Abstract
Embryo survival in birds depends on a controlled transfer of water vapour and respiratory gases through the eggshell, and this exchange is critically sensitive to the surrounding physical environment. As birds breed in most terrestrial habitats worldwide, we proposed that variation in eggshell conductance has evolved to optimize embryonic development under different breeding conditions. This is the first study to take a broad-scale macro-ecological view of avian eggshell conductance, encompassing all key avian taxonomic groups, to assess how life history and climate influence the evolution of this trait. Using whole eggs spanning a wide phylogenetic diversity of birds, we determine that body mass, temperature seasonality and whether both parents attend the nest are the main determinants of eggshell conductance. Birds breeding at high latitudes, where seasonal temperature fluctuations are greatest, will benefit from lower eggshell conductance to combat temporary periods of suspended embryo growth and prevent dehydration during prolonged incubation. The nest microclimate is more consistent in species where parents take turns incubating their clutch, resulting in lower eggshell conductance. This study highlights the remarkable functional qualities of eggshells and their importance for embryo survival in extreme climates.
Keywords: avian eggshells, climate, life history, nest, temperature seasonality, water vapour conductance
1. Introduction
Adaptive diversification across species typically occurs amidst an array of distinct ecological niches and environments and is a key driver in the development of novel functional traits to enhance the fitness of an organism [1]. The evolution of a new trait may provide the adaptive potential to exploit a resource that was not previously possible, or interact with its environment in a new way without a specific change in the external environment [2]. Close association between certain traits and a species environment and life history can therefore point to probable causes of trait divergence [3]. Traits can evolve rapidly over several generations or slowly over millions of years in accordance with environmental rates of change [4]. Species persistence during abrupt climate change will, therefore, depend on their ability to rapidly respond and adapt to novel environmental conditions [5]. Individual species will either move to more favourable conditions, tolerate or adapt to their changed environment, or go extinct [6]. Understanding the evolutionary history and diversification of functional traits closely linked to reproductive success will help predict how species will react to these new environmental pressures.
Foremost, the survival of any species is reliant on having a viable embryo. One crucial step in understanding avian responses to environmental differences over evolutionary time is a better appreciation of factors shaping avian incubation and their subsequent influence on the embryo [7]. Birds have evolved multiple functional traits to improve offspring survival in the nest: arguably one of the most important is the eggshell. Most bird species lose 10–20% of their fresh egg mass over the incubation period through the passive diffusion of water vapour through the eggshell to the ambient air [8]. Eggs that lose too much water during incubation frequently do not hatch due to desiccation [9], while embryos that do not lose enough water from the egg experience respiratory problems or drown [10]. Maintaining a controlled loss of water from inside the egg to the external environment while allowing sufficient exchange of respiratory gases is therefore essential for normal embryo development and hatching.
Birds are highly diversified and widely distributed, occupying every continent on Earth and every terrestrial habitat within it [11]. Some birds breed in extremely inhospitable environments, such as cold and dry regions [12], deserts [13], moist wetlands [14] and high altitudes [15]. Among these are ground-nesting birds in alpine or Arctic/Antarctic regions that must cope with unpredictable wind, precipitation and snow conditions, with ambient temperatures fluctuating from below freezing to over 45°C [16]. Avian embryos in such cold regions will freeze to death if left unattended by their parents [17]. Desert birds that breed in the Sahara, Arabian and Kalahari regions face extreme physiological challenges to conserve water and avoid dehydration for the eggs, adults and hatchlings [18]. In contrast with dry, xeric environments, eggs exposed to high precipitation are prone to rain-induced suffocation [19]. The major challenges for birds breeding in high-altitude regions like the Himalayas is the low barometric pressure and high solar radiation, which can result in desiccation of egg contents and overheating of the embryo [15]. Species living at such environmental extremes must adapt behaviourally or physiologically at each stage of their breeding cycle if they are to produce viable offspring [20].
Water vapour conductance through the avian eggshell, herein referred to as conductance or GH2O, is influenced by the properties of the eggshell (e.g. pore length, functional pore area and eggshell cuticle) and humidity and gas composition of the surrounding environment. Species that incubate their eggs buried [21], in dry [22] or wet environments [23], or at high altitudes [15] have particularly unusual vapour pressure gradients, yet are still able to maintain water loss within acceptable limits. GH2O may be optimized to suit particular environments through changes in nest-site preferences, eggshell structures and incubation behaviours [14], making eggs and their species-specific conductance ideal model systems for understanding how trait selection varies over time during diversification.
Predicting GH2O of a species is not straight forward, as multiple ecological factors must be taken into account. For example, brood-chambers of burrow-nesting birds are often permanently saturated with water vapour, resulting in a low water vapour pressure (favouring enhanced conductance) and longer incubation periods (favouring reduced conductance) [24]. Inter-species differences in GH2O thus can only be untangled by considering the contribution of multiple life-history traits and the phylogenetic history of the lineage. A study across 141 non-passerine species detected differences in GH2O between nest types and parental incubation behaviours [25], emphasizing the importance of maintaining a suitable nest microclimate for optimum egg-water loss. However, it is unknown whether a similar relationship between conductance and nesting behaviour is expected in the passerines, which comprise over 6000 species and represent almost 60% of all living birds [26]. Moreover, previous studies have typically focused on either (i) one group of birds (e.g. gulls), with the goal to look for micro-adaptations between closely related species [27], or (ii) eggs of ‘extreme nesters’ such as desert-nesting Bedouin fowl (Gallus domesticus) [28] and grey gulls (Larus modestus) [29], water-nesting grebes and divers [30] and marsh-nesting black terns (Chlidonias niger) [31]. The role of life-history and environmental factors in the evolution of avian eggshell conductance thus requires a large-scale comparative analysis encompassing all key taxonomic groups.
Our aim was to evaluate how climate and life history influence GH2O across a wide taxonomic distribution of birds spanning 28 avian orders, after accounting for the effects of adult body mass and phylogeny. Previous comparative analyses of eggshell conductance have not corrected for allometric effects of body mass [25], which can hide potentially important adaptive information relating to the environment and nesting behaviour of the species. Based on previous findings, we predicted GH2O would be primarily explained by body mass. By contrast, we predict that mass-independent conductance (RGH2O) would be primarily associated with traits known to affect nest humidity, including climate, nest location and type.
