Abstract
Background
Lung cancer is the neoplasm with the highest prevalence and mortality rates in the world. Most patients with lung cancer that are symptomatic have hemoptysis, coughing, shortness of breath, chest pain and persistent infections. Less than 10% of patients are asymptomatic when the tumor is detected as an incidental finding.
Objective
The present expert review aims to describe the use of radiological imaging modalities for the diagnosis of lung cancer.
Methods
Some papers were selected from the international literature, by using mainly Pubmed as a source.
Results
Chest x-ray (CXR) is the first investigation performed during the workup of suspected lung cancer. In the absence of a rib erosion, CXR cannot distinguish between benign and malignant masses, therefore computed tomography (CT) with contrast enhancement should be performed in order to obtain a correct staging. Magnetic resonance imaging of the chest is considered a secondary approach as the respiratory movement affects the overall results.
Conclusion
Radiological imaging is essential for the management of patients affected by lung cancer.
Keywords: Chest X-ray, computed tomography, MRI, lung cancer, radiological imaging, carcinoid tumour
1. INTRODUCTION
Lung cancer is the neoplasm with the highest prevalence and mortality rates in the world (1.35 million new cases and 1.8 million deaths per year), with a higher frequency in the US and Europe. Epidemiological data indicate that the main risk factor for the development of lung cancer is cigarette smoking. Other risk factors include, but are not limited to, hydrocarbon and industrial pollution, radon, asbestos, uranium chromium, chronic obstructive pulmonary disease (or COPD), and lung fibrosis.
Although the majority of patients present at an advanced stage, those with early-stage lung cancer may be treated with potentially curative intent. Therefore, the importance of early diagnosis and an appropriate radiological staging cannot be underestimated.
Most patients with lung cancer that are symptomatic have hemoptysis, coughing, shortness of breath, chest pain and persistent infections [1]. Symptoms related to skeletal pain and neurological manifestations suggest metastatic involvement. Less than 10% of patients are asymptomatic when the tumor is detected as an incidental finding. The purpose of the present review is to assess the role of radiological imaging for the identification and management of patients with lung cancer.
2. LUNG CANCER CLASSIFICATION
Classifications of lung cancer by the anatomic site are:
- central lung cancer: mostly squamous cell carcinoma and small cell carcinoma.
- peripheral lung cancer: mostly adenocarcinoma and large cell carcinoma.
Classification to Histopathology:
- Small Cell Lung cancer (SCLC 15-20%, small cell carcinoma)
- Non-Small cell Lung Cancer (NSCLC, 80-85%, squamous cell carcinoma 30-40%, adenocarcinoma, large cell carcinoma 10%)
Other malignant pulmonary neoplasms include lymphoma and sarcoma (rare).
The histopathological classification is helpful in defining the prognosis, facilitating the treatment and the prediction of successful results. The effectiveness of screening for lung cancer with computed tomography (CT) in terms of reducing mortality is still a controversial argument. The study observed a growth of the number of cases diagnosed without a consistent reduction of the number of tumors in advanced state and death rates. Infante et al. [2] did not report any benefit of screening versus mortality, meanwhile American results have demonstrated a reduction of 20% of the mortality for lung cancer in the individual under screening with CT with respect to those with a conventional x-ray. Magnetic resonance imaging (MRI) of the chest is considered a secondary approach because of the respiratory movement affecting the overall results.
Small cell lung cancer is strongly associated with cigarette smoking and derived from neuroendocrine cells. May be related to paraneoplastic syndromes. SCLC is among the most aggressive primary lung cancers. More than 70% of patients show evidence of extrathoracic metastatic disease at the time of diagnosis [3].
