Abstract
Thrombosis within the microvasculature and medium to large vessels is a serious and common complication among critically ill individuals with coronavirus disease 2019 (COVID‐19). While children are markedly less likely to develop severe disease than adults, they remain at risk for thrombosis during acute infection and with the post‐acute inflammatory illness termed multisystem inflammatory syndrome in children. Significant knowledge deficits in understanding COVID‐19‐associated coagulopathy and thrombotic risk pose clinical challenges for pediatric providers who must incorporate expert opinion and personal experience to manage individual patients. We discuss clinical scenarios to provide framework for characterizing thrombosis risk and thromboprophylaxis in children with COVID‐19.
Keywords: anticoagulation, COVID‐19, MIS‐C, thrombosis
Abbreviations
- AA
African American
- ACS
acute chest syndrome
- CAC
COVID‐19‐associated coagulopathy
- CDC
Centers for Disease Control and prevention
- COVID‐19
coronavirus disease 2019
- DOAC
direct oral anticoagulant
- DVT
deep venous thrombosis
- LMWH
low molecular weight heparin
- MIS‐C
multisystem inflammatory syndrome in children
- PE
pulmonary embolism
- PICU
pediatric intensive care unit
- PT
prothrombin time
- SARS‐CoV‐2
severe acute respiratory syndrome coronavirus 2
- SCD
sickle cell disease
- UFH
unfractionated heparin
- VTE
venous thromboembolism.
1. INTRODUCTION
Coronavirus disease 2019 (COVID‐19) encompasses the different syndromes associated with severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) infection. 1 , 2 At present, three syndromes have been described: acute infection, post‐acute inflammatory illness, and late sequelae. 3 Acute infection has primary respiratory manifestations and can be characterized as mild, moderate, and severe using a WHO progression scale. 4 Post‐acute inflammatory illness, which was first described in children 5 and termed multisystem inflammatory syndrome in children or adults (MIS‐C/MIS‐A), most often presents few weeks after a recent SARS‐CoV‐2 infection. 6 , 7 This article will focus on thrombosis risk for both acute infection and post‐acute inflammatory illness.
Micro‐ and macrovascular thrombotic events are predominant features of critically ill adults with COVID‐19. 8 , 9 , 10 This adverse outcome results from an immune‐thrombotic phenotype termed COVID‐19‐associated coagulopathy (CAC). 8 , 11 Prominent features of CAC include a marked host inflammatory response with endothelial cell dysfunction characterized by elevation in biomarkers of thrombogenesis specifically D‐dimers. 8 , 11 , 12 , 13 , 14 , 15 , 16 Bleeding is not common and the thrombotic spectrum of CAC ranges from pulmonary embolism (PE), deep venous thrombosis (DVT), thrombotic microangiopathy (TMA), and arterial events including stroke. 9 , 10 , 17 , 18 Thrombotic complications have also been reported in children with COVID‐19, 7 , 19 , 20 but much less is known about its prevalence and risk factors. Anticoagulation therapy has shown to reduce mortality in hospitalized adult patients. 18 Therefore, several scientific societies have recommended pharmacological thromboprophylaxis in adults. 21 , 22 Recently, the International Society of Thrombosis and Haemostasis (ISTH) proposed a set of consensus guidelines for the prevention of venous thromboembolism (VTE) in children with COVID‐19 based mainly on expert opinion and extrapolation from adult studies with inclusion of D‐dimer and prothrombotic risk factors for decision making. 23 Nevertheless, pediatric providers are challenged with using available evidence to implement thromboprophylaxis strategies in children with COVID‐19.
In this article, we discuss specific clinical vignettes to illustrate approaches to assessing thrombosis risk and decision making regarding thromboprophylaxis in children with COVID‐19. Our goal is to use these cases to highlight challenging issues that are not addressed in detail within the consensus guidelines, 23 which are listed in Table 1 Key considerations in our proposed approaches are framed upon severity of COVID‐19, 4 current knowledge about the risk factors of thrombosis in children with and without COVID‐19, 3 , 19 current thromboprophylaxis practices among children without COVID‐19, 24 and current practices in adults with COVID‐1922 (Figure 1). We acknowledge that we have focused primarily on VTE prevention and in many situations, there is insufficient evidence to recommend a treatment strategy.
TABLE 1.
Key management issues in evaluation and management of COVID‐19‐associated thrombosis risk in pediatric population
| Focus | Clinical challenges |
|---|---|
| Assessing thrombotic risk | |
| Clinical evaluation: Identifying high‐risk population | How to risk‐stratify children for pharmacological thromboprophylaxis? |
| How to approach children with specific disease conditions such as Crohn's, sickle cell, cancer? Should they be treated differently? | |
| What other clinical parameters should be taken into account while considering thromboprophylaxis? | |
|
Laboratory evaluation: Assessment of severity of prothrombotic milieu |
Do all children hospitalized with COVID‐19 require CAC evaluation? |
| What CAC workup is needed? | |
| What biomarkers should be evaluated to characterize the severity of COVID‐19? | |
| Should D‐dimer be used as a guide for biomarker for CAC? | |
| Thromboprophylaxis management | |
|
Anticoagulation consideration |
How to determine intensity of anticoagulation: Prophylaxis versus therapeutic regimen? |
| Which anticoagulant to choose? | |
| Is there a need for monitoring? | |
| What is the role of DOACs in children? | |
| How to determine the duration of anticoagulation after discharge? | |
| MIS‐C and thromboprophylaxis considerations | |
| Thromboprophylaxis consideration | Should MIS‐C patients be treated like COVID‐19? |
| What is the role of aspirin? | |
| Research priorities | |
| Risk stratification | Identifying high‐risk population: role of clinical risk factors for VTE, COVID‐19‐related risk factors; role of biomarkers |
| Coagulopathy assessment | Evaluation for severity of CAC: Role of blood type, TEG, VWF, FVIII, lupus anticoagulant, and APS antibodies |
| Outcome assessment |
Vascular Doppler imaging to screen for DVT VTE outcomes of MIS‐C vs. COVID‐19 |
| Other therapies and VTE risk reduction | Therapy intervention: Aspirin, low‐dose t‐PA therapy, inhaled UFH, protein C, and thrombomodulin concentrates, impact of antiviral therapy |
Abbreviations: APS, antiphospholipid antibody syndrome; CAC, COVID‐associated coagulopathy; DOAC, direct oral anticoagulants; DVT, deep venous thrombosis; FVIII, factor VIII; MIS‐C, multisystem inflammatory syndrome in children; TEG, thromboelastography; t‐PA, tissue plasminogen activator; UFH, unfractionated heparin; VTE, venous thromboembolic event; VWF, Von Willebrand's factor.