2. Material and methods
(a) . Egg samples and preparation
In total, 365 bird species were included in this study. Conductance of whole emptied eggs at the Natural History Museum, Tring (NHM, UK) was established using the standard protocol of measuring the decrease in egg mass as a result of water loss over consecutive days, in eggs kept in constant moisture-free conditions [32]. GH2O measured using whole eggshells is preferable to eggshell fragments as shell thickness and porosity varies between different regions of an egg [33]; therefore, we only used values from whole eggs in this study.
Eggs were prepared by gently cleaning the surface, filling the egg with water then sealing the blow hole (see electronic supplementary material). Eggs were placed in an acrylic desiccator cabinet (ThermoFisher Scientific, Nalgene, catalogue number: 5317-0070) inside a constant temperature thermocabinet (Porkka, Hertfordshire, UK) at 30 ± 1°C. Temperature was monitored via a logtag analyser every 10 min (Loggershop, Bournemouth, Dorset, UK). Self-indicating silica gel (Merck, Honenbrunn, Germany, catalogue number: 101 969) were placed in the desiccator to remove all moisture. Any loss in egg mass was entirely due to the diffusion of water vapour via the shell pores [34]. The first 24 h can give unexpectedly high mass loss values as the outer shell surface dries out [35]. Therefore, the eggs were left 24 h before being weighed to the nearest 0.1 mg (Sartorius, Göttingen, Germany), then were returned to the desiccator. Eggs were weighed at the same time of day on 3 successive days to give two values of 24 h mass loss (MH2O). Species GH2O was then calculated, as described in the electronic supplementary material.
Species mean GH2O values of whole eggs reported in the literature (n = 188) were incorporated if specimens had been measured under constant conditions (temperature and humidity) and followed protocols used in the present study. GH2O measures from whole fresh eggs (unemptied or water-filled) and museum (water-filled) eggs were combined as GH2O does not differ significantly between these treatments [36]. Mean GH2O values reported in the literature were corrected to standard barometric pressure (1 ATM) at 30°C (see electronic supplementary material).
(b) . Life-history and ecological data
We collated data on 18 key life-history traits that have previously been hypothesized to play a role in the evolution of avian conductance in addition to climate variables (table 1). These data were extracted from multiple sources detailed in the FigShare repository (doi:10.6084/m9.figshare.12490559). Major sources are detailed in section (e) of electronic supplementary material. Only 13 predictors were included in the analysis due to collinearity (see electronic supplementary material). The phylogenetic generalized least-squares (PGLS) method was used to test the evolutionary association between whole eggshell GH2O life-history traits, within a phylogenetic context [37]. In this procedure, closely related species are assumed to have more similar traits because of their shared ancestry and consequently will produce more similar residuals from the least-squares regression line. By taking into account the expected covariance structure of these residuals, modified slope and intercept estimates are generated that account for interspecific autocorrelation due to phylogeny.
Table 1.
Putative predictions and definitions for 13 possible explanations for variation in water vapour conductance (GH2O) in birds.
| predictor | hypothesis | definition |
|---|---|---|
| body mass | as adult body mass is correlated to egg mass, heavier birds will have higher GH2O due to greater egg surface area | mean body mass (g) of adult birds |
| clutch size | evaporation from multiple eggs will create a nest atmosphere of greater humidity and reduced water vapour transfer, so GH2O should be higher for species with larger clutches | number of eggs per brood, measured as geometric mean of the typical minimum and maximum clutch size |
| calcium content | eggshells of calcium-poor species are expected to be thinner, less dense and more porous than calcium-rich species, and thus facilitate higher GH2O | (1) calcium-rich: species that ingest mollusc shells, fish, shellfish, calcareous grit, calcareous ash or bones |
| (2) calcium-poor: species with primarily insectivorous or granivorous diet | ||
| egg maculation | maculated eggs are expected to have lower GH2O than immaculate eggs to reduce the risk of desiccation | (1) immaculate: no spotting or markings on eggshell surface |
| (2) maculation: maculation present on eggshell surface | ||
| nest type | fully enclosed nests have less air movement than semi-enclosed and exposed nests, facilitating greater GH2O | (1) exposed: nest is open above and has no side walls (no nest, scrape, saucer, platform, heap) |
| (2) semi-enclosed: nest is partially open and has side walls (cup, bowl, pendant, sphere, dome, pouch) | ||
| (3) enclosed: nest is entirely enclosed (cavity, burrow, crevice) | ||
| nest location | nests above ground have lower risk of flooding or water accumulation, therefore will have lower GH2O | (1) ground: nest location in or on the ground, or floating on water |
| (2) tree: nest located in tree, bush, shrub, wall, cave roof, or attached to reed | ||
| (3) cliff: nest located on cliff | ||
| nest lining | incorporation of nest lining will better insulate the egg, therefore will have higher GH2O | (1) lined: nest lining is always or sometimes present |
| (2) not lined: nest lining is absent | ||
| habitat | among open nesting species, more direct sunlight reaches eggs in open habitats and experience greater air movement around the nest than closed habitats; open-nesting species in open habitats will have lower GH2O than in closed habitats | (1) open: breeds in desert, grassland, open water, open moorland, low shrubs, rocky habitats, seashores and cities |
| (2) semi-open: breeds in open shrubland and bushland, scattered bushes, parkland, forest edge | ||
| (3) dense: breeds in forest with a closed canopy, or in the lower vegetation strata of dense thickets, shrubland, mangroves or marshland | ||
| incubating parent | nest vapour pressure will decrease when the parent leaves the nest uncovered, which is more likely to occur if incubation is not shared between parents, resulting in lower GH2O | (1) shared: contact incubation of eggs by two adults |
| (2) not shared: contact incubation of eggs by single adult | ||
| mode of development | higher GH2O may contribute to improving the use of nutritional support by the embryo of precocial species by removing excess water, thus resulting in increased development at hatching | (1) altricial: newly born young are relatively immobile, naked, and usually require care and feeding by the parents |
| (2) precocial: newly born young are relatively mobile, covered in feathers, and independent | ||
| parental care | the eggs of species that provide biparental care are expected to have higher GH2O as nest humidity and temperature can be better maintained when both parents assist | (1) uniparental: the brood is provisioned and/or defended by one adult |
| (2) biparental: the brood is provisioned and/or defended by two adults | ||
| parental contact | the wet incubating parent returning to the nest will increase the nest's humidity, thus are excepted to have higher GH2O | (1) wet plumage: adults returned habitually to the nest with wet plumage; this included species that feed on freshwater or marine prey or use nests built on water |
| (2) dry plumage: adults did not return habitually to the nest with wet plumage | ||
| temperature seasonality | eggs incubated in environments with highly variable temperature will experience lower GH2O as high temperature seasonality occurs in cooler environments | average temperature seasonality (BIO4) of breeding/resident range, based on WorldClim v1 data |
| precipitation seasonality | eggs incubated in environments with highly variable precipitation will experience higher GH2O to combat temporary periods of excessive rain | average precipitation seasonality (BIO15) of breeding/resident range, based on WorldClim v1 data |
Prior to updated avian phylogenies based on genomic DNA, near-passerines was a term given to tree-dwelling birds (within the conventional non-passerines) that were traditionally believed to be related to Passeriformes due to ecological similarities. In this study Pterocliformes (sandgrouse), Columbiformes (pigeons), Cuculiformes (cuckoos), Caprimulgiformes (nightjars) and Apodiformes (swifts, hummingbirds) were defined as near-passerines. All passerines and near-passerines are land birds and have altricial and nidicolous (stay within the nest) chicks, while non-passerine chicks vary in their mode of development and include water and land birds [38]. Sandgrouse are an exception as they have precocial young and are not tree-dwelling [39]. In respect to nest architecture, most passerines build open-cup nests, though some build more elaborate dome structures with roofs [40]. Dome nests, however, are more common among passerines than non-passerines and are particularly frequent among very small passerines [41]. Although these groups are no longer recognized as near-passerines, this definition was used here to distinguish between ecologically profound differences among birds.
