Abstract
Temozolomide (TMZ), an oral alkylating prodrug which delivers a methyl group to purine bases of DNA (O6-guanine; N7-guanine and N3-adenine), is frequently used together with radiotherapy as part of the first-line treatment of high-grade gliomas. The main advantages are its high oral bioavailability (almost 100% although the concentration found in the cerebrospinal fluid was approximately 20% of the plasma concentration of TMZ), its lipophilic properties, and small size that confer the ability to cross the blood-brain barrier. Furthermore, this agent has demonstrated activity not only in brain tumors but also in a variety of solid tumors. However, conventional therapy using surgery, radiation, and TMZ in glioblastoma results in a median patient survival of 14.6 months. Treatment failure has been associated with tumor drug resistance. This phenomenon has been linked to the expression of O6-methylguanine-DNA methyltransferase, but the mismatch repair system and the presence of cancer stem-like cells in tumors have also been related to TMZ resistance. The understanding of these mechanisms is essential for the development of new therapeutic strategies in the clinical use of TMZ, including the use of nanomaterial delivery systems and the association with other chemotherapy agents. The aim of this review is to summarize the resistance mechanisms of TMZ and the current advances to improve its clinical use.
Keywords: Alkylating agents, drug resistance, chemotherapy, nanoparticles, cancer, clinical trials
1. INTRODUCTION
Temozolomide (TMZ) is a small alkylating molecule that introduces methyl groups into DNA. It is an analog of dacarbazine with an antineoplastic activity that was developed by Professor Malcolm Stevens in a multidisciplinary drug discovery laboratory in the pharmacy department at Aston University (UK) at the end of the 1980s. As a small lipophilic molecule, TMZ can penetrate the blood–brain barrier (BBB) and, therefore, is one of the few drugs with central nervous system (CNS) activity. TMZ provides an advantage over other traditional alkylating agents such as carmustine (BCNU) and lomustine (CCNU) (alone or in combination with procarbazine and vincristine), which have shown a very poor survival benefit in patients [1]. In addition, these agents have high associated toxicity (mainly causing myelosuppression and respiratory alterations) that limits their use and even leads to treatment discontinuation. TMZ eludes these problems because cytochrome P450 enzymes and the kidneys are not involved in its metabolism, it has predictable side effects, and its toxic effects are usually reversible and only mild or moderate [2]. TMZ was first licensed in 1999, initially as second-line therapy for glioblastoma (GBM), the most malignant grade of glioma, because it was the first alkylating agent that showed a clear improvement in two-year survival rates, from 10% to 25% in glioma patients, and four- and five-year survival rates, ranging from insignificant to 10%. Since then, the indications for which it is prescribed have increased; it is currently administered with radiotherapy as a first-line treatment for GBM and second-line treatment for other malignant gliomas after relapse, so it is now found in a multitude of clinical trials for use in non-nervous system tumors.
1.1. Chemical Structure and Mechanism of Action
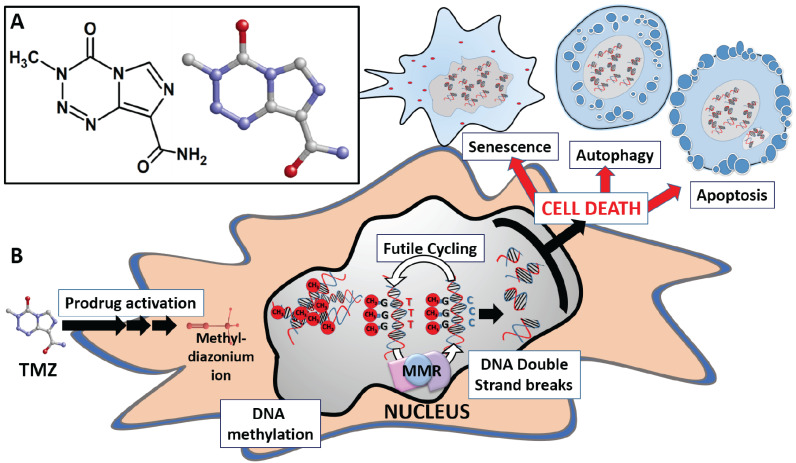
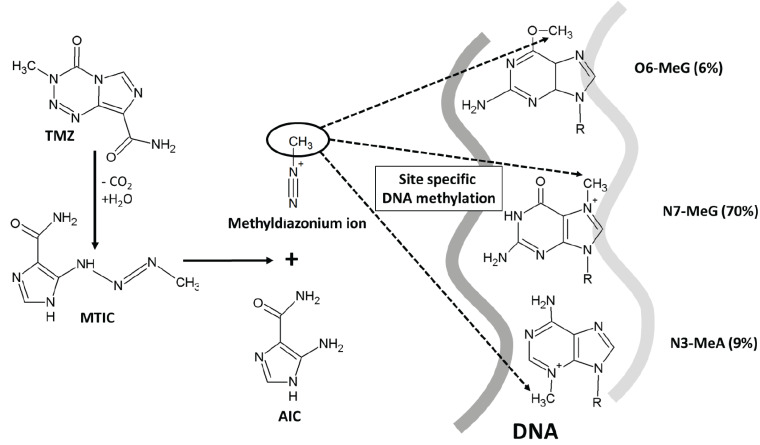
TMZ has a low molecular weight of 194.154 g/mol, a lipophilic character, and acts as a potent alkylating agent. Currently, TMZ represents the standard adjuvant treatment in newly diagnosed GBM patients, along with surgery and radiation therapy [3]. TMZ, whose chemical name is 3,4-dihydro-3-methyl-4-oxoimidazole (Fig. 1A), undergoes spontaneous hydrolysis to generate the active metabolite 5-(3-methyl-1-triazen-1-yl)imidazole-4-carboxamide (MTIC). TMZ is stable under acidic conditions, while this reaction occurs at physiological and basic pH conditions and involves the interaction of H2O molecule with the C4 atom of TMZ in which the heterocyclic ring opens to generate MTIC and a molecule of carbon dioxide [4]. The intrinsic properties of MTIC prevent its efficient interaction with tumor cell membranes, reducing its ability to penetrate target cells. This may be one of the reasons why the effectiveness of TMZ in tumors is lower than expected [5]. MTIC is unstable and converts into a methyldiazonium ion, i.e. the reactive compound that transfers the methyl group to DNA and generates the final degradation product, 4-amino-5-imidazolecarboxamide (AIC), which is eliminated by the organism. The effect of TMZ is highly pH-dependent; it has been shown that slightly more basic intracellular pH values in cancer cells (as compared to normal cells) favor the damage induced by TMZ in tumor cells [3]. In fact, to elucidate the role of pH on the antitumor effect of TMZ, Stéphanou and Ballesta developed a mathematical model using U-87 MG cells. In this model, the cellular, spatial and pH heterogeneity of the tumor microenvironment were examined. The authors found that, in all simulation scenarios, optimal TMZ efficacy was obtained when the pH of tumor cells was close to physiological pH values. Based on these predictions, they postulated that the combination of TMZ with pH-regulating agents could enhance the therapeutic effect of TMZ [6]. The resulting reactive methyldiazonium ion methylates DNA at the N7 position of guanine (N7-MeG; 70%), followed by methylation at the N3 position of adenine (N3-MeA; 9%), and the O6 position of guanine (O6-MeG; 6%) (Fig. 2) [3]. This last type of methylation in the O6 position of guanine is considered the lethal step and mediates the cytotoxic action of TMZ. During the DNA replication phase, thymine, rather than a cytosine, is incorporated in the complementary strand opposite to O6-MeG. This error is recognized by the DNA mismatch repair (MMR) system, which corrects it by removing the thymine from the complementary strand, although the primary error at the guanine O6 position of the main chain remains. When the accumulation of double-strand breaks in the DNA molecule increases, the cell stops in the G1/S or G2/M phase to have more time to proceed with the repair. If the repair ultimately fails, apoptosis, senescence, or autophagy can be induced in the cell [2, 7] (Fig. 1B). Tumor cells have a series of defense mechanisms against the action of TMZ based on the ability to eliminate these methyl groups or alter the mismatch repair system, which makes them resistant to treatment with TMZ. These mechanisms are discussed in detail below.
1.2. Clinical Pharmacokinetics and Dose Recommendations
TMZ has a high penetration capacity in all tissues, including nervous tissue, and its bioavailability is practically 100%, as evidenced in 7-day fecal excretion tests after administration with TMZ marked with 14C, where only 0.8% extraction was detected. It presents a peak plasma concentration after 20 minutes following oral administration, indicating that it undergoes rapid intestinal absorption. The plasma concentration is known to follow a dose-dependent pattern as the administration of a dose of between 150-200 mg/m2/day resulted in peak concentrations of 3 to 15 μg/ml (15-77 μM) [8]. Another characteristic of TMZ is its low protein binding (10-20%) and a half-life in plasma of 74-110 minutes. It is mainly eliminated through the hepatobiliary pathway and approximately 10-15% is excreted via the urine. Regarding its metabolism, after the first 24 hours, 5-10% remains as unmodified TMZ and the rest is TMZ acid, AIC, or unidentified polar metabolites. TMZ biodistribution is another important aspect of its pharmacodynamics. It is found in the cerebrospinal fluid (CSF), which is key in the treatment of glial tumors. Analysis of plasma and CSF samples from 35 patients with newly diagnosed or recurrent malignant gliomas has shown that TMZ behaves as a three-compartment model with first-order absorption and transfer rates between plasma and CSF [9]. After an oral dose of TMZ of 150-200 mg/m2/day, upon analyzing the plasma and CSF at three different time points (between 0.2 and 8.4 hours), TMZ in CSF was 20±5% of the plasma concentration, but the presence of TMZ in CSF was more prolonged. With respect to drug interactions, co-administration with other drugs related to the treatment (like antiemetics, anticonvulsants, corticosteroids, antacids, etc.) did not influence TMZ concentration levels in CSF [1]. According to FDA guidelines, food reduces the rate and degree of TMZ absorption, modifying the parameters of the maximum plasma concentration, for example, these levels decrease by 32% when administered after a modified breakfast with high-fat content. Patients are therefore recommended to fast for one hour before and after taking the drug. Regarding other parameters, age is observed to not affect a range of 19-78 years old, and the pharmacokinetic profile in children does not differ significantly from adults, but in terms of gender, women have an approximately 5% lower TMZ clearance than men.
The recently-developed technique of focused ultrasound exposure with the presence of microbubbles provides a transient disruption of the BBB, thus improving the entry of TMZ. Preclinical trials have been performed on nude mice implanted with U-87 MG human glioma cells treated with TMZ and this technique demonstrated that the localized cerebral accumulation of TMZ increased from 6.98 to 19 ng/mg. The TMZ degradation time in the tumor also increased from 1.02 to 1.56 hours, as did survival in 30% of the mice. Therefore, this technique is quite attractive for its clinical application and to increase the efficacy of TMZ compared to its current use in brain tumor treatments [10].
For clinical use, TMZ appears under the brand name Temodar® and can be administered orally in strengths of 5, 20, 100, 140, 180, and 250 mg, but recently it is also available as a solution for intravenous administration (with a concentration of 100 mg/ml). Initial preclinical studies found that repeated exposure to TMZ produces greater drug activity (apparently by reducing the availability of the tumor cells’ O6-methylguanine-DNA methyltransferase repair enzyme). Therefore, in clinical guidelines for TMZ, chemotherapy regimens used to treat recurrent glioblastoma are 150 mg/m2 (200 mg/m2 if there is no prior thrombocytopenia) for 5 days every 28 days. There is also an extended administration schedule of 50 mg/m2/day continuously and, finally, it can be administered in cycles of 75-100 mg/m2 for 21 days every 28 days or 150 mg/m2 for 7 days every 14 days [10]. These administration regimens should be discontinued if patients present hematologic alterations: absolute neutrophil count (ANC) ≥500/mm3 but <1,500/mm3; National Cancer Institute Common Toxicity Criteria grading system (CTC) ≤ Grade 1; platelets: ≥10,000/mm3 but ≤100,000/mm3; Grade 2 CTC non-hematologic toxicity; or ceased completely if ANC <500/mm3, platelets <10,000/mm3, or there is Grade 3 or 4 CTC non-hematologic toxicity. Patients are usually treated with TMZ until tumor progresses, unacceptable toxicity appears, patients reject the therapy, or after a maximum of 1 year [11]. A phase II clinical trial including two cohorts, has been conducted to try to reduce the side effects of TMZ. The initial cohort comprised 10 patients who received TMZ at 40 mg/m2 per day. This regimen seemed to be safe and effective, so the metronomic schedule was increased to 50 mg/m2 per day. The second cohort, comprising 28 patients, received 50 mg/m2 TMZ per day. In both, treatment with TMZ was effective for recurrent GBM that is even refractory to conventional TMZ treatment and has acceptable toxicity [12].
1.3. Side Effects and Toxicity
The main side effect associated with the use of TMZ that leads to the interruption of treatment is myelosuppression with delayed thrombocytopenia (which is usually reversible). This side effect usually appears 21-28 days after the start of each treatment cycle, improving to grade 1 thrombocytopenia at 7-14 days after stopping treatment. In clinical trials, grade 4 myelosuppression has been noted in 10% of patients following a dose accumulation of more than 1,000 mg/m2 for more than 5 days [1]. A study carried out by the European Organization for Research and Treatment of Cancer and the National Cancer Institute of Canada revealed that when radiotherapy is combined with the standard dose of TMZ (75 mg/m2), then 7% of patients had to interrupt treatment due to severe hematological toxicity. This figure increased to 14% when the TMZ maintenance dose (150-200 mg/m2/day) was initiated [13].
Lymphocytopenia is one of the most frequent side effects observed with this alkylating drug [14]. Most studies find that the main cell populations affected are CD4 T cells and then B cells, without any immune compensation through the increased production of cytokines involved in the proliferation of lymphocytes, such as interleukin 7 and 15 [15]. To counteract the lymphocytopenia, one study carried out a post-treatment adoptive transfer of lymphocytes, but the results were poor as the number of lymphocytes did not increase, demonstrating TMZ’s long-term effect on the lymphocyte population [16]. In continuous regimens, the lymphocytopenia induced by TMZ is dose-dependent. An example of this is the profound and selective CD4+ lymphocytopenia observed in malignant melanoma patients treated with TMZ 75 mg/m2 for 6 weeks every 8 weeks. Of the patients who developed lymphocytopenia, 61% had persistently low lymphocyte counts up to 2 months after the interruption of TMZ [17]. However, other authors found that higher doses of TMZ, such as 150 mg/m2 per day for one week during alternate weeks, did not cause an increase in myelotoxicity or profound lymphocytopenia that are associated with other dose-dense regimens [18]. In some patients, this decrease in the number of lymphocytes has been associated with an increased risk of opportunistic infections, such as pneumonia caused by Pneumocystis carinii and other opportunistic infections, such as Aspergillus pneumonia, Herpes simplex, Herpes zoster, and candidiasis, as reported by different authors [17], besides being a risk in patients with immune diseases such as HIV. One example is the case of a 66-year-old patient treated with TMZ [19], that led to recommendations for the initiation of a primary prophylaxis protocol in at-risk patients if the lymphocyte count falls below 500 cells/µl or the CD4+ count falls below 200 cells/µl. Nevertheless, it seems that this lymphocytopenia provides an advantage in combination with immunotherapies. For example, studies in humans have shown that cellular immunotherapy can be administered successfully together with TMZ, as the presence of lymphocytopenia has been shown to increase the proliferation and function of antigen-specific T cells due to the subsequent homeostatic recovery period [15]. An example of this was provided in a phase I/II trial, wherein TMZ was combined with a dendritic cell vaccine fused with glioma cells taken from surgical patients [20]. The study revealed that surgical specimens from patients with recurrent GBM had a higher expression of tumor-associated antigens (Wilms’ Tumor 1 (WT-1), glycoprotein-100 (gp100), and melanoma-associated antigen gene A3 (MAGE-A3)) and greater amounts were located in the cytoplasm, compared with newly diagnosed patients. This accumulation of immunogenic peptides in the cytoplasm can lead to an improved response to immunotherapy.
Non-hematologic side effects are usually mild to moderate and associated with the dose and TMZ treatment regimen. However, these non-hematologic effects can alter patient quality of life. The main ones are short-term, low-frequency nausea, and vomiting. They are often treated with standard antiemetics such as metoclopramide or 5-hydroxytryptamine. Patients normally respond well to these prophylactic treatments, so approximately half of patients on low and continuous dose regimens do not need any antiemetic treatment after the initial doses of the treatment. Other non-hematological side effects are mostly mild and include fatigue, headache, and in rare cases, skin reactions (urticaria and rash) [1].
2. MECHANISMS OF TEMOZOLOMIDE RESISTANCE
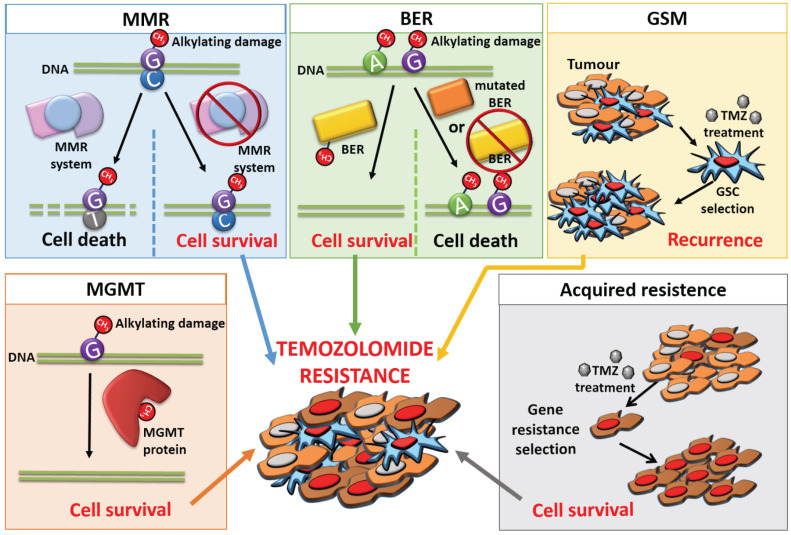
As an oral chemotherapy agent, TMZ has demonstrated efficacy against several types of tumors, including glioma, metastatic melanoma, lung, colon, and ovarian cancer [21]. Despite the treatment’s promise, the use of radiotherapy and chemotherapy has some limitations. The first issue is the initial size of the tumor: tumor volume can be considered by itself a prognostic factor of patient survival, and some authors observed significant overall survival differences in GBM patients with tumor volume less than 15cc [22]. In addition, some patients subsequently suffer recurrences or their tumor continues to develop. This represents a treatment failure that is usually associated with the phenomenon known as drug resistance, which is a major issue with TMZ treatment. Unregulated signaling pathways, DNA repair pathways, persistence of cancer stem cell (CSCs) subpopulations, and self-defense mechanisms are some of the causes of resistance to TMZ [22]. Each mechanism will be explained in more detail in the following sections (Fig. 3).
2.1. Blood-Brain Barrier
TMZ is the main chemotherapy agent used to treat CNS tumors; therefore, the resistance that develops has been studied thoroughly in this type of tumor. In the particular case of brain tumors, there is an important characteristic that contributes to its resistance – their anatomical location. Brain tumors are protected by the BBB which acts as an initial filter in this resistance mechanism [23]. The BBB features some crucial components such as the ABC transporter protein family, which exports foreign substances out of cells, removing a variety of different substances, including drugs such as alkylating agents [24, 25]. It is difficult to choose a systemic antitumor drug for the treatment of GMB because its efficacy is related to its capacity to cross the BBB. TMZ has demonstrated widespread distribution into all tissues, including the brain, thanks to its capacity to cross the BBB. However, only 20% of the levels of this drug in the systemic circulation have been detected in the brain [9]. This, added to the short half-life of this drug, implies the need for higher systemic doses to reach therapeutic levels. This added to a short half-life requires high systemic doses to reach therapeutic levels. In brain tumors, there is an additional barrier in terms of function and morphology, called the blood–brain tumor barrier (BBTB). These tumors feature areas with hypoxia, where the expression of certain angiogenic mediators increases, leading to abnormal neovascularization and therefore a new BBTB. The BBTB contains several new blood vessels that help transfer substances and oxygen to the tumor, thereby promoting its growth and expansion while hindering the entry of chemotherapeutic drugs [26, 27].
