Abstract
Trophic ecology of detrital-based food webs is still poorly understood. Abyssal plains depend entirely on detritus and are among the most understudied ecosystems, with deposit feeders dominating megafaunal communities. We used compound-specific stable isotope ratios of amino acids (CSIA-AA) to estimate the trophic position of three abundant species of deposit feeders collected from the abyssal plain of the Northeast Pacific (Station M; ~ 4000 m depth), and compared it to the trophic position of their gut contents and the surrounding sediments. Our results suggest that detritus forms the base of the food web and gut contents of deposit feeders have a trophic position consistent with primary consumers and are largely composed of a living biomass of heterotrophic prokaryotes. Subsequently, deposit feeders are a trophic level above their gut contents making them secondary consumers of detritus on the abyssal plain. Based on δ13C values of essential amino acids, we found that gut contents of deposit feeders are distinct from the surrounding surface detritus and form a unique food source, which was assimilated by the deposit feeders primarily in periods of low food supply. Overall, our results show that the guts of deposit feeders constitute hotspots of organic matter on the abyssal plain that occupy one trophic level above detritus, increasing the food-chain length in this detritus-based ecosystem.
Subject terms: Stable isotope analysis, Ecology, Ocean sciences
Introduction
Detritus is the main standing stock of organic matter for most ecosystems, both aquatic and terrestrial1, and its importance in ecosystem functioning has long been noted2. Since detritus is the most abundant source of carbon at the base of food webs3, decomposers are the dominant feeding guild on Earth4. Most decomposers are prokaryotes and small invertebrate detritivores5. Thus, detritus-based food webs have highly complex trophic-dynamics and, contrary to primary producer-based food webs, are difficult to compartmentalize into trophic levels. As a consequence, trophic ecology has largely neglected the inclusion of detritus and its pathways into higher trophic levels6,7, and instead they often appear illustrated as a black box in the flow of carbon or energy through food webs.
Most deep-sea ecosystems, where there is no in situ primary production (except for chemoautotrophy at e.g. hydrothermal vents and cold seeps), entirely depend on detritus arriving from surface waters8–10. Detritus is mainly consumed as it sinks through the water column before reaching the deep seafloor (on average only < 5% of surface production reaches abyssal depths9,11), so these ecosystems are largely limited by food, which often arrives with marked seasonality12. The deep ocean is the largest ecosystem on Earth, and within it the abyssal seafloor occurring between 3000 and 6000 m depth, covers 54% of the Earth’s surface13. Abyssal plains can host high faunal diversity and given their vast size are a major reservoir of biodiversity9, which can exert a significant influence on the carbon cycle. Thus, understanding the energy flow within the deep-sea detrital food web and the trophic ecology of abyssal species is of global importance.
Deposit feeders are adapted to live in such food-poor environments and unlike many ecosystems, on the abyssal plains they can be very large, dominating megafaunal communities10,14,15. Deposit feeders play a key role in abyssal ecosystems since they rapidly consume detritus reaching the seafloor and are major sediment bioturbators16–18. They employ an array of feeding and digestive strategies that give them a competitive advantage to cope with food scarcity19,20. Some species of mobile deposit feeders selectively feed on freshly deposited patches of organic matter20–22. In other species the gut anatomy allows for efficient processing of ingested food by gut microbes23. In this sense, the guts of some deep-sea holothurians have been found to hold high abundance of prokaryotes relative to the surrounding sediments23–25. These microbial communities seem to increase the efficiency of deposit feeders to exploit the limiting resources, however, their functional role and whether they are selected from the environment or have a symbiotic origin remain unknown. It has been suggested that these microbial communities act as “commensal” flora to the deposit feeder25, possibly transforming refractory organic matter into compounds easier to digest26, or they might be used as a nutritional source for the deposit feeder25,27. Overall, gut microbial communities might be a crucial yet overlooked component of abyssal food webs.
In recent years, compound-specific stable isotope analysis of amino acids (CSIA-AA) has proven useful in estimating trophic position of metazoans more accurately than bulk isotope analysis28,29. The calculation of trophic position using CSIA-AA is based on the differential enrichment in 15N of amino acids (AAs), which can be grouped into “source” and “trophic” AAs30. “Trophic” AAs, like glutamic acid, increase their δ15N values with trophic level, whereas “source” AAs, like phenylalanine, reflect the δ15N values at the base of the food web, changing little with trophic transfer31,32. The difference in δ15N values between glutamic acid and phenylalanine of an organism is used to estimate its trophic position. Thus, by applying CSIA-AA, information about trophic position and the δ15N values at the base of the food web can be obtained from a single sample. This technique has also been used to prove that in laboratory-cultured heterotrophic bacteria, metabolic reactions can result in changes of δ15N values in AAs similar to metazoans33,34. Moreover, δ15N values of “source” AAs and δ13C values of essential AAs provide information on dietary sources in consumers. CSIA-AA can therefore be used to estimate trophic position of decomposers and can help to disentangle trophic dynamics of detritus-based food webs.
In this study we investigated the trophic ecology of abyssal deposit feeders and the role of microbial assemblages present in their guts. We suggest that gut microbes play an important role in supplying nutrition to deposit feeders. For that, we estimated the trophic position of three species of deposit feeders collected from the abyssal plain of the Northeast Pacific (Station M; ~ 4000 m depth), their gut contents and the surrounding sediments using the nitrogen isotopic composition of amino acids. Furthermore, we investigated the role of gut microbes in deposit feeder nutrition using the carbon isotopic composition of essential amino acids. Our findings contribute to the understanding of the trophic dynamics of this deep-sea ecosystem and reveal that microbes present in the guts of deposit feeders might support a detritus-based food web that is longer than expected.
Results
Trophic position
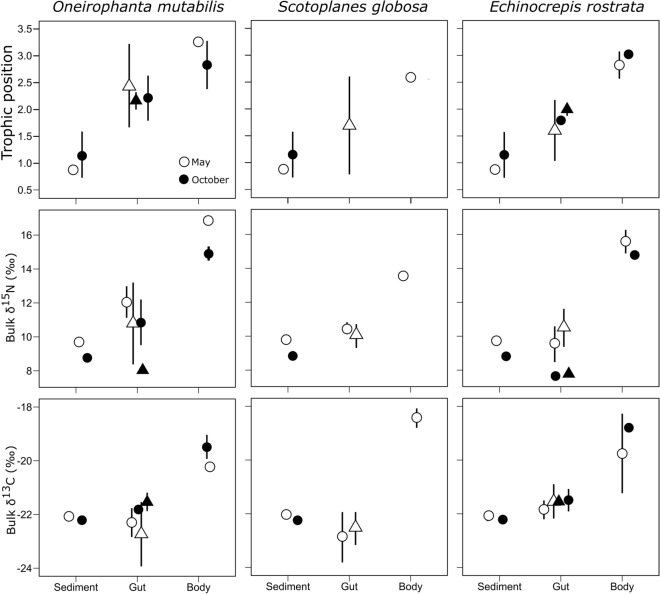
The average trophic position (TP), calculated from the AAs Glx and Phe, was 1.0 ± 0.2 (mean ± SD) for sediments. This confirms that a β value of 3.4‰ used in the calculation of trophic position was appropriate (see “Methods”). TP of deposit feeders (DF) was 2.9 ± 0.6 (Supplementary material, Table S1). Specifically, TP was 1.7 ± 0.1, 2.0 ± 0.2, and 2.4 ± 0.0 higher than that of surface sediments for S. globosa, E. rostrata, and O. mutabilis, respectively. Thus, deposit feeders were secondary consumers of detritus present in the surrounding sediments. Both foregut and hindgut contents were approximately one trophic level higher than sediments (ΔTP [≡ TPgut content – TPsediment]; ΔTPHindgut = 0.8 ± 0.6 for E. rostrata, 0.8 ± 0.9 for S. globosa and 1.3 ± 0.6 for O. mutabilis; ΔTPForegut = 0.65 for E. rostrata, and 1.1 ± 0.4 for O. mutabilis; Fig. 1). Thus, trophic position of foregut contents were not significantly different from those of hindguts (ANOVA, F = 0.057, P > 0.05).
