Abstract
Women with complications of pregnancy such as preeclampsia and preterm birth are at risk for adverse long-term outcomes, including an increased future risk of chronic kidney disease (CKD) and end-stage kidney disease (ESKD). This observational cohort study aimed to examine the risk of CKD after preterm delivery and preeclampsia in a large obstetric cohort in Germany, taking into account preexisting comorbidities, potential confounders, and the severity of CKD. Statutory claims data of the AOK Baden-Wuerttemberg were used to identify women with singleton live births between 2010 and 2017. Women with preexisting conditions including CKD, ESKD, and kidney replacement therapy (KRT) were excluded. Preterm delivery (< 37 gestational weeks) was the main exposure of interest; preeclampsia was investigated as secondary exposure. The main outcome was a newly recorded diagnosis of CKD in the claims database. Data were analyzed using Cox proportional hazard regression models. The time-dependent occurrence of CKD was analyzed for four strata, i.e., births with (i) neither an exposure of preterm delivery nor an exposure of preeclampsia, (ii) no exposure of preterm delivery but exposure of at least one preeclampsia, (iii) an exposure of at least one preterm delivery but no exposure of preeclampsia, or (iv) joint exposure of preterm delivery and preeclampsia. Risk stratification also included different CKD stages. Adjustments were made for confounding factors, such as maternal age, diabetes, obesity, and dyslipidemia. The cohort consisted of 193,152 women with 257,481 singleton live births. Mean observation time was 5.44 years. In total, there were 16,948 preterm deliveries (6.58%) and 14,448 births with at least one prior diagnosis of preeclampsia (5.61%). With a mean age of 30.51 years, 1,821 women developed any form of CKD. Compared to women with no risk exposure, women with a history of at least one preterm delivery (HR = 1.789) and women with a history of at least one preeclampsia (HR = 1.784) had an increased risk for any subsequent CKD. The highest risk for CKD was found for women with a joint exposure of preterm delivery and preeclampsia (HR = 5.227). These effects were the same in magnitude only for the outcome of mild to moderate CKD, but strongly increased for the outcome of severe CKD (HR = 11.90). Preterm delivery and preeclampsia were identified as independent risk factors for all CKD stages. A joint exposure or preterm birth and preeclampsia was associated with an excessive maternal risk burden for CKD in the first decade after pregnancy. Since consequent follow-up policies have not been defined yet, these results will help guide long-term surveillance for early detection and prevention of kidney disease, especially for women affected by both conditions.
Subject terms: Kidney diseases, Health care, Epidemiology
Introduction
Preterm birth is a major global health burden and leading cause of perinatal morbidity and mortality. Approximately 15 million babies are born preterm every year, with rates rising1,2. The majority of preterm deliveries occur spontaneously; approximately 30% are performed for medical reasons, including maternal preeclampsia or fetal growth restriction3,4. Preterm delivery not only poses a significant risk for adverse perinatal outcomes, it is also an important factor among pregnancy complications that predispose the mother to cardiovascular disease (CVD) and chronic hypertension later in life, analogous to preeclampsia5–10.
During the last few decades, there has been increasing evidence that women with a history of adverse pregnancy outcomes also have an elevated risk of developing a chronic kidney disease (CKD) or end-stage kidney disease (ESKD) later in life11–18. The prevalence of CKD is estimated at 10–15% of the world population and, as a major cause of morbidity, CKD is an important global health issue19,20.
Despite the clinical and public health significance, the pathomechanism underlying preterm delivery is not fully understood. Inflammation, infection, and complex immunologic abnormalities represent the main determinants that have been suggested21–24. When preterm birth is associated with placental malperfusion, mechanisms of vascular dysfunction come to the fore, with far-reaching consequences for the renal and cardiovascular system25–27. Two hypotheses have been proposed regarding the association between adverse pregnancy outcomes and the development of CKD and CVD: a pregnancy in itself, but especially if complicated by preeclampsia or preterm delivery, might be an early marker of underlying cardiovascular dysfunction in women with a corresponding risk profile; thereby, pregnancy serves as a “stress test” that exacerbates a preexisting pathologic condition28,29. Indeed, classic modifiable cardiometabolic risk factors such as hypertension, dyslipidemia, and diabetes are likely to play a role and evidently become manifest more often in women after a preterm delivery or preeclampsia30–32. On the other hand, adverse pregnancy outcomes might cause permanent vascular damage, leading to premature aging of the vascular system and thus to an increased risk of subsequent kidney disease and CVD33.
While studies have identified preterm delivery as a strong risk factor for later CVD, only few studies have addressed the association between preterm delivery and subsequent renal failure. Two Norwegian cohort studies demonstrated that having a preterm infant increased the relative risk of later ESKD in a healthy registry cohort18,34. Likewise, two retrospective cohort studies from Canada and Israel found significantly higher rates of ESKD hospitalization in women with either spontaneous or indicated preterm deliveries35,36. A recent Swedish nationwide cohort study demonstrated that women with a history of preterm delivery were at increased risk for subsequent CKD (aHR 1.39, CI 1.32–1.45) and ESKD (aHR 3.61, CI 2.03–6.39), regardless of preeclampsia or whether they delivered small-for-gestational-age children. The highest CKD risk rates were found for women with medically indicated deliveries, including preterm deliveries with preeclampsia (aHR 2.81, CI 2.46–3.20)15,16. Preeclampsia was itself recently identified as a strong risk factor for CKD and ESKD in large cohort studies17,18,35,37.
Despite increasing evidence for adverse renal outcomes, current research revealed that knowledge of long-term risks is relatively low among healthcare professionals; obstetricians tend to have a greater awareness of subsequent kidney disease after a complicated pregnancy, but they are not routinely involved in the long-term aftercare38. A study in Germany showed that, although the majority of obstetricians were aware of an increased renal risk after an unfavorable pregnancy outcome, their knowledge of follow-up care and counseling of these patients was insufficient39.
Using claims data from a statutory health insurance, the aim of our large-scale cohort study was to assess the risk of CKD in women with a history of preterm delivery and to determine how the risk differs depending on CKD severity or when preeclampsia is present concurrently.