2.2. MGMT Can Repair TMZ-Induced DNA Alkylation
O6-methylguanine-DNA methyltransferase (MGMT) protects the cell genome as it repairs damaged DNA by eliminating alkyl groups produced by the action of alkylating agents such as TMZ [28]. TMZ introduces methyl groups at the O6 position of guanine. This group is eliminated from an internal active site represented by a cysteine residue in the amino acid sequence of the protein, which irreversibly inactivates the MGMT molecule. That is why the MGMT protein is also known as a suicide enzyme because it cannot be reused and must be resynthesized de novo. Therefore, MGMT must be continually replenished to ensure the effective repair of O6-MeG [29-31].
The gene that encodes for the MGMT protein is located on chromosome 10. This gene is epigenetically regulated by methylation of the 25 CpG islands that make up its promoter [32]. When the CpG regions of the MGMT promoter are unmethylated, the polymerase can bind and induce the expression of the protein. However, when the CpG regions of the promoter are methylated, the polymerase cannot find the binding sites, so there is no transcription or protein synthesis. This is known as MGMT silencing [33].
As the MGMT protein constitutes a repair mechanism that counteracts TMZ-induced DNA damage, the level of MGMT expression is directly related to TMZ resistance in chemotherapy [34, 35]. Several in vitro studies have observed a correlation between MGMT expression and the response to chemotherapy with TMZ, noting that tumor cells expressing higher MGMT activity are more resistant to the cytotoxic effects of TMZ than those lacking MGMT activity [13, 36, 37]. This suggests that the measurement of MGMT levels could be used as a treatment response predictor and prevent the need for systematic administration of chemotherapy to all patients. Consequently, clinical trials such as that of Hegi et al. [38] have been conducted, confirming that the methylation status of MGMT by itself can predict the patient's response to treatment with alkylating agents such as TMZ. Depending on MGMT methylation status, we could classify patients as candidates for standard treatment with chemo/radiotherapy, or alternatively, for radiation or monotherapy with TMZ [39, 40].
The relationship between MGMT status and patient survival has also been widely studied. Patients with methylated MGMT promoter have shown a greater overall survival compared to those who have an unmethylated MGMT promoter, with median survival of 18.2 months and 12.2 months, respectively [37]. Stupp et al. [13] found a significant correlation between MGMT methylation and survival in patients treated with radiotherapy or radiotherapy and adjuvant TMZ. In this latter study, adjuvant TMZ showed a 13.8% survival after five years. Spiegl-Kreinecker et al. [41] found an increase in survival of 20 months in patients treated with TMZ who did not express MGMT versus those who did express MGMT. Brandes et al. [42] observed a negative (inverse) correlation between MGMT expression and survival. They analyzed the methylation status of MGMT and the survival of 103 patients with GBM. The study revealed that patients with methylation in the MGMT promoter had a mean survival of 46 months compared to 17 months in patients without methylation. They also concluded that methylation in patients receiving radiotherapy and TMZ followed by adjuvant administration of TMZ was an independent prognostic factor for overall survival and disease-free survival. Weller et al. [43], analyzed 189 patients treated with radiotherapy and TMZ and again found that MGMT methylation was a significant prognostic factor, improving patient survival from 6.8 to 12.5 months. In 2018, Dalhrot et al. [44] evaluated 171 samples collected from patients with GBM and concluded that MGMT was an independent prognostic factor of patient survival, with a 5-month increase in patient survival when MGMT was not expressed. Radke et al. [45] also see that the MGMT promoter is predictive for treatment response in glioblastoma patients using samples of 111 patients showing a correlation between MGMT promoter status and the overall survival and the progression-free survival. Recent studies (e.g. Schaff et al. [46]) correlated the methylation of the MGMT promoter with significantly increased patient survival. These authors analyzed 58 patients with glioblastoma and observed a median survival of 19.2 months in patients with methylated MGMT versus 7.6 months in patients with unmethylated MGMT. Similarly, a larger retrospective study including 119 patients analyzed the state of MGMT methylation and survival, among other factors [47]. The results obtained indicate that patients under 50 years old with methylation of the MGMT promoter are those with the greatest survival.
However, although these studies show a better evolution in patients who had epigenetic silencing of MGMT, there are other studies that sometimes contradict this correlation. For example, Hegi et al. [48] observed that not all patients with GBM who presented methylation of MGMT promoter responded satisfactorily to treatment, while others did not find any differences in the tumor recurrence pattern based on the state of the MGMT gene promoter [49]. In addition, Dahlrot et al. [50] showed in a study using 327 samples that MGMT gene promoter methylation was only related to survival during the first 9 months after treatment.
Although there are a greater number of studies that found a relationship between MGMT promoter methylation and increased patient survival, unfortunately, neither MGMT protein expression levels nor MGMT promoter methylation status provide enough information to accurately predict an effective response to TMZ treatment and therefore decide whether it should be administered [31]. Nevertheless, it is apparent that MGMT promoter methylation status remains stable in most patients, which indicates that resistance to TMZ is not associated with changes in the promoter methylation pattern [51].
Methylation of MGMT is currently considered one of the most important factors for predicting the sensitivity of GBM cells to treatment with alkylating agents [52], but the contrasting results imply that it is not the only DNA repair mechanism involved in the tumor patients’ response. Therefore, we need to analyze other DNA repair mechanisms, such as the MMR system or base excision repair (BER) that could explain why TMZ-induced alkylation fails.
2.3. DNA Mismatch Repair (MMR) System
Several tumor-suppressor genes are involved in correcting DNA replication errors that occur during cell division. This mismatching could be due to DNA replication errors during the S phase, including insertion or deletion loops and point mutations, which escape correction by exonucleases or homologous recombination [53, 54].
The MMR system is composed of several subunits that join to form heterodimers. The MMR DNA repair mechanism consists of several consecutive stages [55]. The first stage involves recognition and binding to the mismatch. This step is carried out by MSH2-MSH6 or MSH2-MSH3 subunits, depending on the type of mismatch that is recognized. The corresponding heterodimer recruits the MLH1-PMS2 complex forming a tertiary complex which in turn recruits the proteins required to produce a gap. The second stage comprises excision by the EXO1 exonuclease and the process is concluded with repair and ligation by ligase LIG1 in the third stage [3, 56].
We now know that the MMR system is involved in the processing of DNA damage induced by alkylating agents such as TMZ [47]. In the absence of MGMT, O6-MeG pairs with thymine; the O6-MeG-T mispair is recognized by the MMR system which initiates a futile repair process that results in DNA strand breaks. Attempted repairs and subsequent DNA strand breaks trigger prolonged G2 cell cycle arrest, leading to autophagy, senescence, or apoptotic cell death [7, 57]. Therefore, if the MMR complex is mutated or inactivated, tolerance to TMZ increases in GBMs since the erroneous O6-MeG/thymine pairs may not be recognized, which leads to continuous DNA replication and resistance to TMZ treatment [2, 57, 58].
Several in vitro studies have linked resistance to TMZ therapy with a deficiency in the MMR system in cell lines with MGMT promoter methylation, that is, lines without MGMT expression [36, 59, 60]. Within the MMR system, the MSH6 gene is known to be highly prone to inactivating somatic mutations and even complete suppression of its expression after chemotherapy with TMZ [61]. In about 25% of cases of GBM, the MSH6 gene is altered after treatment with alkylating agents such as TMZ, but not in GBMs without prior treatment or in recurrent tumors after radiotherapy [62]. Therefore, in vitro experiences showed that a deficiency of the MSH6 protein causes resistance to TMZ treatment and that restoration of the expression of this protein leads to a more chemosensitive phenotype. However, Stark et al. [63] demonstrated that MSH6 positivity is a poor prognostic indicator in GBM patients as they observed a decrease in MLH1 immunoexpression in 42 paired GBM samples from patients with recurrence. Furthermore, studies have shown that the loss/reduction of MLH1 expression is related to tumor recurrence and increased resistance to TMZ in different GBM lines [64]. A recent study proposed the use of treatment with a PLK1 inhibitor (Volasertib) since in vitro studies show that it inhibits glioblastoma tumor cell proliferation with MMR deficiency [65]. On the other hand, studies such as that of Maxwell et al. [66] did not find a relationship between an absence of MMR and resistance to TMZ at a clinical level, which suggests that more mechanisms must be involved in TMZ resistance.
2.4. Base Excision Repair (BER) Pathway
The DNA base excision repair (BER) pathway involves the cooperation of several DNA repair proteins and functions by replacing a single nucleotide containing a damaged base in a multistep process [67]. It is the main means of elimination and repair of damaged nucleotides generated by reactive oxygen species, ionizing radiation, and alkylating agents [28, 68], repairing not only base modifications but also single-strand DNA breaks [54]. The BER pathway plays a crucial role in both dividing and non-dividing cells since different types of DNA damage can occur during the cell cycle, such as oxidation, deamination, and spontaneous hydrolysis [69]. More than 90% of the methylations caused by TMZ are N3-MeA and N7-MeG methylations, which are both repaired quickly and efficiently by the BER pathway, thus BER is involved in TMZ resistance mechanisms. Although N3 lesions are fatal if they are not repaired, N7 lesions do not appear to be markedly cytotoxic. N3-MeA and N7-MeG methylations are much more common than O6-meG methylation; however, MGMT and MMR appear to be more important pathways in TMZ resistance than BER [22, 58].
Within the BER pathway, poly(ADP-ribose) polymerase-1 (PARP-1) is a key protein in terms of successful signaling of DNA damage. Although this protein is expressed constitutively, it is activated in response to DNA damage [70]. By binding to DNA, this enzyme synthesizes poly(ADP-ribose) (PAR) from NAD+, which is essential for the recruitment of BER pathway proteins and consequent DNA repair. PARP inhibition increases the frequency of DNA strand breaks, so PARP deficient cells are hypersensitive to carcinogenic agents [71].
PARP inhibition increases the cytotoxicity of the lesions normally repaired by the BER system, thus improving TMZ’s in vitro and in vivo cytotoxicity [54]. Therefore, interrupting the BER pathway by inhibiting PARP is a means of overcoming resistance to TMZ [72, 73]. Many PARP inhibitors have been developed and tested in several tumor types [74], wherein the cytotoxic effect of TMZ was enhanced against colon and glioma tumor cells.
In the case of colon cancer, studies by Calabrese et al. [75] investigated the inhibitor AG14361 in human colon cancer cell lines A549, LoVo, and SW620 using proliferation and survival assays and in xenografts in mice through tumor volume determination. They reported an in vitro increase of TMZ’s antiproliferative activity when combined with the PARP inhibitor and complete regression of SW620 xenograft tumors in in vivo studies. Curtin et al. [72] also studied the inhibitor AG14361 in colon cancer cell lines with and without expression of the genes from the MMR system, resulting in suppression of TMZ resistance. AG14361 can enhance TMZ activity in all cell lines although it is more effective in MMR-deficient cells. In the case of glioma, Miknyoczki et al. [76] studied a different inhibitor, CEP-6800, in U251MG. They carried out in vivo studies with the co-administration of CEP-6800 and TMZ, observing 100% regression of tumors when both drugs were used versus a 60% regression when TMZ was administered alone. Two years later Cheng et al. [77] used INO-1001 as an in vivo PARP inhibitor in malignant glioma xenograft D-245 MG and showed that PARP inhibition may increase the efficacy of TMZ in the treatment of malignant gliomas, particularly in MMR-deficient tumors where the combination of TMZ and INO-1001 extended tumor growth delay by 21.6 days.
2.5. Cancer Stem Cells
Cancer is a heterogeneous disease with most tumors containing phenotypically and functionally distinct subsets of cells [78]. These tumors are known to contain a relatively small population of cancer cells with stem-like properties, or CSCs. The main characteristics of these cells are they can be found in the same tissue, biologically they resemble normal stem cells, and they have the capacity for self-renewal and differentiation [79]. In addition, the CSC model suggests that the growth and progression of many tumors are driven by small subpopulations of CSCs [80]. CSCs have been isolated from many different malignancies, including breast, prostate, colon, brain, pancreas, lung, liver, bladder, ovarian, and others [81, 82].
Glioma was one of the first solid tumors in which CSCs were identified thanks to the expression of the antigen CD133 on their surface and nestin expression, although CD133 is currently being questioned as a CSC marker [83-85]. What seems clear is that GBM treatment resistance can also be explained by the presence of glioma stem cells (GSCs) found among the tumor cell population. GSCs are tumor cells with a higher level of proliferation among all the cells in GBM [86] and which reside in a special microenvironment called the stem cell niche. This niche provides favorable conditions for GSC maintenance and survival and allows them to interact with non-tumor cells and the extracellular matrix without being recognized by the patient’s immune system [87]. What distinguishes these cells from normal stem cells is their high protein content which is associated with proliferation, migration, DNA repair, resistance to radiation and chemotherapy, survival, and invasion [88]. Therefore, their key characteristics include resistance to redox stress, active DNA repair capacity, and the expression of several ABC transporters that can expel antitumor drugs out of the cell. Several in vitro studies have suggested that cells containing GSC markers are better able to withstand TMZ exposure [89, 90].
GSCs are known to express a higher number of ABC transporters than differentiated tumor cells. One of the primary overexpressed transporters is ABCG2, which seems to interact with matrix metalloproteinases and consequently triggers an increase in cell migration and invasion [91, 92]. Different authors have also demonstrated that GSCs present self-renewal in neurosphere assays, have a greater tumorigenic potential in vivo compared to tumor cells, and can be differentiated into cells that express astrocyte, oligodendrocyte, and neuronal markers. They are also reported to play a role in resistance to both chemotherapy and radiotherapy [83, 93, 94].
All these characteristics help GSCs survive chemoradiotherapy in patients with GBM, which means the tumor can continue to proliferate [95] and they may contribute to recurrence, which is a potential target for improving therapeutic efficacy and the prognosis for this tumor [96]. Again, there are also contrasting studies. Beier et al. [85] demonstrated that CD133+ and CD133- GSCs lose proliferative and tumorigenic potential after TMZ treatment, while most of the tumor population seemed to be resistant in comparison with GSCs. These authors noted this effect was independent of MGMT so further studies are needed to fully understand the effects of TMZ and other therapies on different tumor cell populations in this heterogeneous disease.
2.6. Acquired Resistance
Acquired drug resistance develops as a consequence of selective pressure in the presence of a chemotherapeutic agent. Acquired chemoresistance may be a consequence of genetic and epigenetic changes induced by drugs in neoplastic cells. These changes include the induction and selection of genes that confer a survival advantage, or the selection of pre-existing resistant cell clones [97]. Included among the possible alterations are an increase of drug efflux through membrane pumps that actively expel chemotherapeutic agents, inactivation of the intracellular drug, tolerance to DNA lesions, and alteration of apoptosis-related genes [98]. These modulations are important in highly heterogeneous tumors like GBM because treatment may select resistant cells that will later give rise to tumor recurrence [85, 99].
One of the pathways involved in the acquired resistance in GBM is the Src tyrosine kinase pathway, which regulates actin dynamics and the invasion of malignant glial cells [100]. Src mediates signals from the extracellular matrix and interacts with several intracellular proteins, including integrins, Eph kinase, and growth factor receptors. Src tyrosine kinase activity is higher in GBM cells compared to normal brain cells [101]. Eom et al. [102] investigated an Src tyrosine kinase inhibitor (PP2) and its coadministration with TMZ, observing that PP2 enhanced in vitro radiosensitivity of malignant glioma cells and suppressed invasion and migration, while in the in vivo trials the combination resulted in a statistically non-significant tumor volume decrease. However, other authors [103] found that suppressing Src family kinase signaling can inhibit bevacizumab-induced GBM cell invasion, suggesting a possible strategy for overcoming GBM treatment resistance.
Some studies have shown that certain miRNAs are implicated in acquired TMZ resistance. Ujifuku et al. [104] reported that miR-195, miR-455-3p, and miR-10a* were upregulated in a panel of laboratory-generated TMZ resistant human glioma cell lines. Li et al. [105] evidenced that miR-1268a was markedly downregulated in U-87 MGand LN229 cells following TMZ treatment, resulting in the upregulation of ABCC1, thus contributing to acquired TMZ resistance. Another example is the work by Xu et al. [106] who found that TMZ treatment-induced autophagy activation as well as miR-30a downregulation. A study by Slaby et al. [107] showed that GBM patients who responded to concomitant radiotherapy with TMZ presented miR-181b and miR-181c downregulation. All of these studies concluded that miRNA could serve as a predictive marker of TMZ treatment response in glioblastoma patients.
3. TEMOZOLOMIDE: CLINICAL USE
After successful results obtained in phase II clinical trials, TMZ received accelerated approval from the Food and Drug Administration (FDA) and the European Medicines Agency (EMA) for recurrent GBM and anaplastic astrocytoma in 1999 [108, 109]. The National Institute for Health and Care Excellence (NICE) then recommended it as a second-line drug for patients with brain tumors who had suffered a recurrence. The positive data obtained in a large phase III clinical trial [110] prompted the FDA to recommend TMZ as a first-line drug for the treatment of newly diagnosed brain tumors in 2005. More recently, the use of TMZ for the treatment of a variety of solid tumors -in addition to nervous system tumors- is under investigation in a number
of clinical trials. In this review, we searched the ClinicalTrials.gov database for eligible studies since January 2012. Our search strategy included the key terms “Temozolomide”, “TMZ” and “Cancer”. From the clinical trials retrieved, we reviewed the most relevant studies in which TMZ, either in monotherapy or in combined therapy, was employed for cancer treatment regardless of tumor type. Finally, we selected those with results published in the PubMed electronic database (Table 1).
3.1. Temozolomide in Glioblastoma
Gliomas constitute a broad tumor group that develops in glial cells and which affects the brain and spinal cord. Traditionally, the World Health Organization has categorized gliomas according to their histological appearance but a recent update to the classification also incorporated molecular parameters [111]. Gliomas are based on grades that represent their potential for malignant transformation, although low-grade gliomas can develop into high-grade tumors. Grades I and II are considered low-grade gliomas and thus correspond to a better prognosis, while grades III and IV are high-grade gliomas which show a more aggressive behavior [112]. GBM, a grade IV glioma, is the most common and most malignant primary brain tumor [113] and leads to extremely low median survival rates (14.5-16.6 months) with the standard of care treatment (surgery, radiotherapy, and TMZ chemotherapy) [114].
Numerous clinical trials have presented results regarding TMZ activity when administered as a monotherapy or in combination therapy. Recently, trans sodium crocetinate (TSC), a drug that enhances oxygen delivery, was administered in combination with the standard dose TMZ and radiotherapy in a phase I/II trial, including 59 patients with GBM. The results showed that 36% of patients receiving TSC were alive after 2 years compared with 27-30% of those given the standard treatment. The authors suggested that coadministration of TSC with the standard treatment was advantageous in GBM therapy [115]. A large phase III trial with 695 GBM patients and 83 participating centers examined the efficacy of standard TMZ maintenance therapy combined with tumor-treating fields (TTFields) in patients previously treated with TMZ and radiotherapy. They established two treatment groups, patients receiving TMZ plus TTFieds (n = 210) and those given TMZ alone (n = 105). The primary endpoint was progression-free survival (PFS), which increased by 3.1 months in the group treated with TMZ plus TTFieds compared to TMZ alone (PFS of 7.1 months and 4 months, respectively). Similarly, an increase in overall survival was also noted in the TMZ plus TTFieds group (20.5 months) compared with those who received TMZ alone (15.6 months), suggesting the benefits of using TTFields in conjunction with standard therapy [116].
Two landmark trials examined the possible benefit of bevacizumab in the clinical outcomes of GBM patients. In a phase III study, Gilbert et al. [117] assessed 637 patients with newly diagnosed GBM who were administered bevacizumab in combination with the standard chemoradiotherapy and TMZ, observing a longer PFS in the patients receiving bevacizumab than in the placebo group (10.7 months vs. 7.3 months). Unfortunately, no significant difference was observed between the two groups with respect to OS (15.7 and 16.1 months, respectively). Similar results were obtained by Chinot et al. [118] in a trial evaluating the benefits of adding bevacizumab to the TMZ plus radiation-based therapy for GBM.
