Abstract
Introduction
Acetaminophen is a commonly used analgesic and antipyretic, with the potential to cause significant injury when ingested in toxic amounts. Although the antidote n-acetylcysteine (NAC) is available, evidence supporting dose recommendations for patients weighing over 100 kg are lacking. We performed a retrospective, multi-center analysis to determine if a capped NAC dosing scheme is similar to a non-capped dosing scheme in patients weighing over 100 kg.
Methods
Between January 2009 and January 2016, we identified patients presenting to 12 different centers who were evaluated for acetaminophen poisoning treatment. Patients must have weighed greater than 100 kg and were evaluated and identified as needing treatment for acetaminophen-related poisoning with NAC. The primary outcome was occurrence of hepatic injury, defined as an AST or ALT ≥ 100 IU/L. Secondary endpoints included number of drug-related adverse events, occurrence of hepatotoxicity, cumulative NAC dose, regimen cost, length of hospital and intensive care unit stays, and in-hospital mortality.
Results
There were 83 patients identified as meeting the pre-specified inclusion and exclusion criteria. A capped NAC dosing scheme resulted in no difference in hepatic injury when compared to a non-capped regimen (49.4% vs 50%, p = 1.000). The capped dosage regimen was associated with a lower cumulative dose (285.2 mg/kg vs 304.6 mg/kg, p < 0.001) and cost. No other statistically significant differences were identified among the secondary endpoints.
Conclusion
A capped NAC dosing scheme was not associated with higher rates of hepatic injury or hepatotoxicity in obese patients in the setting of acetaminophen poisoning when compared to a non-capped regimen. Further research is needed to verify these results.
Keywords: Acetaminophen, N-acetylcysteine, Toxicity, Obesity, Overdose
Background
Acetaminophen is one of the most common over-the-counter and prescription medications used worldwide. Available alone or in combination products, acetaminophen’s accessibility has led to a number of drug-related adverse events, with acetaminophen ingestions representing one of the top five most common poisonings in the United States [1]. While generally considered a safe medication, ingestion of toxic amounts of acetaminophen (typically doses of 150 mg/kg or greater) is associated with acute liver failure, acute kidney failure, and death [2]. Toxicity is classically identified through aspartate aminotransferase (AST) or alanine aminotransferase (ALT) elevations [2, 3].
During therapeutic dosing of acetaminophen, small amounts of a toxic metabolite, N-acetyl-p-benzoquinone imine (NAPQI), are produced by hepatic cytochrome P450 (CYP) enzymes. Although traditionally hepatotoxic, NAPQI is quickly conjugated with endogenous glutathione to generate nontoxic cysteine and mercapturic acid metabolites, which are further excreted in the urine. Unfortunately, toxic doses of acetaminophen saturate the hepatic glucuronidation and sulfation systems, shunting metabolism to CYP enzymes, yielding higher amounts of the NAPQI metabolite [2]. Thankfully, n-acetylcysteine (NAC) is an antidote that serves as a glutathione precursor, binding NAPQI metabolites to prevent acetaminophen toxicity. In addition to NAPQI detoxification, NAC also serves to limit NAPQI formation by increasing sulfation and even treats fulminant hepatic failure through its antioxidant properties, as well as other mechanisms that are less well-defined [2].
Dosing for NAC is well-protocolized and traditionally based on the patient’s actual body weight [4, 5]. The Food and Drug Administration–approved package insert for intravenous (IV) NAC provides dosing guidance up to 100 kg [6], with recommendations for capping the NAC dose in patients above 100 kg. Unfortunately, data for this rationale is lacking, with this issue specifically being cited within the prescribing information [7]. In addition to potential cost savings, the rationale for capping the dose of NAC at an actual body weight of 100 kg is twofold. First, adverse reactions secondary to NAC tend to happen early in the dosing regimen and are concentration related [8]. Second, although patient’s fat mass and other organs’ mass may increase as weight increases, it is assumed that all patients should have equivalent hepatic volume and metabolism regardless of body weight [9, 10]. Combined, this suggests that additional NAC for patients weighing over 100 kg may not contribute to improved outcomes, with additional drug cost becoming a factor for obese patients.
Data is limited for NAC dosing in patients weighing greater than 100 kg. This study serves to compare two NAC dosing strategies in acetaminophen poisoning: capping doses at an actual body weight of 100 kg versus traditional non-capped dosing.
Methods
Study Design
We performed a multi-center, retrospective review of patients who presented to the emergency department with acetaminophen toxicity and, on evaluation, treated with IV NAC was initiated. Data were collected from patients who presented between January 1, 2009, and January 1, 2016, at twelve collaborating institutions. An Institutional Review Board (IRB) approval was attained at all participating sites.
Patients were identified by the International Classification of Disease (ICD)-9 or ICD-10 codes, suggestive of acetaminophen overdose (Table 1). Charts were then reviewed to ensure patients met the appropriate inclusion criteria before analysis.
Table 1.
