Abstract
Wilson’s disease (WD) is an inherited disease caused by mutations in ATP7B and is characterized by the pathological accumulation of copper in the liver and brain. Common clinical manifestations of WD include a wide range of liver disease and neurological symptoms. In some patients, psychiatric symptoms may be the only manifestation at the time of diagnosis. The clinical features of WD are highly variable and can mimic any disease of internal medicine. Therefore, for unexplained medical diseases, the possibility of WD should not be ignored. Early diagnosis and treatment can improve the prognosis of WD patients and reduce disability and early death. Gene sequencing is becoming a valuable method to diagnose WD, and if possible, all WD patients and their siblings should be genetically sequenced. Copper chelators including D-penicillamine, trientine, and dimercaptosuccinic acid can significantly improve the liver injury and symptoms of WD patients but may have a limited effect on neurological symptoms. Zinc salts may be more appropriate for the treatment of asymptomatic patients or for the maintenance treatment of symptomatic patients. High-quality clinical trials for the drug treatment of WD are still lacking, therefore, individualized treatment options for patients are recommended. Individualized treatment can be determined based on the clinical features of the WD patients, efficacy and adverse effects of the drugs, and the experience of the physician. Liver transplantation is the only effective method to save patients with acute liver failure or with severe liver disease who fail drug treatment.
Keywords: Wilson’s disease, ceruloplasmin, copper homeostasis, genetics, diagnosis, management
1. INTRODUCTION
Copper is an essential trace element for humans and plays an important role in maintaining normal physiological functions. Most of the copper in the body is absorbed from our diet in the upper small intestine. Excess copper is excreted mainly through bile, and a small part (about 3% of the absorbed copper) is excreted from urine [1]. If the homeostasis of copper absorption and excretion is disrupted, copper deficiency or excess accumulation can lead to serious consequences. The most typical example disease of copper accumulation is Wilson’s disease (WD), also known as hepatolenticular degeneration, an autosomal recessive inherited disorder caused by ATP7B mutations on chromosome 13 [2]. The causative gene, ATP7B, encodes a copper-transporting P-type ATPase ATP7B residing in the trans-Golgi network of hepatocytes. ATP7B is a copper transporter required for the bile excretion of copper and the synthesis of functional ceruloplasmin. The dysfunction of ATP7B causes excessive copper accumulation in the liver, and large amounts of non-ceruloplasmin-bound copper in the circulation leading to uptake then subsequent injury and impairment of brain, kidney and other organs [3, 4].
The clinical features of WD are highly variable, although the majority of the symptomatic patients have liver disease and/or neuropsychiatric disturbances. Because of the lack of specific biomarkers, early diagnosis of WD is relatively difficult, especially for asymptomatic patients and patients with atypical symptoms. Although serum ceruloplasmin reduction is one of the most characteristic features of WD, serum ceruloplasmin is within the normal range in approximately 5% of WD patients [5]. We recently treated a female patient who had been diagnosed with autoimmune hepatitis (via a liver biopsy) 20 years ago. Her hepatic lesions continued to deteriorate after more than 10 years of treatment with glucocorticoids and immunosuppressive agents. Eventually, she was diagnosed with WD in our department according to laboratory testing and genetic sequencing. Aside from the most common liver and brain involvement, WD can affect almost any system in the body, including the eyes, musculoskeletal system, endocrine system, hematological system, immunological system, and even the heart [6]. WD is a multi-system disorder that can mimic any internal medical disease. It is difficult to define whether WD is a disease or a series of diseases. The possibility of WD should also be considered for internal medical diseases in which conventional treatment is not effective. We should say: if you would think of WD, don't miss it. This is an important prerequisite for the early diagnosis of WD. A comprehensive understanding of WD is the basis for early diagnosis and effective treatment. The review will focus on the latest advances in the genetics, pathophysiology, and diagnosis of WD, and highlight the importance of early diagnosis and individualized therapy in the management of WD.
2. GENETICS
2.1. Common Mutations in ATP7B
WD is an autosomal recessive inherited disorder caused by mutations in the ATP7B gene. ATP7B is located on the short arm of chromosome 13 and consists of 20 introns and 21 exons. To date, more than 700 mutations have been identified in all exons of this causative gene, and most of those mutations are missense mutations, small deletions or insertions, and splice junction mutations [4, 7]. Due to the differences in genetic background, the ATP7B hotspot mutations vary greatly between different regions (Table 1). The point mutation H1069Q on exon14, causing the replacement of histidine with glutamic acid in the N-domain of ATP7B, is one of the most common mutations in WD patients from European countries. It is estimated that 50–80% of WD patients from central, eastern, and northern Europe carry at least one H1069Q allele [8-11]. H1069Q is also the most common mutation in WD patients from North America with a high allele frequency of 38% [12]. The frequency of H1069Q is only about 19% in WD patients from the United Kingdom (UK), much lower than that in other European countries. The second most common mutation identified in the same UK cohort is the M769V in exon 8 with a frequency of 6% [13]. Other mutations such as 2299insC and 3400delC have been reported as common mutations in the WD patients from Europe, with a frequency of less than 10% [8, 11]. However, the common mutations of ATP7B in Asian populations are quite different from those in European populations. The point mutation R778L in exon 8 is the most common mutation in WD patients from east Asian countries, with an allele frequency of 13–49% [14-17]. In addition to R778L, P992L and T935M are also common ATP7B mutations in the Chinese population, and these three mutations are found in more than 70% of patients with WD [15]. In central and eastern Japan, 2871delC is the most common mutation in WD patients, with an allele frequency similar to that of R778L [17, 18]. In addition, C271X is reported as the most common ATP7B mutation in the eastern Indian patients, and the mutation spectrum between east and west India is quite different [19, 20]. Despite WD as an autosomal recessive disorder, patients with homozygous mutations in ATP7B are not common. Most WD patients are compound heterozygotes with two different mutations on each copy of the chromosome [21]. Conventional gene sequencing-based on hotspot mutations can usually find one mutation in WD patients with compound heterozygotes, but the other mutation is often missing. These rare mutations may be present in siblings or parents of WD patients and become family-specific mutations.
Table 1.
Common ATP7B mutations in Europeans and Asians.
| Variants | Exon | DNA Nucleotide Change | Protein Amino Acid Change | Allele Frequency | Refs. | |
|---|---|---|---|---|---|---|
| Europe | H1069Q | 14 | 3207C > A | His1069Gln | 17–78% | [8-11] |
| 2299insC | 8 | 2298-2299insC | Pro767Pro-fs | 3–11% | [8, 11] | |
| M769V | 8 | 2305A > G | Met769Val | 6–8% | [13, 22] | |
| 3400delC | 15 | 3400delC | Frameshift | 3–7% | [8, 11*] | |
| Asia | R778L | 8 | 2333G > T | Arg778Leu | 13–49% | [14-17, 23] |
| P992L | 13 | 2975C > T | Pro992Leu | 6–16% | [15, 24] | |
| 2871delC | 13 | 2871delC | Frameshift | 16–20% | [17, 18] | |
| C271X | 2 | 813C > A | Cys271Stop | 19–24% | [19, 20] |
*3402delC instead of 3400delC according to the author. 3402delC and 3400delC have the same reference single nucleotide polymorphism (SNP) cluster identification number (rs137853281).
2.2. Genotype-Phenotype Correlation
Mutations in ATP7B gene may result in the absence or complete disablement of the ATP7B protein, although these mutations are fairly rare. These mutations are reported to be associated with an earlier age of onset and lower serum ceruloplasmin oxidase activity [25]. Common missense mutations like H1069Q tend to impair the structural stability of ATP7B protein and are associated with its reduced levels [26, 27]. Recently, Ferenci et al. conducted a large-scale genetic study including more than 1000 European WD patients, but they did not find a genotype-phenotype correlation. Gender and age may affect the phenotype of WD, and cirrhosis is more common in female patients and adult patients [11]. As mentioned above, R778L is the most common mutation in east Asian WD patients. Wu and Cheng et al. found that homozygous R778L mutation was associated with the early onset of WD with hepatic presentation and a lower ceruloplasmin concentration [23, 28]. In addition, young children carrying R778L may present with a higher rate of acute liver failure (ALF) than the patients without R778L [29]. Nevertheless, these results are still controversial [30]. Correlations between genotypes and phenotypes of the common ATP7B mutations have not been clearly established [11].
The lack of genotype–phenotype correlation is partly due to the finding that the majority of patients with WD are compound heterozygotes, and the likely influence of environmental factors such as dietary copper intake and absorption, and due to modifier genes that affect one’s copper tolerance [4]. Modifier genes are a group of genes that alter the expression of a gene at another locus or its phenotypic expression. Several genes such as Apolipoprotein E (APOE) and 5,10-methylenetetrahydrofolate reductase (MTHFR) have been proposed as potential modifier genes of ATP7B [31, 32]. APOE gene is associated with lipid metabolism and has been identified as the major genetic risk factor of late-onset Alzheimer's disease [33]. The presence of the APOE ε3 or ε4 allele may be associated with late and early onset of WD, respectively [31, 34]. However, the association between APOE and WD phenotype was not confirmed in other independent studies [35, 36]. MTHFR gene encodes a key folate–homocysteine pathway enzyme. Gromadzka et al. reported that the polymorphism of the MTHFR gene was associated with the phenotypic variability of WD. Patients with MTHFR C677T allele were more likely to show hepatic signs, while the A1298C allele was associated with an earlier age of onset [32]. Recently, a whole-exome sequencing analysis including 248 WD patients identified esterase D (ESD) and INO80 as two possible modifier genes. Rare mutations in ESD and INO80 increased and reduced the possibility for the neurological phenotype, respectively [37]. Other potential modifier genes, such as human homologue antioxidant 1 copper chaperone (ATOX1), copper metabolism domain-containing 1 (COMMD1), Prion Protein (PRNP), and patatin-like phospholipase domain-containing 3 gene (PNPLA3), may be associated with the phenotype of WD, but have not been confirmed in other independent studies. The potential modifier genes were well summarized by Medici et al. in their recent published review [38]. The role of modifier genes in the disease heterogeneity seems limited but remains to be fully investigated. In addition to modifier genes, gender may also affect the phenotype of WD. Female patients are more likely to show liver symptoms and ALF [11]. In order to establish an accurate genotype–phenotype correlation, potential modifier genes need to be validated in larger independent cohorts.
3. PREVALENCE
The world prevalence of WD had been estimated as 1/30000, based on a heterozygous ATP7B mutation carrier frequency of 1/90 in 1984 [39-41]. However, the true prevalence of WD may be higher than previously thought. The prevalence of WD varies widely in different regions due to differences in genetic backgrounds, and it is higher in relatively isolated populations because of similar genetic backgrounds [42]. In the absence of large-scale epidemiological investigations, the prevalence of WD is often estimated based on laboratory tests and the ATP7B mutation carrier frequency. It is believed that east Asia countries have a higher prevalence of WD than western countries. Two large epidemiological investigations in China identified 9 cases of WD in a population of 153370, with a prevalence rate of 5.87/100000 [30]. Considering that some asymptomatic patients do not have Kayser-Fleischer rings and biochemical abnormalities, the actual prevalence may be higher in China. Mass screening studies performed in South Korea and Japan showed a relatively higher prevalence of WD (1 in 3667 and 2 in 2789, respectively) in children based on serum ceruloplasmin concentrations [43, 44]. Studies based on clinical features tend to miss early asymptomatic patients, and screening of biochemical parameters may lead to more false-positives. ATP7B genotype analysis is a relatively accurate epidemiological research method but still requires a large sample size. Through haplotype analysis of 660 healthy individuals, the prevalence of WD in Hong Kong Chinese is estimated as 1/5400 [45]. Furthermore, a genetic analysis performed in 500 healthy South Korean individuals indicated the ATP7B mutation carrier frequency was 1/27 and the extrapolated prevalence of WD was 1/3000 [46]. Nevertheless, the prevalence predicted based on hotspot mutations analysis is not accurate because most pathogenic mutations in WD are rare mutations. Recently, a large-scale investigation conducted in the UK indicated that the heterozygote ATP7B mutation carrier frequency was 1/40, predicting prevalence of 1/7000 [13]. However, the prevalence of clinically diagnosed WD patients is far less than those reported in the above studies. This is partly due to the difficulty in early diagnosis of WD for its varying clinical features, and the possibility of incomplete penetrance of ATP7B mutations. It is foreseeable that with the further understanding of WD and the development of economical and rapid ATP7B gene sequencing technology, more WD patients will be diagnosed and we will be able to determine the true penetrance of the disease.
4. PATHOGENESIS AND PATHOLOGICAL CHANGES
Mutations in ATP7B result in partial or complete structural and functional abnormalities of ATP7B protein, leading to disturbed copper homeostasis in the body. ATP7B plays a critical role in maintaining copper homeostasis in hepatocytes and neurons mainly through two physiologic processes. Firstly, ATP7B is essential for the transport of copper for the synthesis of functional ceruloplasmin (holoceruloplasmin), which contains six copper atoms per molecule. Secondly, ATP7B is the transporter responsible for the biliary excretion of excess intracellular copper in hepatocytes [47]. This could explain why most WD patients present with low serum ceruloplasmin levels and increased hepatic copper content. Increased intracellular copper accumulates in the liver, and then distributes to brain, kidney, eye, heart, and other organs, leading to oxidative stress and damage of cell components. In turn, a series of clinical symptoms including liver disease, neuropsychiatric symptoms, renal tubular dysfunction, ophthalmologic symptoms, and cardiomyopathy may present in WD patients due to the toxic effects of copper [48].
4.1. Copper Homeostasis
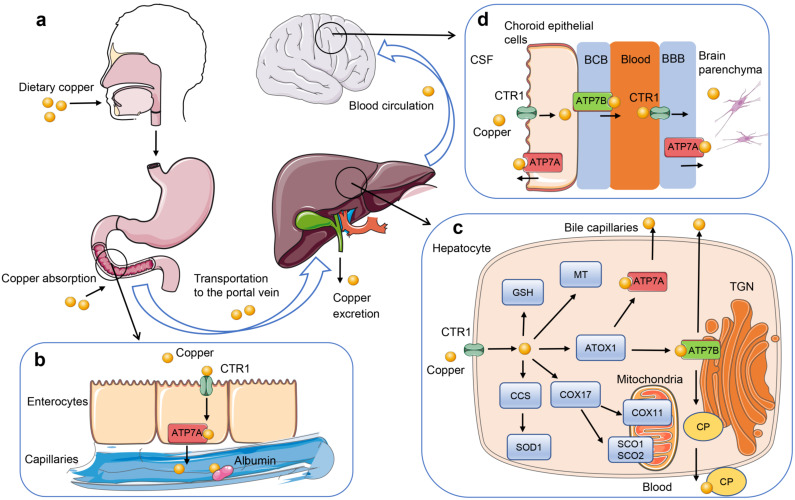
Copper is an essential trace element for humans to maintain normal physiological functions and brain development. Acting as a cofactor or structural component of various enzymes, copper is involved in many physiological processes such as mitochondrial respiratory chains, antioxidant defense, and neurotransmitter synthesis [49]. However, the excess accumulation of copper can cause the generation of toxic reactive oxygen species due to its redox activity, leading to oxidative DNA damage and mitochondria-mediated apoptosis [50]. Furthermore, excess copper may directly inhibit the functions of enzymes [51]. Therefore, the absorption, transport, storage and excretion of copper must be precisely regulated to control the level of copper within a proper range, providing enough copper for the synthesis of copper-containing enzymes while preventing copper-induced oxidative stress (Fig. 1).
Fig. (1).
Regulation of copper homeostasis. (a) Dietary copper enters the digestive tract and is absorbed into the blood mainly through the small intestine. Serum copper is then transported to the liver through the portal vein. (b) Copper is taken up by the intestinal enterocytes through CTR1 on the apical site, and then is released into the blood through ATP7A at the basolateral aspect. Copper loosely binds to proteins such as albumin in the blood and then transported to the liver. (c) Copper enter the hepatocytes via CTR1 and then bind to copper chaperones. Copper chaperone CCS transports copper to SOD1. Copper-specific chaperone ATOX1 transports copper to the copper transporting P-type ATPases ATP7A and ATP7B. COX17, SCO1, SCO2, and COX11 transport copper in the cytoplasm to synthesize cytochrome c oxidase. Intracellular free copper can be stored in combination with MT and GSH. ATP7B transports copper into the TGN to provide copper for the synthesis of CP. In addition, ATP7B is the main copper-transporter for biliary copper excretion in hepatocytes. (d) ATP7A and ATP7B in choroid epithelial cells excrete excess copper into CSF or blood to maintain copper balance in the brain. Brain capillary endothelial cells absorb copper from the blood through CTR1, and then release copper into the brain parenchyma through ATP7A. ATOX1, antioxidant protein 1; BBB, blood-brain barrier; BCB, blood-CSF barrier; CCS, copper chaperone for superoxide dismutase; COX17, Cytochrome c oxidase copper chaperone 17; CP, ceruloplasmin; CSF, cerebrospinal fluid; CTR1, copper transporter 1; GSH, glutathione; MT, metallothionein; SOD1, superoxide dismutase 1; TGN, trans-Golgi network. (A higher resolution / colour version of this figure is available in the electronic copy of the article).