Avian phylogenetic trees were constructed online (http://www.birdtree.org) from the complete avian phylogeny of Jetz et al. [42] and used the primary backbone tree of Hackett et al. [43]. Ten thousand trees were constructed and statistical analyses were performed in the program R, v. 3.6.1 (R Software, Vienna, Austria, http://www.R-project.org). All quantitative variables (except absolute median latitude, annual temperature and temperature range) were log10-transformed prior to phylogenetic analysis to reduce skewness [44].
As body mass affects all aspects of animal biology and ecology [45], our initial set of phylogenetic analysis account for adult body mass by including this variable as a predictor of log(GH2O). We repeated our phylogenetic analysis using mass-corrected GH2O as the response variable, herein called relative GH2O (RGH2O), thereby removing adult body mass as a predictor. RGH2O values were computed as residuals from a PGLS regression of log(GH2O) on log(body mass) (slope = 0.53 ± 0.03 s.e.; intercept = −0.69 ± 0.12 s.e.; λ = 0.68; electronic supplementary material, figure S1). Using this second series of models, we can ask how well one or more life-history traits results in higher or lower GH2O than is expected for a given body mass of the adult bird.
Phylogenetic signal in GH2O and RGH2O was measured by Pagel's lambda (λ) [46] using the phylosig function in the package ‘phytools’ [47] to determine to what extent related species were more likely to share similar conductance values than species drawn randomly from a tree. The phylosig function was used to test the hypothesis that Pagel's λ is different from 0. To test the alternative hypothesis (that Pagel's λ is less than 1), we computed the difference in the log-likelihood ratio of the lambda model (phylosig function) and Brownian motion model (brownie.lite function), then compared it to a chi-squared (χ2) distribution with 1 degree of freedom. PGLS models were fitted using the phylolm function in the package ‘phylolm’ [48]. We ran the full model containing all traits as predictor variables, then used the pdredge function from the package MuMIn [49] to fit all possible model combinations with a maximum of five predictors following protocols by Powney et al. [50], in addition to a null model comprising only the intercept. The best subset of models had an AICc (Akaike's information criterion adjusted for low sample size) within two of the model with the lowest AICc [51]. Conditional model averaging was then used to identify parameter estimates and importance for each trait present in at least one of the subset models [52].
3. Results
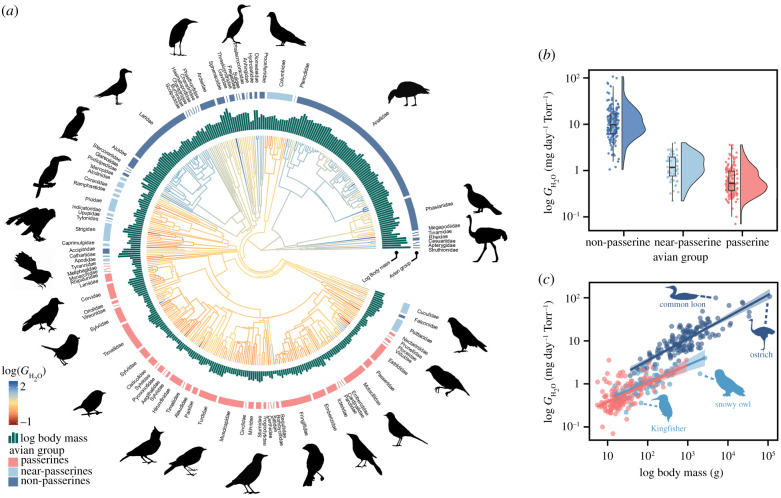
In total, we used over 2533 eggs from 364 species to assess diversification in conductance across the avian phylogeny. These species span across 85 families and represent 28 of the 49 extant avian orders. Overall, bird species in Australia, North America and South America had higher log(GH2O) and RGH2O than species in Africa, Europe and Asia (electronic supplementary material, figure S2). GH2O was highest for large flightless birds (e.g. ostriches (Struthio camelus) (106.99 mg day−1 Torr−1)), nightjars (Caprimulgiformes 0.55 ± 0.19 mg day−1 Torr−1) and songbirds (Passeriformes 0.74 ± 0.05 mg day−1 Torr−1). GH2O was also high for aquatic birds (e.g. common loons (Gavia immer) 98.82 mg day−1 Torr−1), kiwis (Southern brown kiwi (Apteryx australis) 26.22 mg day−1 Torr−1) and penguins (Sphenisciformes 22.66 ± 5.45 mg day−1 Torr−1). Viewing total phylogenetic variation in this trait (figures 1a and 2a) revealed that log(GH2O) and RGH2O were typically lower in passerines and near-passerines, than non-passerines (figure 1b).