Methods
Study design and population
Our analyses were part of a large retrospective observational database study. We obtained a dataset with claims data from the AOK Baden-Wuerttemberg. The AOK Baden-Wuerttemberg is one of the major regional German statutory health insurance companies, covering 4.5 million insured persons. Women with singleton live births between January 1, 2010, and December 31, 2017, were identified. Regarding birth outcome, we analyzed a sample of mothers whose data could be matched with the data of their child/children (222,779 women with 291,091 births). Claims data from the AOK registry were also used to identify women who developed CKD or ESKD during follow-up, until September 30, 2019. Relevant billing coding both in primary care and in the outpatient sector was used to identify exposure and outcome variables. Claims data sets contained outpatient data from 2007, the full dataset including inpatient data was available from 2008 onwards. All diagnoses were encoded by the International Classification of Diseases (ICD-10 German Modification). Additionally, OPS (Operation and Procedure Classification System) and DRG (Diagnosis Related Groups) codes were used. A full list of ICD, OPS, and DRG codes is presented in Table S1 (Appendix).
We considered the births as the statistical subject for the current analyses. Due to miscoding of the quarter of birth in the claims data, we had to exclude pregnancies with implausible delivery times (n = 964) from all analyses. We also excluded births of multiples (n = 5,872) since they are more likely to be delivered preterm than are singleton pregnancies. Moreover, births with implausible records for maternal age (n = 23) were excluded, as were patients who were not insured with the AOK for < 40% of the observation period (n = 26,365). To avoid further potential confounding, births with preexisting maternal diagnoses of CKD, ESKD, or KRT (between January 1, 2007, and September 30, 2019) were excluded at baseline (n = 863). Overall, 33,610 births were excluded (in some births more than one exclusion criterion applied) and the final sample consisted of 193,152 women with 257,481 births.
This study is reported as per the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guideline (see Table S2, Appendix). Patient identification numbers that were originally used for linking files within the insurance databases were encrypted; all data were anonymized and nonidentifiable to the researchers. Ethical approval was obtained from the Ethics Committee of Tuebingen University Medical Faculty and University Hospital.
Study variables
Exposure variables
The main exposure of interest was a history of preterm delivery, defined as any delivery with a gestational age of < 37 weeks. Gestational age was either determined based on the last menstrual period or by measuring the crown-rump length by transvaginal ultrasound. Preterm deliveries were categorized as “extremely preterm” for gestational age of < 28 weeks, “very preterm” for gestational age of 28 to < 32 weeks, and “moderate to late preterm” for gestational age of 32 to < 37 weeks. According to ICD coding for disorders associated with short gestation and low birth weight, gestational age at birth cannot be distinguished in detail, but only between < 28 weeks and ≥ 28 to < 37 weeks of gestation. Due to only a small number of cases in the former group, all preterm deliveries were combined into one single exposure variable “preterm delivery”.
We considered preterm delivery as a time-dependent variable so that a woman could contribute pregnancies and person-time to both unexposed and exposed groups during follow-up. For the main analysis, births were stratified into term birth vs. preterm delivery at gestational age < 37 weeks. Women were considered unexposed (i) if they had never delivered preterm, or (ii) had not delivered preterm from the date of their first term birth until the date of their first preterm delivery, irrespective of subsequent pregnancy outcomes. For example, if a woman had two deliveries during the study period and only her second delivery was preterm, she was considered unexposed between her first and second delivery, but from then on was considered exposed.
The second time-dependent exposure of interest was preeclampsia. Preeclampsia was defined as new onset of hypertension ≥ 140/90 mmHg after 20 weeks of gestation with at least one significant end-organ dysfunction, typically kidney involvement with proteinuria40. On the basis of ICD coding, it was impossible to classify preeclampsia as mild or severe disease. Additionally, we were not able to determine whether a preterm delivery was a spontaneous or medically indicated delivery. Hence, we determined preterm birth with concurrent preeclampsia as a surrogate parameter for a severe form of a medically indicated preterm birth.
Thus, we finally stratified the births into four groups according to the status of the two binary prior risk exposures: (i) neither any exposure to preterm delivery nor any exposure to preeclampsia, (ii) no exposure to preterm delivery but exposure to at least one preeclampsia, (iii) exposure to at least one preterm delivery, but no exposure to preeclampsia, or (iv) joint exposure to preterm delivery and preeclampsia.
Outcome variables
CKD was the main outcome of interest. CKD was defined as a diagnosis of CKD, ESKD, or initiation of dialysis recorded in the health insurance database during follow-up. We categorized CKD according to disease severity. “General CKD” comprised the total quantity of cases with any CKD, including all CKD stages and ESKD cases, and also unspecified renal insufficiency. “Mild to moderate CKD” was defined as CKD stages 1–3, “Severe CKD” included CKD stage 4 and stage 5 (ESKD), initiation of dialysis, and (status after) kidney transplantation. The ICD and OPS codes used for each category are shown in Table 1.
Table 1.
Outcome variables.
| Category | ICD codes | OPS codes |
|---|---|---|
| “mild to moderate CKD” | N18.1, N18.2, N18.3, N18.81, N18.82, N18.83 | |
| “severe CKD” | N18.0, N18.4, N18.5, N18.84, Z49.1, Z49.2, Z94.0, Z99.2 | 5-555, 8-853, 8-854, 8-855, 8-857 |
| “general CKD” | N19, N18.8, N18.80, N18.9, N18.1, N18.2, N18.3, N18.81, N18.82, N18.83, N18.0, N18.4, N18.5, N18.84, Z49.1, Z49.2, Z94.0, Z99.2 | 5-555, 8-853, 8-854, 8-855, 8-857 |
Change of ICD code N18.- in 2010; both versions of ICD coding for CKD (before and after 2010) were used for the analyses.
Covariates
We adjusted for the following confounding covariates: maternal age, diabetes, or gestational diabetes as well as obesity and dyslipidemia. Maternal exposure to diabetes (type 1 and 2) or gestational diabetes was determined by such a diagnosis having been recorded in the claims database. To identify obese women, we used ICD diagnoses of adiposity, overnutrition, and dyslipidemia as recorded. Adiposity was classified according to WHO grades. These preexisting conditions were time-dependent variables, where women were considered exposed from the date of their first delivery with a diagnosis of diabetes, adiposity, or dyslipidemia, respectively.
