Abstract
Introduction
Critically ill COVID-19 patients are at high risk for nosocomial bacterial and fungal infections due to several predisposing factors such as intensive care unit stay, mechanical ventilation, and broad-spectrum antibiotics. Data regarding multidrug resistant (MDR) Candida species in COVID-19 patients is scarce, and nonexistent regarding Candida duobushaemulonii superinfections.
Case description
A 34-year-old male presented to our institution with acute respiratory distress syndrome (ARDS) due to COVID-19 infection and developed Candida duobushaemulonii fungemia after multiple courses of antibiotics and prolonged mechanical ventilation. He died after recurrent pneumothorax led to respiratory failure and cardiac arrest.
Discussion
Bacterial and fungal infections are common complications of viral pneumonia in critically ill patients. Data regarding these infections in COVID-19 patients has been poorly studied with only a few cases reporting secondary infection, mostly without identifying specific pathogens. Prolonged hospital stays, invasive interventions (central venous catheter, mechanical ventilation), and the use of broad-spectrum antibiotics in COVID-19 infections could carry a high risk of bacterial and/or fungal superinfections.
Conclusion
Strategies to improve outcome in COVID-19 ICU patients should include early recognition of candidemia and appropriate antifungal therapy.
Keywords: COVID-19, Candida duobushaemulonii, ICU, Multidrug resistance, Mechanical ventilation, Broad-spectrum antibiotics
Introduction
1.9 million deaths globally have been linked to the COVID-19 pandemic with 88 million cumulative cases reported as of January 12, 2021 [1]. Critically ill patients with COVID-19 are at high risk of developing acute respiratory distress syndrome (ARDS), requiring intensive care and mechanical ventilation, predisposing them to nosocomial bacterial and fungal infections [2,3]. In India, candidemia affected 15 critically ill coronavirus disease patients admitted to an intensive care unit (ICU), with two third of cases attributed to multidrug resistant Candida auris [4]. Data regarding multidrug resistant (MDR) Candida species in COVID-19 patients is scarce, and nonexistent regarding Candida duobushaemulonii superinfections. Candida duobushaemulonii is a yeast that is closely related to Candida auris and is emerging as a rare cause of invasive fungal infections with a multidrug resistant profile [5].
We report a case of Candida duobushaemulonii candidemia in a patient with prolonged ICU stay due to a complicated case of severe COVID-19 infection. To our knowledge, no data exists in the literature in this setting.
Case description
A 34-year-old male without a history of comorbidities presented to our tertiary care center in Beirut, Lebanon from Gabon, Central Africa with severe COVID-19 infection and ARDS. He was hospitalized in Gabon and his medical course was complicated by acute pulmonary embolism treated with recombinant tissue plasminogen activator (rtPA), with subarachnoid hemorrhage (SAH) developing as sequela, and a superimposed bacterial pneumonia for which he received levofloxacin and imipenem.