International Classification of Disease (ICD) codes: ICD-9/ICD-10 codes used to identify patients with potential cases of acetaminophen overdose.
| Diagnostic codes | Definition |
|---|---|
| ICD-9-CM | |
| 965.4 | Poisoning by aromatic analgesics including acetaminophen |
| E850.4 | Accidental poisoning by aromatic analgesics including acetaminophen |
| E935.4 | Adverse effects of therapeutic use of aromatic analgesics including acetaminophen |
| E950.0 | Suicide and self-inflicted poisoning by analgesics, antipyretics, and antirheumatics |
| ICD-10 | |
| T39.1 | Poisoning by 4-aminophenol derivatives |
| X40 | Accidental poisoning by and exposure to non-opioid analgesics, antipyretics, and antirheumatics including acetaminophen |
| Y45.5 | Adverse effects of therapeutic use of 4-aminophenol derivatives |
| X60 | Intentional self-poisoning by and exposure to non-opioid analgesics, antipyretics, and antirheumatics including acetaminophen |
| Y10 | Poisoning by and exposure to non-opioid analgesics, antipyretics, and antirheumatics, including acetaminophen, of undetermined intent |
Data Collection
Study data were collected by investigators at each site and managed using the REDCap (Research Electronic Data Capture) electronic data capture tools hosted at the University of Kentucky. This is a secure, web-based application designed to support data capture for research studies. REDCap was supported by the National Center for Research Resources and the National Center for Advancing Translational Sciences, National Institutes of Health, through Grant 8UL1TR000117-02. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH [11].
Inclusion/Exclusion Criteria
Patients were included in the study if they received the standard three-bag IV NAC treatment for acetaminophen overdose between January 1, 2009, and January 1, 2016, had an actual body weight greater than 100 kg, and were aged greater than or equal to 18 years. Patients were excluded if NAC was used for non-acetaminophen toxicity indications, if no acetaminophen concentrations or liver function tests (LFT) were drawn after 24 h of admission, if the patient had missing data necessary for analysis, or if the patient was transferred from an outside hospital where NAC treatment had already been initiated. Patients receiving oral NAC were also excluded. Data regarding patient demographics, clinical presentation, treatment, and outcome information were collected. Figure 1 demonstrates differences in dosage and estimated cost for the two regimens, as patient weight increases.
Fig. 1.
Dosing of N-acetylcysteine in acetaminophen overdose. Comparison of potential total dosage received dependent on dosing strategy.
Outcome Measures
The primary endpoint was development of hepatic injury, defined as an AST or ALT greater than 100 IU/L. The secondary endpoints included development of hepatotoxicity, defined as an AST or ALT ≥ 1000 IU/L, occurrence of adverse events during NAC administration, patient cumulative dose, regimen cost, hospital length of stay, ICU length of stay (if admitted to the ICU), and in-hospital mortality. For both the primary and secondary outcomes, we intend to test non-inferiority (or equivalence) between capped and uncapped dosing using the Farrington and Manning score test. Based on a 13.5% prevalence rate of hepatic injury, a non-inferiority margin of 8%, an alpha value of 0.05, and a power value of 0.80, a sample size calculation yielded 233 patients per group.
Adverse events during NAC administration were identified through documentation of an event during NAC infusion. These included the development of cutaneous symptoms (flushing, rash, urticaria, wheals), gastrointestinal symptoms (nausea or vomiting), respiratory symptoms (bronchospasms, wheezing, dyspnea, or shortness of breath), cardiovascular instability, or angioedema. Additionally, any discontinuation of NAC due to adverse effects or the use of any rescue medications was also monitored (antiemetics, steroids, antihistamines, bronchodilators, or epinephrine) as a surrogate marker of adverse effects.
Statistical Measures
The primary outcome of interest was a comparison of the rates of hepatic injury development between both capped and non-capped NAC regimens following an acetaminophen overdose. Secondary outcomes included assessment of hepatotoxicity rates between groups, adverse event rates during NAC administration, patient cumulative dose, length of hospital stay, length of ICU stay (if they are admitted to the ICU), and mortality.
Patient characteristics were compared by dosing strategy. For categorical variables, frequencies and column percentages (%) were reported and p values were calculated using χ2 and Fisher’s exact tests, as appropriate. Continuous variables were tested for normality using the Shapiro-Wilk normality test along with histograms. Normally distributed continuous variables were reported using means and standard deviations (SD) and p values were calculated using Welch two-sample t tests; otherwise, medians and first/third quartiles [Q1, Q3] were reported and p values were calculated using Mann-Whitney U tests.
Statistical significance was set at p ≤ 0.05. A power analysis for non-inferiority with respect to the primary outcome resulted in a required sample size of 233 patients per group. Missing observations were reported and all analyses were conducted using all available observations. All analyses were done in R programming language, version 3.6.3 (R Foundation for Statistical Computing, Vienna, Austria). All graphics were produced using the R package ggplot2, version 3.3.0 (Hadley, Wickham).