Daily food is the main source of copper in the body. Some foods such as animal liver, shellfish, beans, nuts, and chocolate are rich in copper. Most copper is absorbed in the upper small intestine and then transported to the liver through the portal vein. Generally, 1.0 mg of copper per day is absorbed into the body through diet but the physiological requirement for copper is only 0.75mg per day, and the remaining copper is excreted by the liver mainly through the bile to maintain the copper balance [52]. Copper homeostasis is regulated by a series of proteins including transmembrane and cytosolic copper transporters, copper storage proteins, and copper-binding enzymes [7]. The copper transport receptor 1 (CTR1) is the main copper transmembrane transporter in normal physiological processes, although other copper transmembrane transporters such as divalent metal transporter 1 (DMT1) have been reported [53, 54]. When copper enters the cells, it binds to the copper chaperones and transports to intracellular biomolecules. Copper chaperone for superoxide dismutase (CCS) transports copper to superoxide dismutase 1 (SOD1), which is involved in free radical defense. Copper-specific chaperone antioxidant protein 1 (ATOX1) transports copper to the copper transporting P-type ATPases ATP7A and ATP7B. Cytochrome c oxidase copper chaperone 17 (COX17), SCO1, SCO2, and COX11 transport copper in the cytoplasm to provide copper for the synthesis of cytochrome c oxidase in mitochondria. In addition, intracellular free copper is controlled at a fairly low concentration by binding to metallothionein (MT) and ligands like glutathione (GSH) [55]. Mitochondria play a critical role in maintaining intracellular copper balance. Copper is an important cofactor of several key enzymes in the oxidative respiratory chain. Furthermore, mitochondria have been suggested as a dynamic copper pool, and the copper level in mitochondria changes with the cytosolic copper level [56, 57]. The excretion of copper mainly relies on the function of ATP7A and ATP7B, which can transport copper across the cell membrane by using the energy of ATP hydrolysis [58]. Dietary copper is mainly absorbed by enterocyte through CTR1 and then transported into the interstitial fluid by ATP7A at the basolateral aspect of the enterocyte. When the concentration of intracellular copper rises above the normal range or the concentration of copper in peripheral blood decreases, hepatocytes release the stored copper into peripheral blood through ATP7A and ATP7B. Similarly, the two ATPases in choroid epithelial cells excrete excess copper into the cerebrospinal fluid (CSF) or blood to maintain copper balance in the brain [49]. ATP7B transports copper into the trans-Golgi network to provide copper for the synthesis of holoceruloplasmin. More importantly, ATP7B is the main copper-transporter for biliary copper excretion in hepatocytes. In WD, the non-function mutations in ATP7B cause disruption of copper excretion from hepatocytes to the bile, leading to excessive accumulation of copper in the liver. When copper accumulates in hepatocytes to its capacity, excess free copper is released into the circulation and distributed to organs such as the kidneys, heart, eyes, and brain. Increased intracellular copper concentration leads to free radical formation and oxidative stress, and mitochondrial dysfunction by mechanisms other than oxidative stress [59].
4.2. Pathological Changes
4.2.1. Liver Pathology
The accumulation of toxic copper is considered as the main cause of hepatic injury in WD. Excess intracellular copper, especially the free copper is the main cause of oxidative stress due to its redox activity, leading to injury of DNA, RNA, lipids, proteins, and other small molecules. Copper can change between cuprous and divalent copper, catalyzing hydrogen peroxide and superoxide to generate highly reactive hydroxyl radicals through the Haber-Weiss reaction [60]. Copper can also split lipid hydroperoxides to generate alkoxyl and peroxyl radicals and accelerate lipid peroxidation via a reaction like the Fenton reaction [61]. In addition, excess copper may induce apoptosis by activating acid sphingomyelinase and releasing ceramide, and inhibits the activities of some enzymes by nonspecific binding to their thiol groups [62, 63]. Compared with other organelles, mitochondria are more sensitive to oxidative stress caused by copper. Copper may induce cardiolipin fragmentation to influence the integrity and function of the mitochondrial membrane, which can lead to mitochondrial damage [64]. Mitochondrial damage has been observed in the hepatocytes of early WD patients, thus it is considered as an initial event of liver injury [65, 66].
The liver is the major organ for ATP7B synthesis and regulation of copper metabolism. The distribution of copper in the liver is inhomogeneous, and copper concentration in hepatocytes changes during disease progression. Typically, the liver copper content (dry weight) in WD patients is five-fold higher or more than that of healthy individuals [67]. In the early stages of WD, cytosolic copper in hepatocytes tends to bind to MT, which are cysteine-rich proteins that can bind and store heavy metals. The MT-bound copper is not histochemically detectable by standard tests, but the Timm stain may detect the copper sulfur complexes. As the disease progresses, more and more copper accumulates in hepatocytes and then enters lysosomes. The lysosomal copper protein complexes are now detectable by rhodamine [68]. When the copper in hepatocytes exceeds their capacity, a large amount of non-ceruloplasmin-bound copper is released into the circulation and gradually accumulates in different organs of the body.
The histologic spectrum of liver injury in WD ranges from normal to very mild changes to severe disease with massive necrosis [69]. In the initial stage of the disease, the liver histology may include nonspecific changes such as mild portal lymphocytic inflammation, simple steatosis, nuclear glycogenation of hepatocytes but no obvious fibrosis [70]. Subsequently, hepatocyte degeneration and ballooning, Mallory hyaline bodies, and hepatocyte necrosis can be observed by light microscopy. However, some of these histologic changes are not specific and are seen in other liver diseases [71]. The typical pathological changes of chronic active viral hepatitis and autoimmune liver disease have been observed in some WD patients [72]. With the progression of the disease, liver disease appears more chronic with steatohepatitis and progressive typical liver fibrosis and eventual micronodular and macronodular cirrhosis. It should be noted that WD is highly suspected in patients presenting unexplained liver disease with multiple pathologic changes, especially in young patients [71]. Acute hepatic necrosis on the background of fibrosis appears in a minority of patients and is associated with ALF.
4.2.2. Brain Pathology
Neurological symptoms and brain damage in WD are the primary extrahepatic manifestations of copper toxicity. The human brain has the highest concentration of copper after the liver [73]. The copper concentration in the brain of WD patients is 10-15 fold higher than in healthy controls, but the distribution of copper is inhomogeneous among different brain regions [74, 75]. Copper concentrations in the brain parenchyma and CSF of WD patients were still higher than normal even after years of chelation treatment [48]. A neuropathological study including 11 WD patients found a correlation between the severity of brain injury and brain copper content [74]. However, Faa et al. reported that the increased copper content had no correlation with the neuropathologic lesions [76]. In another study with 12 WD patients, the brain copper was homogeneously distributed in the examined structures (frontal cortex, putamen, pons, and dentate nucleus) and was increased almost eight times than controls. The copper concentration in putamen correlated positively with the duration of the disease [77]. Astrocytes play a critical role in regulating copper homeostasis and copper toxicity in the brain. Astrocytes are not only a major component of the blood-brain barrier but are essential in metabolic supply to neurons, modulation of synaptic plasticity and functions, and defense against oxidative stress [49, 78]. The toxicity of copper is firstly buffered by astrocytes. Astrocytes are able to store large amounts of copper, which may be achieved by upregulating the synthesis of copper-bounding proteins such as MT and GSH [79, 80]. In response to rising levels of copper in the brain, the number of astrocytes increases (astrogliosis), and at the same time, the morphology of astrocytes changes (cell swelling) [81]. Alzheimer's types of glia and Opalski cells are two types of abnormal astrocytes that are considered to be characteristic pathological changes in the brain of WD. Staining showed that these cells were rich in MT and copper, suggesting their role in copper detoxification [82]. As the intracellular copper accumulates, the buffering effect of astrocytes is depleted, leading to excess copper being distributed to other brain tissues. The increased copper concentration and changes in the cerebral microenvironment caused by astrocyte damage may induce impairment of neurons and oligodendrocytes [83].
Different regions of the brain show distinct susceptibility to copper toxicity, and the most vulnerable areas are basal ganglia, especially the putamen [75]. The basal ganglia in the brain are more sensitive to copper toxicity probably due to its increased blood supply, abundant mitochondria, and high metabolic rate. In addition to the basal ganglia, thalamus, midbrain, and pons are also regions vulnerable to toxic injury, and abnormal signals in these regions are often seen in magnetic resonance imaging (MRI) [84]. Pathological changes including astrogliosis, demyelination, and tissue disintegration in these regions may lead to hyperintensity in T2-weighted images in MRI. Demyelination lesions tend to occur in fiber bundles across the frontal lobe, basal ganglia and pontine regions. Basal ganglia and associated fiber bundle lesions are the pathophysiological basis of dystonia, parkinsonism and dyskinesia in WD [48]. All the cells in the brain will necrose when exposed to high concentrations for long periods of time. Dopaminergic neurons in substantia nigra may be affected, and parkinsonism in WD may be caused by damage to the presynaptic substantia striatum pathway. Cortical and white matter lesions have also been reported in several cases, and these lesions may be related to epilepsy [85, 86].
Besides copper toxicity due to ATP7B dysfunction, other factors such as hepatic encephalopathy and accumulation of iron may contribute to the neuropathological changes in WD [48]. Although ATP7B has been identified in the brain, its role in maintaining brain physiological functions is still not clear. Some WD patients with ATP7B mutations remain to have no neurological symptoms after many years of onset, but other patients with the same genotype may develop neurological symptoms at an earlier age. ATP7B knockout mice showed neuron morphologic abnormalities even without significant copper deposition, raising the possibility that copper deposition might not be the only cause of neuropathologic changes in WD [87]. Hepatic encephalopathy, caused by liver dysfunction, portosystemic shunting or both may contribute to neuropathological changes in WD. Abnormal astrocytes like Alzheimer's type of glia existed in the brain of WD, can also be observed in hepatic encephalopathy. Some WD patients showed bilateral pallidus hyperintensity in T1-weighted images in MRI, which was also observed in patients with portosystemic shunt [7]. It should be noted that in addition to copper, a large amount of iron may be deposited in the brain of WD patients. Postmortem neuropathologic studies found that the amount of detectable iron accumulated in the basal ganglia correlated with the pathological severity of changes due to WD and was accompanied by an increased number of iron-containing macrophages [88, 89]. Positron emission tomography (PET) scans of 52Fe-citrate in vivo also showed increased cerebral iron uptake in WD patients [90]. The mechanism of iron accumulation in WD patients and its effect on brain pathophysiology are still unclear.
5. CLINICAL FEATURES
Although WD patients may show clinical symptoms at any age, most patients develop symptomatic disease between the ages of 5 and 35. The youngest patient diagnosed with WD was an 8-month-old child [91]. In contrast, late-onset patients diagnosed with WD in their 70s have been reported [92]. Therefore, the age of a patient cannot be used to exclude WD. Liver disease and neuropsychiatric disorders are the most common clinical features of WD. The clinical features of WD can be quite complex, including not only liver and neuropsychiatric symptoms, but also symptoms due to injury of other organs as well.
5.1. Liver Disease
WD patients generally develop liver disease in the first or second decade of their lives. Liver disease appears in most WD patients with or without neurological symptoms and may precede neurological symptoms by 10 years [67]. Liver disease presents as the first clinical symptom in 40–60% of WD patients and other symptoms may be present as well [7]. The spectrum of liver disease in WD is highly variable, ranging from asymptomatic to severe cirrhosis with complications of portal hypertension and even ALF. Most asymptomatic WD patients are diagnosed through family screening (siblings of WD patients) by medical examination and biochemical testing, only increased serum transaminases along with disease-specific findings such as the presence of Kayser-Fleischer rings, a low ceruloplasmin level, or the elevated urine copper excretion. In the early stages of the disease, the liver disease slowly progresses from elevated serum transaminases to a state of chronic active hepatitis [93]. In children and adolescents, early liver histology includes mild to moderate steatosis, which can sometimes be detected on ultrasonography as well as liver biopsy. The persistent active inflammation in the liver leads to fibrosis progression, and WD patients gradually develop advanced fibrosis and cirrhosis, initially compensated and later on decompensated with complications of portal hypertension. Similar to the clinical manifestations of chronic active hepatitis caused by other diseases, WD patients with compensated chronic cirrhosis often present with fatigue, jaundice, and abdominal discomfort and have splenomegaly on examination. Portal hypertension with edema, ascites, esophageal or gastric varices and hepatic encephalopathy may occur in patients with decompensated cirrhosis [94]. Jaundice in WD patients may be caused by liver dysfunction in advanced disease; however, it may also be caused or exacerbated by transient or chronic hemolysis. Obvious hemolysis is usually seen in severe ALF. In this setting, massive necrosis of hepatocytes leads to the release of copper into blood, and high levels of serum-free copper may have a direct oxidation effect on hemoglobin and membrane phospholipids, which may be the underlying mechanism of hemolysis [95]. A study including 321 WD patients revealed that 22 of these patients presented with acute hemolysis [96]. In another study including 283 Japanese WD patients, only 3 patients with acute hemolysis were observed [97]. Low-grade hemolysis may also occur in WD patients without any obvious manifestation of liver disease.
ALF caused by WD, also known as fulminant WD or abdominal Wilsonian disease, is the most severe form of liver disease. The most common clinical symptoms of ALF are rapid onset of jaundice, ascites, severe coagulopathy, acute renal failure, and hepatic encephalopathy, similar to ALF caused by other etiologies. ALF due to WD accounts for about 5% of all ALF cases and accounts for 6–12% of all ALF cases requiring emergency liver transplantation [98, 99]. ALF tends to present more frequently in young females (female: male ratio 4: 1) and WD patients who interrupt medical therapy [94]. ALF should be highly suspected when a WD patient presents with deep jaundice, anemia, low level of serum alkaline phosphatase, and only mild transaminase elevation [67]. It should be noted that some WD patients may also have concurrent liver diseases such as autoimmune hepatitis, nonalcoholic steatohepatitis, and viral hepatitis. As the liver histologic changes of WD overlap with these liver diseases, sometimes the diagnosis of WD can be delayed and the disease becomes more severe over time. A retrospective cohort study found that 9 of 42 WD patients had concurrent liver disease, including 4 patients with autoimmune hepatitis, 2 patients with hepatitis C virus, and 1 patient with viral hepatitis progressing to hepatocellular carcinoma (HCC). Patients with concurrent liver disease were significantly older at diagnosis, with more advanced fibrosis and cirrhosis and are associated with higher mortality [100]. Concurrent liver disease may accelerate the deterioration of liver symptoms, delay the diagnosis of WD, and also affect the judgment of whether drug treatment is effective. Therefore, other liver disorders must be excluded in WD patients embarking on disease-specific treatment. Although liver cirrhosis is a common clinical feature in WD patients, HCC is rarely present in WD patients. This phenomenon may be explained by the antitumor effect of copper in hepatocytes [101]. A retrospective study including 130 WD patients found only 2 patients progressing to HCC after a median follow-up of 15 years. The estimated annual HCC incidence in WD patients is 0.09% for all patients and 0.14% for patients with cirrhosis [102]. In another cohort of 363 WD patients, only 2 patients with cirrhosis (0.5%) developed HCC after more than 30 years of treatment [103]. Recently, a large-scale retrospective study found 8 cases of HCC and 6 cases of intrahepatic cholangiocellular carcinomas (ICC) in 1186 WD patients. The low HCC risks in WD did not reach the recommended thresholds for screening and surveillance for HCC [104]. Therefore, regular HCC monitoring may be unnecessary for WD patients without other risk factors.
5.2. Neuropsychiatric Symptoms
Neurological symptoms are also one of the more common and characteristic symptoms of WD. Neurological symptoms generally appear between the ages of 20 and 30, about 10 years after the onset of liver symptoms. It is estimated bout 18–68% of WD patients were diagnosed for their initial neurological symptoms, and these symptoms may also occur without obvious liver disease [105]. There is a wide spectrum of neurological symptoms in WD, and most are extrapyramidal symptoms caused by copper-induced damage to the basal ganglia and associated fiber bundles. The most common neurological symptoms include tremor, dystonia, dysarthria, parkinsonism, chorea, and athetosis [106]. Neurological symptoms can be very subtle and persist for many years, but their onset and progression may also be rapid, leading to complete disability within a few months. In general, patients with neurological symptoms continue to progress and eventually develop severe disabilities if they are not diagnosed or treated promptly [67]. There is no specific neurological manifestation in early WD patients. Neurological WD patients usually begin with mild dysarthria, dysphagia, and finger tremors. These symptoms of WD patients can persist for several years without significant progression, or they can quickly worsen within a few months if not diagnosed and treated properly. The neurological manifestations of WD patients can be classified into three syndromes: dystonic syndrome, parkinsonian syndrome, and ataxia syndrome. However, the neurological symptoms of most WD patients are not isolated but a combination of these features [4].
Tremor is one of the most characteristic symptoms in neurological patients with WD. The characteristic tremor is the wing-beating tremor or flapping tremor, a coarse and irregular limb tremor similar to that of a bird flapping its wings. Wing-beating tremor combined with dysarthria highly suggests the possibility of WD. Rest, action, or intention tremor may occur alone or in combination in WD patients [4]. Tremor may be bilateral or limb asymmetry and may gradually affect other parts of the body with disease progression. Unlike Parkinson's disease, tremor in WD patients is generally not accompanied by hypermyotonia, and some patients with tremor even show hypomyotonia. Wing-beating tremor can be observed in patients with hepatic encephalopathy and it may be a feature of negative myoclonus. Dystonia is a common symptom in WD and affects more than 30% of all patients with neurological symptoms. Dystonia in WD can be focal, segmental, multifocal, or even generalized, ranging from mild to severe contractures. Putamen abnormalities are found in 80% WD patients with dystonia, indicating that damage to the putamen may be associated with dystonia [107]. Dystonia often involves the cranial region and is clinically manifested as abnormal facial expressions (e.g., facial grimacing and risus sardonicus), dysarthria, and oropharyngeal dystonia (e.g., open jaw drooling). Focal dystonia affecting the limbs, neck, tongue, and blepharospasm are often observed in WD [106]. Speech changes and drooling are the most common neurologic symptoms in the early stage of WD. Young patients with tremor and dystonia should be highly suspicious of WD [67]. Like tremors, dystonia can also be asymmetrical, gradually spreading throughout the body as the disease progresses. Severe dystonia can lead to postural abnormalities of the neck, trunk, and limbs.
