Abstract
Introduction
Gallstones are a known adverse effect of somatostatin analogs, but the exact incidence and clinical implications are unknown.
Objectives
The aim of this study was to investigate the incidence of gallstones on imaging and related complications in unbiased trial data.
Methods
Data from the DIPAK 1 trial, in which 305 polycystic kidney disease patients were randomized to standard of care (SoC) or lanreotide for 120 weeks, were used. Magnetic resonance imaging (MRI) was performed at baseline and end of treatment and was assessed for the presence, number, and size of gallstones. For all patients who had gallstones at the end of the trial, we obtained follow-up after the trial.
Results
Of 249 patients with data available, 11 patients randomized to lanreotide and four randomized to SoC had gallstones at baseline. During the study, new gallstones were formed in 19/124 patients using lanreotide (15%) and 1/125 patients receiving SoC (1%). The odds ratio for gallstone formation with lanreotide use was 25.9 (95% confidence interval 3.37–198.8; p < 0.001). Gallstones during lanreotide treatment were multiple (> 20 stones in 69% of patients) and small (≤ 3 mm in 63% of patients). Of the 19 patients with incident gallstones during lanreotide treatment, 9 experienced gallstone-associated complications, 8 of whom experienced gallstone-associated complications after discontinuation of treatment (median time after discontinuation 2.5 years). In patients with gallstones at baseline and in patients receiving SoC, no complications occurred.
Conclusions
Treatment with a somatostatin analog leads to the formation of multiple, small gallstones that are associated with severe complications, especially after discontinuation of therapy.
Clinical Trial Registry Website and Trial Number
ClinicalTrials.gov (https://clinicaltrials.gov); NCT01616927.
Supplementary Information
The online version contains supplementary material available at 10.1007/s40268-021-00342-7.
Key Points
| During 2.3 years of lanreotide use, 15% of patients formed new gallstones, compared with 1% in untreated patients. |
| In most cases, these gallstones were multiple (>20) and small (≤3 mm). |
| About 50% of patients who developed gallstones during treatment experienced complications after discontinuation, while no complications occurred in untreated patients or patients with gallstones present before the start of treatment. |
Introduction
Somatostatin analogs such as octreotide and lanreotide are available as treatment for several conditions such as acromegaly, neuroendocrine tumors, and dumping syndrome [1–6]. We recently executed a large randomized controlled trial and demonstrated that the somatostatin analog lanreotide also reduces liver growth in patients with polycystic liver disease [7].
Gallstones are a known adverse event of somatostatin analog treatment that may cause acute biliary complications such as pancreatitis, cholecystitis, or biliary colics, but there is uncertainty about the incidence of these gallstones and the clinical consequences of this gallstone formation. In the studies that led to marketing authorization of somatostatin analogs as treatment for neuroendocrine tumors (the PROMID and CLARINET trials, including 85 and 204 patients and with a treatment duration of 1.8 and 1.2 years, respectively), incident gallstones were observed in 10% and 12% of patients, respectively [2–5]. The current thinking is that these incident gallstones remain asymptomatic. In line, these landmark trials did not report complications, not even in the open-label follow-up phase [2–5]. However, in a recent retrospective study of somatostatin analogs as treatment for neuroendocrine tumors (n = 300, median treatment duration 3.5 years), a much higher incidence of gallstone formation (37%) and also a high incidence of biliary complications (28%) in patients with incident gallstones were reported [8]. However, this study was retrospective by design and based findings on symptoms only, not on systematic imaging. In a small case series it was suggested that complications of gallstones could also occur after discontinuation of somatostatin analogs [9]. This is important because most studies have no follow-up after discontinuation of treatment. These data show that there is uncertainty about the exact incidence of gallstones and related biliary complications during and after treatment with somatostatin analogs.
We had access to unbiased data from the DIPAK 1 trial, a controlled trial in which patients with autosomal dominant polycystic kidney disease (ADPKD) were randomized to lanreotide use or standard of care (SoC) with a relatively long treatment duration (2.3 years) and in which MR images were performed before and after treatment [10]. In the present study, we used these data to determine the incidence, number, size, and complications of gallstones in a systematic, prospective way.
Methods
The design, methods, and results of the DIPAK 1 trial have been published previously [10, 11] (ClinicalTrials.gov: NCT01616927). In brief, this was an investigator-driven, randomized, open-label clinical trial with blinded endpoint analysis to assess the effect of lanreotide on rates of renal function decline and kidney and liver volume growth in patients with autosomal dominant polycystic kidney disease (ADPKD). The Institutional Review Board at each of the participating hospitals approved the protocol and all patients provided written informed consent. In the study, 305 patients with ADPKD (diagnosed according to the Ravine criteria), aged 18–60 years, and with a kidney function of 30–60 mL/min/1.73m2 were included in four tertiary care hospitals in The Netherlands (Leiden, Rotterdam, Nijmegen, and Groningen). Patients with diabetes mellitus, a history of pancreatitis, known presence of gallstones, or bradycardia were not eligible. Patients were randomized to receive SoC, or SoC and, additionally, lanreotide 120 mg subcutaneously every 4 weeks. If patients did not tolerate this dose, lanreotide was downtitrated to 90 mg or 60 mg, or stopped. Lanreotide injections were continued for 120 weeks. MR images were made at baseline and at the end of treatment. For this post hoc analysis, all patients who previously underwent cholecystectomy, without a baseline or end-of treatment magnetic resonance imaging (MRI), or inability to identify the gallbladder at MRI, were excluded.