Results
We identified 83 patients who met the pre-specified inclusion criteria spanning 12 different study sites: 56 patients in the traditional dosing group and 27 patients in the capped dosing group. Table 2 contains the patient demographics for each cohort. There was a statistically significant difference with respect to patient weight, but no difference in body mass index (BMI), observed between groups. Median weight was higher in the traditional dosage group (120 kg traditional dosage vs. 108.9 kg capped dosage, p = 0.026). The median BMI in the study population was 39.3 kg/m2, consistent with current definitions of obesity. There were no differences in age, race, or past medical history (PMH).
Table 2.
Patient demographics.
| Dosing strategy | ||||
|---|---|---|---|---|
| No. of patients | All Patients 83 |
Traditional 56 |
Capped 27 |
P value |
| Male, N (%) | 43 (51.8) | 30 (53.6) | 13 (48.1) | 0.819 |
| Age (years), median [Q1, Q3] | 40.0 [28.0, 48.0] | 36.0 [28.0, 46.2] | 44.0 [31.0, 50.0] | 0.147 |
| BMI (kg/m2), median [Q1, Q3] (missing observations) | 39.3 [34.2, 44.4] (N = 3) | 40.0 [34.3, 44.7] (N = 3) | 38.8 [34.2, 44.1] (N = 0) | 0.629 |
| Weight (kg), median [Q1, Q3] | 116.0 [106.6, 132.2] | 120.0 [109.7, 135.2] | 108.9 [104.9, 118.6] | 0.026 |
| Height (cm), median [Q1, Q3] (missing observations) | 175.2 [167.0, 182.9] (N = 3) | 175.3 [167.6, 182.9] (N = 3) | 172.7 [165.1, 177.8] (N = 0) | 0.235 |
| Race, N (%) | 0.129 | |||
| African American | 11 (13.3) | 9 (16.1) | 2 (7.4) | |
| Caucasian | 68 (81.9) | 45 (80.4) | 23 (85.2) | |
| Hispanic | 2 (2.4) | 0 (0.0) | 2 (7.4) | |
| Other | 2 (2.4) | 2 (3.6) | 0 (0.0) | |
| History of chronic alcohol abuse, N (%) | 9 (10.8) | 6 (10.7) | 3 (11.1) | 1.000 |
| History of acute alcohol ingestion, N (%) | 11 (13.3) | 8 (14.3) | 3 (11.1) | 1.000 |
| History of chronic liver disease, N (%) | 7 (8.4) | 4 (7.1) | 3 (11.1) | 0.677 |
| LOS (days), median [Q1, Q3] | 3.6 [2.0, 5.5] | 3.7 [2.0, 5.9] | 3.5 [2.3, 5.0] | 0.915 |
| Any ICU stay, N (%) | 41 (49.4) | 24 (42.9) | 17 (63.0) | 0.138 |
| ICU LOS (days), median [Q1, Q3] (missing observations) | 3.0 [2.0, 6.0] (N = 42) | 3.5 [1.8, 7.2] (N = 32) | 3.0 [2.0, 4.0] (N = 10) | 0.406 |
IQR interquartile range = 25th–75th percentiles
Differences in overdose type were identified between groups and are described in Table 3. Furthermore, the traditional dosage group was found to possess more ingestions of an immediate release APAP preparation (58.9% traditional dosage vs. 29.6% capped dosage, p = 0.023). More patients ingested extended-release preparations in the capped dosage group, although this did not reach statistical significance (7.1% traditional dosage vs. 18.5% capped dosage, p = 0.143). Lastly, large burden overdoses were identified in twenty-seven patients and defined as acetaminophen ingestions > 16 g total or > 300 mg/kg or had acetaminophen levels > 300 mcg/ml (n = 17 traditional dosage vs. n = 10 capped dosage). The prevalence of large burden overdoses was not statistically different between groups. There were no statistically significant differences in estimated time from ingestion to presentation, with 30.4% of patients in the traditional dosage group and 22.2% of the capped dosage group presenting within 8 h of ingestion.
Table 3.
Characterization of overdose.
