Scholar]
- 6.Bultink I.E.M. Bone disease in connective tissue disease/systemic lupus erythematosus. Calcif. Tissue Int. 2018;102(5):575–591. doi: 10.1007/s00223-017-0322-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.De Martinis M., Ciccarelli F., Sirufo M.M., Ginaldi L. An overview of environmental risk factors in systemic sclerosis. Expert Rev. Clin. Immunol. 2016;12(4):465–478. doi: 10.1586/1744666X.2016.1125782. [DOI] [PubMed] [Google Scholar]
- 8.Panday K., Gona A., Humphrey M.B. Medication-induced osteoporosis: screening and treatment strategies. Ther. Adv. Musculoskelet. Dis. 2014;6(5):185–202. doi: 10.1177/1759720X14546350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Franceschi C., Garagnani P., Parini P., Giuliani C., Santoro A. Inflammaging: a new immune-metabolic viewpoint for age-related diseases. Nat. Rev. Endocrinol. 2018;14(10):576–590. doi: 10.1038/s41574-018-0059-4. [DOI] [PubMed] [Google Scholar]
- 10.Okamoto K., Nakashima T., Shinohara M., Negishi-Koga T., Komatsu N., Terashima A., Sawa S., Nitta T., Takayanagi H. Osteoimmunology: the conceptual framework unifying the immune and skeletal systems. Physiol. Rev. 2017;97(4):1295–1349. doi: 10.1152/physrev.00036.2016. [DOI] [PubMed] [Google Scholar]
- 11.Ginaldi L., De Martinis M. Osteoimmunology and beyond. Curr. Med. Chem. 2016;23(33):3754–3774. doi: 10.2174/0929867323666160907162546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Harvey N.C.W., McCloskey E.V., Mitchell P.J., Dawson-Hughes B., Pierroz D.D., Reginster J.Y., Rizzoli R., Cooper C., Kanis J.A. Mind the (treatment) gap: a global perspective on current and future strategies for prevention of fragility fractures. Osteoporos. Int. 2017;28(5):1507–1529. doi: 10.1007/s00198-016-3894-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cotts K.G., Cifu A.S. Treatment of osteoporosis. JAMA. 2018;319(10):1040–1041. doi: 10.1001/jama.2017.21995. [DOI] [PubMed] [Google Scholar]
- 14.Kanis J.A., Reginster J.Y., Kaufman J.M., Ringe J.D., Adachi J.D., Hiligsmann M., Rizzoli R., Cooper C. A reappraisal of generic bisphosphonates in osteoporosis. Osteoporos. Int. 2012;23(1):213–221. doi: 10.1007/s00198-011-1796-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Martinkovich S., Shah D., Planey S.L., Arnott J.A. Selective estrogen receptor modulators: tissue specificity and clinical utility. Clin. Interv. Aging. 2014;9:1437–1452. doi: 10.2147/CIA.S66690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Leder B.Z. Parathyroid hormone and parathyroid hormone-related protein analogs in osteoporosis therapy. Curr. Osteoporos. Rep. 2017;15(2):110–119. doi: 10.1007/s11914-017-0353-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lewiecki E.M. Denosumab in postmenopausal osteoporosis: what the clinician needs to know. Ther. Adv. Musculoskelet. Dis. 2009;1(1):13–26. doi: 10.1177/1759720X09343221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kenkre J.S., Bassett J. The bone remodelling cycle. Ann. Clin. Biochem. 2018;55(3):308–327. doi: 10.1177/0004563218759371. [DOI] [PubMed] [Google Scholar]
- 19.Takayanagi H. Osteoimmunology in 2014: Two-faced immunology-from osteogenesis to bone resorption. Nat. Rev. Rheumatol. 2015;11(2):74–76. doi: 10.1038/nrrheum.2014.219. [DOI] [PubMed] [Google Scholar]