This article is being made freely available through PubMed Central as part of the COVID-19 public health emergency response. It can be used for unrestricted research re-use and analysis in any form or by any means with acknowledgement of the original source, for the duration of the public health emergency.
FIGURE 1.
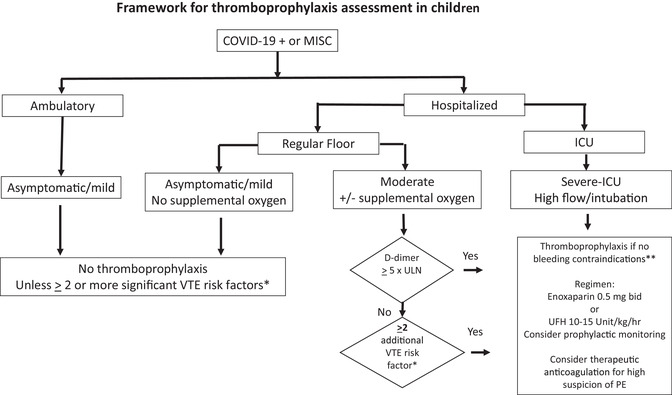
Suggested algorithm for risk stratification and considering thromboprophylaxis for children with COVID‐19 infection. Note that location of patient and oxygen requirement was used for assessment of severity of acute infection. 4 Definitions: *VTE risk examples: MIS‐C, age ≥12 years, obesity, immobilization, CVL, estrogen, asparaginase, malignancy, soft tissue infection, family history of thrombosis; **Bleeding contraindications: active bleeding, significant risk of bleeding, platelet count <20,000 mm 3 . Abbreviations: CVL, central venous catheter; ICU, intensive care unit; O2, oxygen; PE, pulmonary emboli; ULN, upper limit of normal
2. CLINICAL VIGNETTES
2.1. Vignette #1: Assessing thrombotic risk
A 10‐year old Hispanic boy with Crohn's disease with acute infection is admitted under pediatric service with left lower lobe pneumonia and receiving 2 L/min of oxygen via nasal canula. Hematology is consulted to evaluate a need for thromboprophylaxis.
2.1.1. What is the risk for thrombosis in this patient?
Severity assessment of acute COVID‐19 infection is an important initial step to evaluating thrombosis risk. 25 , 26 , 27 , 28 , 29 , 30 In adult patients, hospitalization status and increasing requirement for oxygen support are associated with worse outcomes, including thrombosis. 26 , 28 , 30 An operational approach to initial risk stratification would determine if the patient is ambulatory (asymptomatic/mild symptoms), hospitalized without oxygen (asymptomatic/mild symptoms) or with oxygen support (mild/moderate symptoms), and ICU (severe symptoms) with significant respiratory support (Figure 1). 4 In children, the majority (∼95%) with acute infection have milder clinical course compared to adults, and most recover without any complications. 31 However, similar to adults, children with respiratory symptoms and oxygen requirement have increased VTE risk. 3 A recent study reported a 7% rate of VTE in those hospitalized with COVID‐19 and respiratory symptoms 3 and another study demonstrated a significant risk VTE in those needing high ventilatory support (≥5 L/min oxygen, high‐flow nasal cannula, non‐rebreather mask, or intubation). 19
This patient has at least three additional risk factors for VTE besides oxygen requirement: a pneumonia, 3 underlying comorbid condition with chronic inflammation, and Hispanic race, 2 , 3 increasing thrombotic risk. Based on the consensus guidelines, 23 he would meet criteria for thromboprophylaxis. However, a subsequent study in 814 pediatric patients hospitalized with SARS‐CoV‐2 showed that thrombotic risk was decreased in younger age groups (<12 years). 20 In the absence of bleeding risks, we would consider thromboprophylaxis as inflammatory bowel disease a risk factor for VTE 32 ; however, given the young age, we requested additional laboratory markers in order to aid risk assessment and to make the final determination for prophylaxis.
2.1.2. What laboratory workup is considered for thrombosis risk?
CAC, which typically presents with extremely elevated D‐dimer, modest decrease in platelet count, elevation of fibrinogen, and mild prolongation of prothrombin time (PT), is associated with increased risk of thrombosis. 11 , 14 CAC is distinct from sepsis‐associated coagulopathy where fibrinogen and platelets are significantly low. The pathophysiology of COVID‐19 suggests that cytokine‐mediated endotheliopathy and platelet activation play a central role in CAC. 11 Markers of endothelial and platelet activation, including ultra‐large multimers of von Willebrand factor (VWF) and VWF antigen, factor VIII, and soluble P‐selectin, are higher in adults admitted to the ICU than in those admitted to regular ward. 33 Viscoelastic testing shows elevated maximal clot amplitude or strength consistent with increased platelet activation and increased fibrinogen in both children and adults. 34 , 35 IL‐6, IL‐2, and TNF‐α produced during acute infection are also contributory. Dysregulation in plasma levels of coagulation factors and increased levels of anti‐fibrinolytic factors are correlated with high levels of IL‐6 in adults with acute infection. 36 Imbalance between procoagulant elevation and anticoagulant depletion overwhelms fibrinolysis resulting in a prothrombotic milieu. Elevations in D‐dimer, a biomarker for fibrinolysis, and fibrinogen reflect this prothrombotic milieu. Presence of lupus anticoagulant and antiphospholipid antibodies have also been reported during acute infection. 30 Recently, anti‐A antibodies in individuals with blood type “O” have been shown to have protective effect against SARS‐CoV‐2 invasion. 37
For hospitalized patients with symptomatic acute infection, we typically request complete blood counts, peripheral blood smear, screening coagulation tests (PT, activated partial thromboplastin time [aPTT]), fibrinogen, and D‐Dimer to assess for CAC and disease severity. Inflammatory markers, such as CRP and ferritin, may be used to trend disease progression. In case of prolongation of coagulation tests, lupus anticoagulant testing should be performed to assist the diagnosis of CAC. Other potential biomarkers may be considered for evaluation of children, however their utility to predict clinical outcomes, particularly thrombosis, has not yet been extensively studied.