Figure 1.
Relationship between conductance of whole eggs and ecological variables for 364 bird species. (a) Phylogenetic tree from which water vapour conductance (GH2O) data were obtained. The bar plot around the phylogeny represents the only significant predictors of log(GH2O) in conditionally averaged models. Conditional model averaging was used to obtain a single average model when more than one PGLS model was best ranked (i.e. more than one model with ΔAICc < 2 from the top-ranked model). Branch colours show the diversification in log(GH2O) across the phylogeny and ancestral trait estimates. GH2O is plotted as a function of (b) avian group and (c) adult body mass (g) within each of the three avian groups. Silhouette illustrations came from PhyloPic (http://phylopic.org), contributed by various authors under public domain licence. (Online version in colour.)
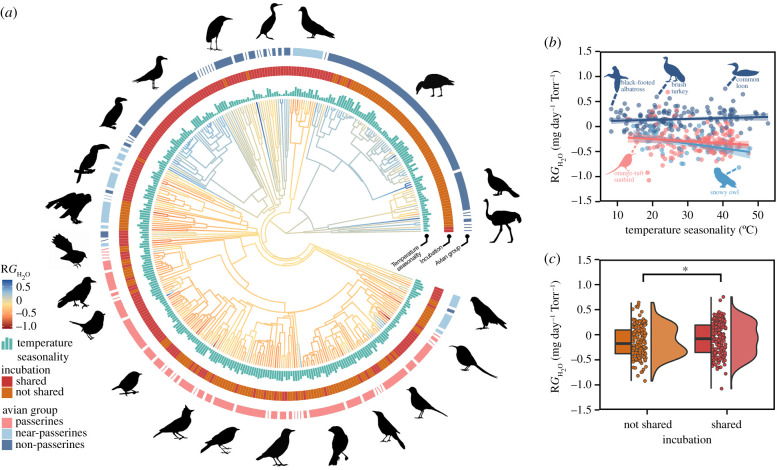
Figure 2.
Relationship between conductance of whole eggs and ecological variables for 364 bird species. (a) Phylogenetic tree of residual water vapour conductance (RGH2O). Bar plots and rings around the phylogeny represent significant predictors of RGH2O in conditionally averaged models. Conditional model averaging was used to obtain a single average model when more than one PGLS model was best ranked (i.e. more than one model with ΔAICc < 2 from the top-ranked model). Branch colours show the diversification in RGH2O across the phylogeny and ancestral trait estimates. RGH2O is plotted as a function of (b) temperature seasonality within each avian group and (c) whether both parents incubate the eggs. In the hybrid box plot, species RGH2O are shown as filled circles, vertical lines indicate the median, box shows the interquartile range (IQR) and the whiskers are 1.5 × IQR (distribution is shown as histograms). p-values are given in asterisks, with *less than 0.05, **less than 0.01 and ***less than 0.001. Silhouette illustrations came from PhyloPic (http://phylopic.org), contributed by various authors under public domain license. (Online version in colour.)
(a) . Phylogenetic correlation
Phylogenetic signal for log(GH2O) and RGH2O (table 2) was significantly different from 0 (i.e. no phylogenetic signal) (p < 0.001) and 1 (i.e. the Brownian explanation) (p < 0.001), meaning that while there is an effect of phylogeny on conductance, it is influenced by evolutionary processes that are weaker than would be seen with a Brownian motion model of trait evolution. Phylogenetic signal was high for Log(GH2O) (λ = 0.96), showing that closely related species exhibit similar eggshell conductance prior to accounting for differences in body mass, and this biological similarity decreases as the evolutionary distance between species increases. Phylogenetic signal was intermediate for RGH2O (λ = 0.55), suggesting that phylogeny and other selective pressures (e.g. those associated with species life history or climate) are important in determining eggshell conductance, after accounting for differences in species body mass.
Table 2.
Estimates of phylogenetic signal in eggshell water vapour conductance (GH2O) in all birds. Phylogenetic signal was analysed separately for log10-transformed GH2O (log(GH2O)) and residual water vapour conductance (RGH2O). The p-value tests the null hypothesis of no phylogenetic signal (λ = 0) and Brownian motion model (λ = 1) of evolution.
| response variable | Pagel's λ | log-likelihood | log-likelihood for λ = 0 | log-likelihood for λ = 1 | p for λ = 0 | p for λ = 1 |
|---|---|---|---|---|---|---|
| log(GH2O) | 0.96 | −74.39 | 590.76 | −125.64 | <0.001 | <0.001 |
| RGH2O | 0.55 | 27.20 | 258.50 | −92.27 | <0.001 | <0.001 |
(b) . Life history and climate influence conductance across birds
Adult body mass and temperature seasonality were the strongest predictors of log(GH2O) across all birds based on conditionally averaged models (electronic supplementary material, table S1). Log(GH2O) was significantly higher among heavier species (z = 18.40, p < 0.001; figures 1c and 3a) since initial egg mass increases with adult body mass (n = 251, r2 = 0.89, p < 0.001 [52]). Log(GH2O) was negatively associated with increased temperature seasonality across all birds (z = 2.13, p = 0.03; figures 2b and 3b). Temperature seasonality is defined here as the amount of temperature variation over a given year (or averaged years) based on the standard deviation of monthly temperature averages [53]. There was also a weaker yet significant effect of dietary calcium, nest location, mode of development, shared incubation and parental contact among top-ranked models (electronic supplementary material, table S2).
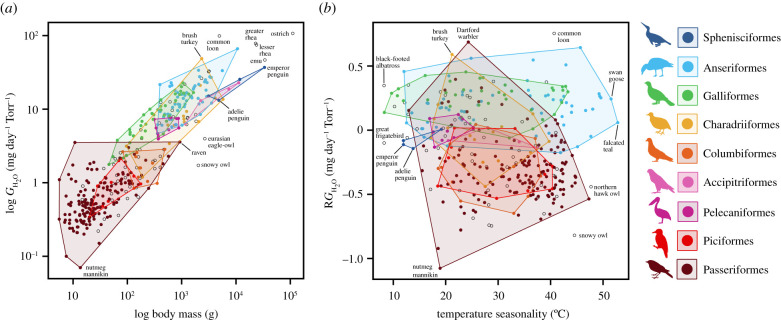
Figure 3.
