Abstract
In the last few years research into Cannabis and its constituent phytocannabinoids has burgeoned, particularly in the potential application of novel cannabis phytochemicals for the treatment of diverse illnesses related to neurodegeneration and dementia, including Alzheimer’s (AD), Parkinson’s (PD) and Huntington’s disease (HD). To date, these neurological diseases have mostly relied on symptomatological management. However, with an aging population globally, the search for more efficient and disease-modifying treatments that could delay or mitigate disease progression is imperative. In this context, this review aims to present state of the art in the research with cannabinoids and novel cannabinoid-based drug candidates that have been emerged as novel promising alternatives for drug development and innovation in the therapeutics of a number of diseases, especially those related to CNS-disturbance and impairment.
Keywords: Phytocannabinoids, endocannabinoid system, neurodegenerative diseases, cannabis sp. cannabinoid receptors, neurological disorders
1. INTRODUCTION
Cannabis has been used since the beginning of human civilization, and it was first described by a Chinese Emperor around 2700 B.C. for the treatment of constipation, gout, beriberi, malaria, rheumatism and menstrual disorders [1]. The use of the plant was disseminated worldwide until the beginning of the twentieth century for a number of medicinal purposes and recreational use [2]. In occidental medicine, the first report of its use was in 1843, by the Irish physician William Brook O’Shaughnessy for the treatment of convulsion and other illnesses [3, 4]. The plant extracts were included in both British and American Pharmacopeias in the 19th Century for their sedative and anticonvulsant effects. However, the use of these extracts was forsaken due to both their chemical variability that gave rise to adverse effects but also the negative social impact as a consequence of recreational use [4]. Only in the 21st Century, in spite of its restrictions, cannabis returned to favor to be studied for therapeutical purposes [4]. Indeed, modern medicinal cannabis is gaining more widespread acceptance as an option in the treatment of a spectrum of illnesses. With a loosening of the regulatory framework for access in many countries, many more people will be expected to seek medicinal cannabis for a variety of conditions beyond its more established use in chemotherapyrelated nausea and vomiting, chronic pain, muscle spasticity and epilepsy [5]. Alternative extraction methods such as cold and supercritical CO2 processes may preserve a range of terpenes, parent cannabinoid carboxylic acids and other phytocannabinoids [6], which also extends to the extraction of hemp for the food and nutraceutical industries [7]. Thus, the changing landscape of both the composition and exposure profile of phytochemicals in medicinal cannabis may be substantively different from what we have experienced to date. This review is therefore timely to describe and discuss a number of major and minor phytocannabinoids and other cannabis phytochemicals in terms of their pharmacology, bioactivity and potential applications in disease, with a focus on novel neurotherapies. It is in this field where the plethora of novel phytochemicals in cannabis may be exploited for the most promise in developing new treatments for neurological diseases [8].
The term cannabinoid is broad, being used for synthetic cannabinoids, as well as for endocannabinoids and phytocannabinoids that act on the cannabinoid receptors. This term was originally used for designating a set of oxygenated aromatic hydrocarbon metabolites from marihuana, constituted by 21 carbon atoms, which are now named phytocannabinoids [9]. Currently, thousands of cannabis strains are known in the market with different composition of phytocannabinoids, which are classified and marketed on the basis of the total amount of THC (Δ9-tetrahydrocannabinol, 13, Fig. 3) and CBD (cannabidiol, 20, Fig. 3) [10, 11]. Nowadays, among the 10 known subclasses of phytocannabinoids, the most common and studied are the psychotropics, where THC is the most notable constituent, followed by CBN (cannabinol, 15, Fig. 3), Δ8-THC (Δ8-tetrahydrocannabinol, 14, Fig. 3) and the non-psychotropics such as CBD (20, Fig. 3), CBC (cannabichromene, 17, Fig. 3) and CBG (cannabigerol, 9, Fig. 3) [11-13]. All these compounds exert their biological effects variably through an interaction with a variety of receptors, including the cannabinoid receptors CB1 and CB2, but notwithstanding a number of other non-cannabinoid receptors, including G-protein coupled receptors (GPR55, GPR3) and ion channels [11]. The ability of phytocannabinoids to bind to these types of orphan GPCRs has been proposed as an important pathway of cannabis in the context of use as alternative treatments of a number of diseases, including chronic pain, nausea, multiple sclerosis, epilepsy, anxiety, AD, PD and HD, among others [14, 15].
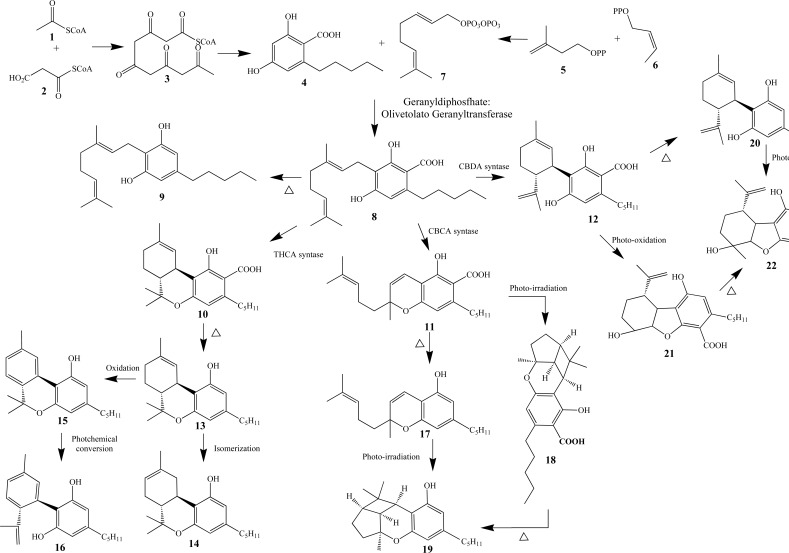
Fig. (3).
Biosynthetic pathway of phytocannabinoids (Adapeted form Refs. [11, 12]).
2. The plant Cannabis and its uses
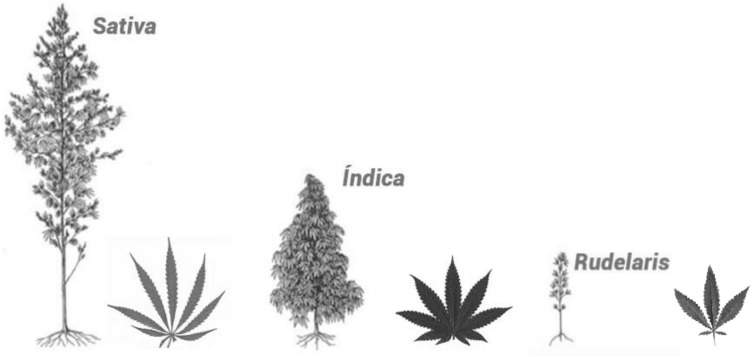
Cannabis is an Asian native species from the Family Cannabaceae, being an annual and dioecious plant (and in rare cases can develop as hermaphrodite) [16]. All species are rich in diverse biologically active chemical constituents divided into 18 chemical classes, including cannabinoids, alkaloids, terpenoids and flavonoids produced by secondary metabolism [17-19]. Based on taxonomy there are three accepted species of Cannabis, known as C. sativa, C. indica and C. ruderalis (Fig. 1) which vary both in physical characteristics such as height but also in chemical composition, including psychoactive componentes [18, 20]. Recreational use of C. sativa is often considered in physiologically generalised terms as uplifting, energetic and eurphoric and is the type more commonly used for recreational purposes, whereas C. indica tends to be described more for promotion of relaxation and sedation [16].
Fig. (1).
Illustration of morphological aspects of Cannabis species.
To date, 538 bioactive compounds have been identified in the most studied species C. sativa, of which more than 100 are phytocanabinoids [18, 21]. Currently, there are around 700 types of Cannabis, with variations in their cannabinoid and terpene composition [16]. The current chemotaxonomic classification of Cannabis establishes a relationship between the amount of THC (13, Fig. 3) and CBD (20, Fig. 3) in the plant due to the natural variability, and broadly this generates 5 Cannabis chemotypes:
Chemotype 1: narcotic, with a higher ratio of THC and THCA than CBD and CBDA (>1).
Chemotype 2: intermediary, with a similar ratio between the relation of THC/THCA and CBD/CBDA.
Chemotypes 3 and 4: fibrous, with a ratio between THC/THCA and CBD/CBDA much smaller than 1, therefore ascribed higher concentrations of CBD.
Chemotype 5: Plants of fiber type, but with a very low amount of cannabinoids [20, 22].
Cannabis has been cultivated since ancient times in some regions of Central and Southeast Asia, being used as a source of fiber, food, oils and medicines, but also used for recreational and religious purposes [13, 18]. Currently, cannabis can be readily cultivated in temperate and tropical climates [16]. Over time its domestication was influenced by human selection factors such as inbreeding, outbreeding and genome mixing [10], with a risk that domestication has reduced both biodiversity and chemical diversity in modern cultivated cannabis strains [10]. Its medicinal use has been reported from over 5000 years ago by a Chinese Emperor to treat malaria, rheumatism, gout, constipation and fatigue [13, 18] and cannabis has been used in Traditional Medicine. However, due to difficulties in the standardization of extracts, their low solubility in water that impedes injectable administration and a slow oral absorption with a consequent irregularity in extract absorption, cannabis use for medicinal purposes declined from 1937 [16]. Additional legal restrictions and cannabis taxation in the United States at that time also further limited its accessibility and use, which spread globally [23]. In 1960, the plant, its extracts and isolated cannabinoids were included in the Single Convention on Narcotic Drugs, in part as a consequence of their adverse side effects not being compensatable by therapeutic benefits [24, 25]. Ten years later, in 1971, by the Convention of Psychotropic Substances (COPS), the use of all THC analogues and derivatives was prohibited, except for medical and scientific purposes when specifically authorized [25, 26]. Despite the legal prohibition, marihuana is the most commonly used illicit drug in occidental countries, causing euphoria and changes in sensorial perception amongst other effects [27]. Many studies report that its intense and recurring recreational use, mainly amongst young people, has enhanced the risk of certain mental disorders, dependence and psychosis [28], which has been related to adverse effects in central GABAergic, glutamatergic and dopaminergic systems. By contrast, the use of Cannabis in the elderly has been reported to improve quality of life, reducing pain and prescribed opioid drug use [14].
The most active constituents, the cannabinoid metabolites, also known as phytocannabinoids, are produced within the glandular trichomes in leaves, bracts and stems, especially in the female plant [18, 29]. These glands are responsible for interactions between the plant and the environment, assisting in defense against pests and interactions with herbivores [18]. Plant genetic modifications have been explored to change the relative proportion of expressed phytocannabinoids and hence the pharmacological effects of cannabis extracts [25, 26]. In C. sativa, the Δ9-THC (13, Fig. 3) class is responsible for 17,3% of the extract’s composition, followed by the class of CBG (16,3%, 9, Fig. 3) and CBD (9,6%, 20, Fig. 3) [30].
3. Phytocannabinoids
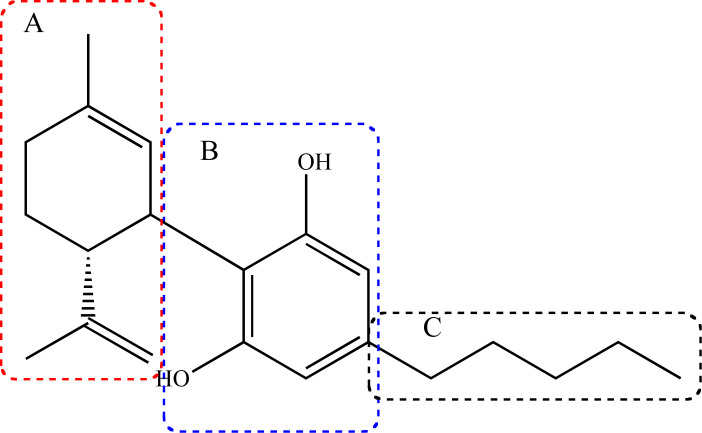
Phytocannabinoids are predominant, but not exclusive constituents of Cannabis species, with some aromatic ketone analogues also found in Radula sp and in fungi Pichia spp [12, 31]. To date, ca. of 120 phytocannabinoids are known, with a chemical structure with an oxygenated 21 carbon atoms skeleton, with a common fragment that includes the dibenzopyran ring and a hydrophobic alkyl chain [32]. This variety of compounds arises from the differences in the substituents and stereochemistry of the 3 structural subunits, namely the isopropenyl residue of the terpenoid moiety (A), the resorcinol nucleous (B) and the alkyl side chain (C, Fig. 2) [12].
Fig. (2).
Schematic general structure of a phytocannabinoid skeleton, with the three variable structural fragments: the isopropenyl residue at the terpenoids moiety (A), the resorcinol nucleous (B) and the alkyl side chain (C).
