Abstract
There are several studies investigating the effects of risperidone on autism, but many of these studies are contradictory or inconclusive. This systematic review and meta-analysis investigated the effects of risperidone on five domains of the Aberrant Behaviour Checklist (ABC) scale on Autism Spectrum Disorder (ASD), as well as weight gain and waist circumference. The protocol for the present systematic review and meta-analysis was registered on the International Prospective Register of Systematic Reviews (PROSPERO). For this study, we analysed articles (2,459), selecting them according to the PICOS strategy (Population, Intervention, Comparison, Outcome, Study design). Although risperidone is effective for the treatment of lethargy and inadequate speech, concerns about the association between weight gain, waist circumference and risperidone require a need for evaluation of the risk-benefit ratio in its use. There was a significant association between weight gain, waist circumference and risperidone. In conclusion, it was possible to suggest the efficacy of risperidone for the treatment of lethargy and inadequate speech. Finally, we emphasize that the risk-benefit in its use should be evaluated (Protocol number CRD42019122316).
Keywords: Weight gain, waist circumference, aberrant behavior checklist, autism spectrum disorder, risperidone, systematic review
1. Introduction
Characterised as a neuronal disorder that appears in early childhood, Autism Spectrum Disorder (ASD) compromises some aspects of the development of sociability and language in children and adolescents. To these, we can add restricted and repetitive patterns of behaviour [1, 2], as well as they can be associated with other comorbidities, including anxiety, intellectual disability, epilepsy, aggression, sleep disorders and gastrointestinal problems [3]. According to the current Diagnostic and Statistical Manual of Mental Disorders (DSM-5) definition, the term ASD encompasses all previous classifications of autism [Autistic Disorder, Asperger Syndrome, Disintegrative Childhood Disorder and Invasive Developmental Disorder - Not Otherwise Specified (PDD-NOS)] [4].
ASD has complex aetiology, which is associated with genetics, environment and neurobiology. Several diagnoses were prescribed with the help of questionnaires that describe clinical pictures of patients with particular characteristics. Behaviour can be assessed by behavioural scales such as the Aberrant Behaviour Checklist (ABC scale) [5, 6]. ABC scale was originally developed to evaluate the efficacy of the psychotropic medication and has been used in behavioural and psychiatric research on children and adults because of its high reliability and validity. The ABC scale is sensitive to both pharmacological and psychosocial treatment effects, and can differentiate between behavioral phenotypes in people with neurodevelopmental disorders [7].
Although little is known about the multiple factors involved in the treatment of ASD, the drugs used are always directed at symptomatic treatment. Several classes are prescribed, especially antipsychotics; however, there are still ongoing discussions about their effectiveness and side effects in ASD [8].
Risperidone is a Second Generation Antipsychotics (SGA), which was initially developed for the treatment of schizophrenia. Since 2006, its use has been approved by the Food and Drug Administration (FDA) for the treatment of irritability associated with autistic disorder. Risperidone is intended to reduce symptoms, such as aggression, and rapid mood swings. Studies report a significant improvement in symptoms associated with autism when risperidone is administered as compared to a placebo. It was possible to observe an improvement of 43% in the mean change in irritability [9-11].
After more than a decade, the adverse effects of risperidone in patients with ASD are still unclear. Its association with the weight gain and development of metabolic syndrome has been well discussed in the scientific world, leading to a fear of its use [12]. Obesity is a common disorder and is associated with several medical complications that may manifest in insulin resistance and metabolic syndrome [12-14]. In addition, inadequate weight gain and metabolic disturbances may lead to Type 2 Diabetes Mellitus [15-17].
There are several studies investigating the efficacy of risperidone, which constitutes a considerable body of evidence to determine its effectiveness in ASD. However, many of these studies are contradictory or inconclusive, especially regarding its efficacy in the treatment of inadequate speech and lethargy [6, 10]. To answer these questions, some authors have performed systematic reviews and meta-analyses. Sharma and Shaw [2] addressed the efficacy of risperidone in treating maladjusted behaviours, such as irritability and aggressiveness. Almandil et al., [18] evaluated weight gain and other metabolic effects of Second General Antipsychotics (SGAs), and the authors observed significant weight gain due to the use of SGAs. However, there were limitations regarding the number and duration of studies. Sochocky and Milin [19] estimated the efficacy of SGAs, without drug-isolated sub-analysis, and showed no real efficacy of risperidone in the treatment of Asperger’s syndrome. Fung et al., [20] conducted a systematic review and meta-analysis of the use of antipsychotics in the treatment of autism, especially aggressiveness and irritability, not performing medication meta-analysis alone. Furthermore, this study used only the ABC scale total score [20]. Thus, a better understanding of the effects of risperidone on the individual with ASD is necessary, such as a joint evaluation of different outcomes and a review of the quality of existing literature. Some of these studies did not describe the quality analysis of the studies used or showed only poor quality. There was also no analysis of the risk of bias.
In this context, the objectives of this study were to perform a systematic review and meta-analysis of the efficacy of risperidone on ASD assessed by the ABC scale and on weight gain and waist circumference and also evaluate the quality and the risk of bias of the publications by means of standardised tools.
2. Methods
2.1. Search Strategy
The protocol for the present systematic review and meta-analysis was registered on the International Prospective Register of Systematic Reviews (PROSPERO; https://www.crd.york.ac.uk/PROSPERO, protocol number CRD42019122316). This study was conducted and reported following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) recommendations. We conducted a search for articles that evaluated the treatment of
risperidone in individuals diagnosed with ASD using the databases Scopus, PubMed and Web of Science without any restrictions of date, type of document or language. The dates of the search and the search criteria by databases are described in Supplementary Table 1 (1.9MB, pdf) . The last search was completed on 04 January 2019. All supplementary materials have been published on Mendeley Data (http://dx.doi.org/10.17632/ktjcf4m58t.2).
2.2. Selection of Relevant Articles
For the study selection, we used the PICOS strategy (Population, Intervention, Comparison, Outcome and Study design):
Population: Individuals with ASD, according to the current DSM-5 definition [4], including males and females of 2 to 17 years old.
Intervention: risperidone therapy.
Comparison: Baseline values were compared with those after risperidone treatment, and/or compared to the placebo group.
Outcome: Risperidone’s effect in each or all domains of ASD (irritability, inappropriate speech, hyperactivity, lethargy and stereotypical behaviour), was evaluated by the ABC scale, as well as for weight gain and waist circumference; and study design: before-and-after studies regardless of the existence of comparison groups. All reviews, case reports, editorials and studies published in summary form and articles that were inaccessible, even after attempts to contact the corresponding author, were excluded.
2.3. Selection of Studies and Data Extraction
Duplicate studies were excluded using reference managers (Mendeley® and JabRef). The eligibility process was conducted in two separate stages. First, three researchers (AMP, BJMS and BCA) independently screened all non-duplicate articles and excluded non-relevant articles based on the title or abstract. A final list was agreed upon, with discrepancies resolved by a fourth researcher (JMDA). Second, full-text versions of the studies selected from stage 1 screening were downloaded and independently assessed for eligibility by the two researchers. Any discrepancies were resolved by a third researcher.
2.4. Risk of Bias of Included Studies
The quality of the studies was evaluated using the Jadad scale [21]. Articles with a Jadad score lower than 3 were classified as low quality, while those higher than 3 were classified as high quality [21]. In addition, the risk of bias for each reference included in the meta-analysis was assessed using the Cochrane Risk of Bias Tool [22]. Risk of bias domains included selection bias (random sequence generation, allocation concealment), performance bias (blinding of participants and personnel), detection bias (blinding of outcome assessment), attrition bias (incomplete outcome data), reporting bias (selective reporting) and other biases. The overall bias risk score was the lowest score for any of the criteria
(if any item was scored as high risk of bias, the whole study was scored as high risk of bias; if all items were scored as low risk, the whole study was classified as low risk).
2.5. Statistical Analyses
Analyses were conducted as per protocol data [23]. All analyses were performed with Comprehensive Meta-analysis software, version 2.0.057 (Biostat, Englewood, NJ, USA).
Independent meta-analyses were performed for each one of the five sub-scales of the ABC scale, as well as for weight gain and waist circumference. The results of each of the primary studies were described by subtracting the mean after the treatment from the mean before treatment. Differences in the means of individual studies were pooled to produce the Standard Paired mean difference. In addition, in comparison group studies, differences between pre and post treatment means in the intervention and placebo groups were combined to produce the standardised mean difference. In both cases, the random effects model was applied; except when the number of studies was less than four, in which case the fixed effects model was employed. For each variable submitted to meta-analysis, the studies were separated according to the follow-up time of participants, into short (up to 8 weeks) and long-term follow-up (more than 8 weeks). Evaluations were performed separately for each group.
The heterogeneity in the primary results was analysed using the Q test and the I2 statistic, which describes the percentage of real dispersion in effect-sizes which is not due to the random error. The possibility of the occurrence of publication bias was analysed through Egger’s tests. In all procedures, the significance level was 5%.
3. Results
3.1. Search Strategy
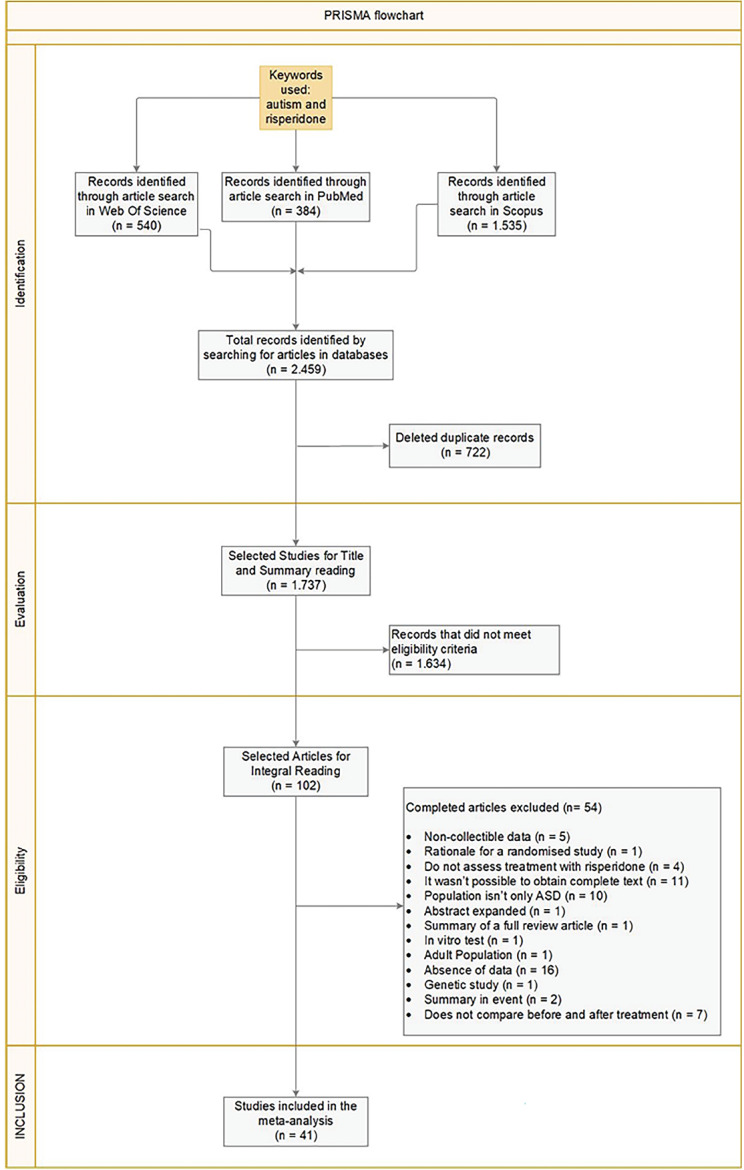
The initial research identified 2,459 articles and 41 were included in the meta-analysis. Fig. 1 reports the PRISMA flowchart detailing the screening process, and Supplementary Table 2 (1.9MB, pdf) reports the studies discarded after reading the full text, together with the specific reasons for exclusion.