Partitioning of variation of water vapour conductance (GH2O) among avian orders. Dots (one per species) (n = 364) show the distribution of log(GH2O) as a function of (a) adult body mass (g) and (b) residual water vapour conductance (RGH2O) as a function of temperature seasonality. The minimum convex hull is plotted for all species within a subset of avian orders. Silhouette illustrations came from PhyloPic (http://phylopic.org), contributed by various authors under public domain license. (Online version in colour.)
Temperature seasonality (z = 2.20, p = 0.03) and whether contact incubation was shared among parents (z = 2.22, p = 0.03) were significant in conditionally averaged models after accounting for adult body mass (RGH2O) (electronic supplementary material, table S3; figure 2). RGH2O overall decreased with temperature seasonality (figures 2b and 3b). RGH2O was higher in species where both parents incubate the clutch (figure 2c). Dietary calcium, mode of development, nest location and parental contact showed weaker but significant correlations with RGH2O among top-ranked models (electronic supplementary material, table S4). RGH2O was higher in species with calcium-rich diets, precocial young, parents that return to the nest with wet plumage and ground nesters compared to tree nesters (electronic supplementary material, figure S6). Based on conditionally averaged models for GH2O and RGH2O, eggshell conductance across birds is primarily influenced by adult body mass, temperature seasonality and parent incubation strategies.
4. Discussion
This study focused on one performance trait—conductance—of modern avian eggshells to better understand how birds have achieved high ecological diversity. We identified the importance of phylogeny, physiology (body mass and mode of development), behaviour (diet, parental incubation strategies and nest location) and climate in the evolution of this trait. This study is the first to identify a broad-scale reduction in eggshell conductance where temperature seasonality increases. Regions with greater temperature seasonality experience a greater range in temperatures over the course of a year and correlate with an organisms' temperature tolerance breadth [54]. Increased temperature seasonality occurs further from the equator and is associated with a decline in annual temperature, precipitation and day length [55]. A comparative study on 139 bird species found that adults inhabiting low and seasonally variable temperatures had lower basal metabolic rate after removing the effects of body mass [56]. In the light of this, it appears possible that eggshells are already preparing the embryo for adulthood, with respect to their environment and breeding biology. Amniotic embryos adjust their metabolic activity and active cell division in response to varying environmental conditions, and by doing so, alter their period of development [57]. Reproductive strategies to prolong the egg state are most diverse in reptiles and less varied in birds and mammals that provide more parental care [58]. Even so, the low metabolic rate expected for embryos incubated in highly seasonal climates would favour a reduction in conductance to prolong their incubation period.
Broad-scale geographical trends in RGH2O identified here may be the result of long-term evolutionary responses or short-term physiological modifications [59]. Evolutionary adaptation would involve changes in GH2O over (rather than within) generations when natural selection acts on genetic variants while acclimatization would involve reversible changes to GH2O that can happen gradually (greater than 1 day) in response to the recent environment [60]. Intraspecific variation in GH2O has been reported across altitude [61,62] and humidity [63,64] gradients of multiple species, but the timeframe in which GH2O diversification has taken place is unknown. Some studies propose that rapid evolution of eggshell structure from exposure to novel environments is unlikely [65,66] and is instead compensated by behavioural modifications of the parents. Other studies find that incubation behaviour does not significantly modulate conductance [67], so adaptive responses must be accomplished by changes in eggshell structure [63].
Birds are seemingly capable of short-term and instantaneous physiological adjustments in shell structure in response to environmental variation. Pigeons (Columba livia) bred for several years within an environmentally controlled room experienced approximately 30% lower GH2O than predicted when exposed to high temperature and low humidity over a short period [68]. Similarly, domestic chickens (Gallus domesticus) bred at high elevation for multiple generations produced eggshells with a 30% higher GH2O within two months of being translocated to low altitudes [69]. In other species, GH2O did not change when individuals were transferred to higher altitudes [70] or were exposed to natural seasonal changes in humidity [71], suggesting there is variation in the plasticity of a species response. Identifying the speed of the response in eggshell parameters to novel environments across multiple species will be very informative in determining climate change effects on bird species and their breeding.
We found that conductance across birds was also dependent on nest location, whether parents alternate nest attendance, and whether the parent returns to the nest with wet plumage, corroborating previous studies [25,72]. Shared incubation between two parents allows one of them to be relieved from incubation to feed while the other incubates the egg, thus allowing the eggs to be covered at all times [73]. Clutches that are incubated by both parents encounter less variation in egg temperature than clutches that are incubated by a single parent [74] and thus, are expected to have higher eggshell conductance. Water added to the nest by parents can be many orders of magnitude higher than water lost by the eggs [75]. Consequently, RGH2O is significantly higher in species where parents return the nest with wet plumage [25]. Eggs laid on the ground, in a burrow, mound or on floating vegetation are subject to higher humidity than arboreal nesters, leading to eggshell adaptations that promote water loss. Common loons (Gavia immer), for example, had the highest RGH2O of the species investigated. This may be attributed to their high eggshell porosity [76] in response to building nests on or near the water where transpiration of water is high, and nest materials can be wet [64]. Nest location and whether parents return to the nest with wet or dry plumage was significant in most top models where these predictors were included, but this effect was weak compared to life-history traits retained in conditionally averaged models. Combined, our results demonstrate that different behavioural strategies used by parents to alter nest humidity have contributed to the evolution of conductance among birds.
Variation in the incubation period across the altricial-precocial spectrum reflects a trade-off between embryo growth rate and degree of maturity when hatched. Precocial species take up to two times longer to incubate an egg of the same size as altricial species, but are far more developed when they hatch [77]. For eggs of the same mass, precocial species incur a higher total energy cost than altricial species because the embryo is larger for a longer period during incubation [78]. Consequently, eggs of species with fast (precocial) growing offspring had significantly higher RGH2O than those of species with slow (altricial) growing offspring based on top-ranked models. As higher conductance enables greater gas exchange, this may optimize embryo access to high energy content in precocial eggs [79], thus resulting in a more developed chick at birth. RGH2O in passerines was found here to be particularly low, likely because they have altricial young, whereas non-passerines consist of precocial and altricial species.
