Abstract
Banana pseudostem, a cellulose-rich by-product, is regarded as an important agricultural waste during the process of banana production. Microcrystalline cellulose was successfully prepared from banana pseudostem using acid hydrolysis method. Microcrystalline cellulose was characterized through various techniques such as XRD, TGA, SEM, FTIR and antioxidant activity to explore the possible applications in the pharmaceutical industries especially as a drug delivery vehicle. The investigation revealed that the derived microcrystalline cellulose is non-aggregated, short rods with high crystallinity index 67% and stable up to 347 °C. FTIR spectroscopy showed that hydrolysis treatments are efficient for the removal of lignin and hemicellulose content. Microcrystalline cellulose exhibited good antioxidant activity 90.29% at 100 μg/ml. In vitro studies for the drug release were carried out in simulated intestinal fluid (SIF) using Isoniazid drug. The study proves that microcrystalline cellulose can be directly obtained from banana pseudostem which is not only beneficial to reduce the cost of traditional microcrystalline cellulose but is also conducive to the value-added utilization of the pseudostem.
Keywords: Banana pseudostem, Microcrystalline cellulose, Thermal stability, Antioxidant activity, Drug delivery
Introduction
Every year, agriculture processing generates large amount of lignocellulosic biomass in millions of ton. These lignocellulosic biomass are generated from corn cobs, rice straw, groundnut shell, sugarcane bagasse and banana pseudostem. Banana (genus Musa) is a monocotyledonous herbaceous plant of Musaceae family (Okareh et al. 2015). It is a second most important fruit crop in the world. It is grown in 130 countries of the world. India contributes 10.4 million tons of banana to the world production. Banana farming generates the pseudostem as a waste in abundance and are widely available as a lignocellulosic waste (Akpabio et al. 2012). The cellulose present is a classic example of renewable and biodegradable structural polymer which can be processed into microfibers. Approximately 220 tons of lignocellulosic waste is generated from each hectare of banana plant.
Banana pseudostem fibers contain high amount of cellulose which is used for the production of microcrystalline cellulose. Microcrystalline cellulose is obtained by the hydrolysis process of cellulose. Microcrystalline cellulose (MCC) is a partially purified depolymerized non-fibrous form of cellulose and it is a fine, white, odorless, crystalline powder and biodegradable material (Das et al. 2010). The recent research is focused on a wide range of underutilized lignocellulosic material as low price by converting waste products into value-added products. The lignocellulosic waste having high content of cellulose such as corn cobs (Shao et al. 2020), cotton wool (Rashid et al. 2017), orange mesocarp (Ejikeme 2008), jute (Jahan et al. 2011), rice straw (Fan et al. 2017), date palm (Abu-Thabit et al. 2020), soybean hulls (Merci et al. 2015), mulberry (Li et al. 2009), sugar beet pulp (Dinand et al. 1999), tea waste (Zhao et al. 2018) and sugarcane bagasse (Zhang et al. 2013) are excellent sources for the production of MCC. The production of MCC can be achieved via mechanical route (Hanna et al. 2001), biological route (Adel et al. 2010) and chemical route (Haafiz et al. 2013). However, chemical method is a more efficient method than the mechanical and biological methods as biological method is very expensive and result in MCC with lower crystallinity (Trache et al. 2014).
Microcrystalline cellulose is well known for its excellent applications in medicine, food, cosmetic and other chemical industries (Parveen et al. 2018; Zuo et al. 2014). Microcrystalline cellulose is non-hazardous, chemically inactive, and has high sorption capacity, biodegradability, biocompatibility and large surface area. These unique characteristics of microcrystalline cellulose are desirable for pharmaceutical industries as it can be utilized as a binder and filler, as a direct compression in design development to increase compressibility and solubility of the drugs (Sun and Sun 2020; Chamsai and Sriamornsak 2013; Othman et al. 2019). Furthermore, microcrystalline cellulose is also useful as a stabilizer, emulsifier and reinforcing agent in polymer matrix composites (Galal et al. 2011; Ng et al. 2015). Microcrystalline cellulose-based hydrogels have been used in delivery of Cephalexin (Kundu and Banerjee 2020). Microcrystalline cellulose and polyvinyl alcohol hydrogel are porous and glossy in nature, and are a good carrier for 5-fluorouracil (Seera et al. 2020). Xu et al. (2019) prepared amylose starch and MCC hybrid gel for gastric floating drug ranitidine for the delivery purpose. The production of MCC using various agricultural waste and use as a green filler are an efficacious way in evolution of green materials for future. Commercially available microcrystalline cellulose is expensive; thus, natural fibers are good alternative for the extraction of MCC form agricultural waste.
The objective of the present study is to prepare and extract the microcrystalline cellulose from banana pseudostem using chemical hydrolysis because the yield of the biomass of banana is comparatively higher than the other biomass and it also contains high amount of cellulose. Furthermore, structure, micromorphology and thermal stability of microcrystalline cellulose were analyzed by Fourier transform infrared spectroscopy (FTIR), thermogravimetric analysis (TGA), and derivative thermogravimetry (DTG), X-ray diffraction, and scanning electron microscopy (SEM). The antioxidant activity and drug release efficiency were also studied. The properties of derived microcrystalline cellulose from banana pseudostem were compared with commercially available microcrystalline cellulose.
Materials and methods
Materials
Banana pseudostem was collected from a local banana plantation at Anand, Gujarat. The experimental chemicals such as sodium hydroxide, sodium chlorite, sulfuric acid and glacial acetic acid, calcium chloride, sodium chloride, potassium chloride, disodium hydrogen phosphate and potassium dihydrogen phosphate were acquired from Himedia, Mumbai, India.
Preparation of microcrystalline cellulose
The banana fibers were derived by mechanical method using Raspador machine. The fibers were cut into the small pieces and washed thoroughly with the water. Figure 1 shows the steps for the extraction of microcrystalline cellulose from banana pseudostem fibers. Pure cellulose fibers were derived by alkaline and acid hydrolysis method. Alkaline solution removes the hemicellulose and pectin from the fibers. The fibers were dried at 60 °C for 24–48 h. The dried banana fibers were dispersed with 4% (w/v) NaOH at 80 °C for 4 h. The solubilized components from the banana fibers were removed by washing with distilled water. Subsequently, bleaching treatment was carried out to remove the phenolic components. The fibers were bleached with sodium chlorite (NaClO2) for 90 min at 80 °C. The bleaching process was repeated twice. The white cellulose pulp obtained was rewashed with deionized water until the pH 7 is obtained. Microcrystalline cellulose was derived from the cellulose fibers through acid hydrolysis method. Sulfuric acid (2.5 N) was used to treat the cellulose pulp at 90 °C for 30 min. The derived microcrystalline cellulose was washed with deionized water until the neutral pH is obtained. MCC was dried at 60 °C for 5–6 h and stored in airtight plastic bags.
Fig. 1.
The process step for the extraction of microcrystalline cellulose
Physicochemical parameters of microcrystalline cellulose
Chemical composition
The chemical composition of the banana pseudostem fibers, alkaline-treated fibers, bleached fibers (cellulose) and acid-treated fibers (MCC) were studied according to TAPPI (Technical Association of Pulp and Paper industries) methods. The ash content was analyzed using the muffle furnace at the temperature 550 °C.