Statistical analysis
The statistical analyses were performed using R version 4.0.2 and R-Studio v. 1.3.1056 for Windows (32/64 bit)41. The date of the first delivery marked the entry in the observation period for each woman. The study cohort was followed up for the earliest diagnosis of CKD from delivery to either death, migration, or end of the study period. Thus, using these survival data (i.e., data regarding the occurrence of time-dependent events and censored data) we aimed to compare risk groups (at least one risk exposure such as preterm birth and/or preeclampsia) with a reference group (no risk exposures), adjusted for defined binary and parametric covariates.
Cox proportional hazard regression was used to compute hazard ratios and 95% confidence intervals. In our proportional hazard model, the risk groups (ii – iv) were compared to the reference group of individuals who were not exposed to preterm delivery or to preeclampsia (i). The HRs were interpreted like relative risks: In our design a HR = 1 meant that there was no difference in the event rate between a risk group and the reference group free of risk. A HR > 1 meant that the event rate of a risk group was > 1 times the event-rate of the reference group without risk. A HR < 1 meant that the event rate of a risk group was < 1 times the event-rate of the reference group without risk. The model was adjusted for confounders, such as maternal age, diabetes or obesity, and dyslipidemia. We set the critical α-error to α ≤ 0.01. However, as the sample size was extensive, and thus the statistical power to detect even small effects (HRs ≈ 1) amounted to nearly 1-β = 1.00, we cannot conclude that any substantial meaning could be attributed to an effect just because of its statistical significance. Thus, in this study, we focused on the interpretation of the effect sizes, i.e., the HRs. In the final study population, there were no missing values regarding the variables used in the models (see Table 2).
Table 2.
Sample characteristics.
| f | % | ||
|---|---|---|---|
| Number of births | 1 | 134,805 | 52.36 |
| 2 | 101,625 | 39.47 | |
| ≥ 3 | 21,051 | 8.18 | |
| Missing | 0 | 0.00 | |
| Birth mode | C-Section | 80,635 | 31.32 |
| Vaginal | 175,679 | 68.23 | |
| Undefined | 85 | 0.03 | |
| Missing | 1,082 | 0.42 | |
| Obesity / dyslipidemia | False | 206,541 | 80.22 |
| True | 50,940 | 19.78 | |
| Missing | 0 | 0.00 | |
| (Gestational) Diabetes | False | 211,730 | 82.23 |
| True | 45,751 | 17.77 | |
| Missing | 0 | 0.00 | |
| Preterms | False | 240,533 | 93.42 |
| True | 16,948 | 6.58 | |
| Missing | 0 | 0.00 | |
| Preeclampsia | False | 243,033 | 94.39 |
| True | 14,448 | 5.61 | |
| Missing | 0 | 0.00 | |
| Mild / moderate CKD | False | 255,904 | 99.39 |
| True | 1,577 | 0.61 | |
| Missing | 0 | 0.00 | |
| Severe CKD | False | 257,291 | 99.93 |
| True | 190 | 0.07 | |
| Missing | 0 | 0.00 |
f = frequency; % = percentage of N = 257,481.
Ethics approval and consent to participate
Ethical approval was obtained from the Ethics Committee of Tuebingen University Medical Faculty and University Hospital. The Ethics Committee of Tuebingen University granted an exemption from requiring informed consent and confirmed that there were no objections to the analysis of sufficiently anonymized aggregated data sets.
Results
Sample characteristics
The study population consisted of 193,152 women with 257,481 singleton live births. Mean maternal age at delivery was 30.68 years (SD 5.27 years). The average interpregnancy intervals amounted to 2.76 years (SD 1.25 years). Mean observation time was 5.44 years. Further characteristics of the study population are listed in Table 2.
There were 16,948 preterm deliveries (6.58%) and 14,448 pregnancies (5.61%) with a diagnosis of preeclampsia. In all, 1,821 women (0.71%) were diagnosed with any CKD, of whom 1,577 were diagnosed with a CKD stage 1–3 (0.61%); 190 (0.07%) women developed a severe CKD, including ESKD. Mean age at any CKD diagnosis was 30.51 years (SD = 5.60 years) and did not differ according to CKD severity (CKD stages 1–3: M = 30.51 years, SD = 5.60 years; CKD stages 4–5 and ESKD: M = 30.51 years, SD = 5.60 years).
Regarding our four strata according to the status of the two binary prior risk exposures, there were:
-
i.
228,214 with neither an exposure of preterm delivery nor an exposure of preeclampsia,
-
ii.
12,319 with no exposure of preterm delivery but exposure of at least one preeclampsia,
-
iii.
14,819 with an exposure of at least one preterm delivery but no exposure of preeclampsia, and
-
iv.
2,129 with a joint exposure of preterm delivery and preeclampsia.
Main analysis
Compared to women with no risk exposure, the CKD risk was significantly increased for women, who had ever given birth affected by preterm delivery (HR 1.789, 95% CI [1.531; 2.091]). Women with a history of preeclampsia occurring at any time during pregnancy also had an increased risk of a subsequent CKD (HR 1.784, 95% CI [1.516; 2.098]), irrespective of CKD severity (see Table 3). The highest increase in risk was observed for women with a combined exposure to preeclampsia and preterm delivery: these women had a more than fivefold risk for subsequent CKD (HR 5.227, 95% CI [4.201; 6.504]) compared to women without any risk exposure. The effects of diabetes and obesity were comparable to the effects of the single risk exposures (diabetes HR = 1.470, obesity HR = 1.609; see Table 3). Only the effect of maternal age within the observation period seemed negligible (HR = 1.051). The cumulative hazard plot regarding the risk of any subsequent CKD is depicted in Fig. 1.
Table 3.
