Abstract
Background
Vitamin E acetate (VEA) has come under significant scrutiny due to its association with E-cigarette or vaping product use–associated lung injury (EVALI). Various theoretical mechanisms have been proposed for toxicity, including tocopherol (vitamin E)–mediated surfactant damage, recruitment of inflammation, and pyrolysis of acetate to the pulmonary irritant ketene.
Objective
Characterize studies in mammals evaluating inhaled VEA, vitamin E analogues, or pyrolyzed acetate that describe subsequent effects on the lung.
Eligibility
Research in all languages from time of inception to October 1, 2020, regarding mammals (human or animal) exposed to inhaled vitamin E analogues, or any compound containing acetate administered via inhalation after pyrolysis, and subsequent description of pulmonary effect.
Sources of evidence
Ovid MEDLINE, Scopus, and Web of Science Core Collection.
Results
In total, 786 unique articles were identified. After duplicate reviewer screening, 16 articles were eligible for inclusion. Tocopherol was evaluated in 68.8% (11/16) of the studies, VEA in 18.8% (3/16), and both VEA and tocopherol were evaluated in 12.5% (2/16). Of the five studies evaluating VEA, it was given by pyrolysis in 60.0% (3/5). No human studies were identified. All included trials were conducted on non-human mammals: 75.0% (12/16) rodent models and 25.0% (4/16) sheep models. Outcomes assessed were heterogeneous and included 57 unique outcomes.
Conclusions
Several questions still exist regarding the pulmonary toxicity of inhaled tocopherol and VEA. More studies are needed to determine whether tocopherol alone (i.e., without acetate) can cause pulmonary injury. Additionally, further studies of VEA should evaluate the impact that pyrolysis and co-administration with other compounds, such as tetrahydrocannabinol, have on the toxic potential of VEA.
Keywords: EVALI, Vitamin E Acetate, Vitamin E, Vaping, E-Cigarette
Introduction
A recent mass poisoning in the United States known as E-cigarette or vaping product use–associated lung injury (EVALI) resulted in 2739 hospitalizations and 68 deaths in a 6-month span [1]. Vitamin E acetate (VEA) has been scrutinized as a potential cause of this outbreak due to being found in both bronchoalveolar lavage (BAL) samples from EVALI patients and as a cutting agent in illicit vaping devices used for tetrahydrocannabinol (THC) [2]. The compound is composed of a molecule of tocopherol (more commonly known as vitamin E) attached via an ester to an acetate molecule (Fig. 1) [3]. While many different isoforms of tocopherol exist, alpha-tocopherol acetate is the primary isoform that has been isolated from EVALI patients [2].
Fig. 1.
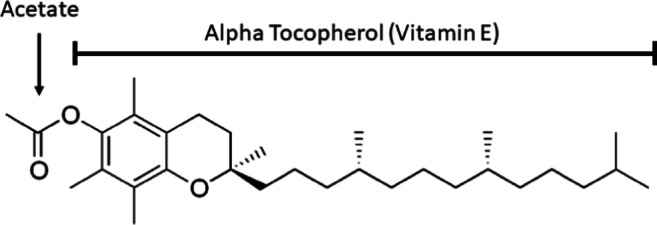
Structure of vitamin E acetate (VEA, also known as alpha-tocopherol acetate).
Various mechanisms have been proposed for the toxicity of VEA. Deleterious pulmonary effects may arise from exposure to the entire molecule (VEA), from one of the individual components (tocopherol or acetate), or from an entirely new compound formed by vaporizing VEA. Some hypothesize that pyrolysis (the process of heating without combustion) of VEA liberates the pulmonary irritant ketene [2, 4]. Others have suggested that new compounds created from VEA hydrogen bonding with THC in vape cartridges may be potential toxins [2, 4, 5]. Moreover, some theories propose that VEA or tocopherol may cause an exogenous lipoid pneumonia, cause damage through inflammatory macrophage recruitment, or interfere with normal surfactant functioning [2, 4].
Little experimental data evaluating the toxicity of VEA or its proposed mechanisms exist. Recent articles have suggested that evidence summaries of vitamin E exposures in biologic tissues may help more easily assess the proposed toxicity of VEA [6]. As many of these studies exist in animals and there is heterogeneity in outcomes assessed, a scoping review is most appropriate for collation of the data. We aim to summarize available literature to better assess what is known and what further study is needed to support the proposed mechanisms of VEA toxicity. As it is unknown which component of the molecule may cause toxicity, the objective of this study is to characterize the current literature evaluating inhaled VEA, tocopherol or its analogues, or pyrolyzed acetate and the subsequent effects on the lungs.
Methods
Protocol and Registration
A protocol was designed utilizing the Preferred Reporting Items for Systematic reviews and Meta-Analyses extension for Scoping Reviews (PRISMA-ScR) Checklist [7]. The protocol was not registered.
Study Eligibility
Peer-reviewed articles evaluating the pulmonary effects of vitamin E or its analogues inhaled via any route (e.g., nebulization, intratracheal injection, aerosolization) and the effects of inhaled acetate compounds only after pyrolysis were included. Only studies with pyrolysis were evaluated for acetate because it is integral to the hypothesis of acetate transformation to the pulmonary irritant ketene [8]. Additionally, other studies have demonstrated that acetate is safe when administered via nebulization [9]. Studies in humans and animals were included if they met both of the following criteria:
Exposed to tocopherol or analogues (including VEA) via inhalational route OR exposed to any compound containing acetate administered via inhalation after pyrolysis.
The study had a subsequent description of the pulmonary effect.
Information Sources
Information sources were Ovid MEDLINE, Scopus, and Web of Science Core Collection.
Search Strategy
Searches were designed and conducted by the medical librarian (ES).
Searches were created using a combination of subject headings and keywords to describe the topics of vitamin E and lung injuries. (See Table 1 for the Ovid MEDLINE search.) A search was conducted for research in all languages from the time of inception to February 26, 2020. Additionally, a monthly PubMed® search alert using the search term “Vitamin E Acetate” was employed until October 1, 2020, to ensure any new articles would be captured. Any duplicate studies were removed.