Procedures
MR images were made according to a standard protocol, including coronal and transversal T2-weighted single-shot fast gradient spin-echo images with fat saturation. All MRIs were scored independently by two trained observers who were blinded for patient identity, treatment allocation, and study phase. If the gallbladder could not be identified by one or both observers, or in case of inconsistencies between observers, a decisive evaluation was performed by an abdominal radiologist.
Patients finished trial treatment between November 2014 and June 2016. For all patients with gallstones on imaging at the end of the treatment period, additional follow-up data until January 2020 were obtained. Follow-up data were extracted from patient files. If these data were incomplete, patients were contacted by phone to obtain follow-up data.
Outcomes
The primary outcome was the incidence of gallstones during the trial, in patients using lanreotide compared with patients receiving SoC. Secondary outcomes were (1) the association of patient characteristics with gallstone formation, including age, sex, body mass index (BMI), estimated glomerular filtration rate (eGFR), liver function tests, and baseline liver volume; (2) the number of gallstones, which was scored in classes of 1–5, 5–10, 10–15, 15–20, 20–25, 25–30, or > 30; (3) the size of the gallstones, which was scored in classes of ≤ 3 mm, > 3 and < 10 mm, and ≥ 10 mm; and (4) the incidence of biliary complications, such as biliary colic, cholecystitis, or biliary pancreatitis during and after the trial. Exploratory outcomes were the association between gallstone formation and (1) response to therapy (indicated by change in liver volume during therapy); (2) hepatic cyst infections; (3) adverse events, such as abdominal pain, abdominal cramps, diarrhea, and discolored stool; and (4) the time period until complications developed.
Statistical Analysis
Analyses were performed using IBM statistics SPSS version 23 (IBM Corporation, Armonk, NY, USA). Continuous data are presented as mean and standard deviation (SD) or as median and interquartile range (IQR) in the case of non-normal distribution. Categorical data are presented as percentages. To test for differences in the presence of gallstones at baseline, end of study, and new gallstones formed during the trial in both groups (lanreotide vs. SoC only), Chi-square and Fisher’s exact tests were used. Logistic regression analyses were performed to test for factors associated with gallstone formation. All authors had access to study data and reviewed and approved the final manuscript.
Results
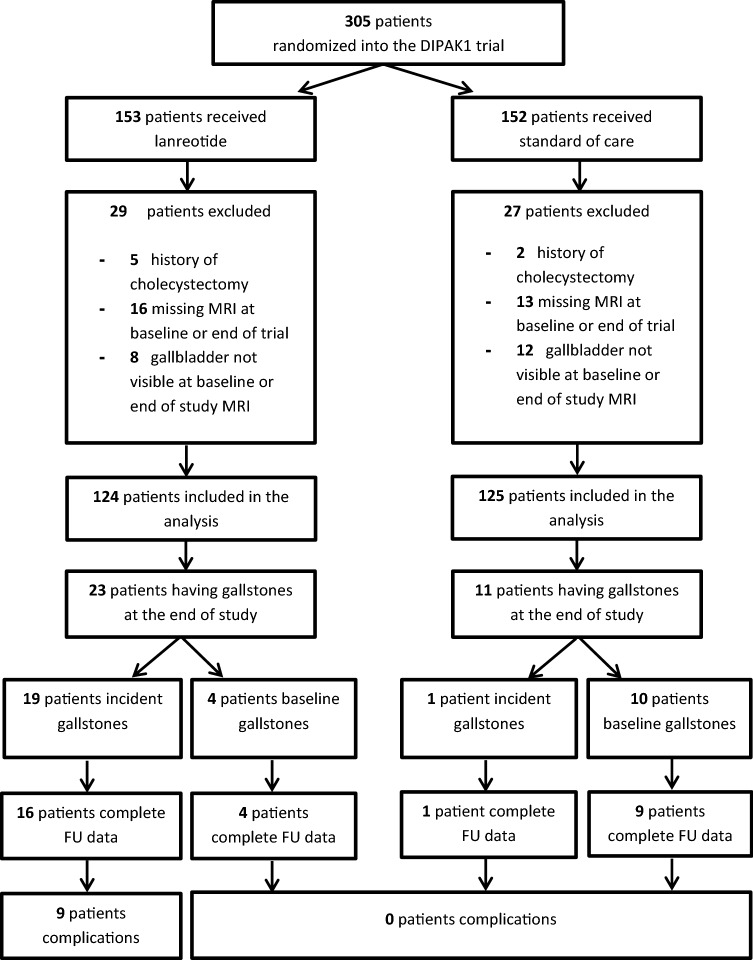
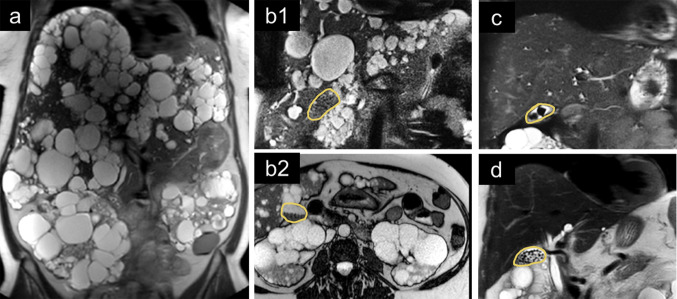
A detailed flowchart of patients in this study is shown in Fig. 1. Of the 305 patients participating in the DIPAK 1 trial, 29 patients were excluded because no MRI was available at baseline or end of treatment, 7 were excluded because of a medical history of cholecystectomy, and 20 patients were excluded due to inability to identify the gallbladder on MRI. Most of these latter patients had severe polycystic liver disease with many cysts in liver segments 4 and 5 adjacent to the gallbladder. An example of such a patient is shown in Fig. 2a.