In the biosynthetic pathway of phytocannabinoids (Fig. 3), Acetyl-CoA (1) and Malonyl-CoA (2) are the precursor building blocks of the aromatic fragment from the polyketide intermediate 3, which undergoes sequential cyclization, aromatization and reduction to originate the olivetolic acid (4). In a parallel route, the formation of geranyl pirophosphate (7) is given from isopentenyl diphosphate (IPP, 5) and dimethylalyl diphosphate (DMAPP, 6) [25]. Under an enzymatic transformation, intermediates 4 and 7 lead to cannabigerolic acid (CBGA, 8), that undergoes decarboxylation to generate CBG (9). Compound 8 is also the key-intermediate in three other distinct ways to form the carboxylated phytocannabinoids THCA (Δ9-tetrahydrocannabinolic acid, 10, Fig. 3), CBCA (cannabichromenic acid 11, Fig. 3) and CBDA (canabidiolic acid, 12, Fig. 3) [12, 25, 33]. Once formed, metabolite 10 undergoes decarboxylation to generate THC (13, Fig. 3) that can, in turn, undergo isomerization to form Δ8-THC (14, Fig. 3) or oxidation to CBN (cannabinol, 15, Fig. 3), which can then be hydrolyzed to CBND (cannabinodiol, 16, Fig. 3). In another biosynthetic cascade, compound 11 can undergo decarboxylation or a cyclization step to form compounds CBC (17, Fig. 3) or cannabicyclic acid (CBLA, 18, Fig. 3), respectively, and both can result in the final product CBL (cannabicyclol, 19, Fig. 3). As a result of the third chemical pathway from CBGA (8, Fig. 3), compound 12 can undergo a decarboxylation step to form CBD (20, Fig. 3) or a cyclization process that leads to cannabielsolic acid (CBEA, 21, Fig. 3). Finally, both metabolites 20 and 21 can generate CBE (cannabielsoin, 22, Fig. 3) [11]. The structural diversity of phytocannabinoids can be explained as a result of non-enzymatic transformations induced by heat, light and oxidation in their acid precursors [12], being classified as neutral cannabinoids, without carboxyl groups and acid cannabinoids [12, 18], besides other polyketides than 6 that can participate in the biosynthetic cascade [10]. Thus, it is presumed that all these phytocannabinoids are firstly generated in the carboxylated form and then can be subjected to decarboxylation by enzyme-assisted reactions or during the plant storage process [34].
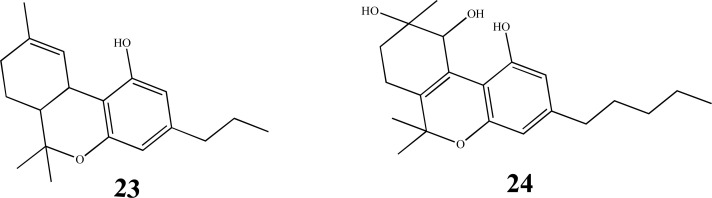
Phytocannabinoids are divided in 10 subclasses, including degradation products, precursors and byproducts [12, 18, 29, 35], such as CBG, (9, Fig. 3), THC (13, Fig. 3), Δ8-THC (14, Fig. 3), CBN (15, Fig. 3), CBC (17, Fig. 3), CBL (19, Fig. 3), CBD (20, Fig. 3), CBE (22, Fig. 3), Δ9-tetrahydrocannabivarine (THCV, 23, Fig. 4) and cannabitriol (CBT, 24, Fig. 4). In addition, these compounds are classified into classic and non-classic cannabinoids, depending on the presence of a tricyclic skeleton or an opened-ring feature, respectively [36]. The proportion of each class of constituent is dependent on cultivation conditions, species, geographic location and processing method [30].
Fig. (4).
Chemical structures of the classic cannabinoids THCV (23) and CBT (24).
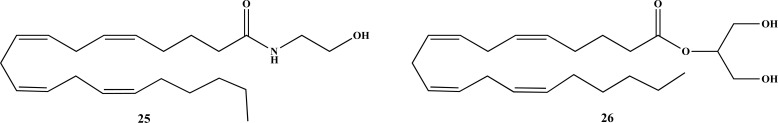
Considering chemical structural similarities, the phytocannabinoids are capable of acting at different receptors and confering a number of pharmacological effects [17], whereas terpenoids and other metabolites can modulate phytocannabinoid activity in the body, such as facilitating blood-brain barrier (BBB) transposition [16, 21]. Originally, phytocannabinoids were termed due to their ability to activate mammalian cannabinoid receptors CB1 and CB2. However, in recent years, other receptors out of the known domain of the endocannabinoid system (ECS) have been proposed, including G-coupled protein receptors (GPCR) (e.g. GPR55, GPR18), opioid and serotonin receptors and transient receptor potential (TRP) ion channels [32]. In their acid form, phytocannabinoids exhibit weak affinity for receptors CB1 and CB2 [11], while in the neutral form there are believed to be able to inhibit anandamide (AEA, 25, Fig. 5) reuptake [37].
Fig. (5).
Chemical structures of endogenous cannabinoids, AEA (25) and 2-AG (26).
3.1. Cannabigerol (CBG)
CBG (9, Fig. 3) was isolated for the first time by Mechoulam and Gaoni in 1964 from the Cannabis resin [30, 38]. This compound is a non-psychotropic phytocannabinoid obtained through decarboxylation of CBGA (8, Fig. 3) [30], and is present in low concentrations in Cannabis because it is the precursor of CBD (20, Fig. 3) and THC (13, Fig. 3) [31]. However, in some commercial hemp varieties, it is the main constituent [39] and can be also found in the aerial parts of Helichrysum umbraculigerum [31]. CBG (9, Fig. 3) has a low affinity for the CB1 receptor, instead acting as CB2 antagonist [27, 39]. In addition, it is a potent TRPM8 antagonist, being capable of activating TRPV1 and TRPA1. It also acts as an agonist of α2-adrenergic receptors, which explains its analgesic properties and as a 5-HT1A anatagonist, blocking the antiemetic and anti-nausea effects caused by CBD (20, Fig. 3) [2]. CBG (9, Fig. 3) is also able to bind peroxisome proliferator-activated receptor gamma (PPARγ) modulating neuroinflammation [40, 41], as well as having anti-inflammatory, anti-apoptotic and anti-proliferative effects [42, 43]. It also has been reported for its antimicrobial activity against gram-negative bactéria [38] and its inhibitory activity in anadamide (AEA) reuptake [2].
CBG has been studied for the treatment of Huntington disease (HD) due to its antioxidant and anti-inflammatory properties [40]. In vivo models of HD, using 3-nitropropionate (3NP)-injured or R6/2 mice at a dose of 10 mg/day showed a protective profile in the disease, preserving striatal neurons against 3NP-induced toxicity. Additionally, it was also active in mitigating mitochondrial dysfunction, calpain activation and 3NP-induced oxidative damage, while the effect on R6/2 mice was only moderate [40]. Compound VCE-003.2, which is a quinone derivative of CBG, was evaluated in 3NP-injured mice at a dose of 20 mg/kg, demonstrating enhanced cerebral antioxidant activity and animal locomotion [44]. The same authors also found a protective effect of this compound in a Parkinson’s disease animal model, belived to occur through PPAR-γ mediated amelioration of inflammation [14]. This group also demonstrated an in vitro pro-neurogenic effect in mice embryonic stem cells [41]. Another study with this CBG derivative (10 mg/kg) administered orally in male C57BL/6N mice showed a neuroprotective effect, increasing neurogenesis in the subventricular region through attenuation in microglial activation [41].
In a neuroinflammatory model used for investigation of amyotrophic lateral sclerosis (ALS), motorneuronal NSC-34 cells were pre-treated with CBG (2.5 and 5 µM) and showed a marked reduction in LPS-induced neuroinflammation [42]. This property was further corroborated by another neuroinflammatory assay, again using NSC-34 cells, where LPS-stimulated RAW-26 macrophages pre-treated with CBG (9, Fig. 3) at a dose of 7.5 µM showed a reduction in neuronal death and inflammation; this occurred via decreasing pro-inflammatory TNF-α, INF-γ cytokine expression and attenuation of oxidative stress due to an increase in Nrf-2 levels [45]. Thus, these findings reinforce the neuroprotective capacity of CBG (9, Fig. 3) particularly against inflammation and oxidative stress.
3.2. Cannabichromene (CBC)
Isolated for the first time in 1966 [46], CBC (17, Fig. 3) is considered one of the main phytocannabinoids along with THC (13, Fig. 3), CBD (20, Fig. 3) and CBG (9, Fig. 3) [34]. However, its concentration in the cannabis plant is often considered to be low (0.2-0.3% of dry weight), although higher amounts are found in chemotype 1 [34]. Isolated CBC (17, Fig. 3) appears as an oil or gum and was not considered to show activity at cannabinoid receptors [30, 34], albeit a recent study identified CB2 receptor agonism [47]. Meanwhile, a study conducted by Rosenthaler and co-workers showed that CBC (17, Fig. 3) has 82.9% of affinity for CB2 in relation to CB1 [48], and in high doses could cause hypothermia, sedation and hypoactivity in mice characteristic of the cannabinoid tetrad [2, 34, 49], albeit one mediated by non-CB1 dependent mechanisms [2]. The most important molecular target of CBC (17, Fig. 3) is believed to be TRPPA1 ion channels, with an IC50 value of 90 nM as a non-covalent modulator [34]. It shows analgesic action by stimulating the descending antinociceptive pathway and activates TRPA1-dependent anti-inflammatory pathways in LPS-induced models [2]. In addition, CBC was capable of reducing carrageenan-induced paw edema in rats in diferent doses (60, 120 and 240 mg/kg), evidence of its anti-inflammatory effect [50]. In vitro studies with human keratinocyte cells HaCaT also revealed that CBC (17, Fig. 3) could reduce the cytokine IL-6 and MCP-2, a pro-inflammatory mediator involved in macrophage and mastocyte recruitment to sites of inflammation [51]. In addition, there are selected studies highlighting the antiproliferative effects of CBC (17, Fig. 3) [43], as well as its comparative antimicrobial and antifungal properties [34]. Importantly, micromolar concentrations of CBC increase endocannabinoid tone by inhibition of AEA (25, Fig. 5) uptake and degradation of 2-arachidonoylglycerol (2-AG, 26, Fig. 5) [34].
There are few studies related to the potential role of CBC (17, Fig. 3) in the management of neurodegenerative diseases (NDs), given its recognised anti-inflammatory and neuroprotective properties from preclinical studies. An in vitro study with C6 glial cells stimulated by amyloid-β protein (Aβ), 10 nM CBC (17, Fig. 3) was capable of reducing the concentration of cellular nitrite, while in Aβ-induced neuronal SH-SY5Y cells, this compound was able to decrease cell death only at the concentration of 10 µM [52]. CBC was also found to enhance the viability of neural stem progenitor cells 47, while Schubert and co-workers demonstrated that CBC could prevent oxytosis in HT-22 cells, which is a type of programmed cell death involving glutamate and, in turn, oxidative stress [53].
3.3. Cannabidiol (CBD)
CBD (20, Fig. 3) was isolated in 1940 by Adams and colleagues [54, 55], but only in 1963 was its chemical structure completely elucidated by Gaoni and Mechoulan [36, 56]. CBD (20, Fig. 3) is the second most abundant chemical constituent in Cannabis, especially in plants of the fiber type [17]. This compound is not psychomimetic, having an acceptable safety profile and tolerability to be proposed for the treatment of several CNS-related diseases [28]. In spite of its relative low oral bioavailability (13-19%), when administered by injection it could promptly pass through the BBB [57]. CBD (20, Fig. 3) has a low affinity for the CB1 and CB2 cannabinoid receptors (CB1-R and CB2-R, respectively) [17], being a negative non-competitive allosteric modulator of CB1-R and capable of reducing the efficacy and potency of THC (13, Fig. 3) and 2-AG (26, Fig. 5) in HEK 293A cells that express CB1-R, without adverse side effects [58, 59]. As a modulator CBD (20, Fig. 3) has a demonstrated capacity to block the psychotropic effects and attenuate anxiogenic effects caused by high doses of THC (13, Fig. 3) [4]. In fact, these properties are in accordance with the known properties of allosteric modulators that have the potential to avoid adverse effects in the central and peripheral nervous system elicited by conventional orthosteric ligands [17, 58]. A study evidenced that CBD (20, Fig. 3) has 74.5% of affinity for CB2-R relative to CB1-R [48]. However, until now no CBD-specific receptor has been identified [60]; instead various receptor activities have been ascribed to CBD (20, Fig. 3), including PPAR-γ and 5HT receptor agonism [61, 62].
Cannabidiol has documented neuroprotective efficacy in in vitro and experimental animal models of Alzheimer’s disease [63-66]. In regards to neuroprotection, in vitro assays demonstrated that CBD (20, Fig. 3) reduced both Aβ production and tau hyperphosphorylation as pathological hallmarks of AD [57, 67-69]. In SH-SY5Y neuronal cells, CBD (20, Fig. 3) neutralized the increase in APP expression through ubiquitinization, leading to a progressive reduction in Aβ and less apoptotic events [69]. The effect in the amyloidogenic pathway could be mediated by peroxisome proliferator-activated receptors (PPARs), where CBD (20, Fig. 3) decreased reactive gliosis and neuronal damage, aside from promoting neurogenesis [70, 71]. A recent study in N13 cells and micróglia showed that at 100 nM CBD (20, Fig. 3) led to a decreasing intracelular calcium concentration as a result of high concentration of ATP, considering that Ca+2 is an important cellular messenger and is involved in several pathologies [72]. In in vivo models of AD it was demonstrated that CBD (20, Fig. 3) could play a central role in attenuation of Aβ-induced inflammation and diminish reactive gliosis [65]. In other studies with in vivo PD models, such as damage induced by the neurotoxin 6-hydroxydopamine (6-OHDA) in rodents, CBD (20, Fig. 3) showed neuroprotective properties, as well as the capacity to modulate other non-motor symptoms such as anxiety, depression and cognition [73, 74]. Brazilian researchers showed that CBD (20, Fig. 3) was able to attenuate pain, a non-motor symptom of PD, in C57/BL6 mice exposed to the neuronal injury from 6-OHDA, reducing allodynia and hyperalgesia [75]. In addition, reserpine-induced motor and cognitive impairmennts in rats were ameliorated by administration of CBD (20, Fig. 3) at the doses of 0.5 mg/kg and 5 mg/kg, being capable of significantly reducing catalepsy and oral movements and, at the lower dose, also improving memory deficits [76]. These findings highlight that CBD (20, Fig. 3) possesses important antioxidant, neuroprotective and anti-inflammatory properites that could be explored in the development of new medicines to treat diverse CNS-related diseases [77]. Limited evidence of elevated serum hepatic markers, CBD-drug interactions and hepatotoxicity has been noted with cannabidiol [78-80], leading to the search for new derivatives with a more favourable activity and safety profile [81]. A clearer understanding of CBD’s somewhat enigmatic pharmacology would assist drug development in this area, particularly with regards to selectivity.
