Summary
Importin 8, encoded by IPO8, is a ubiquitously expressed member of the importin-β protein family that translocates cargo molecules such as proteins, RNAs, and ribonucleoprotein complexes into the nucleus in a RanGTP-dependent manner. Current knowledge of the cargoes of importin 8 is limited, but TGF-β signaling components such as SMAD1–4 have been suggested to be among them. Here, we report that bi-allelic loss-of-function variants in IPO8 cause a syndromic form of thoracic aortic aneurysm (TAA) with clinical overlap with Loeys-Dietz and Shprintzen-Goldberg syndromes. Seven individuals from six unrelated families showed a consistent phenotype with early-onset TAA, motor developmental delay, connective tissue findings, and craniofacial dysmorphic features. A C57BL/6N Ipo8 knockout mouse model recapitulates TAA development from 8–12 weeks onward in both sexes but most prominently shows ascending aorta dilatation with a propensity for dissection in males. Compliance assays suggest augmented passive stiffness of the ascending aorta in male Ipo8−/− mice throughout life. Immunohistological investigation of mutant aortic walls reveals elastic fiber disorganization and fragmentation along with a signature of increased TGF-β signaling, as evidenced by nuclear pSmad2 accumulation. RT-qPCR assays of the aortic wall in male Ipo8−/− mice demonstrate decreased Smad6/7 and increased Mmp2 and Ccn2 (Ctgf) expression, reinforcing a role for dysregulation of the TGF-β signaling pathway in TAA development. Because importin 8 is the most downstream TGF-β-related effector implicated in TAA pathogenesis so far, it offers opportunities for future mechanistic studies and represents a candidate drug target for TAA.
Keywords: TGF-beta, thoracic aortic aneurysm, Loeys-Dietz syndrome, Shprintzen-Goldberg syndrome, importin 8, knockout mouse model
Main text
Thoracic aortic aneurysm (TAA) refers to a pathological and progressive dilatation of the aorta that, if left untreated, imposes a risk for life-threatening aortic dissection or rupture. TAA presents either as an isolated condition (non-syndromic TAA) or as part of a multi-systemic connective tissue disorder (syndromic TAA). Most typically, the inheritance pattern is autosomal dominant, but rare X-linked or autosomal recessive families have also been reported. Because pathogenic variants in the more than 30 known TAA-associated genes explain less than 30% of probands with a positive family history,1 additional TAA-associated genes remain to be identified.
Important mechanistic insights into syndromic TAA formation have largely emanated from elucidation of the etiology of two clinically overlapping autosomal dominant TAA syndromes: Marfan syndrome (MFS [MIM: 154700]) and Loeys-Dietz syndrome (LDS [MIM: 609192, 610168, 613795, 614816, and 615582]).2 Besides TAA, MFS is characterized by ocular (e.g., ectopia lentis), skeletal (e.g., overgrowth, pectus deformity), and cutaneous (e.g., striae, hernia) manifestations. LDS can be distinguished from MFS by the unique presence of hypertelorism, cleft palate or bifid uvula, and prominent arterial tortuosity, as well as by a more widespread and severe aneurysm phenotype. Whereas MFS is caused by dominant-negative or haplo-insufficient variants in the extracellular matrix (ECM) component fibrillin 13 (FBN1 [MIM: 134797]), LDS results from loss-of-function variants in six key components of the canonical transforming growth factor β (TGF-β) signaling pathway (i.e., TGFBR1/2 [MIM: 190181 and 190182], SMAD2/3 [MIM: 601366 and 603109], TGFB2/3 [MIM: 190220 and 190230]) (Figure S1).4, 5, 6, 7, 8, 9, 10 In both conditions, analysis of the aortic wall in mouse models and affected individuals shows a clear tissue signature for enhanced TGF-β signaling, including activation of signaling intermediates and increased output of TGF-β target genes.11 Interestingly, a third condition with extensive phenotypic overlap with MFS and LDS but less severe cardiovascular involvement and the unique presence of neurodevelopmental delay (Shprintzen-Goldberg syndrome [SGS] [MIM: 182212]) is caused by heterozygous missense variants located in the R-SMAD-binding domain of a negative regulator of the TGF-β transcriptional response called SKI (SKI [MIM: 164780]) (Figure S1).12,13
Using exome or genome sequencing in six unrelated probands presenting with an LDS/SGS-like phenotype (for details, see supplemental materials and methods), we identified bi-allelic loss-of-function variants in IPO8 (MIM: 605600; GenBank: NM_006390.3), encoding the nuclear import protein importin 8 (Figures 1A and S2). None of the probands carried a likely pathogenic variant in any of the known TAA-associated genes. Except for p.Leu866Profs∗12 (c.2597_2601delTTTTC) (1/250,920 alleles), all identified variants are absent from the Genome Aggregation Database (gnomAD v.2.1.1). Causality is further supported by segregation analysis, which demonstrated heterozygosity in the unaffected parents and siblings (Figure 1A) as well as homozygosity in one additional affected brother (individual 4-II:3; Figure 1A). Subsequent Sanger sequencing of the coding regions of IPO8 in 50 other genetically unsolved MFS-, LDS-, or SGS-like probands did not reveal additional individuals with homozygous or compound heterozygous variants.
Figure 1.
Familial screening and clinical characterization of individuals with bi-allelic IPO8 variants
(A) Pedigrees of the families with their respective pathogenic variants. Squares represent males, while circles represent females, filled symbols denote affected individuals, a double line connecting spouses symbolizes consanguinity, and m/m1/m2 denote the presence of the respective IPO8 variants and a + sign represents the wild-type IPO8 allele. Variants are annotated against GenBank: NM_006390.3.
(B) Clinical phenotyping. Proband 1-II:3 showing prominent forehead, hypertelorism, mild ptosis left eye, retrognathia, pectus excavatum, umbilical hernia, joint hypermobility with thumb abduction, and camptodactyly of the second toe. CT angiography of proband 2-II:1 demonstrating dilatation of the common carotid arteries along with marked tortuosity of the common carotid and internal carotid artery, mild tortuosity of the vertebral arteries, and enlargement of the anterior and middle cerebral arteries bilaterally. Proband 3-II:3 presenting with frontal bossing with bitemporal flattening, retrognathia, downturned corners of the mouth, and flat feet. Proband 5-II:2 showing prominent forehead, significant hypertelorism with flat nasal bridge, mild ptosis of left eye, and retrognathia. Proband 6-II:1 demonstrating dolichocephaly, retrognathia, malar flattening, downslanting palpebral fissures, and hypertelorism. Magnetic resonance angiography (MRA) revealing tortuous intracranial and extracranial arterial vessels, most prominently involving the superior cervical internal carotid arteries with dilation of the left internal carotid artery at the carotid bifurcation. CT scan (pre-surgical) showing os odontoideum with cervical spinal canal stenosis (arrows).