2.1.3. What is the role of D‐dimer in evaluating CAC in children?
Elevated D‐dimer levels were recognized early in the pandemic as an important biomarker for predicting disease severity state in COVID. 16 , 28 , 29 A recent meta‐analysis showed that baseline D‐dimer was increased by over 3 mcg/ml, more than six times the upper limit of normal (ULN), in adults with VTE. 38 The sensitivity of predicting VTE was 95% when both D‐dimer and CRP were used in decision rule. 39 One pediatric report showed elevation of D‐dimer more than five times ULN increased odds of VTE. 19 While D‐dimer is not specific for COVID‐19, based on adult experience, elevation of D‐dimer has been incorporated into pediatric guidelines for risk assessment. 23 , 40 Recent report from Italy suggested that elevated D‐dimer in children was more pronounced with MIS‐C and did not predict disease severity in acute infection. 40 Based on available data, 19 , 23 we consider elevation of D‐dimer (greater than or equal to five times ULN) as an additional thrombotic risk that should be incorporated into the overall risk assessment in children. This child had a D‐dimer seven times the ULN along with clinical risk factors for VTE, and thromboprophylaxis was recommended.
2.2. Vignette #2: Thromboprophylaxis management
A 16‐year‐old Caucasian girl with history of asthma was admitted in pediatric intensive care unit (PICU) with acute COVID‐19 infection. She has multiorgan failure and acute respiratory distress syndrome (ARDS). PICU would like to start pharmacological thromboprophylaxis along with pneumatic compression stockings. Hematology is consulted to guide about choice and duration of anticoagulation.
2.2.1. What should be the intensity of anticoagulation regimen: Prophylactic versus therapeutic?
This question is under scrutiny as observational studies in adults indicate a high incidence of VTE despite prophylactic anticoagulation. 17 , 19 This has been also recently reported in children. A large multicenter pediatric study by Whitworth and colleagues showed that over two‐thirds of pediatric patients developed thrombotic complications despite prophylactic anticoagulation, 20 while a smaller single‐institution experience by Mitchell et al. reported this occurrence in over one‐third of their patients. 19 However, there is yet no high‐quality evidence demonstrating the safety and efficacy of therapeutic over prophylactic dosing in children. Randomized controlled trials (RCTs) are ongoing to determine the efficacy and safety of therapeutic anticoagulation in adults with acute infection. 41 In pediatrics, “COVAC‐TP” is recruiting acutely ill children with COVID‐19 or MIS‐C (NCT04354155) to answer this question. Some providers consider therapeutic dosing in adolescents with underlying comorbid conditions and severity of lung injury that are associated with breakthrough VTE in adults. 42 This patient is critically ill with high respiratory support and was recommended prophylactic anticoagulation. However, therapeutic anticoagulation should be considered in cases with high suspicion of pulmonary emboli and inability to obtain adequate imaging (e.g., due to risk of infecting health care workers, renal insufficiency for contrast). While the option of therapeutic anticoagulation was strongly considered, this patient was commenced on prophylactic anticoagulation with low molecular weight heparin (LMWH).
2.2.2. What is the choice of anticoagulant for VTE prophylaxis in this patient?
LMWH or unfractionated heparin (UFH) is the anticoagulant of choice in children with acute infection. 24 The dosing and monitoring regimens should be used according to published guidelines. 24 In stable patients without high bleeding risk and with adequate renal function, LMWH is commonly used over UFH due to reliable pharmacokinetics and pharmacodynamic responses and longer half‐life, which allows for twice or once daily regimens. 24 LMWHs may have additional benefits due to its anti‐inflammatory and immunomodulatory properties. 43 , 44 LMWH has shown to have potential antiviral property as it interacts with the SARS‐CoV‐2 Spike S1 protein receptor‐binding domain and interferes with its engagement with receptor. 45 Thus, the consensus guidelines suggest the use of LMWH for thromboprophylaxis for acute infection in children, 23 and COVAC‐TP trial is evaluating LMWH dosing regimen. There is no consensus of monitoring anti‐Xa for prophylaxis dosing, however pediatric experience suggests improved efficacy and safety with target dosing. 19 Higher doses of LMWH/UFH may be needed due to acquired heparin resistance and deficiency of antithrombin as disease progresses. 46 While thrombocytopenia is typically mild and bleeding is uncommon with acute infection, some children may be at high risk of bleeding and preferably prescribed with UFH. Subcutaneous adult UFH dosing can be used, but for pediatric patients (e.g., less than 50 kg), intravenous route is preferred. Anticoagulation with UFH can be reversed by discontinuing the infusion and/or using protamine sulfate. Of note, heparin‐induced thrombocytopenia has been reported in COVID‐19 patients and should be considered with drop in platelet count. 47
Direct oral anticoagulants (DOACs) are increasingly used in the adolescent population with normal renal function even though it is not yet approved by the FDA for this population (<18 years). It is reasonable to use DOAC to treat VTE in adolescents with COVID‐19 as pharmacokinetic and safety data are published. 48 , 49 However, use of antiviral therapies may prohibit its use due to drug interaction and risk of supra‐therapeutic anticoagulation. 21 DOACs are also not recommended for those with triple‐positive high risk for antiphospholipid syndrome (APS). 50 As this patient had normal renal function and no other contraindication for DOAC use, she was transitioned to Apixaban at 2.5 mg by mouth twice daily.