| Dosing strategy | ||||
|---|---|---|---|---|
| No. of patients | All patients 83 |
Traditional 56 |
Capped 27 |
P value |
| Large burden overdose, N (%)* | 27 (32.5) | 17 (30.4) | 10 (37.0) | 0.720 |
| Intentional ingestion, N (%) | 48 (57.8) | 36 (64.3) | 12 (44.4) | 0.140 |
| Accidental ingestion, N (%) | 6 (7.2) | 4 (7.1) | 2 (7.4) | 1.000 |
| Unable to determine intentionality, N (%) | 8 (9.6) | 5 (8.9) | 3 (11.1) | 0.711 |
| Estimated amount of acetaminophen ingested known, N (%) | 37 (44.6) | 30 (53.6) | 7 (25.9) | 0.033 |
| Estimated amount of acetaminophen ingested unknown, mean (SD) | 0.6 (0.5) | 0.5 (0.5) | 0.7 (0.4) | 0.014 |
| Estimated amount of acetaminophen ingested (mg), median [Q1, Q3] (missing observations) | 27500.0 [14000.0, 37500.0] (N = 46) | 28750.0 [14250.0, 37125.0] (N = 26) | 25000.0 [19000.0, 39000.0] (N = 20) | 0.954 |
| Estimated amount of acetaminophen ingested (mg/kg), median [Q1, Q3] (missing observations) | 222.2 [119.4, 341.9] (N = 46) | 209.3 [115.8, 333.5] (N = 26) | 242.0 [178.5, 341.1] (N = 20) | 0.535 |
| Time of ingestion known, N (%) | 34 (41.0) | 27 (48.2) | 7 (25.9) | 0.090 |
| Estimated time from ingestion to presentation (hours), N (%) | 0.097 | |||
| Less than 8 hours | 23 (27.7) | 17 (30.4) | 6 (22.2) | |
| Greater than 8 hours | 11 (13.3) | 10 (17.9) | 1 (3.7) | |
| Unknown | 49 (59.0) | 29 (51.8) | 20 (74.1) | |
| Chronic ingestion, N (%) | 14 (16.9) | 7 (12.5) | 7 (25.9) | 0.209 |
| Acute ingestion within single 8-h period, N (%) | 52 (62.7) | 36 (64.3) | 16 (59.3) | 0.840 |
| Mixed ingestion, N (%) | 43 (51.8) | 29 (51.8) | 14 (51.9) | 1.000 |
| Also ingested alcohol, N (%) | 12 (14.5) | 9 (16.1) | 3 (11.1) | 0.743 |
| Also ingested benzodiazepines, N (%) | 8 (9.6) | 5 (8.9) | 3 (11.1) | 0.711 |
| Also ingested opioids, N (%) | 18 (21.7) | 12 (21.4) | 6 (22.2) | 1.000 |
| Also ingested cocaine, N (%) | 3 (3.6) | 3 (5.4) | 0 (0.0) | 0.547 |
| Also ingested other substances, N (%) | 26 (31.3) | 17 (30.4) | 9 (33.3) | 0.983 |
| Immediate-release acetaminophen, N (%) | 41 (49.4) | 33 (58.9) | 8 (29.6) | 0.023 |
| Extended-release acetaminophen, N (%) | 9 (10.8) | 4 (7.1) | 5 (18.5) | 0.143 |
| Unknown acetaminophen preparation, N (%) | 33 (39.8) | 19 (33.9) | 14 (51.9) | 0.186 |
*Large burden overdose defined as single acute ingestion > 16 g or > 300 mg/kg or serum acetaminophen level > 300 mcg/ml
Primary Objective
The primary endpoint of hepatic injury development was not statistically significantly different among groups (50% traditional dosage vs 48.1% capped dosage, p = 1.000) (Fig. 2). Additionally, no differences were found when individually evaluating AST or ALT values between treatment regimens.
Fig. 2.
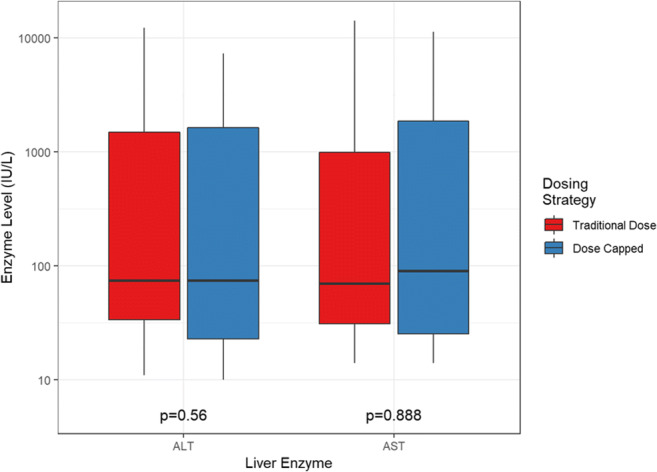
Highest liver enzyme levels.
Secondary Objectives
For the secondary objective of hepatotoxicity rates between groups, we were unable to detect any significant differences among treatment regimens (28.6% vs 33.3%, p = 0.851). There were no differences in mortality between groups (Table 4 and no statistically significant difference noted between the two groups with respect to adverse events or the receipt of rescue medications (Table 5). Statistically significant differences in cumulative dose (304.6 mg/kg traditional dosage vs. 285.2 mg/kg capped dosage, p < 0.001) were observed between regimens. More patients in the traditional dosing group required continuation of NAC treatment, although this was not found to be statistically significant (Table 5). Median duration of hospital stay was 3.6 days (3.7 traditional dosage vs. 3.5 capped dosage, p = 0.915). A large portion of the capped dosing group required ICU admission (42.9% traditional dosage vs. 63% capped dosage, p = 0.138), although this did not reach statistical significance. Lastly, the traditional dosage regimen represented a greater financial burden for patients than the capped regimen ($2174.3 vs $997, p < 0.001) (Table 5).