Recurrent phenotypic manifestations in our series of individuals with bi-allelic IPO8 variants include facial dysmorphism with dolichocephaly (5/7), frontal bossing (6/7), hypertelorism (6/7), eyelid ptosis (4/7), retrognathia (6/7), and a high arched (6/7) or cleft palate/bifid uvula (3/7); skeletal findings with arachnodactyly (6/7), joint hypermobility (7/7), pectus excavatum (7/7), foot deformity (5/7), and scoliosis (3/7); neuromuscular features, including hypotonia (7/7) and developmental delay (7/7); cardiovascular abnormalities with aortic root and/or ascending aortic aneurysm (6/7) and structural heart disease (atrial or ventricular septal defect [ASD or VSD, respectively] and patent ductus arteriosus [PDA]) (7/7); and finally, umbilical and/or inguinal hernia (5/7) (Figure 1B, Table 1). No disproportionate body growth was observed (Figure S3). Of note, despite the severe aneurysm phenotype, none of the affected individuals experienced an arterial or aortic dissection, but this may be due to their young age. Additionally, marked arterial tortuosity, a typical LDS feature, was reported in two affected individuals (2-II:1 and 6-II:1) but might have been overlooked in the others because they have not yet undergone head-to-pelvis arterial imaging. Overall, the phenotype fits in the spectrum of LDS/SGS-like disorders (Table 2).
Table 1.
Detailed overview of the clinical characteristics of individuals with bi-allelic IPO8 variants
| Family 1, proband 1-II:3 | Family 2, proband 2-II:1 | Family 3, proband 3-II:3 | Family 4, proband 4-II:4 | Family 4, individual 4-II:3 | Family 5, proband 5-II:2 | Family 6, proband 6-II:1 | |
|---|---|---|---|---|---|---|---|
| Variant c. annotation | c.1420C>T, homz | c.[770_777delT ATGGTGG]; [1000dupG] |
c.[1428+5G>A]; [2597_2601 delTTTTC] |
c.776G>A, homz | c.776G>A, homz | c. 2347_2369del, homz | c.2900−1G>A, homz |
| Variant p. annotation | p.Arg474∗, homz | p.[Val257Glufs∗3]; [Val334 Glyfs∗19] |
p.[Lys447_Arg 476del]; [Leu866 Profs∗12] |
p.Trp259∗, homz | p.Trp259∗, homz | p.Leu783Valfs∗5, homz | p.Thr967_Glu1006 delinsLys, homz |
| Sex | M | M | F | M | M | F | M |
| Current age | 10 years | 8 years | 8 years | 6 years | 10 years | 3 years 9 months | 19 years |
| Growth | |||||||
| Age at measurement | 7 years 11 months | 8 years | 7 years 4 months | 6 years | 9 years | 3 years 9 months | 19 years |
| Height | 124 cm (P10–P25) | 127 cm (P25) | 118.7 cm (P10–P25) | 121 cm (P75) | 126 cm (P10–P25) | 92 cm (P3) | 175 cm (P25–P50) |
| Weight | 21 kg (P3–P5) | 19.9 kg (P1) | 22 kg (P25–P50) | 18.3 kg (P25–P50) | 17.6 kg (P0.3) | 11 kg (P0.5) | 63 kg (P25) |
| OFC | 55 cm (P97) | ND | 53.5 cm (P50–P75) | ND | ND | 47 cm (P10) | ND |
| Facial features | |||||||
| Dolichocephaly | + | + | − (prominent sutures) | + | + | − | + |
| Frontal bossing | + | + | + | + | + | + | − |
| Hypertelorism | + | + | − | + | + | + | + |
| Ptosis | + (L > R) | + (L > R) | − | − | − | + (L > R) | + |
| Retrognathia | + | + | + | − | − | + | + |
| Submucous cleft palate | − | + and broad uvula | − | − | − | − and bifid uvula | − and bifid uvula |
| High arched palate | + | + | + | + | + | − | + |
| Skeletal findings | |||||||
| Arachnodactyly | + | + | − | + | + | + | + |
| Joint hypermobility | + | + | + | + | + | + | + |
| Pectus excavatum | + | + | + | + | + | + | + |
| Pes planum | + | + | + | + | + | − | − |
| Cervical spine anomalies | ND | + | − | ND | − | − | + |
| Scoliosis | − | + | − | − | + | − | + |
| Other | 2nd toes camptodactyly | kyphosis | recurrent hip and ankle dislocation | talipes equinovarus (L); vertical talus (R) | sagittal clefts of midthoracic vertebrae; talipes equinovarus (R) | − | long toes |
| Neurological findings | |||||||
| Hypotonia | + | + | + | + | + | + | + |
| Developmental delay | + (mild) | + | + (motor) | + (motor) | + (motor) | + (motor) | + |
| Intellectual disability | − | − | − | − | − | mild | +a |
| Cardiovascular findings | |||||||
| Age at finding | 10 years 8 months | 8 years | 7 years 5 months | 1 year 8 months | 9 years | 3 years 6 months | 19 years |
| ASD | + | + | + | − | − | + | + (aneurysmal) |
| VSD | − | − | + (membraneous and muscular) | + (membraneous) | + | + (membraneous) | − |
| PDA | − | + | + (surgical repair) | + | − | − | − |
| Aortic root | 26 mm (Z = 3.5) | 35 mm (Z = 10) | 25 mm (Z = 3.58) | 25 mm (Z = 5.7) | 38 mm (Z = 6.0) | 15 mm (Z = 0,5) | 41 mm (Z = 6.9) |
| Ascending aorta | 28 mm (Z = 5.7) | 28 mm (Z = 8.7) | 21 mm (Z = 2.68) | 17 mm (Z = 3.9) | 23 mm (Z = 2.7) | ND | 31 mm (Z = 3.8) |
| Sinotubular junction | ND | 25 mm (Z = 5.4) | 23 mm (Z = 4.99) | ND | 25 mm (Z = 3.8) | 12 mm (Z = 0.18) | 23 mm (Z = 1.2) |
| Other aneurysms | ND | com/int carotid, cerebral arteries | ND | ND | ND | pulmonary artery, coronary sinus | ND |
| Arterial/aortic tortuosity | ND | + | ND | ND | ND | ND | + |
| Other findings | |||||||
| Hernia | umbilical | umbilical/bilateral inguinal | − | − | umbilical | umbilical | umbilical/inguinal |
| Easy bruising | + | + | − | − | − | − | − |
ND, not determined; L, left; R, right; +, present; −, absent; Z, Z score (calculated according to Lopez et al.);14 P, percentile; com, common; int, internal; ASD, atrial septal defect; VSD, ventricular septal defect; PDA, patent ductus arteriosus; homz, homozygous; OFC, occipitofrontal circumference.
Proband 6:II-1 also has a chromosomal duplication (1.779 Mb gain of 19q13.41), and learning disability is also present in proband’s mother and maternal half-brother.
