Abstract
Background
Accurate staging is crucial for the proper management of patients with non-small cell lung cancer, especially for choosing the best treatment strategy. Different Imaging methods are used to stage patients with non-small cell lung cancer. In the last two decades, FDG PET/CT is carried out in almost all the main Hospitals around the world in this setting.
Objective
The aim of this paper is to focus on the value of integrated FDG PET/CT in the TNM staging of the non-small cell lung cancer.
Methods
A non-systematic revision of the literature was performed in order to identify all papers about the role of FDG PET/CT in the evaluation of non-small cell lung cancer and to highlight the value of FDG PET/CT in this setting.
Results
Many data are now available about this topic, including also randomized controlled trials. FDG PET/CT is of limited added value in the characterization of T status but it increases the diagnostic accuracy for the assessment of the nodal status. The main advantage of FDG PET/CT over conventional imaging methods is its higher sensitivity in identifying extra-thoracic metastases, especially bone and adrenal lesions.
Conclusion
PET/CT with FDG should be included in the diagnostic work-up of patients with lung cancer, because it provides useful information for appropriate therapy.
Keywords: Lung cancer, PET/CT, FDG, staging, therapy, diagnostic workup, imaging
1. Introduction
Lung cancer is the main cause of cancer-related deaths in men and women worldwide [1] and non-small cell lung cancer (NSCLC) accounts for nearly 85% of all lung cancers [2]. Accurate staging is important for the proper management of patients with NSCLC, especially for choosing the best treatment strategy and predicting prognosis. The TNM staging system, which is maintained by the American Joint Committee on Cancer and the International Union Against Cancer, is the established, uniform method of staging lung cancer [3].
The optimal imaging technique for lung cancer staging requires accurate characterization of the primary tumor (T), and precise assessment of local (N) and distant tumor spread (M).
The aim of this paper is to focus on the value of integrated 18F-Fluorodeoxyglucose (FDG) positron emission tomography (PET)/computed tomography (CT) in the TNM staging of the NSCLC. FDG PET/CT has been widely adopted in many international guidelines as a non-invasive technique for lung cancer staging [4, 5]. In a randomized controlled trial, the authors compared the role of conventional staging alone and conventional staging plus FDG-PET/CT in patients with lung cancer. A lower rate of thoracotomies was reported in the PET/CT arm as well as a lower rate of futile thoracotomies [6]. A further prospective, randomized multicenter trial including 337 patients compared staging with conventional imaging versus FDG PET/CT. The authors showed that by staging with FDG PET/CT, 13.8% of patients were correctly upstaged and avoided inappropriate surgery, compared with 6.8% in the conventional imaging group (P=0.046). FDG PET/CT and brain imaging identified more precisely those patients with mediastinal and extra-thoracic disease [7].
Therefore, a widespread consensus exists to use FDG PET/CT for all patients with NSCLC being considered for curative-intent therapy. If patients with early-stage disease are accurately staged using FDG PET/CT, inappropriate surgery is avoided [7-10].
The whole body acquisition is the method of choice for FDG PET/CT Imaging, in order to identify unknown distant metastases. A recent multicenter trial suggested, however “limited-area tumor imaging” (s-PET/CT), in order to reduce patient dose while maintaining diagnostic information. A whole-body FDG PET/CT may be reserved only for high-risk patients with intense FDG uptake and chest node involvement as seen on s-PET/CT [11].
2. T staging
FDG PET/CT is of limited added value in the characterization of T status in patients with NSCLC, already undergoing contrast-enhanced CT for initial staging. In selected cases, FDG PET/CT may be advantageous to CT in delineating tumors associated with extensive atelectasis and may detect areas of invasion not identified by CT.
Some authors state that the standardized uptake value (SUV), the semiquantitative assessment of metabolic activity of the primary tumor seen on FDG-PET may be an independent prognostic factor [12, 13].
In a recent paper published by Cheng G and Huang H, Volume-based PET parameters such as metabolic tumor volume and total lesion glycolysis resulted in reflecting the metabolic status more accurately than SUVmax, and to be better prognostic markers in lung cancer [14]. In contrast to SUVmax, these parameters incorporate both metabolic activity and 3D tumor volume, allowing a more accurate assessment of real tumor burden. The authors stated that the incorporation of quantitative FDG activity in tumor staging may allow better risk stratification and better treatment outcomes in the future. Moreover, a recent paper by Kirienko et al. [15] has demonstrated that the radiomic features can be able to differentiate between primary and metastatic lung lesions and therefore, it can be useful to discriminate also the different histopathological patterns.
3. N staging
After the exclusion of distant metastases, nodal status is the most important determinant of management and prognosis. Patients deemed to have node-negative clinical stage I or II diseases are frequently offered radical curative treatment, while patients with confirmed mediastinal (N2) nodal involvement are considered for a different treatment strategy.
PET has been reported to increase diagnostic accuracy for the assessment of the nodal status. The evaluation of the cancer cell metabolism, which generally precedes anatomic changes, allows FDG PET/CT to determine the nodal status and to detect occult metastatic disease better than CT scanning alone. This data is supported by a meta-analysis of the literature which reveals the high sensitivity (about 81.3%) and specificity (about 79.4%) of FDG-PET/CT for detection of mediastinal lymph node metastasis [16]. However, being aware of the false-positive results of FDG PET/CT, for potential surgical candidates, invasive mediastinal staging is recommended to verify that PET-positive mediastinal lesions are due to cancer. On the other hand, there remains also cases of false-negative results for nodal metastases. Invasive mediastinal staging should be strongly encouraged in central tumors and solid T2 tumors even if the FDG PET/CT scan does not suggest mediastinal node involvement because the risk of occult nodal involvement is relatively high [17]. In one study, patients with clinical N1 disease suggested by integrated FDG PET/CT had a relatively high incidence (17.6% after mediastinoscopy and 23.5% after endoscopic ultrasound-guided fine-needle aspiration (EBUS-FNA) of unsuspected N2 disease [18].