Table 1.
Database(s): Ovid MEDLINE® and Epub ahead of print, in-process and other non-indexed citations, daily and versions® 1946 to February 19, 2020.
| No. | Searches |
|---|---|
| 1 | exp vitamin e/ |
| 2 | (alpha-Tocopherol or alpha-Tocopheryl or d-alpha-tocopheryl or beta-Tocopherol or beta-Tocopheryl or gamma-tocopherol or gamma-tocopheryl or tocoph* or vitamin e or tocotrienol* or (acetate and (pyrolys* or incinerat* or burn* or gasification or thermal decomposition))).mp. |
| 3 | exp acetates/ and (exp incineration/ or exp Inhalation/ or exp Pyrolysis/) |
| 4 | 1 or 2 or 3 |
| 5 | exp Lung Injury/ |
| 6 | ((lung* or pulmonary) adj5 (injur* or damage* or trauma*)).mp. |
| 7 | 5 or 6 |
| 8 | 4 and 7 |
Selection of Sources of Evidence
Two independent reviewers screened articles for relevance by title and abstract using the Rayyan application. Relevant articles included from the title and abstract screen were read in full to determine if they met inclusion criteria. References from included studies were also screened for eligibility.
Data Collection and Charting Process
Two separate reviewers (RF and MS) collected key outcomes independently in a predefined data collection sheet. The first 12% of studies were collected in duplicate to ensure abstracter congruence. After the final article collection, key information regarding study design, substances studied, and outcomes evaluated were abstracted.
Data Items
Study outcomes included the type of study (human or animal), type of animal, number of participants in treatment or control groups, presence of blinding, and presence of randomization. Substance outcomes included which substance was evaluated (VEA, vitamin E isoform, or acetate), vehicles used for administration (e.g., ethanol, saline), duration of administration, dose delivered, route of administration (nebulized, aerosol, intratracheal aspirate, or pyrolysis), and vaporizer power settings used to pyrolyze the substance (if available). An attempt to contact authors via email was done to obtain power settings if not listed in the manuscript. Further substance outcomes included whether the evaluated substance was delivered with other substances (e.g., glutathione) or known toxicants (e.g., smoke) and whether it was given prior to or after exposure to other toxicants. Finally, outcome data extracted included a full list of all measured outcomes, whether the study measured markers of pulmonary damage, and whether authors concluded that tocopherol, VEA, or pyrolyzed acetate decreased markers of lung toxicity compared to groups exposed to a known toxicant.
Data Synthesis
Studies were manually compiled into tables to display results.
Results
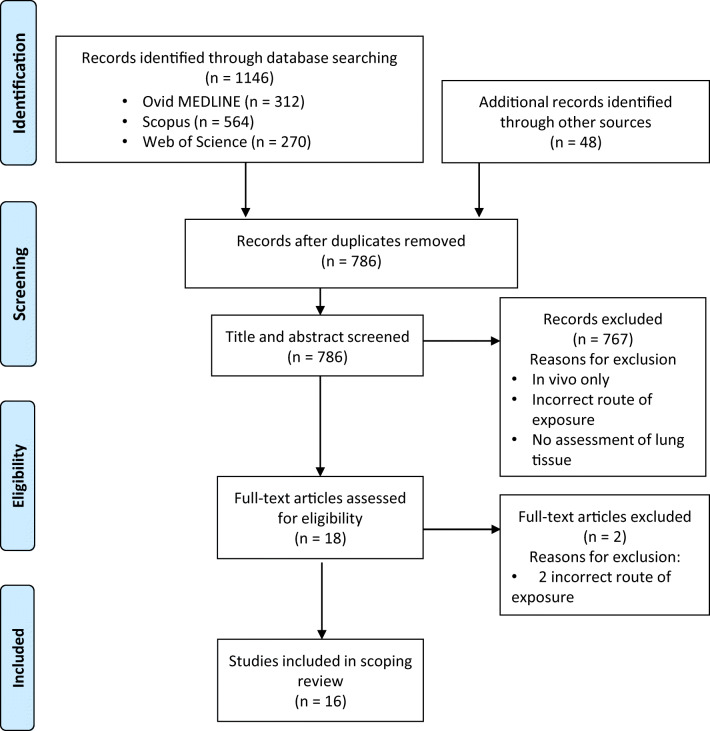
Selection of Sources of Evidence (Fig. 2)
Fig. 2.
Search results.
The initial search and monthly search alerts resulted in 786 unique articles. After abstract and title screening, 17 articles were included for full-text review, one additional study was included from reference screening of full-text articles, and two were excluded due to incorrect route of exposure (intraperitoneal). In total, 16 original research articles were included.
Characteristics of Sources of Evidence
Of the 16 studies (Table 2), tocopherol was evaluated in 68.8% (11/16) of the studies, VEA in 18.8% (3/16), and both VEA and tocopherol were evaluated in 12.5% (2/16). Of the five studies evaluating VEA, it was given by pyrolysis in 60.0% (3/5). No other trials of pyrolyzed acetate were identified. No human studies were identified. All trials were in non-human mammals: 75.0% (12/16) in rodent models and 25.0% (4/16) in sheep models. Outcomes assessed were heterogeneous and included 57 unique outcomes (Table 3).
Table 2.
