Abstract
The use of neuroprotective agents for stroke is pathogenetically justified, but the translation of the results of preclinical studies of neuroprotectors into clinical practice has been a noticeable failure. One of the leading reasons for these failures is the one-target mechanism of their activity. p-Tyrosol (Tyr), a biophenol, is present in a variety of natural sources, mainly in foods, such as olive oil and wine. Tyr has a wide spectrum of biological activity: antioxidant, stress-protective, anti-inflammatory, anticancer, cardioprotective, neuroprotective and many others. This review analyzes data on the neuroprotective, antioxidant, anti-inflammatory, anti-apoptotic and other kinds of Tyr activity as well as data on the pharmacokinetics of the substance. The data presented in the review substantiate the acceptability of tyr as the basis for the development of a new neuroprotective drug with multitarget activity for the treatment of ischemic stroke. Tyr is a promising molecule for the development of an effective neuroprotective agent for use in ischemic stroke.
Keywords: Ischemic stroke, p-tyrosol, neuroprotective activity, antioxidant activity, anti-inflammatory activity, anti-apoptotic activity, pharmacokinetics
1. INTRODUCTION
Acute ischemic stroke is a leading contributor to morbidity and mortality worldwide. Consequently, developing more effective approaches for stroke prevention and therapy is a key objective in medical research [1]. Two main therapeutic strategies are used in the acute phase of stroke: recanalization of the occluded vessel and reperfusion of the microvasculature (thrombolytic therapy/thromboextraction) and blockade of biochemical (metabolic) changes involved in the ischemic cascade (neuroprotection) [2, 3].
Many clinical and preclinical studies of neuroprotection in stroke have been completed; however, few studies have shown clinical benefit [3, 4]. Nevertheless, neuroprotection in stroke remains promising, with many avenues to increase the effectiveness of ischemic stroke therapy [5]. One of the leading reasons that many neuroprotectors have failed is the one-target mechanism of their activity (antioxidant and/or free radical scavenger, Ca2+ channel blocker, N-methyl-d-aspartate (NMDA) receptor antagonist) [5, 6]. Successful future stroke therapy should focus on several pathophysiological mechanisms, including reduction of tissue-type plasminogen activator-related side effects, prevention of cell death, stimulation of neuroregeneration, and plasticity [6, 7]. There is an opinion that this multitarget approach can be achieved with a single neuroprotective drug [6, 8].
A candidate for the role of such a multitarget drug is p-tyrosol (Tyr), which has a wide spectrum of pharmacological activities: stress-protective [9, 10], anti-depressant [9, 10,], antioxidant [see below], anti-inflammatory[see below], anticancer [11], geroprotective [12] cardioprotective [13-15], anti-atherogenic [16, 17], antigenotoxic [18], anti-platelet and hemorheological [19, 20], neuroprotective [see below] and other types of activities.
The purpose of this review is to analyze the scientific research on Tyr related to the properties of this compound that are significant when choosing this substance as the basis for the development of a new neuroprotective agent with multitarget activity.
2. SOURCES OF TYR
Tyr (2-(4-hydroxyphenyl)ethanol) is present in a variety of natural sources [21-25]. The principal sources of Tyr are olive oil and wine [26, 27]. Methods have been developed for the synthesis of Tyr with a high yield of product of high-purity (> 99%) [28], which makes it possible to develop a dosage form of Tyr for injection.
3. NEUROPROTECTIVE ACTIVITY OF TYR
The neuroprotective properties of Tyr have been studied in vitro on cell lines and brain sections and in vivo in rat cerebral ischemic models (Table 1). Pre-incubation with Tyr protected the human astrocytoma U373 MG cell line against oxidative stress following Fenton reaction and increased the catalase (CAT) and glutathione peroxidase (GP) activity in the cells [29]. Tyr protected mouse cortical neuron cultures against injury induced by 5-S-cysteinyl-dopamine, which induces сaspase-3-driven neuronal apoptosis [30]. Tyr decreased the cytotoxicity of peroxynitrite (ONOO-) on primary cultures of mouse cortical neuronal cells [31]. The Tyr-mediated protection was equal to that observed for the flavonoids caffeic acid and p-coumaric acid. However, Tyr was significantly inferior to hydroxyTyr as an inhibitor of α-synuclein protein aggregation and fibril destabilization in PC12 cell cultures [32]. In experiments with neonatal cerebral cortex cell cultures containing both neuronal and glial cells from SD rats, Tyr demonstrated powerful cytoprotective action against the neurotoxic effects of glutamate, comparable with the effects of NMDA receptor antagonists [33]. In a model of brain slice hypoxia-reoxygenation, Tyr ethyl ether administration demonstrated a neuroprotective effect that correlated with inhibition of nitrosative stress and prostaglandin E2 production [34].
Table 1.
Neuroprotective effects of Tyr and its glycoside salidroside*,#.
| Cell Culture/animal | Model | Concentration/dosage | Effect/Result | Refs. |
|---|---|---|---|---|
| Tyrosol | ||||
| Human astrocytoma U373 MG cell line | Fenton reaction | 0.1 and 0.25 mM | ↓ Oxidative stress ↑ CAT and GP activity |
[29] |
| Mouse cortical neurons | 5-S-cysteinyl-dopamine | 0.1–1 µM | ↓ Percentage of neuronal injury | [30] |
| Mouse cortical neurons | ONOO– | 0.1–10 µM | ↓ Cytotoxicity | [31] |
| Neonatal cerebral cortex cell culture | Glutamate | 0.1–10 µM | ↓ Neurotoxic effect | [33] |
| Rats | Focal cerebral ischemia/reperfusion | 3, 10 and 30 mg/kg intraperitoneally | ↓ Infarct volume ↓ Neurological deficit |
[35] |
| Rats | Global cerebral ischemia/reperfusion | 5, 10, and 20 mg/kg daily for 5 days intravenously | ↑ Survival of rats ↓ The neurological deficit, neuronal damage in the hippocampus |
[36] |
| Rats | Global cerebral ischemia/reperfusion | 20 mg/kg daily for 10 days intraperitoneally | ↑ Production of new neurons in the hippocampal CA1 field |
[45] |
| Salidroside | ||||
| Rats | Focal cerebral ischemia/reperfusion | 24 mg/kg daily for 7 days before ischemia per oral | ↓ Ischemic cerebral cortex tissue edema and TNFá | [39] |
| Rat | Focal cerebral ischemia/reperfusion | 12 mg/kg daily for 7 days before ischemia per oral | ↓ Neurological deficit ↑ Normal neurons in the hippocampus and prefrontal cortex |
[40] |
| Rat | Global cerebral ischemia/reperfusion | 12 mg/kg daily for 7 days before ischemia per oral | ↓ Brain edema and MDA in the hippocampus ↑ Cognitive function and activity of SOD |
[41] |
| Rat | Focal cerebral ischemia/reperfusion | 15 and 30 mg/kg before ischemia + after reperfusion intraperitoneally | ↓ Infarct size ↑ Neurological function, activity of SOD and glutathione-S-transferase |
[42] |
*Neuroprotective effects of Tyr and its glycoside salidroside in models of neurodegenerative diseases are not shown. #Neuroprotective effects of salidroside are shown only in vivo.
The neuroprotective properties of Tyr have been proven in in vivo experiments on models of focal cerebral ischemia and global cerebral ischemia, followed by recirculation. Tyr showed a dose-dependent neuroprotective effect in a rat model of transient middle cerebral artery occlusion (2 h of occlusion, 22 h of reperfusion), represented as a reduction of the infarct volume. The Tyr-treated groups showed significant improvement in post-ischemic sensory-motor dysfunction in the rotarod, foot fault, and beam balance tests when compared with the control group [35]. The authors advanced a hypothesis that the ameliorating effects of Tyr against sensory-motor dysfunction might have been due to reduced damage to the cortex and caudoputamen, which is related to synaptic transmission. Neuroprotective effects of Tyr were investigated in a global cerebral ischemia/reperfusion model [36]. The study of Tyr effects on the survival and neurological impairments of rats in this model confirmed the dose-dependency of its neuroprotective action. Administration of intravenous Tyr increased survival, reduced neurological deficits, attenuated neuronal damage in the hippocampus, and attenuated lipid peroxidation in brain tissues of rats after global cerebral ischemia.
Salidroside is a Tyr glycoside. After intravenous injection, salidroside is extensively metabolized into Tyr [37, 38]. Moreover, after intravenous injection, only trace amounts of salidroside are found in the brain tissue, while Tyr, as a deglycosylated metabolite of salidroside, is present in significant concentrations [38]. That is why salidroside may be considered a prodrug whose neuroprotective action may be due to the effects of Tyr. Based on this, to complete the description of the neuroprotective properties of Tyr, Table 1 provides data on the neuroprotective effects of salidroside in vivo.
In a rat model of focal ischemia/reperfusion, pretreatment of salidroside decreased the level of tumor necrosis factor (TNF)-α and edema in the ischemic cerebral cortex tissue [39]. Salidroside and Tyr galactoside significantly prevented cerebral ischemic injury induced by a 2 h middle cerebral artery occlusion and a 24 h reperfusion in rats in vivo. Furthermore, the oxidative stress (H2O2) in cultured rat cortical neurons was markedly attenuated by treatments with Tyr galactoside [40]. Pretreatment with salidroside to rats reduced the degree of cerebral edema, decreased free radical metabolism and improved cognitive function after global cerebral ischemia/reperfusion [41]. In a model of focal cerebral ischemia, salidroside reduced the infarct size, improved neurological function and histological changes, increased the activity of superoxide dismutase (SOD) and glutathione-S-transferase, and reduced malondialdehyde (MDA) levels after cerebral ischemia/reperfusion [42].
The brain can generate new neurons [43]; thus, neurogenesis may be a new therapeutic strategy to improve neurological function in a brain-damaged by stroke [44]. Tyr has a positive effect on neurogenesis. The regenerative effect of Tyr was studied in a model of global cerebral ischemia, followed by recirculation [45]. Tyr administered intraperitoneally at a dose of 20 mg/kg for 10 days after ischemia/recirculation stimulated the production and development of new neurons in the normally nonproliferative CA1 region in the hippocampus and induced a neuroprotective effect on mature neurons.
Tyr exerts neuroprotective properties in models of neurodegenerative diseases (Parkinson’s disease and Alzheimer’s disease) [32, 46-51]. An analysis of publications describing the neuroprotective activity of Tyr in these diseases is beyond the scope of this work.
4. PROPERTIES OF TYR CONTRIBUTING TO ITS NEUROPROTECTIVE ACTIVITY
4.1. Pharmacokinetics of Tyr
Many publications have described the pharmacokinetics of Tyr when taking olive oil, wine, and other foods and nutraceuticals in animals and humans [52-54]. However, within the framework of this review, data on the pharmacokinetics of Tyr per se are of interest, since it is known that the concomitant presence of these plant sources of Tyr can have a modifying effect on its pharmacokinetic parameters [55, 56].
Tyr pharmacokinetics parameters were studied in rats after intravenous and oral administration. According to data by Chernysheva et al. [57], T1/2 was 1.29 ± 0.06 h after intravenous administration of Tyr to rats at a dose of 200 mg/kg. The maximum concentration of Tyr was detected 1 min after intravenous administration. Under similar conditions, with an intravenous administration of Tyr in a dose of 50 mg/kg, the T1/2 value was 1.64 ± 0.30 h [58].
When administered intragastrically, Tyr is rapidly absorbed and enters the bloodstream (Tmax = 10 min) [59]. Tyr bioavailability after intragastric administration was 32% due to pronounced presystemic elimination [60]. Using the human intestinal cell line Caco2, clone TC7, a mechanism of passive diffusion was detected for Tyr, and it was shown that Tyr has the highest bioaccessibility (100%) [61].
In fact, neuroprotective agents must penetrate the blood-brain barrier to attain an effective therapeutic concentration within the cerebral tissue. To recognize Tyr as a promising neuroprotector, its ability to penetrate the blood-brain barrier is very important, since the low neuroprotective activity of previously studied highly active antioxidants, NXY-059 and tirilazad, was associated with weak penetration through the blood-brain barrier [62, 63]. When administered intravenously, Tyr quickly penetrates tissues of different organs, and the tissue bioavailability coefficient for brain tissue was 0.87, exceeding that of other organs [60]. Quantification of Tyr by GC-MS demonstrated the presence of the compound in rat cerebrospinal fluid after oral administration [64].