UV–Vis spectroscopic analysis of aqueous microcrystalline cellulose suspension
The aqueous suspension of different concentration of MCC and sonicated for different time interval (15 and 30 min) at amplitude 75%, frequency 50 Hz, and 1 min. on and 30 s. off. The UV–Vis spectroscopic method was done according to Parveen et al. (2018) to check the effect of microcrystalline concentration and sonication time on the dispersion of MCC. The microcrystalline cellulose at different concentration (1–10 mg/ml) was prepared and evaluated by the UV–Vis spectrophotometer—SHIMARDZU UV-1800 (300 nm) through calibration curve. The dispersed concentration of the MCC was calculated. The extractability (%) was calculated by the following equation as mentioned by Parveen et al. (2018).
| 1 |
Extractability shows the effect to sonication time for the dispersion of MCC in aqueous medium. Extractability evaluates the quality of the dispersed MCC by measuring the agglomerated MCC. The higher extractability represents the higher dispersion of MCC.
Fourier transform infrared spectroscopy
Fourier transform infrared spectroscopy (FTIR) was used to examine the change in the functional group after applying the alkaline and acid treatment. The FTIR spectroscopy was performed by KBr method. The experimental and control samples were mixed with KBr powder in the ratio of 1:500 and prepared the pellets. The spectra of the samples were recorded on a Perkin ELMER-Spectrum GX in the range of 4000–400 cm−1 under transmission mode.
X-ray diffraction
X-ray diffraction was performed to study the crystallinity of the natural banana fibers, cellulose, banana pseudostem–microcrystalline cellulose and commercial microcrystalline cellulose. The Xpert MPD X-ray diffractometer was used for the analysis. The sample was prepared for the investigation by pressing the different sample powder into the cavity of a sample holder on a glass slide. The diffraction pattern was equipped in the (2θ) angle range from 10 to 60° at operating voltage 40 kV and 40 mA current using Cu kα radiation. The crystallinity of the different samples was calculated from the data of diffraction intensity using empirical method. The crystallinity index of the material was determining using the Eq. (2) (Segal et al. 1959).
| 2 |
where CrI is the crystallinity index, I002 is the maximum intensity of the 002 peak and Iam is the intensity of the peak at 2θ = 18. The crystallite size of the microcrystalline cellulose was calculated by Scherrer’s Eq. (3) (Rosa et al. 2012).
| 3 |
where L is the size of crystallite (nm), κ is the Scherrer constant (0.94), λ is the X-ray wavelength (1.54060 nm), β is the full width half maximum (FWHM) of the lattice plan reflection in radian and θ is the Bragg angle.
Thermogravimetry analysis and pyrolysis kinetics
The thermal stability of the MCC and banana pseudostem was characterized using a Perkin Elmer thermogravimetric analyzer. The sample was investigated from 30 to 515 °C at a heating rate of 10 °C/min under nitrogen condition (20 ml/min). To calculate the pyrolysis kinetics of MCC and banana pseudostem, equations of Arrhenius and Coats and Redfern were used to calculate the activation energy as mentioned by Rudakiya and Gupte (2017):
| 4 |
where A is pre-exponential factor (1/s), Ea is activation energy (J/mol), R is universal gas constant (J/(mol K)), T is absolute temperature (K), n is reaction order, t is reaction time (s).
The method described by Rudakiya and Gupte (2017) calculates the asymptotic series expansion for approximating the exponential integral, showing in the following equation:
| 5 |
| 6 |
where β is heating rate (°C/min).
The above-mentioned equations are used to calculate the activation energy and pre-exponential factor.
Scanning electron microscopy
Scanning electron microscopy was carried out to evaluate the structural morphology and particle size of the MCC. The gold layer was prepared on the surface of the sample to generate the conductivity of the surface and it was observed under the Phillips ESEM EDAX XL-30 microscope. The analysis was carried out under 10 kV with the magnification up to 2,50,000X.
Particle size analysis
Particle size of the microcrystalline cellulose was evaluated by Malvern analytical instrument. Three separate samples were analyzed to obtain mean particle size.
Antioxidant assay
The antioxidant activity of microcrystalline cellulose was analyzed using 2,2,diphenyl-1-picryhydrazyl (DPPH) radical scavenging activity. The DPPH solution was prepared by mixing 1 mg (DPPH) in methanol (10 ml). After adding MCC solution to the DPPH solution, the experimental tubes were incubated at room temperature in the dark for 1 h. The absorbance was recorded against the blank (Methanol) at 517 nm. The percentage of the radical scavenging activity was calculated by the following equation:
| 7 |
Ablank is the absorbance of control, containing all the reagent except microcrystalline cellulose.
In vitro drug release study
The drug release efficiency of microcrystalline cellulose was investigated by preparing the sodium alginate beads and simulated intestinal fluid (SIF). The in vitro analysis was performed according to method as described by Kalita et al. (2013). Sodium alginate (5% w/v) beads were prepared by mixing Isoniazid drug and two different concentration of MCC (250 and 500 mg) in beaker and stirred continuously on the magnetic stirrer until the complete homogenize solution is obtained. Sodium alginate beads without MCC containing only Isoniazid were taken as a control. The gelatinous solution was filled in the syringe and dropped down in 0.2 M chilled Calcium Chloride to prepare the small beads. The beads were filtered using Whatman filter paper No. 1 and dried at room temperature. Simulated Intestinal Fluid (SIF) was prepared by mixing of 4 g sodium chloride (NaCl), 100 mg potassium chloride (KCl), 720 mg disodium hydrogen phosphate (Na2HPO4) and 120 mg potassium dihydrogen phosphate (KH2PO4) into 500 ml distilled water and pH was adjusted to 7.4. The drug release absorbance was taken at 262 nm for Stimulated intestinal fluid. The drug release analysis was carried out by placing the experimental beads into the dialysis bag in a beaker containing the simulated intestinal fluid. The beaker was placed on the magnetic stirrer for continuous stirring at 100 rpm for 48 h. The amount of released drug was quantified by taking the absorbance at 262 nm for SIF.
Results and discussion
Characterization of microcrystalline cellulose
Chemical composition
Microcrystalline cellulose was prepared using acid hydrolysis as it is an effective method for dissolving the amorphous group and leaving behind microcrystalline cellulose. Table 1 represents the proximate analysis of banana pseudostem fiber at different chemical treatment. Banana pseudostem fibers contain 12% lignin, 24.7% hemicellulose, 56.4% cellulose content and 2.9% ash. The yield of MCC was obtained 37.5% from banana pseudostem fibers. Such a high yield can be attributed to the crystalline proportion present in the banana pseudostem fibers. The research study of Abu-Thabit et al. (2020) revealed that the yield of the prepared microcrystalline cellulose from date seed (Phoenix dactylifera L.) was only 12.51%. The proportion of cellulose of MCC was reached to 95.4% after the acid hydrolysis from banana fibers. This indicates that the use of alkaline, bleached and acid hydrolysis treatments are effective process for the removal of the non-cellulosic components.
Table 1.
Proximate analysis of fibers and microcrystalline cellulose
| Components | Banana fibers (%) | Alkali-treated fibers (%) | Bleached fibers (%) | Microcrystalline cellulose (%) |
|---|---|---|---|---|
| Cellulose | 56.4 ± 0.4 | 81.5 ± 0.6 | 89.5 ± 0.5 | 95.4 ± 0.6* |
| Hemicellulose | 24.7 ± 0.8 | 11 ± 0.5 | 7.1 ± 0.3 | 1.3 ± 0.3* |
| Lignin | 12 ± 0.4 | 5.4 ± 0.6 | 0 | 0* |
| Ash | 2.9 ± 0.1 | 1.7 ± 0.2 | 0.93 ± 0.1 | 0.84* |
Each value expressed as mean ± SD (n = 3)
*Significant (p < 0.005)
Extractability of microcrystalline cellulose in aqueous medium
Table 2 shows the extractability of microcrystalline cellulose in aqueous medium and it can be noticed that extractability enhanced with time. The maximum extractability of MCC (98.06%) was obtained after 30 min of sonication. Also it is clear that the extractability of MCC was higher at lower concentration, however, at the higher concentration, the extractability reduced. After the 15 min of sonication treatment, 90.44% extractability was obtained which means that lower quantity of dispersed MCC was present. The results suggest that the homogenous solution was achieved by the 30 min of sonication. The similar results have also been reported by the Parveen et al. (2018) who obtained 99% extractability in 30 min.