In contrast to the patients with peripheral T1 tumors or peripheral T2 tumors with a significant ground-glass component, the probability of having a positive node is very low and invasive staging is unlikely to be beneficial. In the latter setting, invasive mediastinal staging may be avoided [17].
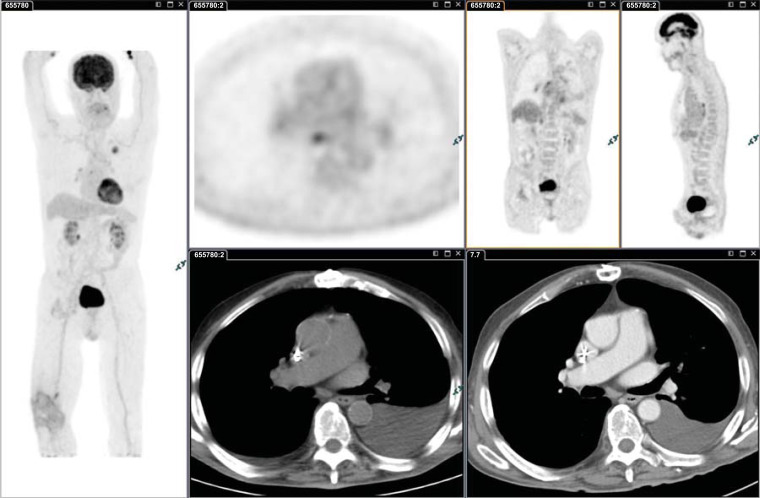
There are also other factors to be considered when interpreting FDG PET/CT findings. The pattern of symmetric FDG uptake in the mediastinum is often more important than absolute SUV value because inflammatory processes usually give rise to symmetric uptake (Fig. 1).
Fig. (1).
Moderately differentiated adenocarcinoma (SUVmax 6.9) of the upper lobe of the left lung with benign bilateral mediastinal nodes: SUVmax 4.5 (SUVmax liver 3) and pleural effusion; even if the SUV value of nodes is higher than the liver uptake, the pattern of symmetric FDG uptake helps to suspect inflammatory condition. In patients with an imaging finding suggestive of metastasis, further evaluation of the abnormality with tissue sampling to pathologically confirm the clinical stage is recommended prior to choosing treatment. (A higher resolution / colour version of this figure is available in the electronic copy of the article).
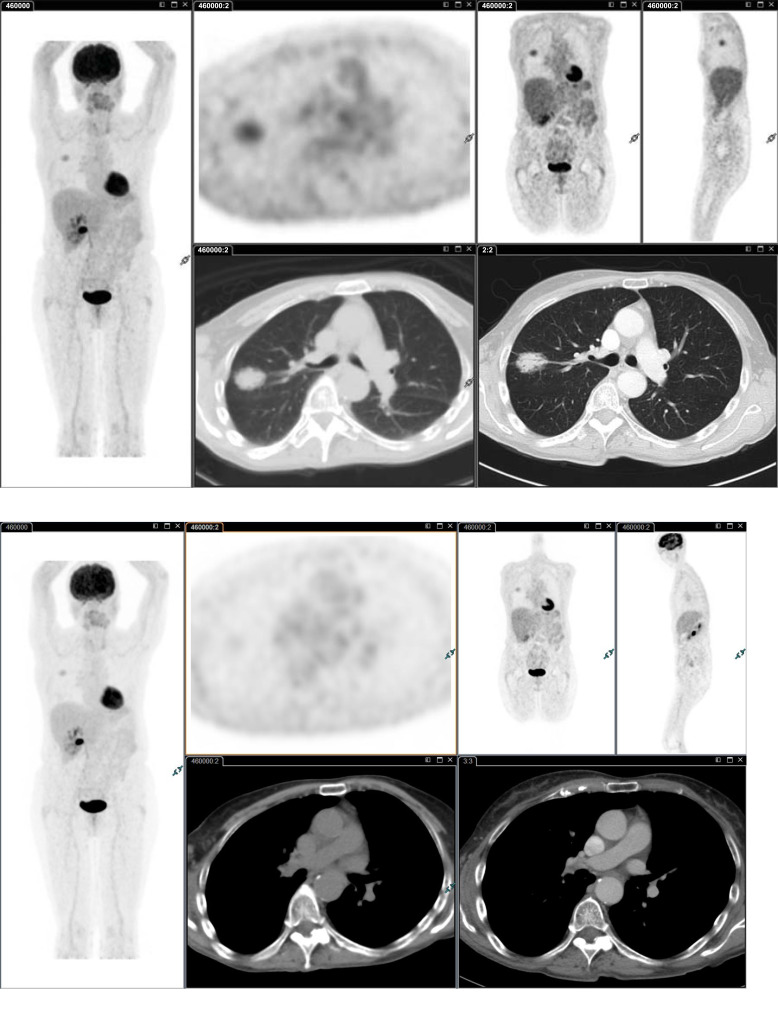
Another consideration is that FDG PET/CT Imaging may fail to stage accurately patients with low FDG uptake primary tumors missing mediastinal node metastases (Fig. 2). Often lymph nodes are likely to have a similar metabolic activity to the primary lesion and therefore, a small intensely avid node is more likely to be malignant if the primary tumor shows high FDG uptake, even if the SUVmax is lower than liver uptake (Fig. 3). In central tumors and solid T2 tumors, even if the nodal uptake is very low or the FDG PET/CT scan does not suggest mediastinal node involvement, invasive mediastinal staging should be carried out, as the risk of occult nodal involvement is relatively high (Fig. 4). These factors should be kept in mind when interpreting FDG PET imaging findings.