Excretion of radiolabeled Tyr was investigated following oral and intravenous administration in rats [65]. It was shown that, with intravenous administration of radiolabeled Tyr, its radiolabeled metabolites are excreted in the urine (45.7 ± 9.3% and 73.9 ± 2.5%, respectively, 1 h and 2 h after administration). With the intragastric administration of an aqueous solution, the rate of Tyr excretion was significantly lower (57.7 ± 3.6% after 4 h). The Tyr molecule is involved in stages I and II of biotransformation in the body. An important feature of stage I of Tyr biotransformation was discovered, namely, its hydroxylation and conversion to hydroxyTyr. This phenomenon has been described in experiments on rats [66] and in clinical trials [67]. Tyr biotransformation into hydroxyTyr occurs in liver microsomes due to the activity of cytochromes CYP2A6 and CYP2D6. HydroxyTyr is a related compound found together with Tyr in natural products (olive oil and wine) [26, 27]. HydroxyTyr has high antioxidant activity [68] and exhibits pronounced neuroprotective properties [34, 68]. The main metabolites in stage II of Tyr biotransformation are glucuronides and sulphates [69-71]. Atzeri et al. [69] assumed that Tyr can exert its biological effects not only directly, but also via its metabolites.
4.2. Antioxidant Activity of Tyr
Numerous experimental and clinical studies have proven the contribution of oxidative and nitrosative stress to the pathophysiology of acute ischemic stroke [72-74]. The neutralization of oxidative and nitrosative stresses is a potential therapeutic strategy because the ischemic brain is highly susceptible to oxidative damage owing to its high consumption of oxygen, its rich content of iron and unsaturated lipids, and its relatively low endogenous antioxidant capacity [75]. A therapeutic approach based on the use of neuroprotectors with antioxidant properties is well-founded, complying with the concepts of the pathogenesis of acute cerebrovascular disorders. Consistent with this idea, many leading laboratories have confirmed the neuroprotective effects of antioxidant agents in cerebral ischemia [76, 77].
The antioxidant properties of Tyr have been studied in vitro in cell-free system (Table 2A), on cell lines, on brain sections and in vivo in rat cerebral ischemic models (Table 2B). Tyr has been shown to be inferior to other simple phenols and polyphenols in antiradical/antioxidant activity when different noncellular systems have been used to evaluate this activity [17, 68, 78-81]. For example, when evaluating the binding of radicals 2,2-diphenyl-1-(2,4,6-trinitrophenyl)hydrazyl (DPPH•), Tyr had the lowest antiradical activity in comparison with polyphenols (catechin, epicatechin, quercetin, procyanidins) and the reference compound vitamin E [29, 80]. Tyr showed weak or intermediate antioxidant activity compared to all other compounds (ferulic acid, quercetin, curcumin, berberine and catechin) as peroxyl, hydrodyl radicals scavenger [82]. In contrast, in other studies with NO generated in vitro, Tyr was not active as a scavenger of reactive nitrogen species [83-86]. An exception is a study in which, using the DPPH• scavenging assay, it was shown that Tyr is superior in antiradical activity to water-soluble phenylpropanoid compounds (salidroside, icariside D2 and others) [87].
The low antiradical/antioxidant activity of Tyr compared with the closely related hydroxyTyr compound is due to the following circumstances: the presence of 1 hydroxyl radical phenol ring did not allow efficient scavenging of radicals; effective Tyr concentrations are in the range of µM concentrations and significantly exceed the effective concentration of hydroxyTyr; Tyr accumulates intracellularly more slowly and probably reaches effective concentrations that have protective effects later [34, 48, 88, 89].
Nevertheless, Tyr exerts more distinct protective effects against oxidative injury in cell models and models of oxidative stress in vivo. Lee et al. [90] demonstrated the protective effects of Tyr against H2O2-induced oxidative damage in L6 muscle cells. In these conditions, Tyr effectively increased the production of ATP and HO-1 (a transcription factor in the nucleus that plays a role in antioxidation). In Caco-2 cell cultures, Tyr exhibited strong antioxidant activities that protect intestinal cells from oxidative stress (action of lipid extract from low-density lipoproteins undergoing oxidative modification) and prevented morphological and functional alterations: membrane damage, modifications of the cytoskeleton network, microtubular disorganization, loss of cell-cell and cell-substrate contacts, cell detachment and cell death [91, 92]. Using Caco-2 cell cultures, Deiana et al. [93] demonstrated that Tyr also exerts a protective effect against fatty acid degradation.
Tyr decreased the phorbol ester-induced production of superoxide anions, hydrogen peroxide, and nitric oxide by RAW 264.7 macrophages [94]. Tyr reduced MDA production in iron-loaded rat hepatocyte cultures; the antioxidant effect of Tyr was similar to the effects of caffeic acid, oleuropein and hydroxyTyr [95]. However, Tyr failed to decrease the MDA production by red blood cells after oxidative stress, which was induced by Fe2+ solution [96].
An important property of Tyr, which can be observed at the level of cellular models, is the ability to weaken the manifestations of oxidative stress and also preserve and/or stimulate antioxidant cell defense system enzymes. In human astrocytoma, U373 MG cells treated with a Fenton reaction, Tyr decreased reactive oxygen species generation and increased the activity and protein expression of the antioxidant enzymes CAT, SOD, glutathione reductase (GR) and GP [29]. Tyr reduced reactive oxygen species, promoted the expression of specific chaperones and antioxidant enzymes, and delayed α-synuclein-dependent degeneration of dopaminergic neurons [50]. Defects in glutathione metabolism might cause oxidative stress, which has been implicated in several neurologic and neurodegenerative diseases [97]. Tyr preserved the cellular activities of antioxidative enzymes, such as GP and GR that were reduced in J774 A.1 cells incubated with low-density lipoproteins in spite of its weak antioxidative effects [89]. Tyr protected CATH.a cells likely via activation of the PI3K/Akt pathway and upregulation of SOD [48].
These data allow us to conclude that Tyr has low or moderate antiradical/antioxidant activity; despite this, Tyr exerts a powerful protective effect against oxidative injury in cell systems and can improve intracellular antioxidant defenses [98]. It can be assumed that the protective role of Tyr under conditions of oxidative stress is due to other mechanisms.
Tyr metabolites can contribute to its antioxidant effect in vivo. Antioxidant effects of hydroxyTyr are well studied and described [17, 52, 68, 80, 81, 83, 84, 86, 89, 93, 95]. Biotransformation of Tyr on stage II may not lead to a change in its activity. Tyr glucuronide and sulphate display antioxidant activity with efficiency comparable to those of the parent compound [69-71]. For example, sulfate metabolites of Tyr protected intestinal cells Caco-2 against the pro-oxidant effect of oxidized cholesterol with an efficiency comparable to that of the parent compound [69]. Tyr and Tyr sulphate prevented the rise of reactive oxygen species, the depletion of glutathione, and the down-regulation of glutathione peroxidase 1, glutamate-cysteine ligase catalytic subunit, and heme oxygenase-1 genes [70].
4.3. Anti-inflammatory Activity of Tyr
The immune response to acute cerebral ischemia is a major factor in stroke pathobiology and outcomes [99]. Inflammation, initiated by stagnant blood flow, activation of intravascular leukocytes, and release of proinflammatory mediators from the ischemic endothelium and brain parenchyma, has the potential to increase tissue injury [99]. Cerebral ischemia induces inflammatory reactions, such as neutrophil infiltration [100], upregulation of cytokines [101], and microglial activation [102]. A growing number of preclinical and clinical studies are providing evidence that immune modulation in patients with acute ischemic stroke may be a new therapeutic strategy in this disease [75, 103-105].
Many studies have shown that Tyr can modulate inflammation. The anti-inflammatory properties of Tyr have been studied in vitro on cell lines and in vivo in models of inflammation (Table 3). Cytokines, such as TNF-α, interleukin (IL)-1 and IL-6, can modulate the brain insult of a stroke at the experimental and clinical levels [106]. Therefore, the effect of Tyr on these cytokines is of particular interest. In an anaphylaxis model, Tyr dose-dependently decreased mast cell degranulation and the expression of inflammatory cytokines (TNF-α, IL-1β, and IL-4) and blocked calcium influx and phosphorylation of the κB kinase complex [107]. Tyr is able to inhibit TNF-α release by lipopolysaccharide (LPS)-stimulated peripheral blood mononuclear cells isolated from healthy volunteers [108]. Tyr reduced nitrite (NO stable metabolites) and prostaglandin E2 production by activated macrophages (RAW 264.7) in a concentration-dependent manner; these inhibitory effects were strictly correlated with a reduction in either inducible nitric oxide synthase (iNOS) or cyclooxygenase (COX)-2 protein as well as mRNA [109]. In these cells, Tyr decreased iNOS and COX-2 gene expression and activated nuclear factor-κB (NF-κB) and signal transducer and activator of transcription (STAT)-1α induced by reactive oxygen species [110]. In rat peritoneal leukocytes stimulated with a calcium ionophore, Tyr exerted selective inhibitory activity against the leukotriene pathway of arachidonate metabolism without affecting the COX pathway [111]. In molecular docking with COX-2, Tyr was found to possess a satisfactory binding affinity compared to Aspirin, Ibuprofen, and Naproxen and a COX-2 selective drug Celecoxib [112]. Experimental evidence for Tyr inhibition of COX-2 production and activity was also obtained [94, 109].
Table 3.
Anti-inflammatory effects of Tyr.
| Cell culture/animal | Model | Concentration/dosage | Effect/Result | Refs. |
|---|---|---|---|---|
| Human umbilical vein endothelial cells (HUVECs) |
TNF-á-stimulation (40 ng/mL) |
2–20 µM | ↓ Intercellular adhesion molecule-1 and vascular cell adhesion molecule-1 | [17] |
| Murine macrophages RAW 264.7 | Phorbol 12-myristate 13-acetate (1 µM) |
100–250 µM | ↓ PGE2, LTB4, and COX-2 expression | [94] |
| Culture of astrocytes | Oxygen-glucose deprivation |
1.6 mM | ↓ TNF-á and IL-6 ↓ GFAP, STAT3, and STAT3-P ↑ IêBá and IêBá-P |
[105] |
| Mast cells | Anaphylaxis model | 1–1000 nM | ↓ Degranulation of cells ↓ TNF-á, IL-1â, and IL-4 expression ↓ Calcium influx, phosphorylation of the êB kinase complex |
[107] |
| Human peripheral blood mononuclear cells | LPS-stimulation | 100 nM | ↓ TNF-á release | [108] |
| Mouse monocyte/macrophage cell line RAW 264.7 | Interferon-ã (25 U/ml) + gliadin (800 ìg/ml) stimulation | 1–4 mM | ↓ NO stable metabolites, PGE2 production ↓ NF-êB, STAT-1á, IRF-1 activation, iNOS, and COX-2 expression |
[109, 110] |
| Rat peritoneal leukocytes | Calcium ionophore A23187 (1 mM) | 100–200 µM | ↓ Leukotriene B4 No effect on TXB2 |
[111] |
| Human colon adenocarcinoma cells (Caco-2) | LPS-stimulation (1 ìg/mL) |
1 ìM | ↓ iNOS expression, NO overproduction, and IĸBá degradation | [113] |
| RAW264.7 cells | LPS-stimulation (100 ng/mL) |
0.3, 0.6, 1.2 mM | ↓ NF-êB, mCD14 expression, and p-I-êB | [114] |
| Human umbilical vein endothelial cells | LPS-stimulation (1 µg/mL) |
1–75 µM | No effect on the expression of vascular cell adhesion molecule-1 | [115] |
| EA.hy.926 cells | Homocysteine (100 ìM) TNF-á-stimulation (10 ng/mL) |
2.5–7.5 µM 2.5–7.5 µM |
↓ Monocyte adhesion, expression of intercellular adhesion molecule-1 No effect on the expression of intercellular adhesion molecule-1 |
[116] |
| RAW 264.7 mouse macrophages |
LPS-stimulation (100 ng/mL) |
1 nM–10 µM | ↓ NO, nuclear translocation of NF-êB | [117] |
| Mice BULB/c | Acute lung injury (intratracheal injection of LPS, 25 ìg/50 ìL) |
0.1, 1, and 10 mg/kg intragastrically | ↓ Myeloperoxidase ↓TNF-á, IL-1â, and IL-6 ↓ iNOS, COX-2, and phosphorylated-IêBá |
[117] |
| Rats | Uveitis (LPS 1 mg/kg intravenously) | 100 mg/kg iv | ↓ TNF-á, PGE2, and NO | [119] |
| Diluted human blood (1:5) | LPS-stimulation (50 g/mL) | 0.1–10 mM | No effect on IL-1â, IL-6, PGE2 and TNF-á | [120] |
| Human THP-1 monocytic leukemia cells | TNF-á-stimulation (10 ng/mL) |
50 ìM | No effect on matrix metalloproteinase-9 secretion | [121] |
Activation of adenocarcinoma Caco-2 cells with LPS leads to stimulation of iNOS with the involvement of NF-κB activation through the inhibitory subunit IĸBα phosphorylation and subsequent degradation induced by Akt or mitogen-activated protein kinase (MAPK)s [113]. In these experiments, Tyr, its glucuronide and sulfate metabolites acted as inhibitors of iNOS expression and IĸBα degradation and as a modulator of MAPK: p38 and extracellular signal-regulated kinase (ERK) 1/2 [113]. In another study [114], Tyr induced lower LPS–macrophage binding and decreased membrane-bound cell surface pattern recognition receptor cluster of differentiation 14 (CD14) expression.