Dysarthria is another neurological symptom present in neurologically affected WD patients, and 85–97% of patients with neurological symptoms have varying degrees of dysarthria [108, 109]. Dysarthria is often combined with other neurological symptoms with spastic, ataxic, hypokinetic, and dystonic components, such as slow tongue movements and orofacial dyskinesias [4, 52].
Seizures are also observed in some patients with WD and may occur at any stage of the disease. While most WD will not have seizures, the prevalence of seizures in WD patients is estimated to be 6–8%, which is ten times higher than that of epilepsy in the general populations [86, 110]. Seizures in WD patients may be related to lesions of the cortical white matter tracts, and are more likely to occur in patients receiving chelators treatment. The overtreatment or abrupt dis-balance of WD and copper deficiency may be one of the causes of seizures [111]. In addition to neurological symptoms discussed above, WD patients may present other rare neurological symptoms including olfactory dysfunction, rapid eye movement sleep behaviour disorder, neuropathy, restless leg syndrome, and headaches [105].
Psychiatric symptoms may occur in WD patients independently or combined with neurological symptoms. According to a recent review, psychiatric symptoms were observed in bout 30–40% of WD patients at the time of their diagnosis [112]. In general, psychiatric symptoms can easily obscure the true condition of WD, leading to delayed diagnosis of WD [112]. The most common psychiatric symptoms include abnormal behavior, personality changes, depression, and anxiety. Children with WD may exhibit decreased academic performance, personality changes, and inappropriate behavior, which are often mistaken for adolescent problems [67]. The possibility of WD should be considered if young patients with psychiatric symptoms are accompanied by liver test abnormalities, sensitivity to neuroleptics, a family history of psychiatric symptoms, and a history of jaundice [113]. Psychiatric features such as depression, paranoia, and schizophrenia can occur in adult patients. Cognitive impairment is one of the neurological features of WD patients and is present in some patients with psychiatric symptoms. In WD patients, executive function or implicit cognition related to the frontostriatal circuits is vulnerable, but episodic memory and verbal intelligence are less affected [114]. WD patients with neurological symptoms may present with impairment of all cognitive functions, including executive function, memory, and visuospatial processing [115]. Furthermore, the attention deficit is more likely to occur in WD patients with neurological symptoms [116].
5.3. Other Clinical Symptoms
Kayser-Fleischer rings are common clinical features of WD caused by abnormal copper deposits in the corneal Descemet's membrane. Kayser-Fleischer rings exist in most WD patients at the time of diagnosis and are more likely to appear in WD patients with neurological symptoms. Approximately 95% of WD patients with neurological symptoms and 50% WD patients without neurological symptoms have Kayser-Fleischer rings, but pediatric WD patients with liver symptoms often lack this feature [67]. On ophthalmological inspection, the Kayser-Fleischer rings are usually brown or golden rings at the edge of the cornea (starting inferiorly and superiorly and expanding to become circumferential with time), and are visible under the slit-lamp. Any patient who suspects WD should have an ophthalmic slit-lamp examination, which is easy to perform. Anterior segment optical coherence tomography (AS-OCT), a new method of detecting copper deposits in cornea, may be more sensitive than slit lamps in detecting Kayser-Fleischer rings [117]. AS-OCT could detect the Kayser-Fleischer rings that could not be found under the slit-lamp in WD patients [118]. In addition, optical coherence tomography (OCT) also found thinning of the total retinal nerve fiber layer and macular thickness, reflecting neurodegeneration in WD patients [119, 120]. However, Kayser-Fleischer rings are not a specific ophthalmological sign of WD, and can rarely be observed in obstructive liver diseases such as cholestasis and primary biliary cirrhosis [52]. Another ophthalmological sign, sunflower cataracts, is less common than Kayser-Fleischer rings and occurs in about 2–20% of WD patients [105].
The cardiovascular system is also affected in some WD patients. The average copper content of myocardium in WD patients (157 μg/g) is about 10-fold higher than that in healthy individuals (17 μg/g) [121]. Cardiac symptoms in WD patients include arrhythmias, cardiomyopathy, and autonomic dysfunction. In a cohort of 125 WD patients, the structural and functional changes of the heart were mainly manifested as mild left ventricular hypertrophy and diastolic function changes, which were more obvious in neurological WD patients [122]. WD patients with severe clinical symptoms may present with cardiac manifestations and have a higher risk of developing structural heart disease [123]. It is estimated that WD patients have a 29% higher risk of atrial fibrillation and a 55% higher risk of incident heart failure compared to the general populations [6]. Other clinical manifestations include renal abnormalities, bone demineralization, endocrine abnormalities, pancreatitis, immunologic abnormalities, skin changes, infertility, and repeated miscarriage [67, 121]. Although these symptoms are not common in WD patients and some are not very specific to WD, they may divert attention to different processes and thereby delay diagnosis, leading to more severe liver and brain damage with time. Therefore, attention should be paid to the diversity of clinical manifestations of WD.
6. DIAGNOSTIC METHODS
6.1. Clinical Diagnosis of WD
The diversity of clinical manifestations of WD often makes it difficult to differentiate WD from other diseases. Therefore, the diagnosis of WD requires a combination of clinical manifestations and laboratory tests. In 2001, a WD diagnostic scoring system was developed at the 8th International Meeting on Wilson's Disease held in Leipzig, Germany [124]. The scoring system is based on clinical features, copper-related laboratory tests, and genetic testing data from WD patients that are used to calculate a total score. This WD scoring system provides excellent diagnostic accuracy and has been incorporated into the European Association for the Study of the Liver (EASL) clinical practice guidelines for WD as part of an algorithm about how to approach disease diagnosis [67]. The diagnosis of WD is established when the total score reaches 4 or more. In contrast, WD is less likely when the total score is only 2 or less. According to this scoring system, the diagnosis of WD can be established if the patient has decreased serum ceruloplasmin and the presence of Kayser-Fleischer rings. However, as previously described, Kayser-Fleischer rings may be absent in some WD patients [67]. Ceruloplasmin is an acute phase reactant that is elevated in acute inflammation. Although serum ceruloplasmin levels decrease in about 95% of WD patients, serum ceruloplasmin may be within the normal range in some WD patients with active liver diseases or who are on estrogen supplements. Low serum ceruloplasmin levels can be observed in severe liver diseases, renal failure, intestinal absorption disorders, and other diseases that cause protein loss. Patients with aceruloplasminemia and Menkes disease also show decreased serum ceruloplasmin levels due to mutations in ceruloplasmin and ATP7A genes, respectively [125, 126]. In addition, 20% of individuals who are carriers with one mutation of ATP7B may present with mildly decreased serum ceruloplasmin levels, but they do not manifest organ injury from these changes [127]. Serum ceruloplasmin levels as a single test have a poor predictive value and therefore should be combined with other laboratory tests to diagnose WD.
Although WD is a copper overload disease, most WD patients have normal or slightly reduced total serum copper levels. The reduction of ceruloplasmin leads to a decrease in ceruloplasmin-bound copper which usually contains ~90% of the total serum copper. By contrast, there is a relative increase in non-ceruloplasmin-bound copper over time as the disease progresses. Serum non-ceruloplasmin-bound copper, also known as free copper, is generally not directly measured but is calculated by subtracting the ceruloplasmin-bound copper from total serum copper. Serum non-ceruloplasmin-bound copper was proposed as a diagnostic test for WD, but it has proved to be unreliable because it relies on methods for measuring both serum ceruloplasmin by immunologic rather than by enzymatic activity due to the copper content, and a separate measurement for copper [67, 128]. A direct measurement of free copper, called exchangeable copper (CuEXC), has been developed in some laboratories and evaluated as a WD diagnostic tool [129]. The ratio of CuEXC to total serum copper is called relative exchange copper (REC), which has excellent sensitivity and specificity for the diagnosis of WD when the REC value was greater than 18.5% [130]. As a new diagnostic test, the role of REC in WD diagnosis requires further testing to confirm its accuracy in wider populations.
The amount of 24-hour urinary copper excretion is very important for the diagnosis and treatment monitoring of WD. In most symptomatic patients, the 24-hour urinary copper excretion is usually more than 100 μg/24 hours (1.64 μmol/24 hours), and this is an important diagnostic criterion for adult WD [67, 124]. The elevated copper content in urine is attributed to the increased non-ceruloplasmin-bound copper in the circulation. Approximately 20% of the WD patients have a basal 24-hour urinary copper excretion less than 100 μg/24 hours, which is more likely to occur in pediatric patients [7]. Urine copper excretion in healthy individuals is negligible; therefore, urine copper excretion in asymptomatic children exceeding 40 μg/24 hours (0.64 μmol/24 hours) suggests the possibility of WD [67, 131]. For some patients suspected of WD but whose urinary copper excretion does not meet the diagnostic threshold, a D-penicillamine challenge test can be performed. The test was standardized only in pediatric patients, starting with 500 mg of D-penicillamine orally and repeating the same dose of D-penicillamine 12 hours later during 24-hour urine collection. The patient is likely to be WD if a 5-fold increase in copper content is detected in the urine collected in the second 12 hours, or a 10-fold increase in copper content in the urine collected within 24 hours [131, 132]. However, the clinical value of the D-penicillamine challenge test is limited. Fecal copper test can better reflect the copper-excretion ability of the liver and may be used in the diagnosis of WD in the coming decades.
Liver biopsy can be utilized to aid the diagnosis of WD in patients who cannot be definitely diagnosed by non-invasive testing and there is also a role for directed biopsy when there is the presence of other liver lesions. Generally speaking, the hepatic copper content is less than 50 μg/g dry weight in healthy individuals. In contrast, hepatic copper content usually exceeds 250 μg/g dry weight in WD patients; however, there are some patients in whom the copper content may not meet this threshold [133]. The sensitivity of the hepatic copper content to diagnosis WD is increased when the threshold is lowered to 75 μg/g dry weight, but the specificity is decreased. The distribution of copper in the liver is extremely inhomogeneous, especially in patients with cirrhosis, so sampling errors in liver biopsy may lead to false-negative results [128]. However, the copper content in these individuals is rarely low enough to be in the normal range, and therefore a normal hepatic copper content usually excludes the diagnosis of WD. It is worth noting that the increase in hepatic copper content is not only found in WD but also found in cholestatic diseases. The disease manifestations and histologic findings on biopsy allow for the differentiation of these individuals from those with WD [133].
6.2. Neuroimaging Findings
Neuroimaging should be evaluated for all WD patients with or without neurological symptoms. Although neuroimaging examinations are not included in the Leipzig scoring system, they can provide much useful information to detect early neurological lesions and exclude other possible diseases. Neuroimaging changes are observed in almost all patients with neurological signs of WD, as well as some presymptomatic patients and patients with liver symptoms
[105]. MRI is more sensitive in detecting brain lesions compared with other neuroimaging examinations, and if possible, all patients should undergo brain MRI. The most common brain MRI findings of WD patients are symmetrical or asymmetric hyperintensities in T2-weighted images in basal ganglia, thalamus, midbrain, and pontine [7, 134]. Cortical regions, corticospinal tracts, cerebellum, and white matter can also be affected [105]. Severe neurological lesions may lead to hypointense changes in T1-weighted images in the bilateral basal ganglia. The most characteristic MRI signs of WD is the so-called “face of the giant panda” in the midbrain, which occurs in 14–20% of WD patients with neurological manifestations [4, 7]. Hyperintensities in T1-weighted images can be observed in the globus pallidum and substantia nigra in patients with severe liver failure or portosystemic shunting, which may be due to the accumulation of manganese [135]. MRI abnormalities in the brain may completely regress after effective treatment, therefore regular MRI examination may help to evaluate the effectiveness of treatment [136]. Recently, Dusek et al. developed a novel brain MRI rating scale for WD that could semi-quantitatively assess the severity of neurological lesions in MRI. This scale includes the acute toxicity score (calculated by T2 or T2-FLAIR hyperintensities) and the chronic damage score (calculated by T2 hypointensity and brain atrophy), which provides a method for classification of radiological severity. The MRI rating scale scores were well correlated with disease severity, both at baseline and after 24 months of treatment [137]. The effectiveness of the MRI rating scale and its role in assessing disease severity still needs to be confirmed in the following studies.
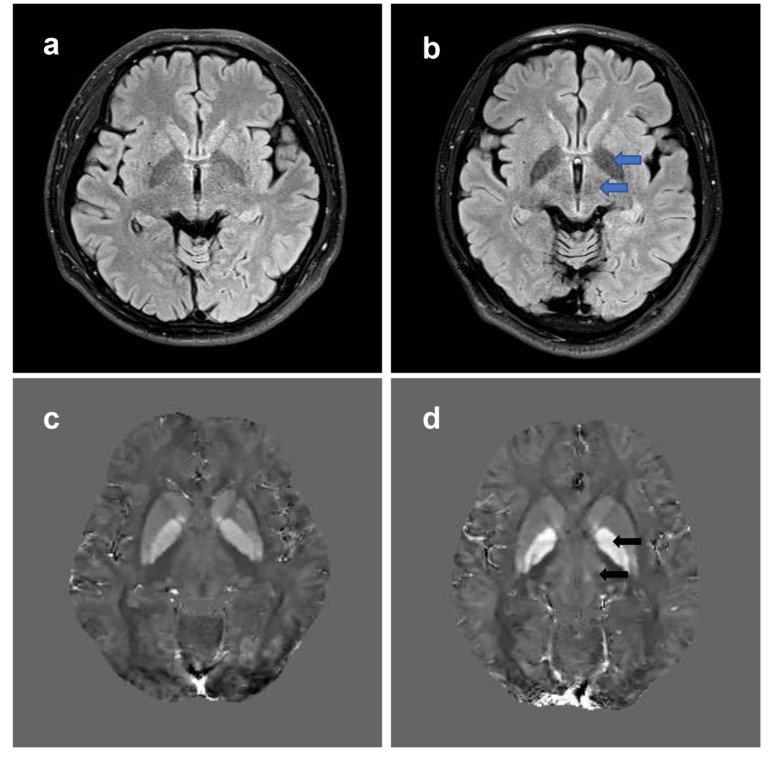
WD is characterized by excess copper deposits in the brain, but iron deposits may be present as well. The magnetic susceptibility of brain tissue changes due to the deposition of copper and iron. T2*-weighted images and susceptibility-weighted imaging (SWI) are superior to conventional MRI sequences in displaying metal deposition and have been used to explore its distribution in WD [138, 139]. A recent postmortem study showed that copper and iron were significantly elevated in the basal ganglia of WD patients, and hypointensities in the basal ganglia in T2*-weighted images were associated with iron rather than copper deposition [89]. Although SWI and T2*-weighted images are able to detect metal deposits in the brain of WD patients, the susceptibility of the deposited metal cannot be quantitatively analyzed and the results were influenced by the imaging parameters. To solve this problem, a new MRI sequence, quantitative susceptibility mapping (QSM), was recently developed. QSM can quantitatively analyze the susceptibility of metal deposition in the brain, so as to reflect the metal content in each brain region [140]. Fritzsch et al. found that susceptibility was significantly increased in the gray matter nuclei of the midbrain and basal ganglia in both neurologically symptomatic and asymptomatic WD patients [141]. The results were also validated in pediatric WD patients [142, 143]. Since QSM is very sensitive to susceptibility changes, it may be used to detect the early stage of copper and iron deposition, providing clues to the diagnosis of asymptomatic WD patients (Fig. 2). Other imaging techniques, such as magnetic resonance spectroscopy (MRS), single-photon emission computed tomography (SPECT), and transcranial parenchymal ultrasound (TCS) are currently only used in WD for research [4, 144, 145].
Fig. (2).
Brain MRI changes in WD. T2-FLAIR weighted images and QSM are used to show brain MRI changes in a 27-year-old WD patient. (a) Normal T2-FLAIR weighted images. (b) Hyperintensities in the bilateral globus pallidus and thalamus (blue arrows) can be observed in T2-FLAIR weighted images in the WD patient. (c) Normal QSM images. (d) QSM images show increased susceptibility (hyperintensities, black arrows) of bilateral globus pallidus and thalamus in the same patient. QSM, quantatitive susceptibility mapping; T2-FLAIR, T2-fluid attenuated inversion recovery; WD, Wilson’s disease. (A higher resolution / colour version of this figure is available in the electronic copy of the article).
6.3. Genetic Sequencing
Direct genetic sequencing of ATP7B is important for the diagnosis of WD, especially if it cannot be confirmed based on clinical manifestations and laboratory tests alone. There are more than 700 mutations identified in ATP7B and most of them are rare mutations. In addition, most WD patients are compound heterozygotes with two different mutations [7]. All these factors make comprehensive genetic screening difficult for the diagnosis of WD due to time can cost, factors that are likely to change in the future. In contrast, allele-specific probes based on hotspot mutations can directly identify causative mutations in ATP7B, which even now can be fast and helpful for the diagnosis of WD [146]. However, allele-specific probes depend on hotspot mutations in specific populations, such as H1069Q in European and R778L in Asian patients, and miss rare mutations. A genetic study performed in the UK showed that 98% of WD patients had two or more pathogenic mutations if the entire ATP7B gene was sequenced [13]. Therefore, with the advancement of technology and the gradual reduction of the cost, comprehensive gene screening such as whole exon sequencing or whole genome sequencing may contribute to the clinical diagnosis of WD. The diagnosis of WD is supported when one pathogenic mutation in ATP7B is found and confirmed when two pathogenic mutations or homozygosity for a pathogenic mutation is found [67]. ATP7B gene sequencing is recommended for any patient with a clinical diagnosis of WD, and it is recommended for the family screening of primary relatives of WD patients. Twenty-five percent of the siblings of WD patients are likely to develop WD, and 0.5–4% of the offspring of WD patients may develop the disease [67, 147]. Early diagnosis of WD is critical to improve the prognosis of WD, reduce liver and brain damage, and reduce disability. Family screening can identify asymptomatic patients for treatment before clinical symptoms appear. In addition, for the diagnosis of children under the age of three, genetic sequencing is far more reliable than the decreased serum ceruloplasmin levels, which generally reach adult levels at the age of three.