2.2.3. Are there alternative therapies/interventions to reduce thrombotic risk?
Considering autopsy findings of widespread microthrombosis, 51 , 52 anticoagulation therapy alone may offer limited efficacy with severe acute infection. 10 Systemic administration of the fibrinolytic therapy with t‐PA 53 and inhaled UFH 54 to adults with ARDS in COVID‐19 have been reported. However, there is yet no experience with thrombolytics in children with COVID‐19 in the absence of acute VTE. The endothelial dysfunction and platelet activation seen with this disease raises the question of the utility of antiplatelet agents or anti‐inflammatory agents such a steroids or anti‐IL6 therapy. Aspirin is shown to have antiviral activity, which could offer potential benefit during acute infection. 55 However, as of today, there is insufficient evidence to use these agents for preventing VTE. Other therapies for consumptive coagulopathies such as protein C concentrates or thrombomodulin are still under evaluation and there are also no data to support its use in children. 56 Direct effect of the virus may contribute to thrombotic risk. We will learn more of the potential information of benefit for antiviral or immune‐based therapies in preventing VTE as more patients are managed with these strategies.
2.2.4. Should this patient receive postdischarge anticoagulation?
The benefit of posthospitalization thromboprophylaxis after acute infection is unclear both in pediatric and adult population. Current adult guidelines suggest that hospitalized patients should not routinely be discharged on thromboprophylaxis. 57 However, anticoagulation should be considered for those with significant persistent risks after discharge, and in adults, D‐dimer has been incorporated into risk assessment to determine the duration of thromboprophylaxis. 57 Anticoagulation is continued until patient is no more exposed to risk factors with normalization of D‐dimers. Based on these data, our current approach is to continue thromboprophylaxis for children with persistent risk factors at discharge, such as continued immobility, presence of central line, significant D‐dimer elevation, for 1–2 weeks and until risk factors are no longer present.
2.3. Vignette #3: VTE risk in sickle cell disease and COVID‐19
A 12‐year‐old African American (AA) male with homozygous sickle cell disease (SCD) on hydroxyurea therapy is evaluated for fever, respiratory distress and oxygen saturations of 80%. He had dry cough and was positive for SARS‐CoV‐2 by PCR. The chest X‐ray showed bilateral infiltrates. White count was 36,000/mm3 with hemoglobin of 7 g/dl and platelet count of 440,000/mm3. The D‐dimer was 11‐times ULN.
2.3.1. What is the VTE risk in pediatric patients with SCD who have COVID‐19?
SCD is a hypercoagulable state. 58 In adults, the incidence of VTE is higher than the general population, affecting over 11% of adults with SCD by age 40 years. 59 In patients 21 years or younger with SCD, the VTE incidence is estimated to be 1.7% compared to a rate of about 5% in children with cancer, and 2.7% in children who have undergone congenital heart surgery. 60 As COVID‐19 and SCD are both thromboinflammatory diseases, acute infection may increase thromboembolic complications. Data from adults with SCD suggest a higher risk (at least doubled for acute chest syndrome [ACS]) for complications with COVID‐19, 61 , 62 , 63 and the Centers for Disease Control and prevention (CDC) considers individuals with SCD at risk to develop severe disease with COVID‐19. There are sparse data for the VTE incidence in children with SCD and COVID‐19. 60 An international registry has been established to collect information on outcomes in patients with SCD (Secure‐SCD covidsicklecell.org). Thus far the registry has reported a low incidence of thromboembolic disease in the pediatric range, however additional shared data from large cohorts will be critical to better understand VTE risks in SCD. Increased D‐dimer levels in adults are associated with acute infection and VTE, but the interpretation in SCD has not been studied. We would consider symptomatic acute infection with COVID‐19 an additional significant VTE risk in patients with SCD. This patient received prophylactic doses of LMWH.
2.3.2. How do we manage ACS in SCD and COVID‐19?
Patients with COVID‐19 and pulmonary findings can develop de novo non‐embolic PE which can precipitate, or exacerbate ACS in SCD. For the same reason imaging for screening for asymptomatic DVT or PE has not been shown to be beneficial. In this patient with ACS, the contributions of acute infection, associated inflammation, possible bacterial superinfection, and ongoing sickling are difficult to parse out, however typical antibiotic coverage is recommended. The potential benefit of antivirals and immune therapies are yet to be studied, specifically in SCD. In addition to antibiotic coverage, red cell transfusions, either simple or exchange, are effective therapies for respiratory compromise with SCD. Dexamethasone has been shown to reduce mortality and duration of ventilatory support in adults with COVID‐19 without SCD due to its anti‐inflammatory properties. 64 , 65 While there may be concerns for use of dexamethasone in patients with SCD and COVID‐19 due to rebound pain risk, dexamethasone has also been shown to be effective for treating ACS. 66 The combination of transfusion therapy may be protective of steroid‐associated pain episodes. 67 In patients with ACS and COVID‐19, we have had low threshold for implementing transfusion therapy. In addition, we have used the recommended COVID‐19 dosing of dexamethasone at 0.15 mg/kg/day (dose equivalent to prednisone 1 mg/kg/day) for several days until improvement in those with moderate to severe disease.
2.3.3. Are patients with SCD and COVID‐19 at increased risk of stroke?
Patients with COVID‐19 have been reported with stroke. The overall incidence of stroke with acute infection is 3% compared the risk of VTE at 20%. 68 The incidence of stroke is increased in SCD pediatric patients who have abnormally elevated transcranial Doppler (TCD) velocities, as well as a previous history of stroke. However, there is yet no data about the additional stroke risk with COVID‐19 in SCD. In SCD patients with abnormal TCD or history of stroke, chronic transfusions are implemented with a goal reduction of sickle hemoglobin to 30% or less for primary and secondary stroke prevention. For acute stroke presentations or transient ischemic attacks in the setting of COVID‐19, the American Society of Hematology guidelines recommend reducing the sickle hemoglobin fraction to 15%. 69
2.4. Vignette #4: VTE risk in malignancy and COVID‐19
A 16‐year‐old male with acute lymphoblastic leukemia (ALL) is currently undergoing treatment and received asparaginase a week ago. The patient comes to clinic with a COVID‐19 exposure in the family and his PCR for SARS‐CoV‐2 is positive. He is asymptomatic and his oxygen saturations are 98% on room air. Chest X‐ray shows no infiltrate.