Data on the effect of Tyr on adhesion molecules are contradictory. Turner et al. [17] revealed that Tyr induced a significant reduction in the secretion of intercellular adhesion molecule-1 and vascular cell adhesion molecule-1 in human umbilical vein endothelial cells measured 24 h after activation with TNF-α. However, followed by co-incubation with bacterial LPS with human umbilical vein endothelial cells, Tyr did not show an effect on the expression of vascular cell adhesion molecule-1 [115]. Tyr reduced homocysteine-induced monocyte adhesion as well as cell surface expression of intercellular adhesion molecule-1 in EA.hy.926 cells but was ineffective in reducing the expression of these molecules induced by TNF-α [116].
Tyr has demonstrated anti-inflammatory activity in in vivo models of various diseases. In acute lung injury Tyr inhibited lung vascular permeability, histopathological changes and pulmonary vascular cell infiltration. Pro-inflammatory cytokines, such as TNF-α, IL-1β, and IL-6, were reduced by Tyr in bronchoalveolar lavage fluid and lung tissue. The activation of inflammatory molecules, including iNOS, COX-2, and phosphorylated nuclear factor κB α (IκBα), was suppressed by Tyr [117]. In this model, Tyr markedly inhibited NF-κB and activator protein-1 activation both in vivo and in vitro. Tyr significantly improved the expression of HO-1 (a transcription factor in the nucleus that plays an antioxidant role) and the activation of Nrf2 (an inducible transcription factor that activates a battery of genes encoding antioxidant proteins) [118]. In the rat model of uveitis, Tyr decreased inflammatory cell number, protein concentration, TNF-α, prostaglandin E2 and NO levels in ocular tissue 24 hr after LPS injection [119].
In the absence of studies of the anti-inflammatory effects of Tyr in in vivo models of cerebral ischemia, research performed on astrocyte cultures in a model of oxygen-glucose deprivation is of particular value [105]. In this condition, Tyr could effectively reverse the loss of cells in culture. Pretreatment of Tyr could decrease the increased TNF-α and IL-6 levels in medium with astrocytes. The reduction of astrocyte cytokine production might be due to its inhibition of astrocyte activation and regulation of signal transducer and activator of transcription 3 (STAT3) (an acute-phase response factor) since Tyr attenuated the expression of the glial fibrillary acidic protein (GFAP) and the phosphorylation of STAT3. The authors demonstrated that Tyr prevented the degradation of IκBα and the increase of IκBα phosphorylation in astrocytes in this model, which led to the suppression of NF-κB function during ischemia. Taken together, these data show that Tyr may be a promising anti-inflammatory compound for the treatment of brain ischemia. Bu et al. [35] believe that the antioxidative and anti-inflammatory effects of Tyr are considered to be the main mechanisms leading to the neuroprotective effect.
Metabolites of Tyr may also be involved in its anti-inflammatory effects. In vitro, Tyr sulphate and Tyr glucuronide prevented the phosphorylation of NF-κB signaling proteins, the over-expression of adhesion molecules at gene, protein, and secretory levels, and the adhesion of human monocytes to TNF-α-treated human endothelial cells [70]. In vivo, Tyr, and, most notably, Tyr sulphate dose-dependently ameliorate plantar and ear edemas in mice models of acute and chronic inflammation [70].
However, few studies have indicated the absence of anti-inflammatory properties of Tyr. There was no effect of Tyr on the concentrations of IL-1β, IL-6, prostaglandin E2, or TNF-α in diluted human blood (1:5) after stimulation with LPS [120]. Tyr was not active on matrix metalloproteinase-9 secretion from human THP-1 monocytic leukemia cells at concentrations as high as 50 μM [121]. At last, Tyr (60-240 µM) increased the intracellular concentration of Ca2+ in human lymphomonocytes through mobilization of calcium from intracellular stores [122].
4.4. Anti-apoptotic Activity of Tyr
Cerebral ischemic injury produces two forms of cell death: necrosis and apoptosis [2]. Apoptosis is the consequence of a genetically regulated program that allows cells to die with minimal inflammation or release of genetic material [90]. Caspase-mediated apoptosis is initiated by the release of cytochrome C from mitochondria through activation of the apoptosome complex, which in turn activates caspase-3 [123]. The MAPK signaling pathway plays an important role in many biological responses, including apoptosis [124]. 5-S-Cysteinyl-dopamine has been shown to be neurotoxic through its ability to induce caspase-3-driven neuronal apoptosis [125].
The anti-apoptotic activity of Tyr has been studied in vitro on cell lines (Table 4). A protective role of Tyr on mouse cortical neuron cultures induced by 5-S-cysteinyl-dopamine was evaluated by Vauzour et al. [30] as a manifestation of the anti-apoptotic activity of Tyr. There are data on the anti-apoptotic activity of Tyr obtained on other cellular objects. Tyr mitigated ischemia/reperfusion-induced apoptosis in H9c2 cells via inhibition of the c-Jun N-terminal kinase (JNK) pathway, protecting the mitochondria from disruption, preventing the release of cytochrome C and decreasing caspase-3 activation [126]. According to the authors, the antioxidant capacity of Tyr may also play a role in the inhibition of JNK activation. An important role of Tyr in preventing apoptotic cell death induced by ultraviolet B radiation was revealed on HaCaT cells [98]. Tyr decreased phosphorylation (activation) of MAPK (ERK, p38, and JNK) in a dose-dependent manner after H2O2-induced oxidative damage in L6 muscle cells [90]. Tyr inhibited LPS-mediated binding of macrophage (RAW 264.7 cells) and decreased phosphorylation of ERK, JNK, and p38 [114]. Tyr dose-dependently protected CATH.a cells from 1-methyl-4-phenylpyridinium-induced cell death this was manifested in the regulation of the gene expression of proapoptotic and anti-apoptolic proteins. Under the influence of Tyr, a decrease in Bax, caspase-3 and -9, the cytosolic fraction of cytochrome C, and an increase in Bcl-2 and Bcl-xl were observed [48]. Tyr treatment increased Akt phosphorylation. It is well-known fact that Akt signalling inhibits apoptosis through up-regulation of anti-apoptotic Bcl-2 family proteins. Further, several studies have suggested that Akt signalling actively participates in the up-regulation of anti-oxidative gene expressions [127].
Table 4.
Anti-apoptotic effects of Tyr.
| Cell Culture/animal | Model | Concentration/dosage | Effect/Result | Refs. |
|---|---|---|---|---|
| Primary cultures of mouse cortical neurons | CysDA (100 µM, 24 h) | 0.1–1 µM | ↑ Cortical neuron viability | [30] |
| CATH.a neuron cell | MPP+-induced cytotoxicity (100 µM – 2 mM) | 100, 200 µM | ↓Bax, caspase-3 and -9, cytochrome C in cytosol ↑ Bcl-2, Bcl-xl, and Akt-P |
[48] |
| L6 muscle cells | H2O2-induced oxidative damage | 30–100 µM | ↓ Caspase-3 level ↓ ERK1/2, JNK and p38 phosphorylation ↑ ATP, HO-1 |
[90] |
| Caco-2 cells | Oxidized LDL (0.2 g/L) treatment | 0.25–1 mM | ↓ Pro-apoptotic processes: membrane damage, modifications of cytoskeleton network, microtubular disorganization, loss of cell-cell and cell-substrate contacts, cell detachment, and cell death ↓ Overproduction and activation of p66Shc |
[91, 92] |
| HaCaT cells | UVB (250 mJ/cm2, 10 min) | 5 mM | ↓ Caspase-3, -8, -9 activity ↓ Quantity of apoptotic and necrotic cells |
[98] |
| RAW264.7 cells | LPS-stimulation (100 ng/mL) | 1.2 mM | ↓ Phosphorylation of ERK, JNK, and p38 | [114] |
| H9c2 cells | Hypoxia (95% nitrogen and 5% CO2, 1 h) / reoxygenation | 250 ìM | ↓ Cytochrome C, caspase-3 activity, and JNK activation | [126] |
MPP+, 1-methyl-4-phenylpyridinium; CysDA, 5-S-cysteinyl-dopamine.
4.5. Other Pleiotropic Effects of Tyr Contributing to its Neuroprotective Activity
Ischemic stroke is characterized by a violation of both the rheological properties of blood and the indicators of cellular and plasma hemostasis. An increase in blood viscosity has been identified in the acute phase of ischemic stroke [128, 129]. Platelets are activated in the acute phase of ischemic stroke, releasing neurotoxic and thrombogenic agents [130]. Ex vivo experiments on rats showed that Tyr (intragastrically 100 mg/kg daily 5 days) inhibited ADP-induced platelet aggregation and limited the increase in blood viscosity at a shear rate of 5–300 sec–1 during thermal exposure of blood [20]. The effects of Tyr are comparable to those of pentoxifylline, a drug known to have hemorheological and antiplatelet properties [131].
CONCLUSION
This review summarizes the scientific literature on the neuroprotective properties of Tyr and its possible mechanisms of action in order to assess the acceptability of Tyr as the basis for the development of a new neuroprotective drug for the treatment of ischemic stroke. In previously published reviews, the neuroprotective properties of Tyr as a corrector for cognitive impairment in neurodegenerative pathology (Parkinson's disease and Alzheimer's disease) is summarized [132-135].
For the treatment of the acute phase of stroke, it requires the use of agents with a wide range of pharmacological actions that can prevent multiple neurochemical cascades involved in ischemic damage [72]. There are at least four fundamental mechanisms underlying cell death during ischemic brain injury: oxidative/nitrosative stress, excitotoxicity and ionic imbalance, local inflammation, and apoptotic-like cell death [2, 72, 136].
The therapeutic approach based on the use of neuroprotectors with antioxidant properties is justified by the well-studied role of free radical processes in the pathogenesis of acute ischemic stroke. Tyr refers to bisphenols, the antioxidant effect of which is due to their “direct” and “indirect” effects [82]. According to the authors, “direct” effects have been proven in vitro and are associated with the metal chelation, scavenging of reactive oxygen and nitrogen species, reduction of hydroperoxide formation and quenching of electronically excited compounds; “indirect” are mainly related to the modulation of cell signalling pathways and gene expression, to the regulation of defence enzymes and changes in nuclear histone acetylation. “Indirect” effects of biophenols are mainly associated with the modulation of cellular signaling pathways and the activation of antioxidant enzymes.
Tyr as an antioxidant, has a weak effect, or it is even completely absent in cell-free systems. There is a study the results of which explain one of the reasons for the unequal antioxidant activity of Tyr under different conditions. MacDonald et al. [137] found that Tyr is effective as an antioxidant in a lipid system, but has very low activity in an aqueous system. Since Tyr is an amphiphilic compound [10], it can be assumed that Tyr is able to exhibit antioxidant activity when distributed in the lipid membranes of cells, preventing the chain reaction of lipid peroxidation. Another significant reason for the differences in antioxidant effects of Tyr in a cell system versus in a cell-free system is its “indirect” effects: increase of antioxidant enzyme expression.