Table 2.
Effect of microcrystalline cellulose and sonication time on extractability of microcrystalline cellulose
| Microcrystalline concentration (%) | Extractability % | |
|---|---|---|
| 15 min | 30 min | |
| 1 | 90.44 ± 0.6*** | 98.06 ± 0.7*** |
| 2 | 83.33 ± 0.8 | 90.95 ± 0.6* |
| 3 | 83.49 ± 0.1 | 91.12 ± 0.8* |
| 4 | 74.05 ± 0.5 | 85.48 ± 0.3 |
| 5 | 69.91 ± 0.1 | 79.06 ± 0.7 |
| 6 | 65.88 ± 0.1 | 77.31 ± 0.7 |
| 7 | 60.82 ± 0.7 | 70.62 ± 0.7 |
| 8 | 57.98 ± 0.4 | 69.42 ± 0.7 |
| 9 | 54.93 ± 0.9 | 63.40 ± 0.3 |
| 10 | 51.72 ± 0.1 | 60.11 ± 0.8 |
Each value expressed as mean ± SD (n = 3)
***Significant (p < 0.001)
Fourier transform infrared spectroscopy (FTIR)
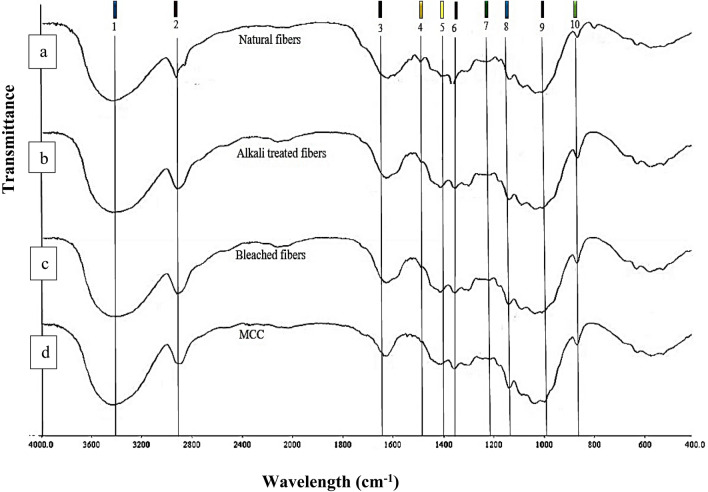
In this work, the FTIR spectra of fibers in different processing stages were investigated. Figure 2a–d demonstrate the IR spectra of the natural banana pseudostem fibers, alkaline-treated, bleached fibers and MCC, respectively. Table 3 shows the wavelength assignments of lignin-, hemicellulose- and cellulose-associated bands and their shift difference. The data obtained from the IR spectra represent that functional group and compositional changes occurred after the different chemical treatment. The broad absorption band observed from 3400 to 3500 cm−1 is attributed to the O–H stretching vibration of hydroxyl group in the cellulose for natural banana fibers, alkaline-treated fibers, bleached fibers and microcrystalline cellulose respectively (Jahan et al. 2011; Rosa et al. 2012; Wang et al. 2017). The band at 2919.66, 2918.18, 2922.18 and 2899.19 cm−1 in all the four samples corresponds to C–H stretching vibration of CH2 group. The absorption band at 1600.19 cm−1 was assigned for C=O in the aldehyde on the terminal anhydroglucose unit. On the basis of data, it is clear that the raw banana pseudostem fibers contain lignin and hemicellulose as amorphous region. Figure 2a shows the sharp absorbance peak at 1507.19 cm−1; lignin which is represented by –C=C– aromatic skeletal vibration (Haafiz et al. 2013; Rosa et al. 2012; Fahma et al. 2010). The peak at 1384 cm−1 is a key wavelength for hemicellulose which corresponds to C–H vibration (Narra et al. 2020). FTIR spectra (Fig. 2b–d) show the absence of peak at 1507.19 and 1384 cm−1 which confirms the removal of amorphous group from banana pseudostem fibers after the alkaline, bleach and hydrolysis treatment. Similar results was reported by Jahan et al. (2011) during the preparation of microcrystalline cellulose from jute fibers.
Fig. 2.
FTIR spectra of banana pseudostem fiber (a), alkaline-treated fiber (b), bleached fiber (c) and microcrystalline cellulose (d) are highlighted with ten different peaks and wavelength assignment of the following peak numbers showed in Table 3
Table 3.
FTIR analysis of banana pseudostem and their shift difference of alkaline-treated, bleached fibers and microcrystalline cellulose
| Peak no | Wavelength assignment | Banana pseudostem fibers | Alkaline treated fibers | Bleached fibers | Microcrystalline cellulose |
|---|---|---|---|---|---|
| 1 | O–H…O intermolecular stretching | 3418.19 | + 0.39 | + 0.23 | + 0.40 |
| 2 | C–H stretching | 2919.96 | − 1.15 | + 2.22 | − 20.05 |
| 3 | C=O stretching | 1608.44 | + 33.05 | + 35.67 | + 42.93 |
| 4 | C=C aromatic skeletal vibration in lignin | 1507.19 | – | – | – |
| 5 | CH2 deformation | 1425.79 | + 3.76 | + 3.71 | + 5.1 |
| 6 | CH3 deformation in lignin | 1384.41 | − 11.64 | − 12.07 | − 10.08 |
| 7 | C–O stretch in lignin and C–O linkage in guaiacyl aromatic group | 1260.14 | – | – | – |
| 8 | C–O–C vibration in cellulose and hemicellulose | 1160.31 | + 2.23 | + 2.25 | − 0.13 |
| 9 | C–O stretching in cellulose and hemicellulose | 1037.83 | + 22.83 | + 22.49 | + 22.49 |
| 10 | C–H deformation in cellulose | 897.50 | + 0.29 | + 0.29 | + 0.26 |
Shift difference (shift increase +, shift decrease/remove −) in the respective wavelength peak of banana pseudostem fiber compared to alkaline-treated fibers, bleached fiber and microcrystalline cellulose
Figure 2d shows the FTIR spectra of microcrystalline cellulose. The absorption peaks at 662.26, 897.76, 1023.93, 1060.32, 1111.99, 1161.22, 1374.32, and 1430.89 cm−1 correspond to structure of cellulose (Liu and Kim 2017). The absorption band at 897.76 cm−1 identified as C-H rocking vibration to β-glycosidic linkage (Sun et al. 2004; Yue et al. 2012; Soni and Mahmoud 2015; Liu and Kim 2017). The absorption peak at 1161.22 cm−1 is core to the stretching of C–O–C (Fahma et al. 2010; Li et al. 2009). The absorption peaks at the 1430.89 cm−1, also known as a “crystallinity band”, is attributed to intermolecular hydrogen at C6 aromatic ring group (Kalita et al. 2013; Kumar et al. 2002; Husin et al. 2017; Trache et al. 2014; Shankar and Rhim 2016). The above study concludes that the alkaline and acid hydrolysis treatment does not alter the chemical structure of the cellulosic fibers; however, the surface of the fibers may be affected. Abu-Thabit et al. (2020) found similar result for microcrystalline cellulose isolated from date seed (Phoenix dactylifera L.).