Table 4.
Patient outcomes hepatotoxicity and hepatic injury.
| Dosing strategy | ||||
|---|---|---|---|---|
| No. of patients | All patients 83 |
Traditional 56 |
Capped 27 |
P value |
| Hepatic injury (AST or ALT >= 100), N (%) | 41 (49.4) | 28 (50.0) | 13 (48.1) | 1.000 |
| Hepatic injury (AST >= 100), N (%) | 39 (47.0) | 26 (46.4) | 13 (48.1) | 1.000 |
| Hepatic injury (ALT >= 100), N (%) | 36 (43.4) | 25 (44.6) | 11 (40.7) | 0.921 |
| Hepatotoxicity (AST or ALT >= 1000), N (%) | 25 (30.1) | 16 (28.6) | 9 (33.3) | 0.851 |
| Hepatotoxicity (AST >= 1000), N (%) | 23 (27.7) | 14 (25.0) | 9 (33.3) | 0.594 |
| Hepatotoxicity (ALT >= 1000), N (%) | 24 (28.9) | 16 (28.6) | 8 (29.6) | 1.000 |
| Deceased during this admission, N (%) | 6 (7.2) | 4 (7.1) | 2 (7.4) | 1.000 |
Table 5.
N-acetylcysteine dosing.
| Dosing strategy | ||||
|---|---|---|---|---|
| No. of patients | All patients 83 |
Traditional 56 |
Capped 27 |
P value |
| Estimated time from ingestion to NAC initiation, N (%) | 0.351 | |||
| Less than 8 hours | 24 (28.9) | 17 (30.4) | 7 (25.9) | |
| Greater than 8 hours | 28 (33.7) | 21 (37.5) | 7 (25.9) | |
| Unknown | 31 (37.3) | 18 (32.1) | 13 (48.1) | |
| NAC dose of standard protocol (mg), median [Q1,Q3] (missing observations) | 31,000.0 [30,000.0, 37953.8] (N = 1) | 36,000.0 [30,775.0, 40,800.0] (N = 1) | 30,000.0 [30,000.0, 30,000.0] (N = 0) | < 0.001 |
| NAC dose of standard protocol (mg/kg), median [Q1, Q3] (missing observations) | 294.0 [262.0, 300.0] (N = 1) | 300.0 [287.1, 300.2] (N = 1) | 275.5 [252.5, 286.0] (N = 0) | < 0.001 |
| Total cumulative NAC dose (mg), median [Q1, Q3] (missing observations) | 37,599.7 [30,000.0, 48,834.8] (N = 1) | 40,800.0 [32,240.0, 62,840.5] (N = 1) | 30,000.0 [30,000.0, 36,000.0] (N = 0) | < 0.001 |
|
Total cumulative NAC dose (mg/kg), median [Q1, Q3] (Missing observations) |
300.0 [275.5, 400.1] (N = 1) | 304.6 [300.0, 462.5] (N = 1) | 285.2 [262.4, 294.1] (N = 1) | < 0.001 |
| Cost of initial NAC dose (US dollars), median [Q1, Q3] (missing observations) | 1432.4 [997.0, 2413.0] (N = 18) | 1524.2 [1326.2, 2778.6] (N = 14) | 732.9 [643.0, 1571.5] (N = 4) | < 0.001 |
| Total NAC cost (US dollars), median [Q1, Q3] (missing observations) | 1777.5 [1029.6, 2911.1] (N = 16) | 2174.3 [1453.6, 3649.3] (N = 12) | 997.0 [667.9, 1810.9] (N = 4) | < 0.001 |
| Continued treatment due to elevated INR, N (%) | 4 (4.8) | 4 (7.1) | 0 (0.0) | 0.299 |
| Continued treatment due to elevated enzymes, N (%) | 16 (19.3) | 13 (23.2) | 3 (11.1) | 0.311 |
| Continued treatment due to elevated APAP levels, N (%) | 7 (8.4) | 6 (10.7) | 1 (3.7) | 0.418 |
| Continued treatment due to unknown reason, N (%) | 9 (10.8) | 6 (10.7) | 3 (11.1) | 1.000 |
| Adverse events of cutaneous symptoms, N (%) | 1 (1.2) | 1 (1.8) | 0 (0.0) | 1.000 |
| Adverse events of gastrointestinal symptoms, N (%) | 3 (3.6) | 3 (5.4) | 0 (0.0) | 0.547 |
| Adverse events of respiratory symptoms, N (%) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1.000 |
| Adverse events of angioedema, N (%) | 1 (1.2) | 1 (1.8) | 0 (0.0) | 1.000 |
| Adverse events of cardiovascular instability, N (%) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1.000 |
| Treatment administered during NAC: antiemetics, N (%) | 11 (13.3) | 7 (12.5) | 4 (14.8) | 0.743 |
| Treatment administered during NAC: epinephrine, N (%) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1.000 |
| Treatment administered during NAC: bronchodilators, N (%) | 2 (2.4) | 0 (0.0) | 2 (7.4) | 0.103 |
| Treatment administered during NAC: antihistamines, N (%) | 2 (2.4) | 2 (3.6) | 0 (0.0) | 1.000 |
| Treatment administered during NAC: steroids, N (%) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1.000 |
| NAC treatment stopped, N (%) | 2 (2.4) | 2 (3.6) | 0 (0.0) | 1.000 |
IQR interquartile range = 25th–75th percentiles
Discussion
Guidance for the dosing of NAC in obese patients is currently limited, with direction provided by the toxicokinetic parameters of acetaminophen and the pharmacokinetics of NAC treatment in obese populations. Findings from our study suggest that dose capping for weights greater than 100 kg reduced the cumulative patient NAC dose, without providing additional risk of hepatic toxicity. Additionally, reductions in cumulative dose may also equate to a decrease in treatment cost. The average wholesale price of NAC obtained from Cumberland Pharmaceuticals was estimated at approximately $16 per 6-g vial. Within our patient cohort, this translates to a total cost savings of $1177.30 per patient hospital stay.