Fig. 1.
Selection of patients from the DIPAK1 trial, used for this analysis. DIPAK Developing Interventions for Polycystic Autosomal Kidney disease, MRI magnetic resonance imaging, FU follow-up
Fig. 2.
Representative magnetic resonance images at the end of the trial. The gallbladder is marked with a yellow line in all panels, except panel a. a An example of a patient with severe polycystic kidney and liver disease in whom the gallbladder could not be identified due to the large number of cysts. b A patient with many small gallstones, treated with lanreotide, in whom the presence of gallstones is easily missed on coronal slices since the intensity of the gallstones is similar to the intensity of the liver parenchyma (b1). However, on axial slices, one can recognize the gallstones more easily because they are located at the dorsal side of the gallbladder due to gravity (b2). c Typical example of gallstones present at baseline (a limited number of larger stones). These stones mostly remained unchanged during the study period. d Typical example of gallstones formed during lanreotide use (multiple small stones)
Demographic and clinical characteristics of the 249 patients at baseline according to randomization group are shown in Table 1. Characteristics of both groups were similar. All patients had ADPKD and thus progressive kidney disease, with a mean eGFR in both groups of approximately 50 mL/1.73m2/min. There was a slight difference in the prevalence of mild, moderate, and severe polycystic liver disease between both groups. Mean aspartate aminotransferase (AST), alanine aminotransferase (ALT), alkaline phosphatase (ALP), γ-glutamyltransferase (GGT), and indirect bilirubin values were comparable between the groups and within normal ranges; direct bilirubin levels differed significantly between the groups.
Table 1.
Baseline characteristics
| Lanreotide [n = 124] |
Standard of care [n = 125] | p value | |
|---|---|---|---|
| Female | 58 (47) | 62 (50) | 0.66 |
| Age, years | 48 ± 7 | 49 ± 7 | 0.54 |
| BMI, kg/m2 | 27.0 ± 4.2 | 27.2 ± 4.8 | 0.74 |
| Height-adjusted total liver volume, mL [median (IQR)] | 1165 (9868–1574) | 1141 (962–1333) | 0.06 |
| Polycystic liver disease classification | |||
| Mild [hTLV <1600 mL/m] | 95 (76.6) | 112 (89.6) | |
| Moderate [hTLV 1600–3200 mL] | 24 (19.4) | 11 (8.8) | |
| Severe [hTLV >3200 mL] | 5 (4.0) | 2 (1.6) | 0.02 |
| eGFR CKD-EPI, mL/1.73m2/min | 50.4 ± 11.3 | 50.1 ± 11.1 | 0.82 |
| AST, U/L [median (IQR)] | 23.0 (19.5–27.5) | 23.0 (20.0–28.0) | 0.58 |
| ALT, U/L [median (IQR)] | 23.0 (18.0–29.0) | 21.0 (16.0–27.0) | 0.15 |
| ALP, U/L [median (IQR)] | 67.0 (56.0–80.0) | 66.0 (55.3–77.8) | 0.58 |
| GGT, µmol/L [median (IQR)] | 33.0 (23.5–49.0) | 29.0 (1.25–47.0) | 0.08 |
| Bilirubin direct, µmol/L [median (IQR)] | 3.0 (2.0–4.0) | 3.0 (2.0–4.0) | 0.008 |
| Bilirubin indirect, µmol/L [median (IQR)] | 6.0 (4.0–8.0) | 6.0 (4.0–7.0) | 0.57 |
Data are expressed as means ± standard deviation or median (interquartile range) in the case of non-normal data distribution, or number (percentage) in the case of categorical data
Significant p-values are highlighted in bold
BMI body mass index, eGFR estimated glomerular filtration rate, hTLV height-adjusted liver volume, AST aspartate aminotransferase, ALT alanine aminotransferase, ALP alkaline phosphatase, GGT γ-glutamyltransferase, IQR interquartile range, CKD-EPI Chronic Kidney Disease Epidemiology Collaboration
Primary Outcome
At baseline, 15 patients (6%) had gallstones on MR imaging, 4 in the lanreotide group and 11 in the SoC group (Table 2). At the end of study (120 weeks), 34 (14%) patients had gallstones on MR imaging, 23 in the lanreotide group and 11 in the SoC group. In the SoC group, gallstones disappeared during the study in one patient and incident gallstones formed in another patient. In the lanreotide group, no gallstones disappeared, and, during the study, 19 patients developed incident gallstones (15%) (Table 2). The difference in gallstone incidence between the two treatment groups was statistically significant (p < 0.0001). In one patient receiving SoC only, a gallstone in the common bile duct was observed; in all other patients, gallstones were observed in the gallbladder only.