Fig. (1).
PRISMA Flowchart reporting the study screening process. ABC Scale: Aberrant Behaviour Checklist scale.
3.2. Characteristics of Studies Included in the Meta-Analysis
Of the 41 studies included in the meta-analysis, only two were recorded on ClinicalTrials.gov [24, 25]. Twenty-four had an open-label design, 2 were cross-sectional, 1 was performed by chart review and the other studies (fourteen – 14) were experimental double-blind studies. In most of the studies, the follow-up time ranged from 8 to 24 weeks, but one study had a follow up time of 72 weeks [26] and another, 84 weeks (Aman et al. 2015). The dosage used was established by weight ranges (mg/day) or dose by weight (mg/Kg/day). For all studies, the ABC scale (baseline and endpoint or mean change), weight gain and waist circumference (baseline and endpoint or mean change) were available. The percentage of males in our meta-analysis was 78.28%. The mean age in years was 8.13±2.68 and the range age was 2 to 17 years. The characteristics of the studies are described in Table 1.
Table 1.
Characteristics of the studies included in this systematic review and meta-analysis.
| Study |
Length
(Weeks) |
Diagnostic Criteria | Population Size | Sample Size |
Age1
(Mean±SD) |
Age Range1 |
Men
(%) |
Quality2 | Risk of Bias3 |
|---|---|---|---|---|---|---|---|---|---|
| Rezaei et al., 2018 [28] | 12 and 24 | DSM-5 | 34 | 17 | 12.41±2.45 | — | 65 | High | Low |
| Firouzabadi et al., 2017 [29] | 8 | DSM-5 | 56 | 56 | 6.9±1.1 | 2.5 a 14 | 71.4 | Low | Some Concerns |
| Nikvarz et al., 2017 [30] | 4 and 8 | DSM-IV | 34 | 16 | 6.56±3.51 | 4 a 17 | 66.7 | High | Some Concerns |
| Lamberti et al., 2016 [31] | 12 and 24 | DSM-5 | 44 | 22 | 7.9±2.3 | 6 a 13 | 79.54 | Low | High |
| Scahill et al., 2016 [12] | 8, 16 and 24 | DSM-IV | 97 | 41 | 6.9±2.35 | 4 a 13 | 84.67 | Low | Low |
| Yoon et al., 2016 [32] | — | — | 202 | 59 | 8.88±3.70 | 2 a 20 | 83.05 | High | High |
| Aman et al., 2015 [27] | 21 | DSM-IV | 84 | 55 | 8.82±2.69 | — | 79.8 | High | High |
| Calarge et al., 2015 [25] | 24 | DSM-IV | 124 | 49 | 7.7±2.4 | 4 a 13 | 85 | High | Some Concerns |
| 72 | DSM-IV | 96 | 96 | 13.2±2.7 | 7 a 17 | 92 | |||
| Boon-Yasidhi et al., 2014 [29] | — | DSM-IV | 45 | 45 | 8.15±2.98 | 2 a 15 | 77.77 | High | Some Concerns |
| Choi et al., 2014 [33] | 8 | DSM-IV | 45 | 45 | 9.5±4.38 | 3 a 15 | 77.77 | Low | High |
| Ghaeli et al., 2014 [34] | 8 | DSM-IV | 15 | 15 | 6.5±3.5 | 4 a 15 | 66.66 | Low | High |
| Ghanizadeh et al., 2014 [24] | 4 and 8 | DSM-IV | 59 | 30 | 9.5±4.6 | 5 a 15 | 76.7 | High | Low |
| Wink et al., 2014 [35] | — | DSM-IV | 142 | 72 | — | 6 a 15 | 81.69 | High | High |
| Kent et al., 2013 [36] | 6 | ADI-R | 77 | 50 | 9±3.1 | 5 a 17 | 88 | High | Low |
| Kent et al., 2013[37] | 24 | DSM-IV ADI-R | 56 | 56 | 9.2±3.1 | 5 a 17 | 89 | High | Some Concerns |
| Arnold et al., 2012 [26] | 24 and 72 | — | 87 | 36 | 7.54±2.33 | 4 a 13 | 83 | High | High |
| Wei et al., 2011 [38] | 8 | — | 40 | 40 | — | 5 a 12 | 92.5 | High | Low |
| Correia et al., 2010 [39] | 1, 3, 6, 12 | ADI-R e ADOS | 45 | 45 | 8.67±4.30 | até 18 | 75.6 | High | High |
| Desousa, 2010 [40] | 16 | ADI-R e ADOS | 40 | 20 | — | 5 a 16 | — | Low | Some Concerns |
| Hoekstra et al., 2010 [41] | 8 | ADI-R | 32 | 32 | 8.74±2.83 | 5 a 16 | 87.5 | High | High |
| Capone et al., 2008 [42] | 240 | DSM-IV | 23 | 23 | 7.8±2.6 | 3 a 13 | 86.95 | Low | High |
| Gencer et al., 2008 [43] | 24 | DSM-IV | 28 | 13 | 10.2±2.8 | 7 a 17 | 32.14 | High | Some Concerns |
| Miral et al., 2008 [44] | 12 | DSM-IV | 30 | 15 | 10.0±2.7 | 7 a 17 | 80 | High | Low |
| Anderson et al., 2007 [45] | 8, 6 e 88 | DSM-IV | 101 | 49 | 8.8±2.7 | 5 a 17 | 81.18 | High | High |
| Pandina et al., 2007 [46] | 8 | DSM-IV | 55 | 27 | 7.4±2.4 | 5 a 12 | 78.78 | High | Low |
| Canitano, 2006 [47] | 24 | DSM-IV | 11 | 11 | 8.7±2.2 | — | 63.63 | Low | High |
| Lindsay et al., 2006 [48] | 8 | DSM-IV | 20 | 20 | — | — | — | Low | Low |
| Luby et al., 2006 [49] | 24 | DSM-IV | 23 | 11 | 4.1±0.90 | 2.5 a 6.0 | 73.91 | Low | Low |
| Nagaraj et al., 2006 [50] | 24 | DSM-IV | 39 | 19 | 5.03±1.70 | 2 a 9 | 87.17 | Low | Low |
| Aman et al., 2005 [51] | 4, 8, 12 and 16 | DSM-IV | 101 | 49 | 8.8±2.7 | 5 a 17 | 81.18 | High | High |
| McCracken, 2005 [52] | 16 | DSM-IV | 63 | 63 | 8.6±2.8 | 5 a 17 | 77.8 | Low | Low |
| Troost et al., 2005 [53] | 24 | DSM-IV | 24 | 12 | 4±3.4 | 5 a 17 | 91.6 | Low | Low |
| Gagliano et al., 2004 [54] | 12 and 24 | DSM-IV | 20 | 20 | 6.0±2.4 | 3 a 10 | 70 | High | High |
| Martín et al., 2004 [55] | 24 | — | 63 | 63 | 8.6±2.9 | 5 a 17 | — | Low | High |
| Shea et al., 2004 [56] | 8 | DSM-IV | 79 | 25] | 7.6±2.3 | 5 a 12 | 77.21 | High | Low |
| Arnold et al., 2003 [57] | 4 and 8 | — | 87 | 44 | 8.8 | 5 a 17 | — | High | High |
| McCracken et al., 2002 [58] | 8 | DSM IV | 101 | 49 | 8.8±2.7 | 5 a 17 | 81.18 | High | Low |
| Masi et al., 2001 [59] | 16 | — | 24 | 24 | 4.6±0.66 | 3.6 a 6.6 | 87.5 | Low | High |
| Masi et al., 2001 [60] | 16 | DSM-IV | 10 | 10 | 4.9±0.8 | 3.9 a 6.5 | 70 | Low | High |
| Zuddas et al., 2000 [61] | 1, 3 and 6 | DSM-IV | 11 | 11 | 12.3±3.8 | 7 a 17 | 72.72 | Low | High |
| Nicolson et al., 1998 [62] | 12 | DSM-IV | 10 | 10 | — | 4.5 a 10.8 | — | Low | High |
DSM – Diagnostic and Statistical Manual of Mental Disorders; ADI-R – Autistic Diagnostic Interview Revised; ADOS – Autism Diagnostic Observation Schedule. 1Age in years. 2The Quality of study was evaluated with Jadad Score. 3Risk of Bias was evaluated by Cochrane Risk of Bias Tool.