The rationale for a specific NAC regimen in obese patients is multifactorial, requiring the clinician to consider the toxicokinetics of acetaminophen in the obese patient, the pharmacokinetics of the NAC antidote, and considering the circumstances behind the acetaminophen ingestion.
Obesity
Studies in adult males have shown that as weight and BMI increase, so does the relative liver and kidney size [12]. Specifically, with relation to acetaminophen toxicity, increases in cytochrome P450 2E1 (CYP2E1) activity are of great interest. As the size of the liver increases so does the fat content and activity of the CYP2E1 enzyme, with varying effects on glutathione levels [13, 14]. Increases in CYP2E1 activity may correlate with increased hepatic toxicity due to increases in the production of NAPQI. The effects of obesity on renal clearance are less well-established; however, renal clearance has not been shown to increase in a linear fashion with body weight [14].
Complicating matters further is the notion that as body size increases, so do the pharmacokinetic and pharmacodynamic parameters of drug behavior in the body. Absorption may be somewhat unchanged by obesity, with product type and amount ingested more likely to influence drug exposure [14]. Additionally, obesity has minimal impact on drugs such as NAC with smaller volumes of distribution, as they concentrate within the vascular system. Protein binding to albumin may not be markedly different between obese and nonobese individuals. However, altered protein binding occurs as triglycerides, lipoproteins, and free fatty acids increase [14, 15].
Toxicokinetics of Acetaminophen in Obesity
Studies in the early 1980s demonstrated that acetaminophen taken at therapeutic doses has a similar half-life when comparing obese and nonobese patients. What differed, however, was the absorption rate, maximal concentrations, and the time to reach those maximal levels, collectively lowering the area under the concentration curve in obese patients [15].
Coccoran and colleagues looked at the implications of acetaminophen toxicity in an overfed rat model and questioned if obesity increased the risk of toxicity. They found ALT released into serum and urine was higher in obese rats than controls. Additionally, cellular necrosis of both the liver and kidney was higher. Of note, the acetaminophen doses were based on total body weight, meaning that obese rats received larger acetaminophen doses potentially confounding the worse outcomes experienced in the obese group [16].
Varney and colleagues retrospectively described demographics, outcomes, and adverse events of 37 cases of acetaminophen overdoses in patients weighing greater than 100 kg [17]. They identified a hepatotoxicity (AST or ALT > 1000 U/L) rate of approximately 33.3%, and a mortality rate of 11.1%, with no serious adverse events reported. Nausea, vomiting, and tachycardia were the most common reactions observed. This study was met with some criticism, as it did not report acetaminophen serum levels and failed to comment on time to NAC treatment, leaving some to question the interpretation of safety and efficacy of dosing in the obese population [18].
Recently, an investigation was completed in the Thai population regarding the impact of being overweight (body mass index 25–29.9 kg/m2) or obese (body mass index ≥ 30 kg/m2) and the prevalence of acetaminophen toxicity-related hepatotoxicity (AST or ALT > 1000 U/L) or acute liver injury (defined as doubling of AST or ALT) [19]. One hundred and ninety-seven patients were included in the analysis, but only a small portion (30%) were overweight (n = 24) or obese (n = 35) [19]. The median weight in the overweight-obese group was 65 kg (IQR 60–69 kg). There were no statistically significant differences in instances of hepatotoxicity (11.9% overweight-obese versus 13% normal, p = 0.926) or ALI (28.8% overweight-obese versus 16.7% normal, p = 0.083). A multinomial logistical analysis was performed which found that being overweight or obese was an independent risk factor for ALI. Taken collectively, these investigations suggest an increased risk of hepatic injury in obese acetaminophen-poisoned patients.
Pharmacokinetics of NAC in Obesity
N-acetylcysteine concentrates well within vasculature due to its small volume of distribution of 0.5 L/kg. Strengthening this assertion is its high degree of protein binding, limiting its distribution into the periphery.