Supplementary Material
Acknowledgements
We are grateful to Douglas Russell at The Natural History Museum Tring for his generous assistance in working with the eggshell collection, and for useful discussions. We thank Craig White for useful discussions and providing code for phylogenetic comparative analysis, and Stephanie McClelland, Jennifer Cantlay and Jack Thirkell for their comments on early drafts.
Data accessibility
Data are publicly available in the FigShare repository, including specimen and species-specific water vapour conductance, life histories and sources used in this study (doi:10.6084/m9.figshare.12490559) [80]. Tables for all PGLS analyses and sources for figure illustrations are available in the electronic supplementary material.
Authors' contributions
M.R.G.A.: conceptualization, data curation, formal analysis, investigation, methodology, project administration, validation, visualization, writing-original draft, writing-review and editing; S.J.P.: conceptualization, formal analysis, funding acquisition, investigation, methodology, project administration, resources, supervision, writing-review and editing
All authors gave final approval for publication and agreed to be held accountable for the work performed therein.
Competing interests
We declare we have no competing interests.
Funding
This project and M.R.G.A. was funded by a Research Project (grant no. RPG-2018-332) from the Leverhulme Trust, awarded to S.J.P.
References
- 1.Losos JB. 2010. Adaptive radiation, ecological opportunity, and evolutionary determinism. Am. Nat. 175, 623-639. ( 10.1086/652433) [DOI] [PubMed] [Google Scholar]
- 2.Yoder JB, et al. 2010. Ecological opportunity and the origin of adaptive radiations. J. Evol. Biol. 23, 1581-1596. ( 10.1111/j.1420-9101.2010.02029.x) [DOI] [PubMed] [Google Scholar]
- 3.Losos JB, Schoener TW, Langerhans RB, Spiller DA. 2006. Rapid temporal reversal in predator-driven natural selection. Science 314, 1111. ( 10.1126/science.1133584) [DOI] [PubMed] [Google Scholar]
- 4.Ho W, Zhang J. 2018. Evolutionary adaptations to new environments generally reverse plastic phenotypic changes. Nat. Commun. 9, 350. ( 10.1038/s41467-017-02724-5) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hendry AP, Gotanda KM, Svensson EI. 2017. Human influences on evolution, and the ecological and societal consequences. Phil. Trans. R. Soc. B 372, 20160028. ( 10.1098/rstb.2016.0028) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hoffmann AA, Hercus MJ. 2000. Environmental stress as an evolutionary force. Bioscience 50, 217-226. ( 10.1641/0006-3568(2000)050[0217:ESAAEF]2.3.CO;2) [DOI] [Google Scholar]
- 7.Durant SE, Willson JD, Carroll RB. 2019. Parental effects and climate change: will avian incubation behavior shield embryos from increasing environmental temperatures? Integr. Comp. Biol. 59, 1068-1080. ( 10.1093/icb/icz083) [DOI] [PubMed] [Google Scholar]
- 8.Ar A, Rahn H. 1980. Water in the avian egg overall budget of incubation. Integr. Comp. Biol. 20, 373-384. ( 10.1093/icb/20.2.373) [DOI] [Google Scholar]
- 9.Carey C. 1986. Tolerance of variation in eggshell conductance, water loss, and water content by red-winged blackbird embryos. Physiol. Zool. 59, 109-122. ( 10.1086/physzool.59.1.30156096) [DOI] [Google Scholar]
- 10.Wangensteen OD, Rahn H. 1970. Respiratory gas exchange by the avian embryo. Respir. Physiol. 11, 31-45. ( 10.1016/0034-5687(70)90100-3) [DOI] [PubMed] [Google Scholar]
- 11.Konishi M, Emlen ST, Ricklefs RE, Wingfield JC. 1989. Contributions of bird studies to biology. Science 246, 465-472. ( 10.1126/science.2683069) [DOI] [PubMed] [Google Scholar]
- 12.Le Maho Y. 1977. The emperor penguin: a strategy to live and breed in the cold. Am. Sci. 65, 680-693. [Google Scholar]
- 13.Carey C. 2002. Incubation in extreme environments. In Avian incubation: behaviour, environment, and evolution (ed. Deeming DC), pp. 238-253. Oxford, UK: Oxford University Press. [Google Scholar]
- 14.Zicus MC, Rave DP, Riggs MR, Zicus MC, Rave DP, Riggs MR. 2003. Mass loss from mallard eggs incubated in nest structures. Wildl. Soc. Bull. 31, 270-278. [Google Scholar]
- 15.Carey C. 1980. Adaptation of the avian egg to high altitude. Am. Zool. 20, 449-459. ( 10.1093/icb/20.2.449) [DOI] [Google Scholar]
- 16.Martin K, Wiebe KL. 2004. Coping mechanisms of alpine and arctic breeding birds: extreme weather and limitations to reproductive resilience. Integr. Comp. Biol. 44, 177-185. ( 10.1093/icb/44.2.177) [DOI] [PubMed] [Google Scholar]
- 17.Spellerberg IF. 1969. Incubation temperatures and thermoregulation in the McCormick Skua. Condor 71, 59-67. ( 10.2307/1366049) [DOI] [Google Scholar]
- 18.McKechnie AE, Wolf BO. 2010. Climate change increases the likelihood of catastrophic avian mortality events during extreme heat waves. Biol. Lett. 6, 253-256. ( 10.1098/rsbl.2009.0702) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Öberg M, Arlt D, Pärt T, Laugen AT, Eggers S, Low M. 2015. Rainfall during parental care reduces reproductive and survival components of fitness in a passerine bird. Ecol. Evol. 5, 345-356. ( 10.1002/ece3.1345) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cloudsley-Thompson JL. 1988. Adaptations to Extreme Environments. In Evolution and adaptation of terrestrial arthropods (ed. Cloudsley-Thompson JL), pp. 80-98. Berlin, Germany: Springer. [Google Scholar]