PARP inhibitors have proven to strongly increase the antitumor activity of TMZ in preclinical tumor models [119]. However, this combination has failed to demonstrate significant efficacy in several clinical trials, e.g. involving pediatric high-grade gliomas (phase I/II), where the addition of veliparib to the TMZ-based treatment did not provide survival benefits [120]. Patient-derived xenograft models have shown that MGMT hypermethylation could be key to increase the efficacy of TMZ plus veliparib [121], suggesting that the lack of stratification of patients according to hypermethylated tumors could explain the disappointing results observed in clinical studies. In this sense, the role of hypermethylated MGMT promoter as a predictive biomarker is currently being investigated in a phase II/III trial involving the combination of TMZ and veliparib (NCT02152982).
Several clinical studies were designed but never undertaken due to a lack of suitable patients. For example, the phase II trial NCT02394665 aimed to evaluate treatment efficacy and toxicity in patients with GBM receiving radiotherapy and TMZ using 3D magnetic resonance spectroscopy imaging (MRSI) and conventional magnetic resonance imaging (MRI). However, the study could not be completed due to an insufficient number of participants. Another projected study intended to explore whether the standard TMZ treatment administered along with stem cell radiation therapy improved survival in GBM patients, but it was not finalized because none of the subjects completed the study (NCT02039778). Treatment with PSMA-ADC, a prostate specific membrane antigen (PSMA) monoclonal antibody targeted tumor angiogenesis, was proposed in the BrUOG 263 study (NCT01856933) to evaluate whether it provided an improved clinical outcome in terms of response rate in GBM patients who progressed after standard therapy with TMZ, radiotherapy, and bevacizumab. Although the study was concluded, it only included a limited number of participants (n = 6) and the statistical analysis has not been reported yet.
Although several programmed studies were eventually discontinued due to a lack of data, there are numerous clinical trials designed to evaluate the clinical impact of TMZ in combination with other agents. One study registered in July 2019 aims to evaluate the efficacy and safety of Δ9-tetrahydrocannabinol (THC) and cannabidiol (CBD) on standard therapy with TMZ and radiotherapy in newly diagnosed GBM patients (NCT03529448). This THC/CBD-based therapy was previously suggested to improve the standard treatment in recurrent GBM patients [122]. Authors showed that the 1-year survival was 83% and 56% in the experimental and placebo groups, respectively; in addition, 6-month PFS and median survival were also higher in the THC/CBD-receiving patients, although these differences were not significant. Another two studies initiated recently are currently recruiting participants to test the monoclonal antibody bavituximab (NCT03139916) and glasdegib (NCT03466450), an inhibitor of the Sonic hedgehog pathway, coadministered with TMZ and radiotherapy. Research into effective therapies for GBM has been limited by the difficulty of treatment considering both the tumor location and aggressiveness, while the results of the numerous clinical trials in progress are not expected for several years.
3.2. Temozolomide in Other Tumors
The use of alkylating agent-based therapies, and especially those incorporating TMZ, is widely documented in patients with gliomas; however, given its broad-spectrum antitumor activity, the efficacy of TMZ, both in monotherapy and combined with other agents, has been tested in numerous trials for other types of cancer, although most of them failed to reveal encouraging anticancer efficacy.
A phase I/II study with 35 patients with neuroendocrine tumors (NETs) analyzed TMZ combined with capecitabine and the targeted radiopeptide 177Lu-octreotate. The authors observed that 15% (5/34) of the patients experienced a complete response (CR), while 38% (13/34) showed a partial response (PR), 38% stable disease (SD), and 9% (3/34) disease progression (DP). Depending on the type of NET, higher response rates were observed in patients with gastropancreatic NETs than those with bowel primary NETs, suggesting TMZ had a synergistic effect in the treatment of these tumors, probably by depleting MGMT levels as a result of prior treatment with capecitabine [123]. More recently, the same authors published results from a phase II study involving 30 patients with advanced NETs after 4 years of follow-up receiving the same therapy. Results showed a CR in 13% of patients, a PR in 70%, and no patients experimented DP [124].
In Ewing’s sarcoma, one of the most common cancers in children and young adults, a phase II study with 24 participants reported that in a group of 12 patients receiving a treatment regimen including TMZ and several other drugs, 25% exhibited PR, 66.7% SD, and 8.3% DP. It should be noted that these data are preliminary results because the study has not finished and most of the outcome measures and the statistical analysis are yet to be reported (NCT01946529).
Incidence rates are remarkably high for brain metastases, representing more than 50% of intracranial tumors in adults [125]. One of the primary tumors that most commonly produces brain metastasis is non-small cell lung cancer (NSCLC) [126, 127]. A phase III study involving 126 patients with NSCLC and brain metastases demonstrated that the combination of TMZ or erlotinib with whole-brain radiation therapy (WBRT) and stereotactic radiosurgery (SRS) did not lead to improved survival rates; on the contrary, the drugs had a negative effect on median survival (13.6 months, 6.3 months, and 6.1 months for WBRT+SRS, WBRT+SRS+TMZ, and WBRT+SRS+erlotinib, respectively) [128]. Similarly, other authors have also published similar survival results in this type of patients receiving TMZ [129, 130]. Taken together, these data suggest that TMZ can provide a minor improvement on current treatments in NSCLC with brain metastases, but further investigations would help clarify its usefulness.
Another type of tumor in which TMZ has been used is malignant melanoma, which is the main cause of death due to cutaneous neoplasms. The combination of the alkylating agent fotemustine and TMZ acting as chemo-modulator, has been proposed as an alternative treatment for melanoma patients who had scarce therapeutic options because previous therapies failed or through non-eligibility for immunotherapy. This phase II clinical trial enrolled 69 patients with melanoma, most of them presenting very poor prognoses (74% of the patients at M1c stage and 15% with brain metastases). The results obtained showed an overall response rate of 30.3%, including three CR, and a disease control rate of 50.5%. PFS and OS data were of 6 and 10 months, respectively [131].
PARP inhibitors have been tested in order to increase TMZ sensitivity in other cancer types such as melanoma [132], liver [133], and colorectal [134] cancers, among others. A phase II study recently published by Han et al. [135], featuring 290 patients with metastatic breast cancer and BRCA1/2 mutations, analyzed the efficacy of a TMZ and veliparib combination compared to carboplatin/paclitaxel administered with veliparib or placebo. The authors found that PFS and OS were inferior in the arm treated with TMZ and veliparib (7.4 months and 19.1 months, respectively) than in the other treatment groups (PFS = 14.1 months and 12.3 months, OS = 28.3 and 25.9 months for carboplatin/paclitaxel plus veliparib or placebo, respectively). Equally, another study conducted in prostate cancer patients evaluating veliparib in combination with TMZ also reported modest antitumor action [136]. This TMZ/veliparib combination was also used in a clinical trial (NCT01638546) with 104 patients with small-cell lung cancer (SCLC) [137, 138]. Interestingly, these authors observed improved PFS and OS results in patients with SLFN11 positive tumors treated with this combination, suggesting its potential use as a predictive biomarker in SCLC. Further studies are needed to identify predictive biomarkers in potentially responding patients to this combined therapy
While MGMT methylation status has long been considered as a biomarker to help predict the response to TMZ in gliomas, and a number of new biomarkers have been proposed for this purpose as epidermal growth factor receptor (EGFR) variant III (C11), MSH2 and MSH6 [37, 48, 139-142], the reality is that their transfer as predictive biomarkers in other types of cancer has been disappointing. A phase I study evaluated the efficacy of radiotherapy and TMZ plus capecitabine-based chemotherapy in patients with locally advanced rectal cancer. Of the 22 patients included in the study, a CR was observed in seven patients (31.8%), of which 6 presented MGMT promoter methylation, suggesting a possible predictive role of MGMT promoter methylation status for TMZ response [143]. The same researchers are currently recruiting participants for a new phase II study (NCT03156036) designed to assess the potential benefit achieved by adding TMZ to this treatment regimen and to validate the predictive value of MGMT status. In contrast, a phase II study recently performed by Calegary et al. [144] evaluated TMZ-based treatment in patients with metastatic colorectal cancer (CRC) and methylated MGMT promoter, but the drug showed limited antitumor activity. Similarly, Amatu et al. [145] also indicated the modest activity of TMZ in metastatic 24 CRC patients previously selected by MGMT promoter methylation
To validate MGMT and mismatch repair (MMR) status as predictive biomarkers for TMZ therapy, Hochhauser et al. [139] analyzed samples from 740 patients with advanced aerodigestive tract cancers and CRCs, of whom 86 had a methylated MGMT promoter. They showed that all cases with a PR to TMZ and most cases with SD (82%) were MMR proficient. Although the observed tendency revealed that MMR-proficient tumors were associated with therapeutic response to TMZ, a correlation could not be assumed due to the low number of patients (only 5 patients with a PR and the absence of MMR-deficient tumors). On the other hand, Pietrantonio et al. [146] proposed RAS or BRAF mutations as potential predictive biomarkers for a response to TMZ treatment, since they only observed an objective response in patients with some of these wild-type genes. In contrast, in their recent study administering a dose-dense TMZ schedule, the same authors reported an increased sensitivity to TMZ in CRC patients with RAS or BRAF mutations [147], while other authors did not observe any significant differences regarding TMZ activity between patients with or without RAS mutations [144].
The antitumor action of TMZ has been evidenced in leukemia cell lines and in vivo models [148]. Unfortunately, as in the case of solid tumors, MGMT expression has a negative effect on TMZ activity in leukemia patients [149] and most cases of acute myeloid leukemia (AML) present unmethylated MGMT promoter, leading to treatment resistance [150]. Several clinical trials involving AML patients have evaluated the effectiveness of TMZ (alone or combined) by analyzing the relevance of MGMT methylation on this therapy [151, 152]. A phase II study tested the clinical response to TMZ in 36 AML patients stratified according to MGMT methylation status. Patients with a methylated promoter (14%, 5/36) received the standard dose of TMZ (200 mg/m2 for 7 days), while patients with unmethylated MGMT (86%, 31/36) were treated with protracted TMZ administration consisting of low-dose TMZ (100 mg/m2 for 14 days) followed by the standard dose (200 mg/m2 for 7 days) in order to inactivate MGMT. The authors observed similar response rates in both patient groups (40% and 29% in the methylated and unmethylated groups). Although overall follow-up was longer in patients with methylated MGMT (32 weeks vs. 6.23 weeks), the authors did not observe significant differences in the OS between both groups (20 weeks in methylated and 10 weeks in unmethylated MGMT) [153].
However, the utility of TMZ in other tumors besides gliomas cannot be definitely ruled out due to serious limitations in the studies, including low numbers of patients. In addition, some studies have demonstrated low MGMT expression regardless of promoter methylation status, both in melanoma [154] and gliomas [155], in which no benefit with TMZ was obtained [156]. Thus, other mechanisms besides promoter methylation could also regulate MGMT expression. In fact, certain microRNAs [157] correlated with MGMT expression, such as an enhancer gene located between a marker of proliferation Ki-67 (MKI67) and MGMT promoters [158]. Besides, other DNA repair systems, such as MMR, BRCA2, and XRCC2 could play a relevant role in repairing alkylation-induced damage [159]. In addition, apoptosis resistance may decrease TMZ efficacy since apoptosis inducers associated with TMZ show a synergistic effect in melanoma [160]. These data suggest the existence of numerous mechanisms involved in the failure of TMZ treatment in melanoma but the ability of TMZ to cross the blood-brain barrier may be useful to reduce brain metastases in high-risk melanoma [161]. In summary, the role of TMZ in tumors other than gliomas is still controversial, yet, further research is needed to clarify the usefulness of this drug.
4. ADVANCES IN THE USE OF TMZ
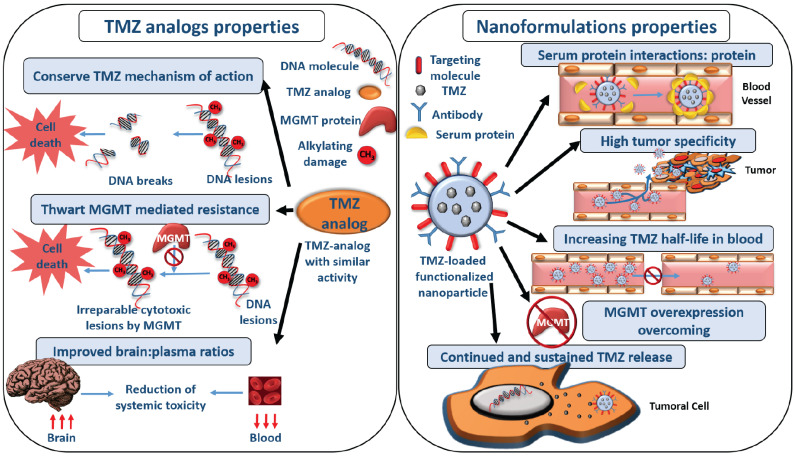
There are some new advances related to the use of TMZ in GBM therapy. One of these new approaches is included in the nanotechnology field. Nanomedicine is based on the use of nanosized materials for the treatment, monitoring, or diagnosis of certain diseases, such as cancer [162]. These nanoformulations can be used to transport different types of a molecule: drugs, proteins, nucleic acids, and fluorophores. Nanoformulations or nanoparticles (NPs) increase the antitumor drug efficacy and reduce their systemic toxicity, enhancing the pharmacokinetic properties of drugs [163]. Nanomedicine could solve the problems associated with TMZ treatment of GBM, such as its rapid degradation, lack of specificity, and the difficulty to obtain an effective dose of TMZ within the tumor tissue (only 20% of the systemic exposure to TMZ penetrates the brain tissue, thus the amount of drug that can reach the tumor is much lower) (Fig. 4) [9, 164]. New nanoformulations are focused on overcoming MDR mechanisms, although most of the studies are preclinical. In addition, the development of TMZ analogs with the same mechanism of action than TMZ but different structure aims to improve its pharmacokinetic profile.
4.1. Nanomedicine and TMZ in Glioblastoma Treatment
New therapeutic strategies based on nanomedicine that increase GBM patient survival have been developed [165]. One strategy is to improve the therapeutic effect of the drugs currently used in chemotherapy, in this case, TMZ in an attempt to erase its limitations. Some examples of TMZ-loaded nanoformulations are summarized in Table 2. In some cases, apolipoprotein E is used to functionalize NPs and facilitate their passage through the BBB [166]. This functionalization can even be carried out indirectly. When NPs are introduced into the bloodstream they can interact with blood proteins absorbing them onto their surface to form what is called the protein corona [167, 168]. Cationic liposomes can form this protein corona when they are exposed to blood, showing improved binding to receptors highly expressed in the BBB. These TMZ-loaded liposomes demonstrated higher cytotoxicity in U-87 MG glioblastoma cells in comparison with liposomes without protein corona [169]. Other targets have been used to promote the passage through the BBB, such as BBB glucose transporters, which promote NP transcytosis, and the transferrin receptor that is overexpressed in brain tumors due to the high metabolic requirements of cancer cells, in this case for iron [170-173]. Anti-transferrin receptor antibody was used to functionalize TMZ-loaded cationic liposomes, resulting in improved survival of mice bearing intracranial GBM tumors compared to non-functionalized liposomes and free TMZ (2.4 and 1.4 times higher, respectively) [174]. Transferrin (Tf)-conjugated solid lipid nanoparticles carrying TMZ showed a better hemocompatibility profile compared to free TMZ (3.5-fold in % of hemolytic toxicity) and a greater accumulation next to the brain blood vessels in comparison with the non-functionalized nanoformulation [175].
Besides, these nanocarriers can be functionalized with certain molecules so they can cross the BBB and specifically target tumor cells such as chlorotoxin peptide [176] and the tripeptide RGD [177]. Lipid carriers functionalized with RGD tripeptide and loaded with TMZ were used to treat BALB/c nude mice with GBM subcutaneous tumors. After the treatment (once every 3 days for 21 days), tumor growth was significantly inhibited with functionalized nanoformulations (83.3%) compared to non-functionalized nanocarriers (66.3%) and free TMZ (20.8%) [178].
Other ligands used to functionalize TMZ-loaded NPs are folic acid. An example of this is magnetite NPs that combine hyperthermia and chemotherapy with TMZ, stimulating the temperature rise the release of the TMZ. Greater cell death rates have been demonstrated in tumor cells that overexpress the folate receptor even greater with the combined treatment of TMZ-loaded magnetic NPs with hyperthermia (23.66% of cell death) compared to treatment with chemotherapy plus hyperthermia (14.32%) or with hyperthermia (12.5%) [179]. Another study used folic acid to functionalize TMZ-loaded poly(ethylene glycol)-poly(butylene-adipate)-poly(ethylene glycol)-coated magnetite NPs. This nanoformulation was tested in cancer C6 cell line showing higher cytotoxic effects compared to free TMZ and non-functionalized NPs, due to the addition of folic acid which increases the drug concentration inside cells [180].
TMZ treatment is sometimes difficult to implement, even in the case of nanomedicine. This is particularly true for poly(lactic-co-glycolic acid) (PLGA) NPs, which is suitable for TMZ vehiculation because GBM tumor cells require high levels of this drug for an adequate cytotoxic effect, while PLGA NPs cannot transport large amounts of a drug (and therefore they might not achieve the desired therapeutic effect) [181]. However, the use of therapies in which TMZ is co-administered with other NP-encapsulated molecules could enhance its antitumor effect. Such is the case of PLGA NPs that transport the antisense microRNA-21 (microRNA-21 is overexpressed in GBM cells) [182]. Functionalized and PEGylated-PLGA NPs were loaded with both antimicroRNA-21 and antimicroRNA-10b and administered before TMZ treatment. The results revealed that TMZ had an increased cytotoxic effect as U-87 MG and Ln229 GBM cell
lines were more sensitive and a potent antitumor effect was observed in tumor-bearing mice 4 days after administering the treatments [183]. Nevertheless, other authors have reported good results with PLGA NPs in terms of TMZ transport, perhaps due to the addition of a functionalizing element. For example, Ramalho et al. [184] encapsulated TMZ in PLGA NPs functionalized with transferrin receptor monoclonal antibody and even though drug encapsulation rates were low (9-10%), they were able to obtain 4.1- and 9.9-fold reductions in TMZ IC50 in U251 and U-87 MG tumor cell lines, respectively. The use of transferrin to functionalize pegylated PLGA NPs has been also studied by Jain et al. [185], who reported higher cytotoxicity in the IMR-32 tumor cell line (neuroblastoma) compared to free TMZ (1.2-fold at 10 µg/ml TMZ); unlike the SK-NS-H (CNS) cell line, in which TMZ was more cytotoxic. Conversely, higher survival rates were observed in glioma-bearing rats treated with PLGA NPs functionalized with ephrin type-A receptor 3 (EPHA3) tyrosine kinase antibody, which increased survival 1.37 times compared to other PLGA formulations [186].
Finally, administration routes other than intravenous have been investigated for the treatment of GBM. One possibility is nasal administration, wherein the drug crosses the mucosa and follows the olfactory pathway to the CSF and is finally transported to the brain [187]. The study used TMZ-loaded nano lipid chitosan hydrogel formulations. They observed that the NPs could cross the nasal mucosa and there was a greater accumulation (2.6 fold) of TMZ in the brain compared with TMZ administered uniquely with chitosan. Furthermore, enhanced cytotoxicity was observed in rat C6 glioma cell line with a reduction of TMZ IC50 from 160 µg/ml for free TMZ to 3.34 μg/ml for TMZ-loaded NPs [188].
4.2. Using Nanomedicine and TMZ to Overcome the MDR Mechanism
NPs can also provide solutions to the numerous TMZ chemoresistance mechanisms of GBMs discussed in previous sections. O6-Benzylguanine (BG) is a potent MGMT inhibitor, so it could improve the efficacy of TMZ treatment in GBM patients with overexpressed MGMT [189]. Nevertheless, the pharmacokinetic properties of BG, such as its short half-life in the bloodstream, and its side effects (e.g. it enhances TMZ-induced myelosuppression), added to its poor BBB permeability limit its use [190, 191]. To solve these problems, BG-loaded iron oxide NPs functionalized with chlorotoxin peptide revealed a potentiation of TMZ antitumor effect and improvement in the survival of mice bearing glioma (3-fold), and a longer half-life in blood (5 hours compared to 1.2 hours for free BG) [191].