X-ray diffraction
The crystallinity estimation of biomass is necessary to obtain the information about the digestibility of sample. Numerous techniques are known to determine the crystallinity index (Cr.I) such as X-ray diffraction (XRD), 13CNMR, Raman spectroscopy and IR. Among these XRD is widely applied to obtain the crystallinity index. In the present, XRD was carried out to study the crystallinity of natural banana pseudostem fibers, cellulose, microcrystalline cellulose and commercial microcrystalline cellulose (Fig. 3). The diffractogram pattern revealed that the crystallinity value of the commercial microcrystalline cellulose (68%) was slightly greater with sharper the main peak (2θ = 22°) compared to prepared microcrystalline cellulose (67%). The peak at 2θ = 22° is less sharp for cellulose which indicates the lower crystallinity of the cellulose 62.60%.
Fig. 3.
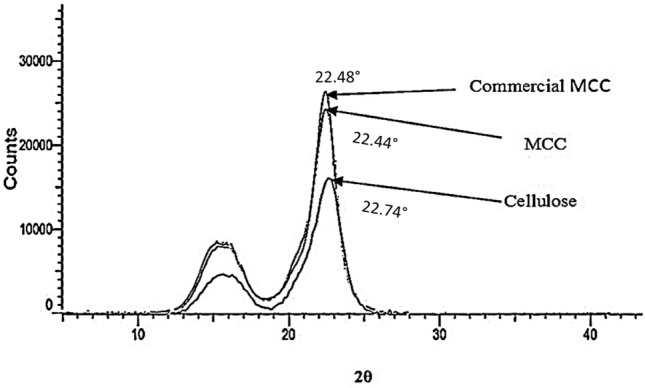
X-ray diffractogram of commercial microcrystalline cellulose and derived microcrystalline cellulose
The XRD diffractogram data represent that all the samples were crystalline in nature with the presence of native cellulose type-Ι and the absence of doublet located on the main peak (22.4°) which indicate the absence of cellulose-ΙΙ. The diffractogram pattern also shows that the crystallinity value of banana pseudostem fiber–MCC was quite similar to the commercial microcrystalline cellulose. On the basis of this, concluding that the acid hydrolysis treatment at high temperature removed the amorphous regions (hemicellulose and lignin) by the cleavage of the glycosidic bond which leads to the release of separate crystallites and it also not destruct the type cellulose-Ι (Chauhan et al. 2009; Haaffiz et al. 2013). High crystallinity attributes to the rigidity of the cellulose structure, which leads to the greater tensile strength. The crystallinity index of 59% was obtained by Fahma et al. (2010) for nanofibers prepared from OPEF using acid hydrolysis process. Ventura-Cruz et al. (2020) derived the microcrystalline cellulose from rose stem and obtained 70.21% crystallinity. The microcrystalline cellulose produced from corncob displayed 52.82% crystallinity index (Shao et al. 2020).
Thermogravimetric analysis
Thermogravimetric analysis (TGA) is generally used to investigate the degradative and thermal stability of microcrystalline cellulose. Thermal properties of MCC are necessary as they have wide variety of applications in different industries. Figure 4 shows TGA and DTG thermograms for banana pseudostem and MCC. The data represent the effect emerging due to the heating and pyrolysis degradation of banana pseudostem–microcrystalline cellulose and natural banana pseudostem fibers. The thermograms obtained for both MCC and natural fibers show a two-step degradation pattern. Initial weight loss 6 and 10% occurred at the temperature range of 72–211 °C for microcrystalline cellulose and banana pseudostem, respectively. The initial weight loss depends on the original moisture content in the sample, and it may vary with different samples (Elanthikkal et al. 2010; Husin et al. 2017; Kalita et al. 2013; Mandal and Chakrabarty 2011). The weight loss of MCC and banana pseudostem fibers was 10 and 12% during the heating to 226 and 230 °C, respectively. The degradation temperature of natural fibers was higher as compared to MCC. The similar results were also detected by Haafiz et al. (2013) for MCC from oil palm empty fruit bunch. Higher degradation rate is due to the presence of lignin in fiber samples (Rudakiya and Gupte 2017).
Fig. 4.
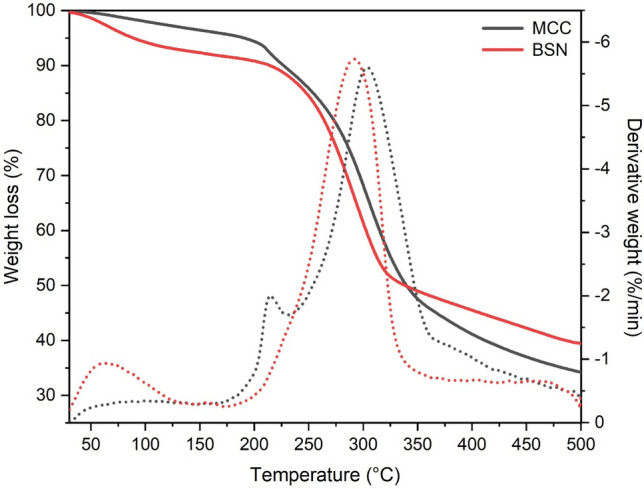
TGA and DTG profile of banana pseudostem fiber and microcrystalline cellulose
The main degradation peak for MCC and natural fibers was observed at the temperature 347 and 331 °C, respectively. On the basis of investigations, these degradation peak was obtained due to the degradation of cellulose and hemicellulose. The difference in the peak of weight loss temperature of MCC and natural fibers was 16 °C and it attributed to the difference in the crystallinity, higher crystallinity of the MCC requires higher degradation temperature (Keshk and Haija 2011). At the maximum temperature (502 °C), 34.13 and 39.36% weight are remaining of MCC and natural fibers, respectively. However, the rate of thermal degradation of MCC is greater than natural fibers. MCC was less thermally stable due to the presence of sulfate group on the surface of the fibers, which split during the thermal degradation (Haafiz et al. 2013; El-sakhawy and Hassan 2007). This thermal degradation study concludes that the MCC sample prepared from banana pseudostem fibers has good thermal stability. MCC was found to be thermally stable up to 347 °C and it will be useful in the preparation of bio-composites and various industrial processing.