Cox regressions for CKD by risk exposures.
| Outcome | Exposure | HR | 95% CI HR | z | P( >|z|) |
|---|---|---|---|---|---|
| Any CKD1,2 | Preeclampsia alone5 | 1.784 | [1.516; 2.098] | 6.981 | < .001 |
| Preterm delivery alone5 | 1.789 | [1.531; 2.091] | 7.316 | < .001 | |
| Both/joint risk exposure(s)5 | 5.227 | [4.201; 6.504] | 14.828 | < .001 | |
| Maternal age | 1.051 | [1.042; 1.060] | 11.619 | < .001 | |
| Diabetes | 1.470 | [1.320; 1.638] | 7.014 | < .001 | |
| Obesity | 1.609 | [1.453; 1.781] | 9.138 | < .001 | |
| Mild to moderate CKD1,3 | Preeclampsia alone5 | 1.753 | [1.471; 2.089] | 6.268 | < .001 |
| Preterm delivery alone5 | 1.806 | [1.529; 2.133] | 6.956 | < .001 | |
| Both risk exposures5 | 5.138 | [4.060; 6.502] | 13.626 | < .001 | |
| Maternal age | 1.056 | [ 1.047; 1.066] | 11.874 | < .001 | |
| Diabetes | 1.483 | [1.322; 1.665] | 6.698 | < .001 | |
| Obesity | 1.635 | [1.465; 1.823] | 8.817 | < .001 | |
| Severe CKD1,4 | Preeclampsia alone5 | 2.971 | [1.874; 4.711] | 4.630 | < .001 |
| Preterm delivery alone5 | 4.705 | [3.259; 6.794] | 8.263 | < .001 | |
| Both risk exposures5 | 11.903 | [6.902; 20.528] | 8.907 | < .001 | |
| Maternal age | 1.021 | [0.995; 1.048] | 1.572 | 0.116 | |
| Diabetes | 1.181 | [0.832; 1.674] | 0.931 | 0.352 | |
| Obesity | 1.479 | [1.074; 2.035] | 2.400 | 0.016 |
z = z-Value; P( >|z|) = empirical significance level.
CKD Chronic kidney disease, HR Hazard ratio, 95% CI HR 95% confidence interval of hazard ratio.
1N = 257,481.
2Number of events = 1.821.
3Number of events = 1.577.
4Number of events = 190.
5Reference group = no exposure either to preterm delivery or to preeclampsia.
Figure 1.
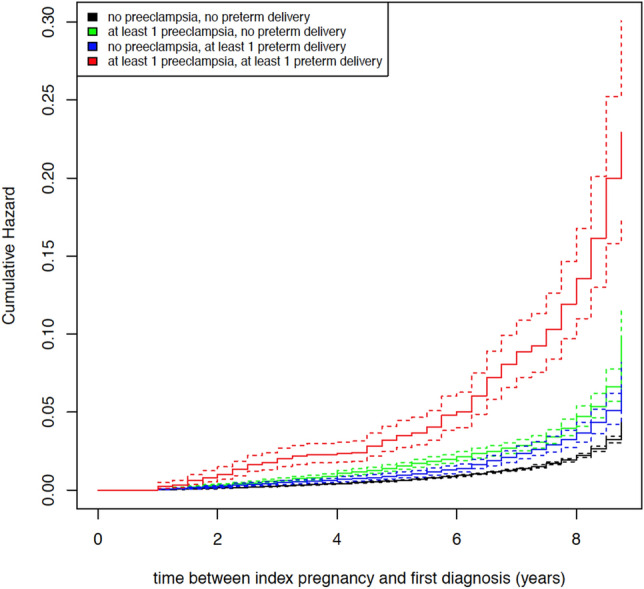
Cumulative hazard plot on risk of any subsequent CKD.
These effects were the same in magnitude only for the outcome of mild to moderate CKD (see Table 3). However, the estimated HRs for the outcome of severe CKD exceeded the respective effects from prior models: Compared to women with no risk exposure, women with a history of at least one preeclampsia alone had an almost threefold risk (HR 2.971, 95% [1.874; 4.711]) for subsequent severe CKD compared to women without any risk exposure (see Table 3). For a history of preterm delivery, the risk was significantly increased (HR 4.705, 95% CI [3.259; 6.794]), with an almost fivefold higher risk compared to women without any risk exposure. Again, women with a combined exposure had the highest risk: Their chance of subsequently developing severe CKD was almost 12 times that of women without any exposure. As shown in Table 3, the CIs of the effects were wider in this model than in the latter two. Maternal age, diabetes, and obesity even failed to reach statistical significance in this model. Figures 2 and 3 depict the cumulative hazard plots regarding the risk of mild to moderate and severe subsequent CKD.
Figure 2.
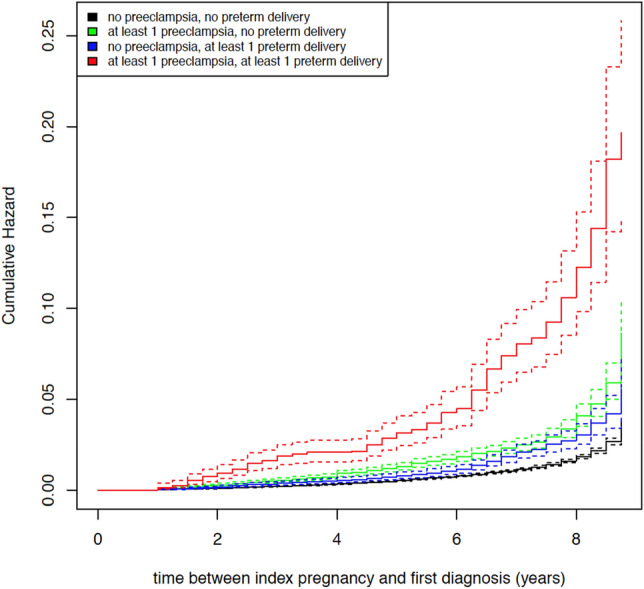
Cumulative hazard plot on risk of mild to moderate subsequent CKD.
Figure 3.
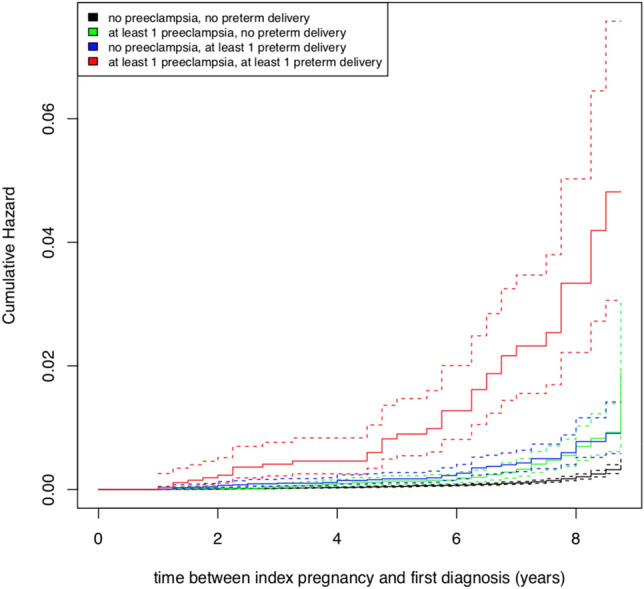
Cumulative hazard plot on risk of severe subsequent CKD.