Fig. (2).
Moderately differentiated adenocarcinoma G2 (70% Acinar, 20% Lepidic, 10% Papillary) of the upper lobe of the right lung (SUVmax 3.5). Surgery identified hilar node metastasis (SUVmax 2.0; SUVmax liver 2.5) not identified by FDG PET/CT Imaging. It should be kept in mind that in patients with less avid primary tumors, node metastases may be missed by FDG PET/CT imaging. (A higher resolution / colour version of this figure is available in the electronic copy of the article).
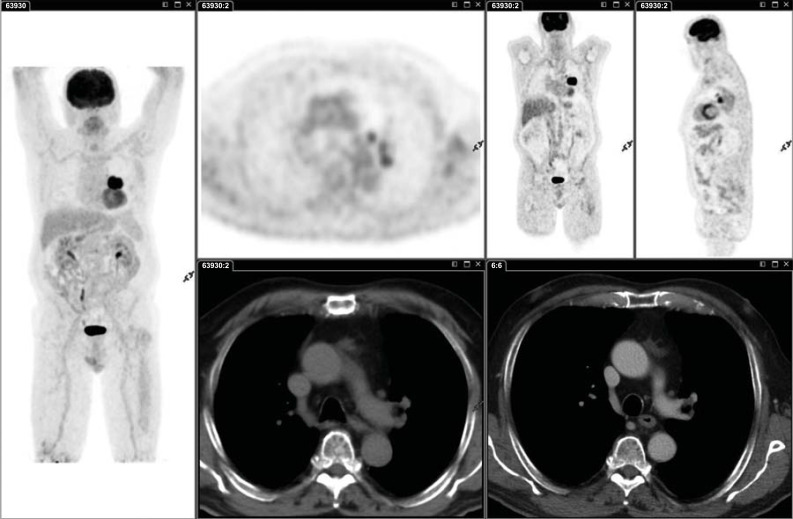
Fig. (3).
Small node metastasis (SUVmax 2.0; SUVmax liver 2.5) in patient with poorly differentiated adenocarcinoma with large cells of the left lung (G3). Nodes are likely to have metabolically similar cells within them to the primary lesion, and consequently, a small intensely avid node, even with an SUV value lower than liver SUV value, is more likely to be malignant. In patients with PET activity in a mediastinal lymph node and normal-appearing nodes by CT (and no distant metastases), an invasive staging of the mediastinum is recommended over staging by imaging alone. (A higher resolution / colour version of this figure is available in the electronic copy of the article).
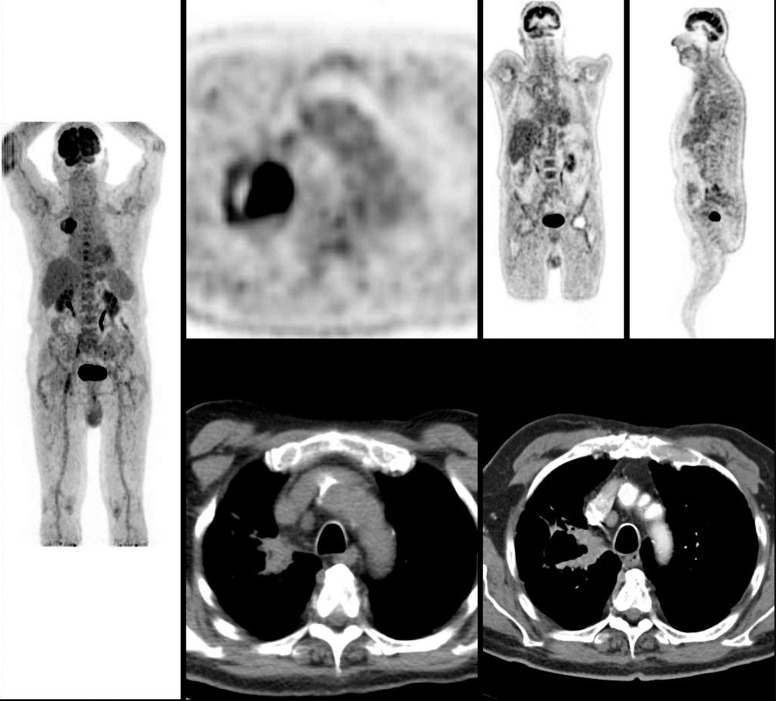
Fig. (4).
Enlarged paratracheal metastasis (4R; SUVmax 1.5; SUVmax liver 2.4) in patient with squamous cell carcinoma of the right lung. FDG PET/CT resulted to be negative with SUV value lower than the liver SUV value. Invasive mediastinal staging should be carried out in central tumors and solid T2 tumors even if the FDG PET/CT scan does not suggest mediastinal node involvement because the risk of occult nodal involvement is relatively high. (A higher resolution / colour version of this figure is available in the electronic copy of the article).