The presence of antioxidant activity is necessary, but insufficient for a molecule to be considered a neuroprotector. Clinical trials of individual drugs with free radical scavenger activity failed [6]. Authors, who analyzed clinical trial failures of antioxidants with expected neuroprotective properties, believe that the compounds of this class are promising only in case when the antioxidant exerts multi-target neuroprotective activity and easily crosses the blood-brain barrier [8]. In this review, the evidence is given of the presence in Tyr of a combination of properties, the manifestation of which provides the neuroprotective activity. Tyr has anti-inflammatory properties, which is demonstrated on a variety of objects in vitro and in vivo, and in particular, on the culture of astrocytes. Tyr blocks the neurotoxic effects of glutamate and comparable in efficacy with NMDA receptor antagonists. Finally, Tyr showed a distinct anti-apoptotic activity on neuronal cells. Tyr induces the up-regulation of anti-apoptotic proteins and inhibits expression of proapoptotic proteins and activity of MAPKs.
Thus, an analysis of the presented scientific literature suggests that Tyr is capable of acting on all the main mechanisms of nerve cell damage during ischemia/reperfusion, as well as additionally exhibiting neuroregenerative properties. Moreover, Tyr has acceptable pharmacokinetic properties, in particular, penetrates well through the BBB.
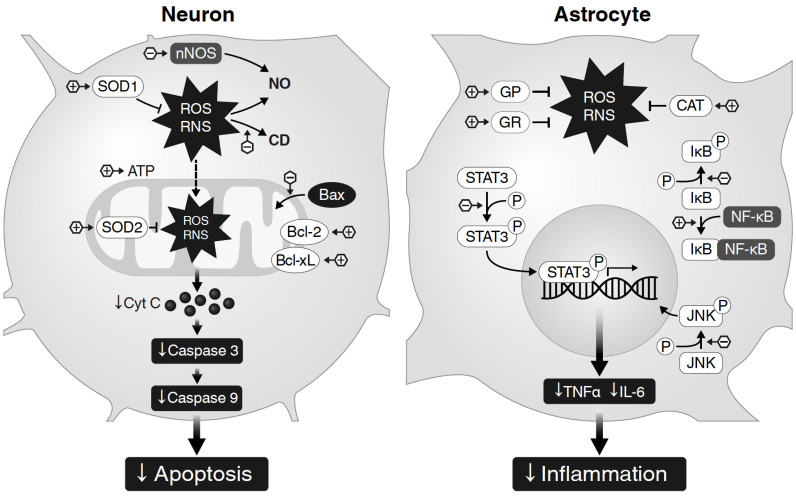
The main mechanisms of Tyr action described for neurons and astrocytes are presented in Fig. 1.
Fig. (1).
Scheme of Tyr action mechanisms based on literature collected for this review. Despite a sufficient number of publications on the study of the antioxidant, anti-inflammatory, anti-apoptotic effects of Tyr, the studies performed on brain cells are sporadic and limited to studies conducted on cultures of neuronal cells [30, 48] and astrocytes [29, 105]. Therefore, the scheme reflecting the mechanisms of action of Tyr contains significant gaps that can be filled with the results of further studies. In neurons under conditions of oxidative stress, the action of Tyr is realized through up-regulation of anti-apoptotic proteins (Bcl-2 and Bcl-xl) и inhibition of apoptotic protein Bax translocation into mitochondria, which limits the release of cytochrome c and subsequent activation of the chain of caspase reactions, including a program of cell apoptosis. The downward trend in nNOS expression is manifested in a regular decrease in NO. Tyr significantly increases the expression of cytosolic SOD-1 and mitochondrial SOD-2, which reduces the severity of oxidative stress, and in an in vivo experiment on a global ischemia model can help reduce the content of lipid peroxidation products (conjugated dienes) in the brain tissue. In astrocytes under hypoxia/reoxygenation (as models of ischemia / recirculation) Tyr protects cultured astrocytes reduces ROS overproduction by increasing the activity of antioxidant enzymes (GR, GP, CAT), attenuates the released TNF-α and IL-6 from astrocyte via regulating of JNK. The reduction of cytokines from astrocyte might be due to its inhibition of astrocyte activation and regulation of the STAT3 signaling pathway since Tyr attenuates the expression level of GFAP and the phosphorylation of STAT3. At last, Tyr prevents the degradation of IκBα, which leads to the suppression of NF-κB function. + or - in the hexagon indicate the direction of Tyr action (activation or inhibition, respectively). (A higher resolution / colour version of this figure is available in the electronic copy of the article).
Tyr has low acute toxicity; the LD50 was 2700 and 1700 mg/kg after intragastric and intraperitoneal injections in mice, respectively; the LD50 was 7079 mg/kg after intragastric administration in rats. No toxicity was observed after 3 months of chronic intragastric administration of Tyr at doses of 200 mg/kg in male rats and 10 mg/kg in dogs [10]. Tyr cytotoxic levels are very low [138]. Tyr did not manifest genotoxic effects [139]. Finally, Absorption, Distribution, Metabolism, Excretion and Toxicity prediction, and bioactivity scores altogether confirm the drug-like potential of Tyr [113].
Therefore, Tyr has a multitarget activity and is a promising compound for the development of an effective neuroprotective agent for use in ischemic stroke.
Table 2A.
Antioxidant effects of Tyr in the cell-free system.
| Model | Concentration/dosage | Effect/Result | Refs. |
|---|---|---|---|
| Ferric reducing ability of plasma | 1–16 µM | No effect | [17] |
| ABTS•+ scavenging | 1–16 µM | Low effect | [17] |
| DPPH• scavenging | 1–16 µM | No effect | [17] |
| DPPH• scavenging | 0.05–0.125 mg/mL | Low effect | [29] |
| ABTS•+ scavenging | 500 µM | Low effect | [68] |
| DPPH• scavenging | LD50 = 86 ± 0.02 µg/mL | Low effect | [78] |
| Lipid matrix | 0.1–1.0 mM/kg | No effect | [79] |
| DPPH• scavenging | 0.01–5/10 µM | No / Low effect | [80] |
| Oxidation of methyl linoleate | 1 · 10–4 M | No effect | [81] |
| HO2-, –OH- and ONOO–-generation | 1 ìM | No effect (HO2, –OH) ONOO–-scavenging | [82] |
| NO scavenging | 5–75 mM | No effect | [83] |
| ABTS•+, C11-BODIPY581/591 and cis-PnA radicals | 1 mM | No effect or low effect | [84] |
| Serum oxidation | 0.2–0.8 mM | ↑ Lag time of oxidation | [85] |
| DPPH• and ABTS•+ scavenging | 10 µg/mL | Low effect | [86] |
| DPPH• scavenging | IC50 = 8.10 ± 0.98 µM | Moderate effect | [87] |
| ABTS•+ scavenging | 1.22 ± 0.03 TEAC | Moderate effect | |
| Hypoxanthine/xanthine oxidase | 100 µM 250 µM | ↓ O2•– Inactive as H2O2 scavenger | [111] |
| Cooper(II)-ascorbate mediated oxidation in an aqueous and a lipid system | 100 mg/L | Inactive in an aqueous system Active in a lipid system |
[137] |
TEAC, trolox equivalent antioxidant capacity; MPP+1, methyl-4-phenylpyridinium; ABTS•+, 2,2'-azinobis-(3-ethylbenzothiazoline-6-sulfonic acid).
Table 2B.
Antioxidant effects of Tyr in the cell culture system and in vivo.
| Cell Culture/animals | Model | Concentration/dosage | Effect/Result | Refs. |
|---|---|---|---|---|
| Cell system in vitro | ||||
| Human endothelial cells EA.hy.926 | H2O2 (100 µM) / oxidized LDL (80 µg protein/mL) |
50, 100 µM | Low effect | [17] |
| Human astrocytoma U373 MG cell line | FeSO4+H2O2-induced oxidative stress |
0.1–0.25 mM | ↓ Oxidative stress ↑ Cell viability, CAT, SOD, GR, and GPx activity |
[29] |
| CATH.a neuron cell | MPP+-induced cytotoxicity (100 µM – 2 mM) |
100, 200 µM | ↑ ATP, SOD-1 and SOD-2 ↓ NO |
[48] |
| Human Cervical Carcinoma cells (HeLa) | Generation of HO2, –OH, and ONOO– |
1, 5, and 10 ìM | ↑ GSH and GHS/GSSG, SIRT1 expression, AMPK activation | [82] |
| J774 A.1 cells | oxLDL-mediated oxidative stress | 1.5 µmol/L–0.5 mmol/L | ↓ MDA ↑ GSH, Gred and GPx activities |
[89] |
| L6 muscle cells | H2O2-induced oxidative damage | 30–100 µM | ↑ Cell viability | [90] |
| Caco-2 cells | Oxidized LDL (0.2 g/L) treatment | 0.25–1 mM | ↓ O2•–, hydrogen peroxide overproduction ↑ GSH |
[92] |
| Caco-2 cells | TBH treatment (2.5 mM) | 5–25 µM | ↓ MDA and fatty acids degradation | [93] |
| Murine macrophages RAW 264.7 | Phorbol 12-myristate 13-acetate (1 µM) |
250 µM | ↓ O2•–, H2O2 and NO2– | [94] |
| Generation of O2•– and hydrogen peroxide | 25, 50 and 100 µM | ↓ MDA, RO• and ROO• | [95] | |
| Red blood cells | Iron-induced oxidative stress (100 µM Fe2+) |
100 µM | No effect on MDA | [96] |
| Rat peritoneal leukocytes | Calcium ionophore A23187 (1 mM) | 100 µM | ↓ ROS generation | [111] |
| In vivo | ||||
| Rats | Global cerebral ischemia/ reperfusion |
5, 10, and 20 mg/kg daily for 5 days intravenously | ↓ Lipid peroxidation in brain tissue, CD | [36] |
| Mice BULB/c | Acute lung injury (intratracheal injection of LPS, 25 ìg/50 ìL) | 0.1, 1, and 10 mg/kg intragastrically | ↑ SOD | [117] |
In vivo, the antioxidant properties of Tyr have been studied in rats using a model of acute global cerebral ischemia/reperfusion. On day 5, after ischemia/reperfusion, the levels of conjugated dienes (CD) and fluorescent products were significantly lower (by 37% and 45%, respectively) in animals treated with Tyr compared with controls [36].
Acknowledgements
The authors thank Anton Osipenko for technical help.
LIST OF ABBREVIATIONS
- CAT
Сatalase
- CD
Conjugated dienes
- CD14
Сell surface pattern recognition receptor cluster of differentiation 14
- COX
Сyclooxygenase
- DPPH
2,2-diphenyl-1-(2,4,6-trinitrophenyl)hydrazyl
- ERK
Extracellular signal-regulated kinase
- GFAP
Glial fibrillary acidic protein
- GP
Glutathione peroxidase
- GR
Glutathione reductase
- HO-1
Transcription factor
- IL
Interleukin
- IκBα
Nuclear factor κBα
- JNK
c-Jun N-terminal kinase
- LPS
Lipopolysaccharide
- LT
Leukotriene
- MAPK
Mitogen-activated protein kinase
- MDA
Malondialdehyde
- NF-κB
Nuclear factor-κB
- NMDA
N-methyl-d-aspartate
- NOS
Nitric oxide synthase
- Nrf2
Inducible transcription factor
- ONOO–
Peroxynitrite
- ROS
Reactive oxygen species
- SOD
Superoxide dismutase
- STAT
Signal transducer and activator of transcription
- TNF-α
Tumor necrosis factor α
- Tyr
p-Tyrosol
AUTHORS’ CONTRIBUTIONS
Conceptualization, Search and Analyze of Literature: P.M.B., P.T.M.; Writing: P.M.B., P.T.M.; Tables: P.T.M; Design of Figure: P.M.B.; Final Editing: P.M.B.
Consent for Publication
Not applicable.
Funding
None.
Conflict of Interest
The authors declare no conflict of interest, financial or otherwise.