Table 2.
Presence of gallstones at baseline and end of study, and incidence of gallstones compared in the lanreotide and control groups
| Lanreotide [n = 124] | Standard of care [n = 125] | p value | |
|---|---|---|---|
| Gallstones at baseline | 4 (3.2) | 11 (8.8) | 0.07 |
| Gallstones at end of study | 23 (18.5) | 11 (8.8) | < 0.05 |
| New gallstones during study | 19 (15.3) | 1a (0.8) | < 0.0001 |
Significant p-values are highlighted in bold
aIn one patient, a gallstone disappeared, and another patient receiving standard of care had incident gallstones
Secondary Outcomes
In univariate logistic regression analysis, lanreotide use was significantly associated with the risk of gallstone formation (odds ratio [OR] 22.5, 95% confidence interval [CI] 2.96–171; p < 0.01). In a multivariate logistic regression, lanreotide was again associated with gallstone formation, whereas other known risk factors for gallstone formation, such as female sex, age, and BMI were not significantly associated nor showed interaction with lanreotide use for the outcome of gallstone formation (Table 3). ALP, GGT, bilirubin level, baseline liver volume, and eGFR were also not significantly related with gallstone formation (Table 3).
Table 3.
Logistic regression to assess the risk associated with lanreotide use for incident gallstones
| OR (95% CI) | p value | |
|---|---|---|
| Univariate | ||
| Lanreotide use | 22.5 (2.96–171) | < 0.01 |
| Multivariate | ||
| Lanreotide use | 25.9 (3.37–199) | < 0.01 |
| Female sexa | 1.66 (0.59–4.66) | 0.33 |
| Age, years | 1.04 (0.97–1.12) | 0.28 |
| BMI, kg/m2a | 0.95 (0.83–1.09) | 0.46 |
| Baseline liver volume (ml) | 1.00 (1.00–1.00) | 0.16 |
| Baseline eGFR (ml/1.73m2/min) | 1.00 (0.96–1.05) | 0.90 |
Significant factors via multivariable analysis are highlighted in bold
OR odds ratio, CI confidence interval, BMI body mass index, eGFR estimated glomerular filtration rate, as calculated using the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation
aInteraction terms for sex * lanreotide and BMI * lanreotide were not significant
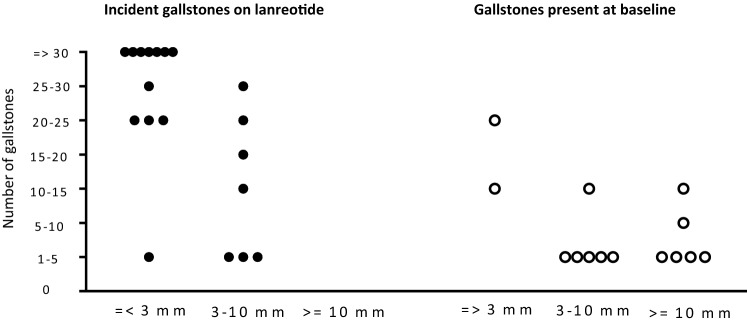
In Table 4 and Fig. 3, the characteristics of incident gallstones during lanreotide treatment are compared with gallstones present at baseline and formed in the SoC group. Patients with gallstones at baseline had, in general, less and larger gallstones, whereas patients with gallstones formed during lanreotide treatment in general had several and small gallstones. Differences in stone size and number were significant (p = 0.001 and p = 0.004, respectively). Representative examples of patients with lanreotide-associated gallstones and gallstones at baseline are shown in Fig. 2.
Table 4.
Characteristics of incident gallstones during lanreotide treatment versus gallstones that were present at baseline and persisted during the trial
| Incident gallstones during lanreotide treatment [n = 19] | Gallstones at baseline [n = 14] | p value | |
|---|---|---|---|
| Number of gallstones | 0.004 | ||
| 1–5 | 4 (21) | 9 (64) | |
| 5–10 | 0 (0) | 1 (7) | |
| 10–15 | 1 (5) | 3 (21) | |
| 15–20 | 1 (5) | 0 (0) | |
| 20–25 | 4 (21) | 1 (7) | |
| 25–30 | 2 (11) | 0 (0) | |
| 30+ | 7 (37) | 0 (0) | |
| Gallstone size, mm | 0.001 | ||
| ≤ 3 | 12 (63) | 2 (14) | |
| 3–10 | 7 (37) | 6 (43) | |
| ≥ 10 | 0 (0) | 6 (43) | |
| Follow-up data available until January 2020 | 16 (84) | 13 (93) | |
| Complications | 9 (56) | 0 | 0.002 |
| Pancreatitisa | 4 (25) | 0 | |
| Cholecystitis | 2 (13) | 0 | |
| Biliary colic | 2 (13) | 0 | |
| Porcelain gallbladder | 1 (6) | 0 |
Data are expressed as n (%)
Significant p-values are highlighted in bold
aOne patient developed pancreatitis during lanreotide treatment; all other complications occurred after the cessation of therapy
Fig. 3.