7. THE MANAGEMENT OF WD
Once the diagnosis of WD is established, treatment should begin immediately and continue for life. Most asymptomatic patients who were diagnosed early and treated had a good prognosis and similar survival to healthy people [148]. Early diagnosis and treatment is the most critical factor to improve the prognosis of WD. Patient compliance with treatment is also required for good outcomes. Treatment of WD includes a low-copper diet, drug treatment, liver transplantation, symptomatic treatment and other therapies. Since most copper enters the body through food, a low-copper diet is recommended for WD patients. Copper-rich foods such as animal liver and other organs, shellfish, mushrooms, nuts, and chocolate should be avoided. Copper containers or cooking utensils should also be avoided [67, 149]. Several drugs have been used for the treatment of WD, including D-penicillamine, trientine, zinc salts, tetrathiomolybdate (TM), and dimercaptosuccinic acid (DMSA). Large-scale randomized controlled trials are still lacking to evaluate the efficacy of these drugs, and the selection of which drugs to use is still based on physician experience and preference. The initial treatment of WD should begin with a low dose of chelators and gradually reach a maintenance dose to prevent excessive copper release into the blood causing exacerbation of symptoms. WD patients receiving medication should be continuously monitored to assess treatment compliance, drug effectiveness, and adverse reactions. Neurological and liver symptoms, copper status, liver function, and other organs that may be affected should be regularly evaluated in WD patients [7].
7.1. Drug Treatments
7.1.1. D-Penicillamine
According to different mechanisms of action, drugs for the treatment of WD can be divided into copper chelators and zinc salts. These drugs can create a negative copper balance, promoting the excretion of excess copper from the body through the urine or feces. D-penicillamine is one of the most effective chelating drugs for WD and is widely used worldwide. D-penicillamine can form complexes with free copper via sulfhydryl groups and excrete them through urine. In addition, D-penicillamine may induce the synthesis of tissue MT, thereby reducing the toxicity of free copper [150]. The maintenance dose in an adult is usually 750–1500 mg/day (or 10–15 mg/kg/day) given in two or three divided doses. Children can also be reduced from an initial 20 mg/kg/day dosage to 10–15 mg/kg/day [67]. Food can inhibit the absorption of D-penicillamine, thus it should be taken 1 hour before or 2 hours after meals. The efficacy of D-penicillamine in the treatment of WD has been reported in numerous studies. The liver symptoms of WD patients generally improve within a few months after treatment and may recover further in the first year of treatment [151]. In contrast, patients with neurological symptoms tend to improve their symptoms more slowly. Most severely, neurological symptoms may worsen in 10–50% of patients after receiving D-penicillamine treatment, and some of them are even irreversible. It has been reported that D-penicillamine was more likely to worsen neurological symptoms than trientine and zinc salts in initial treatment [152]. However, several recent retrospective studies found that copper chelators and zinc salts had similar risks of neurological deterioration [151, 153]. The true risk of D-penicillamine-induced neurological deterioration is difficult to accurately assess. Firstly, it is difficult to tell whether the neurological deterioration is caused by D-penicillamine or the progress of the disease itself. Secondly, it is difficult to determine the cause of neurological deterioration in patients who initially received D-penicillamine and then changed or added other drugs. The mechanism of the paradoxical neurological deterioration in initial treatment may be explained by the release of large amounts of copper from the liver and other organs into the circulation, causing oxidative stress and damaging brain tissue [154]. Slowly increasing the dose of the copper chelators during treatment initiation may reduce the risk of neurological deterioration [67]. D-penicillamine has been considered as the second-line anti-copper drug for neurological WD patients, especially ones with severe disease, and some experts recommend other chelators instead of D-penicillamine for initial treatment to reduce the risk of neurological deterioration [152, 155]. D-penicillamine has more adverse drug reactions than other chelators and zinc salts, causing about 30% of patients to be unable to continue taking this drug [156]. Common adverse drug reactions include fever, cutaneous eruptions, neutropenia, thrombocytopenia, proteinuria, and lymphadenopathy may appear in the first three weeks of D-penicillamine treatment [67]. If severe adverse reactions occur, D-penicillamine should be discontinued immediately and replaced with trientine or zinc salts. Late reactions such as nephrotoxicity, lupus-like syndrome, myasthenia-like syndromes, polymyositis, and loss of taste are infrequent but have been reported [151].
The 24-hour urinary copper excretion should be monitored during the treatment of WD with D-penicillamine. Generally, the 24-hour urinary copper excretion increases rapidly after receiving D-penicillamine and can reach 1000 μg/24 hours (16 μmol/24 hours) or even higher, and decreases over time afterwards. Urinary copper excretion during maintenance treatment is generally between 200–500 μg/24 hours. If urinary copper excretion is less than 200 μg/24 hours, there may be overtreatment or non-compliance of the patients with treatment. The concentration of serum non-ceruloplasmin-bound copper in the serum may also reflect the efficacy of drug treatment. However, both serum non-ceruloplasmin-bound copper and 24-hour urinary copper excretion can only be used as a reference for therapeutic effects, not a definite marker, and clinical correlation is important [67, 151].
7.1.2. Trientine
Similar to D-penicillamine, trientine is a chelator that binds to free copper and is mainly excreted in the urine. Trientine has a polyamine-like structure and lacks sulfhydryl groups. Trientine is an effective chelator for WD and has been used successfully in patients who cannot tolerate D-penicillamine [157]. The adverse reactions disappeared after the substitution of D-penicillamine for trientine and did not recur during subsequent treatment. Due to fewer adverse reactions, trientine has been recommended as an alternative drug to D-penicillamine. Trientine is also effective in the initial treatment of WD, even for patients with decompensated liver diseases [158]. The typical dose of trientine for initial treatment is 900–2700 mg/day (or 20 mg/kg/day), divided into two or three times, and 900–1500 mg/day (or 10–15 mg/kg/day) is used for maintenance treatment. For children, the usual dose is 20 mg/kg/day, rounded off to the nearest 250 mg, and overtime may be decreased to maintenance dosages [67]. The copper-chelating potency of trientine may be less than D-penicillamine from a biochemical perspective on a mole per mole basis, but the dose can be adjusted to make up for this difference. Although trientine has fewer adverse reactions than D-penicillamine, it does not reduce the risk of neurological deterioration in the initial treatment of WD. The study conducted by Brewer et al. showed that 6 of 23 neurological WD patients had neurological deterioration after initial treatment with trientine [159]. In a recent large cohort study, patients initially treated with trientine (4 out of 38) even had a higher risk of neurological deterioration than those initially D-penicillamine (6 out of 295) [160]. Trientine can also chelate iron and should be avoided in combination with iron, which can form a toxic complex. Sideroblastic anemia may occur during trientine treatment over time, indicating overtreatment and resultant copper deficiency [67]. The available data still do not determine whether D-penicillamine or trientine is more suitable for the first-line treatment of WD. The selection of drugs should be based on the needs of patients and tolerance to drugs to develop individualized treatment plans, as well as regional availability [4]. Unfortunately, besides the much expensive cost, the drug is not approved for marketing in many countries such as China.
7.1.3. Tetrathiomolybdate
TM is an effective anti-copper agent that interferes with the absorption of copper in the intestinal tract, copper cellular uptake in the circulation and may promote biliary copper excretion [161]. As an experimental drug, TM has not been officially approved for clinical use. Brewer et al. performed a clinical trial involving 55 neurological WD patients and found that ammonium TM could significantly improve neurological symptoms, with only 2 of 55 patients experiencing neurological deterioration [162]. In their subsequent studies, they found that neurological WD patients treated with ammonium TM (1 of 25) had a significantly lower risk of neurological deterioration than trientine (6 of 23), suggesting that ammonium TM may be more suitable than trientine for the initial treatment of neurological WD [159]. A phase 2 clinical trial found another TM compound, the more stable bis-choline TM, could rapidly lower non-ceruloplasmin-bound copper levels and improve neurological symptoms after 24 weeks of treatment. Paradoxical neurological deterioration was not observed in 28 WD patients in this study, and after some initial elevation of alanine transaminase (ALT) in about a third of patients that was reversible with dose adjustment, their liver function remained stable during treatment, suggesting that the drug was safe and effective [163]. TM is a promising anti-copper drug that may reduce the risk of worsening neurological symptoms in the initial treatment of WD, but side effects such as bone marrow depression and liver toxicity need to be reviewed carefully in the phase 3 study that is ongoing to confirm its efficacy and safety in treating WD [67].
7.1.4. Zinc Salts
The mechanism of zinc salts in WD treatment is quite different from that of copper chelators. Zinc salts can inhibit the absorption of dietary copper in the intestine mainly by inducing the production of MT in enterocytes, which has an affinity for copper. Zinc may also induce hepatocytes to produce MT and reduce the toxicity of excess copper to the liver [164]. Commonly used zinc salt preparations include zinc sulfate, acetate, and gluconate. There is no significant difference in efficacy between different zinc salts, but it may affect patient tolerance. For adult patients, the recommended dose is elemental zinc 150 mg/day in three divided doses. For children weighing less than 50 kg, the recommended dose is 75 mg/day in three divided doses. Zinc salts should be taken 1 hour before or 2 hours after meals because the food may interfere with its absorption and efficacy [67, 149]. The efficacy and safety of zinc salts in treating WD have been confirmed in numerous studies and has been used as the first-line drug for initial and maintenance treatment [165]. Zinc salts seem to be as effective as D-penicillamine in both asymptomatic and symptomatic WD patients, but better tolerated [166, 167]. However, Weiss et al. found that hepatic deterioration was more likely to occur in patients treated with zinc monotherapy than with D-penicillamine [153]. Hepatic deterioration may be due to the fact that zinc salts are less effective than chelators in removing tissue copper. In addition, it takes several months to produce a negative copper balance, which may explain the lack of reaction or symptomatic deterioration during initial treatment with this agent [4, 151]. Some experts suggested that all symptomatic WD patients should receive a chelator at the stage of initial treatment, and zinc salts may have a role in maintenance treatment [67, 149]. For patients whose neurological symptoms continue to worsen after treatment with chelators, replacement with zinc may lead to improvement [168]. Zinc salts have few adverse effects, but the most common one is gastric irritation which can be influenced by the type of zinc salts. Neurological deterioration is not common in zinc salts treatment [67]. There is not enough evidence to show that the combination of zinc salts with chelators can increase the efficacy of treatment. Zinc salts can be bound by chelators in the intestinal tract, so if combination therapy is used, the dosing interval between zinc and chelators should be greater than 2 hours.
7.1.5. Dimercaptosuccinic Acid and Other Drugs
DMSA was first invented as a heavy metal chelator by Ding GS et al. in the 1950s. Since trientine was not available in China, patients who cannot tolerate D-penicillamine needed to find an effective alternative chelator. Therefore, Yang RM et al. explored the role of DMSA in WD treatment in the 1980s. DMSA could increase the biliary copper excretion and significantly improve neurological symptoms after only 4 weeks of treatment [169]. Despite the lack of large-scale randomized controlled trials, years of clinical practice have shown that the efficacy of DMSA is similar to D-penicillamine in treating WD, but with fewer adverse effects [170]. In China, DMSA has been used for patients who are unable to tolerate D-penicillamine or suffer from neurological deterioration after taking D-penicillamine and has been used as a first-line chelator for patients with a neuropsychiatric symptom or mild liver symptoms [30]. Sodium dimercaptosulphonate (Na-DMPS) has a similar structure to DMSA and is used intravenously in the maintenance treatment of WD. The combination of D-penicillamine crossover with intravenous DMPS therapy may be more effective in improving symptoms of WD patients, reducing adverse reactions to continuous drug therapy, and maintaining life-long treatment [171, 172]. In addition, Wang et al. found that captopril, an antihypertensive drug, also had a mild anti-copper effect and could reduce portal hypertension caused by cirrhosis [171]. In China, traditional Chinese medicine (TCM) is used as an adjunct drug for WD treatment. The TCM preparations commonly used to treat WD are Gandou decoction and Gandou tablets, which are composed of Chinese herbal medicines including Radix et Rhizoma rhei, Rhizoma coptidis, Herba andrographidis, Herba scutellariae barbatae and Rhizoma dioscoreae hypoglaucae [173]. TCM combined with chelators may have better efficacy and fewer adverse reactions than chelators alone, especially for patients with liver symptoms [174, 175]. Antioxidants, such as vitamin E and curcumin, can reduce oxidative stress caused by excessive copper deposition and may become adjuvant drugs for the treatment of WD [67].
7.2. Early Neurological Deterioration
Neurologic deterioration is the major challenge for the anti-copper treatment of WD, especially in neurological WD patients. The mechanism of neurological deterioration is still unclear, which may be caused by the transient increase of non-ceruloplasmin-bound copper in the serum and CSF after anti-copper treatment [67]. Early neurological deterioration tends to occur within the first 6 months after anti-copper treatment, especially in patients with neurological symptoms at the time of diagnosis. Just as discussed above, D-penicillamine was once thought to be the drug most likely to cause neurological deterioration. It is estimated that nearly 50% of patients could experience neurological deterioration after D-penicillamine treatment [67, 152]. However, recent studies indicated that all copper chelators and zinc salts may lead to neurological deterioration with a similar frequency of less than 10% [151]. A retrospective performed by Weiss et al. found that neurological deterioration occurred in 9.1% of WD patients on D-penicillamine, 8.8% on trientine, and 7.3% on zinc salts [153]. In subsequent studies, they found that patients who were initially treated with D-penicillamine were even less likely to develop neurological deterioration than those who were initially treated with trientine [160]. Litwin et al. reported that neurological deterioration was observed in 16 of 143 WD patients (about 11%) and involved only patients with neurological symptoms at diagnosis, and there were no significant differences between initial treatment with D-penicillamine or zinc salts [176]. Patients with severe baseline neurological symptoms, thalamic and brainstem lesions, and using dopamine receptor antagonists may have a higher risk of neurological deterioration [176]. In addition, stress events like traumatic, surgical or emotional events may exacerbate neurological symptoms in WD patients [177]. There is no recommended effective treatment for neurological deterioration, and it may be helpful to reduce the dose of the currently used drug or switch to another drug. Slowly increasing the dose of D-penicillamine at the initial treatment may reduce the risk of neurological deterioration [67, 153]. It is worth noting that persistence with treatment is critical to the long-term prognosis of WD patients. Non-persistent WD patients presented more often with neurologic deterioration than persistent patients [178].
7.3. Liver Transplantation for Abdominal WD
Most WD patients can improve their clinical symptoms through medication and achieve long-term survival through maintenance treatment. However, for some patients who are unresponsive or intolerant to the medication, liver function may continue to deteriorate and eventually become decompensated cirrhosis. Approximately 5% of WD patients develop ALF, and another 10% develop chronic end-stage liver disease. Liver transplantation is necessary and life-saving for these patients [179]. Since WD is a disease caused by abnormal copper metabolism in the liver, liver transplantation can restore the copper excretion capacity of the liver and even improve neurological symptoms. Transplantation indications for ALF are usually based on a revised King’s prognostic Wilson Index, and liver transplantation is recommended when the score reaches 11 or higher [67, 180]. The prognosis of liver transplantation is satisfactory. According to two follow-up studies, the one-year survival rate after orthotopic liver transplantation was 79% and the maximum survival time was 20 years. Compared with patients with ALF, graft survival is better for patients with chronic advanced liver cirrhosis due to WD [181, 182]. The outcome of pediatric liver transplantation is likely to be better than that of adult liver transplantation. A recent systematic review of 290 pediatric WD patients showed that the one-year survival rate for children with liver transplantation was 91.9% and the five-year survival rate was 88.2% [183]. Whether liver transplantation can be used as a treatment for severe neurological symptoms remains controversial, and neurological deterioration may still occur after liver transplantation [184]. Liver transplantation is currently the only effective way to save patients with liver failure, but the limited source of liver prevents many patients from receiving the operation in time. Hepatocyte or stem cell transplants are promising treatments that may replace liver transplantation for WD in the future [185].
7.4. Symptomatic Treatment
In addition to anti-copper treatment, symptomatic treatment is also very important for WD patients. Symptomatic impairment of liver function or liver cirrhosis is a common feature of WD and usually appears at the time of diagnosis. WD patients with symptomatic hepatic manifestations are recommended to be treated with copper chelators. Strict adherence to the chelators can partially reverse the liver damage and prevent the progression of cirrhosis, thereby minimizing its complications [153, 186]. WD patients with steatosis, liver fibrosis, or compensated liver cirrhosis should avoid further damage to the liver by exogenous hepatotoxic substances, such as alcohol, drugs with known hepatotoxicity, as well as some herbal supplements [186]. In WD patients with cirrhosis, regular esophagogastroduodenoscopy (EGD) is necessary to screen for esophageal or gastric varices. Generally, after strict anti-copper treatment, liver function improves or remains stable, and rarely continues to deteriorate into decompensated cirrhosis. However, a small number of patients with concomitant liver disease, taking hepatotoxic drugs, or having poor treatment compliance may cause decompensation of liver function [151]. Patients with decompensated cirrhosis have a series of complications caused by portal hypertension (ascites, spontaneous bacterial peritonitis, hepatorenal syndrome, gastroesophageal variceal hemorrhage) and complications caused by liver insufficiency (hepatic encephalopathy, infections, and jaundice) [186]. While WD patients receive anti-copper treatment, the treatment of liver symptoms should be performed under the guidance of a gastroenterologist.