- 21.Seymour RS, Ackerman RA. 1980. Adaptations to underground nesting in birds and reptiles. Am. Zool. 20, 437-447. ( 10.1093/icb/20.2.437) [DOI] [Google Scholar]
- 22.Rahn H, Hammel HT. 1982. Incubation water loss, shell conductance, and pore dimensions in Adelie penguin eggs. Polar Biol. 1, 91-97. ( 10.1007/BF00263805) [DOI] [Google Scholar]
- 23.Adkerman RA, Platter-Rieger M. 1979. Water loss by pied-billed grebe (Podilymbus podiceps) eggs. Arner. Zool. 19, 921. [Google Scholar]
- 24.Lill A, Fell PJ. 2007. Microclimate of nesting burrows of the Rainbow Bee-eater. Emu 107, 108-114. ( 10.1071/MU06046) [DOI] [Google Scholar]
- 25.Portugal SJ, Maurer G, Thomas GH, Hauber ME, Grim T, Cassey P. 2014. Nesting behaviour influences species-specific gas exchange across avian eggshells. J. Exp. Biol. 217, 3326-3332. ( 10.1242/jeb.103291) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ericson PG, Klopfstein S, Irestedt M, Nguyen JM, Nylander JA. 2014. Dating the diversification of the major lineages of Passeriformes (Aves). BMC Evol. Biol. 14, 8. ( 10.1186/1471-2148-14-8) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rahn H, Dawson WR. 1979. Incubation water loss in eggs of Heermann's and western gulls. Physiol. Zool. 52, 451-460. ( 10.1086/physzool.52.4.30155936) [DOI] [Google Scholar]
- 28.Arad Z, Marder J. 1982. Egg-shell water vapour conductance of the domestic fowl: comparison between two breeds and their crosses. Br. Poult. Sci. 23, 325-328. ( 10.1080/00071688208447964) [DOI] [Google Scholar]
- 29.Guerra C, Aguilar R, Fitzpatrick L. 1988. Water vapor conductance in Gray gulls (Larus modestus) eggs: adaptation to desert nesting. Colon. Waterbirds 11, 107-109. ( 10.2307/1521176) [DOI] [Google Scholar]
- 30.Sotherland P, Ashen M, Shuman R, Tracy C. 1984. The water balance of bird eggs incubated in water. Physiol. Zool. 57, 338-348. ( 10.1086/physzool.57.3.30163723) [DOI] [Google Scholar]
- 31.Davis TA, Ackerman RA. 1985. Adaptations of black tern (Chlidonias niger) eggs for water loss in a moist nest. Auk 102, 640-643. ( 10.1093/auk/102.3.640) [DOI] [Google Scholar]
- 32.Portugal SJ, Hauber ME, Maurer G, Stokke BG, Grim T, Cassey P. 2014. Rapid development of brood-parasitic cuckoo embryos cannot be explained by increased gas exchange through the eggshell. J. Zool. 293, 219-226. ( 10.1111/jzo.12144) [DOI] [Google Scholar]
- 33.Rokitka MA, Rahn H. 1987. Regional differences in shell conductance and pore density of avian eggs. Respir. Physiol. 68, 371-376. ( 10.1016/S0034-5687(87)80021-X) [DOI] [PubMed] [Google Scholar]
- 34.Booth DT, Seymour RS. 1987. Effect of eggshell thinning on water vapor conductance of malleefowl eggs. Condor 89, 453-459. ( 10.2307/1368635) [DOI] [Google Scholar]
- 35.Bamelis FR, De Ketelaere B, Mertens K, Kemps BJ, Decuypere EM, De Baerdemaeker JG.. 2008. Measuring the conductance of eggshells using the acoustic resonance technique and optical transmission spectra. Comput. Electron. Agric. 62, 35-40. ( 10.1016/j.compag.2007.08.009) [DOI] [Google Scholar]
- 36.Portugal SJ, Maurer G, Cassey P. 2010. Eggshell permeability: a standard technique for determining interspecific rates of water vapor conductance. Physiol. Biochem. Zool. 83, 1023-1031. ( 10.1086/656287) [DOI] [PubMed] [Google Scholar]
- 37.Symonds MRE, Blomberg SP. 2014. A primer on phylogenetic generalised least squares. In Modern phylogenetic comparative methods and their application in evolutionary biology (ed. Garamszegi L), pp. 105-130. Berlin, Germany: Springer. [Google Scholar]
- 38.Carnaby T. 2010. Beat about the bush, birds. Johannesburg, South Africa: Jacana Media. [Google Scholar]
- 39.Temrin H, Tullberg BS. 1995. A phylogenetic analysis of the evolution of avian mating systems in relation to altricial and precocial young. Behav. Ecol. 6, 296-307. ( 10.1093/beheco/6.3.296) [DOI] [Google Scholar]
- 40.Price JJ, Griffith SC. 2017. Open cup nests evolved from roofed nests in the early passerines. Proc. R. Soc. B 284, 20162708. ( 10.1098/rspb.2016.2708) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Collias NE. 1997. On the origin and evolution of nest building by passerine birds. Condor 99, 253-270. ( 10.2307/1369932) [DOI] [Google Scholar]
- 42.Jetz W, Thomas GH, Joy JB, Hartmann K, Mooers AO. 2012. The global diversity of birds in space and time. Nature 491, 444-448. ( 10.1038/nature11631) [DOI] [PubMed] [Google Scholar]
- 43.Hackett SJ, et al. 2008. A phylogenomic study of birds reveals their evolutionary history. Science 320, 1763-1768. ( 10.1126/science.1157704) [DOI] [PubMed] [Google Scholar]
- 44.Feng C, Wang H, Lu N, Chen T, He H, Lu Y, Tu XM. 2014. Log-transformation and its implications for data analysis. Shanghai Arch. psychiatry 26, 105-109. ( 10.3969/j.issn.1002-0829.2014.02.009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Brown JH. 1995. Macroecology. Chicago, IL: University of Chicago Press. [Google Scholar]
- 46.Pagel M. 1999. Inferring the historical patterns of biological evolution. Nature 401, 877-884. ( 10.1038/44766) [DOI] [PubMed] [Google Scholar]
- 47.Revell LJ. 2012. phytools: an R package for phylogenetic comparative biology (and other things). Methods Ecol. Evol. 3, 217-223. ( 10.1111/j.2041-210X.2011.00169.x) [DOI] [Google Scholar]
- 48.Ho LST, Ané C, Lachlan R, Tarpinian K, Feldman R, Yu Q, Ho MLST. 2014. Phylolm: phylogenetic linear regression. R package version 2.1.
- 49.Barton K, Barton MK. 2019. Package ‘MuMIn’. R package version 1.