Figure 1.
Trophic position, bulk δ15N and δ13C values for O. mutabilis, S. globosa and E. rostrata, their gut contents and the surrounding sediments, collected in the period of low food supply (i.e. May; open symbols) or in the period of high food supply (i.e. October; closed symbols). Within gut contents samples foregut (circles) or hindgut (triangles) are shown separately. Note that the same data from sediments are represented in all columns for comparison.
Bulk δ15N values of body tissue were also higher than those of sediments for the three species (Δδ15N [≡ δ15Ndeposit feeder—δ15Nsediment] = 3.8‰ for S. globosa, 5.9‰ for E. rostrata, and 7.2‰ O. mutabilis); however, bulk δ15N values of gut contents were not different from those of sediments (ANOVA, F = 1.15, P > 0.05). Similarly to δ15N values, bulk δ13C values were comparable between sediments and gut contents but were higher for body tissue (Δδ13C [≡ δ13C deposit feeder—δ13C sediment] = 3.6‰ for S. globosa, 2.8‰ for E. rostrata, and 2.2‰ O. mutabilis). Trophic position was not significantly different between seasons for any species (Tukey test, P > 0.05) although O. mutabilis had a mean TP of 3.3 ± 0.0 and 2.8 ± 0.4 in periods of low and high food supply, respectively. However, bulk δ15N values of gut contents of all individuals collected were lower in the more productive period for both O. mutabilis (ANOVA, F = 8.19, P < 0.05, n = 8) and E. rostrata (ANOVA, F = 11.21, P < 0.05, n = 8).
Elemental composition of sediment and gut contents
The contribution of total nitrogen (TN) to surface sediments was 0.22 weight (wt.) % for both periods. The contribution of TN in the hindgut contents of DFs was twice as high, ranging from 0.32 wt.% to 0.51 wt.%. In the foregut of O. mutabilis it was three and four times higher in periods of low and high food supply, respectively (0.60 wt.% and 0.85 wt.%; Table 1). Within TN, which accounts for both inorganic and organic N, the contribution of N from AAs was between 18 and 35 times higher inside the guts than in the sediments (Table 1).
Table 1.
Total organic carbon (TOC), total nitrogen (TN) and contribution of N (% dry weight) from AAs to TN in surface sediments (0–5 mm) and gut contents (F: foregut; H: hindgut) of deposit feeders (mean ± SD) collected in periods of low food supply (i.e. May) and high food supply (i.e. October). The sample size was two for each mean value.
| %TOC | % TN | % AA/TN | |||||
|---|---|---|---|---|---|---|---|
| Low food supply | High food supply | Low food supply | High food supply | Low food supply | High food supply | ||
| Sediment | 1.4 ± 0.05 | 1.6 ± 0.02 | 0.22 ± 0.01 | 0.22 ± 0.02 | 0.28 ± 0.17 | 0.31 ± 0.09 | |
| O. mutabilis | F | 3.7 ± 1.0 | 5.8 ± 0.07 | 0.60 ± 0.23 | 0.83 ± 0.11 | 5.76 ± 2.05 | |
| H | 3.2 ± 1.4 | 3.5 ± 0.8 | 0.47 ± 0.27 | 0.46 ± 0.14 | 10.83 ± 3.39 | 5.48 ± 0.55 | |
| S. globosa | F | 3.0 ± 1.0 | 0.43 ± 0.15 | ||||
| H | 2.1 ± 0.07 | 0.32 ± 0.03 | 3.88 ± 3.45 | ||||
| E. rostrata | F | 2.3 ± 0.5 | 3.4 ± 0.8 | 0.35 ± 0.01 | 0.42 ± 0.11 | 9.38 ± 3.47 | |
| H | 2.2 ± 0.4 | 2.9 ± 0.3 | 0.39 ± 0.09 | 0.51 ± 0.00 | 2.02 ± 0.34 | 10.97 ± 8.74 | |
δ13C values of essential AA and δ15N values of source AAs
Results of PCA of normalized δ13C values of essential AAs (δ13CEAA) showed that PC1 and PC2 together explained 68.3% of the variation (Fig. 2). δ13CEAA values were not available for gut contents of S. globosa. The normalized δ13CEAA values differed significantly between sediments, gut contents and body tissue (PERMANOVA, P < 0.05). Phe and Val differed significantly between gut contents and body tissue, whereas Lys differed between sediments and gut contents (ANOVA, P < 0.05). After including gut contents and sediments as two food sources in the training set, the LDA grouped all DFs collected during low food supply with gut contents (probability > 99%), whereas during high food supply E. rostrata was grouped with sediments (prob. > 90%) and O. mutabilis showed a mixture of both sources (prob. 30–55% of gut contents).
Figure 2.
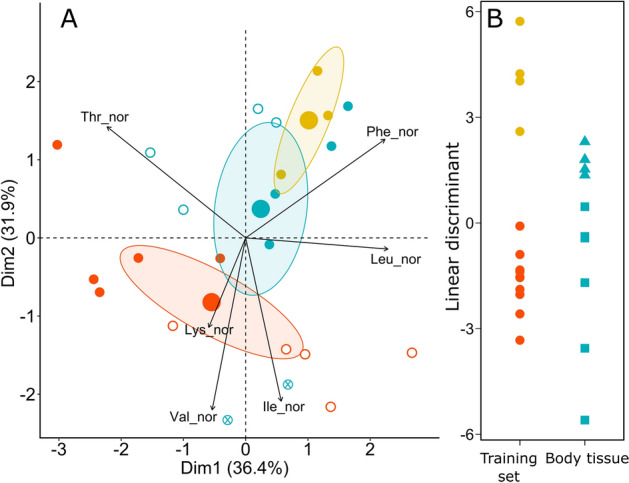
(A) Principal component analysis (PCA) using normalized δ13C values of essential amino acids (δ13CEAA) in surface sediments (yellow), gut contents (red) and body tissue (blue) of O. mutabilis (filled symbols), E. rostrata (open symbols), and body tissue of S. globosa (circled cross). Ellipses outline the 95% confidence region around the mean (large symbols) for each group. (B) Linear Discriminant Analysis (LDA) based on a training set built with δ13CEAA values of surface sediments (yellow) and gut contents (red). Body tissue samples (blue) were collected in the period of low food supply (i.e. May; squares) and in the period of high food supply (i.e. October; triangles).
δ15N values of “source” AAs (δ15NSrc-AA) in gut contents and body tissue in O. mutabilis (11.3 ± 1.3‰; mean ± SD) and S. globosa (11.1 ± 1.9‰) were similar to surrounding sediments (11.6 ± 1.3‰). However, E. rostrata body tissue (15.0 ± 0.3‰) and gut contents in the period of low food supply (18.1 ± 0.5‰) showed higher δ15NSrc-AA values relative to sediments (Fig. 3).
