Abstract
The study aimed to isolate and identify lactic acid bacteria (LAB) from silages and their application to improve the fermentation quality of alfalfa. Forty-nine LAB strains were isolated from silages, and two strains were screened for growth and acid production rates. Then two strains were selected for Physiological and morphological tests and 16S rRNA sequencing. They were Gram-positive and Catalase-negative and were able to grow at pH 3.5 and at 45 °C, were unable to grow different NaCl concentrations as 3.0% and 6.5%. Strain BDy3-10 was identified as Lactobacillus rhamnosus, while TSy1-3 was identified as L. buchneri. The selected strains were evaluated on fermentation of alfalfa silage. The highest crude protein content occurred in the BDy3-10 treatment group. The contents of neutral detergent fiber and acid detergent fiber in the TSy1-3 treatment were significantly lower than other treatment (P < 0.05). Compared to the control treatment, inoculation treatments deceased pH during ensiling (P < 0.001) and provided the most increased lactic acid content after ensiling for 10 days (P < 0.001). The acetic acid contents of all the inoculation groups were significantly increased (P < 0.001) during ensiling, and were lower than that of control group (P < 0.001). So, the TSy1-3 treatment most effectively improved the fermentation quality of alfalfa silage in warm and humid climate area.
Subject terms: Microbiology, Molecular biology
Introduction
Lactic acid bacteria (LAB) can ferment and produce abundant lactic acid, which is used as a silage additive. Inoculation with LAB could increase the content of lactic acid, decrease the pH1, and help improve the silage fermentation profile and enhance feed quality2,3. Previous studies have shown that LAB inoculation in silages can reduce dry matter losses and increase aerobic stability, degradability rate and animal performance4,5. Recently, dual-purpose inoculants containing homo-fermentative and hetero-fermentative bacteria have been developed to overcome the limitations of inoculants containing either type of bacteria alone, and the combination of both types of organisms can improve the speed of fermentation and enhance aerobic stability6–8. Drouin et al. reported that inoculation with a combination of additives effectively improved the fermentation quality and aerobic stability of silage9. Homofermentative LAB fermentation produced low levels of volatile fatty acids that could not effectively inhibit the growth of molds and yeasts, resulting in silage corruption2. However, heterofermentative LAB could produce high levels of acetic acid that inhibited the growth of fungi and increased the aerobic stability of silage2. Several studies have suggested that mixed LAB inoculants can improve aerobic stability6,10,11.
In recent years, increasing work has focused on how to improve fermentation quality by isolating LAB ideally capable of dominating lactic fermentation from forage or silage12,13. Wang et al. discovered that a LAB strain isolated from Leymus chinensis silage could be useful for promoting favourable fermentation of Moringa oleifera leaf silage14. Ennahar et al. found that the presence of L. plantarum dominates in rice silage15, while Ni et al. shown that the presence of Ped. pentosaceus dominates16. This might be due to their different environments. LAB strains isolated from different forages in different environments usually have different effects on silage fermentation17. Thus, it is necessary to screen local lactic acid bacteria resources suitable for the area.
Therefore, the goals of the present study were to isolate, screen and identify the LAB from superior silage in the warm and humid climate of Guizhou karst areas. After that, the evaluated excellent LAB strains were used to inoculate alfalfa silage to determine their effect on the fermentation quality.
Materials and methods
Silage materials and isolation of LAB
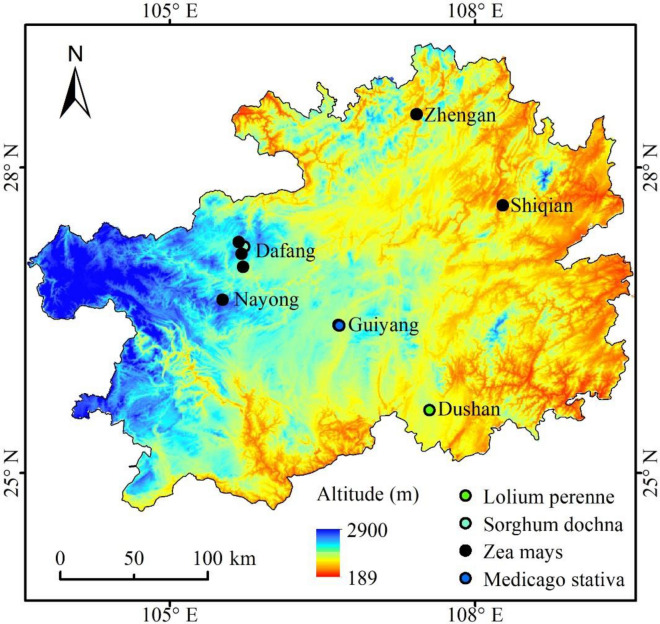
12 silage raw materials that included Lolium perenne, Sorghum dochna, Zea mays and Medicago stative L., were harvested from different sites in Guizhou Province, as shown in Fig. 1 and Table 1. The study comply with the regulation of Agricultural and Rural Bureau of Guizhou for plant research. Also, the samples of plant collected in this experiment were approved by the same department. The silage raw material was chopped to about (1–2) cm using a paper-cutter, the chopped forages were packed into polyethylene plastic bags (dimensions 16 × 25 cm; Embossed Food saver bag; Taizou Wenbwu Soft-Packing Color-Printing Co. Ltd, Zhejiang, China), and approximately 600 g of forage was packed in each polyethylene bag and then vacuum-sealed. The bags were stored at room temperature for 60 d. After the silage was finished, the colour was yellow-green, the sample presented acidity and aroma, and the stem and leaf structure could be clearly identified.
Figure 1.
The silage materials, longitude, latitude and altitude of sampling sites. Note The map was generated by Arcmap 10.8 software (https://links.esri.com/open-source-acknowledgments).
Table 1.