4. M staging
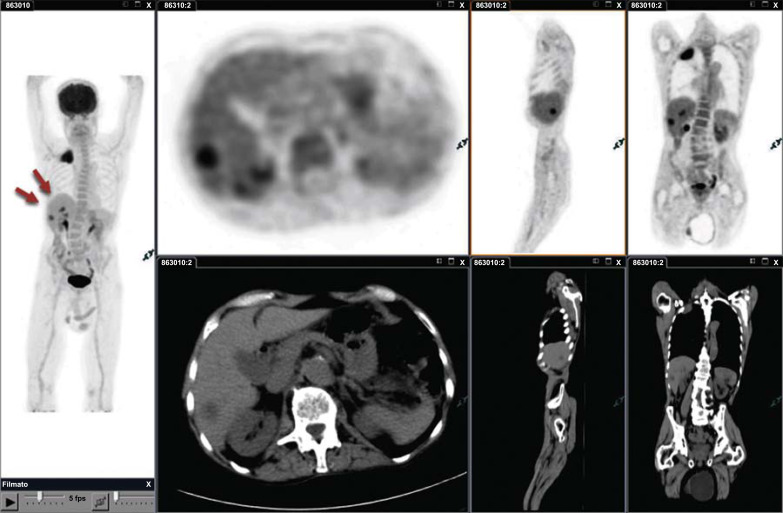
With the new TNM classification [3], M classification is subdivided into different descriptors on the basis of the number and site of extrathoracic metastases, by maintaining the prior M1a category (intrathoracic disease) and adding the categories of M1b disease (single extra-thoracic metastasis) and M1c disease (multiple extra-thoracic metastasis). The timely detection of metastatic disease is by far the most important prognostic factor when faced with newly diagnosed lung cancer (Fig. 5). Most patients with metastatic disease are not suitable for locoregional therapy with curative intent. One of the main advantages of FDG PET/CT over conventional imaging methods is its higher sensitivity in identifying extra-thoracic metastases. A randomized, controlled multicenter trial including 320 patients who were randomized to a PET/CT plus cranial imaging arm (163 patients) and conventional imaging arm (157 patients; CT abdomen, bone scan, and cranial imaging), 23 (14%) of 163 patients in the PET/CT arm were correctly upstaged and avoided inappropriate surgery compared with 11 (7%) of 157 in the conventional imaging arm (0.046). The authors concluded that FDG PET/CT better identifies extra-thoracic metastases in patients with NSCLC, sparing some from stage inappropriate surgery [19].
Fig. (5).
Pancoast tumor of the right lung without nodal involvement. Two hypodense, unknown lesions with high FDG uptake were identified by PET/CT Imaging in the liver (red arrows). The diagnosis of metastases was confirmed by the US and CT extrathoracic metastatic disease, classified as M1b in TNM-7, is now subdivided into M1b for a single metastasis and M1c for multiple extrathoracic metastases (TNM-8), based on the different prognosis. (A higher resolution / colour version of this figure is available in the electronic copy of the article).
A more recent review by the National Institute for Clinical Excellence (NICE) confirmed that in an average of 15% of patients, unexpected extra-thoracic metastases may be detected by FDG PET/CT [20].
It should be underlined that the strength of FDG PET/CT imaging, compared to conventional imaging methods for M staging in lung cancer, is to detect occult metastatic disease of adrenal glands, liver, and skeleton. The main limitation of FDG PET/CT for the detection of distant metastases is the identification of cranial lesions, owing to the high physiologic uptake of FDG in the brain. Therefore, in patients with diagnosed NSCLC, the use of a brain CT scan for complete staging is mandatory and in patients with neurological symptoms, an MRI of the brain may be also performed.
FDG PET/CT is particularly effective for detecting bone metastasis in patients with NSCLC and is more sensitive and accurate than bone scanning. Song and colleagues compared FDG PET/CT and bone scintigraphy and found that the sensitivity, specificity, PPV, and NPV of FDG PET/CT were 94.3%, 98.8%, 90.0%, and 99.3%, respectively; and those of bone scintigraphy were 78.1%, 97.4%, 75.9%, and 97.4%, respectively [21]. Discordant findings of skeletal metastasis between bone scintigraphy and PET/CT occur in approximately 20% of patients with NSCLC [22]. Skeletal metastases occur in the red marrow space and do not alter cortical bone initially. Bone scans relying on phosphate metabolism are therefore insensitive in the detection of the marrow phase of osseous metastasis. FDG PET/CT scan, by imaging the tumor itself rather than the bone’s response to the tumor, detects early neoplastic infiltration and is more sensitive in the detection of skeletal metastases than conventional bone scintigraphy. A meta-analysis of 17 studies demonstrated that the pooled sensitivity and specificity for the detection of bone metastasis were 92% and 98%, respectively, for PET/CT, compared with 86% and 88%, respectively, for bone scintigraphy [23]. Therefore, FDG PET/CT has replaced almost totally bone scintigraphy for the detection of bone metastasis in patients with newly diagnosed NSCLC [24].
Adrenal metastases from lung cancer are common and usually occur with metastases in other organs, although they can also be present as an oligometastatic disease. Early detection of adrenal metastases is of crucial importance as resection of an adrenal oligometastasis from NSCLC has been shown to improve the long-term disease-free survival [25]. In patients with NSCLC, however, many solitary adrenal lesions are not malignant. It is not always reliable enough to distinguish between metastases and benign lesions only on the bases of CT Imaging. Adrenal masses are evaluated by CT based on tumor size, attenuation value and wash-out, which may lead to false-negative or false-positive results.