Figure 3.
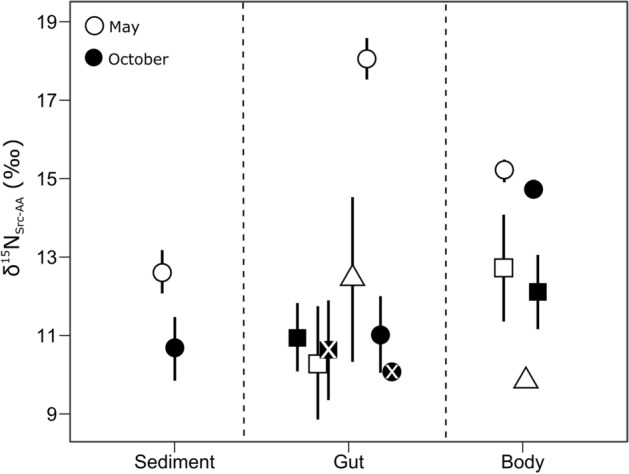
δ15N values of “source” AAs (δ15NSrc-AA; mean ± SD) of surface sediments (0–5 mm), gut contents and body tissue collected in the period of low food supply (i.e. May; open symbols) and in the period of high food supply (i.e. October; closed symbols). Gut contents (frontgut: crossed symbol; hindgut: plain symbol) and body tissue correspond to species of deposit feeders: O. mutabilis (squares), S. globosa (triangles) and E. rostrata (circles).
Degree of degradation of sediments and gut contents
DI values were significantly higher (i.e. less degraded) in gut contents of O. mutabilis (0.34 ± 0.07) and S. globosa (0.43 ± 0.42) than in those of E. rostrata (-0.97 ± 0.31; Tukey test, P < 0.01; Table 2). The lower %Mol Gly in gut contents of O. mutabilis (18.8 ± 1.71%) and S. globosa (13.3 ± 3.3%) also indicated a lower degree of degradation than in those of E. rostrata (45.0 ± 7.0%; Tukey test, P < 0.01; Table 2). DI values in sediments were higher in the period of low food supply (− 0.030) than in that of high food supply (− 0.94). ΣV values ranged between 2.6 and 4.9, indicating a high degree of heterotrophic microbial resynthesis. However, ΣV values were not significantly different among species or between species and sediments (Tukey test, P > 0.05; Table 2).
Table 2.
Degradation index (DI), ΣV parameter, and %Mol Gly in surface sediments (0–5 mm) and gut contents (F: foregut; H: hindgut) of deposit feeders (mean ± SD) collected in periods of low food supply (i.e. May) and high food supply (i.e. October). The sample size was two for each mean value, and for those values not including standard deviation the parameter was calculated from one sample.
| DI | ΣV | %Mol Gly | |||||
|---|---|---|---|---|---|---|---|
| Low food supply | High food supply | Low food supply | High food supply | Low food supply | High food supply | ||
| Sediment | -0.030 ± 0.026 | -0.94 ± 0.18 | 2.7 | 3.2 ± 0.3 | 15.9 ± 10.1 | 32.9 ± 4.5 | |
| O. mutabilis | F | 0.15 ± 0.18 | 2.7 ± 0.01 | 19.2 ± 0.6 | |||
| H | 0.33 ± 0.07 | 0.42 ± 0.05 | 4.0 ± 1.3 | 2.8 ± 0.2 | 20.5 ± 0.6 | 16.9 ± 0.8 | |
| S. globosa | F | ||||||
| H | 0.43 ± 0.42 | 3.2 | 13.3 ± 3.6 | ||||
| E. rostrata | F | -0.88 ± 0.24 | 3.2 | 38.4 ± 0.2 | |||
| H | -0.82 ± 0.38 | -1.21 ± 0.31 | 3.0 ± 0.04 | 49.8 ± 4.5 | 46.9 ± 9.3 | ||
Discussion
Here we used CSIA-AA to show that megafaunal deposit feeders (DF) were secondary consumers of detritus on the abyssal plain at Station M. This was shown by their trophic position, which was two trophic levels higher than the surrounding sediments. To this date, these are the first estimates of trophic position reported for deep-sea DFs using CSIA-AA approaches. These estimates were higher than expected for organisms feeding mostly on detritus, which are assumed to have trophic positions consistent with primary consumers. Previous research using stable isotope analysis of bulk material has reported high δ15N values in DFs relative to sediments, attributing such values to feeding selection35–38, to feeding on older, more recycled material35 or to a different trophic 15N-discrimination of ingested microbial biomass37. However, our results based on CSIA-AA, which are not dependent on assumptions of the δ15N value at the base of the food web, indicate that they are secondary consumers. In fact, this was true for the 3 species analyzed, despite their bulk δ15N values differing by 3.5‰, which typically corresponds to a difference of one trophic level39. Abyssal DFs are, therefore, trophically equivalent to zooplanktivorous fish or insectivorous birds.
Consequently, the main food source of DF must have a trophic position consistent with primary consumers. This agrees with the estimated trophic position of gut contents, which was around one trophic level higher than that of surface sediments (Fig. 1). One possible explanation is that DFs are highly selective of food from the environment, so their diet could be based on selected meiofauna, or metazoan remains, like zooplankton carcasses sinking from the water column. There is an extensive body of research addressing selectivity of abyssal benthic megafauna through time-lapse photography17, presence of phytopigments like chlorophyll a in the guts20,40–42, higher concentrations of organic carbon and nitrogen in their guts compared to surrounding sediments18, or excess 234Th activities in gut contents similar to sediment traps but higher than surface sediments21,43. In our study, the gut contents were 18 to 35 times enriched in N from AAs relative to sediments (Table 1), so the guts of DFs concentrate material highly enriched in AAs. However, despite the differences observed in trophic position (estimated from δ15N values of specific AAs), δ15N and δ13C values of bulk gut contents were not different from those of sediments (but see below for differences among species). If the material present in the guts had been selected and ingested from the environment, their high TP relative to sediments would have been reflected in higher bulk δ15N values as well4. However, 15N was not gained nor lost in the gut. This unchanged isotopic signal of bulk material can only be explained if the increase in TP of gut contents took place after the particles had been ingested. In other words, our results suggest that the estimated TP in the guts corresponded mainly to a living microbial biomass, whereas the bulk δ15N values remained unchanged because they integrated the living biomass with waste products, which became depleted in 15N but do not include AAs.
A remarkable abundance of bacteria in tentacles44 and guts26 of abyssal holothurians has been previously reported, with abundances 1.5-fold to fivefold higher than the surrounding sediments19,23,24. Also, a high RNA:DNA ratio found in the foregut of Oneirophanta mutabilis40 supported the idea of a high proliferation of bacteria in their guts. Although this microbial community could be selected from the environment, our hypothesis that they primarily grow inside the gut was supported by δ13C values of essential AAs (δ13CEAA). We found that surface sediments and gut contents had distinct δ13CEAA patterns (Fig. 2), suggesting that they are composed of different microbial communities because only organisms able to synthesize the essential AAs de novo generate distinct δ13CEAA fingerprints45. In this regard, Amaro et al.25 found that bacteria present in the guts of the abyssal holothurian Molpadia musculus showed a 73% dissimilarity with the surrounding sediments and ca. 40% of bacterial OTUs (Operational Taxonomical Units) were associated uniquely with the guts. Overall, guts of mobile megabenthos concentrate large quantities of organic matter that are largely the living biomass of heterotrophic prokaryotes that form a unique community in the guts.