Annual precipitation and annual mean temperature of sampling sites (data in 2014–2018).
| Sampling sites | Huaxi | Nayong | Shiqian | Zhengan | Dafang | Dushan |
|---|---|---|---|---|---|---|
| Annual precipitation (mm) | 1268.66 | 1278.92 | 1216.36 | 1144.14 | 1169.18 | 1413.54 |
| Annual mean temperature (°C) | 15.84 | 14.54 | 17.86 | 16.36 | 12.8 | 15.92 |
Approximately 10 g of silages were blended with 90 ml of sterilized saline solution (8.50 g L−1 NaCl) and serially diluted from 10–1 to 10–6 in sterilized water. Then, the three dilutions 10–1, 10–3 and 10–5 were taken and coated on LAB medium plates for cultivation. By observing the appearance and morphology of colonies on the solid medium of Lactobacillus, a single colony with different appearances was selected, and each colony was separated and purified twice. The purified colonies were cultured on the solid medium of Lactobacillus under a constant temperature 37 °C and anaerobic conditions for 24 h, and then identified by Gram's staining and catalase activity. Finally, the identified Lactobacillus was added to the liquid nutrient medium containing sterile glycerin and preserved at − 80 °C.
Screening of LAB
Isolation strains were initially screened due to their high acidification ability and growth efficiency at 37 °C18. Gram staining, colony morphology and catalase activity of screening strains were evaluated19. Strains were inoculated in MRS broth with different NaCl (3.0% and 6.5%) to test salt tolerance. Growth in MRS broth at pH 3, 3.5, 4, 4.5, 5, 5.5, 6, 7 and 7.5 and at temperatures 20 °C, 30 °C, 40 °C, 45 °C and 50 °C were tested.
16S rRNA sequencing analysis
A TSINGKE DNA kit (general type) was used to extract the 16S rRNA of screened strains. The 16S rRNA universal amplification primers were: 27F (5, -AGAGTTTGATCCTGGCTCAG-3) and 1492R (5, -GGTTACCTTGTTACGACTT-3)20. Then, the PCR amplification products were sent to Beijing for sequencing at Biotechnology Co., Ltd., Kunming Branch.
After obtaining 16S rRNA sequences, they were compared with the 16S rRNA sequences of type strains in GenBank. The CLUSTALW program was used to assemble and align the sequences of selected strains and typical strains.
Silage and ensiling
Alfalfa (Medicago sativa L.) at early budding was harvested from Guizhou University West Campus experimental site (Guiyang, Guizhou, China) on January 1, 2019, and wilted for 48 h. The chemical and microbial compositions after wilting are shown in Table 2.
Table 2.
Chemical and microbial compositions after alfalfa wilting.
| Items | Content |
|---|---|
| DM (%) | 35.72 ± 2.59 |
| CP (% DM) | 23.16 ± 1.99 |
| NDF (% DM) | 37.97 ± 1.82 |
| ADF (% DM) | 24.83 ± 1.46 |
| WSC (% DM) | 7.51 ± 0.37 |
| CF (% DM) | 29.64 ± 1.21 |
| Ash (% DM) | 11.15 ± 0.83 |
| EE (% DM) | 9.93 ± 0.82 |
| LAB (log cfu g−1 FM) | 3.01 ± 0.56 |
DM dry matter, CP crud protein, NDF neutral detergent fiber, ADF acid detergent fiber, WSC water soluble carbohydrates, CF crud fiber, Ash crud ash, EE ether extract, LAB lactic acid bacteria, cfu colony-forming units, FM fresh matter.
Two selected strains (BDy3-10 and TSy1-3) were used as inoculants for silage preparation. Each strain was dissolved to approximately 106 colony-forming units (cfu) g−1 FM, and 100 mL of inoculant was dissolved and sprayed on 3.2 kg of chopped forage (1–2 cm), which was then mixed thoroughly. The chopped forage was then treated with the same amount of (1) distilled water (control), (2) L. rhamnosus (BDy3-10), (3) L. buchneri (TSy1-3), or (4) BDy3-10 + TSy1-3 at a ratio of 1:1. All the treated forages were packed into polyethylene plastic bags (dimensions 16 × 25 cm; Embossed Food saver bag; Taizou Wenbwu Soft-Packing Color-Printing Co. Ltd, Zhejiang, China), and approximately 200 g of wilted forage was packed in each polyethylene bag and then vacuum-sealed, with three replicates for each treatment. The bags were stored at room temperature and opened after 1, 6, 10, 20 and 40 days of storage, their chemical composition, fermentation quality and aerobic stability were analyzed.
Chemical and fermentation analysis
The dry matter (DM) contents of fresh and ensiled forages were determined by drying the sample in a forced-air oven at 65 °C for 48 h. The dried samples were ground to pass a 1 mm screen by a laboratory knife mill (FW100, Taisite Instrument Co., Ltd., Tianjin, China). Crude protein (CP) was analyzed using a Kjeldahl nitrogen analyzer (Kjeltec 2300 Auto-Analyzer, FOSS Analytical AB, Hoganas, Sweden) and crude fat (EE) was determined by an extraction method21. Crude ash content (Ash) was detected in an ash furnace by burning at 550 °C for 4 h. Crude fiber (CF), neutral detergent fiber (NDF) and acid detergent fiber (ADF) contents were measured by an A220 Fiber Analyzer (ANKOM Technology Corp., Macedon, NY, USA)22. Water soluble carbohydrate (WSC) was determined using the thracenone-sulphuric acid method23.
Twenty grams of each silage sample was mixed with 180 mL of distilled water, stored at 4 °C for 18 h, and then filtered. The pH of this filtrate was measured by a glass electrode pH meter (PHS-3C, INESA Scientific Instrument Co., Ltd, Shanghai, China), and ammonia-N was determined by steam distillation of the filtrates. The concentration of organic acids (lactic acid, acetic acid, propionic acid and butyric acid) was measured using high performance liquid chromatography (column, Shodex RSpak KC-811S-DVB gel C; 8.0 mm × 30 cm; Shimadzu, Tokyo, Japan); oven temperature, 50 °C; mobile phase, 3 mmol/L HClO4; flow rate, 1.0 mL/min; injection volume, 5 μL; and a SPD-M10AVP detector24.
Statistical analysis
The 16S rRNA sequences of the LAB isolates were analyzed by MEGA 6.0 for Windows (The Biodesign Institute, Tempe, AZ). Statistical analyses of data for chemical composition, fermentation characteristics and aerobic stability were performed using one-way ANOVA of the Statistical Package for Social Sciences (SPSS Version 19.0, SPSS Inc., Chicago, IL, USA). Turkey’s honest significant difference (HSD) test was employed for different sample means and the significance was declared at P < 0.05. Sigma Plot 10.0 is plotted.