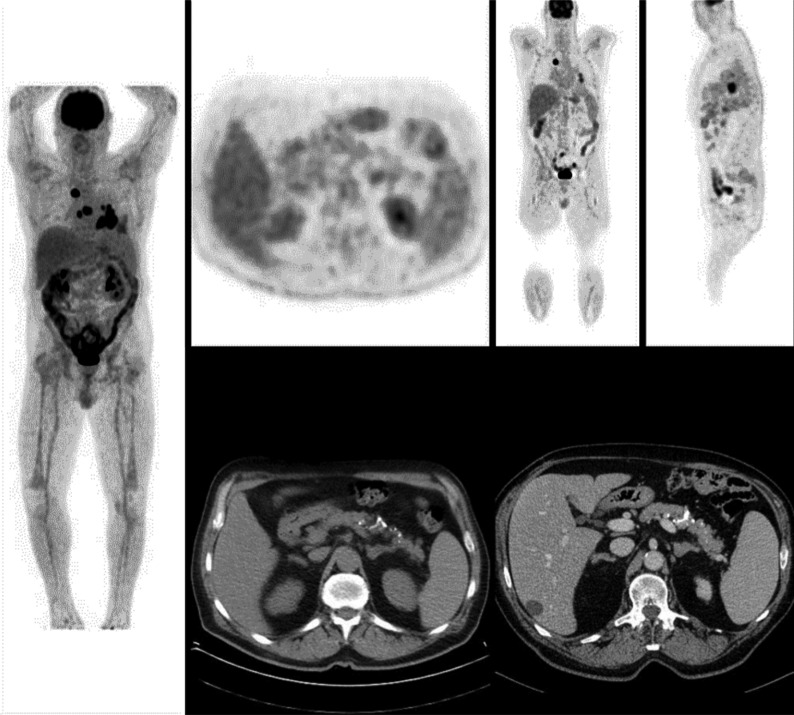
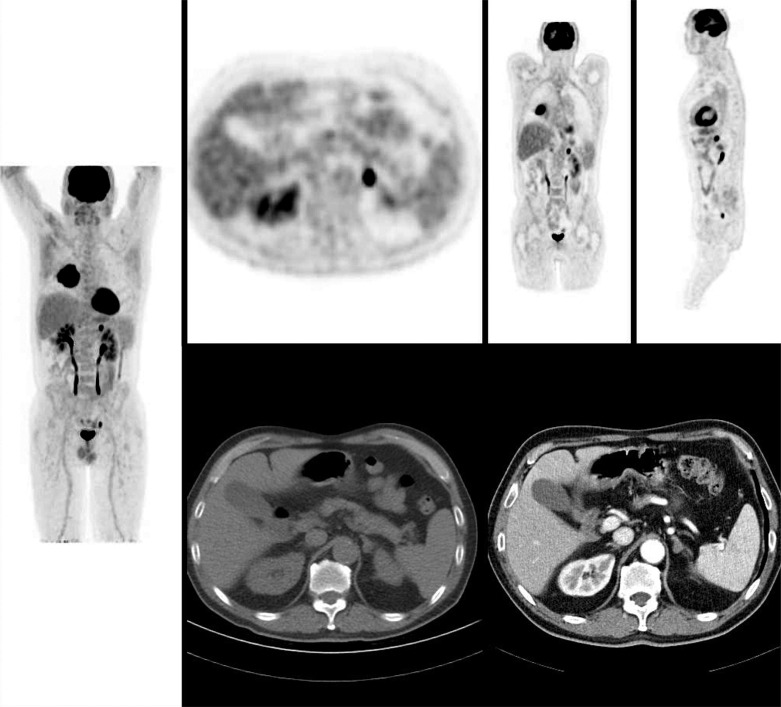
In this setting, FDG PET/CT turns out to be a very useful method to distinguish benign from malignant adrenal lesions [26], since it allows early detection and accurate localization of adrenal lesions and differentiation of metastatic nodules from benign lesions (Figs. 6 and 7). In a retrospective study, the accuracy of FDG PET/CT for characterization of adrenal lesions in patients with proved or suspected malignancy was evaluated, and the authors found that all malignant lesions had SUV values greater than that of the liver with a mean adrenal lesion liver ratio of 4.04 (range 1.53-17.08). Of the 32 benign lesions, 30 had activity ratios that were less than liver activity (mean adrenal lesion-liver activity ratio of 0.66); the maximum adrenal lesion liver ratio of a benign lesion was 1.47 [27].
It should be kept in mind that some benign lesions, like adenomas, may show also intense FDG uptake [28]. However, the combination of SUV values and CT morphologic characteristics (size, shape, attenuation value) as allowed in most of the cases to significantly improve the diagnostic accuracy for characterizing adrenal lesions, leading to a significant reduction in the number of false-positive cases [29].
Boland and colleagues in a meta-analysis showed that adrenal lesions can be characterized with high accuracy with the use of FDG PET/CT Imaging, and subsequent imaging is usually unnecessary [30].
Pleural effusions are common in patients with NSCLC, but only some of these effusions are malignant. Several studies showed that the use of FDG PET/CT Imaging has an added value in distinguishing malignant from benign pleural effusion, improving the accuracy of CT Imaging [31-33]. In those cases, where invasive diagnostic methods like thoracocentesis are considered impossible or of high risk for the elderly, or insufficient quantity of pleural fluid is present, FDG PET/CT may be applied as a reliable noninvasive method for the detection of malignant pleural effusion.
It should be mentioned that several benign conditions (infection or inflammation) or malignant lesions unrelated to the primary NSCLC may show also intense FDG uptake, mimicking distant metastases. Therefore, atypical lesions with pathologic FDG uptake require further testing, particularly where management would be impacted.