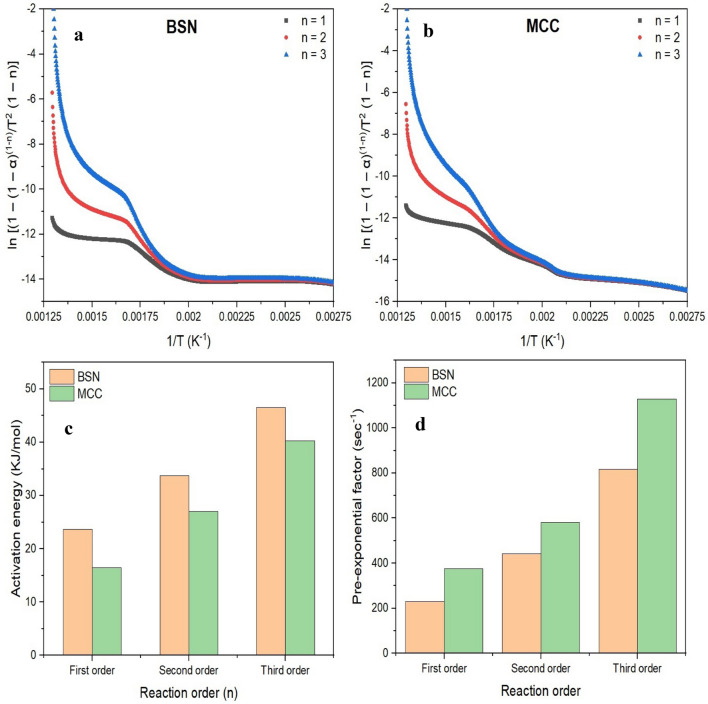
Figure 5 represents the kinetic study of banana pseudostem fiber and microcrystalline cellulose on the basis of weight loss data obtained by TG curve analysis. Figure 5a, b shows the Plots of ln [(1 – (1 – α) (1-n)/T2 (1 – n)] versus T − 1 for banana pseudostem natural fiber and microcrystalline cellulose, respectively. The activation energy of (KJ/mol) of banana pseudostem natural fiber and microcrystalline cellulose is shown in Fig. 5c for first-, second- and third-order reactions, respectively. The activation energy of banana pseudostem and microcrystalline cellulose was found in the range of 23.65–46.50 kJ/mol and 16.47–40.23 kJ/mol, respectively. The activation energy of banana pseudostem natural fiber was higher (0.86 fold) than the microcrystalline cellulose. The activation energy of MCC was lower as compared to natural fibers, it may be due to the crystallinity of cellulose and reduced the ratio of lignin to cellulose in microcrystalline cellulose (Narra et al. 2020). The pre-exponential factor (s−1) of natural fibers and microcrystalline cellulose was found in the range 231.00–816.42 s−1 and 374.87–1128.19 s−1, respectively (Fig. 5d). The pre-exponential factor of the natural banana fiber is lower than the microcrystalline cellulose. Narra et al. (2020) also showed the third-order pyrolysis kinetics of pretreated and untreated banana pseudostem wherein activation energy of the samples was 85.15 and 76.56 kJ/mol, respectively.
Fig. 5.
Plots of ln [(1 – (1 – α) (1 − n)/T2 (1 – n)] versus T − 1 for banana pseudostem fiber (a), and for microcrystalline cellulose (b), activation energy of banana pseudostem fiber and microcrystalline cellulose (c), and pre-exponential factor of banana pseudostem fiber and microcrystalline cellulose (d)
Scanning electron microscopy (SEM)
Microcrystalline cellulose is well known for the strong mechanical properties. Scanning electron microscopic graph (Fig. 6) shows the morphology of natural fibers and microcrystalline cellulose prepared from banana pseudostem fibers. As seen from the (Fig. 6a), the raw fibers exhibited agglomerated with smooth surface morphology. The smooth surface was observed due to the presence of lignin, hemicellulose, wax and oil that attached to the cellulose fibers (Abu-Thabit et al. 2020; Haafiz et al. 2013). The MCC obtained by acid hydrolysis process using 2.5 N H2SO4 showed short rod-like fibers which appeared in the range of 20–100 μm. The acid treatment removes the amorphous region and introduces a sulfate group on the surface, which provide a net negative charge on the surface of the fiber. It enhances the stability of colloidal suspension (MCC) through the repulsion forces of the electrical double layers present on the crystallites. Due to this, after the drying process, the fibers are observed like a short rod fibers (Mandal and Chakrabarty 2011; Li et al. 2009). Figure 6b shows the surface morphology of the microcrystalline cellulose. The image shows that the particles of the MCC are in an irregular shape with rough surface and form a network-like structure. The rough surface of the MCC can be attributed to different chemical treatments during MCC extraction process. During the chemical process, lignin, polysaccharides and other substances remove from the surface. It can be confirmed by the chemical composition. Similar results were also reported by Adel et al. (2011) for microcrystalline cellulose prepared from rice and bean hulls.
Fig. 6.
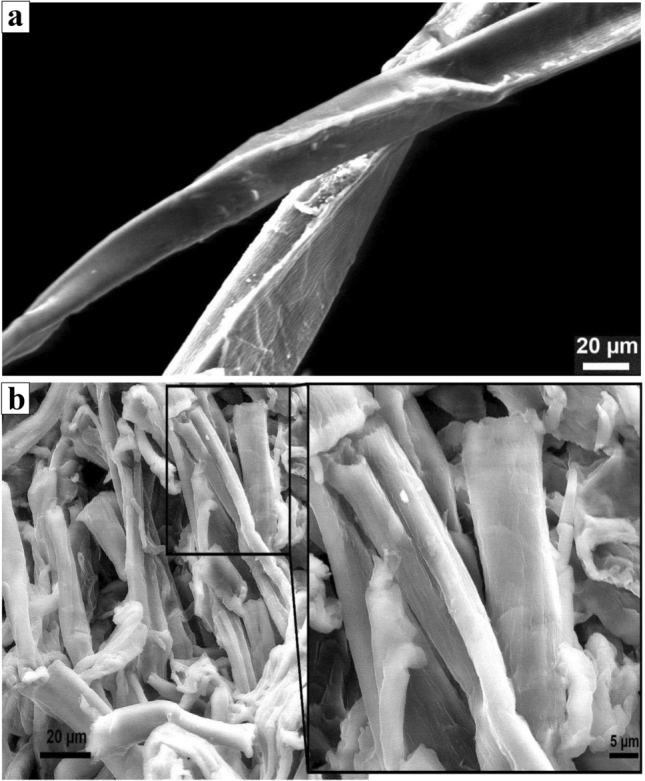
SEM microscopy of the banana pseudostem fiber (a) and microcrystalline cellulose (b)
Particle size analysis
The result revealed that the particle size of the microcrystalline cellulose derived from banana pseudostem fibers is 4.09 μm. The particle size and aggregation of the microcrystalline cellulose depend upon the main biomass substance and chemical treatment. The particle size of the banana pseudostem-derived MCC is similar to MCC prepared from Bagasse (3–6 μm), Rice straw (3–6 μm) and Cotton stalks (4.5–6 μm) (El-Sakhawy and Hassan 2007).
Antioxidant activity
The microcrystalline cellulose has the ability to protect against the free radicals. The free radical scavenging activity of microcrystalline cellulose was evaluated using the DPPH free radicals. When antioxidants scavenged the free radicals, the purple color of the DPPH turns into yellow color and it exhibits the maximum absorbance at 517 nm. Figure 7 reveals that the microcrystalline cellulose at low concentration (10 μg/ml) was able to scavenge 59.86% free radicals. The scavenging activity increased with increase in the concentration of MCC. Microcrystalline cellulose shows maximum 90.29% scavenging activity at 100 μg/ml concentration. Ascorbic acid is a known antioxidant agent; in this experiment, it was taken as a positive control for the comparison analysis. Ascorbic acid shows 97.35% scavenging activity at the concentration of 100 μg/ml. Kalita et al. (2013) reported that the extraction of microcrystalline cellulose from Setaria glauca (L.) P. Beauv had the scavenging activity 78.29% at a concentration of 50 mg. Zhang et al. (2017) reported very low antioxidant activity 6.7% at the concentration of 10 mg. The variation in antioxidant properties of MCC may be due to the difference in the sources as well as the method of MCC preparation. The knowing of antioxidant property of MCC could be beneficial when it is to be used for the drug delivery. As MCC has a good antioxidant property, it improves the shelf life of the drugs and inhibit the oxidation of organic and inorganic molecules (Celestino et al. 2012).