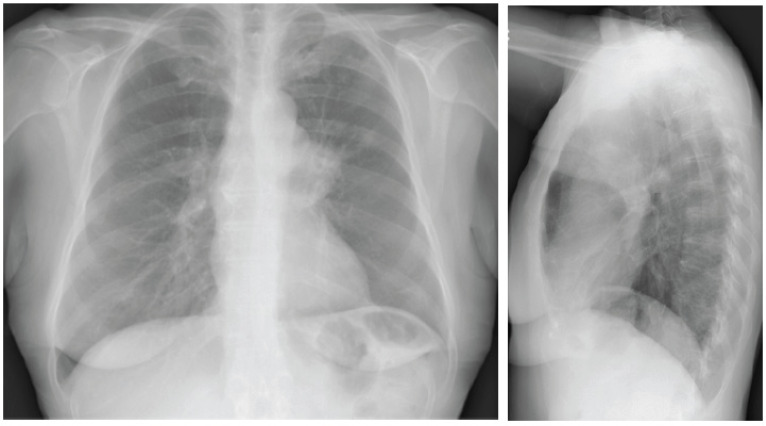
Approximately 90% of SCLCs occur centrally and usually arise in a lobar bronchus like hilar or perihilar masses. Appearance on chest x-ray is non-specific (Fig. 1). They may be seen as a hilar/perihilar mass with mediastinal widening due to lymph node enlargement.
Fig. (1).
Chest X-ray shows the prominence of left hilum and LUL nodular opacity. (A higher resolution / colour version of this figure is available in the electronic copy of the article).
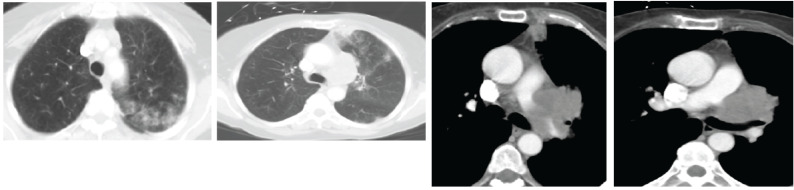
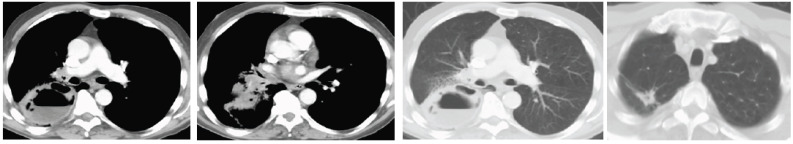
On CT, mediastinal involvement may appear similar to lymphoma (Figs. 2 and 3), with numerous enlarged nodes. In most cases, SCLC produces necrosis or hemorrhages, superior vena cava (SVC) infiltration or SVC obstruction, and paraneoplastic syndromes.
Fig. (2).
CT shows two spiculated nodules in the left upper lobe (LUL). (Left) Diffuse emphysema and patchy infiltrate in the posterior aspect of LUL. (Right) Bulky lymphadenopathy in the left hilum. (A higher resolution / colour version of this figure is available in the electronic copy of the article).
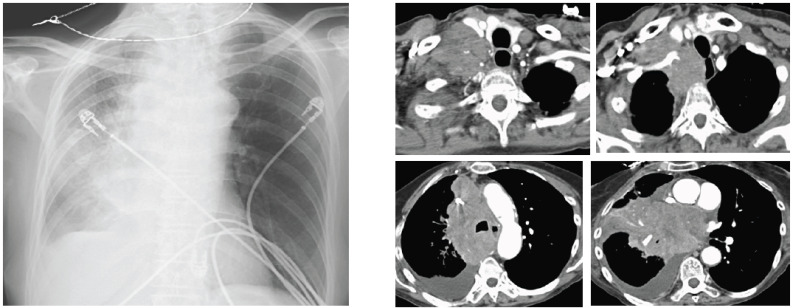
Fig. (3).
(Left) Chest X-ray shows mediastinal enlargement. (Right) CT shows the right mediastinal mass and supraclavicular metastatic lymph nodes. (A higher resolution / colour version of this figure is available in the electronic copy of the article).
Squamous cell carcinoma represents 30% of all lung cancers [4]. It arises from altered bronchial epithelium and growths in situ. It is related to cigarette smoking.