Characteristics of studies of tocopherol (vitamin E) or vitamin E acetate and effect on pulmonary tissue.
| Author year | Animal | Compound evaluated: (tocopherol or VEA) | Route | Total dose (mg) | Substances administered with vitamin E/VEA (vehicles, or other antioxidants that could confound results) | Exposure duration | Toxin used for induced pulmonary damage | Effect on markers of lung toxicity | Evaluated substance administered before or after toxin? |
|---|---|---|---|---|---|---|---|---|---|
| Tocopherol (vitamin E) administered alone | |||||||||
| Suntres et al. 1993 [10] | Rat | alpha-Tocopherol | IT | 2 mg | DPPC (v) | N/A | None | Did not assess markers of injury (assessed lung tissue tocopherol concentration only) | N/A |
| Suntres and Shek 1994 [11] | Rat | alpha-Tocopherol | IT | 2 mg | DPPC (v) glutathione | N/A | None | N/A | |
| Tocopherol (vitamin E) administered with toxin | |||||||||
| Yamamoto et al. 2012 [13] | Sheep | 95% gamma-tocopherol/5% alpha-Tocopherol | N | 11 mg | Ethanol (v) | 45 hours | Smoke | Tocopherol reduced smoke-induced lung injury markers | 3 hours after |
| Yamamoto et al. 2012 [12] | Sheep | gamma-Tocopherol | N | 11 mg | Ethanol (v) | 45 hours | Smoke | Tocopherol reduced smoke-induced lung injury markers | 3 hours after |
| Suntres et al. 1992 [14] | Rat | alpha-Tocopherol | IT | Not listed | DPPC (v) | N/A | Paraquat | Pretreatment with tocopherol reduced paraquat-induced lung injury markers | 24 hours before |
| Suntres and Shek 1995 [15] | Rat | alpha-Tocopherol | IT | 8 mg/kg | DPPC (v) | N/A | Paraquat | Pretreatment with tocopherol reduced paraquat-induced lung injury markers | 24 hours before |
| Suntres and Shek 1995 [17] | Rat | alpha-Tocopherol | IT | 8 mg/kg | DPPC (v) | N/A | Paraquat | Post-treatment with tocopherol reduced paraquat-induced lung injury markers | 24 hours after |
| Suntres and Shek 1996 [18] | Rat | alpha-Tocopherol | IT | 2 mg | DPPC (v) glutathione | N/A | Paraquat | Tocopherol + glutathione pretreatment reduced paraquat-induced lung injury markers | 0.5 hours before |
| Morita et al. 2006 [19] | Sheep | 98% alpha/2% gamma | N | 321.6 mg | Saline (v) | 3 hours | Smoke | Tocopherol reduced smoke-induced lung injury markers | 1 hour after |
| Hamahata et al. 2008 [20] | Sheep | 87% gamma-tocopherol /13% alpha-tocopherol | N | 25.5 mg | Flax Oil (v) | 47 hours | Smoke | Flax oil alone and tocopherol + flax oil reduced smoke-induced lung injury markers | 1 hour after |
| Hybertson et al.a 1998 [22] | Rat | alpha-Tocopherol | A | 500 mg | Super critical CO2 (v) | 0.5 hours | Xanthine oxidase and purine | Tocopherol reduced markers of oxidant-induced injury | Before(time unknown) |
| Hybertson et al. 1995 [21] | Rat | alpha-Tocopherol | A | 14 mg | Super critical CO2 (v) | 0.167 hours | Interleukin-1 | Tocopherol reduced interleukin-1 induced injury | Before (time unknown) |
| Hybertson et al.a 2005 [23] | Rat | alpha-Tocopherol | A | 30 mcg | Super critical CO2 (v) | 0.5 hours | Lipopolysaccharide | Tocopherol reduced markers of lipopolysaccharide-induced injury | After (time unknown) |
| Vitamin E acetate (VEA) administered with toxin | |||||||||
| Hybertson et al.a 1998 [22] | Rat | VEA | A | 500 mg | Super critical CO2 (v) | 0.5 hours | Xanthine oxidase and purine | VEA did not reduce markers of oxidant lung injury compared to toxin alone, though level was not significantly higher than toxin alone | Before (time unknown |
| Hybertson et al.a 200 [23] | Rat | VEA | A | 30 mcg | Super critical CO2 (v) | 0.5 hours | Lipopolysaccharide | VEA reduced lung neutrophil and TNF-α concentrations compared to toxin alone but not it was not statistically significant. Lung CINC levels were the same with VEA + toxin versus toxin alone | After (time unknown) |
| Vitamin E acetate (VEA) administered alone | |||||||||
| Bhat et al. 2020 [24] | Mouse | VEA | P | 77.3–167.5 mcg/g/d | None | 5 hours per day × 14 d | None | Increased markers of lung injury in VEA exposed mice compared to control or PG/VG | N/A |
| Matsumoto et al. 2020 [25] | Mouse | VEA | P | 9600 mL/hour 100% VEA aerosol | None | 2 hours per day × 5–16 d | None | Increased markers of lung injury in VEA exposed mice compared to control, PG/VG, or JUULpods® | N/A |
| Muthumalage et al. 2020 [26] | Mouse | VEA | P | 8400 mL/hour 50% VEA aerosol | MCT | 1 hour per day × 3 days | MCT |
Increased inflammatory cells recruitment in VEA/MCT exposed mice compared to air, however less so than inflammation caused by counterfeit CBD vape cartridge brands reported to be associated with EVALI Decreased inflammatory cytokines except for IL-6 and eotaxin compared to air |
Concurrent |
A aerosolization, N nebulization, d day, CINC cytokine-induced neutrophil chemoattractant, DPPC dipalmitoyl phosphatidyl choline, IT intratracheal, MCT medium chain triglycerides, P pyrolysis/aerosol, PG/VG propylene glycol/vegetable glycerin, VEA vitamin E acetate. v denotes this was a vehicle other substances were suspended in TNF-α (tumor necrosis factor alpha)
aTrials that assessed both tocopherol and VEA were listed in both the tocopherol and VEA sections of this table; this trial assessed both and is listed in duplicate; total studies included in the table n = 16
Table 3.