References
- 1.Hachinski V., Donnan G.A., Gorelick P.B., Hacke W., Cramer S.C., Kaste M., Fisher M., Brainin M., Buchan A.M., Lo E.H., Skolnick B.E., Furie K.L., Hankey G.J., Kivipelto M., Morris J., Rothwell P.M., Sacco R.L., Smith S.C., Jr, Wang Y., Bryer A., Ford G.A., Iadecola C., Martins S.C., Saver J., Skvortsova V., Bayley M., Bednar M.M., Duncan P., Enney L., Finklestein S., Jones T.A., Kalra L., Kleim J., Nitkin R., Teasell R., Weiller C., Desai B., Goldberg M.P., Heiss W.D., Saarelma O., Schwamm L.H., Shinohara Y., Trivedi B., Wahlgren N., Wong L.K., Hakim A., Norrving B., Prudhomme S., Bornstein N.M., Davis S.M., Goldstein L.B., Leys D. Tuomilehto, J. Stroke: working toward a prioritized world agenda. Stroke. 2010;41(6):1084–1099. doi: 10.1161/STROKEAHA.110.586156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brouns R., De Deyn P.P. The complexity of neurobiological processes in acute ischemic stroke. Clin. Neurol. Neurosurg. 2009;111(6):483–495. doi: 10.1016/j.clineuro.2009.04.001. [DOI] [PubMed] [Google Scholar]
- 3.Neuhaus A.A., Couch Y., Hadley G., Buchan A.M. Neuroprotection in stroke: the importance of collaboration and reproducibility. Brain. 2017;140(8):2079–2092. doi: 10.1093/brain/awx126. [DOI] [PubMed] [Google Scholar]
- 4.Sarwal A., Hussain M.S., Shuaib A. In: Neuroprotection in stroke. Translational Stroke Research, Springer Series in Translational Stroke Research; Lapchak, P. Zhang J., editor. New York: Springer; 2012. pp. 79–90. [Google Scholar]
- 5.Rajah G.B., Ding Y. Experimental neuroprotection in ischemic stroke: a concise review. Neurosurg. Focus. 2017;42(4):E2. doi: 10.3171/2017.1.FOCUS16497. [DOI] [PubMed] [Google Scholar]
- 6.Rogalewski A., Schneider A., Ringelstein E.B., Schäbitz W.R. Toward a multimodal neuroprotective treatment of stroke. Stroke. 2006;37(4):1129–1136. doi: 10.1161/01.STR.0000209330.73175.34. [DOI] [PubMed] [Google Scholar]
- 7.Lapchak P.A., Araujo D.M. Advances in ischemic stroke treatment: neuroprotective and combination therapies. Expert Opin. Emerg. Drugs. 2007;12(1):97–112. doi: 10.1517/14728214.12.1.97. [DOI] [PubMed] [Google Scholar]
- 8.Fisher M. New approaches to neuroprotective drug development. Stroke. 2011;42(1) Suppl.:S24–S27. doi: 10.1161/STROKEAHA.110.592394. [DOI] [PubMed] [Google Scholar]
- 9.Panossian A., Nikoyan N., Ohanyan N., Hovhannisyan A., Abrahamyan H., Gabrielyan E., Wikman G. Comparative study of rhodiola preparations on behavioral despair of rats. Phytomedicine. 2008;15(1-2):84–91. doi: 10.1016/j.phymed.2007.10.003. [DOI] [PubMed] [Google Scholar]
- 10.Saratikov A.S., Krasnov E.A. Rhodiola Rosea (Golden Root): A Valuable Medicinal PlantTomsk University Press. Tomsk: Russain; 2004. [Google Scholar]
- 11.Torić J., Marković A.K., Brala C.J., Barbarić M. Anticancer effects of olive oil polyphenols and their combinations with anticancer drugs. Acta Pharm. 2019;69(4):461–482. doi: 10.2478/acph-2019-0052. [DOI] [PubMed] [Google Scholar]
- 12.Moskalev A., Chernyagina E., Tsvetkov V., Fedintsev A., Shaposhnikov M., Krut’ko V., Zhavoronkov A., Kennedy B.K. Developing criteria for evaluation of geroprotectors as a key stage toward translation to the clinic. Aging Cell. 2016;15(3):407–415. doi: 10.1111/acel.12463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Samuel S.M., Thirunavukkarasu M., Penumathsa S.V., Paul D., Maulik N. Akt/FOXO3a/SIRT1-mediated cardioprotection by n-tyrosol against ischemic stress in rat in vivo model of myocardial infarction: switching gears toward survival and longevity. J. Agric. Food Chem. 2008;56(20):9692–9698. doi: 10.1021/jf802050h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chernyshova G.A., Plotnikov M.B., Smol’yakova V.I., Golubeva I.V., Aliev O.I., Tolstikova T.G., Krysin A.P., Sorokina I.V. Antiarrhythmic activity of n-tyrosol during acute myocardial ischemia and reperfusion. Bull. Exp. Biol. Med. 2007;143(6):689–691. doi: 10.1007/s10517-007-0215-7. [DOI] [PubMed] [Google Scholar]
- 15.Plotnikov M.B., Chernysheva G.A., Smol’yakova V.I., Plotnikova T.M., Sysolyatin S.V., Kryukov Y.A. Anti-ischemic activity of p-tyrosol under conditions of repeated transient myocardial ischemia in rats. Bull. Exp. Biol. Med. 2018;165(5):625–628. doi: 10.1007/s10517-018-4228-1. [DOI] [PubMed] [Google Scholar]
- 16.Marrugat J., Covas M.I., Fitó M., Schröder H., Miró-Casas E., Gimeno E., López-Sabater M.C., de la Torre R., Farré M., SOLOS Investigators Effects of differing phenolic content in dietary olive oils on lipids and LDL oxidation--a randomized controlled trial. Eur. J. Nutr. 2004;43(3):140–147. doi: 10.1007/s00394-004-0452-8. [DOI] [PubMed] [Google Scholar]
- 17.Turner R., Etienne N., Alonso M.G., de Pascual-Teresa S., Minihane A.M., Weinberg P.D., Rimbach G. Antioxidant and anti-atherogenic activities of olive oil phenolics. Int. J. Vitam. Nutr. Res. 2005;75(1):61–70. doi: 10.1024/0300-9831.75.1.61. [DOI] [PubMed] [Google Scholar]
- 18.Anter J., Tasset I., Demyda-Peyrás S., Ranchal I., Moreno-Millán M., Romero-Jimenez M., Muntané J., Luque de Castro M.D., Muñoz-Serrano A., Alonso-Moraga Á. Evaluation of potential antigenotoxic, cytotoxic and proapoptotic effects of the olive oil by-product “alperujo”, hydroxytyrosol, tyrosol and verbascoside. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2014;772:25–33. doi: 10.1016/j.mrgentox.2014.07.002. [DOI] [PubMed] [Google Scholar]
- 19.Fragopoulou E., Nomikos T., Karantonis H.C., Apostolakis C., Pliakis E., Samiotaki M., Panayotou G., Antonopoulou S. Biological activity of acetylated phenolic compounds. J. Agric. Food Chem. 2007;55(1):80–89. doi: 10.1021/jf0627221. [DOI] [PubMed] [Google Scholar]
- 20.Plotnikov M.B., Chernysheva G.A., Smol’yakova V.I., Maslov M.Y., Cherkashina I.V., Krysin A.P., Sorokina I.V., Tolstikova T.G. Effect of n-tyrosol on blood viscosity and platelet aggregation. Bull. Exp. Biol. Med. 2007;143(1):61–63. doi: 10.1007/s10517-007-0017-y. [DOI] [PubMed] [Google Scholar]
- 21.Panossian A., Wikman G., Sarris J. Rosenroot (Rhodiola rosea): traditional use, chemical composition, pharmacology and clinical efficacy. Phytomedicine. 2010;17(7):481–493. doi: 10.1016/j.phymed.2010.02.002. [DOI] [PubMed] [Google Scholar]
- 22.Gris E.F., Mattivi F., Ferreira E.A., Vrhovsek U., Filho D.W., Pedrosa R.C., Bordignon-Luiz M.T. Stilbenes and tyrosol as target compounds in the assessment of antioxidant and hypolipidemic activity of Vitis vinifera red wines from southern Brazil. J. Agric. Food Chem. 2011;59(14):7954–7961. doi: 10.1021/jf2008056. [DOI] [PubMed] [Google Scholar]
- 23.Tuck K.L., Hayball P.J. Major phenolic compounds in olive oil: metabolism and health effects. J. Nutr. Biochem. 2002;13(11):636–644. doi: 10.1016/S0955-2863(02)00229-2. [DOI] [PubMed] [Google Scholar]
- 24.Wang S-J., Lee H-J., Cho J-Y., Park K-H., Moon J-H. Isolation and identification of antioxidant from makgeolli. Korean J. Food Sci. Technol. 2012;44:14–20. doi: 10.9721/KJFST.2012.44.1.014. [DOI] [Google Scholar]
- 25.Owen R.W., Giacosa A., Hull W.E., Haubner R., Würtele G., Spiegelhalder B., Bartsch H. Olive-oil consumption and health: the possible role of antioxidants. Lancet Oncol. 2000;1:107–112. doi: 10.1016/S1470-2045(00)00015-2. [DOI] [PubMed] [Google Scholar]
- 26.Bayram B., Ozcelik B., Schultheiss G., Frank J., Rimbach G. A validated method for the determination of selected phenolics in olive oil using high-performance liquid chromatography with coulometric electrochemical detection and a fused-core column. Food Chem. 2013;138(2-3):1663–1669. doi: 10.1016/j.foodchem.2012.11.122. [DOI] [PubMed] [Google Scholar]
- 27.Bevilacqua L., Buiarelli F., Coccioli F., Jasionowska R. Identification of compounds in wine by HPLC-tandem mass spectrometry. Ann. Chim. 2004;94(9-10):679–689. doi: 10.1002/adic.200490085. [DOI] [PubMed] [Google Scholar]
- 28.Sysolyatin S.V., Kryukov Yu.A., Malykhin V.V., Muradov K.K., Chernysheva G.A., Aliev O.I., Smol’yakova V.I., Anishchenko A.M., Sidekhmenova A.V., Shamanaev A.Yu., Plotnikov M.B. p-Tyrosol: a new synthetic method and new types of pharmacological activity. Russ. Chem. Bull. 2015;64:2210–2214. doi: 10.1007/s11172-015-1140-y. [DOI] [Google Scholar]
- 29.Martín S., González-Burgos E., Carretero M.E., Gómez-Serranillos M.P. Neuroprotective properties of Spanish red wine and its isolated polyphenols on astrocytes. Food Chem. 2011;128(1):40–48. doi: 10.1016/j.foodchem.2011.02.074. [DOI] [PubMed] [Google Scholar]
- 30.Vauzour D., Corona G., Spencer J.P. Caffeic acid, tyrosol and p-coumaric acid are potent inhibitors of 5-S-cysteinyl-dopamine induced neurotoxicity. Arch. Biochem. Biophys. 2010;501(1):106–111. doi: 10.1016/j.abb.2010.03.016. [DOI] [PubMed] [Google Scholar]
- 31.Vauzour D., Vafeiadou K., Corona G., Pollard S.E., Tzounis X., Spencer J.P. Champagne wine polyphenols protect primary cortical neurons against peroxynitrite-induced injury. J. Agric. Food Chem. 2007;55(8):2854–2860. doi: 10.1021/jf063304z. [DOI] [PubMed] [Google Scholar]
- 32.Hornedo-Ortega R., Cerezo A.B., Troncoso A.M., Garcia-Parrilla M.C. Corrigendum to “Protective effects of hydroxytyrosol against α-synuclein toxicity on PC12 cells and fibril formation” Food Chem. Toxicol. 120 (2018) 41-49. Food Chem. Toxicol. 2018;121:719. doi: 10.1016/j.fct.2018.09.037. [DOI] [PubMed] [Google Scholar]
- 33.Ma C.J., Kim Y.C., Sung S.H. Compounds with neuroprotective activity from the medicinal plant Machilus thunbergii. J. Enzyme Inhib. Med. Chem. 2009;24(5):1117–1121. doi: 10.1080/14756360802632971. [DOI] [PubMed] [Google Scholar]
- 34.De La Cruz J.P., Ruiz-Moreno M.I., Guerrero A., Reyes J.J., Benitez-Guerrero A., Espartero J.L., González-Correa J.A. Differences in the neuroprotective effect of orally administered virgin olive oil (Olea europaea) polyphenols tyrosol and hydroxytyrosol in rats. J. Agric. Food Chem. 2015;63(25):5957–5963. doi: 10.1021/acs.jafc.5b00627. [DOI] [PubMed] [Google Scholar]
- 35.Bu Y., Rho S., Kim J., Kim M.Y., Lee D.H., Kim S.Y., Choi H., Kim H. Neuroprotective effect of tyrosol on transient focal cerebral ischemia in rats. Neurosci. Lett. 2007;414(3):218–221. doi: 10.1016/j.neulet.2006.08.094. [DOI] [PubMed] [Google Scholar]