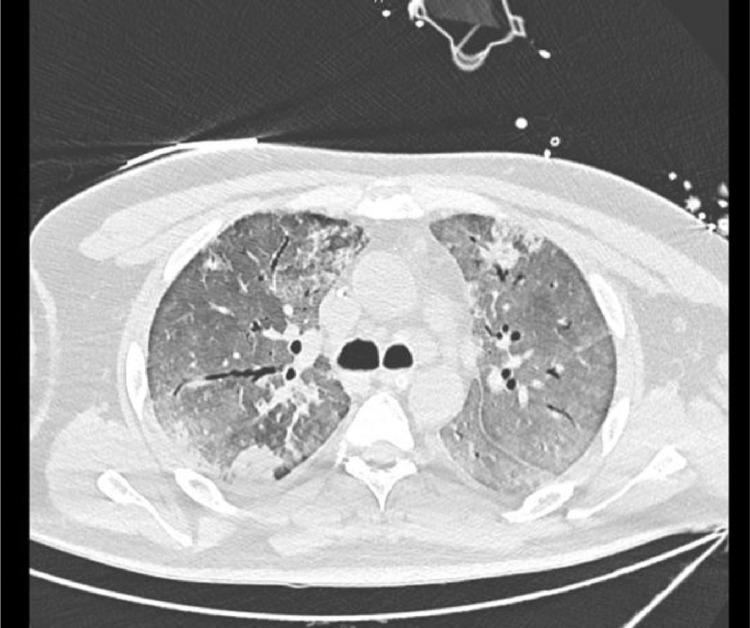
Patient presented 16 days post his COVID-19 infection to our institution. He was intubated, sedated and off vasopressors. His labs are reported and summarized in (Table 1 ) and were significant for elevated pro-inflammatory markers (d-dimer, ferritin, CRP, progressive thrombocytosis) and neutrophilia (80-90%). SARS-CoV-2 PCR was positive on admission. CT chest showed severe ARDS with typical picture of COVID-19 infection (Fig. 1 ). Due to the lack of data regarding local resistance in Gabon and the patient's recent history of multiple antibiotic use and long ICU stay, the decision was made to start meropenem for superimposed pneumonia. Blood, urine, and deep tracheal aspirate (DTA) cultures were taken beforehand. DTA cultures grew Stenotrophomonas maltophilia sensitive to levofloxacin and trimethoprim/sulfamethoxazole (TMP-SMX), and patient was subsequently started on levofloxacin. He was shifted to TMP-SMX after a new isolate of S. maltophilia from DTA was found to be resistant to levofloxacin. He developed catheter acquired urinary tract infection (CAUTI) and ventilator associated pneumonia (VAP) and progressed into septic shock. He was started on amikacin and tigecycline as his cultures grew carbapenem-resistant Enterobacteriaceae (CRE) Enterobacter cloacae with high minimal inhibitory concentrations (MICs) of ceftazidime/avibactam and carbapenems. Five days later, new DTA and urine cultures were taken and were positive for Candida non-albicans, mainly multi-sensitive Candida parapsilosis in the urine and Candida lusitaniae in the DTA. He was subsequently started on intravenous fluconazole.
Table 1.
Brief summary of the lab results during the patient's stay.
| 16/6/2020 | 20/6/2020 | 26/6/2020 | 4/7/2020 | 16/7/2020 | 26/7/2020 | 2/8/2020 | 10/8/2020 | |
|---|---|---|---|---|---|---|---|---|
| WBC (/cu.mm) | 8,600 | 12,100 | 11,900 | 10,300 | 7,600 | 9,200 | 9,200 | 13,000 |
| Neutrophils | 93% | 91% | 85% | 79% | 82% | 90% | 90% | 88% |
| Lymphocytes | 4% | 3% | 6% | 9% | 10% | 7% | 6% | 5% |
| Hb (g/dl) | 10.6 | 10.8 | 10.3 | 9.5 | 8.5 | 8.5 | 8.4 | 9.4 |
| Platelets (/cu.mm) | 164,000 | 194,000 | 366,000 | 360,000 | 355,000 | 352,000 | 401,000 | 562,000 |
| Cr (mg/) | 1 | 0.6 | 0.6 | 0.4 | 0.3 | 0.2 | 0.2 | 0.2 |
| Na (mmol/L) | 144 | 141 | 146 | 141 | 138 | 136 | 135 | 137 |
| K (mmol/L) | 4.8 | 5 | 5.3 | 4.4 | 4.1 | 4.8 | 4 | 4.5 |
| Chloride (mmol/L) | 100 | 102 | 106 | 92 | 90 | 92 | 90 | 93 |
| SGPT (IU/L) | 61 | |||||||
| SGOT (IU/L) | 86 | |||||||
| Alkaline phosphate (IU/L) | 96 | |||||||
| Bilirubin total (mg/dl) | 0.7 | |||||||
| Bilirubin direct (mg/dl) | 0.5 | |||||||
| Ferritin (ng/ml) | 2,207 | 1109 | 820 | 667 | ||||
| D dimer (ng/ml) | 3,862 | 2,160 | 1,825 | |||||
| INR | 1.9 | 1.2 | ||||||
| PTT (seconds) | 29.6 | 27.1 | ||||||
| Procalcitonin (ng/ml) | 6.9 | 0.7 | 0.06 | 0.16 | 0.32 | 0.07 | ||
| CRP (mg/L) | 217.3 | 21.1 | 13.2 | 31 | 27.7 | 24.8 | 43.9 | 85.6 |
| Troponin T (ng/ml) | 0.06 | |||||||
| Blood parasite smear | Negative | |||||||
| COVID-19 PCR | Positive |
Fig. 1.