Fig. 7.
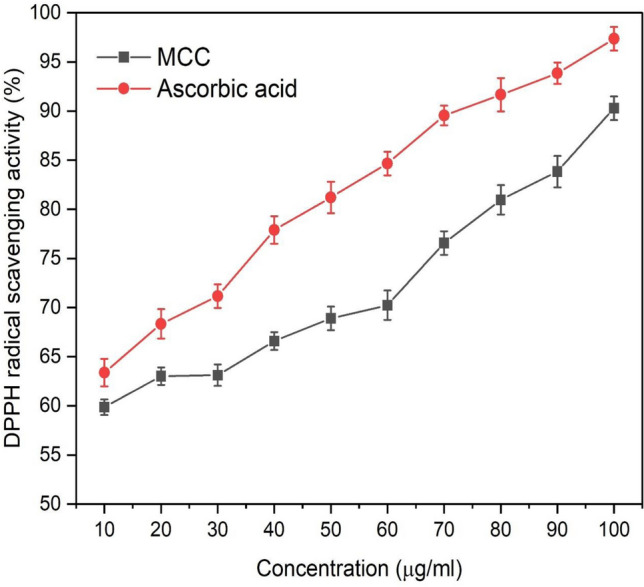
Antioxidant assay of microcrystalline cellulose obtained from banana pseudostem fibers
Drug release study
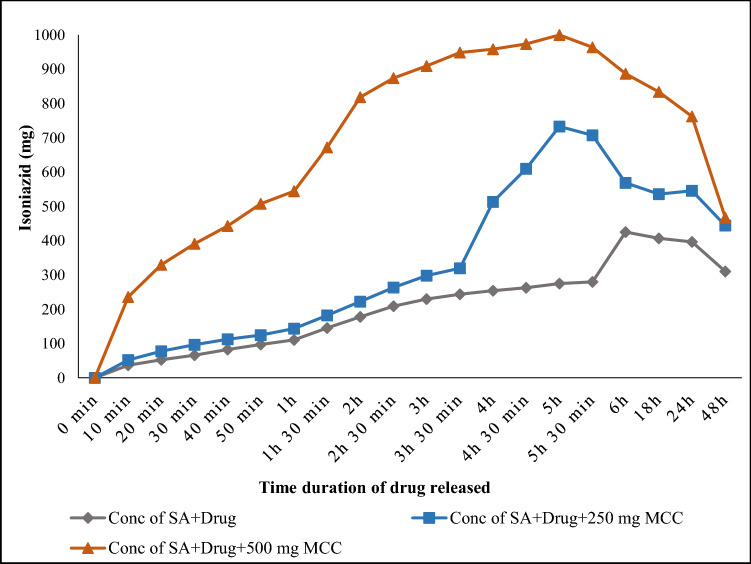
Figure 8 represents the efficiency of microcrystalline cellulose in drug release study using Simulated Intestinal Fluid (SIF) at a pH 7.4 in a cell-free system. In the present study, Isoniazid drug was used and in 10 min, 36.57 mg drug was released from the alginate beads. The amount of drug release gradually enhanced with the time and highest drug release was obtained 425 mg after 6 h. However, any further increase of time led to decline. After 48 h, the release of drug found at lowest 310.33 mg. However, a marked deference in the release of drug pattern was observed when 250 mg of MCC was mixed with Isoniazid drug. The amount of the drug release with MCC was 52 mg in 10 min and it reached to maximum 733.34 mg after 5 h. The release of the drug was much higher than the beads without containing MCC. After 48 h, the rate of drug release declined and only 444.52 mg drug was released.
Fig. 8.
The profile of microcrystalline cellulose for the release of anti-tuberculosis drug in SIF fluid
The concentration of the MCC was increased from 250 mg to 500 mg with Isoniazid drug. The 235.70 mg of drug was released after 10 min, the amount of drug released was very high comparative to the amount of drug release from beads without containing MCC and 250 mg concentration of MCC. Thereafter, the amount of drug released enhanced and maximum Isoniazid released was 999.92 mg after 5 h. After 48 h MCC was able to release 467.12 mg Isoniazid drug. From the above study, it can be concluded that the incorporation of MCC in the alginate beads increased the drug binding properties. A high concentration of MCC stimulates the release of drug (Isoniazid). The maximum drug (Isoniazid) release was obtained from the alginate beads in comparison to that without containing microcrystalline cellulose (Kalita et al. 2013). Table 4 shows the comparison study of Isoniazid drug and microcrystalline cellulose with the other drugs. Kundu and Banerjee (2020) synthesized microcrystalline–carboxymethyl cellulose (MCC–CMC) hydrogel for in vitro study of Cephalexin. They used three different simulated body fluids [artificial gastric fluid (AGF), artificial intestinal fluid (AIF) and phosphate buffer saline (PBS)]. They found that MCC–CMC have the ability to release maximum amount of Cephalexin 98% in PBS followed by 86% in AIF and 15% in AGF. Seera et al. (2020) reported that MCC–PVA (microcrystalline cellulose–polyvinyl alcohol) released 59.13% 5-flurouracil drug in phosphate buffer. Xu et al. (2019) reported 45.87% drug released (Ranitidine) in stimulated gastric fluid.
Table 4.
Comparison study of Isoniazid drug and microcrystalline cellulose with other drug incorporated with microcrystalline cellulose
| Drug | System | % drug released | References |
|---|---|---|---|
| Isoniazid | Stimulated intestinal fluid | 99.9% | Microcrystalline cellulose from banana pseudostem fibers (present study) |
| Cephalexin | Artificial gastric fluid | 15% | Kundu and Banerjee (2020) |
| Cephalexin | Artificial intestinal fluid | 86% | Kundu and Banerjee (2020) |
| Cephalexin | Phosphate buffer saline | 98% | Kundu and Banerjee (2020) |
| 5-Flurouracil | Phosphate buffer | 59.13% | Seera et al. (2020) |
| Ranitidine | Simulated gastric fluid | 45.87% | Xu et al. (2019) |
Conclusion
Microcrystalline cellulose was successfully derived from the banana pseudostem fibers. MCC was found to be with higher crystallinity and thermal stability at higher temperature. Furthermore, XRD data revealed that type Ι cellulose was present in the microcrystalline cellulose. The FTIR study also showed the crystalline and cellulose bond in the microcrystalline cellulose. MCC also show an excellent antioxidant activity. The experimental data indicate that MCC have capability to sustainably disperse Isoniazid medicine, which is used for the treatment of anti-tuberculosis at regular time interval. The present study, thus, manifests that the MCC can be directly prepared from the banana pseudostem fibers, which can be further explored for the applications in the pharmaceutical, food industries and as an excellent reinforced material for the polymeric materials where high thermal stabilities required.
Author contributions
Conceptualization: [MD and AG], Formal analysis and investigation: [MD and AG], Writing-Original draft preparation: [MD], Supervision: [AG].
Funding
Not applicable.
Availability of data and material
Not applicable.
Declarations
Conflict of interest
Authors declare no conflict of interest.
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Contributor Information
Meghna Diarsa, Email: meghnadiarsa19@gmail.com.
Akshaya Gupte, Email: akshaya_gupte@hotmail.com.