It is noteworthy that the TP of foregut contents was similar to that of hindguts (Fig. 1). Moreover, despite the decrease in %TN and %TOC along the digestive tract, the contribution of N from AAs remained unchanged (Table 1). This supports the presence of an important microbial community in different sections of the digestive tract. Previous research also reported higher bacteria counts in the foregut and hindgut of deep-sea DFs, with decreased numbers in the midgut23,24,26, where most absorption of nutrients occurs46. The presence of a flourishing microbial community in hindguts of DFs, later released as fecal pellets to the environment, might create microbial hot spots and contribute to the microbial productivity on the abyssal plain.
The functional role of prokaryotes in the guts of abyssal DFs is largely unknown. It has been suggested that they might be used as a nutritional source since DFs assimilate fatty acids of bacterial origin47–49. However, using counts of bacteria in the guts, the calculated prokaryotic production and the gut transit time, it has been estimated that, overall, prokaryotic biomass contributes only between 0.1–3% of the total protein taken up by deep-sea holothurians24,25,27. In our study the trophic position estimated for the studied DFs, one trophic level above their gut contents, led us to hypothesize that DFs were assimilating AAs from the prokaryotic biomass inhabiting the guts. However, δ13C values of essential AAs in the body tissue of DFs, which come necessarily from the diet, were a combination of those of sediments and gut contents, pointing also to the assimilation of AAs directly from ingested detritus (Fig. 2). It is also possible that the AAs present in the guts could be part of secreted echinoderm digestive enzymes or from the lysis of cells associated with the death of the animals during collection50. However, the differences in δ13CEAA pattern between gut contents and body tissue confirms that only a minority of gut content AAs derived from the DFs. Our results suggest caution on making the assumption that the biomass of a sea cucumber is a good isotopic proxy for detritus51.
We also found differences between the three species analyzed, despite the general patterns described. The elasipod holothurians O. mutabilis and S. globosa are two of the fastest mobile megafauna at Station M52, and they cover a large surface area of the seafloor [294.2 cm2 h−1 by O. mutabilis and 59.8 cm2 h−1 by S. globosa52]. Both species are surface DFs53 that have simple tubular guts which indicate that they feed continuously23,24. On the other hand, the echinoid E. rostrata moves at a lower speed but due to its large size also covers large areas (45.7 cm2 h−1; 52). Echinocrepis rostrata also carves trails at a depth range of 0–2 cm54, so it is likely that they ingest sediment below 0.5 cm. However, this was not reflected in their isotopic composition because sediments showed a similar isotopic composition downcore (Supplementary material, Table S2). E. rostrata, like most echinoids, have compartmentalized digestive tracts55, which allows prolonged residence times and potentially the digestion of more refractory organic matter. Hence, due to its ability to cover larger areas of the seafloor, O. mutabilis was expected to be the most selective species, which was in agreement with our results of the highest %TN and bulk δ15N values in their foreguts compared to the surrounding sediments (Table 1, Fig. 1). In addition, gut contents of O. mutabilis and S. globosa were less degraded (i.e. higher DI and lower Mol% of Gly) than gut contents of E. rostrata, supporting the selection of fresher organic matter by the two holothurians.
The ΣV parameter, which is an index for microbial heterotrophic resynthesis56, was comparable between gut contents of the three species and sediments (Table 2). The broad array of metabolic pathways for AAs used by heterotrophic microbes generate scattered δ15N patterns in “trophic” AAs that can be quantified by increases in the ΣV parameter56. For that reason, it would be expected that microbial resynthesis within the guts would yield higher ΣV values than in sediments. However, the guts might provide a stable environment for a unique microbial community that could be using specific metabolic pathways. Thus, our results suggest that the environment within the guts resembled that of a specific bacterial culture, which led to changes in δ15N values of trophic AAs similar to those of metazoans29,57.
We further investigated potential seasonal changes in the trophic identity of DFs by collecting samples in two periods with contrasting environmental conditions. At Station M, there is a marked seasonality in the flux of particulate organic carbon reaching the abyssal plain, with a higher density of detrital aggregates arriving from June through December58. A higher cover of phytodetritus was also observed on the seafloor in October than in May the year we sampled (Smith, unpublished data). The seasonality of food supply has an effect on the abundance14,59 and reproduction54 of megafauna, but the overall trophic identity of the studied DFs as secondary consumers was maintained. However, we observed some differences in the isotopic composition of gut contents. Gut contents of both O. mutabilis and E. rostrata showed higher bulk δ15N values during the period of low food supply (i.e. May). For O. mutabilis that difference appeared to be trophic (change in “trophic” AAs but not “source” AAs; Fig. 1), probably due to the consistently larger size of specimens collected in May relative to those collected in October (Supplementary material, Table S1). Larger individuals can be more selective by covering larger areas, and their longer gut might allow a more abundant microbial community to grow within it. This difference in TP of gut contents was also reflected in O. mutabilis body tissue, which was slightly higher in the period of low food supply and higher than in the other two species. On the contrary, for E. rostrata the seasonal difference in bulk δ15N values of gut contents was associated with changes in “source” AAs, since the δ15NSrc-AA values of gut contents collected in the period of low food supply were higher than those of sediments (Fig. 3). Increases in δ15NSrc-AA values are usually identified with extracellular enzymatic hydrolysis of proteins from detrital pools by microbes34,60 because 14N-containing bonds are preferentially cleaved, leaving higher δ15N values of AAs in the detrital pool. This suggests that during the period of lower food supply urchins were more dependent on the microbial community within the guts that hydrolyzed proteins from highly degraded detritus.
Despite the higher cover of phytodetritus observed on the seafloor in October than in May (Smith, unpublished data), such differences were not reflected in the elemental or stable isotopic composition of surface sediments. In fact, the DI, the ΣV parameter and the %Mol Gly indicated that sediments collected in the period of high food supply (i.e. October) were slightly more degraded (Table 2). It is likely that our sediment samples, randomly collected from the seafloor, did not comprise patches of freshly deposited aggregates during the more productive period. Nevertheless, the seasonal differences were reflected in the assimilation of essential AAs by DFs. During the period of low food supply (i.e. May) the body tissue of all three species had δ13CEAA values that resembled those of gut contents, whereas during the more productive period (i.e. October) they were more similar to those of sediments in E. rostrata and a mixture of both in O. mutabilis (Fig. 2B). Previous findings showed a shift in the diet of O. mutabilis from fresh to more refractory material in response to seasonal variations in food availability20,61. Our results suggest that when there is a higher abundance of detritus available, the essential AAs can be obtained from the environment, but in periods of food scarcity the microbial community within the guts might be important for the sustenance of DFs. This is in line with findings suggesting that the development of a specific gut flora in a deep-sea holothurian is enhanced by a low organic content in sediments62. Moreover, this was true for E. rostrata and O. mutabilis, which are two species with very different feeding modes, implying that the importance of the microbial community present in the guts of abyssal DFs might be widespread. Despite the consistency in our data, we caution that our results are based on a low number of samples per season and more studies are needed to fully understand how nutritional sources for abyssal megafauna vary due to seasonal differences in the food supply.