- 20.Srivastava R.K., Dar H.Y., Mishra P.K. Immunoporosis: immunology of osteoporosis-role of T cells. Front. Immunol. 2018;9:657. doi: 10.3389/fimmu.2018.00657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Amjadi-Moheb F., Akhavan-Niaki H. Wnt signaling pathway in osteoporosis: Epigenetic regulation, interaction with other signaling pathways, and therapeutic promises. J. Cell. Physiol. 2019;••• doi: 10.1002/jcp.28207. [DOI] [PubMed] [Google Scholar]
- 22.Uehara S., Udagawa N., Kobayashi Y. Non-canonical Wnt signals regulate cytoskeletal remodeling in osteoclasts. Cell. Mol. Life Sci. 2018;75(20):3683–3692. doi: 10.1007/s00018-018-2881-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Witcher P.C., Miner S.E., Horan D.J., Bullock W.A., Lim K.E., Kang K.S., Adaniya A.L., Ross R.D., Loots G.G., Robling A.G. Sclerostin neutralization unleashes the osteoanabolic effects of Dkk1 inhibition. JCI Insight. 2018;3(11):98673. doi: 10.1172/jci.insight.98673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Delgado-Calle J., Sato A.Y., Bellido T. Role and mechanism of action of sclerostin in bone. Bone. 2017;96:29–37. doi: 10.1016/j.bone.2016.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Colditz J., Thiele S., Baschant U., Garbe A.I., Niehrs C., Hofbauer L.C., Rauner M. Osteogenic Dkk1 mediates glucocorticoid-induced but not arthritis-induced bone loss. J. Bone Miner. Res. 2019;34(7):1314–1323. doi: 10.1002/jbmr.3702. [DOI] [PubMed] [Google Scholar]
- 26.Ciccarelli F., De Martinis M., Ginaldi L. Glucocorticoids in patients with rheumatic diseases: friends or enemies of bone? Curr. Med. Chem. 2015;22(5):596–603. doi: 10.2174/0929867321666141106125051. [DOI] [PubMed] [Google Scholar]
- 27.Zhang J., Fu Q., Ren Z., Wang Y., Wang C., Shen T., Wang G., Wu L. Changes of serum cytokines-related Th1/Th2/Th17 concentration in patients with postmenopausal osteoporosis. Gynecol. Endocrinol. 2015;31(3):183–190. doi: 10.3109/09513590.2014.975683. [DOI] [PubMed] [Google Scholar]
- 28.Suzuki T., Nakamura Y., Kato H. Effects of denosumab on bone metabolism and bone mineral density with anti-TNF inhibitors, tocilizumab, or abatacept in osteoporosis with rheumatoid arthritis. Ther. Clin. Risk Manag. 2018;14:453–459. doi: 10.2147/TCRM.S156350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Saribal D., Hocaoglu-Emre F.S., Erdogan S., Bahtiyar N., Caglar Okur S., Mert M. Inflammatory cytokines IL-6 and TNF-α in patients with hip fracture. Osteoporos. Int. 2019;30(5):1025–1031. doi: 10.1007/s00198-019-04874-2. [DOI] [PubMed] [Google Scholar]
- 30.Ginaldi L., De Martinis M., Ciccarelli F., Saitta S., Imbesi S., Mannucci C., Gangemi S. Increased levels of interleukin 31 (IL-31) in osteoporosis. BMC Immunol. 2015;16:60. doi: 10.1186/s12865-015-0125-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Holgado A., Braun H., Van Nuffel E., Detry S., Schuijs M.J., Deswarte K., Vergote K., Haegman M., Baudelet G., Haustraete J., Hammad H., Lambrecht B.N., Savvides S.N., Afonina I.S., Beyaert R. IL-33trap is a novel IL-33-neutralizing biologic that inhibits allergic airway inflammation. J. Allergy Clin. Immunol. 2019;144(1):204–215. doi: 10.1016/j.jaci.2019.02.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ginaldi L., De Martinis M., Saitta S., Sirufo M.M., Mannucci C., Casciaro M., Ciccarelli F., Gangemi S. Interleukin-33 serum levels in postmenopausal women with osteoporosis. Sci. Rep. 2019;9(1):3786. doi: 10.1038/s41598-019-40212-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.De Martinis M., Sirufo M.M., Ginaldi L. Allergy and aging: an old/new emerging health issue. Aging Dis. 2017;8(2):162–175. doi: 10.14336/AD.2016.0831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dar H.Y., Azam Z., Anupam R., Mondal R.K., Srivastava R.K. Osteoimmunology: the Nexus between bone and immune system. Front. Biosci. 2018;23:464–492. doi: 10.2741/4600. [DOI] [PubMed] [Google Scholar]