References
- Abu-Thabit NY, Judeh AA, Hakeem AS, Ul-Hamid A, Umar Y, Ahmad A. Isolation and characterization of microcrystalline cellulose from date seeds (Phoenix dactylifera L.) Int J Biol Macromol. 2020;155:730–739. doi: 10.1016/j.ijbiomac.2020.03.255. [DOI] [PubMed] [Google Scholar]
- Adel AM, Abd El-Wahab ZH, Ibrahim AA, Al-Shemy MT. Characterization of microcrystalline cellulose prepared from lignocellulosic materials. Part I. Acid catalyzed hydrolysis. Bioresour Technol. 2010;101:4446–4455. doi: 10.1016/j.biortech.2010.01.047. [DOI] [PubMed] [Google Scholar]
- Adel AM, Abd El-Wahab ZH, Ibrahim AA, Al-Shemy MT. Characterization of microcrystalline cellulose prepared from lignocellulosic materials. Part II: physicochemical properties. Carbohydr Polym. 2011;83:676–687. doi: 10.1016/j.carbpol.2010.08.039. [DOI] [Google Scholar]
- Akpabio UD, Udiong DS, Akpakpan AE. The physicochemical characteristics of plantain (Musa paradisiaca) and banana (Musa sapientum) pseudostem wastes. Adv Nat Appl Sci. 2012;6:167–172. [Google Scholar]
- Celestino MT, Magalhães UDO, Fraga AGM, Carmo FAD, Lione V, Castro HC, Sousa VP, Rodrigues CR, Cabral LM. Rational use of antioxidants in solid oral pharmaceutical preparations. Braz J Pharm Sci. 2012;48:405–415. doi: 10.1590/S1984-82502012000300007. [DOI] [Google Scholar]
- Chamsai B, Sriamornsak P. Novel disintegrating microcrystalline cellulose pellets with improved drug dissolution performance. Powder Technol. 2013;233:278–285. doi: 10.1016/j.powtec.2012.08.019. [DOI] [Google Scholar]
- Chauhan YP, Sapkal RS, Sapkal VS, Zamre GS. Microcrystalline cellulose from cotton rags (waste from garment and hosiery industries) Int J Chem Sci. 2009;7:681–688. [Google Scholar]
- Das K, Ray D, Bandyopadhyay NR, Sengupta S. Study of the properties of microcrystalline cellulose particles from different renewable resources by XRD, FTIR, nanoindentation, TGA and SEM. J Polym Environ. 2010;18:355–363. doi: 10.1007/s10924-010-0167-2. [DOI] [Google Scholar]
- Dinand E, Chanzy H, Vignon RM. Suspensions of cellulose microfibrils from sugar beet pulp. Food Hydrocoll. 1999;13:275–283. doi: 10.1016/S0268-005X(98)00084-8. [DOI] [Google Scholar]
- Ejikeme PM. Investigation of the physicochemical properties of microcrystalline cellulose from agricultural wastes I: orange mesocarp. Cellulose. 2008;15:141–147. doi: 10.1007/s10570-007-9147-7. [DOI] [Google Scholar]
- Elanthikkal S, Gopalakrishnapanicker U, Varghese S, Guthrie JT. Cellulose microfibres produced from banana plant wastes: isolation and characterization. Carbohydr Polym. 2010;80:852–859. doi: 10.1016/j.carbpol.2009.12.043. [DOI] [Google Scholar]
- El-Sakhawy M, Hassan ML. Physical and mechanical properties of microcrystalline cellulose prepared from agricultural residues. Carbohydr Polym. 2007;67:1–10. doi: 10.1016/j.carbpol.2006.04.009. [DOI] [Google Scholar]
- Fahma F, Iwamoto S, Hori N, Iwata T, Takemura A. Isolation, preparation, and characterization of nanofibers from oil palm empty-fruit-bunch (OPEFB) Cellulose. 2010;17:977–985. doi: 10.1016/j.carbpol.2009.12.043. [DOI] [Google Scholar]
- Fan GZ, Wang YX, Song GS, Yan JT, Li JF. Preparation of microcrystalline cellulose from rice straw under microwave irradiation. J Appl Polym Sci. 2017;134:1–8. doi: 10.1002/app.44901. [DOI] [Google Scholar]
- Galal AMN, Fatma AMH, Ali KE, Jihan MK, Sahar HSM. Utilization of microcrystalline cellulose prepared from rice straw in manufacture of yoghurt. J Am Sci. 2011;6:226–231. [Google Scholar]
- Haafiz MM, Eichhorn SJ, Hassan A, Jawaid M. Isolation and characterization of microcrystalline cellulose from oil palm biomass residue. Carbohydr Polym. 2013;93:628–634. doi: 10.1016/j.carbpol.2013.01.035. [DOI] [PubMed] [Google Scholar]
- Hanna M, Biby G, Miladinov V (2001) Production of microcrystalline cellulose by reactive extrusion. US Patent No. 6228213. US Patent and Trademark Office, Washington, DC
- Husin M, Li AR, Ramli N, Romli AZ, Hakimi MI, Ilham Z. Preparation and characterization of cellulose and microcrystalline cellulose isolated from waste Leucaena leucocephala seeds. Int J Adv Appl Sci. 2017;4:51–58. doi: 10.21833/ijaas.2017.03.009. [DOI] [Google Scholar]
- Jahan MS, Saeed A, He Z, Ni Y. Jute as raw material for the preparation of microcrystalline cellulose. Cellulose. 2011;18:451–459. doi: 10.1007/s10570-010-9481-z. [DOI] [Google Scholar]
- Kalita RD, Nath Y, Ochubiojo ME, Buragohain AK. Extraction and characterization of microcrystalline cellulose from fodder grass; Setaria glauca (L) P. Beauv, and its potential as a drug delivery vehicle for isoniazid, a first line antituberculosis drug. Colloids Surf B Biointerfaces. 2013;108:85–89. doi: 10.1016/j.colsurfb.2013.02.016. [DOI] [PubMed] [Google Scholar]
- Keshk SM, Haija MA. A new method for producing microcrystalline cellulose from Gluconacetobacter xylinus and kenaf. Carbohydr Polym. 2011;84:1301–1305. doi: 10.1016/j.carbpol.2011.01.024. [DOI] [Google Scholar]
- Kumar V, de la Luz R-M, Yang D. Preparation, characterization, and tableting properties of a new cellulose-based pharmaceutical aid. Int J Pharm. 2002;235:129–140. doi: 10.1016/s0378-5173(01)00995-4. [DOI] [PubMed] [Google Scholar]
- Kundu D, Banerjee T. Development of microcrystalline cellulose based hydrogels for the in vitro delivery of Cephalexin. Heliyon. 2020;6:1–10. doi: 10.1016/j.heliyon.2019.e03027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li R, Fei J, Cai Y, Li Y, Feng J, Yao J. Cellulose whiskers extracted from mulberry: a novel biomass production. Carbohydr Polym. 2009;76:94–99. doi: 10.1016/j.carbpol.2008.09.034. [DOI] [Google Scholar]
- Liu Y, Kim HJ. Fourier transform infrared spectroscopy (FT-IR) and simple algorithm analysis for rapid and non-destructive assessment of developmental cotton fibers. Sensors. 2017;17:1–13. doi: 10.3390/s17071469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandal A, Chakrabarty D. Isolation of nanocellulose from waste sugarcane bagasse (SCB) and its characterization. Carbohydr Polym. 2011;86:1291–1299. doi: 10.1016/j.carbpol.2011.06.030. [DOI] [Google Scholar]