Table 2.
Comparison of Marfan, Loeys-Dietz, Sphrintzen-Goldberg, and IPO8 phenotypical characteristics
| MFS | LDS | SGS | IPO8 | |
|---|---|---|---|---|
| Gene(s) | FBN1 | TGFBR1/2, SMAD2/3, TGFB2/3 | SKI | IPO8 |
| Inheritance | AD | AD | AD, de novo | AR |
| Ectopia lentis | +++ | − | − | − |
| Cleft palate/bifid uvula | − | ++ | + | + |
| Hypertelorism | − | ++ | ++ | ++ |
| Proptosis | − | + | ++ | ++ |
| Craniosynostosis | − | + | +++ | − |
| Arachnodactyly | +++ | ++ | ++ | ++ |
| Tall stature | +++ | + | ++ | − |
| Pectus deformity | ++ | ++ | ++ | ++ |
| Club foot | − | ++ | + | + |
| Joint hypermobility | + | ++ | ++ | +++ |
| Cervical spine instability | − | ++ | + | + |
| Osteo-arthritis | + | ++ | + | ? |
| Hernia (umbilical, inguinal …) | + | + | + | + |
| Aortic root aneurysm | +++ | +++ | + | +++ |
| Ascending aneurysm | + | ++ | + | ++ |
| Arterial aneurysm | −/+ | +++ | + | + |
| Arterial tortuosity | − | +++ | + | + |
| Early aortic dissection | + | ++ | − | − |
| BAV/ASD/VSD/PDA | − | + | − | ++ |
| Motor developmental delay | − | − | ++ | ++ |
| Intellectual disability | − | − | ++ | − |
−, absent; +, occasional; ++, common; +++, typical clinical feature; ?, unknown; MFS, Marfan syndrome; LDS, Loeys-Dietz syndrome; SGS, Shprintzen-Goldberg syndrome; TAA, thoracic aortic aneurysm; AD, autosomal dominant; AR, autosomal recessive; BAV, bicuspid aortic valve; ASD, atrial septal defect; VSD, ventricular septal defect; PDA, patent ductus arteriosus.
Six out of eight IPO8 variants are predicted to result in a premature termination codon and, as a result, to induce nonsense-mediated mRNA decay (NMD). Indeed, in fibroblast cDNA of individual 3-II:3, c.2597_2601delTTTTC was only observed upon puromycin treatment (Figure S4A). In blood-derived cDNA of the same child, c.1428+5G>A was found to result in exon 13 skipping (Figures S5A and S5B). In silico protein modeling of its predicted resultant in-frame deletion, p.Lys447_Arg476del (c.1428+5G>A), suggests abnormal folding due to removal of a single helix (Figure S5C). In fibroblast cDNA of individual 1-II:3, the variant allele was seen even in the absence of inhibition of NMD with puromycin, revealing surprising escape from NMD (Figure S4B). No protein was seen in immunoblotting on fibroblast lysates of individuals 1-II:3 and 3-II:3 via an antibody against the N-terminal portion of importin 8, in keeping with a loss-of-function mechanism (Figure S4C). In proband 1-II:3, the lack of importin 8 protein is possibly attributed to translational repression, which previously has been described in other conditions,15 or significant protein instability. For individual 6-II:1, fibroblasts are not available, but in silico modeling of the predicted resultant deletion-insertion, p.Thr967_Glu1006delinsLys (c.2900−1G>A), suggests removal of the last structured part of the protein (Figure S6), which based on this region’s role in controlling the protein conformation in some other β-importins, may significantly affect protein stability.16, 17, 18
Murine importin 8 is 92% identical and 95% similar to its human ortholog, rendering mice a suitable animal model to pursue supportive in vivo evidence for a causal relationship between IPO8 deficiency and TAA. We used a C57BL/6N Ipo8−/− model that was previously only known to present with reduced grip strength and diminished vertical activity, suggesting muscle weakness and decreased locomotor exploration, respectively,19 and thus corroborating with the observed hypotonia and (possibly associated) motor delay in individuals with IPO8 bi-allelic variants. Serial transthoracic echocardiography (age 4–32 weeks) of the aortic root at the level of the sinuses of Valsalva and distal ascending aorta in Ipo8−/− mice and their wild-type (WT) littermates (N = 17/group) revealed statistically significant progressive dilatation in mutant mice at both anatomical locations, and aneurysms of the distal ascending aorta were already becoming visible at the age of 8–12 weeks (proot = 1.3E−3 [Figure 2A]; pasc = 8.4E−9 [Figure 2B]). Intriguingly, sex-stratified analyses demonstrated aortic root enlargement in both mutant females (7 Ipo8−/− versus 8 WT; proot_f = 2.3E−3 [Figure S7A]) and males (10 Ipo8−/− versus 9 WT; proot_m = 2.3E−2 [Figure S7B]), whereas the ascending aortic aneurysm phenotype is very pronounced and only statistically significant in the male Ipo8−/− animals (pasc_f = 6.5E−2 [Figure S7C] versus pasc_m = 8.4E−10 [Figure 2C]). After the last echo at 32 weeks, 14 Ipo8−/− and 17 WT animals were kept alive until the age of 48 weeks. Of these, three homozygous mutant males (3/9, 33.3%) died from an aortic rupture at the age of 32, 36, and 46 weeks, while no aortic rupture-related mortality was seen in the homozygous females (0/5, 0%) or WT animals (0/17, 0%). Sex differences in syndromic TAA penetrance and severity have been reported before, both in mice and humans.20,21 Generally, males are more severely affected, exhibiting larger aortas and experiencing dissection and/or rupture more frequently.22,23 Several studies in TAA mouse models have attempted to define the basis for the observed sex differences, revealing a context-dependent role for female and male hormone signaling, hypertension, and/or exacerbated ERK activation, but no predominant mechanism has been identified.21 The C57BL/6N Ipo8−/− mouse model represents a promising tool to further investigate the TAA sexual dimorphism. Of note, during our echocardiography studies, we did not observe severe structural outflow tract defects. Evaluation of lateral and dorsoventral total body X-rays, which are publicly available through the International Mouse Phenotyping Consortium (IMPC) portal, did not show evidence for scoliosis (visual inspection) or increased kyphosis (quantitative evaluation; p = 2.8E−1) in Ipo8−/− mice as compared to WT animals.
Figure 2.
Progressive TAA development in Ipo8−/− mice
(A) Log of weight-corrected aortic root diameters in male and female mice combined (N = 17/group).
(B) Log of weight-corrected ascending aortic diameters in male and female mice combined (N = 17/group).
(C) Log of weight-corrected ascending aortic diameters in male mice only (10 Ipo8−/− versus 9 WT). The error bars show the standard error of the mean (SEM). p values, which represent the interaction term between genotype and age, were calculated via mixed model analysis. WT, wild-type.