Results
Screening of LAB
As shown in Table 3, forty-nine strains of LAB were isolated from silages. We initially screened two strains by their high growth and acid-producing rates for 24 h at 37 °C, the OD value of strain TSy1-3 at 24 h was significantly largest that other strains (P < 0.05), at 3.02. The lowest pH value occurred in the BDy3-10 treatment group (P < 0.05), at 3.75.
Table 3.
OD and pH values of lactic acid bacteria cultured in MRS medium for 24 h.
| Strain number | OD | pH | Strain number | OD | pH |
|---|---|---|---|---|---|
| TSy1-3 | 3.02 ± 0.05a | 3.94 ± 0.07jk | ZZt1-20 | 1.88 ± 0.09ijkl | 4.14 ± 0.11gh |
| BDy3-10 | 2.75 ± 0.04b | 3.75 ± 0.04q | BDy2-29 | 1.83 ± 0.06jklm | 4.16 ± 0.24g |
| BDy3-41 | 2.69 ± 0.14b | 4.06 ± 0.05g | ZZt1-6 | 1.80 ± 0.10klm | 4.08 ± 0.03gh |
| TSy1-10 | 2.63 ± 0.09bcd | 3.78 ± 0.04p | BDy2-19–1 | 1.80 ± 0.07lm | 4.60 ± 0.10ef |
| BDy3-7 | 2.63 ± 0.07bcd | 3.86 ± 0.03m | TSy1-6 | 1.74 ± 0.09lmn | 4.49 ± 0.06f |
| ZZt1-14 | 2.62 ± 0.04bcd | 3.88 ± 0.11lm | BDy3-16-2 | 1.73 ± 0.16lmn | 4.01 ± 0.00gh |
| TSy1-45 | 2.62 ± 0.12bcd | 4.02 ± 0.01gh | BDy2-50 | 1.71 ± 0.10mn | 4.07 ± 0.05gh |
| BDy3-43 | 2.54 ± 0.07cd | 4.09 ± 0.07gh | BDy3-40 | 1.71 ± 0.10mn | 4.69 ± 0.16de |
| GHy1-2 | 2.53 ± 0.06d | 3.97 ± 0.01hi | BDy3-14 | 1.69 ± 0.14mn | 4.76 ± 0.17e |
| BNy1-12 | 2.53 ± 0.05d | 3.85 ± 0.12mn | BNy1-1 | 1.69 ± 0.10mn | 4.93 ± 0.06bc |
| BDy3-35 | 2.36 ± 0.10e | 3.78 ± 0.04p | BNy1-16 | 1.68 ± 0.10mn | 4.82 ± 0.19d |
| TSy1-46 | 2.35 ± 0.09e | 4.00 ± 0.04h | BDy2-47 | 1.63 ± 0.06no | 4.73 ± 0.13d |
| BNy1-8 | 2.30 ± 0.09ef | 4.03 ± 0.07gh | BDy2-62 | 1.61 ± 0.09no | 5.00 ± 0.07b |
| ZZt1-16 | 2.26 ± 0.08ef | 4.01 ± 0.02gh | GHy1-10 | 1.58 ± 0.06no | 5.00 ± 0.01b |
| BDy3-39 | 2.25 ± 0.03ef | 3.86 ± 0.11m | GHy1-1 | 1.52 ± 0.09o | 5.03 ± 0.04b |
| BDy2-46 | 2.15 ± 0.15fg | 4.04 ± 0.03gh | TSy1-34-1 | 1.51 ± 0.08o | 3.83 ± 0.09o |
| BNy1-2 | 2.14 ± 0.04fg | 4.10 ± 0.08gh | TSy1-39 | 1.49 ± 0.04op | 4.50 ± 0.01f |
| TSy1-33 | 2.06 ± 0.12h | 3.92 ± 0.10kl | GHy1-8 | 1.47 ± 0.10op | 4.98 ± 0.05b |
| GHy1-11 | 2.06 ± 0.06h | 4.06 ± 0.04gh | TSy1-1 | 1.36 ± 0.08pq | 5.07 ± 0.06b |
| ZZt1-18 | 2.06 ± 0.08h | 4.05 ± 0.12gh | BDy2-35 | 1.27 ± 0.01q | 5.08 ± 0.04b |
| ZZt1-22 | 2.02 ± 0.14hi | 3.95 ± 0.03i | BNy1-7 | 1.25 ± 0.09q | 5.09 ± 0.08b |
| ZZt1-3 | 1.97 ± 0.05hij | 4.13 ± 0.16gh | TSy1-30 | 1.22 ± 0.05q | 3.77 ± 0.04p |
| ZZt1-5 | 1.97 ± 0.11hijk | 4.09 ± 0.15gh | BNy1-4 | 1.12 ± 0.08r | 5.55 ± 0.10a |
| ZZt1-7 | 1.95 ± 0.10hijkl | 4.12 ± 0.10gh | BNy1-6 | 0.82 ± 0.04s | 5.56 ± 0.18a |
| BDy2-17 | 1.89 ± 0.06ijkl | 4.08 ± 0.11gh |
Values with different lowercase letters show significant (P < 0.05).
The characteristics and type strains of screened LAB strains are shown in Table 4. Two strains were Gram-positive and Catalase-negative. Strain BDy3-10 was a homofermentative LAB, and TSy1-3 was a heterofermentative LAB. The two strains grew normally in the range of 20–45 °C, but grew weakly at 10 °C and 50 °C. They were able to grow at pH values ranging from 3.5 to 7.0, and grew weakly at pH 3.0 tolerating salt (MRS with 3.0% and 6.5% NaCl concentrations, respectively) which limited their growth.
Table 4.