Outcomes evaluated in studies.
| Author and year | Outcomes evaluated |
|---|---|
| Bhat et al. 2020 [24] | Albumin BAL, CD 45 cell count lung, lung weight |
| Hybertson et al. 1998 [22] | Tocopherol level lung, lung tissue Ficoll retention, lung lavage Ficoll levels |
| Hybertson et al. 1995 [21] | Tocopherol level lung, myeloperoxidase activity |
| Hybertson et al. 2005 [23] | TNF alpha lung mRNA, chemoattractant-1 CINC-1 mRNA, TNF alpha lung lavage, CINC lung lavage, neutrophil lung lavage |
| Hamahata et al.2008 [20] | Tocopherol level lung, lung bloodless wet-to-dry ratio, myeloperoxidase activity, malondialdehyde (lipid peroxidation), 3-nitotyrosine, Il-6, IL-8, Immunohistochemistry of poly ADP |
| Morita et al. 2006 [19] | Tocopherol level lung, pulmonary transvascular fluid flux, shunt fraction, airway obstruction score, PaO2/FiO2, 3-nitrotyrosine, plasma nitrate, and nitrite |
| Matsumoto et al. 2020 [25] | Lung edema, BAL analysis, histology, inflammatory cytokines, plasma surfactant protein D |
| Muthumalage et al. 2020 [26] | Arterial oxygen saturation, immune cell influx, and IL-6 and eotaxin in BAL, monocyte chemoattractant protein 1, regulated on activation, normal T cell expressed and secreted, IL-17A, IL-12p40, and IL-4, surfactant-associated protein A, 6-keto-prostaglandin F1-alpha, LTB4, LTD4, LTE4, 5HETE. 12HETE, diradylglycerols, sterols, glycerophosphocholines |
| Suntres et al. 1992 [14] | Wet lung weight CYP activity, lipid peroxidation, GSH lung concentration |
| Suntres et al. 1993 [10] | Distribution of alpha-tocopherol |
| Suntres and Shek 1994 [11] | Distribution of alpha-tocopherol |
| Suntres and Shek 1995 [15] | Wet lung weight, AKP activity, whole blood neutrophils, pulmonary myeloperoxidase activity |
| Suntres and Shek 1995 [17] | Wet lung weight, AKP activity, lipid conjugated dienes, pulmonary myeloperoxidase activity, glutathione concentration |
| Suntres and Shek 1996 [18] | Glutathione lung concentration, wet lung weight, AKP, ACE |
| Yamamoto et al. 2012 [13] | Tocopherol level lung, DDAH-2, ADMA-Arginine ratio, arginase activity, protein expression density hydroxyproline, lung bloodless wet to dry ratio, diffusing capacity |
| Yamamoto et al. 2012 [12] | Tocopherol level lung, pulmonary shunt fraction, peak and pause pressures, lung bloodless wet-to-dry weight ratios, lung obstruction measurements of bronchus and bronchioles, lung histology and scoring, mechanical vent weaning |
ACE angiotensin-converting enzyme, ADMA asymmetrical dimethyl arginine, AKP alkaline phosphatase, BAL bronchoalveolar lavage, DDAH-2 dimethyl arginine dimethylaminohydrolase-2, GSH glutathione, HETE hydroxyeicosatetraenoic, LT leukotriene, IL interleukin, mRNA messenger ribosomal nucleic acid, TNF tumor necrosis factor
Studies Assessing Tocopherol
In the 13 studies assessing tocopherol, three different combinations of tocopherol isoforms were evaluated: 69.2% (9/13) alpha-tocopherol, 23.1% (3/13) alpha-tocopherol/gamma-tocopherol mix, and 7.7% (1/13) gamma-tocopherol. Only two of these trials (15.4%) evaluated the effects of tocopherol administered to the lungs without other substances [10, 11]. These studies evaluated lung tissue levels of tocopherol but did not assess or report markers of lung damage. The remaining 11 trials (84.6%) evaluated tocopherol administration to lungs that were damaged by a known toxicant [12–23]. All trials demonstrated that co-administration of tocopherol with known toxicant reduced markers of lung injury compared to toxicant alone. No trials assessed tocopherol administered after pyrolysis.
Studies Assessing VEA
All trials evaluating VEA assessed the alpha isoform of tocopherol acetate. In 40.0% (2/5) of studies, VEA was administered to lungs that were damaged by a known toxicant. In each of these studies, pre- or post-treatment with VEA did not significantly change markers of lung toxicity compared to toxicant alone [21, 23].
The remaining 60.0% (3/5) of trials evaluated the effects of VEA after atomization (aerosolization via pyrolysis) in models of vaporization [24, 25]. One trial exposed mice to either 5 hours a day of atomized (4.8 voltage, atomizing coil resistance of 2.8 Ω, 8.2 W) VEA or propylene glycol/vegetable glycerin (PG/VG, a common diluent in e-cigarettes). The estimated VEA dose was 77.3–167.5 mcg/g/day. Lung histology, presence of inflammatory immune cells in BAL, lung weight, and lung albumin concentration (lung weight and albumin concentration are markers of capillary lung leak) were compared to control mice [24]. Compared to control and PG/VG, VEA increased markers of capillary leak and inflammation.
The second trial utilized exposure groups of 100% VEA, JUULpods® (30:70 PG:VG, 5% nicotine salts) or control. In their protocol for VEA exposure, mice were placed in a chamber twice a day for 1 hour while the chamber was exposed to 120 × 80 mL puffs of 100% VEA (3.7 V/1.5 Ω coil/9.4 W) for 6 to 15 days [25]. Mice exposed to VEA demonstrated higher concentrations of inflammatory cells and cytokines in BAL, increased markers of capillary leak (lung edema), and lung injury (plasma surfactant protein D) compared to control or JUULpod® exposures. This study also demonstrated a dose-dependent relationship, where increasing exposure resulted in increasing inflammation and biomarkers of pulmonary damage.