3.3. Results of the Meta-Analysis
3.3.1. Effects of Risperidone on Autistic Behaviour
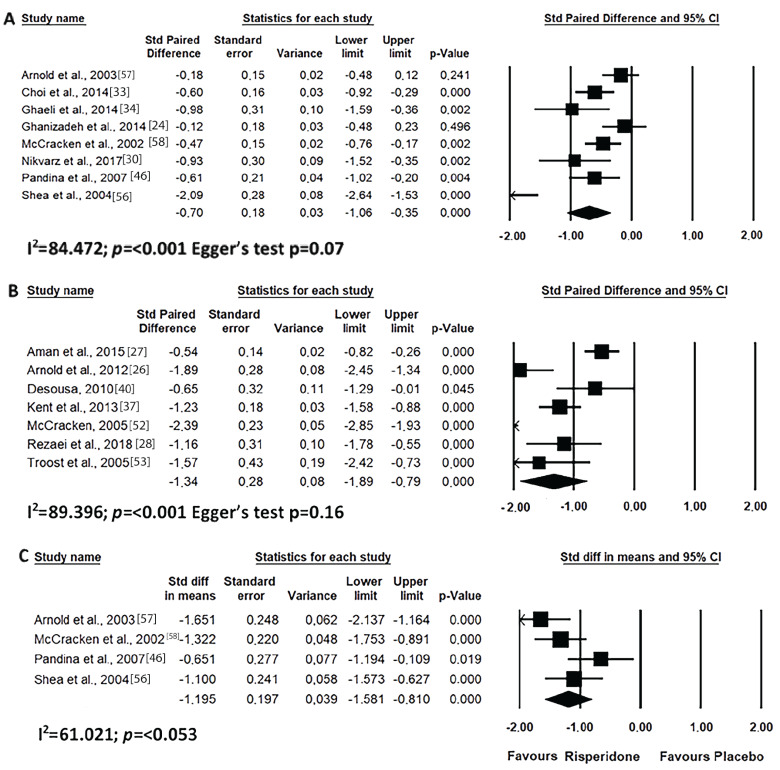
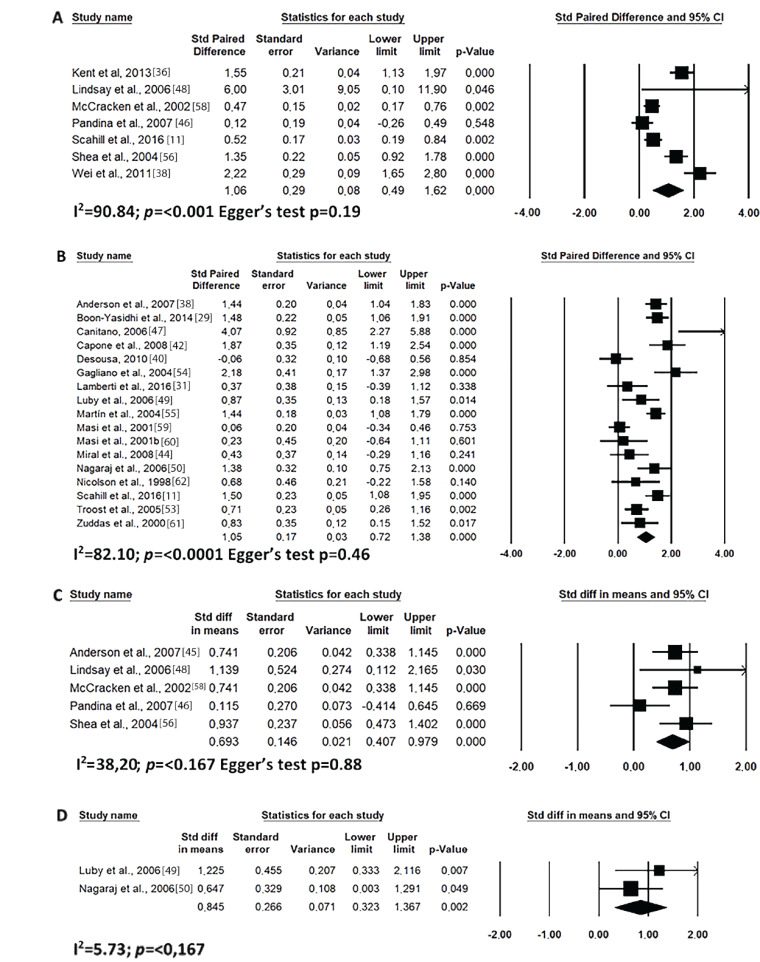
In this study, the studies were grouped into short- (up to 8 weeks) and long- (after 8 weeks) times of use. As shown in Fig. 2, risperidone was effective in improving the overall clinical condition of ABC scale of the autistic individual both for short and long-term uses. This was also demonstrated for treating irritability, although in this case the reduction was greater with long-term use (Fig. 3). In addition, the use of risperidone over short time period reduced irritability, with a large effect over placebo (Fig. 3C).
Fig. (2).
Results of the meta-analysis of the evaluation of ABC scale total score on treatment with risperidone: (A) short-term (until up 8 weeks); (B) long-term (after 8 weeks). Confidence Interval (CI), Heterogeneity (I2) and heterogeneity’s p-value (p).
Fig. (3).
Results of the meta-analysis of the evaluation of irritability in the ABC scale on treatment with risperidone: (A) short-term (until up 8 weeks); (B) long-term (after 8 weeks); (C) versus placebo. Confidence Interval (CI), Heterogeneity (I2) and heterogeneity’s p-value (p).
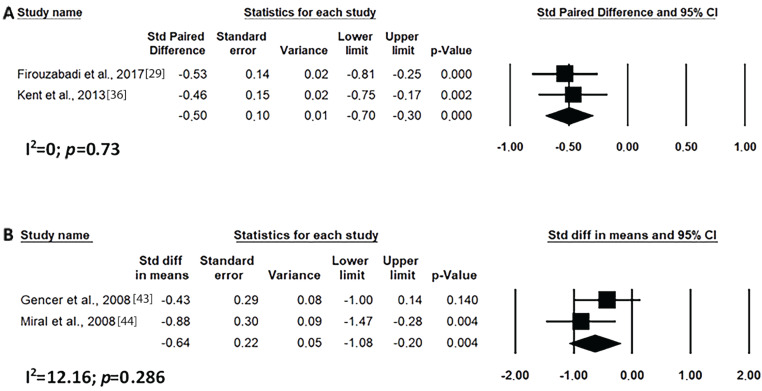
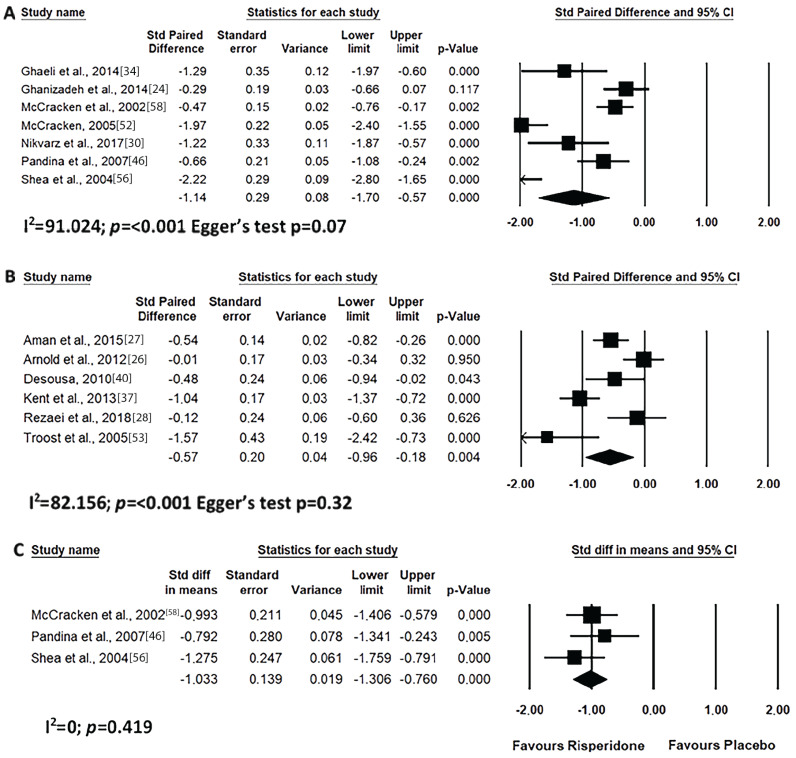
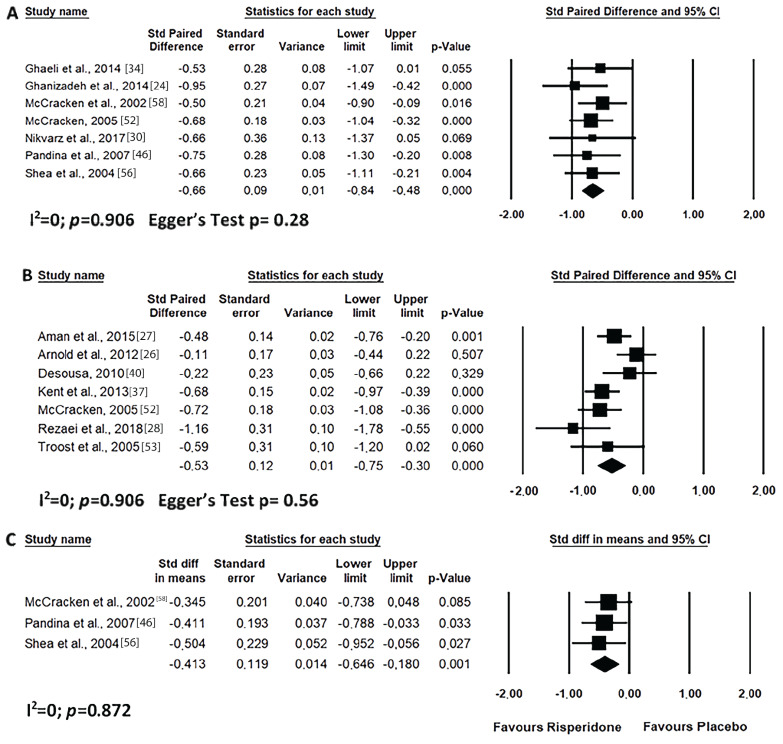
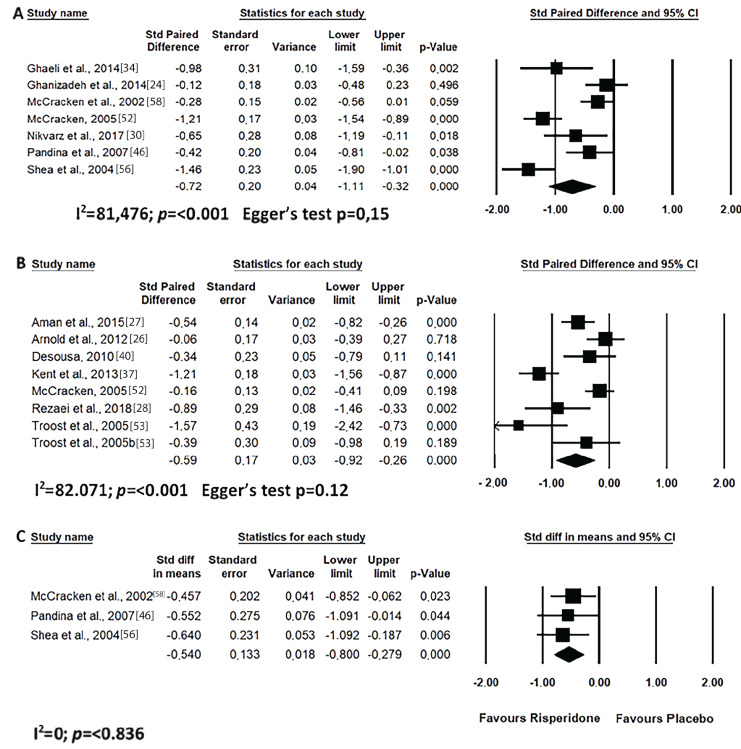
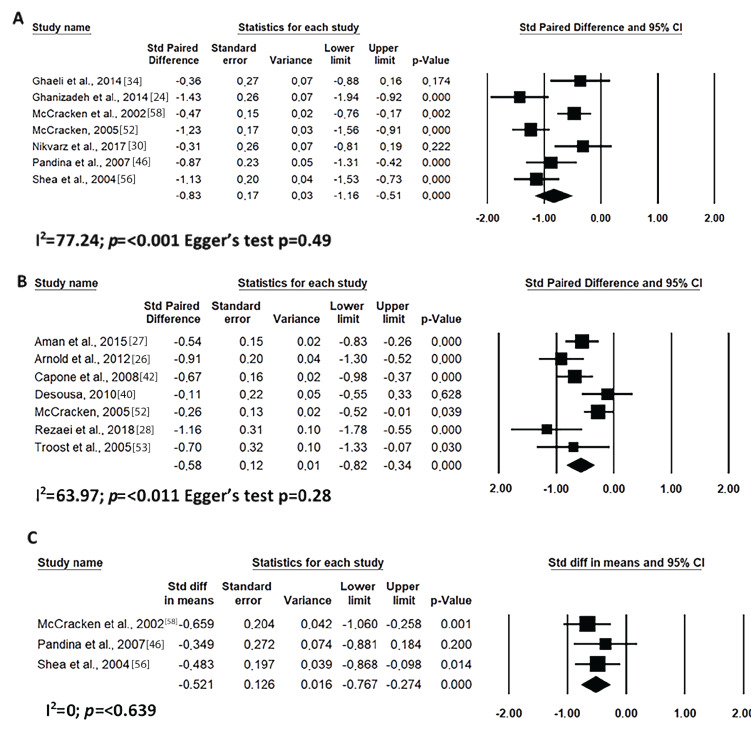
Short-term risperidone treatment decreased hyperactivity, inappropriate speech, lethargy and stereotypic behaviour from ASD individuals as compared to baseline values (Figs. 4A, 5A, 6A and 7A) or to placebo use (Figs. 4C, 5C, 6C and 7C). Similar results were found for long-term risperidone treatment (Figs. 4B, 5B, 6B and 7B). The short-term treatment was more effective in reducing inappropriate speech and lethargy than the long-term treatment. On the other hand, for stereotypic behaviour, the greatest effect was achieved in upto eight-week risperidone treatment.
Fig. (4).
Results of the meta-analysis of the evaluation of hyperactivity in the ABC scale on treatment with risperidone: (A) short-term (until up 8 weeks); (B) long-term (after 8 weeks); (C) versus placebo. Confidence Interval (CI), Heterogeneity (I2) and heterogeneity’s p-value (p).
Fig. (5).
Results of the meta-analysis of the evaluation of inappropriate speech in the ABC scale on treatment with risperidone: (A) short-term (until up 8 weeks); (B) long-term (after 8 weeks); (C) versus placebo. Confidence Interval (CI), Heterogeneity (I2) and heterogeneity’s p-value (p).
Fig. (6).
Results of the meta-analysis of the evaluation of lethargy (social withdrawal) in the ABC scale on treatment with risperidone: (A) short-term (until up 8 weeks); (B) long-term (after 8 weeks); (C) versus placebo. Confidence Interval (CI), Heterogeneity (I2) and heterogeneity’s p-value (p).
Fig. (7).
Results of the meta-analysis of the evaluation of stereotypic behaviour in the ABC scale on treatment with risperidone: (A) short-term (until up 8 weeks); (B) long-term (after 8 weeks); (C) versus placebo. Confidence Interval (CI), Heterogeneity (I2) and heterogeneity’s p-value (p).
3.3.2. Effects on Weight Gain
The effects of risperidone on weight gain in autistic individuals are described in Fig. 8. In the meta-analysis of weight gain for long-term risperidone use, contradictory data was observed among the studies found in the literature, since the value was very close to zero. It can be observed that regardless of the time of risperidone use, there was an increase in patients’ weight compared to baseline values (Fig. 8A and 8B). Similar results were found when the effect of short- and long-term treatment with risperidone on the weight of patients was compared to placebo (Fig. 8C and 8D). In addition, it was possible to associate weight gain with an increased waist circumference over long periods of use (Fig. 9).
Fig. (8).
Results of the meta-analysis of the evaluation of weight gain on treatment with risperidone: (A) short-term (until up 8 weeks); (B) long-term (after 8 weeks); (C) versus placebo in short-term (until up 8 weeks); (D) versus placebo in long-term (after 8 weeks). Confidence Interval (CI), Heterogeneity (I2) and heterogeneity’s p-value (p).
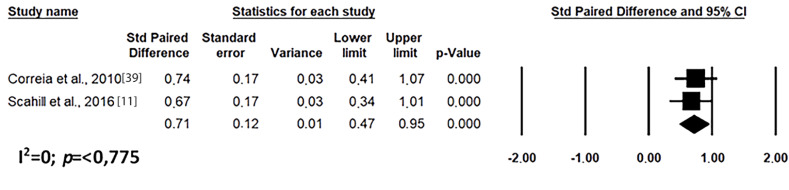
Fig. (9).
Results of the meta-analysis of the evaluation of waist circumference on treatment with risperidone in long-term (after 8 weeks). Confidence Interval (CI), Heterogeneity (I2) and heterogeneity’s p-value (p).
3.3.3. Quality of the Reports (Jadad Score)