2.4.1. What is the VTE risk with malignancy and COVID‐19?
This patient's VTE risks include the diagnosis of active malignancy, central line, and recent asparaginase treatment. Numerous studies have demonstrated an increased rate of VTE in pediatric patients with malignancy. 70 , 71 Pediatric patients with malignancy can develop severe COVID‐19. Current registry data do not show a significantly increased risk for severe infection, and VTE risk is not well studied in this population 72 ; however, a recent study in children shows that malignancy was a risk factor for thrombosis with COVID‐19. 20 Severe disease and need for respiratory support with acute COVID‐19 infection are additional VTE risks, but what is the additional risk with asymptomatic or mild infection? In the adult population, VTE rates in ambulatory patients with mild or asymptomatic SARS‐CoV‐2 infection are lower than those who are hospitalized; however, VTE events, some fatal, do occur in the outpatient setting. 73 Viral shedding has been reported up to 2 months in those with immunosuppression with chemotherapy, so thrombotic risk with the virus may be prolonged, although data are lacking. 74 While this patient is asymptomatic, an important consideration is that the trajectory of the disease over the next several days is not yet known. Severity may progress and thus deserves very close follow‐up and consideration for thromboprophylaxis.
2.4.2. Is there a role for thromboprophylaxis in ambulatory patients with malignancy and COVID‐19?
In ambulatory adult patients with malignancy, VTE prophylaxis is not routinely recommended, but is offered based on cancer type and validated risk‐assessment scores. 75 In children, evidence to support VTE prophylaxis in patients with ALL and other malignancy has been limited due to small sample sizes or retrospective study design. However, a recent prospectively randomized study in pediatric patients with ALL treated on Berlin–Frankfurt–Munich (BFM) protocols in Europe showed a significant reduction in symptomatic VTE rate in patients treated with prophylaxis with LMWH (3.5%) or antithrombin (1.9%) compared to low‐dose UFH (8%). 76 Bleeding rate was low in all groups (∼1%). These patients were treated with asparaginase, and prophylaxis was used only during induction. Current studies in adults with malignancies have shown benefit of DOACs to decrease VTE rate. 77 The current NIH recommendations to manage VTE risk are the same in SARS‐CoV‐2‐infected adults with cancer and the general population and recommends thromboprophylaxis only when VTE risk factors exist. 57 Pediatric patients with ALL and other malignancies have an increased baseline risk of VTE, and acute COVID‐19 adds increasing VTE risk with increasing disease severity. In children with mild to moderate symptoms, we would consider thromboprophylaxis on a case‐by‐case basis if there is high baseline VTE risk. In a completely asymptomatic patient, it is reasonable to defer thromboprophylaxis. However, the patient should be reassessed closely over several weeks for clinical worsening.
2.5. Vignette #5: MIS‐C and thromboprophylaxis consideration
Previously healthy 13‐year‐old AA male presented to the emergency department with fever, abdominal pain, tachycardia, and lower extremity rash. PCR testing for SARS‐CoV‐2 was negative while antibody testing was positive. Patient was admitted to the hospital with a diagnosis of MIS‐C. Soon after admission, he developed shock requiring transfer to the PICU for vasopressor support and mechanical ventilation for respiratory failure. Initial platelet count was 565,000/mm3, D‐dimer was 10 times the ULN, and echocardiography showed slightly depressed left ventricular function and normally sized coronary arteries.
2.5.1. What is the VTE risk in patients with MIS‐C?
The CDC and WHO defines MIS‐C based on fever, laboratory markers of inflammation, organ dysfunction, and temporal relationship to acute infection in children. 78 Several published cohorts of children diagnosed with MIS‐C report elevated rates of VTE. In a multicenter study of children who were admitted to a pediatric ICU in the United States, 8% with MIS‐C developed VTE, with the majority being 13 years or older. 7 In addition to MIS‐C, this patient had multiple VTE risk factors, including postpubertal age, AA race, 2 , 3 ventilatory support, and inflammation. The patient also had elevated D‐dimer levels, which is a feature of CAC and is seen in the majority of patients with MIS‐C. 5 Given the multiple VTE risks and high ventilatory support with MIS‐C, this patient was prescribed LMWH for thromboprophylaxis with prophylactic dosing based on the consensus guidelines. 23
2.5.2. What is the role of antiplatelet therapy for thromboprophylaxis in MIS‐C?
MIS‐C is thought to be the result of a dysregulated host immune response. 79 It has been compared with Kawasaki disease because of reports of coronary artery dilation and prominence of cardiogenic shock with MIS‐C. 80 While differences in age, laboratory parameters, and inflammatory response suggest that the two are different entities, the management of MIS‐C has been patterned after that of Kawasaki disease. 79 , 81 In particular, aspirin is commonly used as thromboprophylaxis in children with Kawasaki disease due to platelet activation, thrombocytosis, altered flow dynamics in abnormal coronary arteries, and endothelial damage. 82 Similarly, the American College of Rheumatology recommends the use of low‐dose aspirin for all children with MIS‐C. Aspirin is continued until the platelet count is normalized and coronary arteries demonstrate to be normal ≥4 weeks after the diagnosis of MIS‐C. 83 Thromboprophylaxis with LMWH is recommended for patients with cardiac ejection fractions less than 35% or with significant coronary artery aneurysms (z‐score over 10, in combination with aspirin). Bleeding risk may be increased with concomitant use of low doses of aspirin (3–5 mg/kg/day) with thromboprophylaxis in children with MIS‐C; however, in the absence of other bleeding risks, is not a contraindication. 23 Given the diagnosis of MIS‐C, low‐dose aspirin was prescribed in addition to LMWH.
3. SUMMARY
Thrombosis is major cause of morbidity with COVID‐19 and is associated with CAC. The paucity of data about VTE risk in children with COVID‐19 poses clinical challenge about considering thromboprophylaxis. Disease severity and exposure to known risk factors for VTE are key elements of this decision making. Laboratory parameters of CAC specifically D‐Dimers may be helpful in determining the intensity and duration of thromboprophylaxis. The pharmacological thromboprophylaxis in children with COVID‐19 is generally limited to those with moderate to severe disease and thus usually limited for hospitalized patients. Each patient will need an individualized approach in the context of his/her clinical course, but improved management and understanding of this disease will need to be informed by results of ongoing clinical studies in this population.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest relevant to this publication.
AUTHOR CONTRIBUTIONS
All authors contributed equally in writing the manuscript.