The final study used an Ooze Slim Twist Vape Pen® (3.8 V) to expose mice to a mixture of 50:50 VEA/medium chain triglyceride (MCT), MCT alone, or aerosol from brands of counterfeit vape cartridges (CVC) reported by EVALI patients [26]. All groups were placed in a chamber for 1 hour per day for 3 days while the chamber was filled with 140 mL per minute of vape aerosol. The study demonstrated mixed results. Only the CVC group demonstrated a statically larger concentration of immune cells in mice BAL compared to control. Exposure to VEA/MCT significantly increased two inflammatory cytokines (IL-6 and eotaxin) compared to control, but it significantly decreased two others (IL-17A, IL12p40). There were also lower concentrations of the inflammatory mediators MCP-1, RANTES, and IL-4 in VEA/MCT-treated rats compared to control, though this observation was not found to be significant. In male rats only, VEA/MCT led to changes in surfactant protein A consistent with excessive lipid accumulation. The contents of the CVC that mice were exposed to are not known, but prior studies suggest they may contain THC and VEA [27]
Discussion
While numerous hypotheses for tocopherol or VEA toxicity have been proposed, very little experimental data exists evaluating their direct pulmonary toxicity. All trials occurred in animals, evaluated heterogeneous routes, doses, duration of exposures, and surrogate outcomes of pulmonary toxicity, creating challenges in extrapolating results to the recent outbreak of EVALI. However, valuable findings regarding the proposed hypothesis of VEA toxicity can be drawn from these trials.
Potential Toxicity of Tocopherol
Only two trials evaluated tocopherol alone (i.e., without the addition of other substances that could also cause lung toxicity) [10, 11]. These trials did report effects on the lung (measurement of lung tissue tocopherol concentrations) and thus met inclusion criteria; however, they unfortunately did not assess markers of lung damage and cannot provide any information on potential tocopherol toxicity. All remaining tocopherol trials assessed lung tissue that underwent damage by other toxins, making it impossible to assess any individual effects tocopherol may have had [12–23]. All trials of tocopherol administered before or after a pulmonary toxin demonstrated that tocopherol reduced markers of lung damage compared to the toxin alone. This observation infers that tocopherol can mitigate markers of pulmonary inflammation and damage, making it unlikely to be a toxicant. In fact, alpha-tocopherol is secreted by type II alveolar pneumocytes with surfactant to protect against oxidant stressors [3]. However, the demonstrated effects of tocopherol on the uninjured lungs are still unknown.
Potential Toxicity of VEA
Trials assessing VEA via standard aerosol (i.e., without pyrolysis) all occurred in the lungs already damaged with an oxidant or inflammatory toxin [22, 23]. Unlike tocopherol, when VEA was administered with an oxidant, it failed to demonstrate significant protective effects [22, 23]. This result makes intuitive sense as VEA lacks the antioxidant properties of tocopherol. The hydroxyl that acts to deprotonate and neutralize free radicals in tocopherol is occupied in VEA by the acetate ester. VEA must undergo hydrolysis of this acetate to become an antioxidant, which is what normally happens when consumed orally [22]. Of note, when VEA was administered to lungs damaged by pseudomonal lipopolysaccharide (LPS), there was a non-statistically significant reduction in lung neutrophil count and TNF-α compared to LPS alone. This observation is interesting as it might be expected that VEA combined with a known toxin would demonstrate worsened markers of damage if each were individually pulmonary toxicants. Yet, this data demonstrates not worsened, but rather improved markers of inflammation, suggesting a possible anti-inflammatory effect. Other reviews have noted that VEA inhibits 5-lipoxygenase activity and prevents leukotriene production similar to tocopherol [3, 28].
The remaining animal models of VEA exposure were designed specifically to answer questions regarding VEA and EVALI and all utilized the route of pyrolysis. Bhat and colleagues importantly demonstrated that mice exposed to vaporized VEA showed more lung capillary leak and pulmonary inflammation than mice exposed to vaporized PG/VG or control [24]. Using a similar method of exposure, Matsumoto and colleagues demonstrated a dose-response relationship to these effects [25]. Additionally, they demonstrated that those with VEA exposure had worsened toxicity markers than those exposed to nicotine only (JUULpods®). While ex vivo evidence was not part of the scope of this review, Matsumoto also demonstrated direct toxic effects of VEA on isolated human alveolar type II cells. Both studies provided supporting evidence of an inflammatory and damaging effect of VEA.
Finally, Muthumalage and colleagues demonstrated conflicting data compared to the previous two studies on the inflammatory effects of pyrolyzed VEA. They assessed BAL inflammatory immune cell and cytokine concentrations in mice exposed to pyrolyzed VEA/MCT, MCT, CVC brands associated with EVALI, or control [26]. Importantly, this study utilized the shortest duration of VEA exposure (1 hour per day for three days), which may play a role in the findings. Only CVC significantly increased inflammatory immune cell recruitment in mouse BAL; VEA/MCT or MCT did not. It should be noted, however, that the CVC themselves may have contained VEA as well as THC/CBD/MCT or numerous other compounds, making the results difficult to interpret [27]. The authors suggested that the multitude of substances in CVC causes higher amounts of volatile organic compounds to be released when pyrolysis occurs and propose this as a possible mechanism for excess inflammation. Interestingly, the effects of exposure on cytokine release were highly variable. Compared to control, VEA/MCT and CVC led to greater IL-6 and eotaxin concentrations. However, VEA/MCT, MCT, and CVC exposures all demonstrated lower BAL concentrations of the five remaining measured cytokines (IL-4, MCP-1, RANTES, IL-17A, 11-12p40). The authors suggest this result is due to a pro-inflammatory, negative feedback effect as opposed to an anti-inflammatory effect. However, as VEA/MCT and MCT did not cause a clear inflammatory increase in immune cells, the rationale behind this explanation is not entirely clear. The mixed inflammatory effects noted may be related to the shorter duration and thus lower dose of exposure, as a dose-response relationship has been previously noted. It should be stated that while the in vivo effects were mixed, the study also conducted an in vitro analysis, which demonstrated cytotoxicity, barrier dysfunction, and inflammation from VEA/MCT.