- 35.van Dam P.A., Verhoeven Y., Trinh X.B., Wouters A., Lardon F., Prenen H., Smits E., Baldewijns M., Lammens M. RANK/RANKL signaling inhibition may improve the effectiveness of checkpoint blockade in cancer treatment. Crit. Rev. Oncol. Hematol. 2019;133:85–91. doi: 10.1016/j.critrevonc.2018.10.011. [DOI] [PubMed] [Google Scholar]
- 36.Massimini M., Palmieri C., De Maria R., Romanucci M., Malatesta D., De Martinis M., Maniscalco L., Ciccarelli A., Ginaldi L., Buracco P., Bongiovanni L., Della Salda L. 17-AAG and apoptosis, autophagy, and mitophagy in canine osteosarcoma cell lines. Vet. Pathol. 2017;54(3):405–412. doi: 10.1177/0300985816681409. [DOI] [PubMed] [Google Scholar]
- 37.Infante M., Fabi A., Cognetti F., Gorini S., Caprio M., Fabbri A. RANKL/RANK/OPG system beyond bone remodeling: involvement in breast cancer and clinical perspectives. J. Exp. Clin. Cancer Res. 2019;38(1):12. doi: 10.1186/s13046-018-1001-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shupp A.B., Kolb A.D., Mukhopadhyay D., Bussard K.M. Cancer metastases to bone: concepts, mechanisms, and interactions with bone osteoblasts. Cancers (Basel) 2018;10:3390. doi: 10.3390/cancers10060182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vishal M., Swetha R., Thejaswini G., Arumugam B., Selvamurugan N. Role of Runx2 in breast cancer-mediated bone metastasis. Int. J. Biol. Macromol. 2017;99:608–614. doi: 10.1016/j.ijbiomac.2017.03.021. [DOI] [PubMed] [Google Scholar]
- 40.Sigl V., Jones L.P., Penninger J.M. RANKL/RANK: from bone loss to the prevention of breast cancer. Open Biol. 2016;6(11):160230. doi: 10.1098/rsob.160230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Baron R., Ferrari S., Russell R.G. Denosumab and bisphosphonates: different mechanisms of action and effects. Bone. 2011;48(4):677–692. doi: 10.1016/j.bone.2010.11.020. [DOI] [PubMed] [Google Scholar]
- 42.Bone H.G., Wagman R.B., Brandi M.L., Brown J.P., Chapurlat R., Cummings S.R., Czerwiński E., Fahrleitner-Pammer A., Kendler D.L., Lippuner K., Reginster J.Y., Roux C., Malouf J., Bradley M.N., Daizadeh N.S., Wang A., Dakin P., Pannacciulli N., Dempster D.W., Papapoulos S. 10 years of denosumab treatment in postmenopausal women with osteoporosis: results from the phase 3 randomised FREEDOM trial and open-label extension. Lancet Diabetes Endocrinol. 2017;5(7):513–523. doi: 10.1016/S2213-8587(17)30138-9. [DOI] [PubMed] [Google Scholar]
- 43.Cummings S.R., San Martin J., McClung M.R., Siris E.S., Eastell R., Reid I.R., Delmas P., Zoog H.B., Austin M., Wang A., Kutilek S., Adami S., Zanchetta J., Libanati C., Siddhanti S., Christiansen C. FREEDOM Trial. Denosumab for prevention of fractures in postmenopausal women with osteoporosis. N. Engl. J. Med. 2009;361(8):756–765. doi: 10.1056/NEJMoa0809493. [DOI] [PubMed] [Google Scholar]
- 44.Lorentzon M. Treating osteoporosis to prevent fractures: current concepts and future developments. J. Intern. Med. 2019;285(4):381–394. doi: 10.1111/joim.12873. [DOI] [PubMed] [Google Scholar]
- 45.Zanchetta M.B., Boailchuk J., Massari F., Silveira F., Bogado C., Zanchetta J.R. Significant bone loss after stopping long-term denosumab treatment: a post FREEDOM study. Osteoporos. Int. 2018;29(1):41–47. doi: 10.1007/s00198-017-4242-6. [DOI] [PubMed] [Google Scholar]