3.4. Δ9-tetrahydrocannabinol (THC)
THC (13, Fig. 3) is the major constituent in Cannabis and was isolated for the first time in 1942 [38], but only had its structure fully elucidated by Mechoulan and colleagues in 1964 [27, 35]. This compound is thermodynamically unstable, undergoing isomerization in the presence of acids to form Δ8-THC (14, Fig. 3) [36]. THC is promptly absorbed and distributed in the body, being metabolized by cytochrome P450 [16]. Due to its lypophilicity, it was thought that its pharmacological properties were resultant of interactions with phospholipid membranes [9] until the discovery of the cannabinoid receptors. Initially, THC (13, Fig. 3) was described as a CB1-R agonist, but in vivo studies evidenced it acts as only a CB1-R partial agonist [28] and exerts its effects by imitating endogenous cannabinoids [82]. Its activity towards CB1 receptors makes this compound unique as the only phytocannabinoid totally active and potent in the four classic assays that evaluate cannabinoid psychotropic capability (ring test, open field, hot plate and tail flick), causing the characteristic tetrad of cannabinoid effects: hypokinesia, hypothermia, antinociception and catalepsy [2]. Activation of CB1-R by THC (13, Fig. 3) also causes local analgesia [83, 84] when intramuscularly injected at the dose of 1mg/mL, and without adverse effects [84].
Recent studies suggest that THC (13, Fig. 3) could play an important role in AD, facilitating Aβ disaggregation, reducing tau hyperphosphorilation and even acting as a competitive AChE inhibitor [19]. In an in vitro PD model using human neuroblastoma SH-SY5Y cells exposed to the toxins MPP+, paraquat and lactacystine, THC showed neuroprotective effects, increasing cell viability and reducing apoptosis and oxidative stress, possibly mediated by PPAR-γ [85]. Neuroprotective capacity of THC was further supported by another study using N18TG2 cells, on which THC attenuated glutamate-induced neurotoxicity mediated through the CB1 receptor [86]. In a 5XFADAPP transgenic AD mice model treated for four weeks with 3 mg/kg THC, a reduction in brain Aβ aggregates and neurodegeneration was observed, associated with an increasing in neprisylin levels, an essential endopeptidase related to Aβ disaggregation [87]. In another study, male AβPP/PS1 mice treated during the initial stages of neurodegeneration with a dose of 0.075 THC mg/kg i.p. showed memory enhancement, in comparison to wild type mice. However, combined treatment with THC/CBD was shown to be more effective [88]. In another in vivo study, 3 mg/kg THC (13, Fig. 3) treatment reversed the age-related decline in cognitive performance in older mice via a CB1-R related pathway, accompanied by enhanced expression of synaptic proteins and increased hippocampal neuronal density [89]. Therefore, in addition to CBD (20, Fig. 3), THC (13, Fig. 3), in spite of its psychotropics effects, has been evaluated for decreasing oxidative stress, neuroinflammation and neuroprotection in illnesses close related to inflammatory cytokine dysregulation and overproduction of free radicals, with evidence of additive effects with CBD (20, Fig. 3) in conferring neuroprotection relevant to neurodegenerative processes in dementia [53].
3.5. Δ8-tetrahydrocannabinol (Δ8-THC)
Δ8-THC (14, Fig. 3) is found only in a few varieties of Cannabis and is a regioisomer of THC [36], with the double bond at the C-8/C9 position being thermodynamically more stable than in the position C9/C10 [38]. Δ8-THC (14, Fig. 3) shows affinity for both CB1 and CB2 receptors in a similar manner as observed for THC (13, Fig. 3) [36]. In vitro studies with HT-22 cells suggested that this phytocannabinoid could prevent oxytosis, mantaining cellular viability, ATP levels and promoting growth factors in embryonic E18 neurons [53]. In addition, evaluation in MC65 cells expressing Aβ showed that Δ8-THC (14, Fig. 3) is efficient in preventing Aβ-induced toxicity with an IC50 of 85 nM [53].
3.6. Δ9-tetrahydrocannabivarine (THCV)
THCV (23, Fig. 3) is a THC analogue with a n-propyl side chain [30]. The mode of action of THCV (23, Fig. 3) is still controversial, with some studies suggesting it acts as an antagonist of both CB1 (Ki= 75.4 nM) and CB2 (Ki= 62.8nM) receptors [36], while other reports describe THCV (23, Fig. 3) as a partial agonist of CB2-R [2]. As a CB1-R antagonist, this compound is capbable of attenuating the effects of the CB1 agonistsWIN55212-2 and CP55,940 [90, 91], and in doses lower than 3 mg/kg it is also capable of reducing hypothermia and nociception and increasing heart rate in human volunteers arising from THC (13, Fig. 3). However, in higher doses (e.g. 10mg/kg) THCV (23, Fig. 3) acts in vivo as a CB1-R agonist [2, 22]. THCV (23, Fig. 3) could also interact with TRP ion channels, being an agonist of TRPA1, TRPV1 and TRPV2-4, while also acting as an antagonist of TRPM8, TRPV5 and TRPV6 [90, 92]. Studies of its pharmacokinetics evidenced a maximum absorption after 30 minutes of oral or intraperitoneal administration in both mice and rats, with a faster elimination and half-life higher than 8 hours from oral administration [93]. In vivo experiments showed neuroprotective effects of THCV (23, Fig. 3) on LPS-injured mice, with the proposed mode of action for neuronal preservation occurring through CB2-R modulation, since knock-out mice were more susceptible to these lesions [2, 91]. Another in vivo study using male Sprague–Dawley rats injured by 6-OHDA showed that THCV (23, Fig. 3) administered at the dose of 2 mg/kg was capable to improve gait and reduce slow movements, while chronic treatment for 14 days was shown to reduce nigrostriatal dopaminergic neuronal loss characteristic of PD [91]. Recently, in another pharmacological approach by using the zebra-fish model, researchers demonstrated that THCV (23, Fig. 3) influenced Ca+2 transport through TRPV5 and TRPV6 receptors [92], which could be an interesting pathway to mitigate neurodegeneration where calcium imbalance is believed to play a key role in excitotoxicity. In addition, Ca+2-dependent process are also involved in decreasing inflammation and pain [22]. Finally, several studies have shown that THCV (23, Fig. 3) could exert anti-convulsant and anti-epileptic properties, while also being investigated for the treatment of obesity and insulin resistance [2, 60].
3.7. Cannabinol (CBN)
CBN (15, Fig. 3) was the first phytocannabinoid to be isolated in the 19th century and its chemical structure was elucidated in 1930 [2]. This metabolite is found in its acid form in C. sativa and C. indica, and it undergoes decarboxylation under heating, being also an oxidation product of THC (13, Fig. 3) [30, 38]. Its concentration increases during storage as levels of THC (13, Fig. 3) decrease [38]. Pharmacologically, CBN (15, Fig. 3) acts as partial agonist of CB1 receptor and exhibits high affinity for CB2, albeit showing lower efficacy than THC (13, Fig. 3) [27]. An in vivo evaluation of the mechanical withdrawal threshold in Von Frey test in rats treated with 1 mg/mL of CBN (15, Fig. 3) evidenced a markedly increasing mechanical threshold, suggesting that peripheral application could provide analgesia with the potential to be used in chronic pain. The mechanism of action is thought to be related to modulation of the CB1 receptor, similarly to THC (13, Fig. 3) [83]. Schubert and co-workers also demonstrated that CBN could act as neuroprotector in HT-22 cells and cortical embryonic E18 neurons, in addition to its ability for stimulating degradation and clearance of pre-formed Aβ aggregates in MC65 cells at the concentration of 100 nM [53]. Interestingly, CBN was found to act synergistically with THC (13, Fig. 3) in conferring neuroprotection in the same study [53].
3.8. Cannabicyclol (CBL), Cannabielsoin (CBE) and Cannabitriol (CBT)
CBL (19, Fig. 3) was firstly isolated by Korte and Sheper in 1964 [94-96] and is the phytocannabinoid found in lowest concentrations in the plant [94], produced by heating from CBC (17, Fig. 3) [38]. CBE (22, Fig. 3), isolated by Shani and Mechoulan as its acid derivative in 1974, is naturally produced by photo-oxidation of CBD (20, Fig. 3) and CBDA (12, Fig. 3) [38] and is also found as a CBD (20, Fig. 3) metabolite in animal studies [97, 98]. In 1965, Obata and Ishikawa discovered CBT (24, Fig. 3) [99], but its chemical structure was elucidated only ten years later [100]. There are nine CBT (24, Fig. 3) isomers, distinguished by the substitution of the hydroxyl groups Interestingly, in spite of their chemical structure and similarity with other bioactive Cannabis-derived constituents, none of these phytocannabinoids have been studied for their potential effects on neurodegenerative diseases.
3.9. Potential Pharmacological Benefits of Cannabinoids in Neurodegeneration Pathogenesis
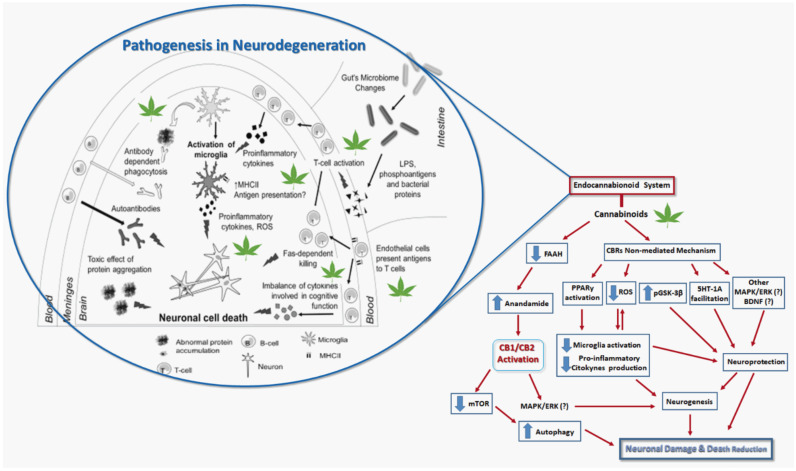
In general, NDs are associated with neuroinflammation as a consequence of the release of cytokines and oxidative stress mediated by overproduction of ROS and RNS, in addition to depression and anxiety [101-105]. In this context, as discussed above, phytocannabinoids could play important modulatory role by either ECS or in other bioreceptors, which may contribute to their potential pharmacological effects against NDs. By acting via ECS, they lead to an increase in endocannabinoids as AEA (25) and 2-AG (26, Fig. 5), which bind to CB1Rs at the nervous terminal of γ-aminobutyric acid (GABA) and, in turn, enhance dopamine concentration and transmission [28, 106]. In addition, an increase in endocannabinoids production could also provide antipsychotic [107] and antidepressive [108] effects observed in animal models, probably by interaction with TRPV1. On the other side, alosteric modulation of serotoninergic receptor (5-HT1A) plays an important role in emotional regulation and response to stress, in depression neurobiology [109] and also neuroprotective effects [102]. Literature data also suggest the effects of cannabinoids in the activation of Peroxisome proliferator-activated receptor gamma (PPARγ), leading to microglial activation and lower expression of inflammatory genes [102], exerting neuroprotective effects [110]. In Fig. 6, there are highlighted some targets and potential pharmacological effects of cannabionids in the pathogenesis of NDs.
Fig. (6).
Some molecular targets and potential pharmacological effects of cannabinoids in Neurodegenerative diseases. Adapted from Refs. [13, 111, 112]. (A higher resolution / colour version of this figure is available in the electronic copy of the article).
4. Cannabinoid receptors and endocanna-binoid system (ECS)
Before the discovery of cannabinoid receptors, it was thought that cannabinoids exert their biological effects mainly by interaction with cellular membranes due to their lipophilicity [113]. However, further studies conducted with Cannabis preparations led to the discovery of the receptors involved in the signalling of their effects on CNS [60]. The CB1 receptor was the first to be identified in the brain in 1988 [31]. It was cloned in 1991, followed by the CB2 receptor two years later [113-115]. Both receptors belong to the GPCR class and are constituted by seven transmembrane domains connected by three extracellular and three intracelular loops, one extracelular N-terminal tail and one intracellular C-terminal tail [59, 116]. These two homologue receptors are expressed in all mammalians, fish, reptiles, birds and in most invertebrates [117]. When activated, due to the coupling with Gi/o-protein, these receptors supress adenylate cyclase and the formation of cyclic adenosine monophosphate (cAMP) [113, 118] and promote activity of mitogen-activated protein kinase (MAPK) [116, 118]. There are three main classes of compounds that act in these receptors: the cannabinoids, eicosanoids (endocannabinoids) and aminoalkylindoles [116].