Production of Ketene as a Mechanism of Toxicity
Pyrolysis is central to some proposed hypothesis of VEA toxicity as a mechanism for converting the acetate molecule of VEA to ketene [29, 30]. Modeling of ketene production from VEA has predicted that temperatures near 700 °C are needed to produce ketene and no appreciable ketene would be produced at temperatures below 500 °C [30]. The heat produced by a vaping device depends greatly on the wetness of the wick drawing fluid to the atomizer, properties of the fluid being vaporized, voltage applied, and resistance [31]. Many of the studies assessing VEA aerosolized after pyrolysis report the power settings that were utilized (Bhat 4.8 V, 2.8 Ω coil, 8.2 W, Matsumoto 3.7 V/1.5 Ω coil/9.4 W, Muthumalage 3.8 v Ooze Slim Twist Vape Pen®, coil resistance not reported). Unfortunately, no studies have evaluated temperatures produced while vaporizing VEA at specific power settings. Therefore, no inference can be made regarding whether these experiments reached temperatures adequate to generate ketene.
Studies of propylene glycol vaporization have found that the highest temperatures are generated with a dry wick, low resistance, and high voltage. For propylene glycol, the temperature produced by “dry hits” where the wick is significantly dry, are within range to produce ketene. This has led some to hypothesize that “dry hits” from vape devices are responsible for ketene production with VEA [30]. Studies of the temperatures produced during VEA vaporization at specific voltages, resistance settings, and wick saturation, such as those done for propylene glycol, may provide information on whether the three studies that have been conducted were capable of generating sufficient heat to produce ketene. The proof of concept study that produced ketene by pyrolyzing VEA was done utilizing a NEXUS P-1 mini Vaping device at 50 W and a coil with a resistance of 0.25 Ω, generating an output of 3.5 V [8, 29]. These settings are noted to be a considerably higher wattage and lower resistance than most commercial vape pens and the prior studies assessing VEA after pyrolysis [3].
Potential Toxicity of VEA or Tocopherol
Another hypothesis for VEA toxicity centers around VEA (or tocopherol) interfering with lung surfactant leading to decreased oxygen diffusion [2]. In models of dipalmitoyl phosphatidylcholine (DPPC), the main constituent of pulmonary surfactant, the introduction of tocopherol leads to a change from a gel to a liquid crystalline phase. It was suggested that this transformation may interfere with oxygen diffusion across surfactant [2, 3, 32–34]. This hypothesis has been contested, as the gel to liquid crystalline transition can only occur in cholesterol depleted models of DPPC, and cholesterol that is normally present in human pulmonary surfactants prevents this transition [3]. Other authors have suggested it is related to the ability of tocopherol to easily incorporate into surfactant membranes (linactant properties). It then leads to a reduction in free volume between particles and increases surfactant viscosity, interfering with its capacity to tolerate compression/expansion cycles in respiration [3]. Regardless of the mechanism, compromised surfactant would lead to impaired oxygen diffusion and tissue hypoxia. Noteworthy in these studies is that animals undergoing continuous arterial oxygenation monitoring did not demonstrate worsening arterial oxygen partial pressure to fraction of inspired oxygen ratios (PaO2/FIO2) ratios or oxyhemoglobin desaturation when exposed to tocopherol or VEA [19, 27]. Additionally, in vitro assessments of RNA expression in isolated human lung cells exposed to VEA did not demonstrate upregulation of genes related to hypoxia [25]. While effects on surfactant and pulmonary compliance may exist, they do not seem to interfere with oxygen exchange observed in animal models [19, 27].
Gaps in Knowledge
While the data available provide valuable insight, there are still many unknowns. Listed below are key areas of future research to aid in answering questions regarding potential toxicity of VEA:
The potential toxic pulmonary effects of tocopherol alone. No trial evaluated markers of pulmonary toxicity after inhaled tocopherol. Its natural function in the body as an antioxidant and the studies showing protection against known toxins are encouraging. However, even VEA has decreased lung injury markers in some trials of injured lungs (though not significantly so) [23]. The demonstrated effects of tocopherol on markers of injury, inflammation, and damage in the uninjured lung remain unknown. Assessment of this data would help determine whether the addition of acetate via an ester linkage to tocopherol was important in the production of its toxic effects.
The effect of inhaled VEA without pyrolysis and its relative toxicity compared to inhalation after pyrolysis. All trials assessing the pulmonary effects of VEA alone utilized pyrolysis prior to exposure. If toxicity is seen in VEA after aerosolization alone, it may suggest alternative toxic mechanisms beyond ketene production. If a study assessed the effects of VEA via standard aerosol compared to vaporization, it may provide insights on whether pyrolysis of VEA is necessary to produce injury.
The temperatures reached when vaporizing mixtures of VEA at reported vaporization settings (volts, resistance, watts, amps, and wick saturation). This data would be relevant in determining if prior studies reached temperature capable of producing ketene. If they did not, it would provide insights into whether VEA possesses toxicity unrelated to ketene production. Future studies should also consider ensuring all electrical settings and wick saturation levels are reported.
The in vivo effect of VEA alone compared to VEA/THC. Only one study (Muthumalage et al.) potentially evaluated the effects of VEA with THC while assessing the effects of counterfeit vape cartridges. While these cartridges may have contained mixtures of THC, CBD, and VEA, or any number of other compounds, it is not known what specifically was inside the cartridges. These unknowns make the results difficult to interpret. Hydrogen bonding between VEA and THC has been discussed as a pathway to create a novel molecular compound. However, it is unknown what effect this compound would have on the lungs. Studies comparing VEA to VEA/THC would demonstrate whether this compound is relevant to toxicity.
The in vivo effects of inhaled tocopherol or VEA in humans. While in vitro effects of tocopherol and VEA on human lung cells have been demonstrated, there is no in vivo data of the pulmonary effects of these substances in humans.
To answer these questions, an ideal study might evaluate three mixtures via two different routes: consistent concentrations of tocopherol, VEA, and VEA/THC given via aerosol or vaporization. Comparison of markers of inflammation, capillary leak, and pulmonary damage among the six trial arms would provide insight into whether tocopherol alone leads to toxicity, if VEA/THC causes more toxicity than VEA alone, and if the route of pyrolysis enhances toxicity.
Limitations
This review is subject to several limitations. First, no assessment of the risk of bias or critical appraisal of evidence sources was performed. Second, due to the manual nature of article selection via title, abstract, and reference screening, it is possible articles may not have been included in the final analysis. Finally, due to the heterogeneous outcomes reported in studies, outcomes were grouped into categories, such as reduced pulmonary toxicity compared to toxin alone, by the authors of this paper interpreting the results of the primary authors. These interpretations have the potential for error in assessment and reporting.