- 46.Leder B.Z., Tsai J.N., Neer R.M., Uihlein A.V., Wallace P.M., Burnett-Bowie S.A. Response to therapy with teriparatide, denosumab, or both in postmenopausal women in the DATA (denosumab and teriparatide administration) study randomized controlled trial. J. Clin. Densitom. 2016;19(3):346–351. doi: 10.1016/j.jocd.2016.01.004. [DOI] [PubMed] [Google Scholar]
- 47.Leder B.Z., Tsai J.N., Jiang L.A., Lee H. Importance of prompt antiresorptive therapy in postmenopausal women discontinuing teriparatide or denosumab: The Denosumab and Teriparatide Follow-up study (DATA-Follow-up). Bone. 2017;98:54–58. doi: 10.1016/j.bone.2017.03.006. [DOI] [PubMed] [Google Scholar]
- 48.Leder B.Z. Optimizing sequential and combined anabolic and antiresorptive osteoporosis therapy. JBMR Plus. 2018;2(2):62–68. doi: 10.1002/jbm4.10041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fusco V., Santini D., Armento G., Tonini G., Campisi G. Osteonecrosis of jaw beyond antiresorptive (bone-targeted) agents: new horizons in oncology. Expert Opin. Drug Saf. 2016;15(7):925–935. doi: 10.1080/14740338.2016.1177021. [DOI] [PubMed] [Google Scholar]
- 50.Suh Y.S., Jang B.W., Nho J.H., Won S.H., Lee W.S. Atypical incomplete femoral neck fracture in patients taking long-term bisphosphonate: Case report, a report of 2 cases. Medicine (Baltimore) 2019;98(9):e14701. doi: 10.1097/MD.0000000000014701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lou S., Lv H., Yin P., Li Z., Tang P., Wang Y. Combination therapy with parathyroid hormone analogs and antiresorptive agents for osteoporosis: a systematic review and meta-analysis of randomized controlled trials. Osteoporos. Int. 2019;30(1):59–70. doi: 10.1007/s00198-018-4790-4. [DOI] [PubMed] [Google Scholar]
- 52.Bhattacharyya S., Pal S., Chattopadhyay N. Targeted inhibition of sclerostin for post-menopausal osteoporosis therapy: A critical assessment of the mechanism of action. Eur. J. Pharmacol. 2018;826:39–47. doi: 10.1016/j.ejphar.2018.02.028. [DOI] [PubMed] [Google Scholar]
- 53.Chang B., Quan Q., Li Y., Qiu H., Peng J., Gu Y. Treatment of osteoporosis, with a focus on 2 monoclonal antibodies. Med. Sci. Monit. 2018;24:8758–8766. doi: 10.12659/MSM.912309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Clarke B.L. Anti-sclerostin antibodies: utility in treatment of osteoporosis. Maturitas. 2014;78(3):199–204. doi: 10.1016/j.maturitas.2014.04.016. [DOI] [PubMed] [Google Scholar]
- 55.van Lierop A.H., Hamdy N.A., Hamersma H., van Bezooijen R.L., Power J., Loveridge N., Papapoulos S.E. Patients with sclerosteosis and disease carriers: human models of the effect of sclerostin on bone turnover. J. Bone Miner. Res. 2011;26(12):2804–2811. doi: 10.1002/jbmr.474. [DOI] [PubMed] [Google Scholar]
- 56.Robling A.G., Drake M.T., Papapoulos S.E. Sclerostin: From bedside to bench, and back to bedside. Bone. 2017;96:1–2. doi: 10.1016/j.bone.2017.01.019. [DOI] [PubMed] [Google Scholar]
- 57.Lewiecki E.M., Dinavahi R.V., Lazaretti-Castro M., Ebeling P.R., Adachi J.D., Miyauchi A., Gielen E., Milmont C.E., Libanati C., Grauer A. One year of romosozumab followed by two years of denosumab maintains fracture risk reductions: results of the FRAME extension study. J. Bone Miner. Res. 2019;34(3):419–428. doi: 10.1002/jbmr.3622. [DOI] [PubMed] [Google Scholar]
- 58.Cosman F., Crittenden D.B., Adachi J.D., Binkley N., Czerwinski E., Ferrari S., Hofbauer L.C., Lau E., Lewiecki E.M., Miyauchi A., Zerbini C.A., Milmont C.E., Chen L., Maddox J., Meisner P.D., Libanati C., Grauer A. Romosozumab treatment in postmenopausal women with osteoporosis. N. Engl. J. Med. 2016;375(16):1532–1543. doi: 10.1056/NEJMoa1607948. [DOI] [PubMed] [Google Scholar]