- 36.Atochin D.N., Chernysheva G.A., Smolyakova V.I., Osipenko A.N., Logvinov S.V., Zhdankina A.A., Sysolyatin S.V., Kryukov Y.A., Anfinogenova Y., Plotnikova T.M., Plotnikov M.B. Neuroprotective effects of p-tyrosol after the global cerebral ischemia in rats. Phytomedicine. 2016;23(7):784–792. doi: 10.1016/j.phymed.2016.03.015. [DOI] [PubMed] [Google Scholar]
- 37.Panossian A., Hovhannisyan A., Abrahamyan H., Gabrielyan E., Wikman G. 2010. [Google Scholar]
- 38.Guo N., Zhu M., Han X., Sui D., Wang Y., Yang Q. The metabolism of salidroside to its aglycone p-tyrosol in rats following the administration of salidroside. PLoS One. 2014;9(8):e103648. doi: 10.1371/journal.pone.0103648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Han T. Effects of salidroside pretreatment on expression of tumor necrosis factor-alpha and permeability of blood brain barrier in rat model of focal cerebral ischemia-reperfusion injury. Asian Pac. J. Trop. Med. 2013;6(2):156–158. doi: 10.1016/S1995-7645(13)60014-0. [DOI] [PubMed] [Google Scholar]
- 40.Shi T.Y., Feng S.F., Xing J.H., Wu Y.M., Li X.Q., Zhang N., Tian Z., Liu S.B., Zhao M.G. Neuroprotective effects of Salidroside and its analogue tyrosol galactoside against focal cerebral ischemia in vivo and H2O2-induced neurotoxicity in vitro. Neurotox. Res. 2012;21(4):358–367. doi: 10.1007/s12640-011-9290-7. [DOI] [PubMed] [Google Scholar]
- 41.Zou Y.Q., Cai Z.Y., Mao Y.F., Li J.B., Deng X.M. Effects of salidroside-pretreatment on neuroethology of rats after global cerebral ischemia-reperfusion. J. Chin. Integr. Med. 2009;7(2):130–134. doi: 10.3736/jcim20090207. [DOI] [PubMed] [Google Scholar]
- 42.Han J., Xiao Q., Lin Y.H., Zheng Z.Z., He Z.D., Hu J., Chen L.D. Neuroprotective effects of salidroside on focal cerebral ischemia/reperfusion injury involve the nuclear erythroid 2-related factor 2 pathway. Neural Regen. Res. 2015;10(12):1989–1996. doi: 10.4103/1673-5374.172317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gage F.H. Neurogenesis in the adult brain. J. Neurosci. 2002;22(3):612–613. doi: 10.1523/JNEUROSCI.22-03-00612.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Koh S.H., Park H.H. Neurogenesis in stroke recovery. Transl. Stroke Res. 2017;8(1):3–13. doi: 10.1007/s12975-016-0460-z. [DOI] [PubMed] [Google Scholar]
- 45.Khodanovich M.Y., Kisel’ A.A., Chernysheva G.A., Smol’yakova V.I., Kudabaeva M.S., Krutenkova E.P., Tyumentseva Y.A., Plotnikov M.B. p-Tyrosol enhances production of new neurons in the hippocampal CA1 field after transient global cerebral ischemia in rats. Bull. Exp. Biol. Med. 2019;168(2):224–228. doi: 10.1007/s10517-019-04679-7. [DOI] [PubMed] [Google Scholar]
- 46.St-Laurent-Thibault C., Arseneault M., Longpré F., Ramassamy C. Tyrosol and hydroxytyrosol, two main components of olive oil, protect N2a cells against amyloid-β-induced toxicity. Involvement of the NF-κB signaling. Curr. Alzheimer Res. 2011;8(5):543–551. doi: 10.2174/156720511796391845. [DOI] [PubMed] [Google Scholar]
- 47.Chen J., Zhou Y., Mueller-Steiner S., Chen L.F., Kwon H., Yi S., Mucke L., Gan L. SIRT1 protects against microglia-dependent amyloid-beta toxicity through inhibiting NF-kappaB signaling. J. Biol. Chem. 2005;280(48):40364–40374. doi: 10.1074/jbc.M509329200. [DOI] [PubMed] [Google Scholar]
- 48.Dewapriya P., Himaya S.W., Li Y.X., Kim S.K. Tyrosol exerts a protective effect against dopaminergic neuronal cell death in in vitro model of Parkinson’s disease. Food Chem. 2013;141(2):1147–1157. doi: 10.1016/j.foodchem.2013.04.004. [DOI] [PubMed] [Google Scholar]
- 49.Taniguchi K., Yamamoto F., Arai T., Yang J., Sakai Y., Itoh M., Mamada N., Sekiguchi M., Yamada D., Saitoh A., Kametani F., Tamaoka A., Araki Y.M., Wada K., Mizusawa H., Araki W. Tyrosol reduces amyloid-β oligomer neurotoxicity and alleviates synaptic, oxidative, and cognitive disturbances in Alzheimer’s disease model mice. J. Alzheimers Dis. 2019;70(3):937–952. doi: 10.3233/JAD-190098. [DOI] [PubMed] [Google Scholar]
- 50.García-Moreno J.C., Porta de la Riva M., Martínez-Lara E., Siles E., Cañuelo A. Tyrosol, a simple phenol from EVOO, targets multiple pathogenic mechanisms of neurodegeneration in a C. elegans model of Parkinson’s disease. Neurobiol. Aging. 2019;82:60–68. doi: 10.1016/j.neurobiolaging.2019.07.003. [DOI] [PubMed] [Google Scholar]
- 51.Qu Z.Q., Zhou Y., Zeng Y.S., Lin Y.K., Li Y., Zhong Z.Q., Chan W.Y. Protective effects of a Rhodiola crenulata extract and salidroside on hippocampal neurogenesis against streptozotocin-induced neural injury in the rat. PLoS One. 2012;7(1):e29641. doi: 10.1371/journal.pone.0029641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Vissers M.N., Zock P.L., Katan M.B. Bioavailability and antioxidant effects of olive oil phenols in humans: a review. Eur. J. Clin. Nutr. 2004;58(6):955–965. doi: 10.1038/sj.ejcn.1601917. [DOI] [PubMed] [Google Scholar]
- 53.Covas M.I., Miró-Casas E., Fitó M., Farré-Albadalejo M., Gimeno E., Marrugat J., De La Torre R. Bioavailability of tyrosol, an antioxidant phenolic compound present in wine and olive oil, in humans. Drugs Exp. Clin. Res. 2003;29(5-6):203–206. [PubMed] [Google Scholar]
- 54.Soldevila-Domenech N., Boronat A., Mateus J., Diaz-Pellicer P., Matilla I., Pérez-Otero M., Aldea-Perona A., de la Torre R. Generation of the antioxidant hydroxytyrosol from tyrosol present in beer and red wine in a randomized clinical trial. Nutrients. 2019;11(9):E2241. doi: 10.3390/nu11092241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Pérez-Mañá C., Farré M., Rodríguez-Morató J., Papaseit E., Pujadas M., Fitó M., Robledo P., Covas M.I., Cheynier V., Meudec E., Escudier J.L., de la Torre R. Moderate consumption of wine, through both its phenolic compounds and alcohol content, promotes hydroxytyrosol endogenous generation in humans. A randomized controlled trial. Mol. Nutr. Food Res. 2015;59(6):1213–1216. doi: 10.1002/mnfr.201400842. [DOI] [PubMed] [Google Scholar]
- 56.Boronat A., Martínez-Huélamo M., Cobos A., de la Torre R. Wine and olive oil phenolic compounds interaction in humans. Diseases. 2018;6(3):E76. doi: 10.3390/diseases6030076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chernysheva G.A., Smol’niakova V.I., Cherkashina I.V., Plotnikov M.B., Tolstikova T.G., Krysin A.P., Sorokina I.V. The main pharmacokinetic parameters of p-tyrosol upon intravenous injection in rats. Eksp. Klin. Farmakol. 2005;68(6):43–44. [PubMed] [Google Scholar]
- 58.Guo N., Hu Z., Fan X., Zheng J., Zhang D., Xu T., Yu T., Wang Y., Li H. Simultaneous determination of salidroside and its aglycone metabolite p-tyrosol in rat plasma by liquid chromatography-tandem mass spectrometry. Molecules. 2012;17(4):4733–4754. doi: 10.3390/molecules17044733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chernysheva G.A., Smol’niakova V.I., Cherkashina I.V., Tolstikova T.G., Krysin A.P., Sorokina I.V. Pharmacokinetics of p-tyrosol when administered orally. Pharmacy (Basel) 2007;5:34–35. [Google Scholar]
- 60.Chernyshova G.A., Plotnikov M.B., Smol’iakova V.I., Krasnov E.A. Main pharmacokinetic parameters of p-tyrosol after intravenous injection in rats. Part III: Distribution of p-tyrosol in rat. Eksp. Klin. Farmakol. 2011;74(7):27–29. [PubMed] [Google Scholar]
- 61.D’Antuono I., Garbetta A., Ciasca B., Linsalata V., Minervini F., Lattanzio V.M., Logrieco A.F., Cardinali A. Biophenols from Table Olive cv Bella di Cerignola: chemical characterization, bioaccessibility, and intestinal absorption. J. Agric. Food Chem. 2016;64(28):5671–5678. doi: 10.1021/acs.jafc.6b01642. [DOI] [PubMed] [Google Scholar]
- 62.Ginsberg M.D. Life after cerovive: a personal perspective on ischemic neuroprotection in the post-NXY-059 era. Stroke. 2007;38(6):1967–1972. doi: 10.1161/STROKEAHA.106.479170. [DOI] [PubMed] [Google Scholar]
- 63.van der Worp H.B., Kappelle L.J., Algra A., Bär P.R., Orgogozo J.M., Ringelstein E.B., Bath P.M., van Gijn J., TESS Investigators TESS II Investigators. The effect of tirilazad mesylate on infarct volume of patients with acute ischemic stroke. Neurology. 2002;58(1):133–135. doi: 10.1212/WNL.58.1.133. [DOI] [PubMed] [Google Scholar]
- 64.Zafra-Gómez A., Luzón-Toro B., Jiménez-Diaz I., Ballesteros O., Navalón A. Quantification of phenolic antioxidants in rat cerebrospinal fluid by GC-MS after oral administration of compounds. J. Pharm. Biomed. Anal. 2010;53(1):103–108. doi: 10.1016/j.jpba.2010.03.003. [DOI] [PubMed] [Google Scholar]
- 65.Tuck K.L., Freeman M.P., Hayball P.J., Stretch G.L., Stupans I. The in vivo fate of hydroxytyrosol and tyrosol, antioxidant phenolic constituents of olive oil, after intravenous and oral dosing of labeled compounds to rats. J. Nutr. 2001;131(7):1993–1996. doi: 10.1093/jn/131.7.1993. [DOI] [PubMed] [Google Scholar]
- 66.Rodríguez-Morató J., Robledo P., Tanner J.A., Boronat A., Pérez-Mañá C., Oliver C.C.Y., Tyndale R.F., de la Torre R. CYP2D6 and CYP2A6 biotransform dietary tyrosol into hydroxytyrosol. Food Chem. 2017;217:716–725. doi: 10.1016/j.foodchem.2016.09.026. [DOI] [PubMed] [Google Scholar]
- 67.Boronat A., Mateus J., Soldevila-Domenech N., Guerra M., Rodríguez-Morató J., Varon C., Muñoz D., Barbosa F., Morales J.C., Gaedigk A., Langohr K., Covas M.I., Pérez-Mañá C., Fitó M., Tyndale R.F., de la Torre R. Cardiovascular benefits of tyrosol and its endogenous conversion into hydroxytyrosol in humans. A randomized, controlled trial. Free Radic. Biol. Med. 2019;143:471–481. doi: 10.1016/j.freeradbiomed.2019.08.032. [DOI] [PubMed] [Google Scholar]
- 68.Young J., Wahle K.W., Boyle S.P. Cytoprotective effects of phenolic antioxidants and essential fatty acids in human blood monocyte and neuroblastoma cell lines: surrogates for neurological damage in vivo. Prostaglandins Leukot. Essent. Fatty Acids. 2008;78(1):45–59. doi: 10.1016/j.plefa.2007.10.005. [DOI] [PubMed] [Google Scholar]