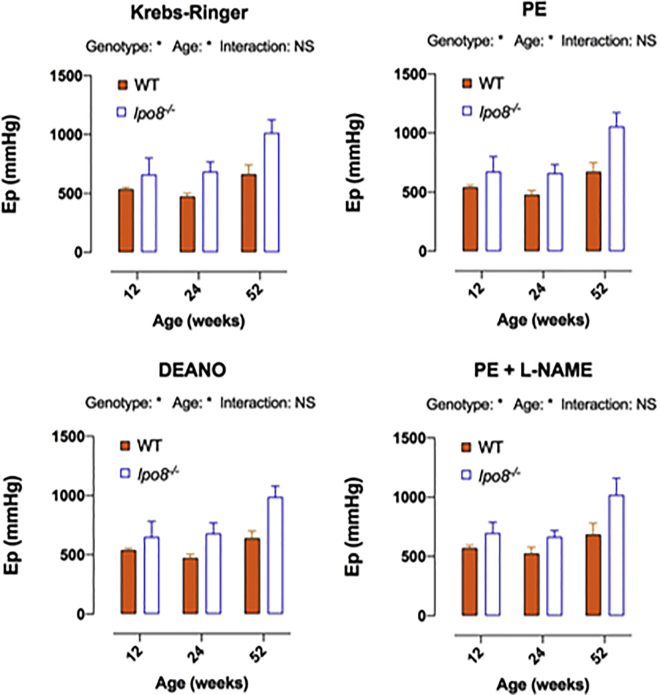
Given the fact that the aneurysmal phenotype is most pronounced in males at the level of the distal ascending aorta, we performed further experiments in male mice only. To study the biomechanical properties of distal ascending aortic rings, we used the “rodent oscillatory tension set-up to study arterial compliance” (ROTSAC) assay.24 More precisely, ex vivo aortic stiffness was assessed at 12 (5 Ipo8−/− versus 4 WT), 24 (4 Ipo8−/− versus 4 WT), and 52 (4 Ipo8−/− versus 2 WT) weeks of age. We used different experimental conditions to evaluate the involvement of vascular smooth muscle cells (VSMCs) and/or endothelial cells. The Peterson modulus (Ep) was first determined in Krebs-Ringer solution at a distention pressure of 80–120 mmHg and 120–160 mmHg, revealing a trend toward higher Ep values and, thus, stiffer ascending aortas at 120–160 mmHg in 12, 24, and 52 week old Ipo8−/− male animals as compared to controls (Figures 3 and S8). Because complete VSMC relaxation by diethylamine NONOate (DEANO) addition or VSMC stimulation with phenylephrine (PE), even upon nitric oxide synthase (NOS) inhibition through N(Ω)-nitro-L-arginine methyl ester (L-NAME) addition, did not considerably alter the Ep increase in Ipo8 null males (Figure 3), neither increased basal tone nor sustained VSMC contraction seem to contribute to the increased aortic stiffness. Our data rather point toward an increased passive stiffness of the ascending aorta in male Ipo8−/− mice throughout life. Increased arterial stiffness, an important marker for cardiovascular disease, has previously been observed in genetic TAA mouse models25 and affected individuals.26 In an established MFS mouse model, i.e., Fbn1mgR/mgR, stiffness was augmented in mutant non-aneurysmal (circa 3-fold) and aneurysmal (circa 4-fold) ascending aortas, which upon histological analysis, was shown to correlate with a diffuse loss in elastic fiber integrity.25 Compared to age-matched controls, TAA-affected individuals exhibit a stiffer mechanical response with aortic biomechanical properties resembling those of a significantly older (“aged”) non-aneurysmal cohort.27 Given the observed trend toward stiffer ascending aortas in Ipo8−/− mice (Figure 3) and recurrent prior associations between aortic ECM deterioration and TAA,2 we evaluated the structural ECM integrity by using histological elastin and collagen staining in ascending aortic sections of 12- (3 Ipo8−/− versus 3 WT), 24- (3 Ipo8−/− versus 3 WT), and 52-week-old (3 Ipo8−/− versus 2 WT) mice. Whereas the collagen content did not differ noticeably (Figure S9A), the elastic fibers were more disorganized and fragmented in mutant males of all age groups as compared to their WT counterparts (page-combined = 5.2E−4) (Figures 4A, 4B, and S9B).
Figure 3.
Trend toward increased ascending aortic passive stiffness in Ipo8−/− mice at a distention pressure of 120–160 mmHg
Age- and genotype-dependency of the Peterson modulus (Ep) of ascending aortic segments of male Ipo8−/− and wild-type mice under control (Krebs-Ringer), maximally relaxed (DEANO), and contracted (PE or PE + L-NAME) conditions at 12 (5 Ipo8−/− versus 4 WT), 24 (4 Ipo8−/− versus 4 WT), and 52 (4 Ipo8−/− versus 2 WT) weeks of age. The error bars show the SEM. Two-way ANOVA p values are shown (∗p < 0.05). Sidak post hoc testing did not reveal statistically significant genotype-based differences in Ep. PE, phenylephrine; DEANO, diethylamine NONOate; L-NAME, N(Ω)-nitro-L-arginine methyl ester; Ep, Peterson modulus; WT, wild-type; NS, non-significant.
Figure 4.
Elastic fiber deterioration and nuclear pSmad2 accumulation in the ascending aorta of Ipo8−/− mice
(A) Histological and immunohistochemistry images demonstrating marked elastin disorganization and fragmentation as well as prominent nuclear pSmad2 accumulation in Ipo8−/− mice. Scale bar represents 50 μm.
(B) Elastic fiber integrity scores and nuclear pSmad2 grades of the ascending aorta of all ages combined (12 [3 Ipo8−/− versus 3 WT], 24 [3 Ipo8−/− versus 3 WT], and 52 weeks [3 Ipo8−/− versus 2 WT]). Elastin grades can range from 1 to 4: grade 1 sections present with continuous and well-organized elastic bundles and grade 4 sections display vastly disorganized fibers, marked fiber fragmentation, and a thickened aortic wall. For pSmad2, grades 1, 2, 3, and 4 denote sections in which respectively <25%, 25%–50%, 50%–75%, and 75%–100% of nuclei stained positive. Averaged age-combined scores of blinded observations of three independent researchers are shown. The error bars depict the SEM. p values were calculated via two-way ANOVA statistics (∗p < 0.05, ∗∗∗p < 0.001). WT, wild-type.