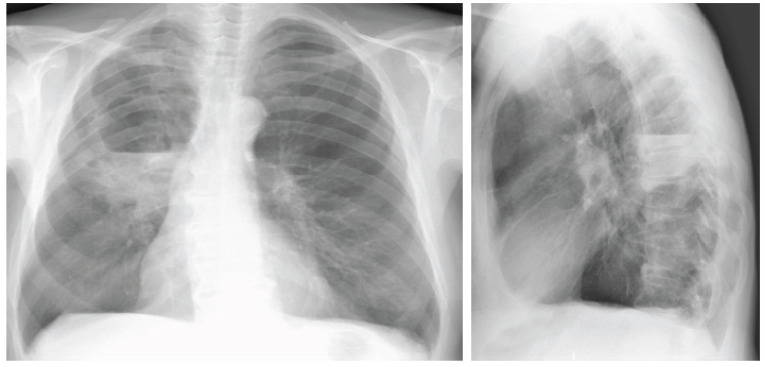
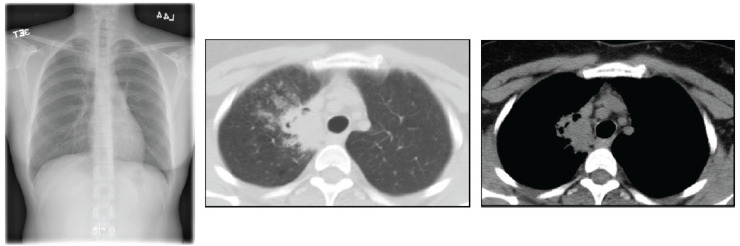
These tumors are more often centrally located within the lung and may grow much larger than 4 cm in diameter. Cavitations are seen in up to 82%. They frequently cause segmental or lobar lung collapse due to their central location and relative frequency (Figs. 4 and 5). They are the most common cause of the Pancost or superior sulcus syndrome.
Fig. (4).
Chest X-ray shows consolidation in the right lung with cavitations. (A higher resolution / colour version of this figure is available in the electronic copy of the article).
Fig. (5).
(left) CT shows a right lower lobe cavitary lesion and a right-sided adenopathy. (Right) In the right upper lobe, there is a metastatic spiculated nodule. (A higher resolution / colour version of this figure is available in the electronic copy of the article).
Adenocarcinoma including AIS (ex bronchoalveolar cell carcinoma) arises from the submucosal glands. It is considered the commonest subtype in a young woman and non-smokers [5]. In the majority of cases, it appears as a parenchymal nodule with dimensions of less than 3 centimeters, less frequently like a mass with a diameter larger than 3 centimeters.
Both types can infiltrate the lymph node. It can also show an opacity that is able to produce a consolidation in a lobe or whole lung. This tumor frequently gives metastasis to the liver, bone, nervous system, within the same lung or the contralateral lung and adrenal glands [6]. There are two different radiological presentations of bronchoalveolar cell carcinomas. The most common is a focal solitary peripheral nodule which is often indistinguishable from other lung cancers. This appearance is more frequently seen with non-mucinous tumours. Thin-section CT may demonstrate a halo of ground-glass opacification surrounding the nodule but overt consolidation is usually absent (Fig. 6). The prognosis of this type of tumour is significantly better than other lung cancers [7].
Fig. (6).
(Left) Chest X-ray shows a consolidation in paramediastinal upper lobe. (right) CT shows a right upper lobe consolidation with a surrounding infiltration. (A higher resolution / colour version of this figure is available in the electronic copy of the article).
Sub-solid lesions can be associated with lung adenocarcinoma [8]. A recently revised version of the International Association for the Study of Lung Cancer (IASLC)/ American Thoracic Society (ATS)/European Respiratory Society (ERS) has been published in order to provide a homogeneous terminology, specific diagnostic criteria, and a better risk stratification. The description of the classification is out of the scope of the present review. For further details, the readers are invited to read the report by Zheng et al. [9]. The terms adenocarcinoma in situ (AIS), minimally invasive carcinoma (MIA), and lepidic predominant adenocarcinoma (LPA) are usually employed for adenocarcinomas with a lepidic or non-invasive growth. However, the term “bronchioloalveolar” (or bronchoalveolar) carcinoma has been eliminated [10].