- 69.Atzeri A., Lucas R., Incani A., Peñalver P., Zafra-Gómez A., Melis M.P., Pizzala R., Morales J.C., Deiana M. Hydroxytyrosol and tyrosol sulfate metabolites protect against the oxidized cholesterol pro-oxidant effect in Caco-2 human enterocyte-like cells. Food Funct. 2016;7(1):337–346. doi: 10.1039/C5FO00074B. [DOI] [PubMed] [Google Scholar]
- 70.Muriana F.J.G., Montserrat-de la Paz S., Lucas R., Bermudez B., Jaramillo S., Morales J.C., Abia R., Lopez S. Tyrosol and its metabolites as antioxidative and anti-inflammatory molecules in human endothelial cells. Food Funct. 2017;8(8):2905–2914. doi: 10.1039/C7FO00641A. [DOI] [PubMed] [Google Scholar]
- 71.Serreli G., Deiana M. Biological relevance of extra virgin olive oil polyphenols metabolites. Antioxidants. 2018;7(12):E170. doi: 10.3390/antiox7120170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Roleira F.M.F., Tavares-da-Silva E.J., Garrido J., Borges F. In: Antioxidants and stroke: success and pitfalls. Translational Stroke Research, Springer Series in Translational Stroke Research; Lapchak, P. Zhang J., editor. New York: Springer; 2012. pp. 117–143. [Google Scholar]
- 73.Barber P.A., Demchuk A.M., Hirt L., Buchan A.M. Biochemistry of ischemic stroke. Adv. Neurol. 2003;92:151–164. [PubMed] [Google Scholar]
- 74.Sanderson T.H., Reynolds C.A., Kumar R., Przyklenk K., Hüttemann M. Molecular mechanisms of ischemia-reperfusion injury in brain: pivotal role of the mitochondrial membrane potential in reactive oxygen species generation. Mol. Neurobiol. 2013;47(1):9–23. doi: 10.1007/s12035-012-8344-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Chamorro Á., Dirnagl U., Urra X., Planas A.M. Neuroprotection in acute stroke: targeting excitotoxicity, oxidative and nitrosative stress, and inflammation. Lancet Neurol. 2016;15(8):869–881. doi: 10.1016/S1474-4422(16)00114-9. [DOI] [PubMed] [Google Scholar]
- 76.Margaill I., Plotkine M., Lerouet D. Antioxidant strategies in the treatment of stroke. Free Radic. Biol. Med. 2005;39(4):429–443. doi: 10.1016/j.freeradbiomed.2005.05.003. [DOI] [PubMed] [Google Scholar]
- 77.Wang C.X., Shuaib A. Neuroprotective effects of free radical scavengers in stroke. Drugs Aging. 2007;24(7):537–546. doi: 10.2165/00002512-200724070-00002. [DOI] [PubMed] [Google Scholar]
- 78.Cerretani L., Bendini A. In: Tyrosol is inferior to other simple phenols and polyphenols in antiradical / antioxidant activity when different non-cellular systems are used to evaluate this activityOlives and Olive Oil in Health and Disease Prevention; Preedy, V. Watson R., editor. Amsterdam: Academic Press; 2010. pp. 625–635. [Google Scholar]
- 79.Mateos R., Domínguez M.M., Espartero J.L., Cert A. Antioxidant effect of phenolic compounds, alpha-tocopherol, and other minor components in virgin olive oil. J. Agric. Food Chem. 2003;51(24):7170–7175. doi: 10.1021/jf034415q. [DOI] [PubMed] [Google Scholar]
- 80.Khymenets O., Fitó M., Touriño S., Muñoz-Aguayo D., Pujadas M., Torres J.L., Joglar J., Farré M., Covas M.I., de la Torre R. Antioxidant activities of hydroxytyrosol main metabolites do not contribute to beneficial health effects after olive oil ingestion. Drug Metab. Dispos. 2010;38(9):1417–1421. doi: 10.1124/dmd.110.032821. [DOI] [PubMed] [Google Scholar]
- 81.Le Tutour B., Guedon D. Antioxidative activities of Olea europaea leaves and related phenolic compounds. Phytochemistry. 1992;31:1173–1178. doi: 10.1016/0031-9422(92)80255-D. [DOI] [Google Scholar]
- 82.Fusi J., Bianchi S., Daniele S., Pellegrini S., Martini C., Galetta F., Giovannini L., Franzoni F. An in vitro comparative study of the antioxidant activity and SIRT1 modulation of natural compounds. Biomed. Pharmacother. 2018;101:805–819. doi: 10.1016/j.biopha.2018.03.006. [DOI] [PubMed] [Google Scholar]
- 83.de la Puerta R., Martínez Domínguez M.E., Ruíz-Gutíerrez V., Flavill J.A., Hoult J.R. Effects of virgin olive oil phenolics on scavenging of reactive nitrogen species and upon nitrergic neurotransmission. Life Sci. 2001;69(10):1213–1222. doi: 10.1016/S0024-3205(01)01218-8. [DOI] [PubMed] [Google Scholar]
- 84.Drummen G.P., Makkinje M., Verkleij A.J., Op den Kamp J.A., Post J.A. Attenuation of lipid peroxidation by antioxidants in rat-1 fibroblasts: comparison of the lipid peroxidation reporter molecules cis-parinaric acid and C11-BODIPY(581/591) in a biological setting. Biochim. Biophys. Acta. 2004;1636(2-3):136–150. doi: 10.1016/j.bbalip.2003.10.013. [DOI] [PubMed] [Google Scholar]
- 85.Vlachogianni I.C., Fragopoulou E., Kostakis I.K., Antonopoulou S. In vitro assessment of antioxidant activity of tyrosol, resveratrol and their acetylated derivatives. Food Chem. 2015;177:165–173. doi: 10.1016/j.foodchem.2014.12.092. [DOI] [PubMed] [Google Scholar]
- 86.Pérez-Bonilla M., Salido S., van Beek T.A., Linares-Palomino P.J., Altarejos J., Nogueras M., Sánchez A. Isolation and identification of radical scavengers in olive tree (Olea europaea) wood. J. Chromatogr. A. 2006;1112(1-2):311–318. doi: 10.1016/j.chroma.2005.12.055. [DOI] [PubMed] [Google Scholar]
- 87.Chen D., Fan J., Wang P., Zhu L., Jin Y., Peng Y., Du S. Isolation, identification and antioxidative capacity of water-soluble phenylpropanoid compounds from Rhodiola crenulata. Food Chem. 2012;134(4):2126–2133. doi: 10.1016/j.foodchem.2012.04.011. [DOI] [PubMed] [Google Scholar]
- 88.Dávalos J.Z., Valderrama-Negrón A.C., Barrios J.R., Freitas V.L.S., Ribeiro da Silva M.D.M.C. Energetic and structural properties of two phenolic antioxidants: tyrosol and hydroxytyrosol. J. Phys. Chem. A. 2018;122(16):4130–4137. doi: 10.1021/acs.jpca.8b00457. [DOI] [PubMed] [Google Scholar]
- 89.Di Benedetto R., Varì R., Scazzocchio B., Filesi C., Santangelo C., Giovannini C., Matarrese P., D’Archivio M., Masella R. Tyrosol, the major extra virgin olive oil compound, restored intracellular antioxidant defences in spite of its weak antioxidative effectiveness. Nutr. Metab. Cardiovasc. Dis. 2007;17(7):535–545. doi: 10.1016/j.numecd.2006.03.005. [DOI] [PubMed] [Google Scholar]
- 90.Lee K.M., Hur J., Lee Y., Yoon B-R., Choi S.Y. Protective effects of tyrosol against oxidative damage in L6 muscle cells. Food Sci. Technol. Res. 2018;24:943–947. doi: 10.3136/fstr.24.943. [DOI] [Google Scholar]
- 91.Giovannini C., Straface E., Modesti D., Coni E., Cantafora A., De Vincenzi M., Malorni W., Masella R. Tyrosol, the major olive oil biophenol, protects against oxidized-LDL-induced injury in Caco-2 cells. J. Nutr. 1999;129(7):1269–1277. doi: 10.1093/jn/129.7.1269. [DOI] [PubMed] [Google Scholar]
- 92.Giovannini C., Scazzocchio B., Matarrese P., Varì R., D’Archivio M., Di Benedetto R., Casciani S., Dessì M.R., Straface E., Malorni W., Masella R. Apoptosis induced by oxidized lipids is associated with up-regulation of p66Shc in intestinal Caco-2 cells: protective effects of phenolic compounds. J. Nutr. Biochem. 2008;19(2):118–128. doi: 10.1016/j.jnutbio.2007.01.010. [DOI] [PubMed] [Google Scholar]
- 93.Deiana M., Corona G., Incani A., Loru D., Rosa A., Atzeri A., Paola Melis M., Assunta Dessì M. Protective effect of simple phenols from extravirgin olive oil against lipid peroxidation in intestinal Caco-2 cells. Food Chem. Toxicol. 2010;48(10):3008–3016. doi: 10.1016/j.fct.2010.07.041. [DOI] [PubMed] [Google Scholar]
- 94.Moreno J.J. Effect of olive oil minor components on oxidative stress and arachidonic acid mobilization and metabolism by macrophages RAW 264.7. Free Radic. Biol. Med. 2003;35(9):1073–1081. doi: 10.1016/S0891-5849(03)00465-9. [DOI] [PubMed] [Google Scholar]
- 95.Chimi H., Morel I., Lescoat G., Pasdeloup N., Cillard P., Cillard J. Inhibition of iron toxicity in rat hepatocyte culture by natural phenolic compounds. Toxicol. In Vitro. 1995;9(5):695–702. doi: 10.1016/0887-2333(95)00060-L. [DOI] [PubMed] [Google Scholar]
- 96.Aissa I., Bouaziz M., Frikha F., Ben Mansourc R., Gargouri Y. Synthesized tyrosyl hydroxyphenylacetate, a novel antioxidant, anti-stress and antibacterial compound. Process Biochem. 2012;47:2356–2364. doi: 10.1016/j.procbio.2012.09.016. [DOI] [Google Scholar]
- 97.Ma C.J., Kim S.R., Kim J., Kim Y.C. Meso-dihydroguaiaretic acid and licarin A of Machilus thunbergii protect against glutamate-induced toxicity in primary cultures of a rat cortical cells. Br. J. Pharmacol. 2005;146(5):752–759. doi: 10.1038/sj.bjp.0706380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Salucci S., Burattini S., Battistelli M., Buontempo F., Canonico B., Martelli A.M., Papa S., Falcieri E. Tyrosol prevents apoptosis in irradiated keratinocytes. J. Dermatol. Sci. 2015;80(1):61–68. doi: 10.1016/j.jdermsci.2015.07.002. [DOI] [PubMed] [Google Scholar]
- 99.Anrather J., Iadecola C. Inflammation and stroke: An overview. Neurotherapeutics. 2016;13(4):661–670. doi: 10.1007/s13311-016-0483-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Barone F.C., Schmidt D.B., Hillegass L.M., Price W.J., White R.F., Feuerstein G.Z., Clark R.K., Lee E.V., Griswold D.E., Sarau H.M. Reperfusion increases neutrophils and leukotriene B4 receptor binding in rat focal ischemia. Stroke. 1992;23(9):1337–1347. doi: 10.1161/01.STR.23.9.1337. [DOI] [PubMed] [Google Scholar]
- 101.Feuerstein G.Z., Wang X., Barone F.C. Inflammatory gene expression in cerebral ischemia and trauma. Potential new therapeutic targets. Ann. N. Y. Acad. Sci. 1997;825:179–193. doi: 10.1111/j.1749-6632.1997.tb48428.x. [DOI] [PubMed] [Google Scholar]
- 102.Gehrmann J., Bonnekoh P., Miyazawa T., Hossmann K.A., Kreutzberg G.W. Immunocytochemical study of an early microglial activation in ischemia. J. Cereb. Blood Flow Metab. 1992;12(2):257–269. doi: 10.1038/jcbfm.1992.36. [DOI] [PubMed] [Google Scholar]
- 103.Liu J., Zhang C., Tao W., Liu M. Systematic review and meta-analysis of the efficacy of sphingosine-1-phosphate (S1P) receptor agonist FTY720 (fingolimod) in animal models of stroke. Int. J. Neurosci. 2013;123(3):163–169. doi: 10.3109/00207454.2012.749255. [DOI] [PubMed] [Google Scholar]