Importin 8 is a nuclear transport receptor belonging to the importin-β protein family, which has not been linked to human diseases before. It is ubiquitously expressed and becomes upregulated upon TGF-β1 stimulation.28 β-importins translocate cargo molecules such as proteins, RNAs, and ribonucleoprotein complexes to the nucleus in a RanGTP-dependent manner. While a specific cargo can be shuttled by multiple β-importins, superior affinity to one of them is often observed. The most established cargoes for human importin 8 are phosphorylated SMADs 1–4 (pSMAD1–4),29 AGO2,30 mature miRNAs,31 EIF4E,32 and SRP19.33 Apart from being a nuclear transport receptor, importin 8 has been implicated in miRNA-guided gene silencing.30 Given that individuals with bi-allelic IPO8 variants phenotypically resemble individuals with TGF-β-related aortopathy syndromes such as LDS and SGS and key effectors of the canonical TGF-β pathway (i.e., pSMAD2–4) have been reported to be shuttled by importin 8,29 a plausible hypothesis is that dysregulated TGF-β signaling is involved in the pathogenesis of IPO8-related disease (Figure S1). We determined the levels of nuclear pSmad2, an effector of canonical TGF-β signaling, in ascending aortic sections of 12- (3 Ipo8−/− versus 3 WT), 24- (3 Ipo8−/− versus 3 WT), and 52-week-old (3 Ipo8−/− versus 2 WT) mice. A larger fraction of nuclei stained positive for pSmad2 in Ipo8−/− mice as compared to WT animals (page-combined = 3.4E−2), suggesting a role for dysregulated TGF-β signaling in the pathogenesis of IPO8-related TAA (Figures 4A, 4B, and S9C). Subsequent RT-qPCR analysis for nine TGF-β superfamily-related genes (i.e., Tgfb1, Tgfb2, Smad4, Smad6, Smad7, Mmp2, Ccn2 [Ctgf], Eln, and Serpine1 [Pai1]) in ascending aortic samples of 16-week old Ipo8−/− and WT males (N = 12/group) revealed significantly reduced Smad6 (p = 6.0E−3) and Smad7 (p = 3.6E−2) mRNA expression in the mutant animals, along with a significant increase in Mmp2 (p = 4.2E−3) and Ccn2 (Ctgf) (p = 7.8E−3) (Figure 5). SMAD6 and 7 inhibit SMAD-dependent and -independent TGF-β family signaling through various mechanisms.34 Whereas SMAD6 preferentially inhibits bone morphogenetic protein (BMP)-related signaling,35 SMAD7 impedes both TGF-β- and BMP-induced signaling.36 In the absence of SMAD7, TGF-β receptor activation is augmented, resulting in excessive SMAD2/3 phosphorylation. The detected decrease in Smad7 mRNA levels in the Ipo8−/− aortic walls might thus be directly linked to the observed increase in nuclear pSmad2 levels. SMAD6, on the other hand, has mostly been linked to BMP signaling, which is less well studied in the context of TAA development. Nonetheless, our group identified loss-of-function SMAD6 variants as a cause of bicuspid aortic valve-related TAA,37,38 demonstrating a mechanistic link between SMAD6 deficiency and TAA development. MMP2 and CCN2 (CTGF) are prototypical downstream transcriptional targets of the TGF-β signaling pathway.39 MMP2 belongs to the family of matrix metalloproteinases, which mediate the physiological turnover of the aortic ECM by degrading structural ECM proteins, including collagen and elastin.40 In TAA-affected individuals and mouse models, MMP2 levels and/or activity are strongly increased.41, 42, 43 Moreover, Mmp2 deletion in Fbn1mgR/mgR mice inhibited TGF-β activation and subsequent Smad2 and Erk1/2 phosphorylation,44 which significantly prolonged the lifespan of the MFS Fbn1mgR/mgR mice.44 As such, increased Mmp2 expression might connect increased TGF-β signaling and impaired elastic fiber integrity in our Ipo8−/− mouse model. CCN2 (CTGF) is a multifunctional protein that is involved in ECM remodeling.39 Overexpression of CCN2 (CTGF) has been proven to be associated with TAA development45 and has previously been shown to be upregulated in the aortic walls of individuals with LDS.4,7 Interestingly, elastic fiber fragmentation but normal collagen content as well as reduced Smad6 and Smad7 mRNA expression levels and higher Mmp activity were also described in aneurysmal aortic tissue specimens and/or VSMCs of Smad3−/− mice, an established LDS model that presents with TAA already at the age of 6 weeks.46 Together, our histological, immunohistochemistry, and RT-qPCR findings suggest a link between IPO8 deficiency and dysregulated TGF-β signaling. Moreover, they recapitulate prior observations in an established LDS mouse model, further relating IPO8-related TAA to the LDS disease spectrum.
Figure 5.
mRNA expression analysis of TGF-β-related genes reveals decreased Smad6 and Smad7 levels as well as increased Mmp2 and Ccn2 (Ctgf) levels in the ascending aorta of Ipo8−/− mice
Ascending aortic samples of 16-week-old Ipo8−/− and WT males were used (N = 12/group). The error bars depict the SEM. p values were calculated via mixed model statistics (∗p < 0.05, ∗∗p < 0.01). WT, wild-type; NS, non-significant.
In conclusion, we describe a syndrome caused by bi-allelic loss-of-function variants in IPO8. The human and mouse phenotypes caused by importin 8 loss of function are characterized by severe early-onset TAA development. Our immunohistochemistry and RT-qPCR studies of murine Ipo8-deficient aortic tissue reveal pathophysiological mechanisms that have previously been described in clinically overlapping TGF-β-related signalopathies. Further research is warranted to obtain more in-depth insight into the disease’s clinical course and mechanisms. First, identification of additional individuals with bi-allelic IPO8 variants will shed better light on the variability with respect to disease expressivity and penetrance. Moreover, longitudinal follow-up of affected individuals will provide information on aortic/arterial dissection or rupture risk. Interestingly, our clinical findings are corroborated by the observations of Ziegler et al.,47 in this issue of The American Journal of Human Genetics, who describe aortic dilatation in 11 out of 12 individuals with bi-allelic IPO8 variants. Second, it remains to be determined whether and how abnormal cytosol-to-nucleus shuttling elicits IPO8-related disease and dysregulated TGF-β signaling in aneurysmal aortic walls. Finally, as we predominantly focused on the TAA phenotype, it would be interesting to have a closer look at the mechanisms involved in the other affected organ systems, especially the neuromuscular system in order to explain the motor developmental delay that was observed in individuals with IPO8 bi-allelic variants.
Consortia
The members of the Genomics England Research Consortium are John C. Ambrose, Prabhu Arumugam, Marta Bleda, Freya Boardman-Pretty, Christopher R. Boustred, Helen Brittain, Mark J. Caulfield, Georgia C. Chan, Tom Fowler, Adam Giess, Angela Hamblin, Shirley Henderson, Tim J.P. Hubbard, Rob Jackson, Louise J. Jones, Dalia Kasperaviciute, Melis Kayikci, Athanasios Kousathanas, Lea Lahnstein, Sarah E.A. Leigh, Ivonne U.S. Leong, Javier F. Lopez, Fiona Maleady-Crowe, Loukas Moutsianas, Michael Mueller, Nirupa Murugaesu, Anna C. Need, Peter O‘Donovan, Chris A. Odhams, Christine Patch, Daniel Perez-Gil, Mariana Buongermino Pereira, John Pullinger, Tahrima Rahim, Augusto Rendon, Tim Rogers, Kevin Savage, Kushmita Sawant, Richard H. Scott, Afshan Siddiq, Alexander Sieghart, Samuel C. Smith, Alona Sosinsky, Alexander Stuckey, Mélanie Tanguy, Ellen R.A. Thomas, Simon R. Thompson, Arianna Tucci, Emma Walsh, Matthew J. Welland, Eleanor Williams, Katarzyna Witkowska, and Suzanne M. Wood.