However, MGMT expression levels usually recover after combined treatment with TMZ and BG, so other strategies have been required to alter MGMT expression [192]. To this end, iron oxide NPs functionalized with chlorotoxin peptide were loaded with a siRNA to silence MGMT expression. The coadministration of these NPs with TMZ in mice with orthotopic brain tumors produced a significant reduction in tumor volume, up to 15-fold compared to mice treated with TMZ alone [193]. Another potential target for improving treatment with TMZ through the regulation of MGMT is the p53 protein, which is normally down-regulated in most GBM. Some studies showed that the restitution of wild-type p53 activity in tumor cells can increase their sensitivity to alkylating agents through a significant reduction in the transcription rate of the MGMT gene via mechanisms that are not yet clear [194, 195]. In another study, cationic liposomes functionalized with a single-chain antibody fragment (scFv) loaded with a wt-p53 plasmid DNA to increase p53 expression showed inhibition of tumor growth and a reduction of tumor volume that persisted until 3 weeks from the end of the treatment [196, 197].
Due to the nature of GBM, it has numerous zones with hypoxia which is believed to contribute to GBM´s resistance to TMZ treatment and its aggressiveness [198]. Therefore, increasing tumor oxygen levels could increase TMZ’s antitumor effect, as in the case of hyperbaric oxygen (HBO) therapy. Xie et al. [199] studied the effect of TMZ loaded in porous silicon NPs combined with hyperbaric HBO therapy in C6 subcutaneous tumor-bearing mice. They observed an enhanced antitumor effect with the combination of HBO and TMZ, but the greatest antitumor effect was detected with the use of TMZ-loaded NPs combined with HBO which produced a decrease in tumor volume of 82% compared to 70% with free TMZ and HBO, and approximately 40% with TMZ alone.
Furthermore, hypoxic tumors are resistant to radiotherapy because the main cause of death produced by this treatment is the generation of reactive oxygen species [200]. Recently, Zong et al., have developed angiopep-2 decorated NPs loaded with TMZ, with a hydrophobic P-(MIs)25 core that can increase cell sensitization to radiotherapy in low oxygen conditions, transforming these hydropic groups into hydrophilic amino groups. The results obtained in glioma-bearing mice showed a strong sensitizing effect against radiotherapy treatment of nanoformulation loaded with TMZ as well as a strong synergistic effect compared to the treatments separately. These mice showed a greater survival (67 days) compared with free TMZ + radiotherapy (43 days) and unloaded NPs with radiotherapy (44 days) [201].
Besides being expressed in the BBB and tumor cells, the transferrin receptor is also overexpressed in glioma CSCs [90, 202]. To act specifically on these cells and prevent treatment resistance, NPs were synthesized to target the transferrin receptor and loaded with TMZ. They were tested in intracranial tumor-bearing mice, revealing colocalization of the NPs and SOX2-labeled CSCs that showed apoptosis and greater antitumor effect with TMZ treatment, thus decreasing the likelihood that this population of cells would redevelop the tumor [203].
4.3. TMZ Analogs
The structure of the TMZ molecule does not offer many possibilities for the generation of analogs without altering its mechanism of action. Rai et al. generated the 8-position N, N-dimethyl carboxamide analogs of TMZ synthetizing γ-carboline, and β-carbolines series. These analogs showed a better DNA-alkylating activity and brain/plasma ratio improvement (up to 30-fold) compared to TMZ. However, this antitumor activity was not observed in the in vivo studies [204]. Some TMZ analogs maintain their mechanism of action but are not affected by the TMZ-resistance mechanisms. TMZ analogs generated by the substitution of N3-methyl with propargyl or sulfoxide showed great antitumor activity in MGMT-positive GBM cells [205, 206] and the TMZ analogs C8-imidazolyl and C8-methylimidazole tetrazines also showed an improved antitumor effect in MGMT-positive GBM cells and MMR-deficient colorectal cancer cells [207]. Finally, nanoformulations including these analogs have also been studied. An N3-propargyl imidazotetrazine analog associated to a targeted-liposomal nanocarrier overcame TMZ resistance in GBM cells [208] and the TMZ analog NEO212, covalent conjugation of TMZ and perillyl alcohol (POH), increased the antitumor activity in nasopharyngeal carcinoma (NPC) cells compared to TMZ. Moreover, this analog made cells more sensitive to the second cycle of drug treatment by MGMT inactivation [209].
4.4. Future Perspectives
Despite a large number of preclinical studies (in vitro and in vivo), TMZ-loaded nanoformulations have not yet entered clinical trials. Consequently, there is still a lot to explore in this field and the evaluation of these nanocarriers in patients will take a long time. There are ongoing clinical trials in which TMZ is administered together with other nanoformulations, usually in combination with other drugs. For instance, a phase II clinical trial (NCT03463265) is exploring the treatment of patients with newly diagnosed or post-treatment (bevacizumab-naïve) GBM using albumin-bound rapamycin or nab-rapamycin (ABI-009), either in monotherapy or in combination with TMZ. In another phase II clinical trial (NCT02340156), patients with recurrent GBM are being treated with combined therapy of oral TMZ and SGT-53, a cationic liposome carrying a plasmid with the DNA sequence of p53 that can induce apoptosis in the tumor cell. In addition, a phase I/II clinical trial is assessing the treatment of patients with recurrent GBM with a prolonged administration of TMZ in combination with doxorubicin-loaded in pegylated liposomes -that enhance its passage across the BBB-, associated with radiotherapy (NCT00944801). In this case, 30.2% of the patients survived more than 12 months, with a median survival of 17.6 months. However, these results are seemingly not superior to the administration of TMZ plus radiotherapy [210]. Moreover, an ongoing phase I-II clinical trial (NCT01044966) aims to evaluate the treatment of patients with GBM and astrocytoma using TMZ combined with cytarabine (Ara-C) loaded in liposomes (DepoCyt). It has been demonstrated that cytarabine can inhibit the proliferation and migration of the subventricular zone progenitor cells (located in the ventricular system of the CNS), which are thought to play a crucial role in GBM recurrence. Finally, a phase I clinical trial is exploring the treatment of pediatric patients with recurrent and refractory solid tumors using nanoparticle albumin-bound rapamycin combined with TMZ and irinotecan hydrochloride (NCT02975882). In summary, despite the increasing number of in vitro and animal studies, the use of TMZ-transporting nanomaterials for the treatment of GBM represents a relatively recent field worthy of exploration. In the same way, TMZ analogs are not being investigated in clinical trials yet. Thus, it will take a long time to use these drugs in patients.
CONCLUSION
Since its approval by the FDA in 2005, TMZ has become the leading first-line chemotherapeutic agent for the treatment of GBM patients. In the clinical context, its use has improved two-year survival rates, PFS, and quality of life compared to radiotherapy alone. However, a large number of patients do not respond satisfactorily to treatment. The analysis of these cases has revealed numerous resistance mechanisms involved in the failure of therapy. One of the most relevant mechanisms involved in TMZ-resistance is the blood-brain barrier, which acts as an initial filter due to the presence of membrane transporters that protect the brain from different substances, such as alkylating agents used in the treatment of brain tumors. Also, different studies reported that the expression of some cellular mechanisms such as MGMT repair protein is related to worse prognosis and lower patient survival. In addition, the MMR system can be altered in GBM and, together with the expression of MGMT, can lead to tumor recurrence. Another mechanism of resistance discussed in this review is the DNA BER pathway. BER eliminates and repairs damaged nucleotides generated by alkylating agents such as TMZ, thus it is highly involved in this drug resistance. In all tumors, a small population of cancer cells with stem-like properties (CSC), i.e. with the capacity for self-renewal and differentiation, can be found. In gliomas, GSCs can form niches for the growth of tumor cells that cannot be detected by the immune system, in addition to presenting a high number of ABC transporters that may be responsible for drug extrusion from the cells. Last but not least, acquired resistance results from the pressure were exerted by the chemotherapeutic agent and can lead to genetic or epigenetic changes which, in turn, can lead to increased cell resistance to treatment. Understanding these resistance mechanisms will be essential to design therapeutic interventions to overcome them. In this context, new tools currently available, such as nanotechnology, could thwart resistance phenomena mediated by the MDR mechanism present in CSCs.
Currently, TMZ is the standard therapy for GBM. However, there are serious drawbacks, mainly related to the invasive nature of this tumor, and to inherent and acquired resistance, that may ultimately lead to treatment failure. Therefore, there is an urgent need for new therapeutic strategies that enhance the benefits of TMZ. In the last decade, the use of TMZ combined with other agents has been increasing in clinical trials, and although most of them have shown survival benefits of only a few months, encouraging results have been reported. Particularly, bortezomib provided a remarkable improvement in OS, revealing the potential therapeutic advantages of combining TMZ with proteasome inhibitors. TMZ is a highly potent drug with a good toxicity profile, thus it has been extensively studied in other tumors, but results are controversial. The combination of TMZ and veliparib showed little or no benefit in different tumor types such as prostate and breast cancer, while certain advantages were observed in small-cell lung cancer. In addition, TMZ provided positive effects on capecitabine treatment in rectal cancer. This scenario reveals the importance of understanding how TMZ interacts with other agents and their impact on the microenvironment of different tumor types, in order to design therapeutic combinations with synergistic effects that minimize treatment resistance.
Thus, despite the fact that TMZ was discovered more than three decades ago, it is a drug that will be present not only for the treatment of GBM but also for the treatment of a large number of tumor pathologies. Further investigations focused on the understanding of mechanisms of action and resistance of TMZ are required to improve its clinical use today and in the future.
Fig. (1).
TMZ. A. Bidimensional and tridimensional structure. B. Illustrative scheme of the mechanism of action of TMZ. (A higher resolution / colour version of this figure is available in the electronic copy of the article).
Fig. (2).
TMZ prodrug activation. Graphic representation of the specific DNA methylation site with TMZ. Only the methylation of O6 guanosine is considered the lethal step and mediates the cytotoxic action of TMZ.
Fig. (3).
Diagram of the mechanisms of resistance to Temozolamide present in Glioblastoma. The MMR system induces the cell to apoptosis by initiating a futile repair process that results in DNA strand breaks. When MMR is inactive, these errors are not corrected and the cell survives, giving rise to drug resistance. The BER system works by repairing the errors caused in the DNA, resulting in treatment resistance. However, some enzymes of the BER system can present mutations even be absent, which leads to increased TMZ sensitivity and cell death. Glioblastoma CSCs can survive the treatment, leading to tumor recurrence. The expression of the MGMT protein counteracts TMZ-induced DNA damage, thus its presence is directly related to drug resistance. Finally, TMZ itself can make tumor cells resistant as this treatment may cause a selection of genes that confer a survival advantage. (A higher resolution / colour version of this figure is available in the electronic copy of the article).
Fig. (4).
Main properties and advantages of the use of nanoparticles and TMZ analogs in cancer treatment. The advantages of the use of nanoformulations in cancer treatment derive from their physicochemical properties, composition, structure, and passive and active targeting to the tumor tissue. Such properties increase their half-life in the bloodstream and their specificity for the tumor tissues, enabling the overcoming of drug resistance mechanisms as well. The analogs of TMZ conserve de mechanism of action of TMZ but provide some advantages such as avoiding the overexpression of MGMT in tumor cells or increasing its concentration in the brain while decreasing the concentration in plasma. (A higher resolution / colour version of this figure is available in the electronic copy of the article).
Table 1.
Trials employing TMZ for the treatment of GBM and other tumors.
| Phase | Tumor Type | Number Patients Enrolled | Treatment Including TMZ |
OS
(Months) |
PFS (Months) | Response n (%) | Refs. (NCT) |
|---|---|---|---|---|---|---|---|
| I-II | GBM | 59 | RT+TMZ+TSC | NR | 3.3 | NR | [115] (NCT01465347) |
| III | GBM | 695 | TMZ+TTFields | 20.5 | 7.1 | NR | [116] (NCT00916409) |
| TMZ | 15.6 | 4 | NR | ||||
| III | GBM | 637 | RT+TMZ+BEV | 15.7 | 10.7 | NR | [117] |
| RT+TMZ+Placebo | 16.1 | 7.3 | NR | ||||
| III | GBM | 921 | TMZ+RT+BEV | 16.8 | 10.6 | NR | [118] |
| TMZ+RT+Placebo | 16.7 | 6.2 | NR | ||||
| I/II | GBM | 21 | TMZ+Sativex | 18.3 | NR | NR | [122] (NCT01812603) |
| TMZ+Placebo | 12.3 | NR | NR | ||||
| I-II | NETs | 35 | Lutetium-177-octreotate+Capecitabine+TMZ | NR | 31 | ORR: 56%; CR: 5 (15%); PR: 13 (38%); SD: 13 (38%) | [123] |
| III | NSCLC | 126 | WBRT+SRS+TMZ | 6.3 | 4.6 | NR | [128] |
| II | NSCLC | 45 | TMZ | 27.1 | 11.7 | NR | [129] |
| II | NSCLC | 60 | WBRT+TMZ+Pemetrexed | 16.9 | 19.3 | ORR: 12 (75%); CR: 6 (21.4%); PR: 15 (53.6%); SD: 4 (14.3%) | [130] (NCT02284490) |
| II | Breast cancer | 284 | Veliparib+TMZ | 19.1 | 7.4 | ORR: 28.6%; CR: 1.4%; PR: 27.1% | [135] (NCT01506609) |
| I | mCRPC | 26 | Veliparib+TMZ | 39.6 (weeks) |
9 (weeks) |
NR | [136] (NCT01085422) |
| II | Aerodigestive tract cancer and CRC | 86 | TMZ | 6.7 | 2.8 | ORR: 5.8%; PR: 5 (6%); SD: 39 (45%) | [139] (NCT00423150) |
| II | SCLC | 64 | TMZ | 5.8 | 1.6 | ORR: 20%; CR: 1; PR: 12; SD: 6 (9%) | [138] |
| I | Rectal cancer | 22 | RT+Capecitabine+TMZ | NR | NR | CR: 7 (31.8%) | [143] (NCT01781403) |
| II | CRC | 41 | TMZ | 5.1 | 1.9 | ORR: 4 (10%); CR: 0; PR: 4 (10%); SD: 9 (22%) | [144] |
| II | CRC | 29 | TMZ | 6.2 | 2.6 | ORR: 3.4%; PR: 1 (3.4%); SD: 13 | [145] |
| II | CRC | 32 | TMZ | 8.4 | 1.8 | ORR: 4 (12%); CR: 0; PR: 4 (12%); SD: 6 (19%) | [146] |
| II | AML and MDS | 45 | TMZ | 6.7 | NR (in entire group) | ORR: 53%; CR: 36% | [151] |
| I | AML | 48 | Veliparib+TMZ | 5.3 | NR (in entire group) | CR: 8 (16.6%) | [152] (NCT01139970) |
AML: acute myeloid leukemia; BEV: bevacizumab; CR: complete response; CRC: metastatic colorectal; GBM: glioblastoma; mCRPC: metastatic castration-resistant prostate cancer; MDS: myelodysplastic syndrome; NETs: neuroendocrine tumors; NR: no reported; NSCLC: non-small cell lung cancer; ORR: overall response rate; OS: overall survival; PFS: progression-free survival; PR: partial response; RT: radiotherapy; SCLC: small cell lung cancer; SD: stable disease; SRS: stereotactic radiosurgery; TMZ: temozolomide; TSC: trans sodium crocetinate; TTFields: tumor-treating fields; WBRT: brain radiation therapy.
Table 2.
Summary of TMZ-loaded transporting nanoformulations.
| Nanomaterial |
Functionalization
to BBB or Tumor Cells |
Agents in Combined Therapy | In Vitro Assay | In Vivo Assay | Refs. |
|---|---|---|---|---|---|
| Poly (butyl cyanoacrylate) (PBCA) NPs | Indirectly by protein corona | - | - | Wistar rats | [168] |
| Cationic liposomes | Indirectly by protein corona | - | Human umbilical vein endothelial cells (HUVECs) and U-87 MG GBM cell line | - | [169] |
| Coumarin-6 labeled liposomes |
Glycosylderivative of cholesterol to target glucose receptors | - | Brain capillary endothelial cells (BCECs) and astrocytes (ACs) of rat, (BBB in vitro) | Kunming mice and Sprague- Dawley rats |
[170] |
| Chitosan-TMZ conjugates plus NA-AF647-CTX conjugates | Chlorotoxin peptide | - | U118 MG, SF767 and GBM6 GBM cell lines | C56BL/6 mice | [164] |
| Pegylated poly (β-L-malic acid) nanoplatforms |
Transferrin receptor monoclonal antibody | - | U-87 MG and T98G primary glioma cell lines and MDA-MB-231 and MDA-MB-468 invasive breast carcinoma cell lines | - | [171] |
| Pegylated NPs (lipidic) |
Transferrin | Bromodoain inhibitor JQ1 | U-87 MG and GL261 glioma cell lines | NCR nude mice and C57/BL6 mice | [173] |
| Cationic liposomes | Anti-transferrin receptor antibody | - | U-87 MG-luc2 luciferase expressing GBM cell line, T98F and U251 GBM cell lines and U-87 MG-R TMZ-resistant cell | BALB/c mice and athymic mice | [174] |
| Solid lipid nanoparticles | Transferrin | - | IMR-32 neuroblastoma cell line and U373 MG CNS | Rats | [175] |
| Nanostructured lipid carriers |
Lactoferrin and the tripeptide arginine-glycine-aspartic acid (RGD) |
Vincristine | U-87 MG and T98G glioma cell lines and A549 lung cancer cell line | BALB/c nude mice | [172] |
| Mesoporous silica NPs coated with polydopamine | RGD tripeptide | - | C6 rat glioma cell line | - | [177] |
| Nanostructured lipid carriers |
RGD tripeptide | - | U-87 MG glioma cell line | BALB/c nude mice | [178] |
| Magnetite triblock copolymers |
Folic acid | Hyperthermia | C6 rat glioma cell line and OLN-93 rat normal glial cells | - | [179] |
| PEG-poly (butylene adipate)-PEG-coated magnetite NPs |
Folic acid | - | C6 rat glioma cell line and OLN-93 rat normal glial cells | - | [180] |
| Pegylated PLGA NPs | Transferrin receptor monoclonal antibody | - | U251 and U-87 MG GBM cell lines, an immortalized human astrocyte cell line (NHA) and human brain-like endothelial cells (HBLECs) | - | [184] |
| Pegylated PLGA NPs | Transferrin | - | Tumor cell lines IMR-32 neuroblastoma cell cline, SK-NS-H CNS cell line, DU145, PC3 prostate, COLO 205, HCT15 colon, MCF7 breast and A549 lung | Albino rats | [185] |
| PAMAM-PEG NPs | Transferrin | - | Primary cell culture of human brain tumors and SU2 stem cell line of glioma origin and 51A glioma stem cell line | BALB/c nude mice | [203] |
| PLGA NPs | Ephrin type-A receptor 3 (EPHA3) tyrosine kinase antibody | - | 16HBE and C6 cell lines | Sprague- Dawley rats |
[186] |
| Nano lipid chitosan hydrogel |
- | - | C6 rat glioma cell line | Wistar rats | [188] |
| Porous silicon NPs | - | Hyperbaric HBO therapy | C6 rat glioma cell line | Nude mice | [199] |
| NPs with a hydrophobic P-(MIs)25 |
Angiopep-2 | Radiotherapy | C6 rat glioma cell line | ICR mice | [201] |
Acknowledgements
This study was supported by Junta de Andalucía (PAIDI group CTS107).
AUTHOR’s CONTRIBUTIONS
JP and CM coordinated the organization of the manuscript. Resistance: RO, GP; Nanomedicine: RO, LC; Trials: CJL, RL; Figures, and Table: LC, GP, CJL, RL. Critical review of the manuscript: RO, JP, CM. Language and editing revision of the manuscript: RO, JP. All the authors approved the final version of the manuscript.
Consent for Publication
Not applicable.
Funding
None.
Conflict of Interest
The authors declare no conflict of interest, financial or otherwise.