Differences in the number and size of incident gallstones in patients receiving lanreotide compared with other gallstones. Size of the gallstones and the number of gallstones differed significantly between patients with incident gallstones receiving lanreotide, and gallstones that were present at baseline and persisted during the trial (p = 0.001 and p = 0.004, respectively)
Biliary complications were observed in nine patients (4%). All complications occurred in patients with incident gallstones during lanreotide treatment. No complications occurred in patients having gallstones at baseline persisting during the study, neither in the lanreotide group nor the control group (p = 0.002) (Table 4). One patient developed a biliary pancreatitis during the treatment period while using lanreotide. This patient was admitted to hospital and the disease course was complicated by pneumonia. A month later, the patient was rehospitalized because of a gastropancreatic fistula, and shortly thereafter, a second readmission followed because of a necrotizing pancreatitis.
After the trial, complications occurred in an additional eight patients. There were no factors significantly associated with complications of incident gallstones. Median time between discontinuation of lanreotide and the development of symptoms or complications was 2.5 years (IQR 1.0–3.6 years). For all patients, the timelines are shown in electronic supplementary Fig. 1.
Three patients developed a biliary pancreatitis, one of whom had to be admitted to an intensive care unit for 4 months because of a severe necrotizing pancreatitis, complicated by multi-organ failure, need for mechanical ventilation, and recurrent bleeding from the distal colon, eventually treated by a colon resection and several reresections leading to a colostoma. A pancreatic deficiency resulted in diabetes mellitus. The second patient who experienced a pancreatitis was admitted to the hospital and developed bleeding after endoscopic retrograde cholangiopancreaticography (ERCP). In a second ERCP procedure, the bleeding focus was coagulated. The laparascopic cholecystectomy had to be converted to an open procedure because of the large kidney cyst volume. The third patient with a pancreatitis had a mild course and could be discharged from the hospital after 3 days. The patient underwent a cholecystectomy but experienced a new episode of pancreatitis a year later, which was this time thought to be caused by propofol. Again, the patient experienced a fast recovery and was discharged after 4 days.
In addition, two patients developed cholecystitis, one a porcelain gallbladder, and another two patients had biliary colics. In all patients, a cholecystectomy was performed, and no further complications occurred.
Exploratory Outcomes
Adverse events that can be related to the presence of gallstones in patients using lanreotide are shown in electronic supplementary Table 1. Complaints such as abdominal pain, abdominal cramps, and discolored stool had a high prevalence, both in subjects with and without incident gallstones, possibly because these complaints can also be adverse effects of lanreotide. None of these symptoms was significantly related to the presence or formation of gallstones. In a previous report on the DIPAK 1 trial, we described an increased incidence of hepatic cyst infections in lanreotide users [10, 12]. In the present analysis, hepatic cyst infections during lanreotide treatment were not related to the presence at baseline or incident gallstones during the study. The response to lanreotide treatment (assessed as the annual rate of liver growth) was not associated with incident gallstones.
Discussion
In the present study, we investigated the incidence of gallstones using systematic imaging in a relatively large-scale cohort of ADPKD patients who were randomized to lanreotide or SoC. At the end of the 120-week lasting trial, 15% of patients in the lanreotide group had developed incident gallstones, compared with 1% of patients in the control group (OR 25.9, 95% CI 3.37–198.81). We showed that lanreotide-associated gallstones were more often multiple (> 20) and small (≤ 3 mm). During the trial, one patient treated with lanreotide experienced a biliary pancreatitis. For patients with gallstones at the end of the trial, additional follow-up data until January 2020 were obtained. After discontinuation of therapy, an additional eight patients experienced acute biliary complications, sometimes even years after discontinuation. Complications only occurred in patients who developed gallstones during lanreotide treatment.
The incidence of gallstones during somatostatin analog treatment was previously systematically assessed using imaging in two studies [2, 13]. In the CLARINET study, the incidence of gallstones in patients treated with lanreotide was 12% in 1.2 years, similar to the 15% that we observed in our 2.3 years lasting trial. The CLARINET study did not report biliary complications [2]. In a prospective registry of patients treated with somatostatin analogs for acromegaly with yearly ultrasound assessments, 33/91 patients formed new gallstones (36%) [13]; however, follow-up time was longer (median 11 years) and acromegaly is a risk factor for gallstone formation [14]. In this study, there was no follow-up after the cessation of treatment [13].
A strength of our study is that we obtained data about gallstone-related complications not only during but also after the end of the formal trial period when medication was withdrawn. During the treatment period, only one patient developed an acute biliary pancreatitis. However, after the formal trial period, an additional three patients experienced a pancreatitis, two patients experienced biliary colic, two patients experienced an acute cholecystitis, and one patient developed a porcelain gallbladder. All nine complications occurred in the 19 patients with incident gallstones during lanreotide treatment. Median time between discontinuation of treatment and the acute biliary complications was 2.5 years.