REMARKS AND CONCLUSION
FDG PET/CT functional imaging has proved an invaluable noninvasive, diagnostic tool, improving the accuracy in the staging of NSCLC. If the CT appearances of a patient with known NSCLC suggest localized disease, an FDG PET/CT imaging should be performed to confirm the absence of mediastinal nodal disease in nonenlarged lymph nodes, and the absence of occult distant metastases. For N staging, FDG PET/CT imaging has high negative predictive value which can reduce unnecessary invasive mediastinal staging. Most used guidelines do not recommend invasive mediastinal staging in cases of normal-sized mediastinal nodes on CT and no uptake with FDG PET/CT in patients with T1 tumors or peripheral T2 tumors with a significant ground-glass component. On the other hand, lymph nodes with pathologic FDG uptake on PET/CT scan should be confirmed histologically by mediastinoscopy or EBUS-FNA before curative surgery is excluded as a treatment option. One of the main advantages of an FDG PET/CT scan over conventional imaging is its higher sensitivity in identifying extra-thoracic metastases. The general consensus is that FDG PET/CT PET can reduce needless thoracotomy rates. For the detection of brain metastasis, FDG PET/CT has limited value, as the high physiologic uptake of FDG in the cerebral cortex may obscure pathological uptake.
In conclusion, FDG PET/CT plays an important role in the staging of NSCLC, providing complementary information to conventional morphological imaging modalities and is considered standard of care for noninvasive mediastinal staging and detection of distant metastasis in NSCLC. Evidence is also accumulating for the prognostic and predictive implications of FDG PET/CT.
Fig. (6).
Poorly differentiated adenocarcinoma with bilateral mediastinal metastasis. Left enlarged adrenal lesion suspected to be metastases on CT scan, turn out to be benign showing no FDG uptake. FDG PET/CT imaging is useful in the differentiation of benign and malignant adrenal lesions in NSCLC patients. (A higher resolution / colour version of this figure is available in the electronic copy of the article).
Fig. (7).
Poorly differentiated adenocarcinoma of the right lung without nodal involvement. Left enlarged adrenal lesion suspected to be metastases on CT scan, showed high FDG uptake. The pathological diagnosis of metastases was confirmed after resection. Adrenalectomy of an isolated adrenal metastasis from NSCLC has been shown to improve the long-term disease-free survival of such patients. (A higher resolution / colour version of this figure is available in the electronic copy of the article).
Acknowledgements
The author would like to thank all his colleagues, in particular Dr. Arber Golemi and Dr. Manuel Tredici for their invaluable professionalism and friendship.
List of ABBREVIATIONS
- CT
Computed Tomography
- EBUS-FNA
Endoscopic Ultrasound-Guided Fine-Needle Aspiration
- FDG
Fluorodeoxyglucose
- NICE
National Institute for Clinical Excellence
- NSCLC
Non-Small Cell Lung Cancer
- PET
Positron Emission Tomography
- SUV
Standardized Uptake Value
Consent for Publication
Not applicable.
Funding
None.
Conflict of Interest
The authors declare no conflict of interest, financial or otherwise.
References
- 1.Bray F., Ferlay J., Soerjomataram I., Siegel R.L., Torre L.A., Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018;68(6):394–424. doi: 10.3322/caac.21492. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 2.American Cancer Society Key statistics for lung cancer. 2018 https://www.cancer.org/cancer/non-small-cell-lung-cancer/ about/key-statistics.html
- 3.Goldstraw P., Chansky K., Crowley J., Rami-Porta R., Asamura H., Eberhardt W.E., Nicholson A.G., Groome P., Mitchell A., Bolejack V. International association for the study of lung cancer staging and prognostic factors committee, advisory boards, and participating institutions; international association for the study of lung cancer staging and prognostic factors committee advisory boards and participating institutions. the iaslc lung cancer staging project: proposals for revision of the tnm stage groupings in the forthcoming (eighth) edition of the tnm classification for lung cancer. J. Thorac. Oncol. 2016;11(1):39–51. doi: 10.1016/j.jtho.2015.09.009. [DOI] [PubMed] [Google Scholar]
- 4.Postmus PE, Kerr KM, Oudkerk M. 2017. [DOI] [PubMed]
- 5.Kalemkerian G.P., Loo B.W., Jr, Akerley W., Attia A., Bassetti M., Boumber Y., Decker R., Dobelbower M.C., Dowlati A., Downey R.J., Florsheim C., Ganti A.K.P., Grecula J.C., Gubens M.A., Hann C.L., Hayman J.A., Heist R.S., Koczywas M., Merritt R.E., Mohindra N., Molina J., Moran C.A., Morgensztern D., Pokharel S., Portnoy D.C., Rhodes D., Rusthoven C., Sands J., Santana-Davila R., Williams C.C., Hoffmann K.G., Hughes M. NCCN guidelines Insights: small cell lung cancer, version 2.2018. J. Natl. Compr. Canc. Netw. 2018;16(10):1171–1182. doi: 10.6004/jnccn.2018.0079. [DOI] [PubMed] [Google Scholar]