- 104.Fu Y., Zhang N., Ren L., Yan Y., Sun N., Li Y.J., Han W., Xue R., Liu Q., Hao J., Yu C., Shi F.D., Hao J., Yu C., Shi F.D. Impact of an immune modulator fingolimod on acute ischemic stroke. Proc. Natl. Acad. Sci. USA. 2014;111(51):18315–18320. doi: 10.1073/pnas.1416166111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Luo G., Huang Y., Mo D., Ma N., Gao F., Song L., Sun X., Xu X., Liu L., Huo X., Wang B., Li X., Jia B., Deng Y., Zhang X., Fernandez-Escobar A., Peng G., Miao Z. Tyrosol attenuates pro-inflammatory cytokines from cultured astrocytes and NF-κB activation in in vitro oxygen glucose deprivation. Neurochem. Int. 2018;121:140–145. doi: 10.1016/j.neuint.2018.10.006. [DOI] [PubMed] [Google Scholar]
- 106.Lambertsen K.L., Biber K., Finsen B. Inflammatory cytokines in experimental and human stroke. J. Cereb. Blood Flow Metab. 2012;32(9):1677–1698. doi: 10.1038/jcbfm.2012.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Je I.G., Kim D.S., Kim S.W., Lee S., Lee H.S., Park E.K., Khang D., Kim S.H. Tyrosol suppresses allergic inflammation by inhibiting the activation of phosphoinositide 3-kinase in mast cells. PLoS One. 2015;10(6):e0129829. doi: 10.1371/journal.pone.0129829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Giovannini L., Migliori M., Filippi C., Origlia N., Panichi V., Falchi M., Bertelli A.A., Bertelli A. Inhibitory activity of the white wine compounds, tyrosol and caffeic acid, on lipopolysaccharide-induced tumor necrosis factor-alpha release in human peripheral blood mononuclear cells. Int. J. Tissue React. 2002;24(2):53–56. [PubMed] [Google Scholar]
- 109.De Stefano D., Maiuri M.C., Carnuccio R. In: Effects of Tyrosol on RAW 264.7 Macrophages activated by interferon-γ and gliadin Olives and Olive Oil in Health and Disease Prevention; Preedy, V.; Watson, R. Carnuccio R., editor. Amsterdam: Academic Press; 2010. pp. 1263–1268. [Google Scholar]
- 110.De Stefano D., Maiuri M.C., Simeon V., Grassia G., Soscia A., Cinelli M.P., Carnuccio R. Lycopene, quercetin and tyrosol prevent macrophage activation induced by gliadin and IFN-gamma. Eur. J. Pharmacol. 2007;566(1-3):192–199. doi: 10.1016/j.ejphar.2007.03.051. [DOI] [PubMed] [Google Scholar]
- 111.de la Puerta R., Ruiz Gutierrez V., Hoult J.R.S. Inhibition of leukocyte 5-lipoxygenase by phenolics from virgin olive oil. Biochem. Pharmacol. 1999;57(4):445–449. doi: 10.1016/S0006-2952(98)00320-7. [DOI] [PubMed] [Google Scholar]
- 112.Yadav T.C., Kumar N., Raj U., Goel N., Vardawaj P.K., Prasad R., Pruthi V. Exploration of interaction mechanism of tyrosol as a potent anti-inflammatory agent. J. Biomol. Struct. Dyn. 2020;38(2):382–397. doi: 10.1080/07391102.2019.1575283. [DOI] [PubMed] [Google Scholar]
- 113.Serreli G., Melis M.P., Corona G., Deiana M. Modulation of LPS-induced nitric oxide production in intestinal cells by hydroxytyrosol and tyrosol metabolites: Insight into the mechanism of action. Food Chem. Toxicol. 2019;125:520–527. doi: 10.1016/j.fct.2019.01.039. [DOI] [PubMed] [Google Scholar]
- 114.Chang C-Y., Huang I-T., Shih H-J., Chang Y-Y., Kao M-C., Shih P-C., Huang C-J. Cluster of differentiation 14 and toll-like receptor 4 are involved in the anti-inflammatory effects of tyrosol. J. Funct. Foods. 2019;53:93–104. doi: 10.1016/j.jff.2018.12.011. [DOI] [Google Scholar]
- 115.Carluccio M.A., Siculella L., Ancora M.A., Massaro M., Scoditti E., Storelli C., Visioli F., Distante A., De Caterina R. Olive oil and red wine antioxidant polyphenols inhibit endothelial activation: antiatherogenic properties of Mediterranean diet phytochemicals. Arterioscler. Thromb. Vasc. Biol. 2003;23(4):622–629. doi: 10.1161/01.ATV.0000062884.69432.A0. [DOI] [PubMed] [Google Scholar]
- 116.Manna C., Napoli D., Cacciapuoti G., Porcelli M., Zappia V. Olive oil phenolic compounds inhibit homocysteine-induced endothelial cell adhesion regardless of their different antioxidant activity. J. Agric. Food Chem. 2009;57(9):3478–3482. doi: 10.1021/jf8037659. [DOI] [PubMed] [Google Scholar]
- 117.Kim Y.Y., Lee S., Kim M.J., Kang B.C., Dhakal H., Choi Y.A., Park P.H., Choi H., Shin T.Y., Choi H.G., Kwon T.K., Khang D., Kim S.H. Tyrosol attenuates lipopolysaccharide-induced acute lung injury by inhibiting the inflammatory response and maintaining the alveolar capillary barrier. Food Chem. Toxicol. 2017;109(Pt 1):526–533. doi: 10.1016/j.fct.2017.09.053. [DOI] [PubMed] [Google Scholar]
- 118.Wang W.C., Xia Y.M., Yang B., Su X.N., Chen J.K., Li W., Jiang T. Protective effects of tyrosol against LPS-induced acute lung injury via inhibiting NF-κB and AP-1 activation and activating the HO-1/Nrf2 pathways. Biol. Pharm. Bull. 2017;40(5):583–593. doi: 10.1248/bpb.b16-00756. [DOI] [PubMed] [Google Scholar]
- 119.Sato K., Mihara Y., Kanai K., Yamashita Y., Kimura Y., Itoh N. Relative potency of tyrosol in the treatment of endotoxin-induced uveitis in rats. J. Vet. Med. Sci. 2016;78(10):1631–1634. doi: 10.1292/jvms.16-0254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Miles E.A., Zoubouli P., Calder P.C. Differential anti-inflammatory effects of phenolic compounds from extra virgin olive oil identified in human whole blood cultures. Nutrition. 2005;21(3):389–394. doi: 10.1016/j.nut.2004.06.031. [DOI] [PubMed] [Google Scholar]
- 121.Dell’Agli M., Fagnani R., Galli G.V., Maschi O., Gilardi F., Bellosta S., Crestani M., Bosisio E., De Fabiani E., Caruso D. Olive oil phenols modulate the expression of metalloproteinase 9 in THP-1 cells by acting on nuclear factor-kappaB signaling. J. Agric. Food Chem. 2010;58(4):2246–2252. doi: 10.1021/jf9042503. [DOI] [PubMed] [Google Scholar]
- 122.Palmerini C.A., Carlini E., Saccardi C., Servili M., Montedoro G., Arienti G. Activity of olive oil phenols on lymphomonocyte cytosolic calcium. J. Nutr. Biochem. 2005;16(2):109–113. doi: 10.1016/j.jnutbio.2004.10.006. [DOI] [PubMed] [Google Scholar]
- 123.Green D.R., Reed J.C. Mitochondria and apoptosis. Science. 1998;281(5381):1309–1312. doi: 10.1126/science.281.5381.1309. [DOI] [PubMed] [Google Scholar]
- 124.Sun Y., Liu W.Z., Liu T., Feng X., Yang N., Zhou H.F. Signaling pathway of MAPK/ERK in cell proliferation, differentiation, migration, senescence and apoptosis. J. Recept. Signal Transduct. Res. 2015;35(6):600–604. doi: 10.3109/10799893.2015.1030412. [DOI] [PubMed] [Google Scholar]
- 125.Mosca L., Lendaro E., d’Erme M., Marcellini S., Moretti S., Rosei M.A. 5-S-Cysteinyl-dopamine effect on the human dopaminergic neuroblastoma cell line SH-SY5Y. Neurochem. Int. 2006;49(3):262–269. doi: 10.1016/j.neuint.2006.01.023. [DOI] [PubMed] [Google Scholar]
- 126.Sun L., Isaak C.K., Zhou Y., Petkau J.C. O, K.; Liu, Y.; Siow, Y.L. Salidroside and tyrosol from Rhodiola protect H9c2 cells from ischemia/reperfusion-induced apoptosis. Life Sci. 2012;91(5-6):151–158. doi: 10.1016/j.lfs.2012.06.026. [DOI] [PubMed] [Google Scholar]
- 127.Chen H.H., Chen Y.T., Huang Y.W., Tsai H.J., Kuo C.C. 4-Ketopinoresinol, a novel naturally occurring ARE activator, induces the Nrf2/HO-1 axis and protects against oxidative stress-induced cell injury via activation of PI3K/AKT signaling. Free Radic. Biol. Med. 2012;52(6):1054–1066. doi: 10.1016/j.freeradbiomed.2011.12.012. [DOI] [PubMed] [Google Scholar]
- 128.Ott E.O., Lechner H., Aranibar A. High blood viscosity syndrome in cerebral infarction. Stroke. 1974;5(3):330–333. doi: 10.1161/01.STR.5.3.330. [DOI] [PubMed] [Google Scholar]
- 129.Fisher M., Meiselman H.J. Hemorheological factors in cerebral ischemia. Stroke. 1991;22(9):1164–1169. doi: 10.1161/01.STR.22.9.1164. [DOI] [PubMed] [Google Scholar]
- 130.van Kooten F., Ciabattoni G., Patrono C., Dippel D.W., Koudstaal P.J. Platelet activation and lipid peroxidation in patients with acute ischemic stroke. Stroke. 1997;28(8):1557–1563. doi: 10.1161/01.STR.28.8.1557. [DOI] [PubMed] [Google Scholar]
- 131.McCarty M.F., O’Keefe J.H., DiNicolantonio J.J. Pentoxifylline for vascular health: a brief review of the literature. Open Heart. 2016;3(1):e000365. doi: 10.1136/openhrt-2015-000365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Karković M.A., Torić J., Barbarić M., Jakobušić B.C., Torić J., Barbarić M., Jakobušić B.C. Hydroxytyrosol, tyrosol and derivatives and their potential effects on human health. Molecules. 2019;24(10):E2001. doi: 10.3390/molecules24102001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Vauzour D. Dietary polyphenols as modulators of brain functions: biological actions and molecular mechanisms underpinning their beneficial effects. Oxid. Med. Cell. Longev. 2012;2012:914273. doi: 10.1155/2012/914273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Obied H.K., Prenzler P.D., Omar S.H., Ismael R., Servili M., Esposto S., Taticchi A., Selvaggini R., Urbani S. Pharmacology of olive biophenols. In: Fishbein J.C., editor. Advances in Molecular Toxicology. Vol. 6. Oxford, UK: Elsevier Science B. V.; 2012. pp. 195–242. [Google Scholar]
- 135.Angeloni C., Malaguti M., Barbalace M.C., Hrelia S. Bioactivity of Olive Oil Phenols in Neuroprotection. Int. J. Mol. Sci. 2017;18(11):E2230. doi: 10.3390/ijms18112230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Khoshnam S.E., Winlow W., Farzaneh M., Farbood Y., Moghaddam H.F. Pathogenic mechanisms following ischemic stroke. Neurol. Sci. 2017;38(7):1167–1186. doi: 10.1007/s10072-017-2938-1. [DOI] [PubMed] [Google Scholar]
- 137.McDonald S., Prenzler P., Antolovich M., Robards K. Phenolic content and antioxidant activity of olive extracts. Food Chem. 2001;73:73–84. doi: 10.1016/S0308-8146(00)00288-0. [DOI] [Google Scholar]
- 138.Anter J., Campos-Sánchez J., Hamss R.E., Rojas-Molina M., Muñoz-Serrano A., Analla M., Alonso-Moraga A. Modulation of genotoxicity by extra-virgin olive oil and some of its distinctive components assessed by use of the Drosophila wing-spot test. Mutat. Res. 2010;703(2):137–142. doi: 10.1016/j.mrgentox.2010.08.012. [DOI] [PubMed] [Google Scholar]
- 139.Nousis L., Doulias P.T., Aligiannis N., Bazios D., Agalias A., Galaris D., Mitakou S. DNA protecting and genotoxic effects of olive oil related components in cells exposed to hydrogen peroxide. Free Radic. Res. 2005;39(7):787–795. doi: 10.1080/10715760500045806. [DOI] [PubMed] [Google Scholar]