Symptomatic treatment should be considered when neurological symptoms are not relieved or neurological deterioration occurs during anti-copper treatment. The symptomatic treatment is mainly based on the predominant neurological symptoms such as tremor, dystonia, and parkinsonism [7]. Treatments for neurological symptoms caused by other etiologies may also be helpful for the symptomatic treatment in WD patients. The choice of drugs for different types of tremor varies greatly. Beta-blockers (e.g., propranolol and atenolol) may be the best treatment option for tremor mimicking essential tremor. Botulinum toxin injection is considered the best treatment option for dystonic tremor involving head and face. High doses of levodopa, anticholinergic drugs (e.g., trihexyphenidyl), benzodiazepines (e.g., clonazepam), and levetiracetam, may be effective for rubral tremor [187, 188]. Focal dystonias can choose botulinum toxin injections, while multisegmental or generalized dystonia should use drugs including anticholinergics, baclofen, benzodiazepines, dopamine receptor antagonists, and dopamine-depleting drugs. Levodopa and dopamine agonists can be used for parkinsonism in WD patients, but the effect may not be as good as in Parkinson’s disease. For patients with persistent or progressive severe dysphagia, percutaneous gastrostomy (PEG) should be considered. Anticholinergic drugs or botulinum toxin injections in the parotid and submandibular glands may be helpful for drooling [187, 188]. For patients with severe neurological symptoms who fail drug treatment, neurosurgical procedures such as deep brain stimulation (DBS) may be the last effective method to relieve neurological symptoms [189].
7.5. Psychiatric Symptom Treatment
Various psychiatric symptoms may occur in WD patients at any stage of the disease [112]. For the treatment of mental symptoms, it should be noted that some psychoactive drugs may affect or even worsen liver function and neurological symptoms in WD patients [7]. Selective serotonin reuptake inhibitors (SSRI) seems to be a reasonable choice for the treatment of depression in WD patients. Neurological deterioration was observed in some patients treated with a tricyclic antidepressant (TCA) [190]. Other antidepressant drugs including selective serotonin reuptake inhibitors (SNRIs), serotonin antagonist and reuptake inhibitors (SARIs), and electroconvulsive therapy (ECT), can be used alternative options for the treatment of depression in WD patients. Antidepressant drugs that may cause liver injuries such as iproniazid, phenelzine, imipramine, amitriptyline, and duloxetine, should be avoided [191]. Mood stabilizers such as lithium and antiepileptic drugs (e.g., carbamazepine and oxcarbazepine) can be used in the treatment of manic/hypomanic syndrome in WD. Valproate should be avoided for its hepatotoxicity [7]. For the treatment of psychosis, the first-generation antipsychotics should be avoided as they may cause neurological deterioration [176]. Clozapine and quetiapine are recommended for the treatment of psychiatric symptoms in Parkinson’s disease, and they are safe options for WD patients for their low risk of extrapyramidal symptoms. However, clozapine treatment may increase the risk of leucopenia and dose-related seizures, therefore regular blood analysis is need during using clozapine. Other antipsychotics such as aripiprazole and olanzapine are alternative options for the treatment of psychosis. Olanzapine and quetiapine have a moderate risk of liver injury and should be used with caution in WD patients with hepatic symptoms. SSRIs, antiepileptics, and benzodiazepines may be used for the treatment of behavioral symptoms and personality disorders in WD patients [7, 191]. More detail about the management of psychiatric symptoms can be found in the recent published review by Litwin et al. [191].
CONCLUSION
It has been more than 100 years since Wilson reported on the disease in his monograph published in 1912. The understanding of WD has expanded from recognizing simple copper deposition to the level of understanding the pathophysiology and genetic pathways controlling copper metabolism. According to the recent large-scale genetic study, the prevalence of WD is higher than previously estimated [13]. The number of WD patients may be significantly underestimated. Many patients are disabled or even die for not receiving a timely diagnosis and disease-specific treatment. Therefore, a comprehensive understanding of WD is necessary for neurologists and gastroenterologists. Early diagnosis of WD is the key to improving its prognosis, and in the future neonatal screening may help with the earliest diagnosis [67, 149]. The diagnosis of WD can be easily established according to the typical neurological or liver symptoms, decreased serum ceruloplasmin levels, the presence of Kayser-Fleischer rings, and abnormal copper metabolism indicated by laboratory tests. But for asymptomatic patients and patients with only one of these manifestations, the diagnosis of WD is often difficult. Gene sequencing can provide direct evidence for the diagnosis of WD, especially in patients with atypical symptoms and in asymptomatic patients. Family screening is recommended if patients are diagnosed with WD. New technologies such as direct measurement of serum non-ceruloplasmin-bound copper concentration and metal-sensitive MRI sequence QSM may also provide clues to the diagnosis of WD [129, 141]. Drug therapy is effective in improving liver symptoms of WD patients but has a limited effect on neurological symptoms. More importantly, paradoxical early neurological deterioration during treatment initiation remains unexplained and is one of the most serious adverse effects of therapy with D-penicillamine and trientine. An individualized treatment plan for WD patients should be developed according to their response and tolerance to the medication. Gene therapy and cell therapy are new directions for the treatment of WD, which are still in the pre-clinical stages, and may be used for the treatment of WD in the future [192, 193].
ACKNOWLEDGEMENTS
The authors express many thanks to Professor Michael Schilsky at Yale University Med Sch.
CONSENT FOR PUBLICATION
Not applicable.
FUNDING
This study was supported by grants from the National Natural Science Foundation of China (81671103 and 8201101090) and Shanghai Jiao Tong University Medical School Innovative Research Plan 2015 (TM201520).
CONFLICT OF INTEREST
The author declares no conflict of interest, financial or otherwise.
REFERENCES
- 1.Barceloux D.G. Copper. J. Toxicol. Clin. Toxicol. 1999;37(2):217–230. doi: 10.1081/CLT-100102421. [DOI] [PubMed] [Google Scholar]
- 2.Tanzi R.E., Petrukhin K., Chernov I., Pellequer J.L., Wasco W., Ross B., Romano D.M., Parano E., Pavone L., Brzustowicz L.M. The Wilson disease gene is a copper transporting ATPase with homology to the Menkes disease gene. Nat. Genet. 1993;5(4):344–350. doi: 10.1038/ng1293-344. [DOI] [PubMed] [Google Scholar]
- 3.Gu M., Cooper J.M., Butler P., Walker A.P., Mistry P.K., Dooley J.S., Schapira A.H. Oxidative-phosphorylation defects in liver of patients with Wilson’s disease. Lancet. 2000;356(9228):469–474. doi: 10.1016/S0140-6736(00)02556-3. [DOI] [PubMed] [Google Scholar]
- 4.Bandmann O., Weiss K.H., Kaler S.G. Wilson’s disease and other neurological copper disorders. Lancet Neurol. 2015;14(1):103–113. doi: 10.1016/S1474-4422(14)70190-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang B., Wang X.P. Does ceruloplasmin defend against neurodegenerative diseases? Curr. Neuropharmacol. 2019;17(6):539–549. doi: 10.2174/1570159X16666180508113025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Grandis D.J., Nah G., Whitman I.R., Vittinghoff E., Dewland T.A., Olgin J.E., Marcus G.M. Wilson’s disease and cardiac myopathy. Am. J. Cardiol. 2017;120(11):2056–2060. doi: 10.1016/j.amjcard.2017.08.025. [DOI] [PubMed] [Google Scholar]
- 7.Członkowska A., Litwin T., Dusek P., Ferenci P., Lutsenko S., Medici V., Rybakowski J.K., Weiss K.H., Schilsky M.L. Wilson disease. Nat. Rev. Dis. Primers. 2018;4(1):21. doi: 10.1038/s41572-018-0018-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ferenci P. Regional distribution of mutations of the ATP7B gene in patients with Wilson disease: impact on genetic testing. Hum. Genet. 2006;120(2):151–159. doi: 10.1007/s00439-006-0202-5. [DOI] [PubMed] [Google Scholar]
- 9.Caca K., Ferenci P., Kühn H.J., Polli C., Willgerodt H., Kunath B., Hermann W., Mössner J., Berr F. High prevalence of the H1069Q mutation in East German patients with Wilson disease: rapid detection of mutations by limited sequencing and phenotype-genotype analysis. J. Hepatol. 2001;35(5):575–581. doi: 10.1016/S0168-8278(01)00219-7. [DOI] [PubMed] [Google Scholar]
- 10.Mihaylova V., Todorov T., Jelev H., Kotsev I., Angelova L., Kosseva O., Georgiev G., Ganeva R., Cherninkova S., Tankova L., Savov A., Tournev I. Neurological symptoms, genotype-phenotype correlations and ethnic-specific differences in Bulgarian patients with Wilson disease. Neurologist. 2012;18(4):184–189. doi: 10.1097/NRL.0b013e31825cf3b7. [DOI] [PubMed] [Google Scholar]
- 11.Ferenci P., Stremmel W., Członkowska A., Szalay F., Viveiros A., Stättermayer A.F., Bruha R., Houwen R., Pop T.L., Stauber R., Gschwantler M., Pfeiffenberger J., Yurdaydin C., Aigner E., Steindl-Munda P., Dienes H.P., Zoller H., Weiss K.H. Age and Sex but Not ATP7B Genotype Effectively Influence the Clinical Phenotype of Wilson Disease. Hepatology. 2019;69(4):1464–1476. doi: 10.1002/hep.30280. [DOI] [PubMed] [Google Scholar]
- 12.Shah A.B., Chernov I., Zhang H.T., Ross B.M., Das K., Lutsenko S., Parano E., Pavone L., Evgrafov O., Ivanova-Smolenskaya I.A., Annerén G., Westermark K., Urrutia F.H., Penchaszadeh G.K., Sternlieb I., Scheinberg I.H., Gilliam T.C., Petrukhin K. Identification and analysis of mutations in the Wilson disease gene (ATP7B): population frequencies, genotype-phenotype correlation, and functional analyses. Am. J. Hum. Genet. 1997;61(2):317–328. doi: 10.1086/514864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Coffey A.J., Durkie M., Hague S., McLay K., Emmerson J., Lo C., Klaffke S., Joyce C.J., Dhawan A., Hadzic N., Mieli-Vergani G., Kirk R., Elizabeth Allen K., Nicholl D., Wong S., Griffiths W., Smithson S., Giffin N., Taha A., Connolly S., Gillett G.T., Tanner S., Bonham J., Sharrack B., Palotie A., Rattray M., Dalton A., Bandmann O. A genetic study of Wilson’s disease in the United Kingdom. Brain. 2013;136(Pt 5):1476–1487. doi: 10.1093/brain/awt035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu X.Q., Zhang Y.F., Liu T.T., Hsiao K.J., Zhang J.M., Gu X.F., Bao K.R., Yu L.H., Wang M.X. Correlation of ATP7B genotype with phenotype in Chinese patients with Wilson disease. World J. Gastroenterol. 2004;10(4):590–593. doi: 10.3748/wjg.v10.i4.590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dong Y., Ni W., Chen W.J., Wan B., Zhao G.X., Shi Z.Q., Zhang Y., Wang N., Yu L., Xu J.F., Wu Z.Y. Spectrum and Classification of ATP7B variants in a large cohort of chinese patients with Wilson’s Disease Guides Genetic Diagnosis. Theranostics. 2016;6(5):638–649. doi: 10.7150/thno.14596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Park S., Park J.Y., Kim G.H., Choi J.H., Kim K.M., Kim J.B., Yoo H.W. Identification of novel ATP7B gene mutations and their functional roles in Korean patients with Wilson disease. Hum. Mutat. 2007;28(11):1108–1113. doi: 10.1002/humu.20574. [DOI] [PubMed] [Google Scholar]
- 17.Okada T., Shiono Y., Hayashi H., Satoh H., Sawada T., Suzuki A., Takeda Y., Yano M., Michitaka K., Onji M., Mabuchi H. Mutational analysis of ATP7B and genotype-phenotype correlation in Japanese with Wilson’s disease. Hum. Mutat. 2000;15(5):454–462. doi: 10.1002/(SICI)1098-1004(200005)15:5<454:AID-HUMU7>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 18.Tatsumi Y., Hattori A., Hayashi H., Ikoma J., Kaito M., Imoto M., Wakusawa S., Yano M., Hayashi K., Katano Y., Goto H., Okada T., Kaneko S. Current state of Wilson disease patients in central Japan. Intern. Med. 2010;49(9):809–815. doi: 10.2169/internalmedicine.49.2931. [DOI] [PubMed] [Google Scholar]
- 19.Gupta A., Aikath D., Neogi R., Datta S., Basu K., Maity B., Trivedi R., Ray J., Das S.K., Gangopadhyay P.K., Ray K. Molecular pathogenesis of Wilson disease: haplotype analysis, detection of prevalent mutations and genotype-phenotype correlation in Indian patients. Hum. Genet. 2005;118(1):49–57. doi: 10.1007/s00439-005-0007-y. [DOI] [PubMed] [Google Scholar]
- 20.Mukherjee S., Dutta S., Majumdar S., Biswas T., Jaiswal P., Sengupta M., Bhattacharya A., Gangopadhyay P.K., Bavdekar A., Das S.K., Ray K. Genetic defects in Indian Wilson disease patients and genotype-phenotype correlation. Parkinsonism Relat. Disord. 2014;20(1):75–81. doi: 10.1016/j.parkreldis.2013.09.021. [DOI] [PubMed] [Google Scholar]
- 21.Chang I.J., Hahn S.H. The genetics of Wilson disease. Handb. Clin. Neurol. 2017;142:19–34. doi: 10.1016/B978-0-444-63625-6.00003-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Curtis D., Durkie M., Balac P., Sheard D., Goodeve A., Peake I., Quarrell O., Tanner S. A study of Wilson disease mutations in Britain. Hum. Mutat. 1999;14(4):304–311. doi: 10.1002/(SICI)1098-1004(199910)14:4<304:AID-HUMU5>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 23.Cheng N., Wang H., Wu W., Yang R., Liu L., Han Y., Guo L., Hu J., Xu L., Zhao J., Han Y., Liu Q., Li K., Wang X., Chen W. Spectrum of ATP7B mutations and genotype-phenotype correlation in large-scale Chinese patients with Wilson Disease. Clin. Genet. 2017;92(1):69–79. doi: 10.1111/cge.12951. [DOI] [PubMed] [Google Scholar]
- 24.Li K., Zhang W.M., Lin S., Wen L., Wang Z.F., Xie D., Wei M., Qiu Z.Q., Dai Y., Lin M.C., Kung H.F., Yao F.X. Mutational analysis of ATP7B in north Chinese patients with Wilson disease. J. Hum. Genet. 2013;58(2):67–72. doi: 10.1038/jhg.2012.134. [DOI] [PubMed] [Google Scholar]