The score of the quality of evidence for the 41 articles used in this study is presented in Table 1, with individual analysis by Jadad. The criteria used in the evaluation of these articles were mainly about randomisation and double-blind studies using the Jadad scale, and the data are presented in Supplementary Table 3 (1.9MB, pdf) . One article [24] had the lowest score (-1) in the Jadad score and 4 presented with a score of zero (0), while 12 presented with the maximum score (5). Among the included studies, 19 presented with high quality, because their Jadad scores were greater than 3 [21].
3.3.4. Risk of Bias
As shown in Table 1, 19 studies were, overall, considered to have a high risk of bias, while 7 presented an unclear risk. The individual analysis by the Cochrane Risk of Bias Tool is shown in Supplementary Fig. 1 (1.9MB, pdf) . All articles that were at
a high risk of bias were due to the randomisation process (Supplementary Fig. 2 (1.9MB, pdf) ). The quantitative evaluation of publication bias, as measured by Egger's intercept (Egger's test), was not significant in any meta-analysis performed.
4. Discussion
The mechanism of action of risperidone is related to its affinity with dopamine D2 receptor and the 5-HT2A serotonin receptor, in addition to binding to α1 e α2 adrenergic and to H1-histaminergic receptors. Risperidone binds by blocking D2 e 5-HT2A receptors and is also an antagonist of adrenergic α1 e α2 and histamine H1 receptors. The active metabolite of risperidone, 9-OH-risperidone (called paliperidone), has the advantage of binding to a lesser extent to H1 histaminergic receptors. These dopamine and serotonin receptors antagonisms are believed to be responsible for the beneficial effects on ASD and for reducing extrapyramidal symptoms. The effects of risperidone on aggression, irritability stereotypes, tantrums and restlessness can be attributed to dopamine antagonism. The effects on communication skills, restricted activity patterns and the ability to respond emotionally and socially (such as detachment, inattention, relationships or eye contact) can be considered as a result of serotonin antagonism [50, 63-65].
Although it has been suggested that appetite stimulation is probably the main mechanism of body weight gain induced by antipsychotic drugs [66], female juvenile rats treated with risperidone also have a decrease in the locomotor activity, in addition to increase in the food intake and body weight gain [67]. These effects are accompanied by elevated mRNA expression of hypothalamic H1 receptor, neuropeptide Y (NPY) and agouti-related peptide (AgRP), without affecting the hypothalamic proopiomelanocortin (POMC) and cocaine- and amphetamine-regulated transcript (CART) mRNA expression [67]. Therefore, these results suggested that risperidone increases the appetite and body weight gain in juveniles via regulation of the hypothalamic H1 receptor, NPY and AgRP pathways, as well as by reducing activity [67]. A study suggests that the effect of antipsychotic drugs is strongly correlated with their affinity for H1 and alpha1 receptors [66]. In fact, H1 receptors knockout mice had hyperphagia, decreased expression of uncoupling protein-1 mRNA and gradually developed mature-onset obesity [68] and intracerebroventricular application of an H1 receptor agonist decreased the food intake, while hypothalamic H1 receptor antagonism increased the food intake [69, 70]. On the other hand, the role of α1 receptors on food intake is not clear, because pretreatment of rodents with phentolamine, a non seletive α-adrenergic antagonist, induces a decrease in the food intake induced by electrical stimulation of the lateral hypothalamic area [71]. Studies suggest that D2 and 5-HT2A receptors can also contribute to these changes in the energy homeostasis [72-75]. Striatal D2 receptor availability is reduced in very obese individuals in proportion to their body mass index [75]. Striatal D2 receptors are downregulated in obese rats, in addition, lentivirus-mediated knockdown of striatal D2 receptors rapidly accelerates the development of addiction-like reward deficits and the onset of compulsive-like food seeking in rats with extended access to palatable high-fat food [74]. In rats, it was demonstrated that 5HT2A, but surprisingly not 5HT2C, receptor is critical for weight-loss, anorexia and fat mass reduction induced by central GLP-1R activation [73]. Central 5HT2A receptors are also required for peripherally injected liraglutide to reduce feeding and weight [73].
Until the present study, there has been no systematic review or meta-analysis that addresses the ASD in individuals assessed on the five domains of the ABC scale. The ABC scale is one of the few scales developed to measure psychiatric symptoms and behavioural disorders exhibited by individuals in five domains: irritability, agitations, lethargy/social withdrawal, hyperactivity and inadequate speech [76].