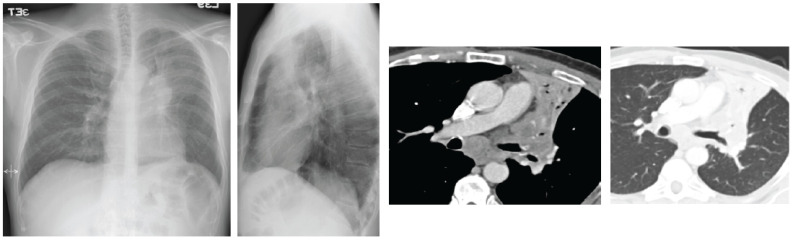
Carcinoid tumours are only 1% of all lung cancer [11] (Fig. 7). Atypical carcinoid tumours are generally large (typically 2.5 cm at CT) and are often carcinoid tumours linked with endobronchial growth and obstructive pneumonia [12]. Carcinoids are often central and their diagnosis is usually made by histological analysis, after excluding an adenocarcinomatous or squamous cell adenocarcinoma [11]. They can grow extremely rapidly and develop early metastases in the mediastinum and in the brain. Notably, two recent trials reported the prevalence of adenocarcinoma of 78% and 58% and squamous cell carcinomas recorded just 4% and 11% [13] as compared to the carcinoid tumour.
Fig. (7).
(left) Chest X-ray shows lobar atelectasis in the left para hilar region. CT shows a consolidation with endo-bronchial growth and mediastinal lymphadenopathy. (A higher resolution / colour version of this figure is available in the electronic copy of the article).
Large Cell carcinoma is strongly associated with smoking. They can be quite large with a rate of excavation of at least 6%. It has the tendency to produce an early large volume hilar (30%) and mediastinal (10%) lymph node enlargement [14].
3. BASELINE RADIOLOGICAL EXAMINATION: CHEST X-RAY
Chest x-ray (CXR) is the first investigation performed during the workup of suspected lung cancer. It has found extensive use in the past for widespread availability, technical feasibility, low risk and low cost. Once a suspicious lesion is detected, more detailed morphological information is required. Lung tumors may present as central or peripheral masses, even those of the in situ adenocarcinoma [15] that may present as an area of chronic air space disease [16]. The central neoplasm may have hilar lymph node enlargement, mediastinal invasion or bronchial obstruction, with partial or total lung collapse; a parenchymal consolidation or super-infection may exist, which may mask or be the first sign of a possible underlying neoplasm.
The persistence over time of such findings should alert the radiologists.
The mediastinal involvement of central neoplasia presents itself as an enlargement of the mediastinal profile on CXR. More invasive features such as the invasion of the phrenic nerve or the superior vena cava cannot be evaluated by CXR. Most asymptomatic pulmonary neoplasms present themselves as single or peripheral nodules; they can have well defined or spiculated margins. Occasionally internal nodularity and air crescents may also be seen.
In absence of rib erosions, CXR cannot distinguish between benign or malignant masses. It can detect the presence of pleural effusions, but even this does not allow determining the benign or malignant nature of the lesion.
4. COMPUTED TOMOGRAPHY
When the CXR raises the suspicion for malignancy, CT with contrast should be performed for complete staging. CT assists in finding abnormalities, highlights signs of disease within the lungs from symptoms like cough, chest pain and fever. It is often used to monitor the response to treatment and as a support to plan the radiation therapy program. CT has recently been indicated as a screening tool in carefully selected populations of patients. CT plays a role of primary importance in the definition of local invasiveness (sensitivity: 62-93% vs. CXR: 1-2,7%; [5-8]), while it has limits in the evaluation of mediastinal and thoracic pleural invasion [14, 15, 17, 18].
Minimum diameter increase over time has been shown to positively correlate with a significant volume increase, an index of malignancy. An increase of about 26% in the diameter of the lesion corresponds to a doubling of the volume and the doubling of the diameter corresponds to an increase in the volume of a factor of 8. If the volume of nodules doubles in less than 7 days, the lesion is going to be benign (usually indicative of inflammation/infection).
Nodal involvement evaluation on CT is based on dimensional criteria. Traditionally lymph node involvement is diagnosed when the short axis is greater than 10 mm [17-19].
The presence of adenopathy less than 10 mm limits the sensitivity of CT (about 57%;18) [20]. From a recent meta-analysis, it emerged that sensitivity, specificity, accuracy, and positive and negative predictive values detective for the staging of mediastinal lymph node involvement in lung cancer were 33-75%; 66-90%; 65-79%; 46-55%; 68-85%, respectively [21].
The evaluation of distant metastasis (in particular of the liver and the adrenal glands) is the rationale behind including the upper abdomen in the staging CT.
Staging with CT allows for the determination of resectability of the lesion (neoplasms with a stage equal or higher than IIIB are not resectable).