The complication rate of these gallstones formed during somatostatin analog treatment is much higher than the complication rate of gallstones identified by routine screening in the general population. Such gallstones in general lead to symptoms in only 1–4% of patients per year, and to complications in only 0.1–0.3% per year in asymptomatic patients and 1–3% per year in symptomatic patients [15]. The high complication rate associated with incident gallstones during lanreotide treatment raises concern because pancreatitis in particular can be a severe complication, with a mortality rate of up to 5–10% [16].
The high complication rate in these patients with incident gallstones during lanreotide treatment may be explained by stone characteristics. Incident gallstones during lanreotide treatment were high in number (> 20 gallstones) and smaller (≤ 3 mm) than gallstones present before the start of treatment (Table 4 and Fig. 3). It has previously been shown that small gallstones in particular are associated with acute biliary complications such as biliary pancreatitis [17]. It is assumed that these smaller stones can migrate more easily into the common bile duct than larger stones and might more often cause a distal obstruction of the pancreatic duct at the level of the sphincter of Oddi [17, 18].
In our study, most acute biliary complications occurred after discontinuation of somatostatin analog therapy. This could potentially be explained by the effect of these drugs on gallbladder motility. Gallstone formation during treatment with somatostatin analogs is promoted by increased formation of cholesterol-rich crystals in the bile and inhibition of postprandial gallbladder motility and impaired relaxation of the sphincter of Oddi, particularly through inhibitory effects on cholecystokinin (CCK) release [18–21]. As a consequence, stasis of bile occurs, leading to increased gallstone formation. After cessation of therapy, gallbladder motility will return, which can lead to expulsion of gallbladder stones, and thereby to complications. This mechanism is known in biliary pancreatitis, where gallbladder motility is an important risk factor [17, 18]. Trials where somatostatin analogs were used did not report these acute biliary complications, which may be caused by the fact that follow-up after discontinuation of treatment was not performed and that in neuroendocrine tumors and acromegaly, most patients continue therapy. In our trial, almost all patients discontinued treatment and follow-up was available after the formal end of the trial.
We showed that complaints caused by gallstones may be difficult to distinguish from lanreotide-related adverse effects. In patients with polycystic kidney or liver disease, gallstones may be even more difficult to recognize because these patients often experience abdominal pain due to their cystic disease.
What may the practical consequences of our findings be? We showed that the asymptomatic gallstones present before the start of treatment do not carry a high complication risk, in contrast to incident gallstones. Therefore, the presence of asymptomatic gallstones before the start of treatment may not necessarily be a contraindication for the start of somatostatin analogs, as is assumed at present. An important finding is that complications of incident gallstones occur mostly after cessation of lanreotide therapy. Therefore, in case of gallstone formation during somatostatin analog therapy, discontinuation of this treatment should be done with caution because discontinuation of therapy seems not to prevent, but may rather provoke, complications. In patients with incident gallstones during somatostatin analog treatment, it may be worthwhile to start ursodeoxycholic acid (UDCA), especially if there is an indication to discontinue lanreotide. This drug does not reduce complaints caused by symptomatic gallstones [22]. However, we showed that the asymptomatic gallstones associated with somatostatin analogs are mostly small and have therefore a large surface area compared with their content. It could well be that UDCA is effective in dissolving these specific gallstones [23]. Another option could be to discuss with a surgeon whether prophylactic cholecystectomy may be indicated and feasible in this specific patient.
The strengths of our study are that we determined the incidence of gallstones not only based on symptoms but also on MR images. Next, we obtained data on the number and size of these gallstones, and obtained data on acute biliary complications, even after discontinuation of therapy, which is mostly not available.
A limitation of this study could be that we studied only lanreotide. Because the mechanism of gallstone formation is similar for all somatostatin analogs [18–21], we expect that our results are not specific for lanreotide but are class-dependent. In this trial, patients with symptomatic gallstones were excluded. This could explain the low prevalence of gallstones at baseline and indicates that we can draw conclusions on patients with baseline asymptomatic gallstones only. Finally, our study was performed in a specific patient population. However, from the literature, it is not known that patients with ADPKD have a higher risk of gallstone formation or complication rate per se. We therefore believe that our results are also applicable to other patient populations.
Conclusion
The present study shows that the somatostatin analog lanreotide led to gallstone formation in about 15% of treated patients, of whom about half developed potentially severe complications. Because patients with asymptomatic gallstones present on imaging before the start of somatostatin analog treatment did not develop complications, the presence of these asymptomatic gallstones may not be a contraindication to the start of somatostatin analogs. Complications mostly occurred after the discontinuation of treatment in patients with incident gallstones during somatostatin analog treatment. In such patients, starting UDCA may be an option before stopping the somatostatin analog. It is important to be aware of the high risk of acute biliary complications of somatostatin analog-associated gallstones, especially after discontinuation of treatment, and to counsel patients carefully.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
The DIPAK (Developing Interventions for Polycystic Autosomal Kidney disease) Consortium is an inter-university collaboration in The Netherlands that was established to study ADPKD. The DIPAK Consortium is sponsored by IPSEN Farmaceutica BV, the Dutch Kidney Foundation (Grants CP10.12 and CP15.01), and the Dutch government (LSHM15018). DIPAK Consortium members are (in alphabetical order): J.P.H. Drenth, J.W. de Fijter, R.T. Gansevoort, E. Meijer, D.J.M. Peters, F.W. Visser, J.F.M. Wetzels, and R. Zietse.