CT chest showing diffuse ground gland abnormalities with peripheral consolidations. Findings suggestive of severe COVID-19 infection.
Antibiotics were discontinued following completion of fourteen days of therapy and clinical improvement. Antifungal therapy with fluconazole was kept. Patient initially improved clinically but he developed hypotension a week later with elevation of his inflammatory markers (Table 1). Blood cultures were taken from his central line and peripheral lines. He was started on inhaled colistin and tigecycline. Central line blood cultures grew Candida non-albicans, and caspofungin was started. Speciation and susceptibility testing revealed Candida duobushaemulonii, susceptible to flucytosine (Table 2 ). Susceptibility data for other anti-fungals is not available. His stay was again complicated by recurrent pneumothoraces leading to respiratory failure followed by cardiac arrest and death.
Table 2.
Fungal susceptibility of Candida duobushaemulonii based on Vitek testing and interpretation.
| Candida duobushaemulonii | Interpretation | |
|---|---|---|
| Amphotericin B | 8 ug/mL | Resistant |
| Flucytosine | ≤ 1 ug/mL | Susceptible |
| Voriconazole | 4 ug/mL | Resistant |
Discussion
Bacterial and fungal infections are common complications of viral pneumonia, especially in critically ill patients, leading to increased mortality rate [6]. Nosocomial fungal infections, particularly Candidiasis and Aspergillosis, are frequently seen in immunocompromised patients that exhibit predisposing risk factors such as neutropenia, compromised neutrophil function, cell-mediated immune dysfunction, and disruption of mucosal integrity [7,8]. In 2017, a team in France analyzed the proportion of fungemia associated with uncommon yeast species and the predisposing factors in 338 cases. The study demonstrated the existence of 35 species with different susceptibility profiles to antifungal drugs and a predisposition to patients who are immunocompromised or have received prior antifungal therapy [9]. COVID-19 has been found to cause immune dysregulation and hyperinflammation in severe cases potentially contributing to the development of nosocomial infections in severely ill patients [10], [11], [12]. Nevertheless, limited data regarding bacterial and fungal infections in COVID-19 patients has been published [6]. Although the mechanism is still unclear, patients with severe COVID-19 are at similar risk of invasive fungal infections as patients with severe influenza [13]. However, a review of the literature showed that even when secondary infection data was available, the antibiotics use rate (94%–100%) was much higher than the reported incidence of secondary infection (10%–15%), potentially increasing the risk of fungal infections due to endogenous fungi such as Candida species [6,7]. A meta-analysis found three studies that reported four fungal pathogens in COVID-19 patients: Candida albicans, Candida glabrata, Aspergillus flavus and Aspergillus fumigatus [14].
No data in the literature currently exists regarding Candida duobushaemulonii superinfection in COVID-19 patients. A member of the Candida haemulonii species complex, Candida duobushaemulonii has been found to be invasive and resistant to drugs [15]. In 2018, a genomic study found that Candida auris and Candida duobushaemulonii were closely related phylogenetically [16]. Recently, a study found that six patients admitted to the ICU for severe COVID-19 were colonized by Candida auris and four of them developed candidemia. Testing showed resistance of all strains to amphotericin-B and azoles but susceptibility to echinocandins [17]. The increased reports of Candida auris co-infection in severely ill COVID-19 patients and the close phylogenetic relation between Candida auris and Candida duobushaemulonii could signify that Candida duobushaemulonii superinfection is underdiagnosed. In fact, in a study done by Jurado-Martin et al, 150 isolates were reanalyzed using novel PCR approaches to identify multidrug-resistant complex of uncommon Candida species that were missed by regular phenotypic testing [18]. The study found that the prevalence of C. duobushaemulonii was likely underestimated and that the species was initially associated with superficial infections before emerging as a cause of invasive candidiasis. The identified isolates also showed reduced susceptibility to fluconazole, itraconazole, and amphotericin B [18]. In our case, the pathogen was found to be susceptible to flucytosine with casponfungin already having been started empirically, but speciation and susceptibility results had come out after the patient had already died and were only available for flucytosine, voriconazole, and amphotericin B. Risk factors for invasive candidemia include prolonged hospital stay, invasive interventions (central venous catheter, mechanical ventilation), and the use of broad-spectrum antibiotics [19,20]. The wide use of empirical antibiotics in COVID-19 ICU patients could be a major cause of both bacterial and fungal superinfections and warrants additional evaluation. The reliance on clinical presentation, inflammatory markers, and radiological findings is insufficient to confirm secondary infections and may lead to overuse of antibiotics empirically, while current data on co-infections is limited [21].