Physiological and biochemical characteristics of LAB.
| Characteristics | BDy3-10 | TSy1-3 |
|---|---|---|
| Gram stain | + | + |
| Fermentation type | Homo | Hetero |
| Catalase | − | − |
| Growth at pH | ||
| 3.0 | w | w |
| 3.5 | + | + |
| 4.0 | + | + |
| 4.5 | + | + |
| 5.0 | + | + |
| 6.0 | + | + |
| 6.5 | + | + |
| 7.0 | + | + |
| Growth at temp | ||
| 10 °C | w | w |
| 20 °C | + | + |
| 35 °C | + | + |
| 40 °C | + | + |
| 45 °C | + | + |
| 50 °C | w | w |
| Growth in NaCl | ||
| 3% | − | − |
| 6.5% | − | − |
+ positive.
− negative.
w weakly positive, Homo homofermentative, Hetero heterofermentative.
16S rRNA analysis of screened LAB
The 16S rRNA sequences were compared using the NCBI database (see Table 5), and the results showed that the similarities between all sequences obtained here and several the known 16S rRNA gene sequences in the database were 99.0–100.0%.
Table 5.
BLAST alignment of 16S rRNA sequences of LAB.
| Strain number | Related species | Gene bank strain number | Similarity (%) |
|---|---|---|---|
| BDy3-10 | Lactobacillus rhamnosus | LR134331.1 | 99.70 |
| TSy1-3 | Lactobacillus buchneri | KR055504.1 | 98.80 |
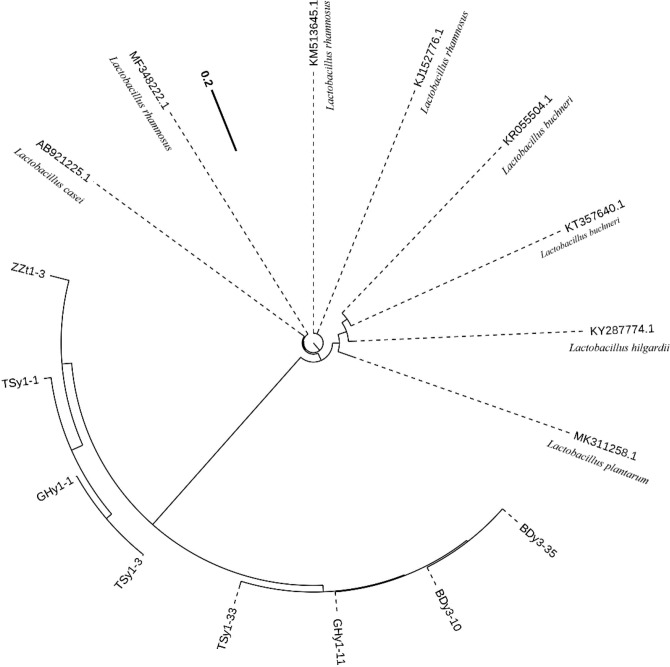
The phylogenetic tree of the partial 16S rDNA sequence of BDy3-10 and TSy1-3 are presented in Fig. 2. The sequence of strain BDy3-10 is closely related to that of L. rhamnosus, with 99.7% similarity in their 16S rRNA gene sequences. The 16S rRNA gene sequence of the TSy1-3 strain showed 98.8% similarity to the corresponding sequence, in L. buchneri, confirming its identity.
Figure 2.
Polygenetic tree showing the relative positions of BDy3-10, TSy1-3 and related species, as inferred by the neighbor-joining method using complete 16S rRNA sequences.
Effect of screened strains on the chemical compositions and aerobic stability of alfalfa silage
The results of the alfalfa silage chemical composition analysis are shown in Table 6. The DM and WSC contents of the TSy1-3 group treatment were significantly higher than that of the CK group (P > 0.05). The CP contents of all additive treatment groups were significantly higher than that of the CK group (P > 0.05), while the BDy3-10 treatment had the highest CP content. The CF content of the combination of TSy1-3 strain and BDy3-10 strain treatments was lower than the CK group, but the alone inoculation of TSy1-3 strain and BDy3-10 strain were higher than the CK group. The NDF and ADF contents in the TSy1-3 treatment group were significantly lower than those in the other groups (P < 0.05), and the EE and Ash contents in all treatments were not significantly different (P > 0.05). After 40 d of ensiling, the aerobic stability of all additive treatment groups was significantly higher than the CK treatment group (P < 0.05), and the TSy1-3 was the highest, at 173 h.
Table 6.
Chemical composition and aerobic stability after 40 days of ensiling.
| Item | CK | BDy3-10 | TSy1-3 | BDy3-10 + TSy1-3 |
|---|---|---|---|---|
| DM (% FM) | 33.49 ± 0.01b | 32.24 ± 0.25d | 34.31 ± 0.31a | 33.04 ± 0.15c |
| CP (% DM) | 20.00 ± 0.55b | 21.86 ± 0.54a | 21.24 ± 0.66a | 21.41 ± 0.48a |
| EE (% DM) | 9.01 ± 0.57 | 9.34 ± 0.24 | 9.26 ± 0.35 | 9.81 ± 1.53 |
| CF (% DM) | 26.21 ± 5.68 | 27.45 ± 1.19 | 27.16 ± 1.23 | 25.69 ± 1.52 |
| NDF (% DM) | 32.14 ± 0.88a | 33.84 ± 0.95a | 28.81 ± 1.23b | 32.93 ± 1.87a |
| ADF (% DM) | 20.26 ± 0.94a | 21.14 ± 0.31a | 17.90 ± 0.88b | 21.14 ± 0.60a |
| WSC (% DM) | 1.47 ± 0.07b | 0.99 ± 0.02c | 1.94 ± 0.05a | 1.06 ± 0.03c |
| Ash (% DM) | 10.85 ± 0.81 | 11.20 ± 0.55 | 10.61 ± 0.57 | 10.55 ± 0.13 |
| Aerobic stability (h) | 121 ± 0.87d | 129 ± 0.59c | 173 ± 0.42a | 144 ± 0.43b |
Values with different lowercase letters show significant (P < 0.05) differences in the same treatment.
CK control, BDy3-10 L. rhamnosus, TSy1-3 L. buchneri.