Declarations
Conflicts of interest
Ron T. Gansevoort received grant support and fees from Galapagos, IPSEN, Otsuka Pharmaceuticals, and Sanofi-Genzyme for serving on advisory boards and steering committees. He also holds the Orphan Medicinal Product Designation status at the European Medicines Agency for lanreotide as treatment for ADPKD (EMA/OD/027/15). Joost P.H. Drenth has received grant support and fees from IPSEN and Novartis for serving on advisory boards and consultancy. Esther Meijer has received consultancy fees from Otuska. All money is paid to their respective institutions. Sophie E. Aapkes, Robbert J. de Haas, Lucas H.P. Bernts, Charles J. Blijdorp, Sosha E.I. Dekker, Maatje D.A. van Gastel, and Abigail Veldman have no other potential conflicts of interest relevant to this article to report.
Ethics approval
The design, methods, and results of the original DIPAK 1 trial have been published previously. The Institutional Review Board at each of the participating hospitals approved the protocol and all patients provided written informed consent. The study was registered at ClinicalTrials.gov (NCT01616927).
Consent to participate
All patients provided written informed consent for this study.
Consent for publication
All patients provided written informed consent for publication.
Availability of data and material
De-identified individual participant data collected during the trial will be made available upon request to researchers who provide a methodologically sound proposal and whose use of the data has been approved by the DIPAK Steering Committee.
Code availability
Not applicable.
Author contributions
All authors contributed to data collection and interpretation. SA and AV scored the MR images for the primary outcome, and, in case of doubt or inconsistency, RdH made the final decision. SA wrote the first draft of the manuscript. LB, CB and SD contributed to the collection of follow-up data after the formal end of the trial. All authors contributed to and approved the final manuscript.
Footnotes
A list of DIPAK consortium members is given in the Acknowledgments section.
Contributor Information
Ron T. Gansevoort, Email: r.t.gansevoort@umcg.nl
The DIPAK consortium:
J. P. H. Drenth, J. W. de Fijter, R. T. Gansevoort, E. Meijer, D. J. M. Peters, F. W. Visser, J. F. M. Wetzels, and R. Zietse
References
- 1.Lamberts SWJ, Hofland LJ. Octreotide, 40 years later. Eur J Endocrinol. 2019;181:173–183. doi: 10.1530/EJE-19-0074. [DOI] [PubMed] [Google Scholar]
- 2.Caplin ME, Pavel M, Ćwikła JB, Phan AT, Raderer M, Sedláčková E, et al. Lanreotide in metastatic enteropancreatic neuroendocrine tumors. N Engl J Med. 2014;371:224–233. doi: 10.1056/NEJMoa1316158. [DOI] [PubMed] [Google Scholar]
- 3.Caplin ME, Pavel M, Ćwikła JB, Phan AT, Raderer M, Sedláčkova E, et al. Anti-tumour effects of lanreotide for pancreatic and intestinal neuroendocrine tumours: the CLARINET open-label extension study. Endocr Relat Cancer. 2016;23:191–199. doi: 10.1530/ERC-15-0490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rinke A, Müller HH, Schade-Brittinger C, Klose KJ, Barth P, Wied M, et al. Placebo-controlled, double-blind, prospective, randomized study on the effect of octreotide LAR in the control of tumor growth in patients with metastatic neuroendocrine midgut tumors: a report from the PROMID study group. J Clin Oncol. 2009;27:4656–4663. doi: 10.1200/JCO.2009.22.8510. [DOI] [PubMed] [Google Scholar]
- 5.Rinke A, Wittenberg M, Schade-Brittinger C, Aminossadati B, Ronicke E, Gress TM, et al. Placebo-controlled, double-blind, prospective, randomized study on the effect of octreotide lar in the control of tumor growth in patients with metastatic neuroendocrine midgut tumors (PROMID): results of long-term survival. Neuroendocrinology. 2016;104:26–32. doi: 10.1159/000443612. [DOI] [PubMed] [Google Scholar]
- 6.van Beek AP, Emous M, Laville M, Tack J. Dumping syndrome after esophageal, gastric or bariatric surgery: pathophysiology, diagnosis, and management. Obes Rev. 2017;18:68–85. doi: 10.1111/obr.12467. [DOI] [PubMed] [Google Scholar]
- 7.van Aerts RMM, Kievit W, D’Agnolo HMA, Blijdorp CJ, Casteleijn NF, Dekker SEI, et al. Lanreotide reduces liver growth in patients with autosomal dominant polycystic liver and kidney disease. Gastroenterology. 2019;157:481–491. doi: 10.1053/j.gastro.2019.04.018. [DOI] [PubMed] [Google Scholar]
- 8.Brighi N, Lamberti G, Maggio I, Manuzzi L, Ricci C, Casadei R, et al. Biliary stone disease in patients receiving somatostatin analogs for neuroendocrine neoplasms. A retrospective observational study. Dig Liver Dis. 2019;51:689–694. doi: 10.1016/j.dld.2018.09.013. [DOI] [PubMed] [Google Scholar]