The studies presented high values of heterogeneity for some variables; however, the direction of the associations was consistent for all variables of the ABC scale. Furthermore, the risk of publication bias (Egger’s test) was not significant (p>0.05), favouring the reliability of the data. In some variables, it was not possible to evaluate the publication bias due to the insufficient number of studies that evaluated the effect of risperidone in each autism domain. In addition, the included studies do not describe factors that may cause heterogeneity and cause bias. Thus, differences were attributed to the clinical and epidemiological specificities of ASD and to the different contexts in which the studies were performed [77, 78]. The heterogeneity may have been caused by the psychometric data of the included studies. According to Doebler et al. [79], in diagnostic meta-analyses, the sensitivities and specificities observed may vary between studies and there may be heterogeneities. Even in randomised trials for therapeutic intervention, there may be heterogeneity because the randomisation was not focused on the hypothesis in question, but on the intervention. Moreover, heterogeneity can be caused by the way the tests were evaluated [80].
In our meta-analysis, the general ABC indicated that prolonged use of risperidone is essential for decreasing the clinical signs of ASD [31, 81]. This data is of great importance for the medical field, since risperidone is prescribed, for the most part, for patients with aggression, self-mutilation and/or excessive irritation and not for treatment of stereotypy, inadequate speech or lethargy [10]. To date, there is no specific drug for ASD; however, risperidone could be this drug according to our observations. Although the improvement in all domains of ABC, including lethargy and speech development, gave rise to the improvement of the total score, irritability was the most apparent one.
The improvement in the total score of the ABC scale is related to an improvement of the individual in all domains evaluated, including lethargy and speech development. However, apparently, the domain that most contributed to the improvement of symptoms was irritability. The behaviour most associated with ASD, and generates more worries for relatives, is aggressiveness. In their daily lives, this behaviour can lead to a reduction of the quality of life, a greater level of stress and less availability of educational and social support. According to our meta-analysis, it is suggested that risperidone has greater efficacy in the treatment of irritability and hyperactivity, both in the short and long term. However, we emphasize that the risk-benefit in its use should be evaluated.
ASD has been known to present with several layers, with each individual presenting with a particular characteristic or group of characteristics. Among these variations, there is hyperactivity associated with autism. The restless and agitated child will have much less capacity for social interaction and eye contact, for example. Through our meta-analysis, it can be concluded that the use of risperidone for a short period of time generates a greater benefit for hyperactive behaviour. The literature has no consensus on the effectiveness of long-term treatment [26, 82], but our meta-analysis was able to demonstrate that risperidone is effective in treating hyperactivity for short and long time periods, as well as demonstrating a large effect as compared to placebo. Lamperti et al., [31] highlighted that aripiprazole is a more effective drug than risperidone in improving inattention in ASD patients, but concludes that risperidone acts more significantly on the hyperkinetic symptoms of Asperger’s Syndrome. Concomitantly, Ghanizadeh et al., [24] have shown that both aripiprazole and risperidone lower all ABC subscale scores, including irritability, agitation, lethargy, social withdrawal, stereotypical behaviour, hyperactivity, nonconformity and inadequate speech.
Of great clinical importance, two new outcomes are presented in this study. Firstly, risperidone was apparently efficacious for the treatment of inappropriate speech and lethargy in individuals with ASD. Secondly, it was possible to infer that risperidone benefits speech structure and development, as well as social interaction/lethargy for short and long-term treatment as compared to baseline, and for short-time treatment, as compared to placebo. Speech is a method of underpinning social interaction. ASD causes a deviation in the child's speech development, leading him/her to regress in the organisation and social intention of speech, or when he/she speaks, the child does not make it socially significant, not in accordance with the context, and can also present echolalia, inversions of jargon and pronouns. To date, there has been no consensus in the literature on the efficacy of risperidone for improving speech [26, 34, 40, 46, 53, 83]; however, this meta-analysis has shown that the use of risperidone leads to symptom improvement.
The data found here corroborate with those found by the study by Nikvarz et al. [30] where an improvement in the speech of children with ASD was observed following short-term treatment with risperidone. An improvement in communication over a short term of 4 weeks was also mentioned. Rezaei et al., [28] observed that risperidone reduces behavioural problems, but also suggested that there be behavioural interventions to further increase drug efficacy and the child’s readiness in the development of speech and language skills.
One of the most significant and worrying behavioural axes in ASD is stereotypy. Stereotyping is a behaviour characterised by repetitive actions and of great interest of the individual, without there being an objective or end purpose. Although some studies report ineffectiveness [40, 46, 84], risperidone is able to reduce short- and long-term ASD stereotypies. Although not a preponderant factor in clinical evaluations, lethargy always showed a decrease when risperidone was used [37, 53]. Our meta-analysis was in disagreement with the literature, as it demonstrated efficacy in lethargy symptoms of the ABC scale, independent of treatment time. Furthermore, there was no consensus on the efficacy of risperidone over placebo [83]. However, it has been possible to evaluate only the short time effect of risperidone compared to placebo, with the meta-analysis showing an effect in favour of risperidone. Pandina et al., [46] demonstrated significant improvements in the ABC “lethargy/social withdrawal” subscale after 8 weeks of risperidone treatment. In contrast, Ghaeli et al., [34] reported a significant improvement in social interactions, based on changes in the ABC “lethargy / social retraction” subscale, after 4 weeks, indicating the efficacy of risperidone in the treatment of autism [34]. These results are consistent with the results obtained by McCracken et al. [58], who demonstrated a significant reduction in the placebo-controlled risperidone assay in the stereotype, hyperactivity and irritability subscales of the ABC scale with 8 weeks of study.
Our meta-analysis revealed that risperidone has the ability to induce weight gain in individuals with ASD. This finding is consistent with previously published studies of risperidone use. In a 12-week prospective study evaluating weight gain in adolescents, the average weight gain was 3.9 kg from baseline to the endpoint [85]. Our data showed a greater weight gain than reported by Fung et al. [20] (3.78 kg vs 1.6 kg, respectively). Regarding the problem of differentiating the weight gain related to the growth of the medication effect, the average annual weight gain of the age group and the time of use must be taken into consideration.
Our study suggested that risperidone treatment increases waist circumference. Fat accumulation in the abdominal region has been described as the type of obesity that poses the greatest health risk to individuals. The incidence of diabetes, atherosclerosis, gout, urinary stones and sudden cardiac death is high in obese people, but one aspect of adiposity that draws attention is the regional distribution of body fat, which is often associated with the abdominal region [86].
The metabolic effects of risperidone should be considered when planning the treatment strategy for these patients. Baseline measurement of weight, height and waist circumference and any monitored changes should be conducted. It has been recommended that plasma glucose, lipid and prolactin be monitored and individualised follow-up conducted [87]. Strategies for managing drug-induced weight gain include therapeutic approaches such as lifestyle changes and pharmaceutical intervention. However, prescribing medications to overcome adverse effects should be avoided whenever possible.
Continuation of treatment with risperidone is essential for the improvement of the symptoms and quality of life of autistic patients. Troost et al., [53] observed that discontinuation of the drug causes a 60% increase in irritability scores on the ABC scale. Another study demonstrated that discontinuation caused an increase in the hyperactivity factor and irritability [84]. Furthermore, Zuddas et al., [61] observed that risperidone contributed to the improvement of sleep disorder in individuals and that the discontinuation of risperidone caused its return.
Risperidone has a large inter-individual variation in pharmacokinetic parameters, such as plasma drug concentration, oral clearances or drug half-life, which can be attributed to several factors, such as age, body weight, medication and genetic variations [88]. Pharmacogenetic studies have focused on the polymorphism of CYP enzymes, with CYP2D6 being the most studied. The CYP2D6 gene is extremely polymorphic with more than 90 known allele variants. The polymorphism of the CYP2D6 gene is responsible for the inter-individual and interracial differences in drug metabolism [89]. The genetic variability in the pharmacodynamics of individuals with identical concentrations of risperidone in plasma and tissue can still vary extensively in their responses. Genetic variants in molecular targets directly involved in the action of the drug may be associated with the response to the drug. Variants of these genes are more likely to have a small effect with several loci involved, each contributing to the phenotype to a small and variable degree. So far, most dopaminergic and serotonergic receptors have been studied as predictors of response to psychotropic drug therapy [90, 91]. We know that genetic factors can influence the efficacy and safety of risperidone, however, due to the protocol established for the search and selection of articles, it was not possible to perform a meta-analysis, considering the pharmacogenetics between inter-individuals and interracial individuals with autism.
Some methodological limitations exist with our analyses. These include the limited number of relevant studies in some domains of ABC scale and waist circumference. This highlights the need for larger and longer trials, using a control group to establish the efficacy of risperidone in improvement in all ASD domains, as well as the association of weight gain and waist circumference.
A limitation of this meta-analysis comes from the methodological limitations of the included studies; 46% of the included studies were assessed to have a high risk of bias in the quality analysis with the Risk of Bias Cochrane Tool, particularly in the domains of randomisation and blinding. We recommend that future studies include a flowchart with information about the method of recruitment and allocation of patients and blinding of patients and researchers, in addition to other details related to the process, to improve replicability and better inform readers about the potential bias.
Conclusion
The reviewed studies indicated that long-term use is not necessary to obtain an improvement in the symptoms of ASD, since it is possible to observe this with 8 weeks of treatment. Apparently, the continuous use of the medicine promotes a better evolution of the clinical condition. Although risperidone has the strongest evidence of reduced irritability and hyperactivity in individuals with ASD, it also was effective in reducing all behavioural axes of ASD. In this study, it was possible to suggest the efficacy of risperidone for the treatment of lethargy and inadequate speech, since there was previously insufficient evidence. However, we emphasize that the risk-benefit in its use should be evaluated.
This paper demonstrated that there is a significant association between weight gain and risperidone administration. Weight gain may be associated with fat accumulation, especially in the abdominal region. The few data available did not allow us to draw definitive conclusions because there are few studies that have evaluated weight gain associated with fat accumulation. Finally, we emphasise that risperidone can be used for a long period.
In summary, this systematic review of interventional studies with meta-analysis suggests that risperidone treatment improves the clinical condition and the quality of life of the autistic individual.
Acknowledgements
The authors thank the Federal University of São João del Rei for the infrastructure, incentive, and collaboration, and Associação Fundo de Incentivo à Pesquisa (AFIP). This study was financed in part by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior – Brasil (CAPES) – Finance Code 001.