Discussion
Principal findings
Our analyses aimed to determine whether preterm birth poses a risk for subsequent CKD, either as a single risk factor or jointly with preeclampsia. Our results revealed that women with a history of preterm delivery had an increased risk for later CKD compared to women with term deliveries. A particularly strong association was found for a history of preterm delivery and subsequent, severe CKD, including ESKD, with a 4.7-fold higher risk. Considering births complicated by preeclampsia, there was also an increased risk for severe CKD and ESKD, but the association was less marked than for preterm births. Women who experienced a preterm delivery concurring with preeclampsia were at the highest risk for developing any CKD with a HR of 5.2. The risk of developing a severe CKD or ESKD after a preterm birth complicated by preeclampsia was even 11-fold higher.
Comparison with previous research
The associations demonstrated are consistent with previous cohort studies which found an increased risk for renal insufficiency after a preterm delivery. A recent meta-analysis suggested that preterm delivery may be independently associated with a higher risk of maternal ESKD. Preterm preeclampsia was associated with the highest risk rates for ESKD (aRR, 5.66), and the association with ESKD persisted for women who had preterm deliveries without preeclampsia (aRR, 2.09)16. Consistently, we found the strongest association for later severe CKD in women with a history of preterm delivery with preeclampsia, with a 11.9-fold increased risk.
Although similar studies have been conducted, the risk rates are not directly comparable. Previous studies failed to adjust for important confounders or explicitly included only women with preexisting conditions. In addition, existing research mainly focused on ESKD incidence and ESKD hospitalization18,34–36. A recent cohort study by Barrett et al. was the first to report an association between preterm delivery and maternal CKD. They found the highest risk of CKD in women who experienced preterm delivery and preeclampsia (aHR 2.81, CI 2.46–3.20). However, spontaneous preterm delivery as a single exposure was also associated with an increased risk of CKD (aHR 1.32, CI 1.25–0.139), independent of preeclampsia or having delivered a small-for-gestational-age baby15. This population-based study from Sweden included 1,943,716 women, as compared to our cohort of 257,481 births. Due to database limitations, we were not able to stratify risk according to gestational age, but we also distinguished preterm deliveries as spontaneous or iatrogenic preterm, which in our case meant a preterm birth complicated by preeclampsia. Our analysis could corroborate previous results and could likewise identify preterm delivery as an important obstetric risk factor for subsequent CKD. As in our study, Barrett et al. also found the highest risk for joint exposure to preterm birth and preeclampsia, regardless of CKD severity15,17. Preeclampsia itself has recently been identified as a strong risk factor for maternal CKD and ESKD14,17,37,42. Our analysis revealed comparable risk rates for preeclampsia or preterm delivery as single risk exposure for any CKD.
With a study period of 10 years and a mean observation time of 5.44 years, the observation period in our study was comparably short. Mean follow-up of previous cohort studies was 7–27 years15,17,18,43. Despite our shorter time span, we were able to demonstrate clear trends for subsequent maternal CKD. Our results showed that after a complicated pregnancy, the first decade plays a particularly important role in the trajectory of renal failure. Kristensen et al. demonstrated a similar tendency in a nationwide cohort study on the risk of later kidney disease after a history of preeclampsia. They found that preeclampsia was especially strongly associated with the risk of CKD within five years of the latest pregnancy; thereafter, the strength of the association decreased43.
Interpretation
The underlying cause of preterm birth is uncertain; often a “proinflammatory phenotype” is assumed27. Similarly, all factors involved in the metabolic syndrome are associated with low-grade inflammation44. Metabolic risk factors such as hypertension, obesity, and insulin resistance have been shown to be associated with an increased risk of preterm delivery and preeclampsia. Placental dysfunction is a key feature of both disease patterns31,45–48. It enhances the release of proinflammatory and antiangiogenic factors in the body33. This could explain the significantly increased risk we found in women with preeclamptic preterm deliveries. Proinflammatory processes contribute to accelerated endothelial dysfunction and systemic atherosclerosis, culminating in impaired end-organ function. In addition to the cardiovascular system, the renal system is particularly affected. Considering the shared underlying pathomechanisms, it seems plausible that preterm birth is a manifestation of a subclinical predisposition to later kidney disease in women with a corresponding risk profile15. However, our findings of severe CKD in women with a history of spontaneous preterm deliveries in the first decade after pregnancy suggest that preterm delivery may also be associated with early kidney damage48.
Strengths and limitations
This study is the first to investigate maternal CKD after a preterm delivery in a German cohort. The key strength is the large cohort: over 190,000 women of childbearing age were followed up for almost a decade. The cohort size provided adequate power to examine the impact of preterm delivery independently of recognized risk factors. The prevalence of CKD was low (0.07–0.61%), which may be due to the fact that our cohort was comparably young for a disease that aggravates gradually over decades. Moreover, we cannot rule out the possibility that cases of early, subclinical CKD might not have been detected.
Regarding the generalizability of our results, we found similar prevalence rates of preterm births and cesarean sections as in national comparisons49,50. However, the study population consisted primarily of white European women living in Southern Germany, which limits the generalizability of our results for other ethnic groups.
To avoid possible confounding, women with pre-pregnancy kidney disease, renal insufficiency, or KRT were excluded from the analyses. Cases of pre-existing CVD or autoimmune diseases without any form of kidney involvement were not excluded, which may have confounded our results; we assume, however, that pre-pregnancy CVD rates were negligible in a cohort of young women of childbearing age, who tend to be healthy. We were also able to adjust for certain metabolic risk factors, such as obesity, diabetes, and dyslipidemia, which have all been associated with both adverse pregnancy outcomes and CKD16,44. However, no data for the exact BMI were available in the claims data registry. It can be assumed, however, that the coding for obesity was conservative since it has little billing relevance. Additionally, we did not have data on smoking, which is a risk factor for preterm delivery and CKD; however, studies have shown an inverse association between cigarette smoking during pregnancy and incidence of preeclampsia51–53. Therefore, we assume that the CKD risk increase cannot be attributed to the smoking status.