Effects of isolated strains on the fermentation quality of alfalfa during ensiling
The changes in LA, AA and PA that occurred during ensiling are shown in Table 7. The LA content reached a maximum value for T10 silages, and then decreased dramatically until T40 in all treatments (P < 0.001). AA and PA contents increased with prolonged fermentation (P < 0.05). The content of LA was generally higher in the BDy3-10 treatment than in the other treatments during ensiling, except at T40 (P < 0.05), and reached a maximum value (57.03 mmol) for the T10 silage. The AA content was significantly higher in the CK silage than in the inoculated silages during ensiling (P < 0.05). The PA contents of all treatment groups were significantly lower than that of the CK treatment group (P < 0.05).
Table 7.
Contents of LA, AA, PA and pH dynamic changes during ensiling.
| Items | Ensiling day | CK | BDy3-10 | TSy1-3 | BDy3-10 + TSy1-3 | P value |
|---|---|---|---|---|---|---|
| LA (mmol) | T1 | 1.05 ± 1.05Bb | 1.70 ± 0.10Da | 1.29 ± 0.38Bab | 1.32 ± 0.20Cab | 0.010 |
| T6 | 39.70 ± 0.79Ab | 45.85 ± 1.07Ba | 44.21 ± 1.61Aa | 44.84 ± 1.11Aa | < 0.001 | |
| T10 | 40.84 ± 3.38Ac | 57.03 ± 0.09Aa | 47.15 ± 1.35Ab | 46.09 ± 0.75Ab | < 0.001 | |
| T20 | 39.54 ± 6.83Ab | 47.18 ± 3.26Ba | 42.4 ± 0.56Aab | 37.31 ± 1.29Bb | 0.023 | |
| T40 | 40.27 ± 4.23A | 42.88 ± 0.35C | 42.98 ± 5.53A | 38.98 ± 2.67B | 0.255 | |
| P value | < 0.001 | < 0.001 | < 0.001 | < 0.001 | ||
| AA (mmol) | T1 | 3.61 ± 1.30Ca | 1.16 ± 0.03Db | 1.50 ± 0.05Eb | 1.90 ± 0.04Cb | 0.002 |
| T6 | 9.77 ± 1.11Ba | 2.93 ± 0.03Cb | 3.58 ± 0.14Db | 3.40 ± 0.33Cb | < 0.001 | |
| T10 | 10.95 ± 1.54ABa | 6.05 ± 0.29Bb | 6.56 ± 0.16Cb | 7.43 ± 1.30Bb | < 0.001 | |
| T20 | 11.45 ± 0.96ABa | 7.22 ± 1.73ABc | 8.59 ± 0.26Bb | 8.30 ± 0.49ABbc | 0.463 | |
| T40 | 13.10 ± 0.99Aa | 8.54 ± 0.22Ac | 11.11 ± 0.01Ab | 9.95 ± 1.78Abc | < 0.001 | |
| P value | < 0.001 | < 0.001 | < 0.001 | < 0.001 | ||
| PA (mmol) | T1 | 0.14 ± 0.04 | 0.11 ± 0.02B | 0.11 ± 0.03B | 0.10 ± 0.01B | 0.202 |
| T6 | 0.21 ± 0.03a | 0.11 ± 0.05Bb | 0.15 ± 0.01Bb | 0.11 ± 0.01Bb | 0.010 | |
| T10 | 0.29 ± 0.05 | 0.33 ± 0.06A | 0.29 ± 0.06A | 0.34 ± 0.01A | 0.624 | |
| T20 | 0.55 ± 0.30 | 0.34 ± 0.05A | 0.30 ± 0.06A | 0.36 ± 0.01A | 0.117 | |
| T40 | 0.60 ± 0.22 | 0.34 ± 0.10A | 0.32 ± 0.07A | 0.40 ± 0.09A | 0.181 | |
| P value | 0.019 | < 0.001 | < 0.001 | < 0.001 | ||
| pH | T1 | 6.42 ± 0.12A | 6.24 ± 0.25A | 6.06 ± 0.08A | 6.19 ± 0.16A | 0.263 |
| T6 | 6.26 ± 0.12Aa | 5.16 ± 0.02Bb | 5.03 ± 0.07Bb | 5.12 ± 0.06Bb | < 0.001 | |
| T10 | 6.24 ± 0.18ABa | 5.05 ± 0.26Bb | 5.10 ± 0.16Bb | 5.01 ± 0.17Bb | < 0.001 | |
| T20 | 5.98 ± 0.11Ba | 5.12 ± 0.02Bb | 4.99 ± 0.03Bb | 5.00 ± 0.08Bb | < 0.001 | |
| T40 | 5.58 ± 0.05Ca | 5.08 ± 0.02Bb | 5.00 ± 0.03Bc | 5.06 ± 0.02Bbc | < 0.001 | |
| P value | < 0.001 | < 0.001 | < 0.001 | < 0.001 |
a–cMeans having different letter superscripts within column are significantly different (P < 0.05).
A–EMeans having different letter superscripts within row are significantly different (P < 0.05).
LA lactic acid, AA acetic acid, PA propionic acid.
The pH values at T1 ensiling time were approximately 6.0 for all treatments and decreased progressively from T1 to T40. The lowest pH value was recorded for the TSy1-3 treatment at T20 (4.99). The pH value of the CK treatment at T40 was 5.58, which was significantly higher than those of all inoculant treatments (P < 0.001). All inoculant treatments were significantly similar in terms of pH values at the T40 ensiling time, and all values tended to be 5.00.