SUPPLEMENTARY MATERIAL
Supplementary material is available on the publisher’s website along with the published article.
Consent for Publication
Not applicable.
Funding
None.
Conflict of Interest
The authors declare no conflict of interest, financial or otherwise.
References
- 1.American Psychiatric Association . Desk reference to the diagnostic criteria from DSM-5. 5th ed. Choice Rev. Online; 2013. [Google Scholar]
- 2.Sharma A., Shaw S.R. Efficacy of risperidone in managing maladaptive behaviors for children with autistic spectrum disorder: a meta-analysis. J. Pediatr. Health Care. 2012;26(4):291–299. doi: 10.1016/j.pedhc.2011.02.008. [DOI] [PubMed] [Google Scholar]
- 3.Lai M., Lombardo M.V., Baron-Cohen S.A., Chan A.S., Sze S.L. Autism and epilepsy. Lancet Publishing Group. 2014;383:1–5. [Google Scholar]
- 4.Associação Psiquiátrica Americana . Manual Diagnóstico e Estatístico de Transtornos Mentais. 5th ed. São Paulo: Artmed; 2014. [Google Scholar]
- 5.Scahill L., Sukhodolsky D.G., Anderberg E., Dimitropoulos A., Dziura J., Aman M.G., McCracken J., Tierney E., Hallett V., Katz K., Vitiello B., McDougle C. Sensitivity of the modified Children’s Yale-Brown Obsessive Compulsive Scale to detect change: Results from two multi-site trials. Autism. 2016;20(2):145–152. doi: 10.1177/1362361315574889. [DOI] [PubMed] [Google Scholar]
- 6.Siegel M., Beaulieu A.A. Psychotropic medications in children with autism spectrum disorders: a systematic review and synthesis for evidence-based practice. J. Autism Dev. Disord. 2012;42(8):1592–1605. doi: 10.1007/s10803-011-1399-2. [DOI] [PubMed] [Google Scholar]
- 7.Halvorsen M., Aman M.G., Mathiassen B., Brøndbo P.H., Steinsvik O.O., Martinussen M. Psychometric properties of the norwegian aberrant behavior checklist and diagnostic relationships in a neuro-pediatric sample. J. Ment. Health Res. Intellect. Disabil. 2019;12:1–22. doi: 10.1080/19315864.2019.1630872. [DOI] [Google Scholar]
- 8.Nikolov R., Jonker J., Scahill L. Autistic disorder: current psychopharmacological treatments and areas of interest for future developments. Br. J. Psychiatry. 2006;28(Suppl. 1):S39–S46. doi: 10.1590/S1516-44462006000500006. [DOI] [PubMed] [Google Scholar]
- 9.Brakoulias V., Stockings E. A systematic review of the use of risperidone, paliperidone and aripiprazole as augmenting agents for obsessive-compulsive disorder. Expert Opin. Pharmacother. 2019;20(1):47–53. doi: 10.1080/14656566.2018.1540590. [DOI] [PubMed] [Google Scholar]
- 10.Jassen-Cillag Pharmaceutical 2016 https://www.janssenlabels.com/package-insert/ product-monograph/prescribing-information/RISPERDAL-pi.pdf
- 11.Nikolov R., Jonker J., Scahill L. Autismo: tratamentos psicofarmacológicos e áreas de interesse para desenvolvimentos futuros. Br. J. Psychiatry. 2006;28(Suppl. 1):S39–S46. doi: 10.1590/S1516-44462006000500006. [DOI] [PubMed] [Google Scholar]
- 12.Scahill L., Jeon S., Boorin S.J., McDougle C.J., Aman M.G., Dziura J., McCracken J.T., Caprio S., Arnold L.E., Nicol G., Deng Y., Challa S.A., Vitiello B. Weight gain and metabolic consequences of risperidone in young children with autism spectrum disorder. J. Am. Acad. Child Adolesc. Psychiatry. 2016;55(5):415–423. doi: 10.1016/j.jaac.2016.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Weiss R., Shaw M., Savoye M., Caprio S. Obesity dynamics and cardiovascular risk factor stability in obese adolescents. Pediatr. Diabetes. 2009;10(6):360–367. doi: 10.1111/j.1399-5448.2008.00504.x. [DOI] [PubMed] [Google Scholar]
- 14.Bocca G., Ongering E.C., Stolk R.P., Sauer P.J.J. Insulin resistance and cardiovascular risk factors in 3- to 5-year-old overweight or obese children. Horm. Res. Paediatr. 2013;80(3):201–206. doi: 10.1159/000354662. [DOI] [PubMed] [Google Scholar]
- 15.De Hert M., Dobbelaere M., Sheridan E.M., Cohen D., Correll C.U. Metabolic and endocrine adverse effects of second-generation antipsychotics in children and adolescents: A systematic review of randomized, placebo controlled trials and guidelines for clinical practice. Eur. Psychiatry. 2011;26(3):144–158. doi: 10.1016/j.eurpsy.2010.09.011. [DOI] [PubMed] [Google Scholar]
- 16.Bobo W.V., Cooper W.O., Stein C.M., Olfson M., Graham D., Daugherty J., Fuchs D.C., Ray W.A. Antipsychotics and the risk of type 2 diabetes mellitus in children and youth. JAMA Psychiatry. 2013;70(10):1067–1075. doi: 10.1001/jamapsychiatry.2013.2053. [DOI] [PubMed] [Google Scholar]
- 17.Srisawasdi P., Vanwong N., Hongkaew Y., Puangpetch A., Vanavanan S., Intachak B., Ngamsamut N., Limsila P., Sukasem C., Kroll M.H. Impact of risperidone on leptin and insulin in children and adolescents with autistic spectrum disorders. Clin. Biochem. 2017;50(12):678–685. doi: 10.1016/j.clinbiochem.2017.02.003. [DOI] [PubMed] [Google Scholar]
- 18.Almandil N.B., Liu Y., Murray M.L., Besag F.M.C., Aitchison K.J., Wong I.C.K. Weight gain and other metabolic adverse effects associated with atypical antipsychotic treatment of children and adolescents: a systematic review and meta-analysis. Paediatr. Drugs. 2013;15(2):139–150. doi: 10.1007/s40272-013-0016-6. [DOI] [PubMed] [Google Scholar]
- 19.Sochocky N., Milin R. Second generation antipsychotics in Asperger’s Disorder and high functioning autism: a systematic review of the literature and effectiveness of meta-analysis. Curr. Clin. Pharmacol. 2013;8(4):370–379. doi: 10.2174/15748847113086660073. [DOI] [PubMed] [Google Scholar]
- 20.Fung L.K., Mahajan R., Nozzolillo A., Bernal P., Krasner A., Jo B., Coury D., Whitaker A., Veenstra-Vanderweele J., Hardan A.Y. Pharmacologic Treatment of Severe Irritability and Problem Behaviors in Autism: A Systematic Review and Meta-analysis. Pediatrics. 2016;137(Suppl. 2):S124–S135. doi: 10.1542/peds.2015-2851K. [DOI] [PubMed] [Google Scholar]
- 21.Jadad A.R., Moore R.A., Carroll D., Jenkinson C., Reynolds D.J., Gavaghan D.J., McQuay H.J. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control. Clin. Trials. 1996;17(1):1–12. doi: 10.1016/0197-2456(95)00134-4. [DOI] [PubMed] [Google Scholar]
- 22.Higgins J.P.T., Thomas J., Chandler J., Cumpston M., Li T., Page M.J., Welch V.A. 2020 www.training.cochrane.org/
- 23.Mano-Sousa B.J., Pedrosa A.M., Alves B.C., Belo V.S., Chaves V.E., Duarte-Almeida J.M. 2019 https://www.crd.york.ac.uk/prospero/display_record.php? ID=CRD42019122316
- 24.Ghanizadeh A., Sahraeizadeh A., Berk M. A head-to-head comparison of aripiprazole and risperidone for safety and treating autistic disorders, a randomized double blind clinical trial. Child Psychiatry Hum. Dev. 2014;45(2):185–192. doi: 10.1007/s10578-013-0390-x. [DOI] [PubMed] [Google Scholar]
- 25.Calarge C.A., Ziegler E.E., Del Castillo N., Aman M., McDougle C.J., Scahill L., McCracken J.T., Arnold L.E. Iron homeostasis during risperidone treatment in children and adolescents. J. Clin. Psychiatry. 2015;76(11):1500–1505. doi: 10.4088/JCP.14m09258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Arnold L.E., Aman M.G., Li X., Butter E., Humphries K., Scahill L., Lecavalier L., McDougle C.J., Swiezy N.B., Handen B., Wilson K., Stigler K.A. Research Units of Pediatric Psychopharmacology (RUPP) autism network randomized clinical trial of parent training and medication: one-year follow-up. J. Am. Acad. Child Adolesc. Psychiatry. 2012;51(11):1173–1184. doi: 10.1016/j.jaac.2012.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Aman M., Rettiganti M., Nagaraja H.N., Hollway J.A., McCracken J., McDougle C.J., Tierney E., Scahill L., Arnold L.E., Hellings J., Posey D.J., Swiezy N.B., Ghuman J., Grados M., Shah B., Vitiello B. Tolerability, safety, and benefits of risperidone in children and adolescents with autism: 21-month follow-up after 8-week placebo-controlled trial. J. Child Adolesc. Psychopharmacol. 2015;25(6):482–493. doi: 10.1089/cap.2015.0005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rezaei M., Moradi A., Tehrani-Doost M., Hassanabadi H., Khosrowabadi R. A pilot study on combining risperidone and pivotal response treatment on communication difficulties in children with autism spectrum disorder. Adv Autism. 2018;4:56–65. doi: 10.1108/AIA-11-2017-0024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Firouzabadi N., Nazariat A., Zomorrodian K. DRD3 Ser9Gly polymorphism and its influence on risperidone response in autistic children. J. Pharm. Pharm. Sci. 2017;20(1):445–452. doi: 10.18433/J3H63T. [DOI] [PubMed] [Google Scholar]
- 30.Nikvarz N., Alaghband-Rad J., Tehrani-Doost M., Alimadadi A., Ghaeli P. Comparing efficacy and side effects of memantine vs. risperidone in the treatment of autistic disorder. Pharmacopsychiatry. 2017;50(1):19–25. doi: 10.1055/s-0042-108449. [DOI] [PubMed] [Google Scholar]