One limitation which applies to most registry-based studies is the questionable validity of recorded diagnoses. Since diagnoses were ascertained by medical professionals and not self-reported, we assumed correct coding in both primary care and specialist settings. However, incomplete or incorrect coding cannot be ruled out, especially for diagnoses with little billing relevance. In addition, claims data based on ICD-10 coding are limited in their ability to assess disease severity. To mitigate this effect, we used surrogate parameters for severity level (e.g., joint exposure of preeclampsia and preterm delivery as a surrogate for a severe form of a medically induced preterm birth).
Since we had no information about parity, we used the first pregnancy during the study period as the index pregnancy. This might have led to misclassification for the proportion of women who gave birth prior to our analysis window.
Regarding the analyses on severe forms of CKD, with a total number of just n = 190 events, the standard errors of the HRs was higher than the analyses regarding general or mild to moderate forms of CKD, making our estimations less exact in that specific model.
In general, the association between preterm delivery and maternal CKD must be considered correlative and not causal because, despite adjustment, we cannot completely rule out the influence of other important confounding factors.
Implications
Despite the highly increased risk rates shown, the absolute risk of developing a CKD or ESKD after a preterm delivery remains low overall. However, the relative increase in risk among women who have been exposed to preterm delivery is clearly relevant from a population-based perspective, as preterm deliveries still account for a significant proportion of all births. Although an increasing number of professional societies recognize that an adverse pregnancy outcome strongly contributes to women’s overall risk profile for future chronic diseases, there is still a lack of structured follow-up. International guidelines show excessive variation in recommendations for long-term surveillance and screening eligibility54. Existing prediction models for CKD and ESKD do not consider obstetric history, although it is easy to assess in the clinical setting55. Identifying certain adverse pregnancy outcomes, particularly preterm delivery, as risk markers for future maternal CKD could provide an opportunity for early preventive measures for women at risk for CKD at a time when it might still be possible to alter the patient’s risk trajectory11,56.
Conclusions
Any history of preterm delivery is associated with an increased risk for subsequent maternal CKD. Women with a history of preterm delivery and preeclampsia as joint exposure represent the main risk group for developing a CKD. So far, regular maternity care does not address this relevant problem adequately. Prevention strategies must be developed and established to reduce adverse long-term outcomes in affected women. Further research investigating the effectiveness and optimal timing of engaging women in systematic renal follow-up and including renal follow-up in existing primary care prevention programs is needed.
Supplementary Information
Acknowledgements
The AOK Baden-Wuerttemberg kindly provided data for the analyses.
Abbreviations
- AOK
Allgemeine Ortskrankenkasse (general local health insurance)
- CKD
Chronic kidney disease
- CVD
Cardiovascular disease
- DRG
Diagnosis related groups
- ESKD
End-stage kidney disease
- ICD
International classification of diseases
- KRT
Kidney replacement therapy
- OPS
Operation and procedure classification system
Author contributions
M.G.: study concept, data analysis, manuscript writing and editing; M.M.: data analysis, manuscript writing and editing; R.G.: data analysis, manuscript writing and editing; T.D.: data analysis, manuscript editing; K.H.: data analysis; manuscript editing; S.Y.B.: study concept, manuscript editing; A.B.: study concept, study management; S.J.: manuscript editing; M.G.C.: manuscript editing; S.H.K.: manuscript editing; A.C.: manuscript editing; G.K.: manuscript editing; F.S.: data management, manuscript editing; S.W.: study concept, data analysis, manuscript writing and editing.
Funding
Open Access funding enabled and organized by Projekt DEAL. This study was funded by the AOK Baden-Wuerttemberg. The funders had no role in study design and analysis, decision to publish or drafting of the manuscript.
Data availability
The data that support the findings of this study are available from AOK Baden-Wuerttemberg but restrictions apply to the availability of these data, which were used under license for the current study, and so are not publicly available. Data are however available from the authors upon reasonable request and with permission of AOK Baden-Wuerttemberg.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-021-92078-2.
References
- 1.Harrison MS, Goldenberg RL. Global burden of prematurity. Semin. Fetal Neonatal Med. 2016;21:74–79. doi: 10.1016/j.siny.2015.12.007. [DOI] [PubMed] [Google Scholar]
- 2.World Health Organisation. Fact Sheet. Preterm Birth. Vol. 2020 (2018).
- 3.Goldenberg RL, Culhane JF, Iams JD, Romero R. Epidemiology and causes of preterm birth. Lancet. 2008;371:75–84. doi: 10.1016/S0140-6736(08)60074-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ananth CV, Vintzileos AM. Medically indicated preterm birth: recognizing the importance of the problem. Clin. Perinatol. 2008;35:53–67. doi: 10.1016/j.clp.2007.11.001. [DOI] [PubMed] [Google Scholar]
- 5.Lykke JA, Langhoff-Roos J, Lockwood CJ, Triche EW, Paidas MJ. Mortality of mothers from cardiovascular and non-cardiovascular causes following pregnancy complications in first delivery. Paediatr. Perinat. Epidemiol. 2010;24:323–330. doi: 10.1111/j.1365-3016.2010.01120.x. [DOI] [PubMed] [Google Scholar]
- 6.Riise HKR, et al. Hypertensive pregnancy disorders increase the risk of maternal cardiovascular disease after adjustment for cardiovascular risk factors. Int. J. Cardiol. 2019;282:81–87. doi: 10.1016/j.ijcard.2019.01.097. [DOI] [PubMed] [Google Scholar]
- 7.Wu P, et al. Preeclampsia and future cardiovascular health: a systematic review and meta-analysis. Circ. Cardiovasc. Qual. Outcomes. 2017;10:150. doi: 10.1161/CIRCOUTCOMES.116.003497. [DOI] [PubMed] [Google Scholar]
- 8.Behrens I, et al. (2017) Risk of post-pregnancy hypertension in women with a history of hypertensive disorders of pregnancy: nationwide cohort study. Bmj. 2017;358:3078. doi: 10.1136/bmj.j3078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.World Health Organisation. Fact Sheet. Cardiovascular diseases (CVDs). Vol. 2020 (2017).