Declaration of interests
The Department of Molecular and Human Genetics at Baylor College of Medicine receives revenue from clinical genetic testing conducted at Baylor Genetics Laboratories. A.B. is an employee of GeneDx, Inc.
Acknowledgments
This research was largely supported by funding from the University of Antwerp (Methusalem-OEC grant “Genomed” FFB190208, Hercules grant 30706), the Research Foundation Flanders (FWO, Belgium, G042321N, G040221N, and G044720N), the Dutch Heart Foundation (2013T093), the Belgian Cardiac Surgery Foundation, and the Marfan Foundation. Also, the research was in part supported by the National Institute for Health Research (NIHR) Oxford Biomedical Research Centre based at Oxford University Hospitals NHS Trust and University of Oxford and the Wellcome Trust (203141/Z/16/Z). The views expressed are those of the authors and not necessarily those of the NHS, the NIHR, or the Department of Health. This research was made possible through access to the data and findings generated by the 100,000 Genomes Project. The 100,000 Genomes Project is managed by Genomics England Limited (a wholly owned company of the Department of Health and Social Care). The 100,000 Genomes Project is funded by the National Institute for Health Research and NHS England. The Wellcome Trust, Cancer Research UK, and the Medical Research Council have also funded research infrastructure. The 100,000 Genomes Project uses data provided by persons and collected by the National Health Service as part of their care and support. B.L.L. holds a consolidator grant from the European Research Council (Genomia—ERC-COG-2017-771945). J.A.N.M. (12X8520N) and D.S. (12R5610N) are post-doctoral FWO fellows. I.V.G. (1S70419N), L.V.D.H. (1S81820N), J.D.V. (11C1721N), and C.H.G.N. (1S24720N) are supported by an FWO PhD fellowship. B.L.L. and A.V. are members of the European Reference Network on rare multisystemic vascular disorders (VASCERN—project ID: 769036, partly co-funded by the European Union Third Health Programme).
Published: May 18, 2021
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.ajhg.2021.04.019.
Contributor Information
Bart L. Loeys, Email: bart.loeys@uantwerpen.be.
Aline Verstraeten, Email: aline.verstraeten@uantwerpen.be.
Data and code availability
The IPO8 variants were submitted to ClinVar (https://www.ncbi.nlm.nih.gov/clinvar/) (GenBank: NM_006390.3; accession numbers SCV001547250–SCV001547257). WES datasets have not been deposited in a public repository because of privacy and ethical restrictions but are available from the corresponding author on request.
Web resources
International Mouse Phenotyping Consortium, https://www.mousephenotype.org/
OMIM, omim.org
PDB, www.rcsb.org
Supplemental information
References
- 1.Faggion Vinholo T., Brownstein A.J., Ziganshin B.A., Zafar M.A., Kuivaniemi H., Body S.C., Bale A.E., Elefteriades J.A. Genes Associated with Thoracic Aortic Aneurysm and Dissection: 2019 Update and Clinical Implications. Aorta (Stamford) 2019;7:99–107. doi: 10.1055/s-0039-3400233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Verstraeten A., Luyckx I., Loeys B. Aetiology and management of hereditary aortopathy. Nat. Rev. Cardiol. 2017;14:197–208. doi: 10.1038/nrcardio.2016.211. [DOI] [PubMed] [Google Scholar]
- 3.Dietz H.C., Cutting G.R., Pyeritz R.E., Maslen C.L., Sakai L.Y., Corson G.M., Puffenberger E.G., Hamosh A., Nanthakumar E.J., Curristin S.M. Marfan syndrome caused by a recurrent de novo missense mutation in the fibrillin gene. Nature. 1991;352:337–339. doi: 10.1038/352337a0. [DOI] [PubMed] [Google Scholar]
- 4.Loeys B.L., Chen J., Neptune E.R., Judge D.P., Podowski M., Holm T., Meyers J., Leitch C.C., Katsanis N., Sharifi N. A syndrome of altered cardiovascular, craniofacial, neurocognitive and skeletal development caused by mutations in TGFBR1 or TGFBR2. Nat. Genet. 2005;37:275–281. doi: 10.1038/ng1511. [DOI] [PubMed] [Google Scholar]
- 5.Cannaerts E., Kempers M., Maugeri A., Marcelis C., Gardeitchik T., Richer J., Micha D., Beauchesne L., Timmermans J., Vermeersch P. Novel pathogenic SMAD2 variants in five families with arterial aneurysm and dissection: further delineation of the phenotype. J. Med. Genet. 2019;56:220–227. doi: 10.1136/jmedgenet-2018-105304. [DOI] [PubMed] [Google Scholar]
- 6.Micha D., Guo D.C., Hilhorst-Hofstee Y., van Kooten F., Atmaja D., Overwater E., Cayami F.K., Regalado E.S., van Uffelen R., Venselaar H. SMAD2 Mutations Are Associated with Arterial Aneurysms and Dissections. Hum. Mutat. 2015;36:1145–1149. doi: 10.1002/humu.22854. [DOI] [PubMed] [Google Scholar]
- 7.van de Laar I.M., Oldenburg R.A., Pals G., Roos-Hesselink J.W., de Graaf B.M., Verhagen J.M., Hoedemaekers Y.M., Willemsen R., Severijnen L.A., Venselaar H. Mutations in SMAD3 cause a syndromic form of aortic aneurysms and dissections with early-onset osteoarthritis. Nat. Genet. 2011;43:121–126. doi: 10.1038/ng.744. [DOI] [PubMed] [Google Scholar]
- 8.Boileau C., Guo D.C., Hanna N., Regalado E.S., Detaint D., Gong L., Varret M., Prakash S.K., Li A.H., d’Indy H., National Heart, Lung, and Blood Institute (NHLBI) Go Exome Sequencing Project TGFB2 mutations cause familial thoracic aortic aneurysms and dissections associated with mild systemic features of Marfan syndrome. Nat. Genet. 2012;44:916–921. doi: 10.1038/ng.2348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lindsay M.E., Schepers D., Bolar N.A., Doyle J.J., Gallo E., Fert-Bober J., Kempers M.J., Fishman E.K., Chen Y., Myers L. Loss-of-function mutations in TGFB2 cause a syndromic presentation of thoracic aortic aneurysm. Nat. Genet. 2012;44:922–927. doi: 10.1038/ng.2349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bertoli-Avella A.M., Gillis E., Morisaki H., Verhagen J.M.A., de Graaf B.M., van de Beek G., Gallo E., Kruithof B.P.T., Venselaar H., Myers L.A. Mutations in a TGF-β ligand, TGFB3, cause syndromic aortic aneurysms and dissections. J. Am. Coll. Cardiol. 2015;65:1324–1336. doi: 10.1016/j.jacc.2015.01.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cannaerts E., van de Beek G., Verstraeten A., Van Laer L., Loeys B. TGF-β signalopathies as a paradigm for translational medicine. Eur. J. Med. Genet. 2015;58:695–703. doi: 10.1016/j.ejmg.2015.10.010. [DOI] [PubMed] [Google Scholar]