- Merci A, Urbano A, Grossmann MVE, Tischer CA, Mali S. Properties of microcrystalline cellulose extracted from soybean hulls by reactive extrusion. Food Res Int. 2015;73:38–43. doi: 10.1016/j.foodres.2015.03.020. [DOI] [Google Scholar]
- Narra M, Rudakiya DM, Macwan K, Patel N. Black liquor: a potential moistening agent for production of cost-effective hydrolytic enzymes by a newly isolated cellulo-xylano fungal strain Aspergillus tubingensis and its role in higher saccharification efficiency. Bioresour Technol. 2020;306:123149. doi: 10.1016/j.biortech.2020.123149. [DOI] [PubMed] [Google Scholar]
- Ng HM, Sin LT, Tee TT, Bee ST, Hui D, Low CY, Rahmat AR. Extraction of cellulose nanocrystals from plant sources for application as reinforcing agent in polymers. Compos B Eng. 2015;75:176–200. doi: 10.1016/j.compositesb.2015.01.008. [DOI] [Google Scholar]
- Okareh OT, Adeolu AT, Adepoju OT. Proximate and mineral composition of plantain (Musa paradisiaca) wastes flour; a potential nutrients source in the formulation of animal feeds. Afr J Food Sci Technol. 2015;6:53–57. doi: 10.14303/ajfst.2015.015. [DOI] [Google Scholar]
- Othman SH, Majid NA, Tawakkal IS, Basha RK, Nordin N, Shapi RA. Tapioca starch films reinforced with microcrystalline cellulose for potential food packaging application. Food Sci Technol. 2019;39:605–612. doi: 10.1590/fst.36017. [DOI] [Google Scholar]
- Parveen S, Rana S, Ferreira S, Fangueiro R. Ultrasonic dispersion of micro crystalline cellulose for developing cementitious composites with excellent strength and stiffness. Ind Crops Prod. 2018;122:156–165. doi: 10.1016/j.indcrop.2018.05.060. [DOI] [Google Scholar]
- Rashid M, Gafur MA, Sharafat MK, Minami H, Miah MAJ, Ahmad H. Biocompatible microcrystalline cellulose particles from cotton wool and magnetization via a simple in situ co-precipitation method. Carbohydr Polym. 2017;170:72–79. doi: 10.1016/j.carbpol.2017.04.059. [DOI] [PubMed] [Google Scholar]
- Rosa SM, Rehman N, de Miranda MIG, Nachtigall SM, Bica CI. Chlorine-free extraction of cellulose from rice husk and whisker isolation. Carbohydr Polym. 2012;87:1131–1138. doi: 10.1016/j.carbpol.2011.08.084. [DOI] [Google Scholar]
- Rudakiya DM, Gupte A. Degradation of hardwoods by treatment of white rot fungi and its pyrolysis kinetics studies. Int Biodeter Biodegrad. 2017;120:21–35. doi: 10.1016/j.ibiod.2017.02.004. [DOI] [Google Scholar]
- Seera SDK, Kundu D, Banerjee T. Physical and chemical crosslinked microcrystalline cellulose-polyvinyl alcohol hydrogel: freeze–thaw mediated synthesis, characterization and in vitro delivery of 5-fluorouracil. Cellulose. 2020;27:6521–6535. doi: 10.1007/s10570-020-03249-9. [DOI] [Google Scholar]
- Segal L, Creely JJ, Martin AE, Jr, Conrad CM. An empirical method for estimating the degree of crystallinity of native cellulose using the X-ray diffractometer. Text Res J. 1959;29:786–794. doi: 10.1177/004051755902901003. [DOI] [Google Scholar]
- Shankar S, Rhim JW. Preparation of nanocellulose from micro-crystalline cellulose: the effect on the performance and properties of agar-based composite films. Carbohydr Polym. 2016;135:1826. doi: 10.1016/j.carbpol.2015.08.082. [DOI] [PubMed] [Google Scholar]
- Shao X, Wang J, Liu Z, Hu N, Liu M, Xu Y. Preparation and characterization of porous microcrystalline cellulose from corncob. Ind Crop Prod. 2020;151:1–6. doi: 10.1016/j.indcrop.2020.112457. [DOI] [Google Scholar]
- Soni B, Mahmoud B. Chemical isolation and characterization of different cellulose nanofibers from cotton stalks. Carbohydr Polym. 2015;134:581–589. doi: 10.1016/j.carbpol.2015.08.031. [DOI] [PubMed] [Google Scholar]
- Sun WJ, Sun CC. A microcrystalline cellulose based drug-composite formulation strategy for developing low dose drug tablets. Int J Pharm. 2020;585:1–5. doi: 10.1016/j.ijpharm.2020.119517. [DOI] [PubMed] [Google Scholar]
- Sun JX, Sun XF, Zhao H, Sun RC. Isolation and characterization of cellulose from sugarcane bagasse. Polym Degrad Stab. 2004;84:331–339. doi: 10.1016/j.polymdegradstab.2004.02.008. [DOI] [Google Scholar]
- Trache D, Donnot A, Khimeche K, Benelmir R, Brosse N. Physico-chemical properties and thermal stability of microcrystalline cellulose isolated from Alfa fibres. Carbohydr Polym. 2014;104:223–230. doi: 10.1016/j.carbpol.2014.01.058. [DOI] [PubMed] [Google Scholar]
- Ventura-Cruz S, Flores-Alamo N, Tecante A. Preparation of microcrystalline cellulose from residual Rose stems (Rosa spp.) by successive delignification with alkaline hydrogen peroxide. Int J Biol Macromol. 2020;155:324–329. doi: 10.1016/j.ijbiomac.2020.03.222. [DOI] [PubMed] [Google Scholar]
- Wang Z, Yao Z, Zhou J, Zhang Y. Reuse of waste cotton cloth for the extraction of cellulose nanocrystals. Carbohydr Polym. 2017;157:945–952. doi: 10.1016/j.carbpol.2016.10.044. [DOI] [PubMed] [Google Scholar]
- Xu J, Tan X, Chen L, Li X, Xie F. Starch/microcrystalline cellulose hybrid gels as gastric-floating drug delivery systems. Carbohydr Polym. 2019;215:151–159. doi: 10.1016/j.carbpol.2019.03.078. [DOI] [PubMed] [Google Scholar]
- Yue Y, Zhou C, French AD, Xia G, Han G, Wang Q, Wu Q. Comparative properties of cellulose nano-crystals from native and mercerized cotton fibers. Cellulose. 2012;19:1173–1187. doi: 10.1007/s10570-012-9714-4. [DOI] [Google Scholar]
- Zhang Z, O’Hara IM, Kent GA, Doherty WO. Comparative study on adsorption of two cationic dyes by milled sugarcane bagasse. Ind Crops Prod. 2013;42:41–49. doi: 10.1016/j.indcrop.2012.05.008. [DOI] [Google Scholar]
- Zhang L, Ge H, Xu M, Cao J, Dai Y. Physicochemical properties, antioxidant and antibacterial activities of dialdehyde microcrystalline cellulose. Cellulose. 2017;24:2287–2298. doi: 10.1007/s10570-017-1255-4. [DOI] [Google Scholar]
- Zhao T, Chen Z, Lin X, Ren Z, Li B, Zhang Y. Preparation and characterization of microcrystalline cellulose (MCC) from tea waste. Carbohydr Polym. 2018;184:164–170. doi: 10.1016/j.carbpol.2017.12.024. [DOI] [PubMed] [Google Scholar]
- Zuo HF, Guo YR, Li SJ, Pan QJ. Application of microcrystalline cellulose to fabricate ZnO with enhanced photocatalytic activity. J Alloys Compound. 2014;617:823–827. doi: 10.1016/j.jallcom.2014.08.071. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.