- 25.Merle U., Weiss K.H., Eisenbach C., Tuma S., Ferenci P., Stremmel W. Truncating mutations in the Wilson disease gene ATP7B are associated with very low serum ceruloplasmin oxidase activity and an early onset of Wilson disease. BMC Gastroenterol. 2010;10:8. doi: 10.1186/1471-230X-10-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dmitriev O.Y., Bhattacharjee A., Nokhrin S., Uhlemann E.M., Lutsenko S. Difference in stability of the N-domain underlies distinct intracellular properties of the E1064A and H1069Q mutants of copper-transporting ATPase ATP7B. J. Biol. Chem. 2011;286(18):16355–16362. doi: 10.1074/jbc.M110.198101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Payne A.S., Kelly E.J., Gitlin J.D. Functional expression of the Wilson disease protein reveals mislocalization and impaired copper-dependent trafficking of the common H1069Q mutation. Proc. Natl. Acad. Sci. USA. 1998;95(18):10854–10859. doi: 10.1073/pnas.95.18.10854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wu Z.Y., Wang N., Lin M.T., Fang L., Murong S.X. Genotype-phenotype correlation of patients with wilson disease in Chinese population. Zhonghua Yi Xue Za Zhi. 2003;83(4):309–311. [PubMed] [Google Scholar]
- 29.Li X., Lu Z., Lin Y., Lu X., Xu Y., Cheng J., Shao Y., Su X., Liu Z., Sheng H., Cai Y., Li T., Zhou Z., Tan J., Liu H., Huang Y., Liu L., Zeng C. Clinical features and mutational analysis in 114 young children with Wilson disease from South China. Am. J. Med. Genet. A. 2019;179(8):1451–1458. doi: 10.1002/ajmg.a.61254. [DOI] [PubMed] [Google Scholar]
- 30.Xie J.J., Wu Z.Y. Wilson’s Disease in China. Neurosci. Bull. 2017;33(3):323–330. doi: 10.1007/s12264-017-0107-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schiefermeier M., Kollegger H., Madl C., Polli C., Oder W., Kühn H., Berr F., Ferenci P. The impact of apolipoprotein E genotypes on age at onset of symptoms and phenotypic expression in Wilson’s disease. Brain. 2000;123(Pt 3):585–590. doi: 10.1093/brain/123.3.585. [DOI] [PubMed] [Google Scholar]
- 32.Gromadzka G., Rudnicka M., Chabik G., Przybyłkowski A., Członkowska A. Genetic variability in the methylenetetrahydrofolate reductase gene (MTHFR) affects clinical expression of Wilson’s disease. J. Hepatol. 2011;55(4):913–919. doi: 10.1016/j.jhep.2011.01.030. [DOI] [PubMed] [Google Scholar]
- 33.Yu J.T., Tan L., Hardy J. Apolipoprotein E in Alzheimer’s disease: an update. Annu. Rev. Neurosci. 2014;37:79–100. doi: 10.1146/annurev-neuro-071013-014300. [DOI] [PubMed] [Google Scholar]
- 34.Litwin T., Gromadzka G., Członkowska A. Apolipoprotein E gene (APOE) genotype in Wilson’s disease: impact on clinical presentation. Parkinsonism Relat. Disord. 2012;18(4):367–369. doi: 10.1016/j.parkreldis.2011.12.005. [DOI] [PubMed] [Google Scholar]
- 35.Gu Y.H., Kodama H., Du S.L. Apolipoprotein E genotype analysis in Chinese Han ethnic children with Wilson’s disease, with a concentration on those homozygous for R778L. Brain Dev. 2005;27(8):551–553. doi: 10.1016/j.braindev.2005.01.006. [DOI] [PubMed] [Google Scholar]
- 36.Kocabay G., Tutuncu Y., Yilmaz H., Demir K. Impact of apolipoprotein E genotypes on phenotypic expression in Turkish patients with Wilson’s disease. Scand. J. Gastroenterol. 2009;44(10):1270–1271. doi: 10.1080/00365520903225908. [DOI] [PubMed] [Google Scholar]
- 37.Kluska A., Kulecka M., Litwin T., Dziezyc K., Balabas A., Piatkowska M., Paziewska A., Dabrowska M., Mikula M., Kaminska D., Wiernicka A., Socha P., Czlonkowska A., Ostrowski J. Whole-exome sequencing identifies novel pathogenic variants across the ATP7B gene and some modifiers of Wilson’s disease phenotype. Liver Int. 2019;39(1):177–186. doi: 10.1111/liv.13967. [DOI] [PubMed] [Google Scholar]
- 38.Medici V., LaSalle J.M. Genetics and epigenetic factors of Wilson disease. Ann. Transl. Med. 2019;7(Suppl. 2):S58. doi: 10.21037/atm.2019.01.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wilson’s disease Br. Med. J. (Clin. Res. Ed.) 1984;288(6431):1689. doi: 10.1136/bmj.288.6431.1689-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Olivarez L., Caggana M., Pass K.A., Ferguson P., Brewer G.J. Estimate of the frequency of Wilson’s disease in the US Caucasian population: a mutation analysis approach. Ann. Hum. Genet. 2001;65(Pt 5):459–463. doi: 10.1046/j.1469-1809.2001.6550459.x. [DOI] [PubMed] [Google Scholar]
- 41.Olsson C., Waldenström E., Westermark K., Landegre U., Syvänen A.C. Determination of the frequencies of ten allelic variants of the Wilson disease gene (ATP7B), in pooled DNA samples. Eur. J. Hum. Genet. 2000;8(12):933–938. doi: 10.1038/sj.ejhg.5200566. [DOI] [PubMed] [Google Scholar]
- 42.Dusek P., Litwin T., Czlonkowska A. Wilson disease and other neurodegenerations with metal accumulations. Neurol. Clin. 2015;33(1):175–204. doi: 10.1016/j.ncl.2014.09.006. [DOI] [PubMed] [Google Scholar]
- 43.Hahn S.H., Lee S.Y., Jang Y.J., Kim S.N., Shin H.C., Park S.Y., Han H.S., Yu E.S., Yoo H.W., Lee J.S., Chung C.S., Lee S.Y., Lee D.H. Pilot study of mass screening for Wilson’s disease in Korea. Mol. Genet. Metab. 2002;76(2):133–136. doi: 10.1016/S1096-7192(02)00026-4. [DOI] [PubMed] [Google Scholar]
- 44.Ohura T., Abukawa D., Shiraishi H., Yamaguchi A., Arashima S., Hiyamuta S., Tada K., Iinuma K. Pilot study of screening for Wilson disease using dried blood spots obtained from children seen at outpatient clinics. J. Inherit. Metab. Dis. 1999;22(1):74–80. doi: 10.1023/A:1005455401076. [DOI] [PubMed] [Google Scholar]
- 45.Mak C.M., Lam C.W., Tam S., Lai C.L., Chan L.Y., Fan S.T., Lau Y.L., Lai J.Y., Yuen P., Hui J., Fu C.C., Wong K.S., Mak W.L., Tze K., Tong S.F., Lau A., Leung N., Hui A., Cheung K.M., Ko C.H., Chan Y.K., Ma O., Chau T.N., Chiu A., Chan Y.W. Mutational analysis of 65 Wilson disease patients in Hong Kong Chinese: identification of 17 novel mutations and its genetic heterogeneity. J. Hum. Genet. 2008;53(1):55–63. doi: 10.1007/s10038-007-0218-2. [DOI] [PubMed] [Google Scholar]
- 46.Park H.D., Ki C.S., Lee S.Y., Kim J.W. Carrier frequency of the R778L, A874V, and N1270S mutations in the ATP7B gene in a Korean population. Clin. Genet. 2009;75(4):405–407. doi: 10.1111/j.1399-0004.2008.01132.x. [DOI] [PubMed] [Google Scholar]
- 47.Ferenci P., Roberts E.A. Defining Wilson disease phenotypes: from the patient to the bench and back again. Gastroenterology. 2012;142(4):692–696. doi: 10.1053/j.gastro.2012.02.035. [DOI] [PubMed] [Google Scholar]
- 48.Scheiber I.F., Brůha R., Dušek P. Pathogenesis of Wilson disease. Handb. Clin. Neurol. 2017;142:43–55. doi: 10.1016/B978-0-444-63625-6.00005-7. [DOI] [PubMed] [Google Scholar]
- 49.Scheiber I.F., Mercer J.F., Dringen R. Metabolism and functions of copper in brain. Prog. Neurobiol. 2014;116:33–57. doi: 10.1016/j.pneurobio.2014.01.002. [DOI] [PubMed] [Google Scholar]
- 50.Chen C., Shen B., Xiao J.J., Wu R., Duff Canning S.J., Wang X.P. Currently Clinical Views on Genetics of Wilson’s Disease. Chin. Med. J. (Engl.) 2015;128(13):1826–1830. doi: 10.4103/0366-6999.159361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Schwerdtle T., Hamann I., Jahnke G., Walter I., Richter C., Parsons J.L., Dianov G.L., Hartwig A. Impact of copper on the induction and repair of oxidative DNA damage, poly(ADP-ribosyl)ation and PARP-1 activity. Mol. Nutr. Food Res. 2007;51(2):201–210. doi: 10.1002/mnfr.200600107. [DOI] [PubMed] [Google Scholar]
- 52.Lorincz M.T. Wilson disease and related copper disorders. Handb. Clin. Neurol. 2018;147:279–292. doi: 10.1016/B978-0-444-63233-3.00018-X. [DOI] [PubMed] [Google Scholar]
- 53.Lee J., Peña M.M., Nose Y., Thiele D.J. Biochemical characterization of the human copper transporter Ctr1. J. Biol. Chem. 2002;277(6):4380–4387. doi: 10.1074/jbc.M104728200. [DOI] [PubMed] [Google Scholar]
- 54.Bulcke F., Dringen R., Scheiber I.F. Neurotoxicity of Copper. Adv. Neurobiol. 2017;18:313–343. doi: 10.1007/978-3-319-60189-2_16. [DOI] [PubMed] [Google Scholar]
- 55.Rae T.D., Schmidt P.J., Pufahl R.A., Culotta V.C., O’Halloran T.V. Undetectable intracellular free copper: the requirement of a copper chaperone for superoxide dismutase. Science. 1999;284(5415):805–808. doi: 10.1126/science.284.5415.805. [DOI] [PubMed] [Google Scholar]
- 56.Cobine P.A., Ojeda L.D., Rigby K.M., Winge D.R. Yeast contain a non-proteinaceous pool of copper in the mitochondrial matrix. J. Biol. Chem. 2004;279(14):14447–14455. doi: 10.1074/jbc.M312693200. [DOI] [PubMed] [Google Scholar]
- 57.Leary S.C., Winge D.R., Cobine P.A. “Pulling the plug” on cellular copper: the role of mitochondria in copper export. Biochim. Biophys. Acta. 2009;1793(1):146–153. doi: 10.1016/j.bbamcr.2008.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tadini-Buoninsegni F., Smeazzetto S. Mechanisms of charge transfer in human copper ATPases ATP7A and ATP7B. IUBMB Life. 2017;69(4):218–225. doi: 10.1002/iub.1603. [DOI] [PubMed] [Google Scholar]
- 59.Zischka H., Lichtmannegger J., Schmitt S., Jägemann N., Schulz S., Wartini D., Jennen L., Rust C., Larochette N., Galluzzi L., Chajes V., Bandow N., Gilles V.S., DiSpirito A.A., Esposito I., Goettlicher M., Summer K.H., Kroemer G. Liver mitochondrial membrane crosslinking and destruction in a rat model of Wilson disease. J. Clin. Invest. 2011;121(4):1508–1518. doi: 10.1172/JCI45401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gunther M.R., Hanna P.M., Mason R.P., Cohen M.S. Hydroxyl radical formation from cuprous ion and hydrogen peroxide: a spin-trapping study. Arch. Biochem. Biophys. 1995;316(1):515–522. doi: 10.1006/abbi.1995.1068. [DOI] [PubMed] [Google Scholar]
- 61.Halliwell B. Oxidative stress and neurodegeneration: where are we now? J. Neurochem. 2006;97(6):1634–1658. doi: 10.1111/j.1471-4159.2006.03907.x. [DOI] [PubMed] [Google Scholar]
- 62.Lang P.A., Schenck M., Nicolay J.P., Becker J.U., Kempe D.S., Lupescu A., Koka S., Eisele K., Klarl B.A., Rübben H., Schmid K.W., Mann K., Hildenbrand S., Hefter H., Huber S.M., Wieder T., Erhardt A., Häussinger D., Gulbins E., Lang F. Liver cell death and anemia in Wilson disease involve acid sphingomyelinase and ceramide. Nat. Med. 2007;13(2):164–170. doi: 10.1038/nm1539. [DOI] [PubMed] [Google Scholar]
- 63.Letelier M.E., Sánchez-Jofré S., Peredo-Silva L., Cortés-Troncoso J., Aracena-Parks P. Mechanisms underlying iron and copper ions toxicity in biological systems: Pro-oxidant activity and protein-binding effects. Chem. Biol. Interact. 2010;188(1):220–227. doi: 10.1016/j.cbi.2010.06.013. [DOI] [PubMed] [Google Scholar]
- 64.Yurkova I.L., Arnhold J., Fitzl G., Huster D. Fragmentation of mitochondrial cardiolipin by copper ions in the Atp7b-/- mouse model of Wilson’s disease. Chem. Phys. Lipids. 2011;164(5):393–400. doi: 10.1016/j.chemphyslip.2011.05.006. [DOI] [PubMed] [Google Scholar]
- 65.Huster D., Finegold M.J., Morgan C.T., Burkhead J.L., Nixon R., Vanderwerf S.M., Gilliam C.T., Lutsenko S. Consequences of copper accumulation in the livers of the Atp7b-/- (Wilson disease gene) knockout mice. Am. J. Pathol. 2006;168(2):423–434. doi: 10.2353/ajpath.2006.050312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zischka H., Lichtmannegger J. Pathological mitochondrial copper overload in livers of Wilson’s disease patients and related animal models. Ann. N. Y. Acad. Sci. 2014;1315:6–15. doi: 10.1111/nyas.12347. [DOI] [PubMed] [Google Scholar]
- 67.European Association for Study of Liver EASL Clinical Practice Guidelines: Wilson’s disease. J. Hepatol. 2012;56(3):671–685. doi: 10.1016/j.jhep.2011.11.007. [DOI] [PubMed] [Google Scholar]
- 68.Mounajjed T., Oxentenko A.S., Qureshi H., Smyrk T.C. Revisiting the topic of histochemically detectable copper in various liver diseases with special focus on venous outflow impairment. Am. J. Clin. Pathol. 2013;139(1):79–86. doi: 10.1309/AJCPDZR4OHDQNG3L. [DOI] [PubMed] [Google Scholar]
- 69.Johncilla M., Mitchell K.A. Pathology of the liver in copper overload. Semin. Liver Dis. 2011;31(3):239–244. doi: 10.1055/s-0031-1286055. [DOI] [PubMed] [Google Scholar]
- 70.Guindi M. Wilson disease. Semin. Diagn. Pathol. 2019;36(6):415–422. doi: 10.1053/j.semdp.2019.07.008. [DOI] [PubMed] [Google Scholar]
- 71.Pronicki M. Wilson disease - liver pathology. Handb. Clin. Neurol. 2017;142:71–75. doi: 10.1016/B978-0-444-63625-6.00007-0. [DOI] [PubMed] [Google Scholar]
- 72.Santos B.C., Guedes L.R., Faria L.C., Couto C.A. Wilson’s disease presentation resembling autoimmune hepatitis. BMJ Case Rep. 2019;12(10):e230721. doi: 10.1136/bcr-2019-230721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lech T., Sadlik J.K. Copper concentration in body tissues and fluids in normal subjects of southern Poland. Biol. Trace Elem. Res. 2007;118(1):10–15. doi: 10.1007/s12011-007-0014-z. [DOI] [PubMed] [Google Scholar]
- 74.Horoupian D.S., Sternlieb I., Scheinberg I.H. Neuropathological findings in penicillamine-treated patients with Wilson’s disease. Clin. Neuropathol. 1988;7(2):62–67. [PubMed] [Google Scholar]
- 75.Poujois A., Mikol J., Woimant F. Wilson disease: brain pathology. Handb. Clin. Neurol. 2017;142:77–89. doi: 10.1016/B978-0-444-63625-6.00008-2. [DOI] [PubMed] [Google Scholar]
- 76.Faa G., Lisci M., Caria M.P., Ambu R., Sciot R., Nurchi V.M., Silvagni R., Diaz A., Crisponi G. Brain copper, iron, magnesium, zinc, calcium, sulfur and phosphorus storage in Wilson’s disease. J. Trace Elem. Med. Biol. 2001;15(2-3):155–160. doi: 10.1016/S0946-672X(01)80060-2. [DOI] [PubMed] [Google Scholar]
- 77.Litwin T., Gromadzka G., Szpak G.M., Jabłonka-Salach K., Bulska E., Członkowska A. Brain metal accumulation in Wilson’s disease. J. Neurol. Sci. 2013;329(1-2):55–58. doi: 10.1016/j.jns.2013.03.021. [DOI] [PubMed] [Google Scholar]
- 78.Hu H.L., Ni X.S., Duff-Canning S., Wang X.P. Oxidative damage of copper chloride overload to the cultured rat astrocytes. Am. J. Transl. Res. 2016;8(2):1273–1280. [PMC free article] [PubMed] [Google Scholar]
- 79.Scheiber I.F., Dringen R. Copper-treatment increases the cellular GSH content and accelerates GSH export from cultured rat astrocytes. Neurosci. Lett. 2011;498(1):42–46. doi: 10.1016/j.neulet.2011.04.058. [DOI] [PubMed] [Google Scholar]
- 80.Tiffany-Castiglioni E., Hong S., Qian Y. Copper handling by astrocytes: insights into neurodegenerative diseases. Int. J. Dev. Neurosci. 2011;29(8):811–818. doi: 10.1016/j.ijdevneu.2011.09.004. [DOI] [PubMed] [Google Scholar]
- 81.Pal A., Badyal R.K., Vasishta R.K., Attri S.V., Thapa B.R., Prasad R. Biochemical, histological, and memory impairment effects of chronic copper toxicity: a model for non-Wilsonian brain copper toxicosis in Wistar rat. Biol. Trace Elem. Res. 2013;153(1-3):257–268. doi: 10.1007/s12011-013-9665-0. [DOI] [PubMed] [Google Scholar]
- 82.Bertrand E., Lewandowska E., Szpak G.M., Hoogenraad T., Blaauwgers H.G., Członkowska A., Dymecki J. Neuropathological analysis of pathological forms of astroglia in Wilson’s disease. Folia Neuropathol. 2001;39(2):73–79. [PubMed] [Google Scholar]
- 83.Pal A., Prasad R. Recent discoveries on the functions of astrocytes in the copper homeostasis of the brain: a brief update. Neurotox. Res. 2014;26(1):78–84. doi: 10.1007/s12640-013-9453-9. [DOI] [PubMed] [Google Scholar]