- 10.Hill NR, et al. Global prevalence of chronic kidney disease-a systematic review and meta-analysis. PLOS ONE. 2016;11:e0158765. doi: 10.1371/journal.pone.0158765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wu P, et al. Pre-eclampsia is associated with a twofold increase in diabetes: a systematic review and meta-analysis. Diabetologia. 2016;59:2518–2526. doi: 10.1007/s00125-016-4098-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Covella B, et al. A systematic review and meta-analysis indicates long-term risk of chronic and end-stage kidney disease after preeclampsia. Kidney Int. 2019;2:16. doi: 10.1016/j.kint.2019.03.033. [DOI] [PubMed] [Google Scholar]
- 13.Wang IK, et al. Association between hypertensive disorders during pregnancy and end-stage renal disease: a population-based study. CMAJ Can. Med. Assoc. J. 2013;185:207–213. doi: 10.1503/cmaj.120230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wu CC, et al. End-stage renal disease after hypertensive disorders in pregnancy. Am. J. Obstet. Gynecol. 2014;210(147):e141–148. doi: 10.1097/AOG.0000000000000057. [DOI] [PubMed] [Google Scholar]
- 15.Barrett PM, et al. Risk of long-term renal disease in women with a history of preterm delivery: a population-based cohort study. BMC Med. 2020;18:66. doi: 10.1186/s12916-020-01534-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Barrett PM, et al. Adverse pregnancy outcomes and long-term maternal kidney disease: a systematic review and meta-analysis. JAMA Netw. Open. 2020;3:e1920964. doi: 10.1001/jamanetworkopen.2019.20964. [DOI] [PubMed] [Google Scholar]
- 17.Khashan AS, et al. Preeclampsia and risk of end stage kidney disease: A Swedish nationwide cohort study. PLoS Med. 2019;16:e1002875. doi: 10.1371/journal.pmed.1002875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vikse BE, Irgens LM, Leivestad T, Skjaerven R, Iversen BM. Preeclampsia and the risk of end-stage renal disease. N. Engl. J. Med. 2008;359:800–809. doi: 10.1056/NEJMoa0706790. [DOI] [PubMed] [Google Scholar]
- 19.Levin A, et al. Global kidney health 2017 and beyond: a roadmap for closing gaps in care, research, and policy. Lancet. 2017;390:1888–1917. doi: 10.1016/S0140-6736(17)30788-2. [DOI] [PubMed] [Google Scholar]
- 20.Ashuntantang GE, Garovic VD, Heilberg IP, Lightstone L. Kidneys and women's health: key challenges and considerations. Nat. Rev. Nephrol. 2018;14:203–210. doi: 10.1038/nrneph.2017.188. [DOI] [PubMed] [Google Scholar]
- 21.Gomez-Lopez N, StLouis D, Lehr MA, Sanchez-Rodriguez EN, Arenas-Hernandez M. Immune cells in term and preterm labor. Cell. Mol. Immunol. 2014;11:571–581. doi: 10.1038/cmi.2014.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cappelletti M, Della Bella S, Ferrazzi E, Mavilio D, Divanovic S. Inflammation and preterm birth. J. Leukoc. Biol. 2016;99:67–78. doi: 10.1189/jlb.3MR0615-272RR. [DOI] [PubMed] [Google Scholar]
- 23.Cnattingius S, et al. Maternal obesity and risk of preterm delivery. JAMA. 2013;309:2362–2370. doi: 10.1001/jama.2013.6295. [DOI] [PubMed] [Google Scholar]
- 24.Lepercq J, Coste J, Theau A, Dubois-Laforgue D, Timsit J. Factors associated with preterm delivery in women with type 1 diabetes. A Cohort Stud. 2004;27:2824–2828. doi: 10.2337/diacare.27.12.2824. [DOI] [PubMed] [Google Scholar]
- 25.Powe CE, Levine RJ, Karumanchi SA. Preeclampsia, a disease of the maternal endothelium: the role of antiangiogenic factors and implications for later cardiovascular disease. Circulation. 2011;123:2856–2869. doi: 10.1161/CIRCULATIONAHA.109.853127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shah DA, Khalil RA. Bioactive factors in uteroplacental and systemic circulation link placental ischemia to generalized vascular dysfunction in hypertensive pregnancy and preeclampsia. Biochem. Pharmacol. 2015;95:211–226. doi: 10.1016/j.bcp.2015.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rodie VA, Freeman DJ, Sattar N, Greer IA. Pre-eclampsia and cardiovascular disease: metabolic syndrome of pregnancy? Atherosclerosis. 2004;175:189–202. doi: 10.1016/j.atherosclerosis.2004.01.038. [DOI] [PubMed] [Google Scholar]
- 28.Williams D. Pregnancy: a stress test for life. Curr. Opin. Obstet. Gynecol. 2003;15:465–471. doi: 10.1097/00001703-200312000-00002. [DOI] [PubMed] [Google Scholar]
- 29.Garovic VD, Hayman SR. Hypertension in pregnancy: an emerging risk factor for cardiovascular disease. Nat. Clin. Pract. Nephrol. 2007;3:613–622. doi: 10.1038/ncpneph0623. [DOI] [PubMed] [Google Scholar]
- 30.Catov JM, Lewis CE, Lee M, Wellons MF, Gunderson EP. Preterm birth and future maternal blood pressure, inflammation, and intimal-medial thickness: the CARDIA study. Hypertension (Dallas, Tex. 1979) 2013;61:641–646. doi: 10.1161/HYPERTENSIONAHA.111.00143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Catov J, et al. Preterm birth with placental evidence of malperfusion is associated with cardiovascular risk factors after pregnancy: a prospective cohort study. BJOG Int. J. Obst. Gynaecol. 2018;125:1009–1017. doi: 10.1111/1471-0528.15040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fraser A, et al. Associations of pregnancy complications with calculated cardiovascular disease risk and cardiovascular risk factors in middle age. Circulation. 2012;125:1367–1380. doi: 10.1161/CIRCULATIONAHA.111.044784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cunningham MW, Jr, LaMarca B. (2018) Risk of cardiovascular disease, end-stage renal disease, and stroke in postpartum women and their fetuses after a hypertensive pregnancy. Am. J. Physiol. 2018;R315:r321–528. doi: 10.1152/ajpregu.00218.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sandvik MK, et al. Are adverse pregnancy outcomes risk factors for development of end-stage renal disease in women with diabetes? Nephrol. Dial. Transplant. 2010;25:3600–3607. doi: 10.1093/ndt/gfq275. [DOI] [PubMed] [Google Scholar]
- 35.Dai L, Chen Y, Sun W, Liu S. Association between hypertensive disorders during pregnancy and the subsequent risk of end-stage renal disease: a population-based follow-up study. J. Obstet. Gynaecol. Can. 2018;40:1129–1138. doi: 10.1016/j.jogc.2018.01.022. [DOI] [PubMed] [Google Scholar]
- 36.Pariente G, Kessous R, Sergienko R, Sheiner E. Is preterm delivery an independent risk factor for long-term maternal kidney disease? J. Matern. Fetal Neonatal Med. 2017;30:1102–1107. doi: 10.1080/14767058.2016.1205022. [DOI] [PubMed] [Google Scholar]
- 37.Barrett PM, et al. Hypertensive disorders of pregnancy and the risk of chronic kidney disease: a Swedish registry-based cohort study. PLoS Med. 2020;17:1003255. doi: 10.1371/journal.pmed.1003255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Roth H, LeMarquand G, Henry A, Homer C. Assessing knowledge gaps of women and healthcare providers concerning cardiovascular risk after hypertensive disorders of pregnancy-a scoping review. Front. Cardiovasc. Med. 2019;6:178. doi: 10.3389/fcvm.2019.00178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Heidrich MB, Wenzel D, von Kaisenberg CS, Schippert C, von Versen-Höynck FM. Preeclampsia and long-term risk of cardiovascular disease: what do obstetrician-gynecologists know? BMC Pregnancy Childbirth. 2013;13:61. doi: 10.1186/1471-2393-13-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.AWMF. S2k-Leitlinie Hypertensive Schwangerschaftserkrankungen: Diagnostik und Therapie. (Praxisleitlinie der Deutschen Gesellschaft für Gynäkologie und Geburtshilfe (DGGG), der Österreichischen Gesellschaft für Gynäkologie und Geburtshilfe (OEGGG) & der Schweizerischen Gesellschaft für Gynäkologie und Geburtshilfe (SGGG)). Berlin, Wien, Bern (2019).