LINKED CONTENT
This article is linked to an article by William B.Mitchell et al. https://onlinelibrary.wiley.com/doi/epdf/10.1002/pbc.28975
Sharathkumar AA, Faustino EVS, Takemoto CM. How we approach thrombosis risk in children with COVID‐19 infection and MIS‐C. Pediatr Blood Cancer. 2021; 68:e29049. 10.1002/pbc.29049.
REFERENCES
- 1. Datta SD, Talwar A, Lee JT. A proposed framework and timeline of the spectrum of disease due to SARS‐CoV‐2 infection: illness beyond acute infection and public health implications. JAMA. 2020;324(22):2251‐2252. [DOI] [PubMed] [Google Scholar]
- 2. Duarte‐Salles T, Vizcaya D, Pistillo A, et al. Baseline characteristics, management, and outcomes of 55,270 children and adolescents diagnosed with COVID‐19 and 1,952,693 with influenza in France, Germany, Spain, South Korea and the United States: an international network cohort study. medRxiv. 10.1101/2020.10.29.20222083 [DOI] [Google Scholar]
- 3. Fernandes DM, Oliveira CR, Guerguis S, et al. Severe acute respiratory syndrome coronavirus 2 clinical syndromes and predictors of disease severity in hospitalized children and youth. J Pediatr. 2020;230:20‐31.e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. WHO Working Group on the Clinical Characterisation and Management of COVID‐19 infection . A minimal common outcome measure set for COVID‐19 clinical research. Lancet Infect Dis. 2020;20(8):e192‐e197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Dufort EM, Koumans EH, Chow EJ, et al. Multisystem inflammatory syndrome in children in New York state. N Engl J Med. 2020;383(4):347‐358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. World Health Organization . Multisystem Inflammatory Syndrome in Children and Adolescents with COVID‐19 . WHO; 2020. https://www.who.int/news‐room/commentaries/detail/multisystem‐inflammatory‐syndrome‐in‐children‐and‐adolescents‐with‐covid‐19. Accessed December 15, 2020. [Google Scholar]
- 7. Feldstein LR, Rose EB, Horwitz SM, et al. Multisystem inflammatory syndrome in U.S. children and adolescents. N Engl J Med. 2020;383(4):334‐346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Iba T, Connors JM, Levy JH. The coagulopathy, endotheliopathy, and vasculitis of COVID‐19. Inflamm Res. 2020;69(12):1181‐1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Nicolai L, Leunig A, Brambs S, et al. Immunothrombotic dysregulation in COVID‐19 pneumonia is associated with respiratory failure and coagulopathy. Circulation. 2020;142(12):1176‐1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wichmann D, Sperhake JP, Lütgehetmann M, et al. Autopsy findings and venous thromboembolism in patients with COVID‐19: a prospective cohort study. Ann Intern Med. 2020;173(4):268‐277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Iba T, Levy JH, Connors JM, Warkentin TE, Thachil J, Levi M. The unique characteristics of COVID‐19 coagulopathy. Crit Care. 2020;24(1):360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ma H, Hu J, Tian J, et al. A single‐center, retrospective study of COVID‐19 features in children: a descriptive investigation. BMC Med. 2020;18(1):123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ranucci M, Sitzia C, Baryshnikova E, et al. Covid‐19‐associated coagulopathy: biomarkers of thrombin generation and fibrinolysis leading the outcome. J Clin Med. 2020;9(11):3487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Tang N, Li D, Wang X, Sun Z. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J Thromb Haemost. 2020;18(4):844‐847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Yin S, Huang M, Li D, Tang N. Difference of coagulation features between severe pneumonia induced by SARS‐CoV‐2 and non‐SARS‐CoV‐2. J Thromb Thrombolysis. 2020:1‐4. 10.1007/s11239-020-02105-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Yu HH, Qin C, Chen M, Wang W, Tian DS. D‐dimer level is associated with the severity of COVID‐19. Thromb Res. 2020;195:219‐225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Middeldorp S, Coppens M, van Haaps TF, et al. Incidence of venous thromboembolism in hospitalized patients with COVID‐19. J Thromb Haemost. 2020;18(8):1995‐2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Tang N, Bai H, Chen X, Gong J, Li D, Sun Z. Anticoagulant treatment is associated with decreased mortality in severe coronavirus disease 2019 patients with coagulopathy. J Thromb Haemost. 2020;18(5):1094‐1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Mitchell W, Davilla J, Keenan J, et al. Children and young adults hospitalized for severe COVID‐19 exhibit thrombotic coagulopathy. Pediatr Blood Cancer. 2021:e28975. 10.1002/pbc.28975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Whitworth H, Sartain SE, Kumar R, et al. Rate of thrombosis in children and adolescents hospitalized with COVID‐19 or MIS‐C. Blood. 2021. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Barnes GD, Burnett A, Allen A, et al. Thromboembolism and anticoagulant therapy during the COVID‐19 pandemic: interim clinical guidance from the anticoagulation forum. J Thromb Thrombolysis. 2020;50(1):72‐81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Thachil J, Tang N, Gando S, et al. ISTH interim guidance on recognition and management of coagulopathy in COVID‐19. J Thromb Haemost. 2020;18(5):1023‐1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Goldenberg NA, Sochet A, Albisetti M, et al. Consensus‐based clinical recommendations and research priorities for anticoagulant thromboprophylaxis in children hospitalized for COVID‐19‐related illness. J Thromb Haemost. 2020;18(11):3099‐3105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Monagle P, Cuello CA, Augustine C, et al. American Society of Hematology 2018 Guidelines for management of venous thromboembolism: treatment of pediatric venous thromboembolism. Blood Adv. 2018;2(22):3292‐3316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Shekerdemian LS, Mahmood NR, Wolfe KK, et al. Characteristics and outcomes of children with coronavirus disease 2019 (COVID‐19) infection admitted to US and Canadian pediatric intensive care units. JAMA Pediatr. 2020;174(9):868‐873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497‐506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Liu W, Zhang Q, Chen J, et al. Detection of COVID‐19 in children in early January 2020 in Wuhan, China. N Engl J Med. 2020;382(14):1370‐1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID‐19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054‐1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Guan WJ, Ni ZY, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382(18):1708‐1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Zhang Y, Xiao M, Zhang S, et al. Coagulopathy and antiphospholipid antibodies in patients with Covid‐19. N Engl J Med. 2020;382(17):e38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. CDC COVID‐19 Response Team . Coronavirus Disease 2019 in children ‐ United States, February 12‐April 2, 2020. MMWR Morb Mortal Wkly Rep. 2020;69(14):422‐426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kappelman MD, Horvath‐Puho E, Sandler RS, et al. Thromboembolic risk among Danish children and adults with inflammatory bowel diseases: a population‐based nationwide study. Gut. 2011;60(7):937‐943. [DOI] [PubMed] [Google Scholar]