- 84.Sinha S., Taly A.B., Ravishankar S., Prashanth L.K., Venugopal K.S., Arunodaya G.R., Vasudev M.K., Swamy H.S. Wilson’s disease: cranial MRI observations and clinical correlation. Neuroradiology. 2006;48(9):613–621. doi: 10.1007/s00234-006-0101-4. [DOI] [PubMed] [Google Scholar]
- 85.Mikol J., Vital C., Wassef M., Chappuis P., Poupon J., Lecharpentier M., Woimant F. Extensive cortico-subcortical lesions in Wilson’s disease: clinico-pathological study of two cases. Acta Neuropathol. 2005;110(5):451–458. doi: 10.1007/s00401-005-1061-1. [DOI] [PubMed] [Google Scholar]
- 86.Prashanth L.K., Sinha S., Taly A.B., Mahadevan A. Vasudev, M.K.; Shankar, S.K. Spectrum of epilepsy in Wilson’s disease with electroencephalographic, MR imaging and pathological correlates. J. Neurol. Sci. 2010;291(1-2):44–51. doi: 10.1016/j.jns.2010.01.007. [DOI] [PubMed] [Google Scholar]
- 87.Dong Y., Shi S.S., Chen S., Ni W., Zhu M., Wu Z.Y. The discrepancy between the absence of copper deposition and the presence of neuronal damage in the brain of Atp7b(-/-) mice. Metallomics. 2015;7(2):283–288. doi: 10.1039/C4MT00242C. [DOI] [PubMed] [Google Scholar]
- 88.Dusek P., Roos P.M., Litwin T., Schneider S.A., Flaten T.P., Aaseth J. The neurotoxicity of iron, copper and manganese in Parkinson’s and Wilson’s diseases. J. Trace Elem. Med. Biol. 2015;31:193–203. doi: 10.1016/j.jtemb.2014.05.007. [DOI] [PubMed] [Google Scholar]
- 89.Dusek P., Bahn E., Litwin T., Jabłonka-Salach K., Łuciuk A., Huelnhagen T., Madai V.I., Dieringer M.A., Bulska E., Knauth M., Niendorf T., Sobesky J., Paul F., Schneider S.A., Czlonkowska A., Brück W., Wegner C., Wuerfel J. Brain iron accumulation in Wilson disease: a post mortem 7 Tesla MRI - histopathological study. Neuropathol. Appl. Neurobiol. 2017;43(6):514–532. doi: 10.1111/nan.12341. [DOI] [PubMed] [Google Scholar]
- 90.Bruehlmeier M., Leenders K.L., Vontobel P., Calonder C., Antonini A., Weindl A. Increased cerebral iron uptake in Wilson’s disease: a 52Fe-citrate PET study. J. Nucl. Med. 2000;41(5):781–787. [PubMed] [Google Scholar]
- 91.Abuduxikuer K., Li L.T., Qiu Y.L., Wang N.L., Wang J.S. Wilson disease with hepatic presentation in an eight-month-old boy. World J. Gastroenterol. 2015;21(29):8981–8984. doi: 10.3748/wjg.v21.i29.8981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Członkowska A., Rodo M., Gromadzka G. Late onset Wilson’s disease: therapeutic implications. Mov. Disord. 2008;23(6):896–898. doi: 10.1002/mds.21985. [DOI] [PubMed] [Google Scholar]
- 93.Schilsky M.L., Scheinberg I.H., Sternlieb I. Prognosis of Wilsonian chronic active hepatitis. Gastroenterology. 1991;100(3):762–767. doi: 10.1016/0016-5085(91)80023-3. [DOI] [PubMed] [Google Scholar]
- 94.Boga S., Ala A., Schilsky M.L. Hepatic features of Wilson disease. Handb. Clin. Neurol. 2017;142:91–99. doi: 10.1016/B978-0-444-63625-6.00009-4. [DOI] [PubMed] [Google Scholar]
- 95.Forman S.J., Kumar K.S., Redeker A.G., Hochstein P. Hemolytic anemia in Wilson disease: clinical findings and biochemical mechanisms. Am. J. Hematol. 1980;9(3):269–275. doi: 10.1002/ajh.2830090305. [DOI] [PubMed] [Google Scholar]
- 96.Walshe J.M. The acute haemolytic syndrome in Wilson’s disease--a review of 22 patients. QJM. 2013;106(11):1003–1008. doi: 10.1093/qjmed/hct137. [DOI] [PubMed] [Google Scholar]
- 97.Saito T. Presenting symptoms and natural history of Wilson disease. Eur. J. Pediatr. 1987;146(3):261–265. doi: 10.1007/BF00716470. [DOI] [PubMed] [Google Scholar]
- 98.Eisenbach C., Sieg O., Stremmel W., Encke J., Merle U. Diagnostic criteria for acute liver failure due to Wilson disease. World J. Gastroenterol. 2007;13(11):1711–1714. doi: 10.3748/wjg.v13.i11.1711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Ostapowicz G., Fontana R.J., Schiødt F.V., Larson A., Davern T.J., Han S.H., McCashland T.M., Shakil A.O., Hay J.E., Hynan L., Crippin J.S., Blei A.T., Samuel G., Reisch J., Lee W.M., Group U.S.A.L.F.S.U.S., Acute Liver Failure Study Group Results of a prospective study of acute liver failure at 17 tertiary care centers in the United States. Ann. Intern. Med. 2002;137(12):947–954. doi: 10.7326/0003-4819-137-12-200212170-00007. [DOI] [PubMed] [Google Scholar]
- 100.Wong R.J., Gish R., Schilsky M., Frenette C. A clinical assessment of Wilson disease in patients with concurrent liver disease. J. Clin. Gastroenterol. 2011;45(3):267–273. doi: 10.1097/MCG.0b013e3181dffaa5. [DOI] [PubMed] [Google Scholar]
- 101.Wilkinson M.L., Portmann B., Williams R. Wilson’s disease and hepatocellular carcinoma: possible protective role of copper. Gut. 1983;24(8):767–771. doi: 10.1136/gut.24.8.767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.van Meer S., de Man R.A., van den Berg A.P., Houwen R.H., Linn F.H., van Oijen M.G., Siersema P.D., van Erpecum K.J. No increased risk of hepatocellular carcinoma in cirrhosis due to Wilson disease during long-term follow-up. J. Gastroenterol. Hepatol. 2015;30(3):535–539. doi: 10.1111/jgh.12716. [DOI] [PubMed] [Google Scholar]
- 103.Walshe J.M., Waldenström E., Sams V., Nordlinder H., Westermark K. Abdominal malignancies in patients with Wilson’s disease. QJM. 2003;96(9):657–662. doi: 10.1093/qjmed/hcg114. [DOI] [PubMed] [Google Scholar]
- 104.Pfeiffenberger J., Mogler C., Gotthardt D.N., Schulze-Bergkamen H., Litwin T., Reuner U., Hefter H., Huster D., Schemmer P., Członkowska A., Schirmacher P., Stremmel W., Cassiman D., Weiss K.H. Hepatobiliary malignancies in Wilson disease. Liver Int. 2015;35(5):1615–1622. doi: 10.1111/liv.12727. [DOI] [PubMed] [Google Scholar]
- 105.Członkowska A., Litwin T., Chabik G. Wilson disease: neurologic features. Handb. Clin. Neurol. 2017;142:101–119. doi: 10.1016/B978-0-444-63625-6.00010-0. [DOI] [PubMed] [Google Scholar]
- 106.Lorincz M.T. Neurologic Wilson’s disease. Ann. N. Y. Acad. Sci. 2010;1184:173–187. doi: 10.1111/j.1749-6632.2009.05109.x. [DOI] [PubMed] [Google Scholar]
- 107.Svetel M., Kozić D., Stefanova E., Semnic R., Dragasevic N., Kostic V.S. Dystonia in Wilson’s disease. Mov. Disord. 2001;16(4):719–723. doi: 10.1002/mds.1118. [DOI] [PubMed] [Google Scholar]
- 108.Soltanzadeh A., Soltanzadeh P., Nafissi S., Ghorbani A., Sikaroodi H., Lotfi J. Wilson’s disease: a great masquerader. Eur. Neurol. 2007;57(2):80–85. doi: 10.1159/000098056. [DOI] [PubMed] [Google Scholar]
- 109.Machado A., Chien H.F., Deguti M.M., Cançado E., Azevedo R.S., Scaff M., Barbosa E.R. Neurological manifestations in Wilson’s disease: Report of 119 cases. Mov. Disord. 2006;21(12):2192–2196. doi: 10.1002/mds.21170. [DOI] [PubMed] [Google Scholar]
- 110.Dening T.R., Berrios G.E., Walshe J.M. Wilson’s disease and epilepsy. Brain. 1988;111(Pt 5):1139–1155. doi: 10.1093/brain/111.5.1139. [DOI] [PubMed] [Google Scholar]
- 111.Benbir G., Gunduz A., Ertan S., Ozkara C. Partial status epilepticus induced by hypocupremia in a patient with Wilson’s disease. Seizure. 2010;19(9):602–604. doi: 10.1016/j.seizure.2010.07.018. [DOI] [PubMed] [Google Scholar]
- 112.Zimbrean P.C., Schilsky M.L. Psychiatric aspects of Wilson disease: a review. Gen. Hosp. Psychiatry. 2014;36(1):53–62. doi: 10.1016/j.genhosppsych.2013.08.007. [DOI] [PubMed] [Google Scholar]
- 113.Srinivas K., Sinha S., Taly A.B., Prashanth L.K., Arunodaya G.R., Janardhana Reddy Y.C., Khanna S. Dominant psychiatric manifestations in Wilson’s disease: a diagnostic and therapeutic challenge! J. Neurol. Sci. 2008;266(1-2):104–108. doi: 10.1016/j.jns.2007.09.009. [DOI] [PubMed] [Google Scholar]
- 114.Wenisch E., De Tassigny A., Trocello J.M., Beretti J., Girardot-Tinant N., Woimant F. Cognitive profile in Wilson’s disease: a case series of 31 patients. Rev. Neurol. (Paris) 2013;169(12):944–949. doi: 10.1016/j.neurol.2013.06.002. [DOI] [PubMed] [Google Scholar]
- 115.Seniów J., Bak T., Gajda J., Poniatowska R., Czlonkowska A. Cognitive functioning in neurologically symptomatic and asymptomatic forms of Wilson’s disease. Mov. Disord. 2002;17(5):1077–1083. doi: 10.1002/mds.10195. [DOI] [PubMed] [Google Scholar]
- 116.Iwański S., Seniów J., Leśniak M., Litwin T., Członkowska A. Diverse attention deficits in patients with neurologically symptomatic and asymptomatic Wilson’s disease. Neuropsychology. 2015;29(1):25–30. doi: 10.1037/neu0000103. [DOI] [PubMed] [Google Scholar]
- 117.Sridhar M.S. Advantages of Anterior Segment Optical Coherence Tomography Evaluation of the Kayser-Fleischer Ring in Wilson Disease. Cornea. 2017;36(3):343–346. doi: 10.1097/ICO.0000000000001126. [DOI] [PubMed] [Google Scholar]
- 118.Broniek-Kowalik K., Dzieżyc K., Litwin T., Członkowska A., Szaflik J.P. Anterior segment optical coherence tomography (AS-OCT) as a new method of detecting copper deposits forming the Kayser-Fleischer ring in patients with Wilson disease. Acta Ophthalmol. 2019;97(5):e757–e760. doi: 10.1111/aos.14009. [DOI] [PubMed] [Google Scholar]
- 119.Langwińska-Wośko E., Litwin T., Szulborski K., Członkowska A. Optical coherence tomography and electrophysiology of retinal and visual pathways in Wilson’s disease. Metab. Brain Dis. 2016;31(2):405–415. doi: 10.1007/s11011-015-9776-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Langwińska-Wośko E., Litwin T., Dzieżyc K., Karlinski M., Członkowska A. Optical coherence tomography as a marker of neurodegeneration in patients with Wilson’s disease. Acta Neurol. Belg. 2017;117(4):867–871. doi: 10.1007/s13760-017-0788-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Dzieżyc K., Litwin T., Członkowska A. Other organ involvement and clinical aspects of Wilson disease. Handb. Clin. Neurol. 2017;142:157–169. doi: 10.1016/B978-0-444-63625-6.00013-6. [DOI] [PubMed] [Google Scholar]
- 122.Buksińska-Lisik M., Litwin T., Pasierski T., Członkowska A. Cardiac assessment in Wilson’s disease patients based on electrocardiography and echocardiography examination. Arch. Med. Sci. 2019;15(4):857–864. doi: 10.5114/aoms.2017.69728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Quick S., Weidauer M., Heidrich F.M., Sveric K., Reichmann H., Ibrahim K., Strasser R.H., Linke A., Speiser U., Reuner U. Cardiac Manifestation of Wilson’s Disease. J. Am. Coll. Cardiol. 2018;72(22):2808–2809. doi: 10.1016/j.jacc.2018.08.2197. [DOI] [PubMed] [Google Scholar]
- 124.Ferenci P., Caca K., Loudianos G., Mieli-Vergani G., Tanner S., Sternlieb I., Schilsky M., Cox D., Berr F. Diagnosis and phenotypic classification of Wilson disease. Liver Int. 2003;23(3):139–142. doi: 10.1034/j.1600-0676.2003.00824.x. [DOI] [PubMed] [Google Scholar]
- 125.Xu X., Pin S., Gathinji M., Fuchs R., Harris Z.L. Aceruloplasminemia: an inherited neurodegenerative disease with impairment of iron homeostasis. Ann. N. Y. Acad. Sci. 2004;1012:299–305. doi: 10.1196/annals.1306.024. [DOI] [PubMed] [Google Scholar]
- 126.Tümer Z., Møller L.B. Menkes disease. Eur. J. Hum. Genet. 2010;18(5):511–518. doi: 10.1038/ejhg.2009.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Gromadzka G., Chabik G., Mendel T., Wierzchowska A., Rudnicka M., Czlonkowska A. Middle-aged heterozygous carriers of Wilson’s disease do not present with significant phenotypic deviations related to copper metabolism. J. Genet. 2010;89(4):463–467. doi: 10.1007/s12041-010-0065-3. [DOI] [PubMed] [Google Scholar]
- 128.Poujois A., Woimant F. Wilson’s disease: A 2017 update. Clin. Res. Hepatol. Gastroenterol. 2018;42(6):512–520. doi: 10.1016/j.clinre.2018.03.007. [DOI] [PubMed] [Google Scholar]
- 129.El Balkhi S., Poupon J., Trocello J.M., Leyendecker A., Massicot F., Galliot-Guilley M., Woimant F. Determination of ultrafiltrable and exchangeable copper in plasma: stability and reference values in healthy subjects. Anal. Bioanal. Chem. 2009;394(5):1477–1484. doi: 10.1007/s00216-009-2809-6. [DOI] [PubMed] [Google Scholar]
- 130.El Balkhi S., Trocello J.M., Poupon J., Chappuis P., Massicot F., Girardot-Tinant N., Woimant F. Relative exchangeable copper: a new highly sensitive and highly specific biomarker for Wilson’s disease diagnosis. Clin. Chim. Acta. 2011;412(23-24):2254–2260. doi: 10.1016/j.cca.2011.08.019. [DOI] [PubMed] [Google Scholar]
- 131.Nicastro E., Ranucci G., Vajro P., Vegnente A., Iorio R. Re-evaluation of the diagnostic criteria for Wilson disease in children with mild liver disease. Hepatology. 2010;52(6):1948–1956. doi: 10.1002/hep.23910. [DOI] [PubMed] [Google Scholar]
- 132.Martins da Costa C., Baldwin D., Portmann B., Lolin Y., Mowat A.P., Mieli-Vergani G. Value of urinary copper excretion after penicillamine challenge in the diagnosis of Wilson’s disease. Hepatology. 1992;15(4):609–615. doi: 10.1002/hep.1840150410. [DOI] [PubMed] [Google Scholar]
- 133.Ferenci P., Steindl-Munda P., Vogel W., Jessner W., Gschwantler M., Stauber R., Datz C., Hackl F., Wrba F., Bauer P., Lorenz O. Diagnostic value of quantitative hepatic copper determination in patients with Wilson’s Disease. Clin. Gastroenterol. Hepatol. 2005;3(8):811–818. doi: 10.1016/S1542-3565(05)00181-3. [DOI] [PubMed] [Google Scholar]
- 134.King A.D., Walshe J.M., Kendall B.E., Chinn R.J., Paley M.N., Wilkinson I.D., Halligan S., Hall-Craggs M.A. Cranial MR imaging in Wilson’s disease. AJR Am. J. Roentgenol. 1996;167(6):1579–1584. doi: 10.2214/ajr.167.6.8956601. [DOI] [PubMed] [Google Scholar]
- 135.Kozić D., Svetel M., Petrović B., Dragasević N., Semnic R., Kostić V.S. MR imaging of the brain in patients with hepatic form of Wilson’s disease. Eur. J. Neurol. 2003;10(5):587–592. doi: 10.1046/j.1468-1331.2003.00661.x. [DOI] [PubMed] [Google Scholar]
- 136.Litwin T., Dzieżyc K., Poniatowska R., Członkowska A. Effect of liver transplantation on brain magnetic resonance imaging pathology in Wilson disease: a case report. Neurol. Neurochir. Pol. 2013;47(4):393–397. doi: 10.5114/ninp.2013.36763. [DOI] [PubMed] [Google Scholar]
- 137.Dusek P., Smolinski L., Redzia-Ogrodnik B., Golebiowski M., Skowronska M., Poujois A., Laurencin C., Jastrzebska-Kurkowska I., Litwin T., Członkowska A. Semiquantitative scale for assessing brain MRI Abnormalities in Wilson Disease: A Validation Study. Mov. Disord. 2020;••• doi: 10.1002/mds.28018. [DOI] [PubMed] [Google Scholar]
- 138.Skowrońska M., Litwin T., Dzieżyc K., Wierzchowska A., Członkowska A. Does brain degeneration in Wilson disease involve not only copper but also iron accumulation? Neurol. Neurochir. Pol. 2013;47(6):542–546. doi: 10.5114/ninp.2013.39071. [DOI] [PubMed] [Google Scholar]