One study has investigated the topic of NAC treatment for acetaminophen toxicity in obesity. Radosevich et al. retrospectively looked at the prevalence of hepatotoxicity (AST or ALT > 1000 U/L) in 40 obese (body mass index > 30 kg/m2) versus 40 nonobese (body mass index < 24.9 kg/m2) patients treated with IV NAC. The median weight in the obese group was 98 kg with an interquartile range of 83.5–108.9; however, only five of the patients weighed greater than 100 kg. Not surprisingly, patients in the obese group received a higher mg/kg total NAC dose. Despite differences in dosing, they found that obese patents did not experience significantly more toxicity than their nonobese counterparts (27.5% obese vs 37.5% nonobese) [10].
Patient-Centered Treatment
Although NAC dosing in obese patients is an important consideration, it represents just one aspect of appropriate care for the acetaminophen-poisoned patient. Obese or not, instituting a proper dose of NAC within 8 h of acetaminophen poisoning drastically reduces the risk for hepatotoxicity [5]. Additionally, adjusting total NAC dosing for large burden overdoses, extending the duration of total NAC therapy, utilizing additional antidotal therapies such as hemodialysis, and modifying the traditional 3-bag regimen to either a 2-bag or 1-bag treatment are all methods hypothesized to improve patient outcomes including mortality in acetaminophen poisoning.
Limitations
Despite the large number of centers participating, this study did not have enough patients to meet the required sample size calculation due to the rarity of this condition. Data collection was retrospective in nature and relied on accurate clinical documentation: our study is limited by charting errors, omissions, and inconsistencies common to other emergency medicine and poison center studies. We also acknowledge that baseline laboratory results were not available for most patients; it is possible that patients with baseline elevated liver enzymes (AST > 100 U/L) would not have been identified.
Although participating medical centers all resided in the United States, we are unable to account for additional practice differences between sites. Patients were identified using the inclusion criteria described above by participating investigators at each site, but verification of treatment appropriateness was not assessed further by the study investigators. More patients in the capped group had unknown ingestion amounts as well as unknown times of ingestions compared to the traditional group making it harder to match groups for exposure severity. Adverse events were characterized from what was charted in the clinical record or by the receipt of rescue medications, but it is possible additional adverse events were not documented or medications administered were not related to NAC infusion. Lastly, although we attempted to account for factors relating to hepatic injury and hepatotoxicity, it is certainly possible that other events or interventions during the patient’s stay confounded our results.
Conclusion
In this study, obese patients treated with a capped NAC dosing regimen for acetaminophen poisoning were not associated with an increased rate of hepatotoxicity or hepatic injury, when compared to a traditional non-capped regimen. Despite the large number of centers participating, this study did not have enough patients to meet the required sample size calculation. Reduced cumulative dose would result in reduction of drug costs. However, a prospective, randomized trial would be needed to verify these results.
Acknowledgements
Collaborators:
A. J. Dugan, L. Atchison, K. M. DeWitt, M. K. Doolin, M. A. Howland, E. Lingenfelter, T. Neitzel, C. Sloan, P. Wong
Authors’ Contributions
• All authors contributed to the study conception and design.
• Material preparation, data collection, and analysis were performed by Regan Baum, Abby Bailey, Molly Howell, Kyle Weant, Sanjay Mohan, LeeAnn Geraghty, Leanne Atchison, Kyle DeWitt, Meagan Doolin, Adam Dugan, Erin Lingenfelter, Sanjay Mohan, Tara Neitzel, Cole Sloan, and Paul Wong.
• The first draft of the manuscript was written by Regan Baum, LeeAnn Geraghty, Ashley Webb, Molly Howell Abby Bailey, Kyle Weant, and Peter Akpunonu.
• All authors commented on previous versions of the manuscript. Formatting, feedback, and revisions are performed by Regan Baum, Abby Bailey, Kyle Webb, Jordan Woolum, Sanjay Mohan, Mark Su, and Peter Akpunonu.
All authors read and approved the final manuscript.
Funding
REDCap used in this study was supported by the National Center for Research Resources and the National Center for Advancing Translational Sciences, National Institutes of Health, through Grant 8UL1TR000117-02. There was no additional funding to support this study.
Declarations
Conflict of Interest
None.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Regan A. Baum, Email: rabaum2@uky.edu
Collaborators:
Adam J. Dugan, Leanne Atchison, Kyle M. DeWitt, Meagan K. Doolin, Mary Ann Howland, Erin Lingenfelter, Tara Neitzel, Cole Sloan, and Paul Wong
References
- 1.Mowry JB, Spyker DA, Cantilena LR, Jr, McMillan N, Ford M. 2013 Annual Report of the American Association of Poison Control Centers’ National Poison Data System (NPDS): 31st Annual Report. Clin Toxicol (Phila). 2014;52(10):1032–1283. doi: 10.3109/15563650.2014.987397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hoffman RS, Howland MA, Lewin NA, Nelson L, Goldfrank LR, Flomenbaum N, Access Pharmacy, and Access Emergency Medicine. Goldfrank's Toxicologic Emergencies. Tenth ed. New York: McGraw-Hill; 2015.