Discussion
In the present study, biochemical and phylogenetic analyses revealed that all of the characterized LAB belong to the genus Lactobacillus. However, previous studies found that the natural fermentation processes in forage crop and grass silages were dominated by Leuconostoc, Lactococcus, Enterococcus, and Pediococcus, not Lactobacillus species25–27. A plausible reason may be that the bacterial colonization of fresh crops and plants is controlled by many factors, such as the plant material. Shah et al. found28 that king grass silage was dominated by P. acidilactici and L. plantarum, while Italian ryegrass (Lolium multiflorum Lam.) silage was dominated by P. acidilactici and L. rhamnosus12. However, Ennahar et al. reported15 that the LAB species of paddy rice silage in Japan, included P. acidilactici and Weissella kimchi. In addition, Ni et al. found16 that the LAB species of rice silage in Henan, China include P. pentosaceus, Enterococcus mundtii and L. garvieae. Hence, the LAB species of the same material in different regions were also different. The LAB characteristics and storage temperature exerted strong effects on the fermentation quality29. The strain BDy3-10 (L. rhamnosus) had a high acid production rate that could result in the inhibition of aerobic microorganism activities and the reduction of fermentation substrates, while the strain TSy1-3 (L. buchneri) had the fastest growth, which that could shorten fermentation time, decrease nutritional loss and improved silage quality. The temperature of silage may rise to above 40 °C at the beginning of ensiling, which is due to the continuous plant respiration and activity of aerobic microorganisms when air still exists in the plant gap, particularly in the tropics and subtropics30. These results were consistent with those of our study, in which the screened strains BDy3-10 and TSy1-3 could grow at 45 °C, but they were both unable to tolerate salt. These results implied that they are very tolerant of acid and high temperature, satisfying the demands for growth in low-pH and high-temperature environments. This is a suitable method for silage preparation in warm and humid areas.
Previous studies reported that LAB inoculation could reduce DM loss during ensiling31,32. Higher residual WSC contents indicate smaller DM losses during fermentation that result in silage with a higher nutritive value33. In our study, lower DM losses and higher residual WSC contents occurred in the TSy1-3 treatment than in the other groups. Because the TSy1-3 strain grew fast in 24 h, it ensured rapid and vigorous LA fermentation and faster reduction of silage pH at earlier stages, which depressed the loss of WSC by fermentation via undesirable bacteria. The low NDF and ADF contents had a positive effect on the silage nutritive value and enhanced digestibility. In our study, the NDF and NDF contents in all inoculant treatments were lower than those in the control. This was similar to the result showing that inoculating LAB results in the highest decline of ADF and NDF, which improves feed intake and digestibility34. CP is the critical factor affecting the quality of commercial feed and roughage for ruminants35. Higher CP contents were obtained by all inoculants compared to the control. This could be related to the rapid reduction in pH caused by the addition of inoculants, which inhibited the growth and proteolytic activity of microorganisms such as Clostridia36. The highest CP content was found in the BDy3-10 treatment. BDy3-10 is a homofermentative LAB that is more efficient in lactic acid production than heterofermentative LAB and can ferment a wide variety of substrates and quickly produce large amounts of lactic acid37.
The pH value is considered a very important indicator for the fermentation profile and fermentation quality of ensiled materials38. The pH values of the control silages were generally higher than those of the inoculated silages. It is generally desired that the pH value is approximately 3.8–4.2 for any high-quality silage. In this study, the measured pH values reached approximately 5.00 at T40 with inoculation. This was because alfalfa is a high-protein forage crop that has a high buffering capacity and a slow rate of pH reduction during ensiling37. This result was similar to results from studies in which the pH value of alfalfa silage inoculated with LAB was 4.8–5.313,39.
Various studies have reported that the application of homo-LAB inoculants can beneficially enhance the LA concentration and decrease AA40. Compared with homo-LAB fermentation, fermentation dominated by hetero-LAB is characterized by a higher pH and AA content but lower LA content34,41. This was consistent with inoculation results in this study. Treatment BDy3-10 had a higher LA content, and treatment TSy1-3 had a higher AA because strain BDy3-10 is a homo-LAB and strain TSy1-3 is a hetero-LAB. Furthermore, the higher AA can increase the aerobic stability of silage8, and it may have inhibited the growth and proteolytic activity of microorganisms such as clostridia42.
Conclusion
The two strains used in this study were suitable as inoculants for alfalfa silage, and the TSy1-3 strains is good choice as an addictive in silage under the warm and humid climate in karst area.
Acknowledgements
This work was supported by the Science and Technology Support (Agriculture) Project of Guizhou Province ([2020]1Y046) and ([2017]2504-2).
Author contributions
C.P. wrote the main manuscript text, W.S. and X.D. provided a few data analysis helps, L.Z. provided academic helps, J.H. provided a lots of writing skills and saved a few difficulty problems in writing manuscript. All authors reviewed the manuscript.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Wang S, Yuan X, Dong Z, Li J, Guo G, Bai Y. Characteristics of isolated lactic acid bacteria and their effects on the silage quality. Asian Australas. J. Anim. Sci. 2017;30:819–827. doi: 10.5713/ajas.16.0589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Muck RE, Nadeau EMG, McAllister TA, Contreras-Govea FE, Santos MC, Kung L., Jr Silage review: recent advances and future uses of silage additives. J. Dairy Sci. 2018;101:3980–4000. doi: 10.3168/jds.2017-13839. [DOI] [PubMed] [Google Scholar]