- 6.Fischer B., Lassen U., Mortensen J., Larsen S., Loft A., Bertelsen A., Ravn J., Clementsen P., Høgholm A., Larsen K., Rasmussen T., Keiding S., Dirksen A., Gerke O., Skov B., Steffensen I., Hansen H., Vilmann P., Jacobsen G., Backer V., Maltbaek N., Pedersen J., Madsen H., Nielsen H., Højgaard L. Preoperative staging of lung cancer with combined PET-CT. N. Engl. J. Med. 2009;361(1):32–39. doi: 10.1056/NEJMoa0900043. [DOI] [PubMed] [Google Scholar]
- 7.Maziak DE, Darling GE, Inculet RI. Positron emission tomography in staging early lung cancer: a randomized trial. 2009. [DOI] [PubMed]
- 8.Viney R.C., Boyer M.J., King M.T., Kenny P.M., Pollicino C.A., McLean J.M., McCaughan B.C., Fulham M.J. Randomized controlled trial of the role of positron emission tomography in the management of stage I and II non-small-cell lung cancer. J. Clin. Oncol. 2004;22(12):2357–2362. doi: 10.1200/JCO.2004.04.126. [DOI] [PubMed] [Google Scholar]
- 9.Herder G.J., Kramer H., Hoekstra O.S., Smit E.F., Pruim J., van Tinteren H., Comans E.F., Verboom P., Uyl-de Groot C.A., Welling A., Paul M.A., Boers M., Postmus P.E., Teule G.J., Groen H.J., POORT Study Group Traditional versus up-front [18F] fluorodeoxyglucose-positron emission tomography staging of non-small-cell lung cancer: a Dutch cooperative randomized study. J. Clin. Oncol. 2006;24(12):1800–1806. doi: 10.1200/JCO.2005.02.4695. [DOI] [PubMed] [Google Scholar]
- 10.van Tinteren H., Hoekstra O.S., Smit E.F., van den Bergh J.H., Schreurs A.J., Stallaert R.A., van Velthoven P.C., Comans E.F., Diepenhorst F.W., Verboom P., van Mourik J.C., Postmus P.E., Boers M., Teule G.J. Effectiveness of positron emission tomography in the preoperative assessment of patients with suspected non-small-cell lung cancer: the PLUS multicentre randomised trial. Lancet. 2002;359(9315):1388–1393. doi: 10.1016/S0140-6736(02)08352-6. [DOI] [PubMed] [Google Scholar]
- 11.Spadafora M., Pace L., Evangelista L., Mansi L., Del Prete F., Saladini G., Miletto P., Fanti S., Del Vecchio S., Guerra L., Pepe G., Peluso G., Nicolai E., Storto G., Ferdeghini M., Giordano A., Farsad M., Schillaci O., Gridelli C., Cuocolo A. Risk-related 18F-FDG PET/CT and new diagnostic strategies in patients with solitary pulmonary nodule: the ITALIAN multicenter trial. Eur. J. Nucl. Med. Mol. Imaging. 2018;45(11):1908–1914. doi: 10.1007/s00259-018-4043-y. [DOI] [PubMed] [Google Scholar]
- 12.Downey R.J., Akhurst T., Gonen M., Vincent A., Bains M.S., Larson S., Rusch V. Preoperative F-18 fluorodeoxyglucose-positron emission tomography maximal standardized uptake value predicts survival after lung cancer resection. J. Clin. Oncol. 2004;22(16):3255–3260. doi: 10.1200/JCO.2004.11.109. [DOI] [PubMed] [Google Scholar]
- 13.Berghmans T., Dusart M., Paesmans M., Hossein-Foucher C., Buvat I., Castaigne C., Scherpereel A., Mascaux C., Moreau M., Roelandts M., Alard S., Meert A.P., Patz E.F., Jr, Lafitte J.J., Sculier J.P. European lung cancer working party for the iaslc lung cancer staging project. primary tumor standardized uptake value (SUVmax) measured on fluorodeoxyglucose positron emission tomography (FDG-PET) is of prognostic value for survival in non-small cell lung cancer (NSCLC): a systematic review and meta-analysis (MA) by the European lung cancer working party for the IASLC lung cancer staging project. J. Thorac. Oncol. 2008;3(1):6–12. doi: 10.1097/JTO.0b013e31815e6d6b. [DOI] [PubMed] [Google Scholar]
- 14.Cheng G., Huang H. Prognostic value of 18F-Fluorodeoxyglucose PET/computed tomography in non-small-cell lung cancer. PET Clin. 2018;13(1):59–72. doi: 10.1016/j.cpet.2017.08.006. [DOI] [PubMed] [Google Scholar]
- 15.Kirienko M., Cozzi L., Rossi A., Voulaz E., Antunovic L., Fogliata A., Chiti A., Sollini M. Ability of FDG PET and CT radiomics features to differentiate between primary and metastatic lung lesions. Eur. J. Nucl. Med. Mol. Imaging. 2018;45(10):1649–1660. doi: 10.1007/s00259-018-3987-2. [DOI] [PubMed] [Google Scholar]
- 16.Schmidt-Hansen M1.; Baldwin, DR.; Hasler, E. PET-CT for assessing mediastinal lymph node involvement in patients with suspected resectable non-small cell lung cancer. Cochrane Database Syst. Rev. 2014;13(11) doi: 10.1002/14651858.CD009519.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gao S.J., Kim A.W., Puchalski J.T., Bramley K., Detterbeck F.C., Boffa D.J., Decker R.H. Indications for invasive mediastinal staging in patients with early non-small cell lung cancer staged with PET-CT. Lung Cancer. 2017;109:36–41. doi: 10.1016/j.lungcan.2017.04.018. [DOI] [PubMed] [Google Scholar]