Radiology plays an extremely important role in the management of lung cancer patients, can accurately detail the extent of cancer, and has a great impact on deciding treatment plans.
CONCLUSION
Radiological imaging is essential for the management of patients affected by lung cancer. It has an important role in the initial staging of disease, able to characterize the spread of the disease, thus guiding the correct therapeutic approach.
Acknowledgements
The authors are thankful to Laura Evangelista MD PhD for her help.
LIST OF ABBREVIATIONS
- ATS
American Thoracic Society
- COPD
Chronic Obstructive Pulmonary Disease
- CT
Computed Tomography
- CXR
Chest X-Ray
- ERS
European Respiratory Society
- IASLC
International Association for the Study of Lung Cancer
- MRI
Magnetic Resonance Imaging
- NSCLC
Non-Small Cell Lung Cancer
- SCLC
Small Cell Lung Cancer
- SVC
Superior Vena Cava
Consent for Publication
Not applicable.
Funding
None.
Conflict of Interest
The authors declare no conflict of interest, financial or otherwise.
REFERENCES
- 1.Buccheri G., Ferrigno D. Lung cancer: clinical presentation and specialist referral time. Eur. Respir. J. 2004;24(6):898–904. doi: 10.1183/09031936.04.00113603. [DOI] [PubMed] [Google Scholar]
- 2.Infante M., Lutman F.R., Cavuto S., Brambilla G., Chiesa G., Passera E., Angeli E., Chiarenza M., Aranzulla G., Cariboni U., Alloisio M., Incarbone M., Testori A., Destro A., Cappuzzo F., Roncalli M., Santoro A., Ravasi G. Lung cancer screening with spiral CT: baseline results of the randomized DANTE trial. Lung Cancer. 2008;59(3):355–363. doi: 10.1016/j.lungcan.2007.08.040. [DOI] [PubMed] [Google Scholar]
- 3.Ioannou G. 1.; Doust, J.; Rockey, D.C. Terlipressin for acute esophageal variceal hemorrhage. Cochrane Database Syst. Rev. 2003;(1):CD00214. doi: 10.1002/14651858.CD002147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dhand R., Tobin M.J. Inhaled bronchodilator therapy in mechanically ventilated patients Respir. Crit. Care Med. 1997;156(1):3–10. doi: 10.1164/ajrccm.156.1.9610025. [DOI] [PubMed] [Google Scholar]
- 5.Owonikoko T.K., Ragin C.C., Belani C.P., Oton A.B., Gooding W.E., Taioli E., Ramalingam S.S. Lung cancer in elderly patients: an analysis of the surveillance, epidemiology, and end results database. J. Clin. Oncol. 2007;25:5570–5577. doi: 10.1200/JCO.2007.12.5435. [DOI] [PubMed] [Google Scholar]
- 6.Parkin D.M., Bray F., Ferlay J., Pisani P. Global cancer statistics, 2002. CA Cancer J. Clin. 2005;55:74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- 7.Okubo K., Mark E.J., Flieder D., Wain J.C., Wright C.D., Moncure A.C., Grillo H.C. Mathisen, D.J. Bronchoalveolar carcinoma: clinical, radiologic, and pathologic factors and survival. J. Thorac. Cardiovasc. Surg. 1999;118:702–709. doi: 10.1016/S0022-5223(99)70016-4. [DOI] [PubMed] [Google Scholar]
- 8.Saito H., Yamada K., Hamanaka N., Oshita F., Ito H., Nakayama H., Yokose T., Kameda Y., Noda K. Initial findings and progression of lung adenocarcinoma on serial computed tomography scans. J. Comput. Assist. Tomogr. 2009;33(1):42–48. doi: 10.1097/RCT.0b013e3181633509. [DOI] [PubMed] [Google Scholar]
- 9.Zheng M. Classification and Pathology of Lung Cancer. Surg. Oncol. Clin. N. Am. 2016;25(3):447–468. doi: 10.1016/j.soc.2016.02.003. [DOI] [PubMed] [Google Scholar]