- 3.Santos AO, Ávila CL, Pinto JC, Carvalho BF, Dias DR, Schwan RF. Fermentative profile and bacterial diversity of corn silages inoculated with new tropical lactic acid bacteria. J. Appl. Microbiol. 2016;120:266–279. doi: 10.1111/jam.12980. [DOI] [PubMed] [Google Scholar]
- 4.Xu S, Yang J, Qi M, Smiley B, Rutherford W, Wang Y, McAllister TA. Impact of Saccharomyces cerevisiae and Lactobacillus buchneri on microbial communities during ensiling and aerobic spoilage of corn silage1. J. Anim. Sci. 2019;97(3):1273–1285. doi: 10.1093/jas/skz021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rabelo CHS, Basso FC, Lara EC, Jorge LGO, Mari LJ, Reis RA. Effects of Lactobacillus buchneri as a silage inoculant and as a probiotic on feed intake, apparent digestibility and ruminal fermentation and microbiology in weathers fed low-dry-matter whole-crop maize silage. Grass Forage Sci. 2017;73:67–77. doi: 10.1111/gfs.12303. [DOI] [Google Scholar]
- 6.Reich LJ, Kung L. Effects of combining lactobacillus buchneri 40788 with various lactic acid bacteria on the fermentation and aerobic stability of corn silage. Anim. Feed Sci. Technol. 2010;159:105–109. doi: 10.1016/j.anifeedsci.2010.06.002. [DOI] [Google Scholar]
- 7.Duniere L, Xu S, Long J, Elekwachi C, Wang Y, Turkington K, Forster R, McAllister TA. Bacterial and fungal core microbiomes associated with small grain silages during ensiling and aerobic spoilage. BMC Microbiol. 2017;17:50. doi: 10.1186/s12866-017-0947-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ferrero F, Piano S, Tabacco E, Borreani G. Effects of conservation period and Lactobacillus hilgardii inoculum on the fermentation profile and aerobic stability of whole corn and sorghum silages. J. Sci. Food Agric. 2018;99:2530–2540. doi: 10.1002/jsfa.9463. [DOI] [PubMed] [Google Scholar]
- 9.Drouin, P., Tremblay, J., Chaucheyras-Durand, F. Dynamic succession of microbiota during ensiling of whole plant corn following inoculation with lactobacillus buchneri and lactobacillus hilgardii alone or in combination. Microorganisms.7, 595 (2019). [DOI] [PMC free article] [PubMed]
- 10.Ferrero F, Tabacco E, Piano S, Casale M, Borreani G. Temperature during conservation in laboratory silos affects fermentation profile and aerobic stability of corn silage treated with Lactobacillus buchneri, Lactobacillus hilgardii, and their combination. J. Dairy Sci. 2021;104(2):1696–1713. doi: 10.3168/jds.2020-18733. [DOI] [PubMed] [Google Scholar]
- 11.Silva NC, Nascimento CF, Nascimento FA, de Resende FD, Daniel JLP, Siqueira GR. Fermentation and aerobic stability of rehydrated corn grain silage treated with different doses of Lactobacillus buchneri or a combination of Lactobacillus plantarum and Pediococcus acidilactici. J. Dairy Sci. 2018;101:4158–4167. doi: 10.3168/jds.2017-13797. [DOI] [PubMed] [Google Scholar]
- 12.Gulfam A, Gou G, Tajebe S, Chen L, Liu QH, Yuan XJ, Bai YF, Saho T. Characteristics of lactic acid bacteria isolate and their effect on the fermentation quality of Napier grass silage at three high temperatures. J. Sci. Food Agric. 2016;97:1931–1938. doi: 10.1002/jsfa.7998. [DOI] [PubMed] [Google Scholar]
- 13.Li DX, Ni KK, Zhang YC, Lin YL, Yang FY. Influence of lactic acid bacteria, cellulase, cellulase-producing Bacillus pumilus and their combinations on alfalfa silage quality. J. Integr. Agric. 2018;17:2768–2782. doi: 10.1016/S2095-3119(18)62060-X. [DOI] [Google Scholar]
- 14.Wang Y, Wang C, Zhou W, Yang FY, Chen XY, Zhang Q. Effects of wilting and Lactobacillus plantarum addition on the fermentation quality and microbial community of Moringa oleifera leaf silage. Front. Microbiol. 2018;9:1817. doi: 10.3389/fmicb.2018.01817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ennahar S, Cai Y, Fujita Y. Phylogenetic diversity of lactic acid bacteria associated with paddy rice silage as determined by 16S Ribosomal DNA analysis. Appl. Environ. Microbiol. 2003;69:444–451. doi: 10.1128/AEM.69.1.444-451.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ni KK, Wang YP, Li DG, Cai YM, Pang HL. Characterization, identification and application of lactic acid bacteria isolated from forage paddy rice silage. PLoS ONE. 2015;10:e0121967. doi: 10.1371/journal.pone.0121967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yan YH, Li XM, Guan H, Huang LK, Ma X, Peng Y, Li Z, Ni G, Zhou JQ, Yang WY, Cai YM, Zhang XQ. Microbial community and fermentation characteristic of Italian ryegrass silage prepared with corn stover and lactic acid bacteria. Bioresour. Technol. 2019;279:166–173. doi: 10.1016/j.biortech.2019.01.107. [DOI] [PubMed] [Google Scholar]
- 18.Guan H, Ke WC, Yan YH, Shuai Y, Li XL, Ran QF, Yang ZF, Wang X, Cai YM, Zhang XQ. Screening of natural lactic acid bacteria with potential effect on silage fermentation, aerobic stability and aflatoxin B1 in hot and humid area. J. Appl. Microbiol. 2020;128:1301–1311. doi: 10.1111/jam.14570. [DOI] [PubMed] [Google Scholar]
- 19.Cai Y, Ohmomo S, Ogawa M, Kumai S. Effect of applying lactic acid bacteria isolated from forage crops on fermentation characteristics and aerobic deterioration of silage. J. Dairy Sci. 1999;82:520–526. doi: 10.3168/jds.S0022-0302(99)75263-X. [DOI] [PubMed] [Google Scholar]
- 20.Bao W, Mi ZH, Xu HY, Zheng Y, Kwok LY, Zhang HY. Assessing quality of Medicago sativa silage by monitoring bacterial composition with single molecule, real-time sequencing technology and various physiological parameters. Sci. Rep. 2016;6:28358. doi: 10.1038/srep28358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.AOAC . Official Method of Analysis. 15. Association of Official Analytical Chemists; 1990. [Google Scholar]