The first crystalline structure of CB1-R with a stabilizing antagonist was performed in 2016, revealing that the N-terminal region plays a fundamental role in ligand recognition, leading to a better comprehension of the native state of this receptor [59, 119, 120]. At the beginning of 2019, the first crystalline structure of CB2-R with the antagonist AM10257 was reported [121]. Until then, all computational studies with CB2 were based on homology models, providing limited understanding about its structure and conformation [59].
CB1-R is the most expressed GPCR in brain, especially in the olfactory bulb, hippocampus, basal ganglia and cerebellum [113]; this is in contrast to other regions such as the thalamus, cerebral stem and medulla, where it is absent [122]. It is present in pre-synaptic neurons [123, 124], mainly in glutamatergic and GABAergic neurons [116], and in peripheral sensorial neurons as part of the pain pathway [125]. In addition, CB1-R is also found in astrocytes where is activates Gs protein [48], leading to the release of neurotransmitters such as glutamate. This can elicit an increase in the concentration of intracellular Ca2+ [116] and may also be involved in the regulation of local cerebral blood flow and neuronal energy supply [126]. On the other hand, in post-synaptic neurons, these receptors control the activity of ion channels against excitotoxicity [126]. Besides being expressed in CNS, CB1-R can be also found in other tissues of heart, urinary bladder, small bowel, reproductive system [116, 125, 127] and in the mitochondria of striated muscle, suggesting a role in cell respiration [59]. CB1-R participates in the modulation of a variety of neurocerebral functions, including executive processing, emotional state (anxiety and depression), reward, modulation of nociception and memory through the endocannabinoid, GABAergic, dopaminergic and glutamatergic systems [123, 126]. Once activated, CB1-R can lead to retrograde release of neurotransmitters that could be inhibitory or excitatory, depending on the cerebral region [125]. CB1 receptors also play a role in regulation of sleep, appetite, short term memory and motor coordination [16]. According to the most recent findings about CB1-related pharmacological activites, its activation by agonists could lead to beneficial effects towards diverse pathologies that include pain, anxiety, depression and neurodegenerative diseases like HD [59, 60]. Meanwhile, polymorphisms in the CNR1 gene have been associated with diseases like schizophrenia and depression in PD [118]. The development of CB1 receptor-selective agonists has been hampered by pharmacologically predictable CNS side effects related to intoxication, while limitations related to CB1 receptor antagonists are best exemplified by the history of rimonabant, which had limited availability as an anti-obesity medication prior to withdrawal due to side effects such as depression and suicidal ideation [60].
The CB2 receptor is expressed by the CNR2 gene in chromosome 1 with 44% homology at the protein level with CB1 [125, 128, 129]. It is coupled to heterotrimeric proteins Gi/o, through which triggers multiple signal transduction pathways involved in cell proliferation, differentiation and survival [115]. CB2-R is located peripherally in imunomodulating cells in organs such as spleen, tonsils and liver [116, 126], and in microglia from post-synaptic areas [123, 125]. Its function is mostly to modulate the release of cytokines and migration of immune cells [125]. When activated, these receptors also act to decrease the release of pro-inflammatory cytokines and other neurotoxic factors with the resultant attenuation of damage in neurons [116, 126], in addition to blocking the differentiation of microglia into a neurotoxic phenotype [57]. CB2-R is lesser expressed in the CNS than CB1-R, but in some pathological conditions (e.g. anxiety, epilepsy and AD) its expression can increase, suggesting its involvement in diverse psychiatric illnesses [19, 123, 126]. CB2-R agonist activation causes inactivation of voltage-dependent calcium channels [116], which leads to a decline in cAMP levels through the MAPK pathway and, in turn, influences cell survival, proliferation and response to stress [126]. Considering that CB2-R is capable of promoting homeostasis and neuron survival by inhibition of excitotoxicity, apoptosis and oxidative stress, it has been considered a promising target for the treatment of autoimune and inflammatory diseases [60, 115, 126]. Indeed, recent exploratory clincial trials for a CB2-R selective agonist, olorinab, in the treatment of inflammatory and irritable gastrointestinal disorders [25] provide some degree of both proof-of-concept and target validation to an immunomodulatory role that may further translate to CNS neuro-inflammatory disorders in the future.
Based on the above-mentioned features, the ECS is an endogenous signalling system cosntituted by CB receptors, metabolic and catabolic enzymes, endocannabinoids and transporters that control diverse actions of cannabinoids both in the CNS and peripheral nervous system (PNS) [21]. The endocannabinoids are amides, ethers or esters of fatty acids with polyunsaturated long chains [37, 130] acting as retrograde transmitters, since they are released in post-synaptic neurons and act in pre-synaptic cells where the receptor expression is highest [16], They have with a half-life of approximately 15 minutes [21]. Based on the literature, the most studied endocannabinoids are 2-AG (26, Fig. 5) and AEA (25, Fig. 5), with the latter being the first endogenous cannabinoid to be discovered after the characterization of CB1-R in 1992 [37, 130]. These two compounds belong to two different classes of lipids, the 2-acylglycerols (2-AcGs) and the N-acylethanolamines (NAEs), respectively [60], and are stored in cytosolic adiposomes which play a kay role in metabolism and cell sigmalling [21, 131]. Besides acting on CB1-R, these compounds act on channels of the TRP class, including TRPV1, TRPA1 and TRPM8 [37], orphan G protein-coupled receptors like GPCRs: GPR18, GPR55, GPR119 but also on PPARs [21, 59]. The main enzyme in the biosynthesis of AEA (25, Fig. 5) is N-acylphosphatidilethanolamine (NAPE-PLS), whereas diacylgliyerol lipases (DAGL)-α and β are responsible for the production of 2-AG (26, Fig. 5) [60, 127, 130]. Once produced, their uptake from the extracellular environment to the intracellular medium occurs through facilitated diffusion by the action of endocannabinoid membrane transporters (EMTs) [60, 126, 130], whereas their intracellular transport is promoted by fatty acid binding proteins (FABPS). FABP1 is also a THC transporter, and its extracellular transport is operated by microvesicles [21]. The endocannabinoids are synthesized on-demand and are biologically inactivated by degradation through the specific enzymes fatty acid amide hydrolase (FAAH) and monoacylglycerol lipase (MAGL), for AEA and 2-AG (26, Fig. 5) respectively [124, 127, 130]. In addition, other enzymes haver been shown to utilize the endocannabinoids as substrates, such as COX-2 and cytochrome P450 [130]. Currently, the ECS possess 37 known components that include GPCRs and other receptors, enzymes for synthesis and degradation, transporters and the endocannabinoids themselves [21, 116]. It is suggested that membrane lipids like cholesterol could affect ECS-related receptors and enzymes, being responsible for its fine adjustment [21]. Recent findings point out that GPR55, an orphan GCPR, could be a new cannabinoid receptor (CB3) [118, 130, 132], with only a 14-15% homology with CB1 and CB receptors [59].
More specifically, the ECS is a neuromodulator system acting on diverse neuronal populations [16], influencing the regulation of fundamental processes in the CNS and PNS [130], including hormetic and homeostatic processes [126]. In a pleiotropic manner therefore, an ECS imbalance may result in aberrant enodcannabinoid tone flowing on to influence the pathophysiology of several diseases [116]. For these reasons, it has received focused attention as a promising target for the modulation of a diverse set of neurodegenerative diseases in which oxidative damage and inflammation play a central role. However known limitations in targeting ECS function for clinical benefit include the recognised and aforementioned adverse side effects of CB1-R antagonists and a lack of efficacy of FAAH blockers that have hindered clinical utility, as two examples of where to reduce or enhance ECS tone are not without their risks [133, 134].
5. Other cannabis phytochemicals: bioactivity and neuroprotection
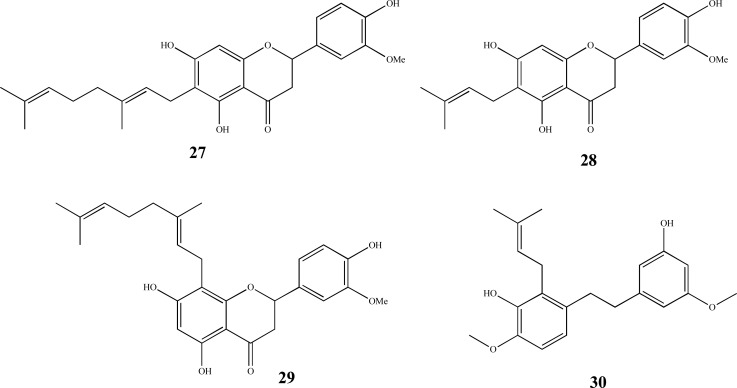
Additionally, relatively novel and minor non-cannabinoid phytochemicals found in cannabis include the cannabis flavonoids cannflavin A-C (Fig. 7), canniprene (30, Fig. 7) and selected terpenes [135]. The stilbenoid canniprene shares anti-inflammatory properties targeting 5-lipoxygenase [136], a target for neuroprotection in a transgenic AD mouse model [137], while the cannflavins are prenylated flavonoids that can constitute up to 1% of leaf material, are also abundant in inflorescences [80] and possess demonstrated anti-inflammatory profiles in vitro [138, 139].
Fig. (7).
Chemical structures of cannabis flavonoids; cannflavin A (27), cannflavin B (28), cannflavin C (29) and stilbenoidand canniprene (30).
They remain largely unstudied when compared to conventional flavonoids, which have well documented neuroprotective and anti-aggregatory activity against neurotoxic amyloidogenic proteins in experimental models [7, 140, 141]. Recent evidence of a relatively potent neuroprotective effect of cannflavin A was described against β-amyloid toxicity, associated with inhibition of aggregation of this hallmark and toxic AD-related protein [142]. Of recent note, an unnatural isomer derivative of cannflavin B has demonstrated pancreatic anti-cancer activity in vivo [143] and may enter clinical trials soon, highlighting the very real potential of novel drug development based on the chemical diversity of the cannabis plant.Terpenes form a diverse array of compounds found in many plants, with over 200 expressed in a range of cannabis chemovars [144, 145]. They represent a major class of chemicals that give cannabis its scent and aroma, which is often a reference that breeders use to cultivate particular types of cannabis [6]. Common terpenes found across the varying cannabis chemotypes include limonene, α-pinene, humulene, β-carophyllene and myrcene [146]. Terpenes are also believed to partly contribute to the synergistic efficacy of medicinal cannabis strains, known as the entourage effect [145], although the basis of this interaction has been difficult to elucidate given the sheer number of compounds in cannabis and the chemical volatility of many of its terpenes. This also does not take into account the loss or alteration in terpene composition during various extraction processes for a range of proprietary medicinal cannabis formulations. Some studies also indicate that such synergy with the major cannabinoids may be lacking when cannabinoid receptor activity has been investigated as the underlying basis of the interaction [147].
In isolated bioactivity studies however, terpenes are neuroprotective in the presence of oxidative stress, stimulating antioxidant defences, limiting ROS-induced apoptosis [148] and inhibiting Aβ aggregation in neuronal cells [149]. There is also evidence that terpene hydroperoxides can cleave advanced gylcation end products formed in neurodegenerative disease [150]. The cannabis terpene β-caryophyllene mediates CB receptor-independent neuritogenesis in PC-12 cells [151] as well as neuroprotection in transgenic APP/PS1 mice via CB2 receptor and PPAR-γ pathways [152]. Additionally, the sesquiterpene α-bisabolol has been shown to be neuroprotective in cellular models of amyloid β-mediated neurodegeneration associated with inhibition of fibrillization [149, 153], while limonene displays similar efficacy in a transgenic Drosophila amyloid β-expressing model [154].
Collectively there is a wealth of preclinical evidence that demonstrates that the ‘other’ cannabis consitituents are clearly important, comprising up to 5-10% of total cannabis phytochemicals and potentially imparting significant biological activity to medicinal cannabis [144, 145] Interestingly, carboxylated progenitor forms of the major neutral phytocannabinoids have also emerged as possessing a diverse and favourable bioactivity [155], including neuroprotection [110]. This raises the prospect of additional formulations of non-heated or raw medicinal cannabis extracts being used potentially for different therapeutic applications. Clearly much further research is required, particularly clinical trials, to sort through the realisation of this cannabis chemical ‘pot-pourri’ towards clinical translation.
6. Clinical studies in dementia
To date there have only been limited clinical studies on the use of medical cannabis extracts or phytocannabinoid formulations in various forms for the treatment of dementia. There is much variability in trail design and phytocannabinoids used, including synthetic forms of THC such as dronabinol or nabilone, off-label use of Sativex® (THC: CBD 1:1) normally indicated for the relief of multiple sclerosis-related muscle spasms and pain, as well as whole medical cannabis extracts. Distinctions should be made between disease-modifying and symptomatic benefits, where generally cannabinoids have been more likely to provide variable benefits in the latter. For example, in clinical dementia trials reporting treatment of patients with phytocannabinoids, some trials reported significant improvements in a range of neuropsychiatric symptoms, whereas other trials returned no significant benefit [156]. Where symptomatic benefit is observed, it is more commonly reflected in decreased agitation and aggression, increased appetite, sleep quality, objective mood and pain control [8]. Generally however, systematic reviews in this field indicate a higher benchmark in clinical trial design is required before objective assessments of benefit and risk can be made, including cannabinoid dosing and cohort numbers [157].
In Parkinson’s disease, orally administered cannabis extract is well tolerated but resulted in no objective or subjective improvement in dyskinesias or Parkinsonism [158]. However some studies point to improvements in sleep and overall quality of life measures in PD patients using CBD [159, 160]. To date there is very little information on medical cannabis efficacy and safety in Alzheimer’s disease, although one small cohort study reported a benefit in CGI (clinical global impression) scores using medical cannabis oil [161], that similarly attest to the abovementioned symptomatic benefits of phytocannabinoids [8]. There are currently a number of clinical trials underway in Alzheimer’s disease that will undoubtedly shed more light on effectiveness and safety of medical cannabis as either or both a disease modifier and a symptomatic treatment.