- 59.Cosman F., Crittenden D.B., Ferrari S., Khan A., Lane N.E., Lippuner K., Matsumoto T., Milmont C.E., Libanati C., Grauer A. FRAME study: the foundation effect of building bone with 1 year of romosozumab leads to continued lower fracture risk after transition to denosumab. J. Bone Miner. Res. 2018;33(7):1219–1226. doi: 10.1002/jbmr.3427. [DOI] [PubMed] [Google Scholar]
- 60.Langdahl B.L., Libanati C., Crittenden D.B., Bolognese M.A., Brown J.P., Daizadeh N.S., Dokoupilova E., Engelke K., Finkelstein J.S., Genant H.K., Goemaere S., Hyldstrup L., Jodar-Gimeno E., Keaveny T.M., Kendler D., Lakatos P., Maddox J., Malouf J., Massari F.E., Molina J.F., Ulla M.R., Grauer A. Romosozumab (sclerostin monoclonal antibody) versus teriparatide in postmenopausal women with osteoporosis transitioning from oral bisphosphonate therapy: a randomised, open-label, phase 3 trial. Lancet. 2017;390(10102):1585–1594. doi: 10.1016/S0140-6736(17)31613-6. [DOI] [PubMed] [Google Scholar]
- 61.Saag K.G., Petersen J., Brandi M.L., Karaplis A.C., Lorentzon M., Thomas T., Maddox J., Fan M., Meisner P.D., Grauer A. Romosozumab or alendronate for fracture prevention in women with osteoporosis. N. Engl. J. Med. 2017;377(15):1417–1427. doi: 10.1056/NEJMoa1708322. [DOI] [PubMed] [Google Scholar]
- 62.Genant H.K., Engelke K., Bolognese M.A., Mautalen C., Brown J.P., Recknor C., Goemaere S., Fuerst T., Yang Y.C., Grauer A., Libanati C. Effects of romosozumab compared with teriparatide on bone density and mass at the spine and hip in postmenopausal women with low bone mass. J. Bone Miner. Res. 2017;32(1):181–187. doi: 10.1002/jbmr.2932. [DOI] [PubMed] [Google Scholar]
- 63.Wu M., Chen G., Li Y.P. TGF-β and BMP signaling in osteoblast, skeletal development, and bone formation, homeostasis and disease. Bone Res. 2016;4:16009. doi: 10.1038/boneres.2016.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Xian L., Wu X., Pang L., Lou M., Rosen C.J., Qiu T., Crane J., Frassica F., Zhang L., Rodriguez J.P., Jia X., Yakar S., Shouhong X., Efstratiadis A., Wan M., Cao X. IGF-1 released from bone matrix stimulates osteoblastic differentiation of MSCs by activation of mTOR during bone remodeling. Nat. Med. 2012;18(7):1095–1101. doi: 10.1038/nm.2793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Irelli A., Sirufo M.M., Scipioni T., De Pietro F., Pancotti A., Ginaldi L., De Martinis M. mTOR links tumor immunity and bone metabolism: what are the clinical implications? Int. J. Mol. Sci. 2019;20(23):5841. doi: 10.3390/ijms20235841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.de Queiroz Fernandes J., de Lima V.N., Bonardi J.P., Filho O.M., Queiroz S.B.F. Bone regeneration with recombinant human bone morphogenetic protein 2: a systematic review. J. Maxillofac. Oral Surg. 2018;17(1):13–18. doi: 10.1007/s12663-016-0988-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Oryan A., Kamali A., Moshiri A. Potential mechanisms and applications of statins on osteogenesis: Current modalities, conflicts and future directions. J. Control. Release. 2015;215:12–24. doi: 10.1016/j.jconrel.2015.07.022. [DOI] [PubMed] [Google Scholar]
- 68.Ruaro B., Casabella A., Paolino S., Pizzorni C., Ghio M., Seriolo C., Molfetta L., Odetti P., Smith V., Cutolo M. Dickkopf-1 (Dkk-1) serum levels in systemic sclerosis and rheumatoid arthritis patients: correlation with the Trabecular Bone Score (TBS). Clin. Rheumatol. 2018;37(11):3057–3062. doi: 10.1007/s10067-018-4322-9. [DOI] [PubMed] [Google Scholar]