Our study revealed for the first time that DFs inhabiting the abyssal plain are secondary consumers of detritus, in contrast to the common assumption that their nutrition comes directly from detritus. The gut contents of the three species analyzed are trophically distinct from the surrounding surface sediment and their mix of prokaryotic biomass and detritus form a unique food source, which is one trophic level higher than sediments. Our results suggest that gut contents are mainly composed of a heterotrophic microbial community, which is assimilated as a food source by the DFs most importantly in periods of low food supply. In summary, we found that microbial communities in the guts of deposit feeders play a key role as primary consumers in the food web of the abyssal plain. The trophic dynamics in this deep-sea ecosystem are bottom-up controlled by the flux of organic matter9. In this ecological framework, the role of prokaryotes as primary consumers might be crucial in providing stability to the food web. As a result, gut microbes deserve attention in future studies analyzing energy flows in the food web of abyssal plains.
Methods
Sample collection
Samples were collected from the abyssal plain (~ 4000 m) in the Eastern North Pacific off of the California coast (34.50° N, 123.06° W; Station M). Sediment cores and megafauna were collected in May and October 2019 using the HOV Alvin and the ROV Doc Ricketts, respectively. Upon retrieval to the surface, samples were placed in a cool room (5 °C) for their further processing.
Sediment cores of 7 cm diameter were sliced in depth intervals (0–0.5, 0.5–1, 1–2, 2–3, 3–4, 4–5 and 5–10 cm), placed in petri dishes and stored frozen at − 80 °C. Specimens of holothurians Oneirophanta mutabilis complex, Scotoplanes globosa and echinoid Echinocrepis rostrata were weighed and measured, then they were dissected using an scalpel. We chose for this study three of the most abundant species of deposit feeders at Station M 14,17,54,59 with a large size so that we could sample the amount of gut contents required for the analyses (~ 150 mg in dry weight). We made a longitudinal cut along the digestive tract and took a sample of the foregut and hindgut contents, avoiding gut tissue. Then we removed the remaining guts and took a sample from cleaned body tissue, or in the case of echinoids, from the test. All samples were placed in cryovials and frozen in liquid nitrogen, and subsequently stored at − 80 °C.
Stable isotope analyses
Samples of sediments, gut contents and body tissue were freeze dried and ground to a homogenous powder using mortar and pestle. For analysis of bulk nitrogen and carbon isotopic composition, TN and TOC, ~ 0.7 and 3 mg of body tissue from holothurians and echinoids, respectively, ~ 5 mg of gut content and ~ 20 mg of sediments were placed in silver capsules. Samples were acidified to remove carbonates with 1 M HCl, which was added dropwise until bubbling ceased, then dried at 60 ºC and packed. Nitrogen and carbon elemental composition and isotopic composition were determined simultaneously using an isotope ratio mass spectrometer (DeltaPlusXP or Delta-V-Advantage) coupled to an elemental analyzer (Costech Model 4010). Measurement error based on within-run replicates of reference materials (glycine and homogenized fish tissue, both extensively characterized with NIST-certified reference materials and their δ13C and δ15N values verified independently in other laboratories) were typically of the order of less than ± 0.2‰ for δ15N and δ13C values. In elemental analyses, the variation of repeated measures performed in a subset of samples was < 10%.
For δ15N and δ13C analysis of individual AAs, we analyzed a subset of two replicates for each type of sample and each species from both cruises, except for S. globosa, which was not found in October (sediments: n = 4, gut content: n = 14, body tissue: n = 10). Samples were analyzed following the methods of Hannides et al.60. Briefly, samples were hydrolyzed using trace metal-grade 6 N HCl and then purified using cation exchange chromatography. Samples were then esterified using 4:1 isopropanol:acetyl chloride and derivatized using 3:1 methylene chloride:trifluoroacetyl anhydride. To account for carbon added during derivatization and variability of isotope fractionation during analysis, we also derivatized and analyzed a sample containing a set of 14 pure AAs (all amino acids analyzed in the samples, see list below) purchased commercially (Sigma-Aldrich, St. Louis, Missouri, USA). The resulting trifluoroacetyl and isopropyl ester derivatives were purified using chloroform extraction and stored at − 20 °C until analysis.
Carbon isotope composition was measured using a Thermo-Fisher Scientific MAT 253 isotope ratio mass spectrometer interfaced with a Trace Ultra GC-III via ConFlo IV. δ13C values were corrected based on the analysis of the set of pure AAs prepared under the same conditions following the approach by Silfer et al.63. Nitrogen isotopic composition of AAs was analyzed using a Thermo Scientific Delta V Plus IRMS interfaced to a trace gas chromatograph (GC) fitted with a 60 m BPx5 capillary column through a GC-C III combustion furnace (980 °C), reduction furnace (680 °C) and liquid nitrogen cold trap.
When possible, each sample was measured on 3 replicate injections (but sediments were only injected once due to their low AA content), with internal reference materials norleucine and aminoadipic acid with known isotopic composition co-injected on each run. The accuracy of CSIA-AA measurements, as the difference between the isotopic composition of the internal reference materials co-injected on each run and their known δ15N and δ13C values, was typically < 1‰. For replicate injections, δ15N and δ13C standard deviations averaged 0.4‰ and 0.5‰, respectively. Results for each sample are given as the mean values of all injections. We obtained information for the following AAs: alanine (Ala), aspartic acid (Asx; included the contribution of asparagine), glycine (Gly), glutamic acid (Glx; included the contribution of glutamine), isoleucine (Ile), leucine (Leu), lysine (Lys), methionine (Met), phenylalanine (Phe), proline (Pro), serine (Ser), threonine (Thr), tyrosine (Tyr), and valine (Val). Full AA reference suites of known weight were injected every 3 sample injections. The corresponding response factors (Vs [nmol AA]− 1) were used to determine total AA concentration and the contribution of each AA to the total AA pool (i.e. Mol% AA; mol AAi/Σ mol AA × 100% for each AA i). δ15N values were normalized using the fitted regression line between the known δ15NAA values and the measured δ15NAA values of the AA reference suite injected prior and after every 3 sample injections.
Stable isotopes data analysis
δ15N values of “source” AAs (δ15NSrc-AA) were calculated as the average δ15N value of: serine, phenylalanine, lysine and glycine. Trophic position (TP) was calculated using the equation from Chikaraishi et al.31: TP = (δ15Nglx – δ15Nphe – 3.4)/7.6 + 1, where δ15Nglx and δ15Nphe are the δ15N values of glutamic acid and phenylalanine, respectively, 3.4 ± 1.0‰ is the difference between glutamic acid and phenylalanine in primary producers (β), and 7.6 ± 1.2‰ is the trophic discrimination factor (TDFAA). Uncertainty in the calculation of δ15NSrc-AA and TP due to analytical error and uncertainties in TDF and β values was calculated by propagation of errors (64; Supplementary material, Table S1). To determine whether sediments and gut contents constituted distinct food sources we focused on δ13C values of essential AAs (Val, Thr, Ile, Leu, Phe and Lys). We normalized the δ13C values of each essential AA to the mean value of all essential AAs for each sample. Algae and bacteria have highly conserved modes of amino acid biosynthesis that produce unique patterns of carbon isotope fractionation. This allows the origins of essential amino acids to be determined from a comparison of the distribution patterns of δ13C values between samples since they fall on the same scale around an average equal to zero45.