- 18.Cerfolio R.J., Bryant A.S., Eloubeidi M.A. Routine mediastinoscopy and esophageal ultrasound fine-needle aspiration in patients with non-small cell lung cancer who are clinically N2 negative: a prospective study. Chest. 2006;130(6):1791–1795. doi: 10.1378/chest.130.6.1791. [DOI] [PubMed] [Google Scholar]
- 19.Maziuk D., Darling G.E., Inculet R.I. A randomized controlled trial (RCT) of 18F-fluorodeoxyglucose (FDG) positron emission tomography (PET) versus conventional imaging (CI) in staging potentially resectable non-small cell lung cancer (NSCLC). J. Clin. Oncol. 2008;26(Suppl.):739s. doi: 10.1200/jco.2008.26.15_suppl.7502. [DOI] [Google Scholar]
- 20.2011 http://www.nice.org.uk/nicemedia/pdf/ CG024niceguideline.pdf
- 21.Song J.W., Oh Y.M., Shim T.S., Kim W.S., Ryu J.S., Choi C.M. Efficacy comparison between (18)F-FDG PET/CT and bone scintigraphy in detecting bony metastases of non-small-cell lung cancer. Lung Cancer. 2009;65(3):333–338. doi: 10.1016/j.lungcan.2008.12.004. [DOI] [PubMed] [Google Scholar]
- 22.Ak I., Sivrikoz M.C., Entok E., Vardareli E. Discordant findings in patients with non-small-cell lung cancer: absolutely normal bone scans versus disseminated bone metastases on positron-emission tomography/computed tomography. Eur. J. Cardiothorac. Surg. 2010;37(4):792–796. doi: 10.1016/j.ejcts.2009.11.011. [DOI] [PubMed] [Google Scholar]
- 23.Qu X., Huang X., Yan W., Wu L., Dai K. A meta-analysis of 18FDG-PET-CT, 18FDG-PET, MRI and bone scintigraphy for diagnosis of bone metastases in patients with lung cancer. Eur. J. Radiol. 2012;81(5):1007–1015. doi: 10.1016/j.ejrad.2011.01.126. [DOI] [PubMed] [Google Scholar]
- 24.Boellaard R., Delgado-Bolton R., Oyen W.J., Giammarile F., Tatsch K., Eschner W., Verzijlbergen F.J., Barrington S.F., Pike L.C., Weber W.A., Stroobants S., Delbeke D., Donohoe K.J., Holbrook S., Graham M.M., Testanera G., Hoekstra O.S., Zijlstra J., Visser E., Hoekstra C.J., Pruim J., Willemsen A., Arends B., Kotzerke J., Bockisch A., Beyer T., Chiti A., Krause B.J. European Association of Nuclear Medicine (EANM). FDG PET/CT: EANM procedure guidelines for tumour imaging: version 2.0. Eur. J. Nucl. Med. Mol. Imaging. 2015;42(2):328–354. doi: 10.1007/s00259-014-2961-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Luketich J.D., Burt M.E. Does resection of adrenal metastases from non-small cell lung cancer improve survival? Ann. Thorac. Surg. 1996;62(6):1614–1616. doi: 10.1016/S0003-4975(96)00611-X. [DOI] [PubMed] [Google Scholar]
- 26.Ansquer C., Scigliano S., Mirallié E., Taïeb D., Brunaud L., Sebag F., Leux C., Drui D., Dupas B., Renaudin K., Kraeber-Bodéré F. 18F-FDG PET/CT in the characterization and surgical decision concerning adrenal masses: a prospective multicentre evaluation. Eur. J. Nucl. Med. Mol. Imaging. 2010;37(9):1669–1678. doi: 10.1007/s00259-010-1471-8. [DOI] [PubMed] [Google Scholar]
- 27.Blake M.A., Slattery J.M., Kalra M.K., Halpern E.F., Fischman A.J., Mueller P.R., Boland G.W. Adrenal lesions: characterization with fused PET/CT image in patients with proved or suspected malignancy--initial experience. Radiology. 2006;238(3):970–977. doi: 10.1148/radiol.2383042164. [DOI] [PubMed] [Google Scholar]
- 28.Chong S., Lee K.S., Kim H.Y., Kim Y.K., Kim B.T., Chung M.J., Yi C.A., Kwon G.Y. Integrated PET-CT for the characterization of adrenal gland lesions in cancer patients: diagnostic efficacy and interpretation pitfalls. Radiographics. 2006;26(6):1811–1824. doi: 10.1148/rg.266065057. [DOI] [PubMed] [Google Scholar]
- 29.Perri M., Erba P., Volterrani D., Guidoccio F., Lazzeri E., Caramella D., Mariani G. Adrenal masses in patients with cancer: PET/CT characterization with combined CT histogram and standardized uptake value PET analysis. AJR Am. J. Roentgenol. 2011;197(1):209–216. doi: 10.2214/AJR.10.5342. [DOI] [PubMed] [Google Scholar]
- 30.Boland GW, Dwamena BA, Jagtiani Sangwaiya M. Characterization of adrenal masses by using FDG PET: a systematic review and meta-analysis of diagnostic. [DOI] [PubMed]
- 31.Gupta N.C., Rogers J.S., Graeber G.M., Gregory J.L., Waheed U., Mullet D., Atkins M. Clinical role of F-18 fluorodeoxyglucose positron emission tomography imaging in patients with lung cancer and suspected malignant pleural effusion. Chest. 2002;122(6):1918–1924. doi: 10.1378/chest.122.6.1918. [DOI] [PubMed] [Google Scholar]
- 32.Sun Y., Yu H., Ma J., Lu P. The Role of 18F-FDG PET/CT integrated imaging in distinguishing malignant from benign pleural effusion. PLoS One. 2016;11(8):e0161764. doi: 10.1371/journal.pone.0161764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kim B.S., Kim I.J., Kim S.J., Pak K., Kim K. Predictive value of F-18 FDG PET/CT for malignant pleural effusion in non-small cell lung cancer patients. Onkologie. 2011;34(6):298–303. doi: 10.1159/000328793. [DOI] [PubMed] [Google Scholar]