Conclusion
Several questions still exist regarding the pulmonary toxicity of inhaled tocopherol or VEA. More studies are needed to determine whether tocopherol alone (i.e., without acetate) can cause pulmonary injury. Additionally, further studies of VEA should evaluate the impact that pyrolysis and co-administration with other compounds (like tetrahydrocannabinol) have on the ability to produce toxicity.
Author Contributions
RF was responsible for conception and design of the study. ES was responsible for literature searches and acquisition of data. RF and MS performed data collection and analysis. RF drafted the manuscript. Revision of manuscript for intellectual content was performed by RF (all), MS (all), and ES (methods).
Source of Funding
None
Compliance with Ethical Standards
Conflicts of Interest
None.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.CDC. Outbreak of lung injury associated with the use of e-cigarette, or vaping, products CDC.Gov 2020 [Available from: https://www.cdc.gov/tobacco/basic_information/e-cigarettes/severe-lung-disease.html. Accessed 14 Oct 2020.
- 2.Blount BC, Karwowski MP, Shields PG, et al. Vitamin E acetate in bronchoalveolar-lavage fluid associated with EVALI. N Engl J Med. 2020;382(8):697–705. doi: 10.1056/NEJMoa1916433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lee H. Vitamin E acetate as linactant in the pathophysiology of EVALI. Med Hypotheses. 2020;144:110182. doi: 10.1016/j.mehy.2020.110182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lal A, Mishra AK, Sahu KK. Vitamin E acetate and e-cigarette or vaping product-associated lung injury (EVALI): an update. Am J Med. 2020;133(5):e204. doi: 10.1016/j.amjmed.2019.11.005. [DOI] [PubMed] [Google Scholar]
- 5.Lanzarotta A, Falconer TM, Flurer R, Wilson RA. Hydrogen bonding between tetrahydrocannabinol and vitamin E acetate in unvaped, aerosolized, and condensed aerosol e-liquids. Anal Chem. 2020;92(3):2374–2378. doi: 10.1021/acs.analchem.9b05536. [DOI] [PubMed] [Google Scholar]
- 6.Feldman R, Meiman J, Stanton M, Gummin DD. Culprit or correlate? An application of the Bradford Hill Criteria to vitamin E acetate. Arch Toxicol. 2020;94(6):2249–2254. doi: 10.1007/s00204-020-02770-x. [DOI] [PubMed] [Google Scholar]
- 7.Tricco AC, Lillie E, Zarin W, O’Brien KK, Colquhoun H, Levac D, et al. PRISMA extension for scoping reviews (PRISMA-ScR): checklist and explanation. Ann Intern Med. 2018;169(7):467–473. doi: 10.7326/M18-0850. [DOI] [PubMed] [Google Scholar]
- 8.Wu D, O’Shea DP. Potential for release of pulmonary toxic ketene from vaping pyrolysis of vitamin E acetate. Proc Natl Acad Sci U S A. 2020;117(12):6349–6355. doi: 10.1073/pnas.1920925117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Skovdal SM, Christiansen SH, Johansen KS, Viborg O, Bruun NH, Jensen-Fangel S, Holm IE, Vorup-Jensen T, Petersen E. Inhaled nebulized glatiramer acetate against Gram-negative bacteria is not associated with adverse pulmonary reactions in healthy, young adult female pigs. PLoS One. 2019;14(10):e0223647. doi: 10.1371/journal.pone.0223647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Suntres ZE, Hepworth SR, Shek PN. Pulmonary uptake of liposome-associated α-tocopherol following intratracheal instillation in rats. J Pharm Pharmacol. 1993;45(6):514–520. doi: 10.1111/j.2042-7158.1993.tb05590.x. [DOI] [PubMed] [Google Scholar]
- 11.Suntres ZE, Shek PN. Incorporation of alpha-tocopherol in liposomes promotes the retention of liposome-encapsulated glutathione in the rat lung. J Pharm Pharmacol. 1994;46(1):23–28. doi: 10.1111/j.2042-7158.1994.tb03714.x. [DOI] [PubMed] [Google Scholar]
- 12.Yamamoto Y, Enkhbaatar P, Sousse LE, Sakurai H, Rehberg SW, Asmussen S, Kraft ER, Wright CL, Bartha E, Cox RA, Hawkins HK, Traber LD, Traber MG, Szabo C, Herndon DN, Traber DL. Nebulization with gamma-tocopherol ameliorates acute lung injury after burn and smoke inhalation in the ovine model. Shock. 2012;37(4):408–414. doi: 10.1097/SHK.0b013e3182459482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yamamoto Y, Sousse LE, Enkhbaatar P, Kraft ER, Deyo DJ, Wright CL, et al. gamma-Tocopherol nebulization decreases oxidative stress, arginase activity, and collagen deposition after burn and smoke inhalation in the ovine model. Shock. 2012;38(6):671–676. doi: 10.1097/SHK.0b013e3182758759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Suntres ZE, Hepworth SR, Shek PN. Protective effect of liposome-associated alpha-tocopherol against paraquat-induced acute lung toxicity. Biochem Pharmacol. 1992;44(9):1811–1818. doi: 10.1016/0006-2952(92)90076-U. [DOI] [PubMed] [Google Scholar]
- 15.Suntres ZE, Shek PN. Intratracheally administered liposomal alpha-tocopherol protects the lung against long-term toxic effects of paraquat. Biomed Environ Sci. 1995;8(4):289–300. [PubMed] [Google Scholar]
- 16.Suntres ZE, Shek PN. Prevention of phorbol myristate acetate-induced acute lung injury by alpha-tocopherol liposomes. J Drug Target. 1995;3(3):201–208. doi: 10.3109/10611869509015946. [DOI] [PubMed] [Google Scholar]