- 31.Lamberti M., Siracusano R., Italiano D., Alosi N., Cucinotta F., Di Rosa G., Germanò E., Spina E., Gagliano A. Head-to-Head comparison of aripiprazole and risperidone in the treatment of ADHD symptoms in children with autistic spectrum disorder and ADHD: A pilot, open-label, randomized controlled study. Paediatr. Drugs. 2016;18(4):319–329. doi: 10.1007/s40272-016-0183-3. [DOI] [PubMed] [Google Scholar]
- 32.Yoon Y., Wink L.K., Pedapati E.V., Horn P.S., Erickson C.A. Weight gain effects of second-generation antipsychotic treatment in autism spectrum disorder. J. Child Adolesc. Psychopharmacol. 2016;26(9):822–827. doi: 10.1089/cap.2016.0049. [DOI] [PubMed] [Google Scholar]
- 33.Choi J.E., Widjaja F., Careaga M., Bent S., Ashwood P., Hendren R.L. Change in plasma cytokine levels during risperidone treatment in children with autism. J. Child Adolesc. Psychopharmacol. 2014;24(10):586–589. doi: 10.1089/cap.2013.0108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ghaeli P., Nikvarz N., Alaghband-Rad J., Alimadadi A., Tehrani-Doost M. Effects of risperidone on core symptoms of autistic disorder based on childhood autism rating scale: an open label study. Indian J. Psychol. Med. 2014;36(1):66–70. doi: 10.4103/0253-7176.127254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wink L.K., Early M., Schaefer T., Pottenger A., Horn P., McDougle C.J., Erickson C.A. Body mass index change in autism spectrum disorders: comparison of treatment with risperidone and aripiprazole. J. Child Adolesc. Psychopharmacol. 2014;24(2):78–82. doi: 10.1089/cap.2013.0099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kent J.M., Hough D., Singh J., Karcher K., Pandina G. An open-label extension study of the safety and efficacy of risperidone in children and adolescents with autistic disorder. J. Child Adolesc. Psychopharmacol. 2013;23(10):676–686. doi: 10.1089/cap.2012.0058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kent J.M., Kushner S., Ning X., Karcher K., Ness S., Aman M., Singh J., Hough D. Risperidone dosing in children and adolescents with autistic disorder: a double-blind, placebo-controlled study. J. Autism Dev. Disord. 2013;43(8):1773–1783. doi: 10.1007/s10803-012-1723-5. [DOI] [PubMed] [Google Scholar]
- 38.Wei B.Y., Huang F., Qin X.T., Liang Q.Q. Treatment of behavioral disorders by risperidone in children with autism. Zhongguo Dang Dai Er Ke Za Zhi. 2011;13(3):216–218. [PubMed] [Google Scholar]
- 39.Correia C.T., Almeida J.P., Santos P.E., Sequeira A.F., Marques C.E., Miguel T.S., Abreu R.L., Oliveira G.G., Vicente A.M. Pharmacogenetics of risperidone therapy in autism: association analysis of eight candidate genes with drug efficacy and adverse drug reactions. Pharmacogenomics J. 2010;10(5):418–430. doi: 10.1038/tpj.2009.63. [DOI] [PubMed] [Google Scholar]
- 40.Desousa A. An open-label trial of risperidone and fluoxetine in children with autistic disorder. Indian J. Psychol. Med. 2010;32(1):17–21. doi: 10.4103/0253-7176.70522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hoekstra P.J., Troost P.W., Lahuis B.E., Mulder H., Mulder E.J., Franke B., Buitelaar J.K., Anderson G.M., Scahill L., Minderaa R.B. Risperidone-induced weight gain in referred children with autism spectrum disorders is associated with a common polymorphism in the 5-hydroxytryptamine 2C receptor gene. J. Child Adolesc. Psychopharmacol. 2010;20(6):473–477. doi: 10.1089/cap.2009.0071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Capone G.T., Goyal P., Grados M., Smith B., Kammann H. Risperidone use in children with Down syndrome, severe intellectual disability, and comorbid autistic spectrum disorders: a naturalistic study. J. Dev. Behav. Pediatr. 2008;29(2):106–116. doi: 10.1097/DBP.0b013e318165c100. [DOI] [PubMed] [Google Scholar]
- 43.Gencer O., Emiroglu F.N.I., Miral S., Baykara B., Baykara A., Dirik E. Comparison of long-term efficacy and safety of risperidone and haloperidol in children and adolescents with autistic disorder. An open label maintenance study. Eur. Child Adolesc. Psychiatry. 2008;17(4):217–225. doi: 10.1007/s00787-007-0656-6. [DOI] [PubMed] [Google Scholar]
- 44.Miral S., Gencer O., Inal-Emiroglu F.N., Baykara B., Baykara A., Dirik E. Risperidone versus haloperidol in children and adolescents with AD: a randomized, controlled, double-blind trial. Eur. Child Adolesc. Psychiatry. 2008;17(1):1–8. doi: 10.1007/s00787-007-0620-5. [DOI] [PubMed] [Google Scholar]
- 45.Anderson G.M., Scahill L., McCracken J.T., McDougle C.J., Aman M.G., Tierney E., Arnold L.E., Martin A., Katsovich L., Posey D.J., Shah B., Vitiello B. Effects of short- and long-term risperidone treatment on prolactin levels in children with autism. Biol. Psychiatry. 2007;61(4):545–550. doi: 10.1016/j.biopsych.2006.02.032. [DOI] [PubMed] [Google Scholar]
- 46.Pandina G.J., Bossie C.A., Youssef E., Zhu Y., Dunbar F. Risperidone improves behavioral symptoms in children with autism in a randomized, double-blind, placebo-controlled trial. J. Autism Dev. Disord. 2007;37(2):367–373. doi: 10.1007/s10803-006-0234-7. [DOI] [PubMed] [Google Scholar]
- 47.Canitano R. Self injurious behavior in autism: clinical aspects and treatment with risperidone. J. Neural Transm. (Vienna) 2006;113(3):425–431. doi: 10.1007/s00702-005-0337-x. [DOI] [PubMed] [Google Scholar]
- 48.Lindsay R.L., Eugene Arnold L., Aman M.G., Vitiello B., Posey D.J., McDougle C.J., Scahill L., Pachler M., McCracken J.T., Tierney E., Bozzolo D. Dietary status and impact of risperidone on nutritional balance in children with autism: a pilot study. J. Intellect. Dev. Disabil. 2006;31(4):204–209. doi: 10.1080/13668250601006924. [DOI] [PubMed] [Google Scholar]
- 49.Luby J., Mrakotsky C., Stalets M.M., Belden A., Heffelfinger A., Williams M., Spitznagel E. Risperidone in preschool children with autistic spectrum disorders: an investigation of safety and efficacy. J. Child Adolesc. Psychopharmacol. 2006;16(5):575–587. doi: 10.1089/cap.2006.16.575. [DOI] [PubMed] [Google Scholar]
- 50.Nagaraj R., Singhi P., Malhi P. Risperidone in children with autism: randomized, placebo-controlled, double-blind study. J. Child Neurol. 2006;21(6):450–455. doi: 10.1177/08830738060210060801. [DOI] [PubMed] [Google Scholar]
- 51.Aman M.G., Arnold L.E., McDougle C.J., Vitiello B., Scahill L., Davies M., McCracken J.T., Tierney E., Nash P.L., Posey D.J., Chuang S., Martin A., Shah B., Gonzalez N.M., Swiezy N.B., Ritz L., Koenig K., McGough J., Ghuman J.K., Lindsay R.L. Acute and long-term safety and tolerability of risperidone in children with autism. J. Child Adolesc. Psychopharmacol. 2005;15(6):869–884. doi: 10.1089/cap.2005.15.869. [DOI] [PubMed] [Google Scholar]
- 52.Research Units on Pediatric Psychopharmacology Autism Network Risperidone treatment of autistic disorder: longer-term benefits and blinded discontinuation after 6 months. Am. J. Psychiatry. 2005;162(7):1361–1369. doi: 10.1176/appi.ajp.162.7.1361. [DOI] [PubMed] [Google Scholar]
- 53.Troost P.W., Lahuis B.E., Steenhuis M.P., Ketelaars C.E.J., Buitelaar J.K., van Engeland H., Scahill L., Minderaa R.B., Hoekstra P.J. Long-term effects of risperidone in children with autism spectrum disorders: a placebo discontinuation study. J. Am. Acad. Child Adolesc. Psychiatry. 2005;44(11):1137–1144. doi: 10.1097/01.chi.0000177055.11229.76. [DOI] [PubMed] [Google Scholar]
- 54.Gagliano A., Germanò E., Pustorino G., Impallomeni C., D’Arrigo C., Calamoneri F., Spina E. Risperidone treatment of children with autistic disorder: effectiveness, tolerability, and pharmacokinetic implications. J. Child Adolesc. Psychopharmacol. 2004;14(1):39–47. doi: 10.1089/104454604773840472. [DOI] [PubMed] [Google Scholar]
- 55.Martín A., Scahill L., Anderson G.M., Aman M., Arnold L.E., McCracken J., McDougle C.J., Tierney E., Chuang S., Vitiello B. Weight and leptin changes among risperidone-treated youths with autism: 6-month prospective data. Am. J. Psychiatry. 2004;161(6):1125–1127. doi: 10.1176/appi.ajp.161.6.1125. [DOI] [PubMed] [Google Scholar]
- 56.Shea S., Turgay A., Carroll A., Schulz M., Orlik H., Smith I., Dunbar F. Risperidone in the treatment of disruptive behavioral symptoms in children with autistic and other pervasive developmental disorders. Pediatrics. 2004;114(5):e634–e641. doi: 10.1542/peds.2003-0264-F. [DOI] [PubMed] [Google Scholar]
- 57.Arnold L.E., Vitiello B., McDougle C., Scahill L., Shah B., Gonzalez N.M., Chuang S., Davies M., Hollway J., Aman M.G., Cronin P., Koenig K., Kohn A.E., McMahon D.J., Tierney E. Parent-defined target symptoms respond to risperidone in RUPP autism study: customer approach to clinical trials. J. Am. Acad. Child Adolesc. Psychiatry. 2003;42(12):1443–1450. doi: 10.1097/00004583-200312000-00011. [DOI] [PubMed] [Google Scholar]