- 9.Paisley AN, Roberts ME, Trainer PJ. Withdrawal of somatostatin analogue therapy in patients with acromegaly is associated with an increased risk of acute biliary problems. Clin Endocrinol. 2007;66:723–726. doi: 10.1111/j.1365-2265.2007.02811.x. [DOI] [PubMed] [Google Scholar]
- 10.Meijer E, Visser FW, Van Aerts RMM, Blijdorp CJ, Casteleijn NF, D’Agnolo HMA, et al. Effect of lanreotide on kidney function in patients with autosomal dominant polycystic kidney disease the DIPAK 1 randomized clinical trial. JAMA. 2018;320:2010–2019. doi: 10.1001/jama.2018.15870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Meijer E, Drenth JP, D’Agnolo HMA, Casteleijn NF, de Fijter JW, Gevers TJG, et al. Rationale and design of the DIPAK1 study: a randomized controlled clinical trial assessing the efficacy of lanreotide to halt disease progression in autosomal dominant polycystic kidney disease. Am J Kidney Dis. 2014;63:446–455. doi: 10.1053/j.ajkd.2013.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lantinga MA, D’Agnolo HMA, Casteleijn NF, de Fijter JW, Meijer E, Messchendorp AL, et al. Hepatic cyst infection during use of the somatostatin analog lanreotide in autosomal dominant polycystic kidney disease: an interim analysis of the randomized open-label multicenter DIPAK-1 study. Drug Saf. 2017;40:153–167. doi: 10.1007/s40264-016-0486-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Prencipe N, Bona C, Cuboni D, Parasiliti-Caprino M, Berton AM, Fenoglio LM, et al. Biliary adverse events in acromegaly during somatostatin receptor ligands: predictors of onset and response to ursodeoxycholic acid treatment. Pituitary. 2021;24(2):242–251. doi: 10.1007/s11102-020-01102-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Montini M, Gianola D, Pagani MD, Pedroncelli A, Caldara R, Gherardi F, et al. Cholelithiasis and acromegaly: therapeutic strategies. Clin Endocrinol. 1994;40:401–406. doi: 10.1111/j.1365-2265.1994.tb03938.x. [DOI] [PubMed] [Google Scholar]
- 15.Lammert F, Acalovschi M, Ercolani G, van Erpecum KJ, Gurusamy KS, van Laarhoven CJ, et al. EASL Clinical Practice Guidelines on the prevention, diagnosis and treatment of gallstones. J Hepatol. 2016;65:146–181. doi: 10.1016/j.jhep.2016.03.005. [DOI] [PubMed] [Google Scholar]
- 16.Uhl W, Isenmann R, Curti G, Vogel R, Beger HG, Büchler MW. Influence of etiology on the course and outcome of acute pancreatitis. Pancreas. 1996;13:335–343. doi: 10.1097/00006676-199611000-00002. [DOI] [PubMed] [Google Scholar]
- 17.Venneman NG, Renooij W, Rehfeld JF, Van Berge-Henegouwen GP, Go PMNYH, Breeders IAMJ, et al. Small gallstones, preserved gallbladder motility, and fast crystallization are associated with pancreatitis. Hepatology. 2005;41:738–746. doi: 10.1002/hep.20616. [DOI] [PubMed] [Google Scholar]
- 18.Venneman NG, van Erpecum KJ. Gallstone disease: primary and secondary prevention. Best Pract Res Clin Gastroenterol. 2006;20:1063–1073. doi: 10.1016/j.bpg.2006.03.008. [DOI] [PubMed] [Google Scholar]
- 19.Moschetta A, Stolk MFJ, Rehfeld JF, Portincasa P, Slee PHTHJ, Koppeschaar HPF, et al. Severe impairment of postprandial cholecystokinin release and gall-bladder emptying and high risk of gallstone formation in acromegalic patients during Sandostatin LAR. Aliment Pharmacol Ther. 2001;15:181–185. doi: 10.1046/j.1365-2036.2001.00924.x. [DOI] [PubMed] [Google Scholar]
- 20.Hussaini SH, Pereira SP, Veysey MJ, Kennedy C, Jenkins P, Murphy GM, et al. Roles of gall bladder emptying and intestinal transit in the pathogenesis of octreotide induced gall bladder stones. Gut. 1996;38:775–783. doi: 10.1136/gut.38.5.775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Grasso LFS, Auriemma RS, Pivonello R, Colao A. Adverse events associated with somatostatin analogs in acromegaly. Expert Opin Drug Saf. 2015;14:1213–1226. doi: 10.1517/14740338.2015.1059817. [DOI] [PubMed] [Google Scholar]
- 22.Venneman NG, Besselink MGH, Keulemans YCA, VanBerge-Henegouwen GP, Boermeester MA, Broeders IAMJ, et al. Ursodeoxycholic acid exerts no beneficial effect in patients with symptomatic gallstones awaiting cholecystectomy. Hepatology. 2006;43:1276–1283. doi: 10.1002/hep.21182. [DOI] [PubMed] [Google Scholar]
- 23.Podda M, Zuin M, Battezzati PM, Ghezzi C, deFazio C, Dioquardi ML. Oral dissolution therapy for cholelithiasis: mix and match. Gastroenterology. 1989;96:222–229. doi: 10.1016/0016-5085(89)90784-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.