- 12.Doyle A.J., Doyle J.J., Bessling S.L., Maragh S., Lindsay M.E., Schepers D., Gillis E., Mortier G., Homfray T., Sauls K. Mutations in the TGF-β repressor SKI cause Shprintzen-Goldberg syndrome with aortic aneurysm. Nat. Genet. 2012;44:1249–1254. doi: 10.1038/ng.2421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schepers D., Doyle A.J., Oswald G., Sparks E., Myers L., Willems P.J., Mansour S., Simpson M.A., Frysira H., Maat-Kievit A. The SMAD-binding domain of SKI: a hotspot for de novo mutations causing Shprintzen-Goldberg syndrome. Eur. J. Hum. Genet. 2015;23:224–228. doi: 10.1038/ejhg.2014.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lopez L., Colan S., Stylianou M., Granger S., Trachtenberg F., Frommelt P., Pearson G., Camarda J., Cnota J., Cohen M., Pediatric Heart Network Investigators∗ Relationship of Echocardiographic Z Scores Adjusted for Body Surface Area to Age, Sex, Race, and Ethnicity: The Pediatric Heart Network Normal Echocardiogram Database. Circ Cardiovasc Imaging. 2017;10:e006979. doi: 10.1161/CIRCIMAGING.117.006979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.You K.T., Li L.S., Kim N.G., Kang H.J., Koh K.H., Chwae Y.J., Kim K.M., Kim Y.K., Park S.M., Jang S.K., Kim H. Selective translational repression of truncated proteins from frameshift mutation-derived mRNAs in tumors. PLoS Biol. 2007;5:e109. doi: 10.1371/journal.pbio.0050109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zachariae U., Grubmüller H. A highly strained nuclear conformation of the exportin Cse1p revealed by molecular dynamics simulations. Structure. 2006;14:1469–1478. doi: 10.1016/j.str.2006.08.001. [DOI] [PubMed] [Google Scholar]
- 17.Yamazawa R., Jiko C., Choi S., Park I.Y., Nakagawa A., Yamashita E., Lee S.J. Structural Basis for Selective Binding of Export Cargoes by Exportin-5. Structure. 2018;26:1393–1398.e2. doi: 10.1016/j.str.2018.06.014. [DOI] [PubMed] [Google Scholar]
- 18.Koyama M., Shirai N., Matsuura Y. Structural insights into how Yrb2p accelerates the assembly of the Xpo1p nuclear export complex. Cell Rep. 2014;9:983–995. doi: 10.1016/j.celrep.2014.09.052. [DOI] [PubMed] [Google Scholar]
- 19.Dickinson M.E., Flenniken A.M., Ji X., Teboul L., Wong M.D., White J.K., Meehan T.F., Weninger W.J., Westerberg H., Adissu H. High-throughput discovery of novel developmental phenotypes. Nature. 2016;537:508–514. doi: 10.1038/nature19356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Robinet P., Milewicz D.M., Cassis L.A., Leeper N.J., Lu H.S., Smith J.D. Consideration of Sex Differences in Design and Reporting of Experimental Arterial Pathology Studies-Statement From ATVB Council. Arterioscler. Thromb. Vasc. Biol. 2018;38:292–303. doi: 10.1161/ATVBAHA.117.309524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Anderson N.K., Juzwiak E.E., Dietz H.C. A seX(X/Y) Article on Marfan Syndrome. J. Am. Heart Assoc. 2020;9:e018814. doi: 10.1161/JAHA.120.018814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Roman M.J., Devereux R.B., Preiss L.R., Asch F.M., Eagle K.A., Holmes K.W., LeMaire S.A., Maslen C.L., Milewicz D.M., Morris S.A., GenTAC Investigators∗ Associations of Age and Sex With Marfan Phenotype: The National Heart, Lung, and Blood Institute GenTAC (Genetically Triggered Thoracic Aortic Aneurysms and Cardiovascular Conditions) Registry. Circ Cardiovasc Genet. 2017;10:e001647. doi: 10.1161/CIRCGENETICS.116.001647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Renard M., Muiño-Mosquera L., Manalo E.C., Tufa S., Carlson E.J., Keene D.R., De Backer J., Sakai L.Y. Sex, pregnancy and aortic disease in Marfan syndrome. PLoS ONE. 2017;12:e0181166. doi: 10.1371/journal.pone.0181166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Leloup A.J., Van Hove C.E., Kurdi A., De Moudt S., Martinet W., De Meyer G.R., Schrijvers D.M., De Keulenaer G.W., Fransen P. A novel set-up for the ex vivo analysis of mechanical properties of mouse aortic segments stretched at physiological pressure and frequency. J. Physiol. 2016;594:6105–6115. doi: 10.1113/JP272623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bellini C., Korneva A., Zilberberg L., Ramirez F., Rifkin D.B., Humphrey J.D. Differential ascending and descending aortic mechanics parallel aneurysmal propensity in a mouse model of Marfan syndrome. J. Biomech. 2016;49:2383–2389. doi: 10.1016/j.jbiomech.2015.11.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Humphrey J.D., Tellides G. Central artery stiffness and thoracic aortopathy. Am. J. Physiol. Heart Circ. Physiol. 2019;316:H169–H182. doi: 10.1152/ajpheart.00205.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sulejmani F., Pokutta-Paskaleva A., Ziganshin B., Leshnower B., Iannucci G., Elefteriades J., Sun W. Biomechanical properties of the thoracic aorta in Marfan patients. Ann. Cardiothorac. Surg. 2017;6:610–624. doi: 10.21037/acs.2017.09.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hu X., Kan H., Boye A., Jiang Y., Wu C., Yang Y. Mitogen-activated protein kinase inhibitors reduce the nuclear accumulation of phosphorylated Smads by inhibiting Imp 7 or Imp 8 in HepG2 cells. Oncol. Lett. 2018;15:4867–4872. doi: 10.3892/ol.2018.7926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yao X., Chen X., Cottonham C., Xu L. Preferential utilization of Imp7/8 in nuclear import of Smads. J. Biol. Chem. 2008;283:22867–22874. doi: 10.1074/jbc.M801320200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Weinmann L., Höck J., Ivacevic T., Ohrt T., Mütze J., Schwille P., Kremmer E., Benes V., Urlaub H., Meister G. Importin 8 is a gene silencing factor that targets argonaute proteins to distinct mRNAs. Cell. 2009;136:496–507. doi: 10.1016/j.cell.2008.12.023. [DOI] [PubMed] [Google Scholar]