- 58.McCracken J.T., McGough J., Shah B., Cronin P., Hong D., Aman M.G., Arnold L.E., Lindsay R., Nash P., Hollway J., McDougle C.J., Posey D., Swiezy N., Kohn A., Scahill L., Martin A., Koenig K., Volkmar F., Carroll D., Lancor A., Tierney E., Ghuman J., Gonzalez N.M., Grados M., Vitiello B., Ritz L., Davies M., Robinson J., McMahon D. Research Units on Pediatric Psychopharmacology Autism Network. Risperidone in children with autism and serious behavioral problems. N. Engl. J. Med. 2002;347(5):314–321. doi: 10.1056/NEJMoa013171. [DOI] [PubMed] [Google Scholar]
- 59.Masi G., Cosenza A., Mucci M., Brovedani P. Open trial of risperidone in 24 young children with pervasive developmental disorders. J. Am. Acad. Child Adolesc. Psychiatry. 2001;40(10):1206–1214. doi: 10.1097/00004583-200110000-00015. [DOI] [PubMed] [Google Scholar]
- 60.Masi G., Cosenza A., Mucci M., De Vito G. Risperidone monotherapy in preschool children with pervasive developmental disorders. J. Child Neurol. 2001;16(6):395–400. doi: 10.1177/088307380101600602. [DOI] [PubMed] [Google Scholar]
- 61.Zuddas A., Di Martino A., Muglia P., Cianchetti C. Long-term risperidone for pervasive developmental disorder: efficacy, tolerability, and discontinuation. J. Child Adolesc. Psychopharmacol. 2000;10(2):79–90. doi: 10.1089/cap.2000.10.79. [DOI] [PubMed] [Google Scholar]
- 62.Nicolson R., Awad G., Sloman L. An open trial of risperidone in young autistic children. J. Am. Acad. Child Adolesc. Psychiatry. 1998;37(4):372–376. doi: 10.1097/00004583-199804000-00014. [DOI] [PubMed] [Google Scholar]
- 63.Scott L.J., Dhillon S. Risperidone: a review of its use in the treatment of irritability associated with autistic disorder in children and adolescents. Paediatr. Drugs. 2007;9(5):343–354. doi: 10.2165/00148581-200709050-00006. [DOI] [PubMed] [Google Scholar]
- 64.Moura F.C. de, Caminha, J. Risperidone and aripiprazole playing a important role in pharmacological therapy of autism spectrum disorders: a global overview. Intl. J. Res. Eng. Sci. 2017;5:56–60. [Google Scholar]
- 65.Schmitz A.P., Kreutz O.C., Suyenaga E.S. Antipsicóticos atípicos versus efeito obesogênico sob a óptica da química medicinal. Rev. Eletrônica Farmácia. 2015;12:23. doi: 10.5216/ref.v12i3.33714. [DOI] [Google Scholar]
- 66.Baptista T., Zárate J., Joober R., Colasante C., Beaulieu S., Páez X., Hernández L. Drug induced weight gain, an impediment to successful pharmacotherapy: focus on antipsychotics. Curr. Drug Targets. 2004;5(3):279–299. doi: 10.2174/1389450043490514. [DOI] [PubMed] [Google Scholar]
- 67.Lian J., De Santis M., He M., Deng C. Risperidone-induced weight gain and reduced locomotor activity in juvenile female rats: The role of histaminergic and NPY pathways. Pharmacol. Res. 2015;95-96(96):20–26. doi: 10.1016/j.phrs.2015.03.004. [DOI] [PubMed] [Google Scholar]
- 68.Masaki T., Chiba S., Yasuda T., Noguchi H., Kakuma T., Watanabe T., Sakata T., Yoshimatsu H. Involvement of hypothalamic histamine H1 receptor in the regulation of feeding rhythm and obesity. Diabetes. 2004;53(9):2250–2260. doi: 10.2337/diabetes.53.9.2250. [DOI] [PubMed] [Google Scholar]
- 69.Sakata T., Yoshimatsu H., Kurokawa M. Hypothalamic neuronal histamine: implications of its homeostatic control of energy metabolism. Nutrition. 1997;13(5):403–411. doi: 10.1016/S0899-9007(97)91277-6. [DOI] [PubMed] [Google Scholar]
- 70.Han M., Deng C., Burne T.H.J., Newell K.A., Huang X-F. Short- and long-term effects of antipsychotic drug treatment on weight gain and H1 receptor expression. Psychoneuroendocrinology. 2008;33(5):569–580. doi: 10.1016/j.psyneuen.2008.01.018. [DOI] [PubMed] [Google Scholar]
- 71.Homrich J.G., Gentil C.G., Peres-Polon V.L., Covian M.R. Feeding behavior elicited by electrical stimulation of the lateral hypothalamic area in the rat: role of the adrenergic pathways and receptors. Braz. J. Med. Biol. Res. 1985;18(1):15–27. [PubMed] [Google Scholar]
- 72.DiFeliceantonio A.G., Small D.M. Dopamine and diet-induced obesity. Nat. Neurosci. 2019;22(1):1–2. doi: 10.1038/s41593-018-0304-0. [DOI] [PubMed] [Google Scholar]
- 73.Anderberg R.H., Richard J.E., Eerola K., López-Ferreras L., Banke E., Hansson C., Nissbrandt H., Berqquist F., Gribble F.M., Reimann F., Wernstedt A.I., Lamy C.M., Skibicka K.P. Glucagon-like peptide 1 and its analogs act in the dorsal raphe and modulate central serotonin to reduce appetite and body weight. Diabetes. 2017;66(4):1062–1073. doi: 10.2337/db16-0755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Johnson P.M., Kenny P.J. Dopamine D2 receptors in addiction-like reward dysfunction and compulsive eating in obese rats. Nat. Neurosci. 2010;13(5):635–641. doi: 10.1038/nn.2519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wang G.J., Volkow N.D., Logan J., Pappas N.R., Wong C.T., Zhu W., Netusil N., Fowler J.S. Brain dopamine and obesity. Lancet. 2001;357(9253):354–357. doi: 10.1016/S0140-6736(00)03643-6. [DOI] [PubMed] [Google Scholar]
- 76.Miranda-Linné F.M., Melin L. A factor analytic study of the autism behavior checklist. J. Autism Dev. Disord. 2002;32(3):181–188. doi: 10.1023/A:1015519413133. [DOI] [PubMed] [Google Scholar]
- 77.Betancur C. Etiological heterogeneity in autism spectrum disorders: more than 100 genetic and genomic disorders and still counting. Brain Res. 2011;1380:42–77. doi: 10.1016/j.brainres.2010.11.078. [DOI] [PubMed] [Google Scholar]
- 78.Lau W.K.W., Leung M.K., Lau B.W.M. Resting-state abnormalities in Autism Spectrum Disorders: A meta-analysis. Sci. Rep. 2019;9(1):3892. doi: 10.1038/s41598-019-40427-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Doebler P., Holling H., Böhning D. A mixed model approach to meta-analysis of diagnostic studies with binary test outcome. Psychol. Methods. 2012;17(3):418–436. doi: 10.1037/a0028091. [DOI] [PubMed] [Google Scholar]
- 80.de Sousa M.R., Ribeiro A.L.P. Revisão sistemática e meta-análise de estudos de diagnóstico e prognóstico: um tutorial. Arq. Bras. Cardiol. 2009;92:241–251. doi: 10.1590/S0066-782X2009000300013. [DOI] [Google Scholar]
- 81.Masi G., Cosenza A., Mucci M., Brovedani P. A 3-year naturalistic study of 53 preschool children with pervasive developmental disorders treated with risperidone. J. Clin. Psychiatry. 2003;64(9):1039–1047. doi: 10.4088/JCP.v64n0909. [DOI] [PubMed] [Google Scholar]
- 82.Rezaei M., Moradi A., Tehrani-Doost M., Hassanabadi H., Khosroabadi R. Effects of combining medication and pivotal response treatment on aberrant behavior in children with Autism Spectrum Disorder. Children (Basel) 2018;5(2):19. doi: 10.3390/children5020019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Nikoo M., Radnia H., Farokhnia M., Mohammadi M-R., Akhondzadeh S. N-acetylcysteine as an adjunctive therapy to risperidone for treatment of irritability in autism: a randomized, double-blind, placebo-controlled clinical trial of efficacy and safety. Clin. Neuropharmacol. 2015;38(1):11–17. doi: 10.1097/WNF.0000000000000063. [DOI] [PubMed] [Google Scholar]
- 84.McCracken J.T. Safety issues with drug therapies for autism spectrum disorders. J. Clin. Psychiatry. 2005;66(Suppl. 10):32–37. [PubMed] [Google Scholar]
- 85.Beasley C.M., Jr, Sutton V.K., Hamilton S.H., Walker D.J., Dossenbach M., Taylor C.C., Alaka K.J., Bykowski D., Tollefson G.D., Olanzapine Relapse Prevention Study Group A double-blind, randomized, placebo-controlled trial of olanzapine in the prevention of psychotic relapse. J. Clin. Psychopharmacol. 2003;23(6):582–594. doi: 10.1097/01.jcp.0000095348.32154.ec. [DOI] [PubMed] [Google Scholar]
- 86.Gondim F.J., Francisco C., Gondim J., Luiz P-A. Indicadores Antropométricos de Obesidade como Instrumento de Triagem para Risco Coronariano Elevado em Adultos na Cidade de Salvador-Bahia Anthropometric. Arq. Bras. Cardiol. 2005 doi: 10.1590/s0066-782x2005001400006. [DOI] [PubMed] [Google Scholar]
- 87.Findling R.L., Kusumakar V., Daneman D., Moshang T., De Smedt G., Binder C. Prolactin levels during long-term risperidone treatment in children and adolescents. J. Clin. Psychiatry. 2003;64(11):1362–1369. doi: 10.4088/JCP.v64n1113. [DOI] [PubMed] [Google Scholar]
- 88.Balant-Gorgia A.E., Gex-Fabry M., Genet C., Balant L.P. Therapeutic drug monitoring of risperidone using a new, rapid HPLC method: reappraisal of interindividual variability factors. Ther. Drug Monit. 1999;21(1):105–115. doi: 10.1097/00007691-199902000-00017. [DOI] [PubMed] [Google Scholar]
- 89.Ingelman-Sundberg M. The human genome project and novel aspects of cytochrome P450 research. Toxicol. Appl. Pharmacol. 2005;207(2) Suppl.:52–56. doi: 10.1016/j.taap.2005.01.030. [DOI] [PubMed] [Google Scholar]
- 90.Correia C., Vicente A.M. Pharmacogenetics of risperidone response and induced side effects. Per. Med. 2007;4(3):271–293. doi: 10.2217/17410541.4.3.271. [DOI] [PubMed] [Google Scholar]
- 91.Mano-Sousa B.J., Pedrosa A.M., Alves B.C., Belo V.S., Chaves V.E., Duarte-Almeida J.M. Efficacy and side effects of risperidone use in autism spectrum disorder. 2020.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material is available on the publisher’s website along with the published article.