- 10.Travis W.D. Pathology of lung cancer. Clin. Chest Med. 2011;32(4):669–692. doi: 10.1016/j.ccm.2011.08.005. [DOI] [PubMed] [Google Scholar]
- 11.The American Thoracic Society and The European Respiratory Society Pretreatment evaluation of non-small-cell lung cancer. Am. J. Respir. Crit. Care Med. 1997;156(1):320–332. doi: 10.1164/ajrccm.156.1.ats156.1. [DOI] [PubMed] [Google Scholar]
- 12.Forster B.B., Müller N.L., Miller R.R., Nelems B., Evans K.G. Neuroendocrine carcinomas of the lung: clinical, radiologic, and pathologic correlation. Radiology. 1989;170(2):441–445. doi: 10.1148/radiology.170.2.2536187. [DOI] [PubMed] [Google Scholar]
- 13.Henschke C.I., McCauley D.I., Yankelevitz D.F., Naidich D.P., McGuinness G., Henschke C.I. McCauley. D.I.; Yankelevitz. D.F.; Naidich. D.P.; McGuinness. G.; Miettinen, O.S.; Libby, D.M.; Pasmantier, M.W.; Koizumi, J.; Altorki, N.K.; Smith, J.P. Early lung cancer action project: overall design and findings from baseline screening. Lancet. 1999;354:99–105. doi: 10.1016/S0140-6736(99)06093-6. [DOI] [PubMed] [Google Scholar]
- 14.Martini N., Kris M.G., Flehinger B.J., Gralla R.J., Bains M.S., Burt M.E., Heelan R., McCormack P.M., Pisters K.M., Rigas J.R. Preoperative chemotherapy for stage IIIa (N2) lung cancer: the Sloan-Kettering experience with 136 patients. Ann. Thorac. Surg. 1993;55:1365–1374. doi: 10.1016/0003-4975(93)91072-u. [DOI] [PubMed] [Google Scholar]
- 15.Travis W.D., Brambilla E., Nicholson A.G., Yatabe Y., Austin J.H.M., Beasley M.B., Chirieac L.R., Dacic S., Duhig E., Flieder D.B., Geisinger K., Hirsch F.R., Ishikawa Y., Kerr K.M. 14, Noguchi, M.; Pelosi, G.; Powell, C.A.; Tsao, M.S.; Wistuba, I.; WHO Panel. The 2015 World Health organization classification of lung tumors: impact of genetic, clinical and radiologic advances since the 2004 classification. J. Thorac. Oncol. 2015;10:1243–1260. doi: 10.1097/JTO.0000000000000630. [DOI] [PubMed] [Google Scholar]
- 16.Onitsuka H., Tsukuda M., Araki A., Murakami J., Torii Y., Masuda K. Differentiation of central lung tumor from postobstructive lobar collapse by rapid sequence computed tomography. J. Thorac. Imaging. 1991;6:28–31. doi: 10.1097/00005382-199104000-00007. [DOI] [PubMed] [Google Scholar]
- 17.Silvestri G.A., Gould M.K., Margolis M.L., Tanoue L.T., McCrory D., Toloza E., Detterbeck F. 2007. American College of Chest Physicians. [DOI] [PubMed] [Google Scholar]
- 18.Detterbeck F.C., Jantz M.A., Wallace M., Vansteenkiste J., Silvestri G.A. 2007. [DOI] [PubMed] [Google Scholar]
- 19.Lewis J.W., Jr, Pearlberg J.L., Beute G.H., Alpern M., Kvale P.A., Gross B.H., Magilligan D.J., Jr Can computed tomography of the chest stage lung cancer? Yes and no. Ann. Thorac. Surg. 1990;49(4):591–595. doi: 10.1016/0003-4975(90)90306-Q. [DOI] [PubMed] [Google Scholar]
- 20.Glazer G.M., Gross B.H., Quint L.E., Francis I.R., Bookstein F.L., Orringer M.B. Normal mediastinal lymph nodes: number and size according to American Thoracic Society mapping. AJR Am. J. Roentgenol. 1985;144(2):261–265. doi: 10.2214/ajr.144.2.261. [DOI] [PubMed] [Google Scholar]
- 21.Kramer H., Groen H.J. Current concepts in the mediastinal lymph node staging of nonsmall cell lung cancer. Ann. Surg. 2003;238(2):180–188. doi: 10.1097/01.SLA.0000081086.37779.1a. [DOI] [PMC free article] [PubMed] [Google Scholar]