- 22.Van SPJ, Robertson JB, Lewis BA. Methods for dietary fiber, neutral detergent fiber and nonstarch polysaccharides in relation to animal nutrition. J. Dairy Sci. 1991;74:3583–3597. doi: 10.3168/jds.S0022-0302(91)78551-2. [DOI] [PubMed] [Google Scholar]
- 23.Ke WC, Ding WR, Xu DM, Ding LM, Zhang P, Li FH, Li FD, Guo XS. Effects of addition of malic or citric acids on fermentation quality and chemical characteristics of alfalfa silage. J. Dairy Sci. 2017;100:8958–8966. doi: 10.3168/jds.2017-12875. [DOI] [PubMed] [Google Scholar]
- 24.Zhang Q, Li XJ, Zhao MM, Yu Z. Lactic acid bacteria strains for enhancing the fermentation quality and aerobic stability of Leymus chinensis silage. Grass Forage Sci. 2015;71:472–481. doi: 10.1111/gfs.12190. [DOI] [Google Scholar]
- 25.Filya I, Ashbell G, Hen Y, Weinberg ZG. The effect of bacterial inoculants on the fermentation and aerobic stability of whole crop wheat silage. Anim. Feed Sci. Technol. 2000;88:39–46. doi: 10.1016/S0377-8401(00)00214-5. [DOI] [Google Scholar]
- 26.Kleinschmit DH, Kung L. A meta-analysis of the effects of Lactobacillus buchneri on the fermentation and aerobic stability of corn and grass and small-grain silages. J. Dairy Sci. 2006;89:4005–4013. doi: 10.3168/jds.S0022-0302(06)72444-4. [DOI] [PubMed] [Google Scholar]
- 27.Rossi F, Dellaglio F. Quality of silages from Italian farms as attested by number and identify of microbial indicators. J. Appl. Microbiol. 2007;103:1707–1715. doi: 10.1111/j.1365-2672.2007.03416.x. [DOI] [PubMed] [Google Scholar]
- 28.Shah AA, Xianjun Y, Zhihao D, Junfeng L, Shao T. Isolation and molecular identification of lactic acid bacteria from King grass and their application to improve the fermentation quality of sweet Sorghum. World J. Microbiol. Biotechnol. 2017;34:4. doi: 10.1007/s11274-017-2387-2. [DOI] [PubMed] [Google Scholar]
- 29.Weinberg ZG, Szakacs G, Ashbell G, Hen Y. The effect of temperature on the ensiling process of corn and wheat. J. Appl. Microbiol. 2001;90:561–566. doi: 10.1046/j.1365-2672.2001.01276.x. [DOI] [PubMed] [Google Scholar]
- 30.Bernardes TF, Daniel JLP, Adesogan AT, Drouin P, Nussio LG, Tremblay GF, Bélanger G, Cai Y. Silage review: unique challenges of silages made in hot and cold regions. J. Dairy Sci. 2018;101:4001–4019. doi: 10.3168/jds.2017-13703. [DOI] [PubMed] [Google Scholar]
- 31.Zhao C, Wang L, Ma G, Jiang X, Yang J, Lv J, Zhang Y. Cellulase interacts with lactic acid bacteria to affect fermentation quality, microbial community, and ruminal degradability in mixed silage of soybean residue and corn stover. Animals Basel. 2021;11:334. doi: 10.3390/ani11020334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Oliveira AS, Weinberg ZG, Ogunade IM, Arriola KG, Jiang Y, Kim D, Li X, Gonçalves MCM, Vyas D, Adesogan AT. Meta-analysis of effects of inoculation with homofermentative and facultative heterofermentative lactic acid bacteria on silage fermentation, aerobic stability, and the performance of dairy cows. J. Dairy Sci. 2017;100:4587–4603. doi: 10.3168/jds.2016-11815. [DOI] [PubMed] [Google Scholar]
- 33.Weinberg ZG, Shatz O, Chen Y. Effect of lactic acid bacteria inoculants on in vitro digestibility of wheat and corn silages. J. Dairy Sci. 2007;90:4754–4762. doi: 10.3168/jds.2007-0176. [DOI] [PubMed] [Google Scholar]
- 34.Hu W, Schmidt RJ, Mcdonell EE, Klingerman CM, Kung L. The effect of Lactobacillus buchneri 40788 or Lactobacillus plantarum MTD-1 on the fermentation and aerobic stability of corn silages ensiled at two dry matter contents. J. Dairy Sci. 2009;92:3907–3914. doi: 10.3168/jds.2008-1788. [DOI] [PubMed] [Google Scholar]
- 35.Silva VP, Pereira OG, Leandro ES, Ribeiro KG, Pieper R, Hackl W, Korn U, Zeyner A, Souffrant W, Pieper B. Effect of ensiling triticale, barley and wheat grains at different moisture content and addition of Lactobacillus plantarum (DSMZ 8866 and 8862) on fermentation characteristics and nutrient digestibility in pigs. Anim. Feed Sci. Technol. 2011;164:96–105. doi: 10.1016/j.anifeedsci.2010.11.013. [DOI] [Google Scholar]
- 36.Arriola KG, Kim SC, Adesogan AT. Effect of applying inoculants with hetero-lactic or homolactic and hetero-lactic bacteria on the fermentation and quality of corn silage. J. Dairy Sci. 2011;94:1511–1516. doi: 10.3168/jds.2010-3807. [DOI] [PubMed] [Google Scholar]
- 37.McDonald, P., Henderson, A. R. & Heron, S. J. E. The Biochemistry of Silage 2nd edn. (Chalcombe Publications, Marlow, Bucks, UK, 1991).
- 38.Denek N, Can A, Avci M, Aksu T, Durmaz H. The effect of molasses-based pre-fermented juice on the fermentation quality of first-cut lucerne silage. Grass Forage Sci. 2011;66:243–250. doi: 10.1111/j.1365-2494.2011.00783.x. [DOI] [Google Scholar]
- 39.Ertekin I, Kızılşimşek M. Effects of lactic acid bacteria inoculation in pre-harvesting period on fermentation and feed quality properties of alfalfa silage. Asian Australas. J. Anim. Sci. 2019;2:245–253. doi: 10.5713/ajas.18.0801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Contreras-Govea FE, Muck RE, Broderick GA, Weimer PJ. Lactobacillus plantarum effects on silage fermentation and in vitro microbial yield. Anim. Feed Sci. Technol. 2013;179:61–68. doi: 10.1016/j.anifeedsci.2012.11.008. [DOI] [Google Scholar]
- 41.Tabacco E, Piano S, Revello-Chion A, Borreani G. Effect of Lactobacillus buchneri LN4637 and Lactobacillus buchneri LN40177 on the aerobic stability, fermentation products, and microbial populations of corn silage under farm conditions. J. Dairy Sci. 2011;94:5589–5598. doi: 10.3168/jds.2011-4286. [DOI] [PubMed] [Google Scholar]
- 42.Heinritz SN, Martens SD, Avila P, Hoedtke S. The effect of inoculant and sucrose addition on the silage quality of tropical forage legumes with varying ensilability. Animal Feed Sci. Technol. 2012;174:201–210. doi: 10.1016/j.anifeedsci.2012.03.017. [DOI] [Google Scholar]