In other types of neurodegenerative diseases, trials include the use of Sativex in Huntington’s disease, which demonstrated a lack of improvement in motor, cognitive (p = 0.824) and behavioural effects, although no additional adverse side effects were noted in a small cohort of 24 patients using Sativex [162]. The combination of THC and CBD is often purported to be more effective than THC alone, believed to be at least in part due to CBD mitigating the negative psychotropic effects of THC. This may occur through a recognised molecular action of CBD in the ventral hippocampus, downregulating pERK1-2 signalling modulated by THC [163]. However adverse psychotropism with THC is more apparent in recreational consumption [164] than in medicinal use, where phytocannabinoid dosing is lower and this is reasonably concordant with the lower reported incidence of side effects in clinical trials.
conclusion
Phytocannabinoids have emerged in recent years to be viewed as promising targets for the treatment of diseases where existing pharmacotherapeutic options may be limited to symptomatological treatment, such as in neurodegenerative disease. AD and PD have occupied a highlighted position globally due to the known rapidly aging world population, where The World Health Organization (WHO) has projected that by 2050, 152 million people will be affected by some type of dementia [165]. Phytocannabinoids are chemically and biologically diverse and possess interesting bioactive properties well suited to their development as novel treatments of such diseases. This includes both general antioxidant and anti-inflammatory, but also directly neuroprotective properties mediated via several distinct biochemical pathways. In many respects, cannabis and its constituent phytochemicals may only interact with limited elements of the ECS at the receptor level, and many potential interactions with the ECS are still to be determined. However, the in vitro complexity of interactions between cannabis phytocannabinoids hinted at in preclinical studies outlined here are likely to be even more complex in vivo, and likely to occur at both pharmacodynamic and pharmacokinetic levels, meaning there is still much research required to translate both effectiveness and safety clinically to dementia and other neurodegenerative disorders.
Acknowledgements
The authors are grateful to the Brazilian Agencies CNPq (#454088/2014-0, #400271/2014-1, #310082/2016-1), FAPEMIG (#CEX-APQ-00241-15), FINEP, INCT-INOFAR (#465.249/2014-0), PRPPG-UNIFAL for financial support and fellowships.
Consent for Publication
Not applicable.
Funding
None.
Conflict of Interest
The authors declare no conflict of interest, financial or otherwise.
REFERENCES
- 1.Robison R. O Grande Livro Da Cannabis. 1st ed. Vermont, USA: 1999. [Google Scholar]
- 2.Ligresti A., De Petrocellis L., Di Marzo V. From phytocannabinoids to cannabinoid receptors and endocannabinoids: Pleiotropic physiological and pathological roles through complex pharmacology. Physiol. Rev. 2016;96(4):1593–1659. doi: 10.1152/physrev.00002.2016. [DOI] [PubMed] [Google Scholar]
- 3.Gontijo C., Castro G.L., De Castro A.D. Canabidiol e suas aplicações terapêuticas. Refacer. 2016;5(1):1–9. [Google Scholar]
- 4.Scherma M., Masia P., Deidda M., Fratta W., Tanda G., Fadda P. New perspectives on the use of cannabis in the treatment of psychiatric disorders. Medicines (Basel) 2018;5:107. doi: 10.3390/medicines5040107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.National Academies of Sciences, Engineering, and Medicine. The Health Effects of Cannabis and Cannabinoids: The Current State of Evidence and Recommendations for Research. 1st ed. Washington, DC: The National Academies Press; 2017. [PubMed] [Google Scholar]
- 6.Sexton M., Shelton K., Haley P., West M. Evaluation of cannabinoid and terpenoid content: Cannabis flower compared to supercritical CO 2 concentrate. Planta Med. 2018;84:4. doi: 10.1055/s-0043-119361. [DOI] [PubMed] [Google Scholar]
- 7.Das S., Stark L., Musgrave I.F., Pukala T., Smid S.D. Bioactive polyphenol interactions with β amyloid: a comparison of binding modelling, effects on fibril and aggregate formation and neuroprotective capacity. Food Funct. 2016;7(2):1138–1146. doi: 10.1039/C5FO01281C. [DOI] [PubMed] [Google Scholar]
- 8.Russo E.B. Cannabis therapeutics and the future of neurology. Front. Integr. Nuerosci. 2018;12(October):51. doi: 10.3389/fnint.2018.00051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pertwee R. Pharmacological actions of cannabinoids. Eur. Neuropsychopharmacol. 2010;20:S205. doi: 10.1016/S0924-977X(10)70232-7. [DOI] [Google Scholar]
- 10.Mudge E.M., Murch S.J., Brown P.N. Chemometric analysis of cannabinoids: chemotaxonomy and domestication syndrome. Sci. Rep. 2018;8(1):13090. doi: 10.1038/s41598-018-31120-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lewis M.M., Yang Y., Wasilewski E., Clarke H.A., Kotra L.P. Chemical profiling of medical cannabis extracts. ACS Omega. 2017;2(9):6091–6103. doi: 10.1021/acsomega.7b00996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hanuš L.O., Meyer S.M., Muñoz E., Taglialatela-Scafati O., Appendino G. Phytocannabinoids: a unified critical inventory. Nat. Prod. Rep. 2016;33(12):1357–1392. doi: 10.1039/C6NP00074F. [DOI] [PubMed] [Google Scholar]
- 13.Franco R.R.G., Viegas C., Junior The contribution of studies with canabidiol and synthetic analogues in the design of new drug candidates for neuropsychiatric disorders and neurodegenerative diseases. Rev Virtual Química. 2017;9(4):1773–1798. doi: 10.21577/1984-6835.20170103. [DOI] [Google Scholar]
- 14.Abuhasira R., Schleider L.B.L., Mechoulam R., Novack V. Epidemiological characteristics, safety and efficacy of medical cannabis in the elderly. Eur. J. Intern. Med. 2018;49:44–50. doi: 10.1016/j.ejim.2018.01.019. [DOI] [PubMed] [Google Scholar]
- 15.Rosenberg E.C., Tsien R.W., Whalley B.J., Devinsky O. Cannabinoids and epilepsy. Neurotherapeutics. 2015;12(4):747–768. doi: 10.1007/s13311-015-0375-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Klumpers L.E., Thacker D.L. A brief background on cannabis: From plant to medical indications. J. AOAC Int. 2019;102(2):412–420. doi: 10.5740/jaoacint.18-0208. [DOI] [PubMed] [Google Scholar]
- 17.Pisanti S., Malfitano A.M., Ciaglia E., Lamberti A., Ranieri R., Cuomo G., Abate M., Faggiana G., Proto M.C., Fiore D., Laezza C., Bifulco M. Cannabidiol: State of the art and new challenges for therapeutic applications. Pharmacol. Ther. 2017;175:133–150. doi: 10.1016/j.pharmthera.2017.02.041. [DOI] [PubMed] [Google Scholar]
- 18.Bonini S.A., Premoli M., Tambaro S., Kumar A., Maccarinelli G., Memo M., Mastinu A. Cannabis sativa: A comprehensive ethnopharmacological review of a medicinal plant with a long history. J. Ethnopharmacol. 2018;227:300–315. doi: 10.1016/j.jep.2018.09.004. [DOI] [PubMed] [Google Scholar]
- 19.Páez J.A., Campillo N.E. Innovative therapeutic potential of cannabinoid receptors as targets in Alzheimer’s disease and less well-known diseases. Curr. Med. Chem. 2019;26(18):3300–3340. doi: 10.2174/0929867325666180226095132. [DOI] [PubMed] [Google Scholar]
- 20.Pellati F., Borgonetti V., Brighenti V., Biagi M., Benvenuti S., Corsi L. Cannabis sativa L. and nonpsychoactive cannabinoids : their chemistry and role against oxidative stress, inflammation, and cancer. BioMed Res. Int. 2018;2018:1691428. doi: 10.1155/2018/1691428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Maccarrone M. Missing pieces to the endocannabinoid puzzle. Trends Mol. Med. 2019;26(3):263–272. doi: 10.1016/j.molmed.2019.11.002. [DOI] [PubMed] [Google Scholar]
- 22.Ożarowski M., Mikolajczak P.Ł., Bogacz A., Bartkowiak-Wieczorek J., Kujawski R., Majchrzycki M., Wielgus K., Seremak-Mrozikiewicz A., Czerny B. Progress in study of Cannabis sativa leaves extracts without psychotropic cannabinoids in animal model of neuropathic pain. J Med Sci. 2016;83:328–335. [Google Scholar]
- 23.Zuardi A.W. History of cannabis as a medicine: a review. Br. J. Psychiatry. 2006;28(2):153–157. doi: 10.1590/S1516-44462006000200015. [DOI] [PubMed] [Google Scholar]
- 24.United Nations Single Convention on Narcotic Drugs. 1961.
- 25.Reekie T.A., Scott M.P., Kassiou M. The evolving science of phytocannabinoids. Nat. Rev. Chem. 2018;2(1):1–12. doi: 10.1038/s41570-017-0101. [DOI] [Google Scholar]
- 26.Nations U. Convention on Psychotropic Substances. 1971;1971:41. [Google Scholar]
- 27.Maurya N., Velmurugan B.K. Therapeutic applications of cannabinoids. Chem. Biol. Interact. 2018;293:77–88. doi: 10.1016/j.cbi.2018.07.018. [DOI] [PubMed] [Google Scholar]
- 28.Bloomfield M.A.P., Hindocha C., Green S.F., Wall M.B., Lees R., Petrilli K., Costello H., Ogunbiyi M.O., Bossong M.G., Freeman T.P. The neuropsychopharmacology of cannabis: A review of human imaging studies. Pharmacol. Ther. 2019;195:132–161. doi: 10.1016/j.pharmthera.2018.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Matos R.L.A., Spinola L.A., Barboza L.L., Garcia D.R., França T.C.C., Affonso R.S.O. Uso do canabidiol no tratamento da epilepsia. Rev Virtual Quim. 2017;9(2):786–814. doi: 10.21577/1984-6835.20170049. [DOI] [Google Scholar]
- 30.Turner S.E., Williams C.M., Iversen L., Whalley B.J. In: Phytocannabinoids. Progress in the Chemistry of Organic Natural Products; Kinghorn, A.; Falk, H.; Gibbons, S. Kobayashi J., editor. Vol. 103. Cham: Springer; 2017. pp. 62–91. [DOI] [PubMed] [Google Scholar]
- 31.Russo E.B. Beyond cannabis: plants and the endocannabinoid system. Trends Pharmacol. Sci. 2016;37(7):594–605. doi: 10.1016/j.tips.2016.04.005. [DOI] [PubMed] [Google Scholar]
- 32.Morales P., Hurst D.P., Reggio P.H. Molecular targets of the phytocannabinoids: a complex picture. Prog. Chem. Org. Nat. Prod. 2017;103:103–131. doi: 10.1007/978-3-319-45541-9_4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Russo E.B. Cannabidiol claims and misconceptions. Trends Pharmacol. Sci. 2017;38(3):198–201. doi: 10.1016/j.tips.2016.12.004. [DOI] [PubMed] [Google Scholar]
- 34.Pollastro F., Caprioglio D., Del Prete D., Rogati F., Minassi A., Taglialatela-Scafati O., Munoz E., Appendino G. Cannabichromene. Nat. Prod. Commun. 2018;13:1189–1194. doi: 10.1177/1934578X1801300922. [DOI] [Google Scholar]
- 35.Mechoulam R., Shani A., Edery H., Grunfeld Y. Chemical basis of hashish activity. Science. 1970;169(3945):611–612. doi: 10.1126/science.169.3945.611. [DOI] [PubMed] [Google Scholar]
- 36.Stern E., Lambert D.M. Medicinal chemistry endeavors around the phytocannabinoids. Chem. Biodivers. 2007;4(8):1707–1728. doi: 10.1002/cbdv.200790149. [DOI] [PubMed] [Google Scholar]
- 37.Di Marzo V., Piscitelli F. The endocannabinoid system and its modulation by phytocannabinoids. Neurotherapeutics. 2015;12(4):692–698. doi: 10.1007/s13311-015-0374-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Capasso A., Sobarzo-Sánchez E., Nabavi S.F., Rastrelli L. Cannabinoids for the treatment of schizophrenia: an overview. Curr. Top. Med. Chem. 2016;16(17):1916–1923. doi: 10.2174/1568026616666160204122033. [DOI] [PubMed] [Google Scholar]
- 39.Navarro G., Varani K., Reyes-Resina I., Sánchez de Medina V., Rivas-Santisteban R., Sánchez-Carnerero Callado C., Vincenzi F., Casano S., Ferreiro-Vera C., Canela E.I., Borea P.A., Nadal X., Franco R. Cannabigerol action at cannabinoid CB 1 and CB 2 receptors and at CB1-CB2 heteroreceptor complexes. Front. Pharmacol. 2018;9:632. doi: 10.3389/fphar.2018.00632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Valdeolivas S., Navarrete C., Cantarero I., Bellido M.L., Muñoz E., Sagredo O. Neuroprotective properties of cannabigerol in Huntington’s disease: studies in R6/2 mice and 3-nitropropionate-lesioned mice. Neurotherapeutics. 2015;12(1):185–199. doi: 10.1007/s13311-014-0304-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Aguareles J., Paraíso-Luna J., Palomares B., Bajo-Grañeras R., Navarrete C., Ruiz-Calvo A., García-Rincón D., García-Taboada E., Guzmán M., Muñoz E., Galve-Roperh I. Oral administration of the cannabigerol derivative VCE-003.2 promotes subventricular zone neurogenesis and protects against mutant huntingtin-induced neurodegeneration. Transl. Neurodegener. 2019;8:9. doi: 10.1186/s40035-019-0148-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mammana S., Cavalli E., Gugliandolo A., Silvestro S., Pollastro F., Bramanti P., Mazzon E. Could the combination of two non-psychotropic cannabinoids counteract neuroinflammation? Effectiveness of cannabidiol associated with cannabigerol. Medicina (Kaunas) 2019;55(11):1–14. doi: 10.3390/medicina55110747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pinzi L., Lherbet C., Baltas M., Pellati F., Rastelli G. In silico repositioning of cannabigerol as a novel inhibitor of the enoyl acyl carrier protein (ACP) reductase (INHA). Molecules. 2019;24(14):1–9. doi: 10.3390/molecules24142567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Díaz-Alonso J., Paraíso-Luna J., Navarrete C., Del Río C., Cantarero I., Palomares B., Aguareles J., Fernández-Ruiz J., Bellido M.L., Pollastro F., Appendino G., Calzado M.A., Galve-Roperh I., Muñoz E. VCE-003.2, a novel cannabigerol derivative, enhances neuronal progenitor cell survival and alleviates symptomatology in murine models of Huntington’s disease. Sci. Rep. 2016;6:29789. doi: 10.1038/srep29789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gugliandolo A., Pollastro F., Grassi G., Bramanti P., Mazzon E. In vitro model of neuroinflammation: Efficacy of cannabigerol, a non-psychoactive cannabinoid. Int. J. Mol. Sci. 2018;19(7):1–16. doi: 10.3390/ijms19071992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mechoulam R., Gaoni Y. Cannabichromene, a new active principle in hashish. Chem. Commun. 1966;1:20–21. [Google Scholar]
- 47.Udoh M., Santiago M., Devenish S., McGregor I.S., Connor M. Cannabichromene is a cannabinoid CB2 receptor agonist. Br. J. Pharmacol. 2019;176(23):4537–4547. doi: 10.1111/bph.14815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rosenthaler S., Pöhn B., Kolmanz C., Huu C.N., Krewenka C., Huber A., Kranner B., Rausch W.D., Moldzio R. Differences in receptor binding affinity of several phytocannabinoids do not explain their effects on neural cell cultures. Neurotoxicol. Teratol. 2014;46:49–56. doi: 10.1016/j.ntt.2014.09.003. [DOI] [PubMed] [Google Scholar]
- 49.Hatoum N.S., Davis W.M., Elsohly M.A., Turner C.E. Cannabichromene and delta 9-tetrahydrocannabinol: interactions relative to lethality, hypothermia and hexobarbital hypnosis. Gen. Pharmacol. 1981;12(5):357–362. doi: 10.1016/0306-3623(81)90090-2. [DOI] [PubMed] [Google Scholar]
- 50.Wirth P.W., Watson E.S., ElSohly M.A., Seidel R., Murphy J.C., Turner C.E. Anti-inflammatory activity of cannabichromene homologs. J. Pharm. Sci. 1980;69(11):1359–1360. doi: 10.1002/jps.2600691136. [DOI] [PubMed] [Google Scholar]
- 51.Petrosino S., Verde R., Vaia M., Allarà M., Iuvone T., Di Marzo V. Anti-inflammatory properties of cannabidiol, a nonpsychotropic cannabinoid, in experimental allergic contact dermatitis. J. Pharmacol. Exp. Ther. 2018;365(3):652–663. doi: 10.1124/jpet.117.244368. [DOI] [PubMed] [Google Scholar]
- 52.Iuvone T., Di Marzo V., Guy G., Wright S., Stott C. 2012.