REFERENCES
- 1.Mutter N., Stupp R. Temozolomide: a milestone in neuro-oncology and beyond? Expert Rev. Anticancer Ther. 2006;6(8):1187–1204. doi: 10.1586/14737140.6.8.1187. [DOI] [PubMed] [Google Scholar]
- 2.Thomas A., Tanaka M., Trepel J., Reinhold W.C., Rajapakse V.N., Pommier Y. Temozolomide in the era of precision medicine. Cancer Res. 2017;77(4):823–826. doi: 10.1158/0008-5472.CAN-16-2983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhang J., Stevens M.F.G., Bradshaw T.D. Temozolomide: mechanisms of action, repair and resistance. Curr. Mol. Pharmacol. 2012;5(1):102–114. doi: 10.2174/1874467211205010102. [DOI] [PubMed] [Google Scholar]
- 4.Denny B.J., Wheelhouse R.T., Stevens M.F.G., Tsang L.L.H., Slack J.A. NMR and molecular modeling investigation of the mechanism of activation of the antitumor drug temozolomide and its interaction with DNA. Biochemistry. 1994;33(31):9045–9051. doi: 10.1021/bi00197a003. [DOI] [PubMed] [Google Scholar]
- 5.Ramalho M.J., Andrade S., Coelho M.A.N., Loureiro J.A., Pereira M.C. Biophysical interaction of temozolomide and its active metabolite with biomembrane models: The relevance of drug-membrane interaction for glioblastoma multiforme therapy. Eur. J. Pharm. Biopharm. 2019;136:156–163. doi: 10.1016/j.ejpb.2019.01.015. [DOI] [PubMed] [Google Scholar]
- 6.Stéphanou A., Ballesta A. pH as a potential therapeutic target to improve temozolomide antitumor efficacy: A mechanistic modeling study. Pharmacol. Res. Perspect. 2019;7(1):e00454. doi: 10.1002/prp2.454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pawlowska E., Szczepanska J., Szatkowska M., Blasiak J. An Interplay between senescence, apoptosis and autophagy in glioblastoma multiforme-role in pathogenesis and therapeutic perspective. Int. J. Mol. Sci. 2018;19(3):889. doi: 10.3390/ijms19030889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Patel M., McCully C., Godwin K., Balis F.M. Plasma and cerebrospinal fluid pharmacokinetics of intravenous temozolomide in non-human primates. J. Neurooncol. 2003;61(3):203–207. doi: 10.1023/A:1022592913323. [DOI] [PubMed] [Google Scholar]
- 9.Ostermann S., Csajka C., Buclin T., Leyvraz S., Lejeune F., Decosterd L.A., Stupp R. Plasma and cerebrospinal fluid population pharmacokinetics of temozolomide in malignant glioma patients. Clin. Cancer Res. 2004;10(11):3728–3736. doi: 10.1158/1078-0432.CCR-03-0807. [DOI] [PubMed] [Google Scholar]
- 10.Liu H.L., Huang C.Y., Chen J.Y., Wang H.Y., Chen P.Y., Wei K.C. Pharmacodynamic and therapeutic investigation of focused ultrasound-induced blood-brain barrier opening for enhanced temozolomide delivery in glioma treatment. PLoS One. 2014;9(12):e114311. doi: 10.1371/journal.pone.0114311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Martínez-Garcia M., Álvarez-Linera J., Carrato C., Ley L., Luque R., Maldonado X., Martínez-Aguillo M., Navarro L.M., Vaz-Salgado M.A., Gil-Gil M. SEOM clinical guidelines for diagnosis and treatment of glioblastoma (2017). Clin. Transl. Oncol. 2018;20(1):22–28. doi: 10.1007/s12094-017-1763-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kong D.S., Lee J.I., Kim J.H., Kim S.T., Kim W.S., Suh Y.L., Dong S.M., Nam D.H. Phase II trial of low-dose continuous (metronomic) treatment of temozolomide for recurrent glioblastoma. Neuro-oncol. 2010;12(3):289–296. doi: 10.1093/neuonc/nop030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stupp R., Hegi M.E., Mason W.P., van den Bent M.J., Taphoorn M.J.B., Janzer R.C., Ludwin S.K., Allgeier A., Fisher B., Belanger K., Hau P., Brandes A.A., Gijtenbeek J., Marosi C., Vecht C.J., Mokhtari K., Wesseling P., Villa S., Eisenhauer E., Gorlia T., Weller M., Lacombe D., Cairncross J.G., Mirimanoff R.O. European organisation for research and treatment of cancer brain tumour and radiation oncology groups; National Cancer Institute of Canada Clinical Trials Group. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol. 2009;10(5):459–466. doi: 10.1016/S1470-2045(09)70025-7. [DOI] [PubMed] [Google Scholar]
- 14.Lin A.J., Campian J.L., Hui C., Rudra S., Rao Y.J., Thotala D., Hallahan D., Huang J. Impact of concurrent versus adjuvant chemotherapy on the severity and duration of lymphopenia in glioma patients treated with radiation therapy. J. Neurooncol. 2018;136(2):403–411. doi: 10.1007/s11060-017-2668-5. [DOI] [PubMed] [Google Scholar]
- 15.Karachi A., Dastmalchi F., Mitchell D.A., Rahman M. Temozolomide for immunomodulation in the treatment of glioblastoma. Neuro-oncol. 2018;20(12):1566–1572. doi: 10.1093/neuonc/noy072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Campian J.L., Ye X., Gladstone D.E., Ambady P., Nirschl T.R., Borrello I., Golightly M., King K.E., Holdhoff M., Karp J., Drake C.G., Grossman S.A. Pre-radiation lymphocyte harvesting and post-radiation reinfusion in patients with newly diagnosed high grade gliomas. J. Neurooncol. 2015;124(2):307–316. doi: 10.1007/s11060-015-1841-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Su Y.B., Sohn S., Krown S.E., Livingston P.O., Wolchok J.D., Quinn C., Williams L., Foster T., Sepkowitz K.A., Chapman P.B. Selective CD4+ lymphopenia in melanoma patients treated with temozolomide: a toxicity with therapeutic implications. J. Clin. Oncol. 2004;22(4):610–616. doi: 10.1200/JCO.2004.07.060. [DOI] [PubMed] [Google Scholar]
- 18.Wick W., Steinbach J.P., Küker W.M., Dichgans J., Bamberg M., Weller M. One week on/one week off: a novel active regimen of temozolomide for recurrent glioblastoma. Neurology. 2004;62(11):2113–2115. doi: 10.1212/01.WNL.0000127617.89363.84. [DOI] [PubMed] [Google Scholar]
- 19.Khan B.A., Khan S., White B., Eranki A. Severe pneumocystis jiroveci pneumonia in a patient on temozolomide therapy: A case report and review of literature. Respir. Med. Case Rep. 2017;22:179–182. doi: 10.1016/j.rmcr.2017.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Akasaki Y., Kikuchi T., Homma S., Koido S., Ohkusa T., Tasaki T., Hayashi K., Komita H., Watanabe N., Suzuki Y., Yamamoto Y., Mori R., Arai T., Tanaka T., Joki T., Yanagisawa T., Murayama Y. Phase I/II trial of combination of temozolomide chemotherapy and immunotherapy with fusions of dendritic and glioma cells in patients with glioblastoma. Cancer Immunol. Immunother. 2016;65(12):1499–1509. doi: 10.1007/s00262-016-1905-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Geffen D.B., Man S. New drugs for the treatment of cancer, 1990-2001. Isr. Med. Assoc. J. 2002;4(12):1124–1131. [PubMed] [Google Scholar]
- 22.Yan Y., Xu Z., Dai S., Qian L., Sun L., Gong Z. Targeting autophagy to sensitive glioma to temozolomide treatment. J. Exp. Clin. Cancer Res. 2016;35:23. doi: 10.1186/s13046-016-0303-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Abbott N.J. Blood-brain barrier structure and function and the challenges for CNS drug delivery. J. Inherit. Metab. Dis. 2013;36(3):437–449. doi: 10.1007/s10545-013-9608-0. [DOI] [PubMed] [Google Scholar]
- 24.Hartz A.M.S., Bauer B. ABC transporters in the CNS - an inventory. Curr. Pharm. Biotechnol. 2011;12(4):656–673. doi: 10.2174/138920111795164020. [DOI] [PubMed] [Google Scholar]
- 25.Miller D.S. Regulation of ABC transporters blood-brain barrier: the good, the bad, and the ugly. Adv. Cancer Res. 2015;125:43–70. doi: 10.1016/bs.acr.2014.10.002. [DOI] [PubMed] [Google Scholar]
- 26.Su B., Wang R., Xie Z., Ruan H., Li J., Xie C., Lu W., Wang J., Wang D., Liu M. Effect of retro-inverso isomer of bradykinin on size-dependent penetration of blood-brain tumor barrier. Small. 2018;14(7) doi: 10.1002/smll.201702331. [DOI] [PubMed] [Google Scholar]
- 27.van Tellingen O., Yetkin-Arik B., de Gooijer M.C., Wesseling P., Wurdinger T., de Vries H.E. Overcoming the blood-brain tumor barrier for effective glioblastoma treatment. Drug Resist. Updat. 2015;19:1–12. doi: 10.1016/j.drup.2015.02.002. [DOI] [PubMed] [Google Scholar]
- 28.Marchesi F., Turriziani M., Tortorelli G., Avvisati G., Torino F., De Vecchis L. Triazene compounds: mechanism of action and related DNA repair systems. Pharmacol. Res. 2007;56(4):275–287. doi: 10.1016/j.phrs.2007.08.003. [DOI] [PubMed] [Google Scholar]
- 29.Blough M.D., Zlatescu M.C., Cairncross J.G. O6-methylguanine-DNA methyltransferase regulation by p53 in astrocytic cells. Cancer Res. 2007;67(2):580–584. doi: 10.1158/0008-5472.CAN-06-2782. [DOI] [PubMed] [Google Scholar]
- 30.Esteller M., Hamilton S.R., Burger P.C., Baylin S.B., Herman J.G. Inactivation of the DNA repair gene O6-methylguanine-DNA methyltransferase by promoter hypermethylation is a common event in primary human neoplasia. Cancer Res. 1999;59(4):793–797. [PubMed] [Google Scholar]
- 31.Kitange G.J., Carlson B.L., Schroeder M.A., Grogan P.T., Lamont J.D., Decker P.A., Wu W., James C.D., Sarkaria J.N. Induction of MGMT expression is associated with temozolomide resistance in glioblastoma xenografts. Neuro-oncol. 2009;11(3):281–291. doi: 10.1215/15228517-2008-090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Weller M., Stupp R., Reifenberger G., Brandes A.A., van den Bent M.J., Wick W., Hegi M.E. MGMT promoter methylation in malignant gliomas: ready for personalized medicine? Nat. Rev. Neurol. 2010;6(1):39–51. doi: 10.1038/nrneurol.2009.197. [DOI] [PubMed] [Google Scholar]
- 33.Esteller M., Garcia-Foncillas J., Andion E., Goodman S.N., Hidalgo O.F., Vanaclocha V., Baylin S.B., Herman J.G. Inactivation of the DNA-repair gene MGMT and the clinical response of gliomas to alkylating agents. N. Engl. J. Med. 2000;343(19):1350–1354. doi: 10.1056/NEJM200011093431901. [DOI] [PubMed] [Google Scholar]
- 34.Thon N., Kreth S., Kreth F.W. Personalized treatment strategies in glioblastoma: MGMT promoter methylation status. OncoTargets Ther. 2013;6:1363–1372. doi: 10.2147/OTT.S50208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Parker N.R., Khong P., Parkinson J.F., Howell V.M., Wheeler H.R. Molecular heterogeneity in glioblastoma: potential clinical implications. Front. Oncol. 2015;5:55. doi: 10.3389/fonc.2015.00055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Perazzoli G., Prados J., Ortiz R., Caba O., Cabeza L., Berdasco M., Gónzalez B., Melguizo C. Temozolomide resistance in glioblastoma cell lines: implication of MGMT, MMR, P-Glycoprotein and CD133 expression. PLoS One. 2015;10(10):e0140131. doi: 10.1371/journal.pone.0140131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hegi M.E., Diserens A.C., Gorlia T., Hamou M.F., de Tribolet N., Weller M., Kros J.M., Hainfellner J.A., Mason W., Mariani L., Bromberg J.E., Hau P., Mirimanoff R.O., Cairncross J.G., Janzer R.C., Stupp R. MGMT gene silencing and benefit from temozolomide in glioblastoma. N. Engl. J. Med. 2005;352(10):997–1003. doi: 10.1056/NEJMoa043331. [DOI] [PubMed] [Google Scholar]
- 38.Hegi M.E., Diserens A.C., Godard S., Dietrich P.Y., Regli L., Ostermann S., Otten P., Van Melle G., de Tribolet N., Stupp R. Clinical trial substantiates the predictive value of O-6-methylguanine-DNA methyltransferase promoter methylation in glioblastoma patients treated with temozolomide. Clin. Cancer Res. 2004;10(6):1871–1874. doi: 10.1158/1078-0432.CCR-03-0384. [DOI] [PubMed] [Google Scholar]
- 39.Taylor J.W., Schiff D. Treatment considerations for MGMT-unmethylated glioblastoma. Curr. Neurol. Neurosci. Rep. 2015;15(1):507. doi: 10.1007/s11910-014-0507-z. [DOI] [PubMed] [Google Scholar]
- 40.Weller M., van den Bent M., Hopkins K., Tonn J.C., Stupp R., Falini A., Cohen-Jonathan-Moyal E., Frappaz D., Henriksson R., Balana C., Chinot O., Ram Z., Reifenberger G., Soffietti R., Wick W. European Association for Neuro-Oncology (EANO) Task Force on Malignant Glioma. EANO guideline for the diagnosis and treatment of anaplastic gliomas and glioblastoma. Lancet Oncol. 2014;15(9):e395–e403. doi: 10.1016/S1470-2045(14)70011-7. [DOI] [PubMed] [Google Scholar]
- 41.Spiegl-Kreinecker S., Pirker C., Filipits M., Lötsch D., Buchroithner J., Pichler J., Silye R., Weis S., Micksche M., Fischer J., Berger W. O6-Methylguanine DNA methyltransferase protein expression in tumor cells predicts outcome of temozolomide therapy in glioblastoma patients. Neuro-oncol. 2010;12(1):28–36. doi: 10.1093/neuonc/nop003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Brandes A.A., Tosoni A., Franceschi E., Reni M., Gatta G., Vecht C. Glioblastoma in adults. Crit. Rev. Oncol. Hematol. 2008;67(2):139–152. doi: 10.1016/j.critrevonc.2008.02.005. [DOI] [PubMed] [Google Scholar]
- 43.Weller M., Felsberg J., Hartmann C., Berger H., Steinbach J.P., Schramm J., Westphal M., Schackert G., Simon M., Tonn J.C., Heese O., Krex D., Nikkhah G., Pietsch T., Wiestler O., Reifenberger G., von Deimling A., Loeffler M. Molecular predictors of progression-free and overall survival in patients with newly diagnosed glioblastoma: a prospective translational study of the German Glioma Network. J. Clin. Oncol. 2009;27(34):5743–5750. doi: 10.1200/JCO.2009.23.0805. [DOI] [PubMed] [Google Scholar]
- 44.Dahlrot R.H., Dowsett J., Fosmark S., Malmström A., Henriksson R., Boldt H., de Stricker K., Sørensen M.D., Poulsen H.S., Lysiak M., Söderkvist P., Rosell J., Hansen S., Kristensen B.W. Prognostic value of O-6-methylguanine-DNA methyltransferase (MGMT) protein expression in glioblastoma excluding nontumour cells from the analysis. Neuropathol. Appl. Neurobiol. 2018;44(2):172–184. doi: 10.1111/nan.12415. [DOI] [PubMed] [Google Scholar]
- 45.Radke J., Koch A., Pritsch F., Schumann E., Misch M., Hempt C., Lenz K., Löbel F., Paschereit F., Heppner F.L., Vajkoczy P., Koll R., Onken J. Predictive MGMT status in a homogeneous cohort of IDH wildtype glioblastoma patients. Acta Neuropathol. Commun. 2019;7(1):89. doi: 10.1186/s40478-019-0745-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schaff L.R., Yan D., Thyparambil S., Tian Y., Cecchi F., Rosenblum M., Reiner A.S., Panageas K.S., Hembrough T., Lin A.L. Characterization of MGMT and EGFR protein expression in glioblastoma and association with survival. J. Neurooncol. 2020;146(1):163–170. doi: 10.1007/s11060-019-03358-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Marton E., Giordan E., Siddi F., Curzi C., Canova G., Scarpa B., Guerriero A., Rossi S., D’ Avella D., Longatti P., Feletti A. Over ten years overall survival in glioblastoma: A different disease? J. Neurol. Sci. 2020;408:116518. doi: 10.1016/j.jns.2019.116518. [DOI] [PubMed] [Google Scholar]
- 48.Hegi M.E., Liu L., Herman J.G., Stupp R., Wick W., Weller M., Mehta M.P., Gilbert M.R. Correlation of O6-methylguanine methyltransferase (MGMT) promoter methylation with clinical outcomes in glioblastoma and clinical strategies to modulate MGMT activity. J. Clin. Oncol. 2008;26(25):4189–4199. doi: 10.1200/JCO.2007.11.5964. [DOI] [PubMed] [Google Scholar]
- 49.Wick W., Stupp R., Beule A.C., Bromberg J., Wick A., Ernemann U., Platten M., Marosi C., Mason W.P., van den Bent M., Weller M., Rorden C., Karnath H.O., European Organisation for Research and Treatment of Cancer and the National Cancer Institute of Canada Clinical Trials Group A novel tool to analyze MRI recurrence patterns in glioblastoma. Neuro-oncol. 2008;10(6):1019–1024. doi: 10.1215/15228517-2008-058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dahlrot R.H., Larsen P., Boldt H.B., Kreutzfeldt M.S., Hansen S., Hjelmborg J.B., Kristensen B.W. Post treatment effect of MGMT methylation level on glioblastoma survival. J. Neuropathol. Exp. Neurol. 2019;78(7):633–640. doi: 10.1093/jnen/nlz032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Felsberg J., Thon N., Eigenbrod S., Hentschel B., Sabel M.C., Westphal M., Schackert G., Kreth F.W., Pietsch T., Löffler M., Weller M., Reifenberger G., Tonn J.C. German Glioma Network. Promoter methylation and expression of MGMT and the DNA mismatch repair genes MLH1, MSH2, MSH6 and PMS2 in paired primary and recurrent glioblastomas. Int. J. Cancer. 2011;129(3):659–670. doi: 10.1002/ijc.26083. [DOI] [PubMed] [Google Scholar]
- 52.Karsy M., Neil J.A., Guan J., Mahan M.A., Colman H., Jensen R.L., Jensen R.L. A practical review of prognostic correlations of molecular biomarkers in glioblastoma. Neurosurg. Focus. 2015;38(3):E4. doi: 10.3171/2015.1.FOCUS14755. [DOI] [PubMed] [Google Scholar]
- 53.Jacob S., Praz F. DNA mismatch repair defects: role in colorectal carcinogenesis. Biochimie. 2002;84(1):27–47. doi: 10.1016/S0300-9084(01)01362-1. [DOI] [PubMed] [Google Scholar]
- 54.Kinsella T.J. Coordination of DNA mismatch repair and base excision repair processing of chemotherapy and radiation damage for targeting resistant cancers. Clin. Cancer Res. 2009;15(6):1853–1859. doi: 10.1158/1078-0432.CCR-08-1307. [DOI] [PubMed] [Google Scholar]
- 55.Jeppesen D.K., Bohr V.A., Stevnsner T. DNA repair deficiency in neurodegeneration. Prog. Neurobiol. 2011;94(2):166–200. doi: 10.1016/j.pneurobio.2011.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Liu L., Gerson S.L. Targeted modulation of MGMT: clinical implications. Clin. Cancer Res. 2006;12(2):328–331. doi: 10.1158/1078-0432.CCR-05-2543. [DOI] [PubMed] [Google Scholar]