Antimicrobial stewardship programs aim to optimize antimicrobial use, improve patient outcomes, and reduce harms from excess use, such as antimicrobial resistance [22]. However, due to the lack of stewardship programs targeted at pandemics such as COVID-19, inpatient antibiotic use may have proceeded unchecked for several months, potentially contributing to antimicrobial resistance and the development of secondary bacterial and/or fungal infections from unnecessary empirical use of broad-spectrum antibiotics [20,23,24].
Conclusion
Severely ill COVID-19 are at high risk of developing nosocomial infections associated with mechanical ventilation and the use of broad-spectrum antibiotics. Medical and invasive procedures are potential routes of bacterial and fungal infections, with the latter though rare, is associated with considerable mortality in critically ill patients. Strategies to improve outcome in COVID-19 ICU patients should, therefore, include early recognition of candidemia and appropriate antifungal therapy.
Authors’ contribution
Dr. Bassem Awada contributed to writing the manuscript and data collection.
Dr. Walid Alam contributed to writing the manuscript, literature review, data analysis, and revision of the manuscript.
Maria Chalfoun contributed to writing the manuscript.
Dr. George Araj contributed to writing the manuscript and data analysis.
Dr. Abdul Rahman Bizri contributed to writing the manuscript, data analysis, and revision of the manuscript.
Funding
No funding was received for this work.
Ethics and patient consent
We acknowledge that approval from the American University of Beirut ethical committee was sought where necessary, and guidelines on consent were followed. Informed consent was obtained from the patient's family.
Conflict of Interest
The authors have no conflict of interest.
Acknowledgment
None
References
- 1.https://www.who.int/publications/m/item/weekly-epidemiological-update—12-january-2021.
- 2.Chen N., Zhou M., Dong X., et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395(10223):507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arastehfar A., Carvalho A., Nguyen M.H., et al. COVID-19-Associated Candidiasis (CAC): an underestimated complication in the absence of immunological predispositions? J Fungi. 2020;6(4):211. doi: 10.3390/jof6040211. PublishedOct 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chowdhary A., Tarai B., Singh A., Sharma A. Multidrug-resistant Candida auris infections in critically ill coronavirus disease patients, India, April-July 2020. Emerg Infect Dis. 2020;26(11):2694–2696. doi: 10.3201/eid2611.203504. NovEpub 2020 Aug 27. PMID: 32852265; PMCID: PMC7588547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ramos R., Caceres D.H., Perez M., et al. Emerging multidrug-resistant Candida duobushaemulonii infections in panama hospitals: importance of laboratory surveillance and accurate identification. J Clin Microbiol. 2018;56(7):e00371–e00378. doi: 10.1128/JCM.00371-18. Published 2018 Jun 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhou P., Liu Z., Chen Y., Xiao Y., Huang X., Fan X.G. Bacterial and fungal infections in COVID-19 patients: a matter of concern. Infect Control Hosp Epidemiol. 2020;41(9):1124–1125. doi: 10.1017/ice.2020.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vazquez J.A., Miceli M.H., Alangaden G. Invasive fungal infections in transplant recipients. Ther Adv Infect Dis. 2013;1(3):85–105. doi: 10.1177/2049936113491936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Suleyman G., Alangaden G.J. Nosocomial fungal infections: epidemiology, infection control, and prevention. Infect Dis Clin North Am. 2016;30(4):1023–1052. doi: 10.1016/j.idc.2016.07.008. [DOI] [PubMed] [Google Scholar]