- 69.Wu M., Chen M., Ma Y., Yang J., Han R., Yuan Y., Hu X., Wang M., Zhang X., Xu S., Liu R., Jiang G., Xu J., Shuai Z., Zou Y., Pan G., Pan F. Dickkopf-1 in ankylosing spondylitis: Review and meta-analysis. Clin. Chim. Acta. 2018;481:177–183. doi: 10.1016/j.cca.2018.03.010. [DOI] [PubMed] [Google Scholar]
- 70.Jin Y., Xu L., Wu X., Feng J., Shu M., Gu H., Gao G., Zhang J., Dong B., Chen X. Synergistic efficacy of the demethylation agent decitabine in combination with the protease inhibitor bortezomib for treating multiple myeloma through the Wnt/βcatenin pathway. Oncol. Res. 2019;27(6):729–737. doi: 10.3727/096504018X15443011011637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wang M., Park S., Nam Y., Nielsen J., Low S.A., Srinivasarao M., Low P.S. Bone-fracture-targeted dasatinib-oligoaspartic acid conjugate potently accelerates fracture repair. Bioconjug. Chem. 2018;29(11):3800–3809. doi: 10.1021/acs.bioconjchem.8b00660. [DOI] [PubMed] [Google Scholar]
- 72.Gaur T., Wixted J.J., Hussain S., O’Connell S.L., Morgan E.F., Ayers D.C., Komm B.S., Bodine P.V., Stein G.S., Lian J.B. Secreted frizzled related protein 1 is a target to improve fracture healing. J. Cell. Physiol. 2009;220(1):174–181. doi: 10.1002/jcp.21747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Schweiger J.U., Schweiger U., Hüppe M., Kahl K.G., Greggersen W., Jauch-Chara K., Fassbinder E. The use of antidepressive agents and bone mineral density in women: a meta-analysis. Int. J. Environ. Res. Public Health. 2018;15(7):15. doi: 10.3390/ijerph15071373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Yadav V.K., Balaji S., Suresh P.S., Liu X.S., Lu X., Li Z., Guo X.E., Mann J.J., Balapure A.K., Gershon M.D., Medhamurthy R., Vidal M., Karsenty G., Ducy P. Pharmacological inhibition of gut-derived serotonin synthesis is a potential bone anabolic treatment for osteoporosis. Nat. Med. 2010;16(3):308–312. doi: 10.1038/nm.2098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Bone H.G., Dempster D.W., Eisman J.A., Greenspan S.L., McClung M.R., Nakamura T., Papapoulos S., Shih W.J., Rybak-Feiglin A., Santora A.C., Verbruggen N., Leung A.T., Lombardi A. Odanacatib for the treatment of postmenopausal osteoporosis: development history and design and participant characteristics of LOFT, the long-term odanacatib fracture trial. Osteoporos. Int. 2015;26(2):699–712. doi: 10.1007/s00198-014-2944-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Antebi B., Pelled G., Gazit D. Stem cell therapy for osteoporosis. Curr. Osteoporos. Rep. 2014;12(1):41–47. doi: 10.1007/s11914-013-0184-x. [DOI] [PubMed] [Google Scholar]
- 77.Kim G., Park Y.S., Lee Y., Jin Y.M., Choi D.H., Ryu K.H., Park Y.J., Park K.D., Jo I. Tonsil-derived mesenchymal stem cell embedded in situ crosslinkable gelatin hydrogel therapy recovers postmenopausal osteoporosis through bone regeneration. PLoS One. 2018;13(7):e0200111. doi: 10.1371/journal.pone.0200111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Yang Y., Fang S. Small non-coding RNAs-based bone regulation and targeting therapeutic strategies. Mol. Cell. Endocrinol. 2017;456:16–35. doi: 10.1016/j.mce.2016.