- 57.Messaoudi K., Clavreul A., Lagarce F. Toward an effective strategy in glioblastoma treatment. Part I: resistance mechanisms and strategies to overcome resistance of glioblastoma to temozolomide. Drug Discov. Today. 2015;20(7):899–905. doi: 10.1016/j.drudis.2015.02.011. [DOI] [PubMed] [Google Scholar]
- 58.Johannessen T.C.A., Bjerkvig R., Tysnes B.B. DNA repair and cancer stem-like cells--potential partners in glioma drug resistance? Cancer Treat. Rev. 2008;34(6):558–567. doi: 10.1016/j.ctrv.2008.03.125. [DOI] [PubMed] [Google Scholar]
- 59.Cancer Genome Atlas Research Network Comprehensive genomic characterization defines human glioblastoma genes and core pathways. Nature. 2008;455(7216):1061–1068. doi: 10.1038/nature07385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Happold C., Roth P., Wick W., Schmidt N., Florea A.M., Silginer M., Reifenberger G., Weller M. Distinct molecular mechanisms of acquired resistance to temozolomide in glioblastoma cells. J. Neurochem. 2012;122(2):444–455. doi: 10.1111/j.1471-4159.2012.07781.x. [DOI] [PubMed] [Google Scholar]
- 61.Cahill D.P., Levine K.K., Betensky R.A., Codd P.J., Romany C.A., Reavie L.B., Batchelor T.T., Futreal P.A., Stratton M.R., Curry W.T., Iafrate A.J., Louis D.N. Loss of the mismatch repair protein MSH6 in human glioblastomas is associated with tumor progression during temozolomide treatment. Clin. Cancer Res. 2007;13(7):2038–2045. doi: 10.1158/1078-0432.CCR-06-2149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yip S., Miao J., Cahill D.P., Iafrate A.J., Aldape K., Nutt C.L., Louis D.N. MSH6 mutations arise in glioblastomas during temozolomide therapy and mediate temozolomide resistance. Clin. Cancer Res. 2009;15(14):4622–4629. doi: 10.1158/1078-0432.CCR-08-3012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Stark A.M., Doukas A., Hugo H.H., Mehdorn H.M. The expression of mismatch repair proteins MLH1, MSH2 and MSH6 correlates with the Ki67 proliferation index and survival in patients with recurrent glioblastoma. Neurol. Res. 2010;32(8):816–820. doi: 10.1179/016164110X12645013515052. [DOI] [PubMed] [Google Scholar]
- 64.Shinsato Y., Furukawa T., Yunoue S., Yonezawa H., Minami K., Nishizawa Y., Ikeda R., Kawahara K., Yamamoto M., Hirano H., Tokimura H., Arita K. Reduction of MLH1 and PMS2 confers temozolomide resistance and is associated with recurrence of glioblastoma. Oncotarget. 2013;4(12):2261–2270. doi: 10.18632/oncotarget.1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Higuchi F., Fink A.L., Kiyokawa J., Miller J.J., Koerner M.V.A., Cahill D.P., Wakimoto H. PLK1 inhibition targets myc-activated malignant glioma cells irrespective of mismatch repair deficiency-mediated acquired resistance to temozolomide. Mol. Cancer Ther. 2018;17(12):2551–2563. doi: 10.1158/1535-7163.MCT-18-0177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Maxwell J.A., Johnson S.P., McLendon R.E., Lister D.W., Horne K.S., Rasheed A., Quinn J.A., Ali-Osman F., Friedman A.H., Modrich P.L., Bigner D.D., Friedman H.S. Mismatch repair deficiency does not mediate clinical resistance to temozolomide in malignant glioma. Clin. Cancer Res. 2008;14(15):4859–4868. doi: 10.1158/1078-0432.CCR-07-4807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Fu D., Calvo J.A., Samson L.D. Balancing repair and tolerance of DNA damage caused by alkylating agents. Nat. Rev. Cancer. 2012;12(2):104–120. doi: 10.1038/nrc3185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wood R.D., Mitchell M., Sgouros J., Lindahl T. Human DNA repair genes. Science. 2001;291(5507):1284–1289. doi: 10.1126/science.1056154. [DOI] [PubMed] [Google Scholar]
- 69.Iyama T., Wilson D.M. III DNA repair mechanisms in dividing and non-dividing cells. DNA Repair (Amst.) 2013;12(8):620–636. doi: 10.1016/j.dnarep.2013.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Dantzer F., Amé J.C., Schreiber V., Nakamura J., Ménissier-de Murcia J., de Murcia G. Poly(ADP-ribose) polymerase-1 activation during DNA damage and repair. Methods Enzymol. 2006;409:493–510. doi: 10.1016/S0076-6879(05)09029-4. [DOI] [PubMed] [Google Scholar]
- 71.Aguilar-Quesada R., Muñoz-Gámez J.A., Martín-Oliva D., Peralta-Leal A., Quiles-Pérez R., Rodríguez-Vargas J.M., Ruiz de Almodóvar M., Conde C., Ruiz-Extremera A., Oliver F.J. Modulation of transcription by PARP-1: consequences in carcinogenesis and inflammation. Curr. Med. Chem. 2007;14(11):1179–1187. doi: 10.2174/092986707780597998. [DOI] [PubMed] [Google Scholar]
- 72.Curtin N.J., Wang L.Z., Yiakouvaki A., Kyle S., Arris C.A., Canan-Koch S., Webber S.E., Durkacz B.W., Calvert H.A., Hostomsky Z., Newell D.R. Novel poly(ADP-ribose) polymerase-1 inhibitor, AG14361, restores sensitivity to temozolomide in mismatch repair-deficient cells. Clin. Cancer Res. 2004;10(3):881–889. doi: 10.1158/1078-0432.CCR-1144-3. [DOI] [PubMed] [Google Scholar]
- 73.Tentori L., Graziani G. Recent approaches to improve the antitumor efficacy of temozolomide. Curr. Med. Chem. 2009;16(2):245–257. doi: 10.2174/092986709787002718. [DOI] [PubMed] [Google Scholar]
- 74.Ratnam K., Low J.A. Current development of clinical inhibitors of poly(ADP-ribose) polymerase in oncology. Clin. Cancer Res. 2007;13(5):1383–1388. doi: 10.1158/1078-0432.CCR-06-2260. [DOI] [PubMed] [Google Scholar]
- 75.Calabrese C.R., Almassy R., Barton S., Batey M.A., Calvert A.H., Canan-Koch S., Durkacz B.W., Hostomsky Z., Kumpf R.A., Kyle S., Li J., Maegley K., Newell D.R., Notarianni E., Stratford I.J., Skalitzky D., Thomas H.D., Wang L.Z., Webber S.E., Williams K.J., Curtin N.J. Anticancer chemosensitization and radiosensitization by the novel poly(ADP-ribose) polymerase-1 inhibitor AG14361. J. Natl. Cancer Inst. 2004;96(1):56–67. doi: 10.1093/jnci/djh005. [DOI] [PubMed] [Google Scholar]
- 76.Miknyoczki S.J., Jones-Bolin S., Pritchard S., Hunter K., Zhao H., Wan W., Ator M., Bihovsky R., Hudkins R., Chatterjee S., Klein-Szanto A., Dionne C., Ruggeri B. Chemopotentiation of temozolomide, irinotecan, and cisplatin activity by CEP-6800, a poly(ADP-ribose) polymerase inhibitor. Mol. Cancer Ther. 2003;2(4):371–382. [PubMed] [Google Scholar]
- 77.Cheng C.L., Johnson S.P., Keir S.T., Quinn J.A., Ali-Osman F., Szabo C., Li H., Salzman A.L., Dolan M.E., Modrich P., Bigner D.D., Friedman H.S. Poly(ADP-ribose) polymerase-1 inhibition reverses temozolomide resistance in a DNA mismatch repair-deficient malignant glioma xenograft. Mol. Cancer Ther. 2005;4(9):1364–1368. doi: 10.1158/1535-7163.MCT-05-0128. [DOI] [PubMed] [Google Scholar]
- 78.Shackleton M., Quintana E., Fearon E.R., Morrison S.J. Heterogeneity in cancer: cancer stem cells versus clonal evolution. Cell. 2009;138(5):822–829. doi: 10.1016/j.cell.2009.08.017. [DOI] [PubMed] [Google Scholar]
- 79.Tang D.G. Understanding cancer stem cell heterogeneity and plasticity. Cell Res. 2012;22(3):457–472. doi: 10.1038/cr.2012.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Reya T., Morrison S.J., Clarke M.F., Weissman I.L. Stem cells, cancer, and cancer stem cells. Nature. 2001;414(6859):105–111. doi: 10.1038/35102167. [DOI] [PubMed] [Google Scholar]
- 81.Visvader J.E., Lindeman G.J. Cancer stem cells: current status and evolving complexities. Cell Stem Cell. 2012;10(6):717–728. doi: 10.1016/j.stem.2012.05.007. [DOI] [PubMed] [Google Scholar]
- 82.Kreso A., Dick J.E. Evolution of the cancer stem cell model. Cell Stem Cell. 2014;14(3):275–291. doi: 10.1016/j.stem.2014.02.006. [DOI] [PubMed] [Google Scholar]
- 83.Singh S.K., Hawkins C., Clarke I.D., Squire J.A., Bayani J., Hide T., Henkelman R.M., Cusimano M.D., Dirks P.B. Identification of human brain tumour initiating cells. Nature. 2004;432(7015):396–401. doi: 10.1038/nature03128. [DOI] [PubMed] [Google Scholar]
- 84.Kania G., Corbeil D., Fuchs J., Tarasov K.V., Blyszczuk P., Huttner W.B., Boheler K.R., Wobus A.M. Somatic stem cell marker prominin-1/CD133 is expressed in embryonic stem cell-derived progenitors. Stem Cells. 2005;23(6):791–804. doi: 10.1634/stemcells.2004-0232. [DOI] [PubMed] [Google Scholar]
- 85.Beier D., Hau P., Proescholdt M., Lohmeier A., Wischhusen J., Oefner P.J., Aigner L., Brawanski A., Bogdahn U., Beier C.P. CD133(+) and CD133(-) glioblastoma-derived cancer stem cells show differential growth characteristics and molecular profiles. Cancer Res. 2007;67(9):4010–4015. doi: 10.1158/0008-5472.CAN-06-4180. [DOI] [PubMed] [Google Scholar]
- 86.Abou-Antoun T.J., Hale J.S., Lathia J.D., Dombrowski S.M. Brain Cancer Stem Cells in Adults and Children: Cell Biology and Therapeutic Implications. Neurotherapeutics. 2017;14(2):372–384. doi: 10.1007/s13311-017-0524-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Plaks V., Kong N., Werb Z. The cancer stem cell niche: how essential is the niche in regulating stemness of tumor cells? Cell Stem Cell. 2015;16(3):225–238. doi: 10.1016/j.stem.2015.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Bryukhovetskiy A., Shevchenko V., Kovalev S., Chekhonin V., Baklaushev V., Bryukhovetskiy I., Zhukova M. To the novel paradigm of proteome-based cell therapy of tumors: through comparative proteome mapping of tumor stem cells and tissue-specific stem cells of humans. Cell Transplant. 2014;23(Suppl. 1):S151–S170. doi: 10.3727/096368914X684907. [DOI] [PubMed] [Google Scholar]
- 89.Alcantara Llaguno S., Chen J., Kwon C.H., Jackson E.L., Li Y., Burns D.K., Alvarez-Buylla A., Parada L.F. Malignant astrocytomas originate from neural stem/progenitor cells in a somatic tumor suppressor mouse model. Cancer Cell. 2009;15(1):45–56. doi: 10.1016/j.ccr.2008.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Chen J., Li Y., Yu T.S., McKay R.M., Burns D.K., Kernie S.G., Parada L.F. A restricted cell population propagates glioblastoma growth after chemotherapy. Nature. 2012;488(7412):522–526. doi: 10.1038/nature11287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Auffinger B., Spencer D., Pytel P., Ahmed A.U., Lesniak M.S. The role of glioma stem cells in chemotherapy resistance and glioblastoma multiforme recurrence. Expert Rev. Neurother. 2015;15(7):741–752. doi: 10.1586/14737175.2015.1051968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Jackson M., Hassiotou F., Nowak A. Glioblastoma stem-like cells: at the root of tumor recurrence and a therapeutic target. Carcinogenesis. 2015;36(2):177–185. doi: 10.1093/carcin/bgu243. [DOI] [PubMed] [Google Scholar]
- 93.Bao S., Wu Q., McLendon R.E., Hao Y., Shi Q., Hjelmeland A.B., Dewhirst M.W., Bigner D.D., Rich J.N. Glioma stem cells promote radioresistance by preferential activation of the DNA damage response. Nature. 2006;444(7120):756–760. doi: 10.1038/nature05236. [DOI] [PubMed] [Google Scholar]
- 94.Galli R., Binda E., Orfanelli U., Cipelletti B., Gritti A., De Vitis S., Fiocco R., Foroni C., Dimeco F., Vescovi A. Isolation and characterization of tumorigenic, stem-like neural precursors from human glioblastoma. Cancer Res. 2004;64(19):7011–7021. doi: 10.1158/0008-5472.CAN-04-1364. [DOI] [PubMed] [Google Scholar]
- 95.Kim S.S., Harford J.B., Pirollo K.F., Chang E.H. Effective treatment of glioblastoma requires crossing the blood-brain barrier and targeting tumors including cancer stem cells: The promise of nanomedicine. Biochem. Biophys. Res. Commun. 2015;468(3):485–489. doi: 10.1016/j.bbrc.2015.06.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Yoshida G.J., Saya H. Therapeutic strategies targeting cancer stem cells. Cancer Sci. 2016;107(1):5–11. doi: 10.1111/cas.12817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Alvino E., Castiglia D., Caporali S., Pepponi R., Caporaso P., Lacal P.M., Marra G., Fischer F., Zambruno G., Bonmassar E., Jiricny J., D’Atri S. A single cycle of treatment with temozolomide, alone or combined with O(6)-benzylguanine, induces strong chemoresistance in melanoma cell clones in vitro: role of O(6)-methylguanine-DNA methyltransferase and the mismatch repair system. Int. J. Oncol. 2006;29(4):785–797. doi: 10.3892/ijo.29.4.785. [DOI] [PubMed] [Google Scholar]
- 98.Bradshaw T.D., Stone E.L., Trapani V., Leong C-O., Matthews C.S., te Poele R., Stevens M.F.G. Mechanisms of acquired resistance to 2-(4-Amino-3-methylphenyl)benzothiazole in breast cancer cell lines. Breast Cancer Res. Treat. 2008;110(1):57–68. doi: 10.1007/s10549-007-9690-9. [DOI] [PubMed] [Google Scholar]
- 99.Huse J.T., Holland E.C. Targeting brain cancer: advances in the molecular pathology of malignant glioma and medulloblastoma. Nat. Rev. Cancer. 2010;10(5):319–331. doi: 10.1038/nrc2818. [DOI] [PubMed] [Google Scholar]
- 100.Angers-Loustau A., Hering R., Werbowetski T.E., Kaplan D.R., Del Maestro R.F. SRC regulates actin dynamics and invasion of malignant glial cells in three dimensions. Mol. Cancer Res. 2004;2(11):595–605. [PubMed] [Google Scholar]
- 101.Du J., Bernasconi P., Clauser K.R., Mani D.R., Finn S.P., Beroukhim R., Burns M., Julian B., Peng X.P., Hieronymus H., Maglathlin R.L., Lewis T.A., Liau L.M., Nghiemphu P., Mellinghoff I.K., Louis D.N., Loda M., Carr S.A., Kung A.L., Golub T.R. Bead-based profiling of tyrosine kinase phosphorylation identifies SRC as a potential target for glioblastoma therapy. Nat. Biotechnol. 2009;27(1):77–83. doi: 10.1038/nbt.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Eom K-Y., Cho B.J., Choi E.J., Kim J-H., Chie E.K., Wu H-G., Kim I.H., Paek S.H., Kim J-S., Kim I.A. The Effect of chemoradiotherapy with SRC tyrosine kinase inhibitor, pp2 and temozolomide on malignant glioma cells in vitro and in vivo. Cancer Res. Treat. 2016;48(2):687–697. doi: 10.4143/crt.2014.320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Huveldt D., Lewis-Tuffin L.J., Carlson B.L., Schroeder M.A., Rodriguez F., Giannini C., Galanis E., Sarkaria J.N., Anastasiadis P.Z. Targeting Src family kinases inhibits bevacizumab-induced glioma cell invasion. PLoS One. 2013;8(2):e56505. doi: 10.1371/journal.pone.0056505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Ujifuku K., Mitsutake N., Takakura S., Matsuse M., Saenko V., Suzuki K., Hayashi K., Matsuo T., Kamada K., Nagata I., Yamashita S. miR-195, miR-455-3p and miR-10a(*) are implicated in acquired temozolomide resistance in glioblastoma multiforme cells. Cancer Lett. 2010;296(2):241–248. doi: 10.1016/j.canlet.2010.04.013. [DOI] [PubMed] [Google Scholar]
- 105.Li Y., Liu Y., Ren J., Deng S., Yi G., Guo M., Shu S., Zhao L., Peng Y., Qi S. miR-1268a regulates ABCC1 expression to mediate temozolomide resistance in glioblastoma. J. Neurooncol. 2018;138(3):499–508. doi: 10.1007/s11060-018-2835-3. [DOI] [PubMed] [Google Scholar]
- 106.Xu J., Huang H., Peng R., Ding X., Jiang B., Yuan X., Xi J. MicroRNA-30a increases the chemosensitivity of U251 glioblastoma cells to temozolomide by directly targeting beclin 1 and inhibiting autophagy. Exp. Ther. Med. 2018;15(6):4798–4804. doi: 10.3892/etm.2018.6007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Slaby O., Lakomy R., Fadrus P., Hrstka R., Kren L., Lzicarova E., Smrcka M., Svoboda M., Dolezalova H., Novakova J., Valik D., Vyzula R., Michalek J. MicroRNA-181 family predicts response to concomitant chemoradiotherapy with temozolomide in glioblastoma patients. Neoplasma. 2010;57(3):264–269. doi: 10.4149/neo_2010_03_264. [DOI] [PubMed] [Google Scholar]
- 108.Yung A., Levin V.A., Albright R., Olson J., Fredericks R., Fink K., Prados M., Brada M., Spence A., Brunner J., Yue N., Dugan M. Zakneon. S. Randomized trial of Temodal (TEM) vs. Procarbazine (PCB) in glioblastoma multiforme (GBM) at first relapse. Proc. Am. Soc. Clin. Oncol. 1999;18:139a. [Google Scholar]
- 109.Yung W.K., Prados M.D., Yaya-Tur R., Rosenfeld S.S., Brada M., Friedman H.S., Albright R., Olson J., Chang S.M., O’Neill A.M., Friedman A.H., Bruner J., Yue N., Dugan M., Zaknoen S., Levin V.A., Temodal Brain Tumor Group Multicenter phase II trial of temozolomide in patients with anaplastic astrocytoma or anaplastic oligoastrocytoma at first relapse. J. Clin. Oncol. 1999;17(9):2762–2771. doi: 10.1200/JCO.1999.17.9.2762. [DOI] [PubMed] [Google Scholar]
- 110.Stupp R., Mason W.P., van den Bent M.J., Weller M., Fisher B., Taphoorn M.J.B., Belanger K., Brandes A.A., Marosi C., Bogdahn U., Curschmann J., Janzer R.C., Ludwin S.K., Gorlia T., Allgeier A., Lacombe D., Cairncross J.G., Eisenhauer E., Mirimanoff R.O. European Organisation for Research and Treatment of Cancer Brain Tumor and Radiotherapy Groups; National Cancer Institute of Canada Clinical Trials Group. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N. Engl. J. Med. 2005;352(10):987–996. doi: 10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]
- 111.Louis D.N., Perry A., Reifenberger G., von Deimling A., Figarella-Branger D., Cavenee W.K., Ohgaki H., Wiestler O.D., Kleihues P., Ellison D.W. The 2016 World Health Organization Classification of Tumors of the Central Nervous System: a summary. Acta Neuropathol. 2016;131(6):803–820. doi: 10.1007/s00401-016-1545-1. [DOI] [PubMed] [Google Scholar]
- 112.Mieskolainen M. Molecular characteristics of malignant gliomas and their future perspectives - a review. Eesti Arst; 2017. [Google Scholar]