- 41.R Core Team. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria (2013).
- 42.Vikse BE, Irgens LM, Leivestad T, Skjærven R, Iversen BM. Preeclampsia and the risk of end-stage renal disease. N. Engl. J. Med. 2008;359:800–809. doi: 10.1056/NEJMoa0706790. [DOI] [PubMed] [Google Scholar]
- 43.Kristensen JH, Basit S, Wohlfahrt J, Damholt MB, Boyd HA. Pre-eclampsia and risk of later kidney disease: nationwide cohort study. BMJ. 2019;365:1516. doi: 10.1136/bmj.l1516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Niyaty S, Moghaddam-Banaem L, Sourinejad H, Mokhlesi S. (2020) Are maternal metabolic syndrome and lipid profile associated with preterm delivery and preterm premature rupture of membranes? Arch. Gynecol. Obst. 2020;2:10. doi: 10.1007/s00404-020-05738-5. [DOI] [PubMed] [Google Scholar]
- 45.Granger JP, Alexander BT, Llinas MT, Bennett WA, Khalil RA. Pathophysiology of preeclampsia: linking placental ischemia/hypoxia with microvascular dysfunction. Microcirculation (New York, N.Y. 1994) 2002;9:147–160. doi: 10.1038/sj.mn.7800137. [DOI] [PubMed] [Google Scholar]
- 46.Kelly R, et al. Placental vascular pathology findings and pathways to preterm delivery. Am. J. Epidemiol. 2009;170:148–158. doi: 10.1093/aje/kwp131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Misra VK, Hobel CJ, Sing CF. Placental blood flow and the risk of preterm delivery. Placenta. 2009;30:619–624. doi: 10.1016/j.placenta.2009.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Vikse BE, Hallan S, Bostad L, Leivestad T, Iversen BM. Previous preeclampsia and risk for progression of biopsy-verified kidney disease to end-stage renal disease. Nephrol. Dial. Transpl. 2010;25:3289–3296. doi: 10.1093/ndt/gfq169. [DOI] [PubMed] [Google Scholar]
- 49.Berger R, et al. Reducing the Risk of Preterm Birth by Ambulatory Risk Factor Management. Deutsches Arzteblatt Int. 2019;116:858–864. doi: 10.3238/arztebl.2019.0858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Simoes E, Kunz S, Bosing-Schwenkglenks M, Schmahl FW. Occupation and risk of cesarean section: study based on the perinatal survey of Baden-Württemberg, Germany. Arch. Gynecol. Obstet. 2005;271:338–342. doi: 10.1007/s00404-004-0616-z. [DOI] [PubMed] [Google Scholar]
- 51.Jaddoe VW, et al. Active and passive maternal smoking during pregnancy and the risks of low birthweight and preterm birth: the Generation R Study. Paediatr. Perinat. Epidemiol. 2008;22:162–171. doi: 10.1111/j.1365-3016.2007.00916.x. [DOI] [PubMed] [Google Scholar]
- 52.Magnussen EB, et al. Prepregnancy cardiovascular risk factors as predictors of pre-eclampsia: population based cohort study. BMJ. 2007;335:978. doi: 10.1136/bmj.39366.416817.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Conde-Agudelo A, Althabe F, Belizán JM, Kafury-Goeta AC. Cigarette smoking during pregnancy and risk of preeclampsia: a systematic review. Am. J. Obstet. Gynecol. 1999;181:1026–1035. doi: 10.1016/S0002-9378(99)70341-8. [DOI] [PubMed] [Google Scholar]
- 54.Barrett PM, Khashan AS, McCarthy FP, Kublickiene K. Adverse pregnancy outcomes and maternal health: action needed for long-term benefit. Acta obstetricia et gynecologica Scandinavica. 2020;2:16. doi: 10.1111/aogs.13945. [DOI] [PubMed] [Google Scholar]
- 55.Collins GS, Omar O, Shanyinde M, Yu LM. A systematic review finds prediction models for chronic kidney disease were poorly reported and often developed using inappropriate methods. J. Clin. Epidemiol. 2013;66:268–277. doi: 10.1016/j.jclinepi.2012.06.020. [DOI] [PubMed] [Google Scholar]
- 56.Andersgaard AB, et al. Recurrence and long-term maternal health risks of hypertensive disorders of pregnancy: a population-based study. Am J Obstet Gynecol. 2012;206(143):e141–148. doi: 10.1016/j.ajog.2011.09.032. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from AOK Baden-Wuerttemberg but restrictions apply to the availability of these data, which were used under license for the current study, and so are not publicly available. Data are however available from the authors upon reasonable request and with permission of AOK Baden-Wuerttemberg.