- 3.Rumack BH, Bateman DN. Acetaminophen and acetylcysteine dose and duration: past, present and future. Clin Toxicol (Phila). 2012;50(2):91–98. doi: 10.3109/15563650.2012.659252. [DOI] [PubMed] [Google Scholar]
- 4.Vale JA, Meredith TJ, Crome P, Helliwell M, Volans GN, Widdop B, Goulding R. Intravenous N-acetylcysteine: the treatment of choice in paracetamol poisoning? Br Med J. 1979;2(6202):1435–1436. doi: 10.1136/bmj.2.6202.1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Smilkstein MJ, Knapp GL, Kulig KW, Rumack BH. Efficacy of oral N-acetylcysteine in the treatment of acetaminophen overdose. Analysis of the national multicenter study (1976 to 1985) N Engl J Med. 1988;319(24):1557–1562. doi: 10.1056/NEJM198812153192401. [DOI] [PubMed] [Google Scholar]
- 6.ACETADOTE (acetylcysteine) injection (package insert). Nashville, TN; Cumberland Pharmaceuticals Inc; 2019. https://www.acetadote.com/New_Acetadote_Labeling_25OCT2019.pdf. Accessed 25 Sept 2020.
- 7.Cumberland Pharmaceuticals Inc. ACETADOTE (acetylcysteine) injection (package insert). U.S. Food and Drug Administration. website: https://www.accessdata.fda.gov/drugsatfda_docs/nda/2004/21-539_Acetadote.cfm. Revised January 2004. Accessed October 2020.
- 8.Chiew AL, Isbister GK, Duffull SB, Buckley NA. Evidence for the changing regimens of acetylcysteine. Br J Clin Pharmacol. 2016;81(3):471–481. doi: 10.1111/bcp.12789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fromenty B. Drug-induced liver injury in obesity. J Hepatol. 2013;58(4):824–826. doi: 10.1016/j.jhep.2012.12.018. [DOI] [PubMed] [Google Scholar]
- 10.Radosevich JJ, Patanwala AE, Erstad BL. Hepatotoxicity in obese versus nonobese patients with acetaminophen poisoning who are treated with intravenous N-acetylcysteine. Am J Ther. 2016;23(3):e714–e719. doi: 10.1097/01.mjt.0000434043.62372.00. [DOI] [PubMed] [Google Scholar]
- 11.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377–381. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Molina DK, DiMaio VJ. Normal organ weights in men: part II-the brain, lungs, liver, spleen, and kidneys. Am J Forensic Med Pathol. 2012;33(4):368–372. doi: 10.1097/PAF.0b013e31823d29ad. [DOI] [PubMed] [Google Scholar]
- 13.Emery MG, Fisher JM, Chien JY, Kharasch ED, Dellinger EP, Kowdley KV, Thummel KE. CYP2E1 activity before and after weight loss in morbidly obese subjects with nonalcoholic fatty liver disease. Hepatology. 2003;38(2):428–435. doi: 10.1053/jhep.2003.50342. [DOI] [PubMed] [Google Scholar]
- 14.Hanley MJ, Abernethy DR, Greenblatt DJ. Effect of obesity on the pharmacokinetics of drugs in humans. Clin Pharmacokinet. 2010;49(2):71–87. doi: 10.2165/11318100-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 15.Lee WH, Kramer WG, Granville GE. The effect of obesity on acetaminophen pharmacokinetics in man. J Clin Pharmacol. 1981;21(7):284–287. doi: 10.1002/j.1552-4604.1981.tb01768.x. [DOI] [PubMed] [Google Scholar]
- 16.Corcoran GB, Wong BK. Obesity as a risk factor in drug-induced organ injury: increased liver and kidney damage by acetaminophen in the obese overfed rat. J Pharmacol Exp Ther. 1987;241(3):921–927. [PubMed] [Google Scholar]
- 17.Varney SM, Buchanan JA, Kokko J, Heard K. Acetylcysteine for acetaminophen overdose in patients who weigh >100 kg. Am J Ther. 2014;21(3):159–163. doi: 10.1097/MJT.0b013e3182459c40. [DOI] [PubMed] [Google Scholar]
- 18.Tillmann BW, Takematsu M, Lugassy DM. Acetylcysteine for acetaminophen overdose in patients who weigh >100 kg. Am J Ther. 2016;23(1):e244–e245. doi: 10.1097/MJT.0b013e31827f9cbf. [DOI] [PubMed] [Google Scholar]
- 19.Chomchai S, Chomchai C. Being overweight or obese as a risk factor for acute liver injury secondary to acute acetaminophen overdose. Pharmacoepidemiol Drug Saf. 2018;27(1):19–24. doi: 10.1002/pds.4339. [DOI] [PubMed] [Google Scholar]