- 31.Wei Y., Li L., Wang D., Zhang C.Y., Zen K. Importin 8 regulates the transport of mature microRNAs into the cell nucleus. J. Biol. Chem. 2014;289:10270–10275. doi: 10.1074/jbc.C113.541417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Volpon L., Culjkovic-Kraljacic B., Osborne M.J., Ramteke A., Sun Q., Niesman A., Chook Y.M., Borden K.L. Importin 8 mediates m7G cap-sensitive nuclear import of the eukaryotic translation initiation factor eIF4E. Proc. Natl. Acad. Sci. USA. 2016;113:5263–5268. doi: 10.1073/pnas.1524291113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dean K.A., von Ahsen O., Görlich D., Fried H.M. Signal recognition particle protein 19 is imported into the nucleus by importin 8 (RanBP8) and transportin. J. Cell Sci. 2001;114:3479–3485. doi: 10.1242/jcs.114.19.3479. [DOI] [PubMed] [Google Scholar]
- 34.Miyazawa K., Miyazono K. Regulation of TGF-β Family Signaling by Inhibitory Smads. Cold Spring Harb. Perspect. Biol. 2017;9:a022095. doi: 10.1101/cshperspect.a022095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Goto K., Kamiya Y., Imamura T., Miyazono K., Miyazawa K. Selective inhibitory effects of Smad6 on bone morphogenetic protein type I receptors. J. Biol. Chem. 2007;282:20603–20611. doi: 10.1074/jbc.M702100200. [DOI] [PubMed] [Google Scholar]
- 36.Hanyu A., Ishidou Y., Ebisawa T., Shimanuki T., Imamura T., Miyazono K. The N domain of Smad7 is essential for specific inhibition of transforming growth factor-beta signaling. J. Cell Biol. 2001;155:1017–1027. doi: 10.1083/jcb.200106023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gillis E., Kumar A.A., Luyckx I., Preuss C., Cannaerts E., van de Beek G., Wieschendorf B., Alaerts M., Bolar N., Vandeweyer G., Mibava Leducq Consortium Candidate Gene Resequencing in a Large Bicuspid Aortic Valve-Associated Thoracic Aortic Aneurysm Cohort: SMAD6 as an Important Contributor. Front. Physiol. 2017;8:400. doi: 10.3389/fphys.2017.00400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Luyckx I., MacCarrick G., Kempers M., Meester J., Geryl C., Rombouts O., Peeters N., Claes C., Boeckx N., Sakalihasan N. Confirmation of the role of pathogenic SMAD6 variants in bicuspid aortic valve-related aortopathy. Eur. J. Hum. Genet. 2019;27:1044–1053. doi: 10.1038/s41431-019-0363-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lipson K.E., Wong C., Teng Y., Spong S. CTGF is a central mediator of tissue remodeling and fibrosis and its inhibition can reverse the process of fibrosis. Fibrogenesis Tissue Repair. 2012;5(Suppl 1):S24. doi: 10.1186/1755-1536-5-S1-S24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Van Doren S.R. Matrix metalloproteinase interactions with collagen and elastin. Matrix Biol. 2015;44-46:224–231. doi: 10.1016/j.matbio.2015.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Meffert P., Tscheuschler A., Beyersdorf F., Heilmann C., Kocher N., Uffelmann X., Discher P., Rylski B., Siepe M., Kari F.A. Characterization of serum matrix metalloproteinase 2/9 levels in patients with ascending aortic aneurysms. Interact. Cardiovasc. Thorac. Surg. 2017;24:20–26. doi: 10.1093/icvts/ivw309. [DOI] [PubMed] [Google Scholar]
- 42.Wang C., Chang Q., Sun X., Qian X., Liu P., Pei H., Guo X., Liu W. Angiotensin II Induces an Increase in Matrix Metalloproteinase 2 Expression in Aortic Smooth Muscle Cells of Ascending Thoracic Aortic Aneurysms Through JNK, ERK1/2, and p38 MAPK Activation. J. Cardiovasc. Pharmacol. 2015;66:285–293. doi: 10.1097/FJC.0000000000000276. [DOI] [PubMed] [Google Scholar]
- 43.Chung A.W., Au Yeung K., Sandor G.G., Judge D.P., Dietz H.C., van Breemen C. Loss of elastic fiber integrity and reduction of vascular smooth muscle contraction resulting from the upregulated activities of matrix metalloproteinase-2 and -9 in the thoracic aortic aneurysm in Marfan syndrome. Circ. Res. 2007;101:512–522. doi: 10.1161/CIRCRESAHA.107.157776. [DOI] [PubMed] [Google Scholar]
- 44.Xiong W., Meisinger T., Knispel R., Worth J.M., Baxter B.T. MMP-2 regulates Erk1/2 phosphorylation and aortic dilatation in Marfan syndrome. Circ. Res. 2012;110:e92–e101. doi: 10.1161/CIRCRESAHA.112.268268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Branchetti E., Poggio P., Sainger R., Shang E., Grau J.B., Jackson B.M., Lai E.K., Parmacek M.S., Gorman R.C., Gorman J.H. Oxidative stress modulates vascular smooth muscle cell phenotype via CTGF in thoracic aortic aneurysm. Cardiovasc. Res. 2013;100:316–324. doi: 10.1093/cvr/cvt205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.van der Pluijm I., van Vliet N., von der Thusen J.H., Robertus J.L., Ridwan Y., van Heijningen P.M., van Thiel B.S., Vermeij M., Hoeks S.E., Buijs-Offerman R.M.G.B. Defective Connective Tissue Remodeling in Smad3 Mice Leads to Accelerated Aneurysmal Growth Through Disturbed Downstream TGF-β Signaling. EBioMedicine. 2016;12:280–294. doi: 10.1016/j.ebiom.2016.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ziegler A., Duclaux-Loras R., Revenu C., Charbit-Henrion F., Begue B., Duroure K., Grimaud L., Guihot A.L., Dumas V.D., Zarhrate M. Bi-allelic variants in IPO8 cause a connective tissue disorder associated with cardiovascular defects, skeletal abnormalities, and immune dysregulation. Am. J. Hum. Genet. 2021 doi: 10.1016/j.ajhg.2021.04.020. Published online May 18, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The IPO8 variants were submitted to ClinVar (https://www.ncbi.nlm.nih.gov/clinvar/) (GenBank: NM_006390.3; accession numbers SCV001547250–SCV001547257). WES datasets have not been deposited in a public repository because of privacy and ethical restrictions but are available from the corresponding author on request.