- 53.Schubert D., Kepchia D., Liang Z., Dargusch R., Goldberg J., Maher P. Efficacy of cannabinoids in a pre-clinical drug-screening platform for Alzheimer’s disease. Mol. Neurobiol. 2019;56(11):7719–7730. doi: 10.1007/s12035-019-1637-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Breuer A., Haj C.G., Fogaça M.V., Gomes F.V., Silva N.R., Pedrazzi J.F., Del Bel E.A., Hallak J.C., Crippa J.A., Zuardi A.W., Mechoulam R., Guimarães F.S. Fluorinated cannabidiol derivatives: Enhancement of activity in mice models predictive of anxiolytic, antidepressant and antipsychotic effects. PLoS One. 2016;11:1–19. doi: 10.1371/journal.pone.0158779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Adams R., Hunt M., Clark J.H. Structure of cannabidiol, a product isolated from the marihuana extract of minnesota wild hemp. I. J. Am. Chem. Soc. 1939;62:196–200. doi: 10.1021/ja01858a058. [DOI] [Google Scholar]
- 56.Mechoulam R., Shvo Y., Hashish I. The structure of cannabidiol. Tetrahedron. 1963;19(12):2073–2078. doi: 10.1016/0040-4020(63)85022-X. [DOI] [PubMed] [Google Scholar]
- 57.Karl T., Garner B., Cheng D. The therapeutic potential of the phytocannabinoid cannabidiol for Alzheimer’s disease. 2017. [DOI] [PubMed]
- 58.Laprairie R.B., Bagher A.M., Kelly M.E.M., Denovan-Wright E.M. Cannabidiol is a negative allosteric modulator of the cannabinoid CB1 receptor. Br. J. Pharmacol. 2015;172(20):4790–4805. doi: 10.1111/bph.13250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ye L., Cao Z., Wang W., Zhou N. New insights in cannabinoid receptor structure and signaling. Curr. Mol. Pharmacol. 2019;12(3):239–248. doi: 10.2174/1874467212666190215112036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Di Marzo V. New approaches and challenges to targeting the endocannabinoid system. Nat. Rev. Drug Discov. 2018;17(9):623–639. doi: 10.1038/nrd.2018.115. [DOI] [PubMed] [Google Scholar]
- 61.O’Sullivan S.E. An update on PPAR activation by cannabinoids. Br. J. Pharmacol. 2016;173(12):1899–1910. doi: 10.1111/bph.13497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Resstel L.B.M., Tavares R.F., Lisboa S.F.S., Joca S.R.L., Corrêa F.M.A., Guimarães F.S. 5-HT1A receptors are involved in the cannabidiol-induced attenuation of behavioural and cardiovascular responses to acute restraint stress in rats. Br. J. Pharmacol. 2009;156(1):181–188. doi: 10.1111/j.1476-5381.2008.00046.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Cheng Y., Dong Z., Liu S. β-Caryophyllene ameliorates the Alzheimer-like phenotype in APP/PS1 Mice through CB2 receptor activation and the PPARγ pathway. Pharmacology. 2014;94(1-2):1–12. doi: 10.1159/000362689. [DOI] [PubMed] [Google Scholar]
- 64.Iuvone T., Esposito G., Esposito R., Santamaria R., Di Rosa M., Izzo A.A. Neuroprotective effect of cannabidiol, a non-psychoactive component from Cannabis sativa, on β-amyloid-induced toxicity in PC12 cells. J. Neurochem. 2004;89(1):134–141. doi: 10.1111/j.1471-4159.2003.02327.x. [DOI] [PubMed] [Google Scholar]
- 65.Watt G., Karl T. In vivo evidence for therapeutic properties of cannabidiol (CBD) for Alzheimer’s disease. Front. Pharmacol. 2017;8:20. doi: 10.3389/fphar.2017.00020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Janefjord E., Mååg J.L.V., Harvey B.S., Smid S.D. Cannabinoid effects on β amyloid fibril and aggregate formation, neuronal and microglial-activated neurotoxicity in vitro. Cell. Mol. Neurobiol. 2014;34(1):31–42. doi: 10.1007/s10571-013-9984-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Libro R., Diomede F., Scionti D., Piattelli A., Grassi G., Pollastro F., Bramanti P., Mazzon E., Trubiani O. Cannabidiol modulates the expression of alzheimer’s disease-related genes in mesenchymal stem cells. Int. J. Mol. Sci. 2016;18(1):1–19. doi: 10.3390/ijms18010026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Costiniuk C.T., Jenabian M.A. Cannabinoids and inflammation: implications for people living with HIV. AIDS. 2019;33(15):2273–2288. doi: 10.1097/QAD.0000000000002345. [DOI] [PubMed] [Google Scholar]
- 69.Scuderi C., Steardo L., Esposito G. Cannabidiol promotes amyloid precursor protein ubiquitination and reduction of beta amyloid expression in SHSY5YAPP+ cells through PPARγ involvement. Phytother. Res. 2014;28(7):1007–1013. doi: 10.1002/ptr.5095. [DOI] [PubMed] [Google Scholar]
- 70.Esposito G., Scuderi C., Valenza M., Togna G.I., Latina V., de Filippis D., Cipriano M., Carratù M.R., Iuvone T., Steardo L. Cannabidiol reduces Aβ-induced neuroinflammation and promotes hippocampal neurogenesis through PPARγ involvement. PLoS One. 2011;6:1–8. doi: 10.1371/journal.pone.0028668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Vallée A., Lecarpentier Y., Guillevin R., Vallée J.N. Effects of cannabidiol interactions with Wnt/β-catenin pathway and PPARγ on oxidative stress and neuroinflammation in Alzheimer’s disease. Acta Biochim. Biophys. Sin. (Shanghai) 2017;49(10):853–866. doi: 10.1093/abbs/gmx073. [DOI] [PubMed] [Google Scholar]
- 72.Martín-Moreno A.M., Reigada D., Ramírez B.G., Mechoulam R., Innamorato N., Cuadrado A., de Ceballos M.L. Cannabidiol and other cannabinoids reduce microglial activation in vitro and in vivo: relevance to Alzheimer’s disease. Mol. Pharmacol. 2011;79(6):964–973. doi: 10.1124/mol.111.071290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Crippa J.A.S., Hallak J.E.C., Zuardi A.W., Guimarães F.S., Tumas V., Dos Santos R.G. Is cannabidiol the ideal drug to treat non-motor Parkinson’s disease symptoms? Eur. Arch. Psychiatry Clin. Neurosci. 2019;269(1):121–133. doi: 10.1007/s00406-019-00982-6. [DOI] [PubMed] [Google Scholar]
- 74.Premoli M., Aria F., Bonini S.A., Maccarinelli G., Gianoncelli A., Pina S.D., Tambaro S., Memo M., Mastinu A. Cannabidiol: Recent advances and new insights for neuropsychiatric disorders treatment. Life Sci. 2019;224:120–127. doi: 10.1016/j.lfs.2019.03.053. [DOI] [PubMed] [Google Scholar]
- 75.Crivelaro do Nascimento G., Ferrari D.P., Guimaraes F.S., Del Bel E.A., Bortolanza M., Ferreira-Junior N.C. Cannabidiol increases the nociceptive threshold in a preclinical model of Parkinson’s disease. Neuropharmacology. 2020;163:107808. doi: 10.1016/j.neuropharm.2019.107808. [DOI] [PubMed] [Google Scholar]
- 76.Peres F.F., Levin R., Suiama M.A., Diana M.C., Gouvêa D.A., Almeida V., Santos C.M., Lungato L., Zuardi A.W., Hallak J.E.C., Crippa J.A., Vânia D., Silva R.H., Abílio V.C. Cannabidiol prevents motor and cognitive impairments induced by reserpine in rats. Front. Pharmacol. 2016;7:343. doi: 10.3389/fphar.2016.00343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hacke A.C.M., Lima D., de Costa F., Deshmukh K., Li N., Chow A.M., Marques J.A., Pereira R.P., Kerman K. Probing the antioxidant activity of Δ9-tetrahydrocannabinol and cannabidiol in Cannabis sativa extracts. Analyst (Lond.) 2019;144(16):4952–4961. doi: 10.1039/C9AN00890J. [DOI] [PubMed] [Google Scholar]
- 78.Huestis M.A., Solimini R., Pichini S., Pacifici R., Carlier J., Busardò F.P. Cannabidiol adverse effects and toxicity. Curr. Neuropharmacol. 2019;17(10):974–989. doi: 10.2174/1570159X17666190603171901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lucas C.J., Galettis P., Schneider J. The pharmacokinetics and the pharmacodynamics of cannabinoids. Br. J. Clin. Pharmacol. 2018;84(11):2477–2482. doi: 10.1111/bcp.13710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Izzo L., Castaldo L., Narváez A., Graziani G., Gaspari A., Rodríguez-Carrasco Y., Ritieni A. Analysis of phenolic compounds in commercial Cannabis sativa L. Inflorescences Using UHPLC-Q-Orbitrap HRMS. Molecules. 2020;25(3):1–12. doi: 10.3390/molecules25030631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kinney W.A., McDonnell M.E., Zhong H.M., Liu C., Yang L., Ling W., Qian T., Chen Y., Cai Z., Petkanas D., Brenneman D.E. Discovery of KLS-13019, a cannabidiol-derived neuroprotective agent, with improved potency, safety, and permeability. ACS Med. Chem. Lett. 2016;7(4):424–428. doi: 10.1021/acsmedchemlett.6b00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Velasco G., Hernández-Tiedra S., Dávila D., Lorente M. The use of cannabinoids as anticancer agents. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2016;64:259–266. doi: 10.1016/j.pnpbp.2015.05.010. [DOI] [PubMed] [Google Scholar]
- 83.Wong H., Cairns B.E. Cannabidiol, cannabinol and their combinations act as peripheral analgesics in a rat model of myofascial pain. Arch. Oral Biol. 2019;104:33–39. doi: 10.1016/j.archoralbio.2019.05.028. [DOI] [PubMed] [Google Scholar]
- 84.Wong H., Hossain S., Cairns B.E. Delta-9-tetrahydrocannabinol decreases masticatory muscle sensitization in female rats through peripheral cannabinoid receptor activation. Eur. J. Pain. 2017;21(10):1732–1742. doi: 10.1002/ejp.1085. [DOI] [PubMed] [Google Scholar]
- 85.Carroll C.B., Zeissler M.L., Hanemann C.O., Zajicek J.P.Δ.Δ. 9-tetrahydrocannabinol (Δ9-THC) exerts a direct neuroprotective effect in a human cell culture model of Parkinson’s disease. Neuropathol. Appl. Neurobiol. 2012;38(6):535–547. doi: 10.1111/j.1365-2990.2011.01248.x. [DOI] [PubMed] [Google Scholar]
- 86.Nguyen C.H., Krewenka C., Radad K., Kranner B., Huber A., Duvigneau J.C., Miller I., Moldzio R. THC (Δ9-Tetrahydrocannabinol) exerts neuroprotective effect in glutamate-affected murine primary mesencephalic cultures through restoring mitochondrial membrane potential and anti-apoptosis involving CB1 receptor-dependent mechanism. Phytother. Res. 2016;30(12):2044–2052. doi: 10.1002/ptr.5712. [DOI] [PubMed] [Google Scholar]
- 87.Chen R., Zhang J., Fan N., Teng Z.Q., Wu Y., Yang H., Tang Y.P., Sun H., Song Y., Chen C.Δ. 9-THC-caused synaptic and memory impairments are mediated through COX-2 signaling. Cell. 2013;155(5):1154–1165. doi: 10.1016/j.cell.2013.10.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Aso E., Sánchez-Pla A., Vegas-Lozano E., Maldonado R., Ferrer I. Cannabis-based medicine reduces multiple pathological processes in AβPP/PS1 mice. J. Alzheimers Dis. 2015;43(3):977–991. doi: 10.3233/JAD-141014. [DOI] [PubMed] [Google Scholar]
- 89.Bilkei-Gorzo A., Albayram O., Draffehn A., Michel K., Piyanova A., Oppenheimer H., Dvir-Ginzberg M., Rácz I., Ulas T., Imbeault S., Bab I., Schultze J.L., Zimmer A. A chronic low dose of Δ9-tetrahydrocannabinol (THC) restores cognitive function in old mice. Nat. Med. 2017;23(6):782–787. doi: 10.1038/nm.4311. [DOI] [PubMed] [Google Scholar]