The ΣV parameter is a proxy for heterotrophic resynthesis of AAs and is defined as the average deviation of δ15N values of individual trophic AAs from the mean δ15N value of those AAs56. The ΣV was calculated as: ΣV = 1/n Σ Abs (χi), where n is the total number of AAs used for the calculation and χi is the deviation of the δ15N value of amino acid i from the mean δ15N value of the n amino acids [ δ15Ni – (Σ δ15Ni /n)]. We used five AAs to calculate ΣV (Ala, Leu, Pro, Asx and Glx), excluding Ile because its δ15N value was missing in a few samples. Nevertheless, for those samples with estimated δ15N values of the six AAs the ΣV parameter was not significantly different when estimated with and without Ile since the slope of the ΣV6-AA vs. ΣV5-AA relationship (n = 18, R = 0.96, P < 0.001) was not significantly different from 1 [slope = 0.98 (95% confidence interval 0.87, 1.09)].
The degradation index (DI) is based on the selective preservation of AAs, so that AAs that comprise refractory material like cell walls will be more abundant in degraded material56,65. In this sense, Gly is abundant in cell walls so its high molar abundance (Mol% Gly) also indicates higher degradation state of particles or sediments65,66. The DI was calculated following the formula proposed by Dauwe et al.65: DI = Σ [(vari – AVG vari )/STD vari] × fac.coefi, where vari is the Mol% of amino acid i in our dataset, AVG vari and STD vari are its mean and standard deviation in the reference dataset (based on a variety of samples from marine phytoplankton to deep-sea sediments) and fac.coefi the factor coefficient from amino acid i based on the first axis of the PCA. AVG vari , STD vari and fac.coefi were obtained from Table 1 in Dauwe et al.65.
Data analysis
We used analysis of variance (ANOVA) to study differences in TP increment or bulk δ15N values between types of samples (i.e. sediments, foregut, hindgut, and body tissue). To disentangle the differences in patterns of δ13C values of EAAs (Val, Thr, Ile, Leu, Phe and Lys) we used multivariate statistics. We performed a principal component analysis (PCA) to the normalized δ13C values of each AA, then we used the permutational analysis of variance (PERMANOVA) to find statistically significant differences in δ13C values of EAAs between groups. We also performed a Linear Discriminant Analysis (LDA) using δ13CEAA values of sediments and gut contents as a training set to determine whether δ13CEAA values of body tissue grouped more closely with sediments or gut contents. All analyses were performed using R version 3.6.367.
Supplementary Information
Acknowledgements
We thank Astrid Leitner for helping to collect and process samples at sea. Special thanks to Ken Smith and Chrissy Huffard (MBARI) for their generosity with time at sea, assistance and mentorship on cruise Pulse 72, and for the valuable data from the Station M time series. We also thank the captain and crew of the R/V Atlantis and R/V Western Flyer and HOV Alvin and ROV Doc Ricketts. We also thank Natalie Wallsgrove for assistance in the laboratory. SRR is supported by a Marie Curie-COFUND Grant (ACA17-07) from the Government of Principado de Asturias and the European Commission. Funding for field and laboratory work was provided by NSF Grant #1829612. This is the School of Ocean and Earth Science and Technology contribution number 11358.
Author contributions
J.C.D., B.N.P. and S.R.R. designed the study and J.C.D., B.N.P., S.R.R., and J.A.B. collected the samples. S.R.R. and E.C.M. processed the samples in the laboratory. S.R.R. carried out the statistical analyses and led the writing of the manuscript and J.C.D., B.N.P., E.C.M. and J.A.B. contributed substantially to the manuscript.
Data availability
Data for individual AAs are available at BCO-DMO: https://www.bco-dmo.org/dataset/840749.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-021-91927-4.
References
- 1.Polis GA, Strong DR. Food web complexity and community dynamics. Am. Nat. 1996;147:813–846. doi: 10.1086/285880. [DOI] [Google Scholar]
- 2.Lindeman RL. The trophic-dynamic aspect of ecology. Ecology. 1942;23:399–417. doi: 10.2307/1930126. [DOI] [Google Scholar]
- 3.Coleman DC, Andrews R, Ellis JE, Singh JS. Energy flow and partitioning in selected man-managed and natural ecosystems. Agro-Ecosyst. 1976;3:45–54. doi: 10.1016/0304-3746(76)90099-8. [DOI] [Google Scholar]
- 4.Steffan SA, et al. Unpacking brown food-webs: Animal trophic identity reflects rampant microbivory. Ecol. Evol. 2017;7:3532–3541. doi: 10.1002/ece3.2951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Coleman DC. Energetics of detritivory and microbivory in soil in theory and practice. In: Polis GA, Winemiller KO, editors. Food Webs. Springer; 1996. pp. 39–50. [Google Scholar]
- 6.Moore JC, et al. Detritus, trophic dynamics and biodiversity. Ecol. Lett. 2004;7:584–600. doi: 10.1111/j.1461-0248.2004.00606.x. [DOI] [Google Scholar]
- 7.Hagen EM, et al. A meta-analysis of the effects of detritus on primary producers and consumers in marine, freshwater, and terrestrial ecosystems. Oikos. 2012;121:1507–1515. doi: 10.1111/j.1600-0706.2011.19666.x. [DOI] [Google Scholar]
- 8.Danovaro R, Snelgrove PVR, Tyler P. Challenging the paradigms of deep-sea ecology. Trends Ecol. Evol. 2014;29:465–475. doi: 10.1016/j.tree.2014.06.002. [DOI] [PubMed] [Google Scholar]
- 9.Smith CR, De Leo FC, Bernardino AF, Sweetman AK, Arbizu PM. Abyssal food limitation, ecosystem structure and climate change. Trends Ecol. Evol. 2008;23:518–528. doi: 10.1016/j.tree.2008.05.002. [DOI] [PubMed] [Google Scholar]
- 10.Gage JD, Tyler PA. Deep-Sea Biology: A Natural History of Organisms at the Deep-Sea Floor. Cambridge University Press; 1991. [Google Scholar]
- 11.De La Rocha CL, Passow U. Factors influencing the sinking of POC and the efficiency of the biological carbon pump. Top. Stud. Oceanogr. 2007;54:639–658. doi: 10.1016/j.dsr2.2007.01.004. [DOI] [Google Scholar]
- 12.Smith KL, Ruhl HA, Huffard CL, Messié M, Kahru M. Episodic organic carbon fluxes from surface ocean to abyssal depths during long-term monitoring in NE Pacific. Proc. Natl. Acad. Sci. USA. 2018;115:12235–12240. doi: 10.1073/pnas.1814559115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ramirez-Llodra E, et al. Deep, diverse and definitely different: Unique attributes of the world’s largest ecosystem. Biogeosciences. 2010;7:2851–2899. doi: 10.5194/bg-7-2851-2010. [DOI] [Google Scholar]
- 14.Ruhl HA. Abundance and size distribution dynamics of abyssal epibenthic megafauna in the northeast Pacific. Ecology. 2007;88:1250–1262. doi: 10.1890/06-0890. [DOI] [PubMed] [Google Scholar]
- 15.Billett DSM. Deep-sea holothurians. Oceanogr. Mar. Biol. An Annu. Rev. 1991;29:259–317. [Google Scholar]
- 16.Bett BJ, Malzone MG, Narayanaswamy BE, Wigham BD. Temporal variability in phytodetritus and megabenthic activity at the seabed in the deep northeast Atlantic. Prog. Oceanogr. 2001;50:349–368. doi: 10.1016/S0079-6611(01)00066-0. [DOI] [Google Scholar]
- 17.Durden, J. M. etal. Response of deep-sea deposit-feeders to detrital inputs: A comparison of two abyssal time-series sites. Deep.Res.PartIITop.Stud.Oceanogr.173, 104677 (2020).