- 139.Bai X., Wang G., Wu L., Liu Y., Cui L., Shi H., Guo L. Deep-gray nuclei susceptibility-weighted imaging filtered phase shift in patients with Wilson’s disease. Pediatr. Res. 2014;75(3):436–442. doi: 10.1038/pr.2013.239. [DOI] [PubMed] [Google Scholar]
- 140.Wang Y., Spincemaille P., Liu Z., Dimov A., Deh K., Li J., Zhang Y., Yao Y., Gillen K.M., Wilman A.H., Gupta A., Tsiouris A.J., Kovanlikaya I., Chiang G.C., Weinsaft J.W., Tanenbaum L., Chen W., Zhu W., Chang S., Lou M., Kopell B.H., Kaplitt M.G., Devos D., Hirai T., Huang X., Korogi Y., Shtilbans A., Jahng G.H., Pelletier D., Gauthier S.A., Pitt D., Bush A.I., Brittenham G.M., Prince M.R. Clinical quantitative susceptibility mapping (QSM): Biometal imaging and its emerging roles in patient care. J. Magn. Reson. Imaging. 2017;46(4):951–971. doi: 10.1002/jmri.25693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Fritzsch D., Reiss-Zimmermann M., Trampel R., Turner R., Hoffmann K.T., Schäfer A. Seven-tesla magnetic resonance imaging in Wilson disease using quantitative susceptibility mapping for measurement of copper accumulation. Invest. Radiol. 2014;49(5):299–306. doi: 10.1097/RLI.0000000000000010. [DOI] [PubMed] [Google Scholar]
- 142.Saracoglu S., Gumus K., Doganay S., Koc G., Kacar Bayram A., Arslan D., Gumus H. Brain susceptibility changes in neurologically asymptomatic pediatric patients with Wilson’s disease: evaluation with quantitative susceptibility mapping. Acta Radiol. 2018;59(11):1380–1385. doi: 10.1177/0284185118759821. [DOI] [PubMed] [Google Scholar]
- 143.Doganay S., Gumus K., Koc G., Bayram A.K., Dogan M.S., Arslan D., Gumus H., Gorkem S.B., Ciraci S., Serin H.I., Coskun A. Magnetic susceptibility changes in the basal ganglia and brain stem of patients with Wilson’s Disease: evaluation with quantitative susceptibility mapping. Magn. Reson. Med. Sci. 2018;17(1):73–79. doi: 10.2463/mrms.mp.2016-0145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Martínez-Fernández R., Caballol N., Gómez-Choco M. MRI and transcranial sonography findings in Wilson’s disease. Mov. Disord. 2013;28(6):740. doi: 10.1002/mds.25492. [DOI] [PubMed] [Google Scholar]
- 145.Tarnacka B., Szeszkowski W., Golebiowski M., Czlonkowska A. MR spectroscopy in monitoring the treatment of Wilson’s disease patients. Mov. Disord. 2008;23(11):1560–1566. doi: 10.1002/mds.22163. [DOI] [PubMed] [Google Scholar]
- 146.Ferenci P. Diagnosis of Wilson disease. Handb. Clin. Neurol. 2017;142:171–180. doi: 10.1016/B978-0-444-63625-6.00014-8. [DOI] [PubMed] [Google Scholar]
- 147.Dzieżyc K., Litwin T., Chabik G., Gramza K., Członkowska A. Families with Wilson’s disease in subsequent generations: clinical and genetic analysis. Mov. Disord. 2014;29(14):1828–1832. doi: 10.1002/mds.26057. [DOI] [PubMed] [Google Scholar]
- 148.Dzieżyc K., Karliński M., Litwin T., Członkowska A. Compliant treatment with anti-copper agents prevents clinically overt Wilson’s disease in pre-symptomatic patients. Eur. J. Neurol. 2014;21(2):332–337. doi: 10.1111/ene.12320. [DOI] [PubMed] [Google Scholar]
- 149.Roberts E.A., Schilsky M.L. American Association for Study of Liver Diseases (AASLD). Diagnosis and treatment of Wilson disease: an update. Hepatology. 2008;47(6):2089–2111. doi: 10.1002/hep.22261. [DOI] [PubMed] [Google Scholar]
- 150.Heilmaier H.E., Jiang J.L., Greim H., Schramel P., Summer K.H. D-penicillamine induces rat hepatic metallothionein. Toxicology. 1986;42(1):23–31. doi: 10.1016/0300-483X(86)90089-2. [DOI] [PubMed] [Google Scholar]
- 151.Członkowska A., Litwin T. Wilson disease - currently used anticopper therapy. Handb. Clin. Neurol. 2017;142:181–191. doi: 10.1016/B978-0-444-63625-6.00015-X. [DOI] [PubMed] [Google Scholar]
- 152.Merle U., Schaefer M., Ferenci P., Stremmel W. Clinical presentation, diagnosis and long-term outcome of Wilson’s disease: a cohort study. Gut. 2007;56(1):115–120. doi: 10.1136/gut.2005.087262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Weiss K.H., Gotthardt D.N., Klemm D., Merle U., Ferenci-Foerster D., Schaefer M., Ferenci P., Stremmel W. Zinc monotherapy is not as effective as chelating agents in treatment of Wilson disease. Gastroenterology. 2011;140(4):1189–1198.e1181. doi: 10.1053/j.gastro.2010.12.034. [DOI] [PubMed] [Google Scholar]
- 154.Brewer G.J., Terry C.A., Aisen A.M., Hill G.M. Worsening of neurologic syndrome in patients with Wilson’s disease with initial penicillamine therapy. Arch. Neurol. 1987;44(5):490–493. doi: 10.1001/archneur.1987.00520170020016. [DOI] [PubMed] [Google Scholar]
- 155.Ala A., Walker A.P., Ashkan K., Dooley J.S., Schilsky M.L. Wilson’s disease. Lancet. 2007;369(9559):397–408. doi: 10.1016/S0140-6736(07)60196-2. [DOI] [PubMed] [Google Scholar]
- 156.Medici V., Trevisan C.P., D’Incà R., Barollo M., Zancan L., Fagiuoli S., Martines D., Irato P., Sturniolo G.C. Diagnosis and management of Wilson’s disease: results of a single center experience. J. Clin. Gastroenterol. 2006;40(10):936–941. doi: 10.1097/01.mcg.0000225670.91722.59. [DOI] [PubMed] [Google Scholar]
- 157.Walshe J.M. Treatment of Wilson’s disease with trientine (triethylene tetramine) dihydrochloride. Lancet. 1982;1(8273):643–647. doi: 10.1016/S0140-6736(82)92201-2. [DOI] [PubMed] [Google Scholar]
- 158.Santos Silva E.E., Sarles J., Buts J.P., Sokal E.M. Successful medical treatment of severely decompensated Wilson disease. J. Pediatr. 1996;128(2):285–287. doi: 10.1016/S0022-3476(96)70412-2. [DOI] [PubMed] [Google Scholar]
- 159.Brewer G.J., Askari F., Lorincz M.T., Carlson M., Schilsky M., Kluin K.J., Hedera P., Moretti P., Fink J.K., Tankanow R., Dick R.B., Sitterly J. Treatment of Wilson disease with ammonium tetrathiomolybdate: IV. Comparison of tetrathiomolybdate and trientine in a double-blind study of treatment of the neurologic presentation of Wilson disease. Arch. Neurol. 2006;63(4):521–527. doi: 10.1001/archneur.63.4.521. [DOI] [PubMed] [Google Scholar]
- 160.Weiss K.H., Thurik F., Gotthardt D.N., Schafer M., Teufel U., Wiegand F., Merle U., Ferenci-Foerster D., Maieron A., Stauber R., Zoller H., Schmidt H.H., Reuner U., Hefter H., Trocello J.M., Houwen R.H., Ferenci P., Stremmel W., Consortium E. 2013. [DOI] [PubMed]
- 161.Brewer G.J., Dick R.D., Johnson V., Wang Y., Yuzbasiyan-Gurkan V., Kluin K., Fink J.K., Aisen A. Treatment of Wilson’s disease with ammonium tetrathiomolybdate. I. Initial therapy in 17 neurologically affected patients. Arch. Neurol. 1994;51(6):545–554. doi: 10.1001/archneur.1994.00540180023009. [DOI] [PubMed] [Google Scholar]
- 162.Brewer G.J., Hedera P., Kluin K.J., Carlson M., Askari F., Dick R.B., Sitterly J., Fink J.K. Treatment of Wilson disease with ammonium tetrathiomolybdate: III. Initial therapy in a total of 55 neurologically affected patients and follow-up with zinc therapy. Arch. Neurol. 2003;60(3):379–385. doi: 10.1001/archneur.60.3.379. [DOI] [PubMed] [Google Scholar]
- 163.Weiss K.H., Askari F.K., Czlonkowska A., Ferenci P., Bronstein J.M., Bega D., Ala A., Nicholl D., Flint S., Olsson L., Plitz T., Bjartmar C., Schilsky M.L. Bis-choline tetrathiomolybdate in patients with Wilson’s disease: an open-label, multicentre, phase 2 study. Lancet Gastroenterol. Hepatol. 2017;2(12):869–876. doi: 10.1016/S2468-1253(17)30293-5. [DOI] [PubMed] [Google Scholar]
- 164.Schilsky M.L., Blank R.R., Czaja M.J., Zern M.A., Scheinberg I.H., Stockert R.J., Sternlieb I. Hepatocellular copper toxicity and its attenuation by zinc. J. Clin. Invest. 1989;84(5):1562–1568. doi: 10.1172/JCI114333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Hoogenraad T.U. Paradigm shift in treatment of Wilson’s disease: zinc therapy now treatment of choice. Brain Dev. 2006;28(3):141–146. doi: 10.1016/j.braindev.2005.08.008. [DOI] [PubMed] [Google Scholar]
- 166.Czlonkowska A., Gajda J., Rodo M. Effects of long-term treatment in Wilson’s disease with D-penicillamine and zinc sulphate. J. Neurol. 1996;243(3):269–273. doi: 10.1007/BF00868525. [DOI] [PubMed] [Google Scholar]
- 167.Członkowska A., Litwin T., Karliński M., Dziezyc K., Chabik G., Czerska M. D-penicillamine versus zinc sulfate as first-line therapy for Wilson’s disease. Eur. J. Neurol. 2014;21(4):599–606. doi: 10.1111/ene.12348. [DOI] [PubMed] [Google Scholar]
- 168.Zaino D., Chiarotti I., Battisti C., Salvatore S., Federico A., Cerase A. Six-year clinical and MRI quantitative susceptibility mapping (QSM) follow-up in neurological Wilson’s disease under zinc therapy: a case report. Neurol. Sci. 2019;40(1):199–201. doi: 10.1007/s10072-018-3557-1. [DOI] [PubMed] [Google Scholar]
- 169.Ren M., Yang R. Clinical curative effects of dimercaptosuccinic acid on hepatolenticular degeneration and the impact of DMSA on biliary trace elements. Chin. Med. J. (Engl.) 1997;110(9):694–697. [PubMed] [Google Scholar]
- 170.Li W.J., Chen C., You Z.F., Yang R.M., Wang X.P. Current Drug Managements of Wilson’s Disease: From West to East. Curr. Neuropharmacol. 2016;14(4):322–325. doi: 10.2174/1570159X14666151130222427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Wang X.P., Yang R.M., Ren M.S., Sun B.M. Anticopper efficacy of captopril and sodium dimercaptosulphonate in patients with Wilson’s disease. Funct. Neurol. 2003;18(3):149–153. [PubMed] [Google Scholar]
- 172.Xu S.Q., Li X.F., Zhu H.Y., Liu Y., Fang F., Chen L. Clinical efficacy and safety of chelation treatment with typical penicillamine in cross combination with DMPS repeatedly for Wilson’s disease. J. Huazhong Univ. Sci. Technolog. Med. Sci. 2013;33(5):743–747. doi: 10.1007/s11596-013-1190-z. [DOI] [PubMed] [Google Scholar]
- 173.Wang X.P., Zhang W.F., Huang H.Y., Preter M. Neurology in the People’s Republic of China--an update. Eur. Neurol. 2010;64(6):320–324. doi: 10.1159/000321648. [DOI] [PubMed] [Google Scholar]
- 174.Wang Y., Xie C.L., Fu D.L., Lu L., Lin Y., Dong Q.Q., Wang X.T., Zheng G.Q. Clinical efficacy and safety of Chinese herbal medicine for Wilson’s disease: a systematic review of 9 randomized controlled trials. Complement. Ther. Med. 2012;20(3):143–154. doi: 10.1016/j.ctim.2011.12.004. [DOI] [PubMed] [Google Scholar]
- 175.Li W.J., Wang J.F., Wang X.P. Wilson’s disease: update on integrated Chinese and Western medicine. Chin. J. Integr. Med. 2013;19(3):233–240. doi: 10.1007/s11655-012-1089-8. [DOI] [PubMed] [Google Scholar]
- 176.Litwin T., Dzieżyc K., Karliński M., Chabik G., Czepiel W., Członkowska A. Early neurological worsening in patients with Wilson’s disease. J. Neurol. Sci. 2015;355(1-2):162–167. doi: 10.1016/j.jns.2015.06.010. [DOI] [PubMed] [Google Scholar]
- 177.Li L.Y., Zhu X.Q., Tao W.W., Yang W.M., Chen H.Z., Wang Y. Acute onset neurological symptoms in Wilson disease after traumatic, surgical or emotional events: A cross-sectional study. Medicine (Baltimore) 2019;98(26):e15917. doi: 10.1097/MD.0000000000015917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Masełbas W., Członkowska A., Litwin T., Niewada M. Persistence with treatment for Wilson disease: a retrospective study. BMC Neurol. 2019;19(1):278. doi: 10.1186/s12883-019-1502-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Schilsky M.L. Liver transplantation for Wilson’s disease. Ann. N. Y. Acad. Sci. 2014;1315:45–49. doi: 10.1111/nyas.12454. [DOI] [PubMed] [Google Scholar]
- 180.Dhawan A., Taylor R.M., Cheeseman P., De Silva P., Katsiyiannakis L., Mieli-Vergani G. Wilson’s disease in children: 37-year experience and revised King’s score for liver transplantation. Liver Transpl. 2005;11(4):441–448. doi: 10.1002/lt.20352. [DOI] [PubMed] [Google Scholar]
- 181.Schilsky M.L., Scheinberg I.H., Sternlieb I. Liver transplantation for Wilson’s disease: indications and outcome. Hepatology. 1994;19(3):583–587. doi: 10.1002/hep.1840190307. [DOI] [PubMed] [Google Scholar]
- 182.Bellary S., Hassanein T., Van Thiel D.H. Liver transplantation for Wilson’s disease. J. Hepatol. 1995;23(4):373–381. doi: 10.1016/0168-8278(95)80194-4. [DOI] [PubMed] [Google Scholar]
- 183.Garoufalia Z., Prodromidou A., Machairas N., Kostakis I.D., Stamopoulos P., Zavras N., Fouzas I., Sotiropoulos G.C. Liver Transplantation for Wilson’s Disease in Non-adult Patients: A Systematic Review. Transplant. Proc. 2019;51(2):443–445. doi: 10.1016/j.transproceed.2019.01.017. [DOI] [PubMed] [Google Scholar]
- 184.Litwin T., Gromadzka G., Członkowska A. Neurological presentation of Wilson’s disease in a patient after liver transplantation. Mov. Disord. 2008;23(5):743–746. doi: 10.1002/mds.21913. [DOI] [PubMed] [Google Scholar]
- 185.Ahmad A., Torrazza-Perez E., Schilsky M.L. Liver transplantation for Wilson disease. Handb. Clin. Neurol. 2017;142:193–204. doi: 10.1016/B978-0-444-63625-6.00016-1. [DOI] [PubMed] [Google Scholar]
- 186.Pfeiffenberger J., Weiss K.H., Stremmel W. Wilson disease: symptomatic liver therapy. Handb. Clin. Neurol. 2017;142:205–209. doi: 10.1016/B978-0-444-63625-6.00017-3. [DOI] [PubMed] [Google Scholar]
- 187.Litwin T., Dušek P., Członkowska A. Symptomatic treatment of neurologic symptoms in Wilson disease. Handb. Clin. Neurol. 2017;142:211–223. doi: 10.1016/B978-0-444-63625-6.00018-5. [DOI] [PubMed] [Google Scholar]
- 188.Dusek P., Litwin T., Członkowska A. Neurologic impairment in Wilson disease. Ann. Transl. Med. 2019;7(Suppl. 2):S64. doi: 10.21037/atm.2019.02.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Hedera P. Treatment of Wilson’s disease motor complications with deep brain stimulation. Ann. N. Y. Acad. Sci. 2014;1315:16–23. doi: 10.1111/nyas.12372. [DOI] [PubMed] [Google Scholar]
- 190.Litwin T., Chabik G., Członkowska A. Acute focal dystonia induced by a tricyclic antidepressant in a patient with Wilson disease: a case report. Neurol. Neurochir. Pol. 2013;47(5):502–506. doi: 10.5114/ninp.2013.38230. [DOI] [PubMed] [Google Scholar]
- 191.Litwin T., Dusek P., Szafrański T., Dzieżyc K., Członkowska A., Rybakowski J.K. Psychiatric manifestations in Wilson’s disease: possibilities and difficulties for treatment. Ther. Adv. Psychopharmacol. 2018;8(7):199–211. doi: 10.1177/2045125318759461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Murillo O., Luqui D.M., Gazquez C., Martinez-Espartosa D., Navarro-Blasco I., Monreal J.I., Guembe L., Moreno-Cermeño A., Corrales F.J., Prieto J., Hernandez-Alcoceba R., Gonzalez-Aseguinolaza G. Long-term metabolic correction of Wilson’s disease in a murine model by gene therapy. J. Hepatol. 2016;64(2):419–426. doi: 10.1016/j.jhep.2015.09.014. [DOI] [PubMed] [Google Scholar]
- 193.Gupta S. Cell therapy to remove excess copper in Wilson’s disease. Ann. N. Y. Acad. Sci. 2014;1315:70–80. doi: 10.1111/nyas.12450. [DOI] [PMC free article] [PubMed] [Google Scholar]