- 90.McPartland J.M., Duncan M., Di Marzo V., Pertwee R.G. Are cannabidiol and Δ(9) -tetrahydrocannabivarin negative modulators of the endocannabinoid system? A systematic review. Br. J. Pharmacol. 2015;172(3):737–753. doi: 10.1111/bph.12944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.García C., Palomo-Garo C., García-Arencibia M., Ramos J., Pertwee R., Fernández-Ruiz J. Symptom-relieving and neuroprotective effects of the phytocannabinoid Δ9-THCV in animal models of Parkinson’s disease. Br. J. Pharmacol. 2011;163(7):1495–1506. doi: 10.1111/j.1476-5381.2011.01278.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Janssens A., Silvestri C., Martella A., Vanoevelen J.M., Di Marzo V., Voets T.Δ. 9-tetrahydrocannabivarin impairs epithelial calcium transport through inhibition of TRPV5 and TRPV6. Pharmacol. Res. 2018;136:83–89. doi: 10.1016/j.phrs.2018.08.021. [DOI] [PubMed] [Google Scholar]
- 93.Deiana S., Watanabe A., Yamasaki Y., Amada N., Arthur M., Fleming S., Woodcock H., Dorward P., Pigliacampo B., Close S., Platt B., Riedel G. Plasma and brain pharmacokinetic profile of cannabidiol (CBD), cannabidivarine (CBDV), Δ9-tetrahydrocannabivarin (THCV) and cannabigerol (CBG) in rats and mice following oral and intraperitoneal administration and CBD action on obsessive-compulsive behaviour. Psychopharmacology (Berl.) 2012;219(3):859–873. doi: 10.1007/s00213-011-2415-0. [DOI] [PubMed] [Google Scholar]
- 94.Vree T.B., Breimer D.D., van Ginneken C.A.M., van Rossum J.M. Identification of cannabicyclol with a pentyl or propyl side-chain by means of combined gas chromatography-mass spectrometry. J. Chromatogr. A. 1972;74(1):124–127. doi: 10.1016/S0021-9673(01)94980-5. [DOI] [PubMed] [Google Scholar]
- 95.Kane V.V. Structure of cannabicyclol, a detailed NMR study of a synthetic analog. Tetrahedron Lett. 1971;216:4101–4104. doi: 10.1016/S0040-4039(01)97472-6. [DOI] [Google Scholar]
- 96.Korte F., Sieper H. Zur chemischen Klassifizierung von Pflanzen, XX Isolierung von Haschisch‐inhaltsstoffen Aus Cannabis Sativa non Indica. Justus Liebigs Ann. Chem. 1964;13:90–98. doi: 10.1016/s0021-9673(01)95077-0. [DOI] [PubMed] [Google Scholar]
- 97.Yamamoto I., Gohda H., Narimatsu S., Yoshimura H. Identification of cannabielsoin, a new metabolite of cannabidiol formed by guinea-pig hepatic microsomal enzymes, and its pharmacological activity in mice. Chem. Pharm. Bull. (Tokyo) 1988;34:833–838. doi: 10.1248/bpb1978.11.833. [DOI] [PubMed] [Google Scholar]
- 98.Shani A., Mechoulam R. Cannabielsoic acids. Isolation and synthesis by a novel oxidative cyclization. Tetrahedron. 1974;30:2437–2446. doi: 10.1016/S0040-4020(01)97114-5. [DOI] [Google Scholar]
- 99.Obata Y., Ishikawa Y. Studies on the constituents of hemp plant (cannabis sativa l.). Agric. Biol. Chem. 1966;30:619–620. doi: 10.1271/bbb1961.30.619. [DOI] [Google Scholar]
- 100.Chan W.R., Magnus K.E., Watson H.A. The strucuture of cannabitriol. Specialia. 1975;7:283–284. doi: 10.1007/BF01940792. [DOI] [PubMed] [Google Scholar]
- 101.Pacher P., Kogan N.M., Mechoulam R. Beyond THC and endocannabinoids. Annu. Rev. Pharmacol. Toxicol. 2020;60:637–659. doi: 10.1146/annurev-pharmtox-010818-021441. [DOI] [PubMed] [Google Scholar]
- 102.Cassano T., Villani R., Pace L., Carbone A., Bukke V.N., Orkisz S., Avolio C., Serviddio G. From Cannabis sativa to Cannabidiol: Promising therapeutic candidate for the treatment of neurodegenerative diseases. Front. Pharmacol. 2020;11:124. doi: 10.3389/fphar.2020.00124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Aso E., Juvés S., Maldonado R., Ferrer I. CB2 cannabinoid receptor agonist ameliorates Alzheimer-like phenotype in AβPP/PS1 mice. J. Alzheimers Dis. 2013;35(4):847–858. doi: 10.3233/JAD-130137. [DOI] [PubMed] [Google Scholar]
- 104.Zuardi A.W. Cannabidiol: from an inactive cannabinoid to a drug with wide spectrum of action. Br. J. Psychiatry. 2008;30(3):271–280. doi: 10.1590/S1516-44462008000300015. [DOI] [PubMed] [Google Scholar]
- 105.Campos H.C., da Rocha M.D., Viegas F.P.D., Nicastro P.C., Fossaluzza P.C., Fraga C.A.M., Barreiro E.J., Viegas C., Jr The role of natural products in the discovery of new drug candidates for the treatment of neurodegenerative disorders I: Parkinson’s disease. CNS Neurol. Disord. Drug Targets. 2011;10(2):239–250. doi: 10.2174/187152711794480483. [DOI] [PubMed] [Google Scholar]
- 106.Bloomfield M.A.P., Ashok A.H., Volkow N.D., Howes O.D. The effects of Δ9-tetrahydrocannabinol on the dopamine system. Nature. 2016;539(7629):369–377. doi: 10.1038/nature20153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Deiana S. Medical use of cannabis. Cannabidiol: a new light for schizophrenia? Drug Test. Anal. 2013;5(1):46–51. doi: 10.1002/dta.1425. [DOI] [PubMed] [Google Scholar]
- 108.Sartim A.G., Moreira F.A., Joca S.R.L. Involvement of CB1 and TRPV1 receptors located in the ventral medial prefrontal cortex in the modulation of stress coping behavior. Neuroscience. 2017;340:126–134. doi: 10.1016/j.neuroscience.2016.10.031. [DOI] [PubMed] [Google Scholar]
- 109.Sales A.J., Crestani C.C., Guimarães F.S., Joca S.R.L. Antidepressant-like effect induced by Cannabidiol is dependent on brain serotonin levels. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2018;86:255–261. doi: 10.1016/j.pnpbp.2018.06.002. [DOI] [PubMed] [Google Scholar]
- 110.Nadal X., Del Río C., Casano S., Palomares B., Ferreiro-Vera C., Navarrete C., Sánchez-Carnerero C., Cantarero I., Bellido M.L., Meyer S., Morello G., Appendino G., Muñoz E. Tetrahydrocannabinolic acid is a potent PPARγ agonist with neuroprotective activity. Br. J. Pharmacol. 2017;174(23):4263–4276. doi: 10.1111/bph.14019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Doty K.R., Guillot-Sestier M.V., Town T. The role of the immune system in neurodegenerative disorders: Adaptive or maladaptive? Brain Res. 2015;1617:155–173. doi: 10.1016/j.brainres.2014.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Aliseychik M.P., Andreeva T.V., Rogaev E.I. Immunogenetic Factors of Neurodegenerative Diseases: The Role of HLA Class II. Biochemistry (Mosc.) 2018;83(9):1104–1116. doi: 10.1134/S0006297918090122. [DOI] [PubMed] [Google Scholar]
- 113.Zou S., Kumar U. Cannabinoid receptors and the endocannabinoid system: Signaling and function in the central nervous system. Int. J. Mol. Sci. 2018;19(3):1–23. doi: 10.3390/ijms19030833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Zou M., Li D., Li L., Wu L., Sun C. Role of the endocannabinoid system in neurological disorders. Int. J. Dev. Neurosci. 2019;76:95–102. doi: 10.1016/j.ijdevneu.2019.03.002. [DOI] [PubMed] [Google Scholar]
- 115.Bisogno T., Oddi S., Piccoli A., Fazio D., Maccarrone M. Type-2 cannabinoid receptors in neurodegeneration. Pharmacol. Res. 2016;111:721–730. doi: 10.1016/j.phrs.2016.07.021. [DOI] [PubMed] [Google Scholar]
- 116.Iannotti F.A., Di Marzo V., Petrosino S. Endocannabinoids and endocannabinoid-related mediators: Targets, metabolism and role in neurological disorders. Prog. Lipid Res. 2016;62:107–128. doi: 10.1016/j.plipres.2016.02.002. [DOI] [PubMed] [Google Scholar]
- 117.Lossignol D. Cannabinoids: a new approach for pain control? Curr. Opin. Oncol. 2019;31(4):275–279. doi: 10.1097/CCO.0000000000000523. [DOI] [PubMed] [Google Scholar]
- 118.Pertwee R.G., Howlett A.C., Abood M.E., Alexander S.P.H., Di Marzo V., Elphick M.R., Greasley P.J., Hansen H.S., Kunos G. Cannabinoid receptors and their ligands : beyond cb 1 and cb 2. Pharmacol. Rev. 2010;62:588–631. doi: 10.1124/pr.110.003004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Shao Z., Yin J., Chapman K., Grzemska M., Clark L., Wang J., Rosenbaum D.M. High-resolution crystal structure of the human CB1 cannabinoid receptor. Nature. 2016;540(7634):602–606. doi: 10.1038/nature20613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Hua T., Vemuri K., Pu M., Qu L., Han G.W., Wu Y., Zhao S., Shui W., Li S., Korde A., Laprairie R.B., Stahl E.L., Ho J.H., Zvonok N., Zhou H., Kufareva I., Wu B., Zhao Q., Hanson M.A., Bohn L.M., Makriyannis A., Stevens R.C., Liu Z.J. Crystal structure of the human cannabinoid receptor CB1. Cell. 2016;167(3):750–762.e14. doi: 10.1016/j.cell.2016.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Li X., Hua T., Vemuri K., Ho J.H., Wu Y., Wu L., Popov P., Benchama O., Zvonok N., Locke K., Qu L., Han G.W., Iyer M.R., Cinar R., Coffey N.J., Wang J., Wu M., Katritch V., Zhao S., Kunos G., Bohn L.M., Makriyannis A., Stevens R.C., Liu Z.J. Crystal structure of the human cannabinoid receptor cb2. Cell. 2019;176(3):459–467.e13. doi: 10.1016/j.cell.2018.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Temple L.M. Medical marijuana and pain management. Dis. Mon. 2016;62(9):346–352. doi: 10.1016/j.disamonth.2016.05.014. [DOI] [PubMed] [Google Scholar]
- 123.Wu J. Cannabis, cannabinoid receptors, and endocannabinoid system: yesterday, today, and tomorrow. Acta Pharmacol. Sin. 2019;40(3):297–299. doi: 10.1038/s41401-019-0210-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.D’Addario C., Di Francesco A., Pucci M., Finazzi Agrò A., Maccarrone M. Epigenetic mechanisms and endocannabinoid signalling. FEBS J. 2013;280(9):1905–1917. doi: 10.1111/febs.12125. [DOI] [PubMed] [Google Scholar]
- 125.Chanda D., Neumann D., Glatz J.F.C. The endocannabinoid system: Overview of an emerging multi-faceted therapeutic target. Prostaglandins Leukot. Essent. Fatty Acids. 2019;140:51–56. doi: 10.1016/j.plefa.2018.11.016. [DOI] [PubMed] [Google Scholar]
- 126.Paloczi J., Varga Z.V., Hasko G., Pacher P. Neuroprotection in Oxidative stress-related neurodegenerative diseases: role of endocannabinoid system modulation. Antioxid. Redox Signal. 2018;29(1):75–108. doi: 10.1089/ars.2017.7144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Nielsen J.E., Rolland A.D., Rajpert-De Meyts E., Janfelt C., Jørgensen A., Winge S.B., Kristensen D.M., Juul A., Chalmel F., Jégou B., Skakkebaek N.E. Characterisation and localisation of the endocannabinoid system components in the adult human testis. Sci. Rep. 2019;9(1):12866. doi: 10.1038/s41598-019-49177-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Malfitano A.M., Basu S., Maresz K., Bifulco M., Dittel B.N. What we know and do not know about the cannabinoid receptor 2 (CB2). Semin. Immunol. 2014;26(5):369–379. doi: 10.1016/j.smim.2014.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Luis J., Costa G.P., Maia L.O., Villares J.C., Fernandez M. Neurobiology of cannabis: from the endocannabinoid system to cannabis-related disorder. J. Bras. Psiquiatr. 2011;60:111–122. doi: 10.1590/S0047-20852011000200006. [DOI] [Google Scholar]