- 17.Suntres ZE, Shek PN. Liposomal alpha-tocopherol alleviates the progression of paraquat-induced lung damage. J Drug Target. 1995;2(6):493–500. doi: 10.3109/10611869509015919. [DOI] [PubMed] [Google Scholar]
- 18.Suntres ZE, Shek PN. Alleviation of paraquat-induced lung injury by pretreatment with bifunctional liposomes containing alpha-tocopherol and glutathione. Biochem Pharmacol. 1996;52(10):1515–1520. doi: 10.1016/S0006-2952(96)89626-2. [DOI] [PubMed] [Google Scholar]
- 19.Morita N, Traber MG, Enkhbaatar P, Westphal M, Murakami K, Leonard SW, Cox RA, Hawkins HK, Herndon D, Traber LD, Traber DL. Aerosolized alpha-tocopherol ameliorates acute lung injury following combined burn and smoke inhalation injury in sheep. Shock. 2006;25(3):277–282. doi: 10.1097/01.shk.0000208805.23182.a7. [DOI] [PubMed] [Google Scholar]
- 20.Hamahata A, Enkhbaatar P, Kraft ER, Lange M, Leonard SW, Traber MG, Cox RA, Schmalstieg FC, Hawkins HK, Whorton EB, Horvath EM, Szabo C, Traber LD, Herndon DN, Traber DL. gamma-Tocopherol nebulization by a lipid aerosolization device improves pulmonary function in sheep with burn and smoke inhalation injury. Free Radic Biol Med. 2008;45(4):425–433. doi: 10.1016/j.freeradbiomed.2008.04.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hybertson BM, Leff JA, Beehler CJ, Barry PC, Repine JE. Effect of vitamin E deficiency and supercritical fluid aerosolized vitamin E supplementation on interleukin-1-induced oxidative lung injury in rats. Free Radic Biol Med. 1995;18(3):537–542. doi: 10.1016/0891-5849(94)00180-R. [DOI] [PubMed] [Google Scholar]
- 22.Hybertson BM, Kitlowski RP, Jepson EK, Repine JE. Supercritical fluid-aerosolized vitamin E pretreatment decreases leak in isolated oxidant-perfused rat lungs. J Appl Physiol (1985) 1998;84(1):263–268. doi: 10.1152/jappl.1998.84.1.263. [DOI] [PubMed] [Google Scholar]
- 23.Hybertson BM, Chung JH, Fini MA, Lee YM, Allard JD, Hansen BN, Cho OJ, Shibao GN, Repine JE. Aerosol-administered alpha-tocopherol attenuates lung inflammation in rats given lipopolysaccharide intratracheally. Exp Lung Res. 2005;31(3):283–294. doi: 10.1080/01902140590918560. [DOI] [PubMed] [Google Scholar]
- 24.Bhat TA, Kalathil SG, Bogner PN, Blount BC, Goniewicz ML, Thanavala YM. An animal model of inhaled vitamin E acetate and EVALI-like lung injury. N Engl J Med. 2020;382(12):1175–1177. doi: 10.1056/NEJMc2000231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Matsumoto S, Fang X, Traber MG, Jones KD, Langelier C, Hayakawa Serpa P, et al. Dose-dependent pulmonary toxicity of aerosolized vitamin E acetate. Am J Respir Cell Mol Biol. 2020;63(6):748–757. doi: 10.1165/rcmb.2020-0209OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Muthumalage T, Lucas JH, Wang Q, Lamb T, McGraw MD, Rahman I. Pulmonary toxicity and inflammatory response of e-cigarettes containing medium-chain triglyceride oil and vitamin E acetate: implications in the pathogenesis of EVALI but independent of SARS-COV-2 COVID-19 related proteins. Toxics. 2020;8(3):46. doi: 10.3390/toxics8030046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Muthumalage T, Friedman MR, McGraw MD, Ginsberg G, Friedman AE, Rahman I. Chemical constituents involved in E-cigarette, or vaping product use-associated lung injury (EVALI) Toxics. 2020;8(2):25. doi: 10.3390/toxics8020025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Reddanna P, Rao MK, Reddy CC. Inhibition of 5-lipoxygenase by vitamin E. FEBS Lett. 1985;193(1):39–43. doi: 10.1016/0014-5793(85)80075-2. [DOI] [PubMed] [Google Scholar]
- 29.Wu D, O’Shea DF. Potential for release of pulmonary toxic ketene from vaping pyrolysis of vitamin E acetate. Proc Natl Acad Sci U S A. 2020;117(12):6349–6355. doi: 10.1073/pnas.1920925117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Narimani M, da Silva G. Does ‘dry hit’ vaping of vitamin E acetate contribute to EVALI? Simulating toxic ketene formation during e-cigarette use. PLoS One. 2020;15(9):e0238140. doi: 10.1371/journal.pone.0238140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chen W, Wang P, Ito K, Fowles J, Shusterman D, Jaques PA, Kumagai K. Measurement of heating coil temperature for e-cigarettes with a “top-coil” clearomizer. PLoS One. 2018;13(4):e0195925. doi: 10.1371/journal.pone.0195925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kamal MA, Raghunathan VA. Modulated phases of phospholipid bilayers induced by tocopherols. Biochim Biophys Acta. 2012;1818(11):2486–2493. doi: 10.1016/j.bbamem.2012.06.016. [DOI] [PubMed] [Google Scholar]
- 33.Massey JB, She HS, Pownall HJ. Interaction of vitamin E with saturated phospholipid bilayers. Biochem Biophys Res Commun. 1982;106(3):842–847. doi: 10.1016/0006-291X(82)91787-9. [DOI] [PubMed] [Google Scholar]
- 34.DiPasquale M, Gbadamosi O, Nguyen MHL, Castillo SR, Rickeard BW, Kelley EG, Nagao M, Marquardt D. A Mechanical mechanism for vitamin E acetate in e-cigarette/vaping-associated lung injury. Chem Res Toxicol. 2020;33(9):2432–2440. doi: 10.1021/acs.chemrestox.0c00212. [DOI] [PubMed] [Google Scholar]