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Feng Q., Zheng S., Zheng J. The emerging role of microRNAs in bone remodeling and its therapeutic implications for osteoporosis. Biosci. Rep. 2018;38(3):BSR20180453. doi: 10.1042/BSR20180453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Liang C., Guo B., Wu H., Shao N., Li D., Liu J., Dang L., Wang C., Li H., Li S., Lau W.K., Cao Y., Yang Z., Lu C., He X., Au D.W., Pan X., Zhang B.T., Lu C., Zhang H., Yue K., Qian A., Shang P., Xu J., Xiao L., Bian Z., Tan W., Liang Z., He F., Zhang L., Lu A., Zhang G. Aptamer-functionalized lipid nanoparticles targeting osteoblasts as a novel RNA interference-based bone anabolic strategy. Nat. Med. 2015;21(3):288–294. doi: 10.1038/nm.3791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Zhao R., Xie P., Zhang K., Tang Z., Chen X., Zhu X., Fan Y., Yang X., Zhang X. Selective effect of epigenetic regulation of osteoblastic cell function by HANPs has significant implication on defining design parameters for a potential therapeutic use of nanomaterials. Acta Biomater. 2017;59:338–350. doi: 10.1016/j.actbio.2017.07.009. [DOI] [PubMed] [Google Scholar]
- 82.Tucker W.O., Kinghorn A.B., Fraser L.A., Cheung Y.W., Tanner J.A. Selection and characterization of a DNA aptamer specifically targeting human HECT ubiquitin ligase WWP1. Int. J. Mol. Sci. 2018;19(3):19. doi: 10.3390/ijms19030763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Lee M.S., Su C.M., Yeh J.C., Wu P.R., Tsai T.Y., Lou S.L. Synthesis of composite magnetic nanoparticles Fe3O4 with alendronate for osteoporosis treatment. Int. J. Nanomedicine. 2016;11:4583–4594. doi: 10.2147/IJN.S112415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Abdelkarem H.M., Fadda L.H., El-Sayed E.M., Radwan O.K. Potential role of L-arginine and vitamin E against bone loss induced by nano-zinc oxide in rats. J. Diet. Suppl. 2018;15(3):300–310. doi: 10.1080/19390211.2017.1343889. [DOI] [PubMed] [Google Scholar]
- 85.Moon N., Effiong L., Song L., Gardner T.R. Soung, DY Tart Cherry prevents bone loss through inhibition of RANKL in TNF-overexpressing mice. Nutrients. 2019;11:63. doi: 10.3390/nu11010063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Chai L.J., Zhang Y., Zhang P.Y., Bi Y.N., Yuan X.M., Li Y.H., Wang Y.Y., Song L., Sun L.K., Zhou K. The antiosteoporosis effects of Zhuanggu Guanjie Pill in vitro and in vivo. BioMed Res. Int. 2018;5:1–11. doi: 10.1155/2018/9075318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Cho Y., Lee S., Kim J., Kang J.W., Baek Y.H., Seo B.K., Lee J.D. The efficacy and safety of herbal medicine BHH10 in postmenopausal women with osteoporosis: study protocol for a phase II, multicenter, randomized, double-blinded, placebo-controlled clinical trial. Trials. 2018;19(1):482. doi: 10.1186/s13063-018-2854-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Tabatabaei-Malazy O., Salari P., Khashayar P., Larijani B. New horizons in treatment of osteoporosis. Daru. 2017;25(1):2. doi: 10.1186/s40199-017-0167-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ciccarelli F., De Martinis M., Sirufo M.M., Ginaldi L. Psoriasis induced by anti-Tumor Necrosis Factor-alpha agents: a comprehensive review of the literature. Acta Dermatovenerol. Croat. 2016;24(3):169–174. [PubMed] [Google Scholar]
- 90.Moseley K.F., Naidoo J., Bingham C.O., Carducci M.A., Forde P.M., Gibney G.T., Lipson E.J., Shah A.A., Sharfman W.H., Cappelli L.C. Immune-related adverse events with immune checkpoint inhibitors affecting the skeleton: a seminal case series. J. Immunother. Cancer. 2018;6(1):104. doi: 10.1186/s40425-018-0417-8. [DOI] [PMC free article] [PubMed] [Google Scholar]