- 18.Khripounoff A, Sibuet ML. nutrition d’echinodermes abyssaux I. Alimentation des holothuries. Mar. Biol. 1980;60:17–26. doi: 10.1007/BF00395602. [DOI] [Google Scholar]
- 19.Roberts D, Gebruka A, Levin V, Manship BAD. Feeding and digestive strategies in deposit-feeding holothurians. Oceanogr. Mar. Biol. Annu. Rev. 2000;38:257–310. [Google Scholar]
- 20.FitzGeorge-Balfour, T., Billett, D. S. M., Wolff, G. A., Thompson, A. & Tyler, P. A. Phytopigments as biomarkers of selectivity in abyssal holothurians; interspecific differences in response to a changing food supply. Deep.Res.PartIITop.Stud.Oceanogr.57, 1418–1428 (2010).
- 21.Miller RJ, Smith CR, Demaster DJ, Fornes WL. Feeding selectivity and rapid particle processing by deep-sea megafaunal deposit feeders : A 234 Th tracer approach. J. Mar. Res. 2000;58:653–673. doi: 10.1357/002224000321511061. [DOI] [Google Scholar]
- 22.Witte U, et al. In situ experimental evidence of the fate of a phytodetritus pulse at the abyssal sea floor. Lett. Nat. 2003;424:763–766. doi: 10.1038/nature01799. [DOI] [PubMed] [Google Scholar]
- 23.Moore, H., Manship, B. & Roberts, D. Gut structure and digestive strategies in three species of abyssal holothurians. in EchinodermResearch 111–119 (Balkema, 1995).
- 24.Deming JW, Colwell RR. Barophilic bacteria associated with digestive tracts of abyssal holothurians. Appl. Environ. Microbiol. 1982;44:1222–1230. doi: 10.1128/aem.44.5.1222-1230.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Amaro, T., Luna, G. M., Danovaro, R., Billett, D. S. M. & Cunha, M. R. High prokaryotic biodiversity associated with gut contents of the holothurian Molpadiamusculus from the Nazaré Canyon (NE Atlantic). Deep.Res.PartIOceanogr.Res.Pap.63, 82–90 (2012).
- 26.Roberts D, et al. Sediment distribution, hydrolytic enzyme profiles and bacterial activities in the guts of Oneirophantamutabilis,Psychropoteslongicauda and Pseudostichopusvillosus: What do they tell us about digestive strategies of abyssal holothurians? Prog. Oceanogr. 2001;50:443–458. doi: 10.1016/S0079-6611(01)00065-9. [DOI] [Google Scholar]
- 27.Sibuet M, Khripounoff A, Deming J, Colwell R, Dinet A. Modification of the gut contents in the digestive tract of abyssal holothurians. In: Lawrence JM, editor. Proceedings of the International Echinoderm Conference. Balkema: Tampa Bay; 1982. pp. 421–428. [Google Scholar]
- 28.Bradley CJ, et al. Trophic position estimates of marine teleosts using amino acid compound specific isotopic analysis. Limnol. Oceanogr. Methods. 2015;13:476–493. doi: 10.1002/lom3.10041. [DOI] [Google Scholar]
- 29.Ohkouchi N, et al. Advances in the application of amino acid nitrogen isotopic analysis in ecological and biogeochemical studies. Org. Geochem. 2017;113:150–174. doi: 10.1016/j.orggeochem.2017.07.009. [DOI] [Google Scholar]
- 30.Popp BN, et al. Stable isotopes as indicators of ecological change. Terr. Ecol. 2007;1:173–190. [Google Scholar]
- 31.Chikaraishi Y, et al. Determination of aquatic food-web structure based on compound-specific nitrogen isotopic composition of amino acids. Limnol. Oceanogr. Methods. 2009;7:740–750. doi: 10.4319/lom.2009.7.740. [DOI] [Google Scholar]
- 32.McClelland JW, Montoya JP. Trophic relationships and the nitrogen isotopic composition of amino acids in plankton. Ecology. 2002;83:2173–2180. doi: 10.1890/0012-9658(2002)083[2173:TRATNI]2.0.CO;2. [DOI] [Google Scholar]
- 33.Steffan SA, et al. Microbes are trophic analogs of animals. Proc. Natl. Acad. Sci. U. S. A. 2015;112:15119–15124. doi: 10.1073/pnas.1508782112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yamaguchi YT, et al. Fractionation of nitrogen isotopes during amino acid metabolism in heterotrophic and chemolithoautotrophic microbes across Eukarya, Bacteria, and Archaea: Effects of nitrogen sources and metabolic pathways. Org. Geochem. 2017;111:101–112. doi: 10.1016/j.orggeochem.2017.04.004. [DOI] [Google Scholar]
- 35.Iken K, Brey T, Wand U, Voigt J, Junghans P. Food web structure of the benthic community at the Porcupine Abyssal Plain (NE Atlantic): A stable isotope analysis. Prog. Oceanogr. 2001;50:383–405. doi: 10.1016/S0079-6611(01)00062-3. [DOI] [Google Scholar]
- 36.Romero-Romero S. Seasonal pathways of organic matter within the Avilés submarine canyon: Food web implications. Deep. Res. Part I. Oceanogr. Res. Pap. 2016;117:1–10. doi: 10.1016/j.dsr.2016.09.003. [DOI] [Google Scholar]
- 37.Reid W, Wigham B, McGill R, Polunin N. Elucidating trophic pathways in benthic deep-sea assemblages of the Mid-Atlantic Ridge north and south of the Charlie-Gibbs fracture zone. Mar. Ecol. Prog. Ser. 2012;463:89–103. doi: 10.3354/meps09863. [DOI] [Google Scholar]
- 38.Drazen J, et al. Bypassing the abyssal benthic food web: Macrourid diet in the eastern North Pacific inferred from stomach content and stable isotopes analyses. Limnol. Oceanogr. 2008;53:2644–2654. doi: 10.4319/lo.2008.53.6.2644. [DOI] [Google Scholar]
- 39.Post D. Using stable isotopes to estimate trophic position: Models, methods, and assumptions. Ecology. 2002;83:703–718. doi: 10.1890/0012-9658(2002)083[0703:USITET]2.0.CO;2. [DOI] [Google Scholar]
- 40.Witbaard R, Duineveld GCA, Kok A, Van Der Weele J, Berghuis EM. The response of Oneirophantamutabilis (Holothuroidea) to the seasonal deposition of phytopigments at the Porcupine Abyssal Plain in the Northeast Atlantic. Prog. Oceanogr. 2001;50:423–441. doi: 10.1016/S0079-6611(01)00064-7. [DOI] [Google Scholar]
- 41.Wigham BD, Hudson IR, Billett DSM, Wolff GA. Is long-term change in the abyssal Northeast Atlantic driven by qualitative changes in export flux? Evidence from selective feeding in deep-sea holothurians. Prog. Oceanogr. 2003;59:409–441. doi: 10.1016/j.pocean.2003.11.003. [DOI] [Google Scholar]
- 42.Hudson IR, Wigham BD, Billett DSM, Tyler PA. Seasonality and selectivity in the feeding ecology and reproductive biology of deep-sea bathyal holothurians. Prog. Oceanogr. 2003;59:381–407. doi: 10.1016/j.pocean.2003.11.002. [DOI] [Google Scholar]
- 43.Lauerman LML, Smoak JM, Shaw TJ, Moore WS, Smith KL. 234Th and 210Pb evidence for rapid ingestion of settling particles by mobile epibenthic megafauna in the abyssal NE Pacific. Limnol. Oceanogr. 1997;42:589–595. doi: 10.4319/lo.1997.42.3.0589. [DOI] [Google Scholar]
- 44.Roberts D, Billett DSM, McCartney G, Hayes GE. Procaryotes on the tentacles of deep-sea holothurians: A novel form of dietary supplementation. Limnol. Oceanogr. 1991;36:1447–1451. doi: 10.4319/lo.1991.36.7.1447. [DOI] [Google Scholar]
- 45.Larsen, T., Lee Taylor, D., Leigh, M. B. & O’Brien, D. M. Stable isotope fingerprinting: A novel method for identifying plant, fungal, or bacterial origins of amino acids. Ecology90, 3526–3535 (2009). [DOI] [PubMed]
- 46.Plante CJ, Jumars PA, Baross JA. Digestive associations between marine detritivores and bacteria. Annu. Rev. Ecol. Syst. 1990;21:93–127. doi: 10.1146/annurev.es.21.110190.000521. [DOI] [Google Scholar]
- 47.Drazen, J. C., Phleger, C. F., Guest, M. A. & Nichols, P. D. Lipid, sterols and fatty acid composition of abyssal holothurians and ophiuroids from the North-East Pacific Ocean: Food web implications. Comp.Biochem.Physiol.-BBiochem.Mol.Biol.151, 79–87 (2008). [DOI] [PubMed]
- 48.Amaro, T. etal. Possible links between holothurian lipid compositions and differences in organic matter (OM) supply at the western Pacific abyssal plains. Deep.Res.PartIOceanogr.Res.Pap.152, (2019).