- 33. Goshua G, Pine AB, Meizlish ML, et al. Endotheliopathy in COVID‐19‐associated coagulopathy: evidence from a single‐centre, cross‐sectional study. Lancet Haematol. 2020;7(8):e575‐e582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Al‐Ghafry M, Aygun B, Appiah‐Kubi A, et al. Are children with SARS‐CoV‐2 infection at high risk for thrombosis? Viscoelastic testing and coagulation profiles in a case series of pediatric patients. Pediatr Blood Cancer. 2020;67(12):e28737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Panigada M, Bottino N, Tagliabue P, et al. Hypercoagulability of COVID‐19 patients in intensive care unit: a report of thromboelastography findings and other parameters of hemostasis. J Thromb Haemost. 2020;18(7):1738‐1742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. D'Alessandro A, Thomas T, Dzieciatkowska M, et al. Serum proteomics in COVID‐19 patients: altered coagulation and complement status as a function of IL‐6 level. J Proteome Res. 2020;19(11):4417‐4427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Fan Q, Zhang W, Li B, Li DJ, Zhang J, Zhao F. Association between ABO blood group system and COVID‐19 susceptibility in Wuhan. Front Cell Infect Microbiol. 2020;10:404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Nopp S, Moik F, Jilma B, Pabinger I, Ay C. Risk of venous thromboembolism in patients with COVID‐19: a systematic review and meta‐analysis. Res Pract Thromb Haemost. 2020;4(7):1178‐1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Dujardin RWG, Hilderink BN, Haksteen WE, et al. Biomarkers for the prediction of venous thromboembolism in critically ill COVID‐19 patients. Thromb Res. 2020;196:308‐312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Del Borrello G, Giraudo I, Bondone C, et al. SARS‐CoV‐2 associated coagulopathy and thromboembolism prophylaxis in children: a single centre observational study. J Thromb Haemost. 2021;19(2):522‐530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Lemos ACB, do Espírito Santo DA, Salvetti MC, et al. Therapeutic versus prophylactic anticoagulation for severe COVID‐19: a randomized phase II clinical trial (HESACOVID). Thromb Res. 2020;196:359‐366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Hasan SS, Radford S, Kow CS, Zaidi STR. Venous thromboembolism in critically ill COVID‐19 patients receiving prophylactic or therapeutic anticoagulation: a systematic review and meta‐analysis. J Thromb Thrombolysis. 2020;50(4):814‐821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Thachil J. Clinical differentiation of anticoagulant and non‐anticoagulant properties of heparin. J Thromb Haemost. 2020;18(9):2424‐2425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Lisman T, Thachil J. Differentiating biochemical from clinical heparin resistance in COVID‐19. J Thromb Thrombolysis. 2020;50(4):1015‐1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Kim SY, Jin W, Sood A, et al. Characterization of heparin and severe acute respiratory syndrome‐related coronavirus 2 (SARS‐CoV‐2) spike glycoprotein binding interactions. Antiviral Res. 2020;181:104873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Beun R, Kusadasi N, Sikma M, Westerink J, Huisman A. Thromboembolic events and apparent heparin resistance in patients infected with SARS‐CoV‐2. Int J Lab Hematol. 2020;42(Suppl 1):19‐20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Warkentin TE, Kaatz S. COVID‐19 versus HIT hypercoagulability. Thromb Res. 2020;196:38‐51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Male C, Lensing AWA, Palumbo JS, et al. Rivaroxaban compared with standard anticoagulants for the treatment of acute venous thromboembolism in children: a randomised, controlled, phase 3 trial. Lancet Haematol. 2020;7(1):e18‐e27. [DOI] [PubMed] [Google Scholar]
- 49. Halton J, Brandão LR, Luciani M, et al. Dabigatran etexilate for the treatment of acute venous thromboembolism in children (DIVERSITY): a randomised, controlled, open‐label, phase 2b/3, non‐inferiority trial. Lancet Haematol. 2021;8(1):e22‐e33. [DOI] [PubMed] [Google Scholar]
- 50. Arachchillage DRJ, Laffan M. What is the appropriate anticoagulation strategy for thrombotic antiphospholipid syndrome? Br J Haematol. 2020;189(2):216‐227. [DOI] [PubMed] [Google Scholar]
- 51. Ackermann M, Verleden SE, Kuehnel M, et al. Pulmonary vascular endothelialitis, thrombosis, and angiogenesis in Covid‐19. N Engl J Med. 2020;383(2):120‐128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Barton LM, Duval EJ, Stroberg E, Ghosh S, Mukhopadhyay S. COVID‐19 autopsies, Oklahoma, USA. Am J Clin Pathol. 2020;153(6):725‐733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Wang J, Hajizadeh N, Moore EE, et al. Tissue plasminogen activator (tPA) treatment for COVID‐19 associated acute respiratory distress syndrome (ARDS): a case series. J Thromb Haemost. 2020;18(7):1752‐1755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. van Haren FMP, Page C, Laffey JG, et al. Nebulised heparin as a treatment for COVID‐19: scientific rationale and a call for randomised evidence. Crit Care. 2020;24(1):454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Bianconi V, Violi F, Fallarino F, Pignatelli P, Sahebkar A, Pirro M. Is acetylsalicylic acid a safe and potentially useful choice for adult patients with COVID‐19? Drugs. 2020;80(14):1383‐1396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Mazzeffi M, Chow JH, Amoroso A, Tanaka K. Revisiting the protein C pathway: an opportunity for adjunctive intervention in COVID‐19? Anesth Analg. 2020;131(3):690‐693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. National Institutes of Health . https://www.covid19treatmentguidelines.nih.gov/adjunctive‐therapy/antithrombotic‐therapy/. Accessed December 15, 2020.