- 113.Westphal M., Lamszus K. Circulating biomarkers for gliomas. Nat. Rev. Neurol. 2015;11(10):556–566. doi: 10.1038/nrneurol.2015.171. [DOI] [PubMed] [Google Scholar]
- 114.Wen P.Y., Reardon D.A. Neuro-oncology in 2015: Progress in glioma diagnosis, classification and treatment. Nat. Rev. Neurol. 2016;12(2):69–70. doi: 10.1038/nrneurol.2015.242. [DOI] [PubMed] [Google Scholar]
- 115.Gainer J.L., Sheehan J.P., Larner J.M., Jones D.R. Trans sodium crocetinate with temozolomide and radiation therapy for glioblastoma multiforme. J. Neurosurg. 2017;126(2):460–466. doi: 10.3171/2016.3.JNS152693. [DOI] [PubMed] [Google Scholar]
- 116.Stupp R., Taillibert S., Kanner A.A., Kesari S., Steinberg D.M., Toms S.A., Taylor L.P., Lieberman F., Silvani A., Fink K.L., Barnett G.H., Zhu J.J., Henson J.W., Engelhard H.H., Chen T.C., Tran D.D., Sroubek J., Tran N.D., Hottinger A.F., Landolfi J., Desai R., Caroli M., Kew Y., Honnorat J., Idbaih A., Kirson E.D., Weinberg U., Palti Y., Hegi M.E., Ram Z. Maintenance therapy with tumor-treating fields plus temozolomide vs temozolomide alone for glioblastoma: A Randomized Clinical Trial. JAMA. 2015;314(23):2535–2543. doi: 10.1001/jama.2015.16669. [DOI] [PubMed] [Google Scholar]
- 117.Gilbert M.R., Dignam J.J., Armstrong T.S., Wefel J.S., Blumenthal D.T., Vogelbaum M.A., Colman H., Chakravarti A., Pugh S., Won M., Jeraj R., Brown P.D., Jaeckle K.A., Schiff D., Stieber V.W., Brachman D.G., Werner-Wasik M., Tremont-Lukats I.W., Sulman E.P., Aldape K.D., Curran W.J., Jr, Mehta M.P. A randomized trial of bevacizumab for newly diagnosed glioblastoma. N. Engl. J. Med. 2014;370(8):699–708. doi: 10.1056/NEJMoa1308573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Chinot O.L., Wick W., Mason W., Henriksson R., Saran F., Nishikawa R., Carpentier A.F., Hoang-Xuan K., Kavan P., Cernea D., Brandes A.A., Hilton M., Abrey L., Cloughesy T. Bevacizumab plus radiotherapy-temozolomide for newly diagnosed glioblastoma. N. Engl. J. Med. 2014;370(8):709–722. doi: 10.1056/NEJMoa1308345. [DOI] [PubMed] [Google Scholar]
- 119.Donawho C.K., Luo Y., Luo Y., Penning T.D., Bauch J.L., Bouska J.J., Bontcheva-Diaz V.D., Cox B.F., DeWeese T.L., Dillehay L.E., Ferguson D.C., Ghoreishi-Haack N.S., Grimm D.R., Guan R., Han E.K., Holley-Shanks R.R., Hristov B., Idler K.B., Jarvis K., Johnson E.F., Kleinberg L.R., Klinghofer V., Lasko L.M., Liu X., Marsh K.C., McGonigal T.P., Meulbroek J.A., Olson A.M., Palma J.P., Rodriguez L.E., Shi Y., Stavropoulos J.A., Tsurutani A.C., Zhu G.D., Rosenberg S.H., Giranda V.L., Frost D.J. ABT-888, an orally active poly(ADP-ribose) polymerase inhibitor that potentiates DNA-damaging agents in preclinical tumor models. Clin. Cancer Res. 2007;13(9):2728–2737. doi: 10.1158/1078-0432.CCR-06-3039. [DOI] [PubMed] [Google Scholar]
- 120.Baxter P.A., Su J.M., Onar-Thomas A., Billups C.A., Li X.N., Poussaint T.Y., Smith E.R., Thompson P., Adesina A., Ansell P., Giranda V., Paulino A., Kilburn L., Quaddoumi I., Broniscer A., Blaney S.M., Dunkel I.J., Fouladi M. A phase I/II study of veliparib (ABT-888) with radiation and temozolomide in newly diagnosed diffuse pontine glioma: a Pediatric Brain Tumor Consortium study. Neuro-oncol. 2020;22(6):875–885. doi: 10.1093/neuonc/noaa016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Twelves C., Short S., Wright S. A two-part safety and exploratory efficacy randomized double-blind, placebo-controlled study of a 1:1 ratio of the cannabinoids cannabidiol and delta-9-tetrahydrocannabinol (CBD:THC) plus dose-intense temozolomide in patients with recurrent glioblastoma multiforme (GBM). J. Clin. Oncol. 2017;35(15):2046–2046. doi: 10.1200/JCO.2017.35.15_suppl.2046. [DOI] [Google Scholar]
- 122.Gupta S.K., Kizilbash S.H., Carlson B.L., Mladek A.C., Boakye-Agyeman F., Bakken K.K., Pokorny J.L., Schroeder M.A., Decker P.A., Cen L., Eckel-Passow J.E., Sarkar G., Ballman K.V., Reid J.M., Jenkins R.B., Verhaak R.G., Sulman E.P., Kitange G.J., Sarkaria J.N. Delineation of MGMT hypermethylation as a biomarker for veliparib-mediated temozolomide-sensitizing therapy of glioblastoma. J. Natl. Cancer Inst. 2015;108(5):djv369. doi: 10.1093/jnci/djv369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Claringbold P.G., Price R.A., Turner J.H. Phase I-II study of radiopeptide 177Lu-octreotate in combination with capecitabine and temozolomide in advanced low-grade neuroendocrine tumors. Cancer Biother. Radiopharm. 2012;27(9):561–569. doi: 10.1089/cbr.2012.1276. [DOI] [PubMed] [Google Scholar]
- 124.Claringbold P.G., Turner J.H. Pancreatic neuroendocrine tumor Control: durable objective response to combination 177lu-octreotate-capecitabine-temozolomide radiopeptide chemotherapy. Neuroendocrinology. 2016;103(5):432–439. doi: 10.1159/000434723. [DOI] [PubMed] [Google Scholar]
- 125.Tatar Z., Thivat E., Planchat E., Gimbergues P., Gadea E., Abrial C., Durando X. Temozolomide and unusual indications: review of literature. Cancer Treat. Rev. 2013;39(2):125–135. doi: 10.1016/j.ctrv.2012.06.002. [DOI] [PubMed] [Google Scholar]
- 126.Owen S., Souhami L. The management of brain metastases in non-small cell lung cancer. Front. Oncol. 2014;4:248. doi: 10.3389/fonc.2014.00248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Greenspoon J.N., Ellis P.M., Pond G., Caetano S., Broomfield J., Swaminath A. Comparative survival in patients with brain metastases from non-small-cell lung cancer treated before and after implementation of radiosurgery. Curr. Oncol. 2017;24(2):e146–e151. doi: 10.3747/co.24.3420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Sperduto P.W., Wang M., Robins H.I., Schell M.C., Werner-Wasik M., Komaki R., Souhami L., Buyyounouski M.K., Khuntia D., Demas W., Shah S.A., Nedzi L.A., Perry G., Suh J.H., Mehta M.P. A phase 3 trial of whole brain radiation therapy and stereotactic radiosurgery alone versus WBRT and SRS with temozolomide or erlotinib for non-small cell lung cancer and 1 to 3 brain metastases: Radiation Therapy Oncology Group 0320. Int. J. Radiat. Oncol. Biol. Phys. 2013;85(5):1312–1318. doi: 10.1016/j.ijrobp.2012.11.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Boggs D.H., Robins H.I., Langer C.J., Traynor A.M., Berkowitz M.J., Mehta M.P. Strategies to prevent brain metastasis in high-risk non-small-cell lung cancer: lessons learned from a randomized study of maintenance temozolomide versus observation. Clin. Lung Cancer. 2014;15(6):433–440. doi: 10.1016/j.cllc.2014.06.008. [DOI] [PubMed] [Google Scholar]
- 130.He Q., Bi X., Ren C., Wang Y., Zou P., Zhang H., Chi N., Xiu C., Wang Y., Tao R. Phase II study of the efficacy and safety of high-dose pemetrexed in combination with cisplatin versus temozolomide for the treatment of non-small cell lung cancer with brain metastases. Anticancer Res. 2017;37(8):4711–4716. doi: 10.21873/anticanres.11877. [DOI] [PubMed] [Google Scholar]
- 131.Guida M., Tommasi S., Strippoli S., Natalicchio M.I., De Summa S., Pinto R., Cramarossa A., Albano A., Pisconti S., Aieta M., Ridolfi R., Azzariti A., Guida G., Lorusso V., Colucci G. The search for a melanoma-tailored chemotherapy in the new era of personalized therapy: a phase II study of chemo-modulating temozolomide followed by fotemustine and a cooperative study of GOIM (Gruppo Oncologico Italia Meridionale). BMC Cancer. 2018;18(1):552. doi: 10.1186/s12885-018-4479-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Middleton M.R., Friedlander P., Hamid O., Daud A., Plummer R., Falotico N., Chyla B., Jiang F., McKeegan E., Mostafa N.M., Zhu M., Qian J., McKee M., Luo Y., Giranda V.L., McArthur G.A. Randomized phase II study evaluating veliparib (ABT-888) with temozolomide in patients with metastatic melanoma. Ann. Oncol. 2015;26(10):2173–2179. doi: 10.1093/annonc/mdv308. [DOI] [PubMed] [Google Scholar]
- 133.Gabrielson A., Tesfaye A.A., Marshall J.L., Pishvaian M.J., Smaglo B., Jha R., Dorsch-Vogel K., Wang H., He A.R. Phase II study of temozolomide and veliparib combination therapy for sorafenib-refractory advanced hepatocellular carcinoma. Cancer Chemother. Pharmacol. 2015;76(5):1073–1079. doi: 10.1007/s00280-015-2852-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Pishvaian M.J., Slack R.S., Jiang W., He A.R., Hwang J.J., Hankin A., Dorsch-Vogel K., Kukadiya D., Weiner L.M., Marshall J.L., Brody J.R. A phase 2 study of the PARP inhibitor veliparib plus temozolomide in patients with heavily pretreated metastatic colorectal cancer. Cancer. 2018;124(11):2337–2346. doi: 10.1002/cncr.31309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Han H.S., Diéras V., Robson M., Palácová M., Marcom P.K., Jager A., Bondarenko I., Citrin D., Campone M., Telli M.L., Domchek S.M., Friedlander M., Kaufman B., Garber J.E., Shparyk Y., Chmielowska E., Jakobsen E.H., Kaklamani V., Gradishar W., Ratajczak C.K., Nickner C., Qin Q., Qian J., Shepherd S.P., Isakoff S.J., Puhalla S. Veliparib with temozolomide or carboplatin/paclitaxel versus placebo with carboplatin/paclitaxel in patients with BRCA1/2 locally recurrent/metastatic breast cancer: randomized phase II study. Ann. Oncol. 2018;29(1):154–161. doi: 10.1093/annonc/mdx505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Hussain M., Carducci M.A., Slovin S., Cetnar J., Qian J., McKeegan E.M., Refici-Buhr M., Chyla B., Shepherd S.P., Giranda V.L., Alumkal J.J. Targeting DNA repair with combination veliparib (ABT-888) and temozolomide in patients with metastatic castration-resistant prostate cancer. Invest. New Drugs. 2014;32(5):904–912. doi: 10.1007/s10637-014-0099-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Pietanza M.C., Waqar S.N., Krug L.M., Dowlati A., Hann C.L., Chiappori A., Owonikoko T.K., Woo K.M., Cardnell R.J., Fujimoto J., Long L., Diao L., Wang J., Bensman Y., Hurtado B., de Groot P., Sulman E.P., Wistuba I.I., Chen A., Fleisher M., Heymach J.V., Kris M.G., Rudin C.M., Byers L.A. Randomized, double-blind, phase ii study of temozolomide in combination with either veliparib or placebo in patients with relapsed-sensitive or refractory small-cell lung cancer. J. Clin. Oncol. 2018;36(23):2386–2394. doi: 10.1200/JCO.2018.77.7672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Pietanza M.C., Kadota K., Huberman K., Sima C.S., Fiore J.J., Sumner D.K., Travis W.D., Heguy A., Ginsberg M.S., Holodny A.I., Chan T.A., Rizvi N.A., Azzoli C.G., Riely G.J., Kris M.G., Krug L.M. Phase II trial of temozolomide in patients with relapsed sensitive or refractory small cell lung cancer, with assessment of methylguanine-DNA methyltransferase as a potential biomarker. Clin. Cancer Res. 2012;18(4):1138–1145. doi: 10.1158/1078-0432.CCR-11-2059. [DOI] [PubMed] [Google Scholar]
- 139.Hochhauser D., Glynne-Jones R., Potter V., Grávalos C., Doyle T.J., Pathiraja K., Zhang Q., Zhang L., Sausville E.A. A phase II study of temozolomide in patients with advanced aerodigestive tract and colorectal cancers and methylation of the O6-methylguanine-DNA methyltransferase promoter. Mol. Cancer Ther. 2013;12(5):809–818. doi: 10.1158/1535-7163.MCT-12-0710. [DOI] [PubMed] [Google Scholar]
- 140.Struve N., Binder Z.A., Stead L.F., Brend T., Bagley S.J., Faulkner C., Ott L., Müller-Goebel J., Weik A.S., Hoffer K., Krug L., Rieckmann T., Bußmann L., Henze M., Morrissette J.J.D., Kurian K.M., Schüller U., Petersen C., Rothkamm K., Rourke O. D.M.; Short, S.C.; Kriegs, M. EGFRvIII upregulates DNA mismatch repair resulting in increased temozolomide sensitivity of MGMT promoter methylated glioblastoma. Oncogene. 2020;39(15):3041–3055. doi: 10.1038/s41388-020-1208-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.McFaline-Figueroa J.L., Braun C.J., Stanciu M., Nagel Z.D., Mazzucato P., Sangaraju D., Cerniauskas E., Barford K., Vargas A., Chen Y., Tretyakova N., Lees J.A., Hemann M.T., White F.M., Samson L.D. Minor changes in expression of the mismatch repair protein MSH2 exert a major impact on glioblastoma response to temozolomide. Cancer Res. 2015;75(15):3127–3138. doi: 10.1158/0008-5472.CAN-14-3616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Yoshimoto K., Mizoguchi M., Hata N., Murata H., Hatae R., Amano T., Nakamizo A., Sasaki T. Complex DNA repair pathways as possible therapeutic targets to overcome temozolomide resistance in glioblastoma. Front. Oncol. 2012;2:186. doi: 10.3389/fonc.2012.00186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Jeong J.H., Hong Y.S., Park Y., Kim J., Kim J.E., Kim K.P., Kim S.Y., Park J.H., Kim J.H., Park I.J., Lim S.B., Yu C.S., Kim J.C., Kim T.W. Phase 1 Study of preoperative chemoradiation therapy with temozolomide and capecitabine in patients with locally advanced rectal cancer. Int. J. Radiat. Oncol. Biol. Phys. 2016;96(2):289–295. doi: 10.1016/j.ijrobp.2016.05.009. [DOI] [PubMed] [Google Scholar]
- 144.Calegari M.A., Inno A., Monterisi S., Orlandi A., Santini D., Basso M., Cassano A., Martini M., Cenci T., de Pascalis I., Camarda F., Barbaro B., Larocca L.M., Gori S., Tonini G., Barone C. A phase 2 study of temozolomide in pretreated metastatic colorectal cancer with MGMT promoter methylation. Br. J. Cancer. 2017;116(10):1279–1286. doi: 10.1038/bjc.2017.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Amatu A., Barault L., Moutinho C., Cassingena A., Bencardino K., Ghezzi S., Palmeri L., Bonazzina E., Tosi F., Ricotta R., Cipani T., Crivori P., Gatto R., Chirico G., Marrapese G., Truini M., Bardelli A., Esteller M., Di Nicolantonio F., Sartore-Bianchi A., Siena S. Tumor MGMT promoter hypermethylation changes over time limit temozolomide efficacy in a phase II trial for metastatic colorectal cancer. Ann. Oncol. 2016;27(6):1062–1067. doi: 10.1093/annonc/mdw071. [DOI] [PubMed] [Google Scholar]
- 146.Pietrantonio F., Perrone F., de Braud F., Castano A., Maggi C., Bossi I., Gevorgyan A., Biondani P., Pacifici M., Busico A., Gariboldi M., Festinese F., Tamborini E., Di Bartolomeo M. Activity of temozolomide in patients with advanced chemorefractory colorectal cancer and MGMT promoter methylation. Ann. Oncol. 2014;25(2):404–408. doi: 10.1093/annonc/mdt547. [DOI] [PubMed] [Google Scholar]
- 147.Pietrantonio F., de Braud F., Milione M., Maggi C., Iacovelli R., Dotti K.F., Perrone F., Tamborini E., Caporale M., Berenato R., Leone G., Pellegrinelli A., Bossi I., Festinese F., Federici S., Di Bartolomeo M. Dose-dense temozolomide in patients with mgmt-silenced chemorefractory colorectal cancer. Target. Oncol. 2016;11(3):337–343. doi: 10.1007/s11523-015-0397-2. [DOI] [PubMed] [Google Scholar]
- 148.Tentori L., Graziani G., Gilberti S., Lacal P.M., Bonmassar E., D’Atri S. Triazene compounds induce apoptosis in O6-alkylguanine-DNA alkyltransferase deficient leukemia cell lines. Leukemia. 1995;9(11):1888–1895. [PubMed] [Google Scholar]
- 149.Brandwein J.M., Yang L., Schimmer A.D., Schuh A.C., Gupta V., Wells R.A., Alibhai S.M., Xu W., Minden M.D. A phase II study of temozolomide therapy for poor-risk patients aged >or=60 years with acute myeloid leukemia: low levels of MGMT predict for response. Leukemia. 2007;21(4):821–824. doi: 10.1038/sj.leu.2404545. [DOI] [PubMed] [Google Scholar]
- 150.Lenz G., Hutter G., Hiddemann W., Dreyling M. Promoter methylation and expression of DNA repair genes hMLH1 and MGMT in acute myeloid leukemia. Ann. Hematol. 2004;83(10):628–633. doi: 10.1007/s00277-004-0925-0. [DOI] [PubMed] [Google Scholar]
- 151.Brandwein J.M., Kassis J., Leber B., Hogge D., Howson-Jan K., Minden M.D., Galarneau A., Pouliot J.F. Phase II study of targeted therapy with temozolomide in acute myeloid leukaemia and high-risk myelodysplastic syndrome patients pre-screened for low O(6) -methylguanine DNA methyltransferase expression. Br. J. Haematol. 2014;167(5):664–670. doi: 10.1111/bjh.13094. [DOI] [PubMed] [Google Scholar]
- 152.Gojo I., Beumer J.H., Pratz K.W., McDevitt M.A., Baer M.R., Blackford A.L., Smith B.D., Gore S.D., Carraway H.E., Showel M.M., Levis M.J., Dezern A.E., Gladstone D.E., Ji J.J., Wang L., Kinders R.J., Pouquet M., Ali-Walbi I., Rudek M.A., Poh W., Herman J.G., Karnitz L.M., Kaufmann S.H., Chen A., Karp J.E. A Phase 1 Study of the PARP inhibitor veliparib in combination with temozolomide in acute myeloid leukemia. Clin. Cancer Res. 2017;23(3):697–706. doi: 10.1158/1078-0432.CCR-16-0984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Medeiros B.C., Kohrt H.E., Gotlib J., Coutre S.E., Zhang B., Arber D.A., Zehnder J.L. Tailored temozolomide therapy according to MGMT methylation status for elderly patients with acute myeloid leukemia. Am. J. Hematol. 2012;87(1):45–50. doi: 10.1002/ajh.22191. [DOI] [PubMed] [Google Scholar]