- 49.Kharlamenko, V. I., Maiorova, A. S. & Ermolenko, E. V. Fatty acid composition as an indicator of the trophic position of abyssal megabenthic deposit feeders in the Kuril Basin of the Sea of Okhotsk. Deep.Res.PartIITop.Stud.Oceanogr.154, 374–382 (2018).
- 50.Ginger ML, et al. Organic matter assimilation and selective feeding by holothurians in the deep sea: Some observations and comments. Prog. Oceanogr. 2001;50:407–421. doi: 10.1016/S0079-6611(01)00063-5. [DOI] [Google Scholar]
- 51.McMahon KW, Thorrold SR, Houghton LA, Berumen ML. Tracing carbon flow through coral reef food webs using a compound-specific stable isotope approach. Oecologia. 2016;180:809–821. doi: 10.1007/s00442-015-3475-3. [DOI] [PubMed] [Google Scholar]
- 52.Kaufmann, R. S. & Smith, K. L. Activity patterns of mobile epibenthic megafauna at an abyssal site in the eastern North Pacific: Results from a 17-month time-lapse photographic study. Deep.Res.PartIOceanogr.Res.Pap.44, 559–579 (1997).
- 53.Kuhnz LA, Ruhl HA, Huffard CL, Smith KL. Rapid changes and long-term cycles in the benthic megafaunal community observed over 24 years in the abyssal northeast Pacific. Prog. Oceanogr. 2014;124:1–11. doi: 10.1016/j.pocean.2014.04.007. [DOI] [Google Scholar]
- 54.Vardaro MF, Ruhl HA, Smith KL. Climate variation, carbon flux, and bioturbation in the abyssal north pacific. Limnol. Oceanogr. 2009;54:2081–2088. doi: 10.4319/lo.2009.54.6.2081. [DOI] [Google Scholar]
- 55.Ziegler A, Mooi R, Rolet G, De Ridder C. Origin and evolutionary plasticity of the gastric caecum in sea urchins (Echinodermata: Echinoidea) BMC Evol. Biol. 2010;10:313. doi: 10.1186/1471-2148-10-313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.McCarthy MD, Benner R, Lee C, Fogel ML. Amino acid nitrogen isotopic fractionation patterns as indicators of heterotrophy in plankton, particulate, and dissolved organic matter. Geochim. Cosmochim. Acta. 2007;71:4727–4744. doi: 10.1016/j.gca.2007.06.061. [DOI] [Google Scholar]
- 57.Calleja ML, Batista F, Peacock M, Kudela R, McCarthy MD. Changes in compound specific δ15N amino acid signatures and d/l ratios in marine dissolved organic matter induced by heterotrophic bacterial reworking. Mar. Chem. 2013;149:32–44. doi: 10.1016/j.marchem.2012.12.001. [DOI] [Google Scholar]
- 58.Smith KL, Ruhl HA, Kaufmann RS, Kahru M. Tracing abyssal food supply back to upper-ocean processes over a 17-year time series in the northeast Pacific. Limnol. Oceanogr. 2008;53:2655–2667. doi: 10.4319/lo.2008.53.6.2655. [DOI] [Google Scholar]
- 59.Huffard, C. L., Kuhnz, L. A., Lemon, L., Sherman, A. D. & Smith, K. L. Demographic indicators of change in a deposit-feeding abyssal holothurian community (Station M, 4000 m). Deep.Res.PartIOceanogr.Res.Pap.109, 27–39 (2016).
- 60.Hannides, C. C. S., Popp, B. N., Anela Choy, C. & Drazen, J. C. Midwater zooplankton and suspended particle dynamics in the North Pacific Subtropical Gyre: A stable isotope perspective. Limnol.Oceanogr.58, 1931–1936 (2013).
- 61.Neto, R. R., Wolff, G. A., Billett, D. S. M., Mackenzie, K. L. & Thompson, A. The influence of changing food supply on the lipid biochemistry of deep-sea holothurians. Deep.Res.PartIOceanogr.Res.Pap.53, 516–527 (2006).
- 62.Amaro T, Witte H, Herndl GJ, Cunha MR, Billett DSM. Deep-sea bacterial communities in sediments and guts of deposit-feeding holothurians in Portuguese canyons (NE Atlantic) Deep Res. Part I Oceanogr. Res. Pap. 2009;56:1834–1843. doi: 10.1016/j.dsr.2009.05.014. [DOI] [Google Scholar]
- 63.Silfer JA, Engel MH, Macko SA, Jumeau EJ. Stable carbon isotope analysis of amino acid enantiomers by conventional isotope ratio mass spectrometry and combined gas chromatography/isotope ratio mass spectrometry. Anal. Chem. 1991;63:370–374. doi: 10.1021/ac00004a014. [DOI] [Google Scholar]
- 64.Jarman CL, et al. Diet of the prehistoric population of Rapa Nui (Easter Island, Chile) shows environmental adaptation and resilience. Am. J. Phys. Anthropol. 2017;164:343–361. doi: 10.1002/ajpa.23273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Dauwe B, Middelburg JJ, Herman PMJ, Heip CHR. Linking diagenetic alteration of amino acids and bulk organic matter reactivity. Limnology. 1999;44:1809–1814. [Google Scholar]
- 66.McCarthy MD, Benner R, Lee C, Hedges JI, Fogel ML. Amino acid carbon isotopic fractionation patterns in oceanic dissolved organic matter: An unaltered photoautotrophic source for dissolved organic nitrogen in the ocean? Mar. Chem. 2004;92:123–134. doi: 10.1016/j.marchem.2004.06.021. [DOI] [Google Scholar]
- 67.R:ALanguageandEnvironmentforStatisticalComputing. https://www.R-project.org (R Core Team. R Foundation for Statistical Computing, 2020).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data for individual AAs are available at BCO-DMO: https://www.bco-dmo.org/dataset/840749.