- 9.Bretagne S., Renaudat C., Desnos-Ollivier M., et al. Predisposing factors and outcome of uncommon yeast species-related fungaemia based on an exhaustive surveillance programme (2002-14) J Antimicrob Chemother. 2017;72(6):1784–1793. doi: 10.1093/jac/dkx045. [DOI] [PubMed] [Google Scholar]
- 10.Bardi T., Pintado V., Gomez-Rojo M., et al. Nosocomial infections associated to COVID-19 in the intensive care unit: clinical characteristics and outcome. Eur J Clin Microbiol Infect Dis. 2021;40(3):495–502. doi: 10.1007/s10096-020-04142-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Alam W. Hypercoagulability in COVID-19: a review of the potential mechanisms underlying clotting disorders. SAGE Open Med. 2021;9 doi: 10.1177/20503121211002996. 20503121211002996. Published 2021 Mar 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Reusch N., De Domenico E., Bonaguro L., et al. Neutrophils in COVID-19. Front Immunol. 2021;12 doi: 10.3389/fimmu.2021.652470. Published 2021 Mar 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gangneux J.P., Bougnoux M.E., Dannaoui E., Cornet M., Zahar J.R. Invasive fungal diseases during COVID-19: we should be prepared. J Mycol Med. 2020;30(2) doi: 10.1016/j.mycmed.2020.100971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lansbury L., Lim B., Baskaran V., Lim W.S. Co-infections in people with COVID-19: a systematic review and meta-analysis. J Infect. 2020;81(2):266–275. doi: 10.1016/j.jinf.2020.05.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hou X., Xiao M., Chen S.C., Wang H., Cheng J.W., Chen X.X., Xu Z.P., Fan X., Kong F., Xu Y.C. Identification and antifungal susceptibility profiles of Candida haemulonii species complex clinical isolates from a multicenter study in China. J Clin Microbiol. 2016;54:2676–2680. doi: 10.1128/JCM.01492-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Muñoz J.F., Gade L., Chow N.A., et al. Genomic insights into multidrug-resistance, mating and virulence in Candida auris and related emerging species. Nat Commun. 2018;9(1):5346. doi: 10.1038/s41467-018-07779-6. Published 2018 Dec 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Magnasco L., Mikulska M., Giacobbe D.R., et al. Spread of Carbapenem-resistant gram-negatives and Candida auris DURING the COVID-19 pandemic in critically ill patients: one step back in antimicrobial stewardship? Microorganisms. 2021;9(1):95. doi: 10.3390/microorganisms9010095. Published 2021 Jan 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jurado-Martín I., Marcos-Arias C., Tamayo E., et al. Candida duobushaemulonii: an old but unreported pathogen. J Fungi. 2020;6(4):374. doi: 10.3390/jof6040374. Published 2020 Dec 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Al-Hatmi A.M.S., Mohsin J., Al-Huraizi A., Khamis F. COVID-19 associated invasive candidiasis [published online ahead of print, 2020 Aug 7] J Infect. 2020 doi: 10.1016/j.jinf.2020.08.005. S0163-4453(20)30539-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ben-Ami R., Olshtain-Pops K., Krieger M., et al. Antibiotic exposure as a risk factor for fluconazole-resistant Candida bloodstream infection. Antimicrob Agents Chemother. 2012;56(5):2518–2523. doi: 10.1128/AAC.05947-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Verroken A., Scohy A., Gérard L., et al. Co-infections in COVID-19 critically ill and antibiotic management: a prospective cohort analysis. Crit Care. 2020;24:410. doi: 10.1186/s13054-020-03135-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.https://www.cdc.gov/antibiotic-use/healthcare/evidence/asp-int-am-resistance.html
- 23.Langford B.J., So M., Raybardhan S., et al. Bacterial co-infection and secondary infection in patients with COVID-19: a living rapid review and meta-analysis. Clin Microbiol Infect. 2020;26(12):1622–1629. doi: 10.1016/j.cmi.2020.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.https://www.idsociety.org/covid-19-real-time-learning-network/disease-manifestations--complications/co-infection-and-Antimicrobial-Stewardship/