- 58. Shet AS, Lizarralde‐Iragorri MA, Naik RP. The molecular basis for the prothrombotic state in sickle cell disease. Haematologica. 2020;105(10):2368‐2379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Naik RP, Streiff MB, Haywood C Jr, Segal JB, Lanzkron S. Venous thromboembolism incidence in the Cooperative Study of Sickle Cell Disease. J Thromb Haemost. 2014;12(12):2010‐2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Kumar R, Stanek J, Creary S, Dunn A, O'Brien SH. Prevalence and risk factors for venous thromboembolism in children with sickle cell disease: an administrative database study. Blood Adv. 2018;2(3):285‐291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Telfer P, De la Fuente J, Sohal M, et al. Real‐time national survey of COVID‐19 in hemoglobinopathy and rare inherited anemia patients. Haematologica. 2020;105(11):2651‐2654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. McCloskey KA, Meenan J, Hall R, Tsitsikas DA. COVID‐19 infection and sickle cell disease: a UK centre experience. Br J Haematol. 2020;190(2):e57‐e58. [DOI] [PubMed] [Google Scholar]
- 63. Arlet JB, de Luna G, Khimoud D, et al. Prognosis of patients with sickle cell disease and COVID‐19: a French experience. Lancet Haematol. 2020;7(9):e632‐e634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Tomazini BM, Maia IS, Cavalcanti AB, et al. Effect of dexamethasone on days alive and ventilator‐free in patients with moderate or severe acute respiratory distress syndrome and COVID‐19: the CoDEX randomized clinical trial. JAMA. 2020;324(13):1307‐1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Horby P, Lim WS, Emberson JR, et al. Dexamethasone in hospitalized patients with Covid‐19 ‐ preliminary report. N Engl J Med. 2021;384(8):693‐704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Quinn CT, Stuart MJ, Kesler K, et al. Tapered oral dexamethasone for the acute chest syndrome of sickle cell disease. Br J Haematol. 2011;155(2):263‐267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Strouse JJ, Takemoto CM, Keefer JR, Kato GJ, Casella JF. Corticosteroids and increased risk of readmission after acute chest syndrome in children with sickle cell disease. Pediatr Blood Cancer. 2008;50(5):1006‐1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Al‐Ani F, Chehade S, Lazo‐Langner A. Thrombosis risk associated with COVID‐19 infection. A scoping review. Thromb Res. 2020;192:152‐160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. National Institutes of Health . Evidence‐Based Management of Sickle Cell Disease: Expert Panel Report, 2014. NIH; 2014. https://www.nhlbi.nih.gov/health‐topics/evidence‐based‐management‐sickle‐cell‐disease. Accessed December 15, 2020. [Google Scholar]
- 70. Ko RH, Thornburg CD. Venous thromboembolism in children with cancer and blood disorders. Front Pediatr. 2017;5:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Piovesan D, Attard C, Monagle P, Ignjatovic V. Epidemiology of venous thrombosis in children with cancer. Thromb Haemost. 2014;111(6):1015‐1021. [DOI] [PubMed] [Google Scholar]
- 72. Sullivan M, Bouffet E, Rodriguez‐Galindo C, et al. The COVID‐19 pandemic: a rapid global response for children with cancer from SIOP, COG, SIOP‐E, SIOP‐PODC, IPSO, PROS, CCI, and St Jude Global. Pediatr Blood Cancer. 2020;67(7):e28409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Overstad S, Tjonnfjord E, Garabet L, et al. Venous thromboembolism and coronavirus disease 2019 in an ambulatory care setting ‐ a report of 4 cases. Thromb Res. 2020;194:116‐118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Aydillo T, Gonzalez‐Reiche AS, Aslam S, et al. Shedding of viable SARS‐CoV‐2 after immunosuppressive therapy for cancer. N Engl J Med. 2020;383(26):2586‐2588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Key NS, Khorana AA, Kuderer NM, et al. Venous thromboembolism prophylaxis and treatment in patients with cancer: ASCO clinical practice guideline update. J Clin Oncol. 2020;38(5):496‐520. [DOI] [PubMed] [Google Scholar]
- 76. Klaassen ILM, Lauw MN, van de Wetering MD, et al. TropicALL study: thromboprophylaxis in children treated for acute lymphoblastic leukemia with low‐molecular‐weight heparin: a multicenter randomized controlled trial. BMC Pediatr. 2017;17(1):122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Li A, Garcia DA, Lyman GH, Carrier M. Direct oral anticoagulant (DOAC) versus low‐molecular‐weight heparin (LMWH) for treatment of cancer associated thrombosis (CAT): a systematic review and meta‐analysis. Thromb Res. 2019;173:158‐163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. www.who.int/publicationsdetail/multisystem‐inflammatory‐syndrome‐in‐children‐and‐adolescents‐withcovid‐19. Accessed December 15, 2020.
- 79. Rowley AH. Understanding SARS‐CoV‐2‐related multisystem inflammatory syndrome in children. Nat Rev Immunol. 2020;20(8):453‐454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Whittaker E, Bamford A, Kenny J, et al. Clinical characteristics of 58 children with a pediatric inflammatory multisystem syndrome temporally associated with SARS‐CoV‐2. JAMA. 2020;324(3):259‐269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Consiglio CR, Cotugno N, Sardh F, et al. The immunology of multisystem inflammatory syndrome in children with COVID‐19. Cell. 2020;183(4):968‐981.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. McCrindle BW, Rowley AH, Newburger JW, et al. Diagnosis, treatment, and long‐term management of Kawasaki disease: a scientific statement for health professionals from the American Heart Association. Circulation. 2017;135(17):e927‐e999. [DOI] [PubMed] [Google Scholar]
- 83. Henderson LA, Canna SW, Friedman KG, et al. American College of Rheumatology clinical guidance for pediatric patients with multisystem inflammatory syndrome in children (mis‐c) associated with SARS‐CoV‐2 and hyperinflammation in COVID‐19. Version 2. Arthritis Rheumatol. 2021;73(4):e13‐e29. [DOI] [PMC free article] [PubMed] [Google Scholar]