- 130.Battista N., Di Tommaso M., Bari M., Maccarrone M. The endocannabinoid system: an overview. Front. Behav. Neurosci. 2012;6:9. doi: 10.3389/fnbeh.2012.00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Fezza F., Maccarrone M. 2014. [Google Scholar]
- 132.Han Q-W., Yuan Y-H., Chen N-H. The therapeutic role of cannabinoid receptors and its agonists or antagonists in Parkinson’s disease. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2020;96:109745. doi: 10.1016/j.pnpbp.2019.109745. [DOI] [PubMed] [Google Scholar]
- 133.Di Marzo V. Targeting the endocannabinoid system: to enhance or reduce? Nat. Rev. Drug Discov. 2008;7(5):438–455. doi: 10.1038/nrd2553. [DOI] [PubMed] [Google Scholar]
- 134.Fowler C.J., Doherty P., Alexander S.P.H. 2017. [DOI] [PubMed]
- 135.Andre C.M., Hausman J.F., Guerriero G. Cannabis sativa: The plant of the thousand and one molecules. Front. Plant Sci. 2016;7:19. doi: 10.3389/fpls.2016.00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Allegrone G., Pollastro F., Magagnini G., Taglialatela-Scafati O., Seegers J., Koeberle A., Werz O., Appendino G. The Bibenzyl canniprene inhibits the production of pro-inflammatory eicosanoids and selectively accumulates in some cannabis sativa strains. J. Nat. Prod. 2017;80(3):731–734. doi: 10.1021/acs.jnatprod.6b01126. [DOI] [PubMed] [Google Scholar]
- 137.Valera E., Dargusch R., Maher P.A., Schubert D. Modulation of 5-lipoxygenase in proteotoxicity and Alzheimer’s disease. J. Neurosci. 2013;33(25):10512–10525. doi: 10.1523/JNEUROSCI.5183-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Barrett M.L., Gordon D., Evans F.J. Isolation from Cannabis sativa L. of cannflavin--a novel inhibitor of prostaglandin production. Biochem. Pharmacol. 1985;34(11):2019–2024. doi: 10.1016/0006-2952(85)90325-9. [DOI] [PubMed] [Google Scholar]
- 139.Werz O., Seegers J., Schaible A.M., Weinigel C., Barz D., Koeberle A., Allegrone G., Pollastro F., Zampieri L., Grassi G., Appendino G. Cannflavins from hemp sprouts, a novel cannabinoid-free hemp food product, target microsomal prostaglandin E2 synthase-1 and 5-lipoxygenase. PharmaNutrition. 2014;2:53–60. doi: 10.1016/j.phanu.2014.05.001. [DOI] [Google Scholar]
- 140.Marsh D.T., Das S., Ridell J., Smid S.D. Structure-activity relationships for flavone interactions with amyloid β reveal a novel anti-aggregatory and neuroprotective effect of 2′,3′,4′-trihydroxy-flavone (2-D08). Bioorg. Med. Chem. 2017;25(14):3827–3834. doi: 10.1016/j.bmc.2017.05.041. [DOI] [PubMed] [Google Scholar]
- 141.Bieschke J., Russ J., Friedrich R.P., Ehrnhoefer D.E., Wobst H., Neugebauer K., Wanker E.E. EGCG remodels mature α-synuclein and amyloid-β fibrils and reduces cellular toxicity. Proc. Natl. Acad. Sci. USA. 2010;107(17):7710–7715. doi: 10.1073/pnas.0910723107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Eggers C., Fujitani M., Kato R., Smid S. Novel cannabis flavonoid, cannflavin A displays both a hormetic and neuroprotective profile against amyloid β-mediated neurotoxicity in PC12 cells: Comparison with geranylated flavonoids, mimulone and diplacone. Biochem. Pharmacol. 2019;169:113609. doi: 10.1016/j.bcp.2019.08.011. [DOI] [PubMed] [Google Scholar]
- 143.Moreau M., Ibeh U., Decosmo K., Bih N., Yasmin-Karim S., Toyang N., Lowe H., Ngwa W. Flavonoid derivative of cannabis demonstrates therapeutic potential in preclinical models of metastatic pancreatic cancer. Front. Oncol. 2019;9:660. doi: 10.3389/fonc.2019.00660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Lewis M.A., Russo E.B., Smith K.M. Pharmacological foundations of cannabis chemovars. Planta Med. 2018;84(4):225–233. doi: 10.1055/s-0043-122240. [DOI] [PubMed] [Google Scholar]
- 145.Russo E.B. Taming THC: potential cannabis synergy and phytocannabinoid-terpenoid entourage effects. Br. J. Pharmacol. 2011;163(7):1344–1364. doi: 10.1111/j.1476-5381.2011.01238.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Russo E.B., Marcu J. Cannabis pharmacology: the usual suspects and a few promising leads. Adv. Pharmacol. 2017;80:67–134. doi: 10.1016/bs.apha.2017.03.004. [DOI] [PubMed] [Google Scholar]
- 147.Santiago M., Sachdev S., Arnold J.C., McGregor I.S., Connor M. Absence of entourage: terpenoids commonly found in cannabis sativa do not modulate the functional activity of Δ9-THC at Human CB1 and CB2 receptors. Cannabis Cannabinoid Res. 2019;4(3):165–176. doi: 10.1089/can.2019.0016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Porres-Martínez M., González-Burgos E., Carretero M.E., Gómez-Serranillos M.P. In vitro neuroprotective potential of the monoterpenes α-pinene and 1,8-cineole against H2O2-induced oxidative stress in PC12 cells. Z. Natforsch. C J. Biosci. 2016;71(7-8):191–199. doi: 10.1515/znc-2014-4135. [DOI] [PubMed] [Google Scholar]
- 149.Shanmuganathan B., Suryanarayanan V., Sathya S., Narenkumar M., Singh S.K., Ruckmani K., Pandima Devi K. Anti-amyloidogenic and anti-apoptotic effect of α-bisabolol against Aβ induced neurotoxicity in PC12 cells. Eur. J. Med. Chem. 2018;143:1196–1207. doi: 10.1016/j.ejmech.2017.10.017. [DOI] [PubMed] [Google Scholar]
- 150.Nagamatsu R., Mitsuhashi S., Shigetomi K., Ubukata M. Cleavage of α-dicarbonyl compounds by terpene hydroperoxide. Biosci. Biotechnol. Biochem. 2012;76(10):1904–1908. doi: 10.1271/bbb.120378. [DOI] [PubMed] [Google Scholar]
- 151.Santos N.A.G., Martins N.M., Sisti F.M., Fernandes L.S., Ferreira R.S., de Freitas O., Santos A.C. The cannabinoid beta-caryophyllene (BCP) induces neuritogenesis in PC12 cells by a cannabinoid-receptor-independent mechanism. Chem. Biol. Interact. 2017;261:86–95. doi: 10.1016/j.cbi.2016.11.015. [DOI] [PubMed] [Google Scholar]
- 152.Cheng D., Spiro A.S., Jenner A.M., Garner B., Karl T. Long-term cannabidiol treatment prevents the development of social recognition memory deficits in Alzheimer’s disease transgenic mice. J. Alzheimers Dis. 2014;42(4):1383–1396. doi: 10.3233/JAD-140921. [DOI] [PubMed] [Google Scholar]
- 153.Jeyakumar M., Sathya S., Gandhi S., Tharra P., Suryanarayanan V., Singh S.K., Baire B., Pandima Devi K. α-bisabolol β-D-fucopyranoside as a potential modulator of β-amyloid peptide induced neurotoxicity: An in vitro &in silico study. Bioorg. Chem. 2019;88:102935. doi: 10.1016/j.bioorg.2019.102935. [DOI] [PubMed] [Google Scholar]
- 154.Shin M., Liu Q.F., Choi B., Shin C., Lee B., Yuan C., Song Y.J., Yun H.S., Lee I-S., Koo B-S., Cho K.S. Neuroprotective effects of limonene (+) against Aβ42-induced neurotoxicity in a Drosophila model of Alzheimer’s disease. Biol. Pharm. Bull. 2020;43(3):409–417. doi: 10.1248/bpb.b19-00495. [DOI] [PubMed] [Google Scholar]
- 155.Moreno-Sanz G. Can you pass the acid test? critical review and novel therapeutic perspectives of Δ9-Tetrahydrocannabinolic acid A. Cannabis Cannabinoid Res. 2016;1(1):124–130. doi: 10.1089/can.2016.0008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Hillen J.B., Soulsby N., Alderman C., Caughey G.E. Safety and effectiveness of cannabinoids for the treatment of neuropsychiatric symptoms in dementia: a systematic review. Ther. Adv. Drug Saf. 2019;102:042098619846993. doi: 10.1177/2042098619846993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Lim K., See Y.M., Lee J. A systematic review of the effectiveness of medical cannabis for psychiatric, movement and neurodegenerative disorders. Clin. Psychopharmacol. Neurosci. 2017;15(4):301–312. doi: 10.9758/cpn.2017.15.4.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Carroll CB, Bain PG, Teare L, Liu X, Joint C, Wroath C, Parkin S.G, Fox P, Wright D, Hobart J. 2004. [DOI] [PubMed]
- 159.Chagas M.H., Zuardi A.W., Tumas V., Pena-Pereira M.A., Sobreira E.T., Bergamaschi M.M., dos Santos A.C., Teixeira A.L., Hallak J.E., Crippa J.A. Effects of cannabidiol in the treatment of patients with Parkinson’s disease: an exploratory double-blind trial. J. Psychopharmacol. (Oxford) 2014;28(11):1088–1098. doi: 10.1177/0269881114550355. [DOI] [PubMed] [Google Scholar]
- 160.Elsaid S., Kloiber S., Le Foll B. Effects of cannabidiol (CBD) in neuropsychiatric disorders: A review of pre-clinical and clinical findings. Prog. Mol. Biol. Transl. Sci. 2019;167:25–75. doi: 10.1016/bs.pmbts.2019.06.005. [DOI] [PubMed] [Google Scholar]
- 161.Shelef A., Barak Y., Berger U., Paleacu D., Tadger S., Plopsky I., Baruch Y. Safety and efficacy of medical cannabis oil for behavioral and psychological symptoms of dementia: an-open label, add-on, pilot study. J. Alzheimers Dis. 2016;51(1):15–19. doi: 10.3233/JAD-150915. [DOI] [PubMed] [Google Scholar]
- 162.López-Sendón Moreno J.L., García Caldentey J., Trigo Cubillo P., Ruiz Romero C., García Ribas G., Alonso Arias M.A.A., García de Yébenes M.J., Tolón R.M., Galve-Roperh I., Sagredo O., Valdeolivas S., Resel E., Ortega-Gutierrez S., García-Bermejo M.L., Fernández Ruiz J., Guzmán M., García de Yébenes Prous J. A double-blind, randomized, cross-over, placebo-controlled, pilot trial with Sativex in Huntington’s disease. J. Neurol. 2016;263(7):1390–1400. doi: 10.1007/s00415-016-8145-9. [DOI] [PubMed] [Google Scholar]
- 163.Hudson R., Renard J., Norris C., Rushlow W.J., Laviolette S.R. Cannabidiol counteracts the psychotropic side-effects of Δ9-tetrahydrocannabinol in the ventral hippocampus through bidirectional control of ERK1–2 phosphorylation. J. Neurosci. 2019;39(44):8762–8777. doi: 10.1523/JNEUROSCI.0708-19.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Niesink R.J.M., van Laar M.W. Does cannabidiol protect against adverse psychological effects of THC? Front. Psychiatry. 2013;4:130–130. doi: 10.3389/fpsyt.2013.00130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.OPAS/OMS Brasil Demência: número de pessoas afetadas triplicará nos próximos 30 anos. https://www.paho.org/bra/index.php? option=com_content&view=article&id=5560:demencia-numero-de-pessoas-afetadas-triplicara-nos-proximos-30-anos&Itemid=839