- 154.Hassel J.C., Sucker A., Edler L., Kurzen H., Moll I., Stresemann C., Spieth K., Mauch C., Rass K., Dummer R., Schadendorf D. MGMT gene promoter methylation correlates with tolerance of temozolomide treatment in melanoma but not with clinical outcome. Br. J. Cancer. 2010;103(6):820–826. doi: 10.1038/sj.bjc.6605796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Kreth S., Thon N., Eigenbrod S., Lutz J., Ledderose C., Egensperger R., Tonn J.C., Kretzschmar H.A., Hinske L.C., Kreth F.W. O-methylguanine-DNA methyltransferase (MGMT) mRNA expression predicts outcome in malignant glioma independent of MGMT promoter methylation. PLoS One. 2011;6(2):e17156. doi: 10.1371/journal.pone.0017156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Ranson M., Hersey P., Thompson D., Beith J., McArthur G.A., Haydon A., Davis I.D., Kefford R.F., Mortimer P., Harris P.A., Baka S., Seebaran A., Sabharwal A., Watson A.J., Margison G.P., Middleton M.R. Randomized trial of the combination of lomeguatrib and temozolomide compared with temozolomide alone in chemotherapy naive patients with metastatic cutaneous melanoma. J. Clin. Oncol. 2007;25(18):2540–2545. doi: 10.1200/JCO.2007.10.8217. [DOI] [PubMed] [Google Scholar]
- 157.Kushwaha D., Ramakrishnan V., Ng K., Steed T., Nguyen T., Futalan D., Akers J.C., Sarkaria J., Jiang T., Chowdhury D., Carter B.S., Chen C.C. A genome-wide miRNA screen revealed miR-603 as a MGMT-regulating miRNA in glioblastomas. Oncotarget. 2014;5(12):4026–4039. doi: 10.18632/oncotarget.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Chen X., Zhang M., Gan H., Wang H., Lee J.H., Fang D., Kitange G.J., He L., Hu Z., Parney I.F., Meyer F.B., Giannini C., Sarkaria J.N., Zhang Z. A novel enhancer regulates MGMT expression and promotes temozolomide resistance in glioblastoma. Nat. Commun. 2018;9(1):2949. doi: 10.1038/s41467-018-05373-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Roos W.P., Nikolova T., Quiros S., Naumann S.C., Kiedron O., Zdzienicka M.Z., Kaina B. Brca2/Xrcc2 dependent HR, but not NHEJ, is required for protection against O(6)-methylguanine triggered apoptosis, DSBs and chromosomal aberrations by a process leading to SCEs. DNA Repair (Amst.) 2009;8(1):72–86. doi: 10.1016/j.dnarep.2008.09.003. [DOI] [PubMed] [Google Scholar]
- 160.Reuland S.N., Goldstein N.B., Partyka K.A., Cooper D.A., Fujita M., Norris D.A., Shellman Y.G. The combination of BH3-mimetic ABT-737 with the alkylating agent temozolomide induces strong synergistic killing of melanoma cells independent of p53. PLoS One. 2011;6(8):e24294. doi: 10.1371/journal.pone.0024294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Li R.H., Hou X.Y., Yang C.S., Liu W.L., Tang J.Q., Liu Y.Q., Jiang G. Temozolomide for treating malignant melanoma. J. Coll. Physicians Surg. Pak. 2015;25(9):680–688. [PubMed] [Google Scholar]
- 162.Tong R., Kohane D.S. New strategies in cancer nanomedicine. Annu. Rev. Pharmacol. Toxicol. 2016;56:41–57. doi: 10.1146/annurev-pharmtox-010715-103456. [DOI] [PubMed] [Google Scholar]
- 163.Shi J., Kantoff P.W., Wooster R., Farokhzad O.C. Cancer nanomedicine: progress, challenges and opportunities. Nat. Rev. Cancer. 2017;17(1):20–37. doi: 10.1038/nrc.2016.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Fang C., Wang K., Stephen Z.R., Mu Q., Kievit F.M., Chiu D.T., Press O.W., Zhang M. Temozolomide nanoparticles for targeted glioblastoma therapy. ACS Appl. Mater. Interfaces. 2015;7(12):6674–6682. doi: 10.1021/am5092165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Ortiz R., Cabeza L., Perazzoli G., Jimenez-Lopez J., García-Pinel B., Melguizo C., Prados J. Nanoformulations for glioblastoma multiforme: a new hope for treatment. Future Med. Chem. 2019;11(18):2459–2480. doi: 10.4155/fmc-2018-0521. [DOI] [PubMed] [Google Scholar]
- 166.Wagner S., Zensi A., Wien S.L., Tschickardt S.E., Maier W., Vogel T., Worek F., Pietrzik C.U., Kreuter J., von Briesen H. Uptake mechanism of ApoE-modified nanoparticles on brain capillary endothelial cells as a blood-brain barrier model. PLoS One. 2012;7(3):e32568. doi: 10.1371/journal.pone.0032568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Durán N., Silveira C.P., Durán M., Martinez D.S.T. Silver nanoparticle protein corona and toxicity: a mini-review. J. Nanobiotechnology. 2015;13:55. doi: 10.1186/s12951-015-0114-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Tian X-H., Lin X-N., Wei F., Feng W., Huang Z.C., Wang P., Ren L., Diao Y. Enhanced brain targeting of temozolomide in polysorbate-80 coated polybutylcyanoacrylate nanoparticles. Int. J. Nanomedicine. 2011;6:445–452. doi: 10.2147/IJN.S16570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Arcella A., Palchetti S., Digiacomo L., Pozzi D., Capriotti A.L., Frati L., Oliva M.A., Tsaouli G., Rota R., Screpanti I., Mahmoudi M., Caracciolo G. Brain targeting by liposome-biomolecular corona boosts anticancer efficacy of temozolomide in glioblastoma cells. ACS Chem. Neurosci. 2018;9(12):3166–3174. doi: 10.1021/acschemneuro.8b00339. [DOI] [PubMed] [Google Scholar]
- 170.Qin Y., Fan W., Chen H., Yao N., Tang W., Tang J., Yuan W., Kuai R., Zhang Z., Wu Y., He Q. In vitro and in vivo investigation of glucose-mediated brain-targeting liposomes. J. Drug Target. 2010;18(7):536–549. doi: 10.3109/10611861003587235. [DOI] [PubMed] [Google Scholar]
- 171.Patil R., Portilla-Arias J., Ding H., Inoue S., Konda B., Hu J., Wawrowsky K.A., Shin P.K., Black K.L., Holler E., Ljubimova J.Y. Temozolomide delivery to tumor cells by a multifunctional nano vehicle based on poly(β-L-malic acid). Pharm. Res. 2010;27(11):2317–2329. doi: 10.1007/s11095-010-0091-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Zhang J., Xiao X., Zhu J., Gao Z., Lai X., Zhu X., Mao G. Lactoferrin- and RGD-comodified, temozolomide and vincristine-coloaded nanostructured lipid carriers for gliomatosis cerebri combination therapy. Int. J. Nanomedicine. 2018;13:3039–3051. doi: 10.2147/IJN.S161163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Lam F.C., Morton S.W., Wyckoff J., Vu Han T-L., Hwang M.K., Maffa A., Balkanska-Sinclair E., Yaffe M.B., Floyd S.R., Hammond P.T. Enhanced efficacy of combined temozolomide and bromodomain inhibitor therapy for gliomas using targeted nanoparticles. Nat. Commun. 2018;9(1):1991. doi: 10.1038/s41467-018-04315-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Kim S.S., Rait A., Kim E., DeMarco J., Pirollo K.F., Chang E.H. Encapsulation of temozolomide in a tumor-targeting nanocomplex enhances anti-cancer efficacy and reduces toxicity in a mouse model of glioblastoma. Cancer Lett. 2015;369(1):250–258. doi: 10.1016/j.canlet.2015.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Jain A., Singhai P., Gurnany E., Updhayay S., Mody N. Transferrin-tailored solid lipid nanoparticles as vectors for site-specific delivery of temozolomide to brain. J. Nanopart. Res. 2013;15(1518):1–9. [Google Scholar]
- 176.Lyons S.A., O’Neal J., Sontheimer H. Chlorotoxin, a scorpion-derived peptide, specifically binds to gliomas and tumors of neuroectodermal origin. Glia. 2002;39(2):162–173. doi: 10.1002/glia.10083. [DOI] [PubMed] [Google Scholar]
- 177.Zhang P., Tang M., Huang Q., Zhao G., Huang N., Zhang X., Tan Y., Cheng Y. Combination of 3-methyladenine therapy and Asn-Gly-Arg (NGR)-modified mesoporous silica nanoparticles loaded with temozolomide for glioma therapy in vitro. Biochem. Biophys. Res. Commun. 2019;509(2):549–556. doi: 10.1016/j.bbrc.2018.12.158. [DOI] [PubMed] [Google Scholar]
- 178.Song S., Mao G., Du J., Zhu X. Novel RGD containing, temozolomide-loading nanostructured lipid carriers for glioblastoma multiforme chemotherapy. Drug Deliv. 2016;23(4):1404–1408. doi: 10.3109/10717544.2015.1064186. [DOI] [PubMed] [Google Scholar]
- 179.Minaei S.E., Khoei S., Khoee S., Vafashoar F., Mahabadi V.P. In vitro anti-cancer efficacy of multi-functionalized magnetite nanoparticles combining alternating magnetic hyperthermia in glioblastoma cancer cells. Mater. Sci. Eng. C. 2019;101:575–587. doi: 10.1016/j.msec.2019.04.007. [DOI] [PubMed] [Google Scholar]
- 180.Emamgholizadeh Minaei S., Khoei S., Khoee S., Karimi M.R. Tri-block copolymer nanoparticles modified with folic acid for temozolomide delivery in glioblastoma. Int. J. Biochem. Cell Biol. 2019;108:72–83. doi: 10.1016/j.biocel.2019.01.010. [DOI] [PubMed] [Google Scholar]
- 181.Ananta J.S., Paulmurugan R., Massoud T.F. Temozolomide-loaded PLGA nanoparticles to treat glioblastoma cells: a biophysical and cell culture evaluation. Neurol. Res. 2016;38(1):51–59. doi: 10.1080/01616412.2015.1133025. [DOI] [PubMed] [Google Scholar]
- 182.Ananta J.S., Paulmurugan R., Massoud T.F. Nanoparticle-delivered antisense microrna-21 enhances the effects of temozolomide on glioblastoma cells. Mol. Pharm. 2015;12(12):4509–4517. doi: 10.1021/acs.molpharmaceut.5b00694. [DOI] [PubMed] [Google Scholar]
- 183.Malhotra M., Sekar T.V., Ananta J.S., Devulapally R., Afjei R., Babikir H.A., Paulmurugan R., Massoud T.F. Targeted nanoparticle delivery of therapeutic antisense microRNAs presensitizes glioblastoma cells to lower effective doses of temozolomide in vitro and in a mouse model. Oncotarget. 2018;9(30):21478–21494. doi: 10.18632/oncotarget.25135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Ramalho M.J., Sevin E., Gosselet F., Lima J., Coelho M.A.N., Loureiro J.A., Pereira M.C. Receptor-mediated PLGA nanoparticles for glioblastoma multiforme treatment. Int. J. Pharm. 2018;545(1-2):84–92. doi: 10.1016/j.ijpharm.2018.04.062. [DOI] [PubMed] [Google Scholar]
- 185.Jain A., Chasoo G., Singh S.K., Saxena A.K., Jain S.K. Transferrin-appended PEGylated nanoparticles for temozolomide delivery to brain: in vitro characterisation. J. Microencapsul. 2011;28(1):21–28. doi: 10.3109/02652048.2010.522257. [DOI] [PubMed] [Google Scholar]
- 186.Chu L., Wang A., Ni L., Yan X., Song Y., Zhao M., Sun K., Mu H., Liu S., Wu Z., Zhang C. Nose-to-brain delivery of temozolomide-loaded PLGA nanoparticles functionalized with anti-EPHA3 for glioblastoma targeting. Drug Deliv. 2018;25(1):1634–1641. doi: 10.1080/10717544.2018.1494226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Illum L. Is nose-to-brain transport of drugs in man a reality? J. Pharm. Pharmacol. 2004;56(1):3–17. doi: 10.1211/0022357022539. [DOI] [PubMed] [Google Scholar]
- 188.Khan A., Aqil M., Imam S.S., Ahad A., Sultana Y., Ali A., Khan K. Temozolomide loaded nano lipid based chitosan hydrogel for nose to brain delivery: Characterization, nasal absorption, histopathology and cell line study. Int. J. Biol. Macromol. 2018;116:1260–1267. doi: 10.1016/j.ijbiomac.2018.05.079. [DOI] [PubMed] [Google Scholar]
- 189.Tomaszowski K.H., Hellmann N., Ponath V., Takatsu H., Shin H.W., Kaina B. Uptake of glucose-conjugated MGMT inhibitors in cancer cells: role of flippases and type IV P-type ATPases. Sci. Rep. 2017;7(1):13925. doi: 10.1038/s41598-017-14129-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Roy S.K., Gupta E., Dolan M.E. Pharmacokinetics of O6-benzylguanine in rats and its metabolism by rat liver microsomes. Drug Metab. Dispos. 1995;23(12):1394–1399. [PubMed] [Google Scholar]
- 191.Stephen Z.R., Kievit F.M., Veiseh O., Chiarelli P.A., Fang C., Wang K., Hatzinger S.J., Ellenbogen R.G., Silber J.R., Zhang M. Redox-responsive magnetic nanoparticle for targeted convection-enhanced delivery of O6-benzylguanine to brain tumors. ACS Nano. 2014;8(10):10383–10395. doi: 10.1021/nn503735w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Vlachostergios P.J., Hatzidaki E., Papandreou C.N. MGMT repletion after treatment of glioblastoma cells with temozolomide and O6-benzylguanine implicates NFκB and mutant p53. Neurol. Res. 2013;35(8):879–882. doi: 10.1179/1743132813Y.0000000191. [DOI] [PubMed] [Google Scholar]
- 193.Yoo B., Ifediba M.A., Ghosh S., Medarova Z., Moore A. Combination treatment with theranostic nanoparticles for glioblastoma sensitization to TMZ. Mol. Imaging Biol. 2014;16(5):680–689. doi: 10.1007/s11307-014-0734-3. [DOI] [PubMed] [Google Scholar]
- 194.Shchors K., Persson A.I., Rostker F., Tihan T., Lyubynska N., Li N., Swigart L.B., Berger M.S., Hanahan D., Weiss W.A., Evan G.I. Using a preclinical mouse model of high-grade astrocytoma to optimize p53 restoration therapy. Proc. Natl. Acad. Sci. USA. 2013;110(16):E1480–E1489. doi: 10.1073/pnas.1219142110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Srivenugopal K.S., Shou J., Mullapudi S.R., Lang F.F., Jr, Rao J.S., Ali-Osman F. Enforced expression of wild-type p53 curtails the transcription of the O(6)-methylguanine-DNA methyltransferase gene in human tumor cells and enhances their sensitivity to alkylating agents. Clin. Cancer Res. 2001;7(5):1398–1409. [PubMed] [Google Scholar]
- 196.Kim S.S., Rait A., Kim E., Pirollo K.F., Nishida M., Farkas N., Dagata J.A., Chang E.H. A nanoparticle carrying the p53 gene targets tumors including cancer stem cells, sensitizes glioblastoma to chemotherapy and improves survival. ACS Nano. 2014;8(6):5494–5514. doi: 10.1021/nn5014484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Kim S.S., Rait A., Kim E., Pirollo K.F., Chang E.H.A. A tumor-targeting p53 nanodelivery system limits chemoresistance to temozolomide prolonging survival in a mouse model of glioblastoma multiforme. Nanomedicine (Lond.) 2015;11(2):301–311. doi: 10.1016/j.nano.2014.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Dagıstan Y., Karaca I., Bozkurt E.R., Ozar E., Yagmurlu K., Toklu A., Bilir A. Combination hyperbaric oxygen and temozolomide therapy in C6 rat glioma model. Acta Cir. Bras. 2012;27(6):383–387. doi: 10.1590/S0102-86502012000600005. [DOI] [PubMed] [Google Scholar]
- 199.Xie Y., Zeng X., Wu X., Hu J., Zhu Y., Yang X. Hyperbaric oxygen as an adjuvant to temozolomide nanoparticle inhibits glioma growth by inducing G2/M phase arrest. Nanomedicine (Lond.) 2018;13(8):887–898. doi: 10.2217/nnm-2017-0395. [DOI] [PubMed] [Google Scholar]
- 200.Graham K., Unger E. Overcoming tumor hypoxia as a barrier to radiotherapy, chemotherapy and immunotherapy in cancer treatment. Int. J. Nanomedicine. 2018;13:6049–6058. doi: 10.2147/IJN.S140462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Zong Z., Hua L., Wang Z., Xu H., Ye C., Pan B., Zhao Z., Zhang L., Lu J., Mei L.H., Rutong Y. Self-assembled angiopep-2 modified lipid-poly (hypoxic radiosensitized polyprodrug) nanoparticles delivery TMZ for glioma synergistic TMZ and RT therapy. Drug Deliv. 2019;26(1):34–44. doi: 10.1080/10717544.2018.1534897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Vescovi A.L., Galli R., Reynolds B.A. Brain tumour stem cells. Nat. Rev. Cancer. 2006;6(6):425–436. doi: 10.1038/nrc1889. [DOI] [PubMed] [Google Scholar]
- 203.Sun T., Wu H., Li Y., Huang Y., Yao L., Chen X., Han X., Zhou Y., Du Z. Targeting transferrin receptor delivery of temozolomide for a potential glioma stem cell-mediated therapy. Oncotarget. 2017;8(43):74451–74465. doi: 10.18632/oncotarget.20165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Rai R., Banerjee M., Wong D.H., McCullagh E., Gupta A., Tripathi S., Riquelme E., Jangir R., Yadav S., Raja M., Melkani P., Dixit V., Patil U., Shrivastava R., Middya S., Olivares F., Guerrero J., Surya A., Pham S.M., Bernales S., Protter A.A., Hung D.T., Chakravarty S. Temozolomide analogs with improved brain/plasma ratios - Exploring the possibility of enhancing the therapeutic index of temozolomide. Bioorg. Med. Chem. Lett. 2016;26(20):5103–5109. doi: 10.1016/j.bmcl.2016.08.064. [DOI] [PubMed] [Google Scholar]
- 205.Zhang J., Hummersone M., Matthews C.S., Stevens M.F., Bradshaw T.D. N3-substituted temozolomide analogs overcome methylguanine-DNA methyltransferase and mismatch repair precipitating apoptotic and autophagic cancer cell death. Oncology. 2015;88(1):28–48. doi: 10.1159/000366131. [DOI] [PubMed] [Google Scholar]
- 206.Cousin D., Zhang J., Hummersone M.G., Matthews C.S., Frigerio M., Bradshaw T.D. Stevens. M.F.G. Antitumor imidazo[5,1-d]-1,2,3,5-tetrazines: compounds modified at the 3-position overcome resistance in human glioblastoma cell lines. MedChemComm. 2016;7:2332–2343. doi: 10.1039/C6MD00384B. [DOI] [Google Scholar]
- 207.Yang Z., Wei D., Dai X., Stevens M.F.G., Bradshaw T.D., Luo Y., Zhang J. C8-Substituted imidazotetrazine analogs overcome temozolomide resistance by inducing DNA adducts and DNA damage. Front. Oncol. 2019;9:485. doi: 10.3389/fonc.2019.00485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Bouzinab K., Summers H.S., Stevens M.F.G., Moody C.J., Thomas N.R., Gershkovich P., Weston N., Ashford M.B., Bradshaw T.D., Turyanska L. Delivery of temozolomide and N3-propargyl analog to brain tumors using an apoferritin nanocage. ACS Appl. Mater. Interfaces. 2020;12(11):12609–12617. doi: 10.1021/acsami.0c01514. [DOI] [PubMed] [Google Scholar]
- 209.Chen T.C., Cho H.Y., Wang W., Wetzel S.J., Singh A., Nguyen J., Hofman F.M., Schönthal A.H. Chemotherapeutic effect of a novel temozolomide analog on nasopharyngeal carcinoma in vitro and in vivo. J. Biomed. Sci. 2015;22:71. doi: 10.1186/s12929-015-0175-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Beier C.P., Schmid C., Gorlia T., Kleinletzenberger C., Beier D., Grauer O., Steinbrecher A., Hirschmann B., Brawanski A., Dietmaier C., Jauch-Worley T., Kölbl O., Pietsch T., Proescholdt M., Rümmele P., Muigg A., Stockhammer G., Hegi M., Bogdahn U., Hau P. RNOP-09: pegylated liposomal doxorubicine and prolonged temozolomide in addition to radiotherapy in newly diagnosed glioblastoma--a phase II study. BMC Cancer. 2009;9(308):308. doi: 10.1186/1471-2407-9-308. [DOI] [PMC free article] [PubMed] [Google Scholar]