- 50.Powney GD, Rapacciuolo G, Preston CD, Purvis A, Roy DB. 2014. A phylogenetically-informed trait-based analysis of range change in the vascular plant flora of Britain. Biodivers. Conserv. 23, 171-185. ( 10.1007/s10531-013-0590-5) [DOI] [Google Scholar]
- 51.Angert AL, Crozier LG, Rissler LJ, Gilman SE, Tewksbury JJ, Chunco AJ. 2011. Do species' traits predict recent shifts at expanding range edges? Ecol. Lett. 14, 677-689. ( 10.1111/j.1461-0248.2011.01620.x) [DOI] [PubMed] [Google Scholar]
- 52.Deeming DC. 2007. Effects of phylogeny and hatchling maturity on allometric relationships between female body mass and the mass and composition of bird eggs. Avian Poult. Biol. Rev. 18, 21-37. ( 10.3184/147020607X245039) [DOI] [Google Scholar]
- 53.O'Donnell MS, Ignizio DA. 2012. Bioclimatic predictors for supporting ecological applications in the conterminous United States. US Geol. Surv. Data Ser. 691, 4-9. ( 10.3133/ds691) [DOI] [Google Scholar]
- 54.Sunday J, et al. 2019. Thermal tolerance patterns across latitude and elevation. Phil. Trans. R. Soc. B 374, 20190036. ( 10.1098/rstb.2019.0036) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.De Frenne P, et al. 2013. Latitudinal gradients as natural laboratories to infer species' responses to temperature. J. Ecol. 101, 784-795. ( 10.1111/1365-2745.12074) [DOI] [Google Scholar]
- 56.White CR, Blackburn TM, Martin GR, Butler PJ. 2007. Basal metabolic rate of birds is associated with habitat temperature and precipitation, not primary productivity. Proc. R. Soc. B 274, 287-293. ( 10.1098/rspb.2006.3727) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rafferty AR, Reina RD. 2012. Arrested embryonic development: a review of strategies to delay hatching in egg-laying reptiles. Proc. R. Soc. B 279, 2299-2308. ( 10.1098/rspb.2012.0100) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Maurer G, Portugal SJ, Cassey P. 2011. Review: an embryo's eye view of avian eggshell pigmentation. J. Avian Biol. 42, 494-504. ( 10.1111/j.1600-048X.2011.05368.x) [DOI] [Google Scholar]
- 59.Walsberg GE, Voss-Roberts KA. 1983. Incubation in desert-nesting doves: mechanisms for egg cooling. Physiol. Zool. 56, 88-93. ( 10.1086/physzool.56.1.30159969) [DOI] [Google Scholar]
- 60.Llewelyn J, Macdonald SL, Moritz C, Martins F, Hatcher A, Phillips BL. 2018. Adjusting to climate: acclimation, adaptation and developmental plasticity in physiological traits of a tropical rainforest lizard. Integr. Zool. 13, 411-427. ( 10.1111/1749-4877.12309) [DOI] [PubMed] [Google Scholar]
- 61.Carey C, Garber SD, Thompson EL, James FC. 1983. Avian reproduction over an altitudinal gradient. II. Physical characteristics and water loss of eggs. Physiol. Zool. 56, 340-352. ( 10.1086/physzool.56.3.30152599) [DOI] [Google Scholar]
- 62.Sotherland PR, Packard GC, Taigen TL, Thomas J. 1980. An altitudinal cline in conductance of cliff swallow (Petrochelidon pyrrhonota) eggs to water vapor. Auk 97, 177-185. ( 10.1093/auk/97.1.177) [DOI] [Google Scholar]
- 63.Stein LR, Badyaev AV. 2011. Evolution of eggshell structure during rapid range expansion in a passerine bird. Funct. Ecol. 25, 1215-1222. ( 10.1111/j.1365-2435.2011.01887.x) [DOI] [Google Scholar]
- 64.Davis A, Platter-Reiger MF, Ackerman RA. 1984. Incubation water loss by pied-billed grebe eggs: adaptation to a hot, wet nest. Physiol. Zool. 57, 384-391. ( 10.1086/physzool.57.4.30163340) [DOI] [Google Scholar]
- 65.Simkiss K. 1980. Eggshell porosity and the water metabolism of the chick embryo. J. Zool. 192, 1-8. ( 10.1111/j.1469-7998.1980.tb04213.x) [DOI] [Google Scholar]
- 66.Board R. 1982. Properties of avian eggshells and their adaptive value. Biol. Rev. 57, 1-28. ( 10.1111/j.1469-185X.1982.tb00362.x) [DOI] [Google Scholar]
- 67.Walsberg GE. 1983. A test for regulation of nest humidity in two bird species. Physiol. Zool. 56, 231-235. ( 10.1086/physzool.56.2.30156054) [DOI] [Google Scholar]
- 68.Arad Z, Gavrieli-Levin I, Marder J. 1988. Adaptation of the pigeon egg to incubation in dry hot environments. Physiol. Zool. 61, 293-300. ( 10.1086/physzool.61.4.30161246) [DOI] [Google Scholar]
- 69.Rahn H, Ledoux T, Paganelli CV, Smith AH. 1982. Changes in eggshell conductance after transfer of hens from an altitude of 3800 to 1200 m. J. Appl. Physiol. 53, 1429-1431. ( 10.1152/jappl.1982.53.6.1429) [DOI] [PubMed] [Google Scholar]
- 70.Carey C, Hoyt DF, Bucher TL, Larson DL. 1984. Eggshell conductances of avian eggs at different altitudes. In Respiration and metabolism of embryonic vertebrates (ed. Seymour RS), pp. 259-270. Berlin, Germany: Springer. [Google Scholar]
- 71.Walsberg GE. 1985. A test for regulation of egg dehydration by control of shell conductance in Mourning Doves. Physiol. Zool. 58, 473-477. [Google Scholar]
- 72.Vleck CM, Vleck D, Rahn H, Paganelli CV. 1983. Nest microclimate, water-vapor conductance, and water loss in heron and tern eggs. Auk 100, 76-83. ( 10.1093/auk/100.1.76) [DOI] [Google Scholar]
- 73.Seddon P. 1989. Patterns of nest relief during incubation, and incubation period variability in the yellow-eyed penguin (Megadyptes antipodes). New Zeal. J. Zool. 16, 393-400. ( 10.1080/03014223.1989.10422905) [DOI] [Google Scholar]
- 74.Reneerkens J, Grond K, Schekkerman H, Tulp I, Piersma T. 2011. Do uniparental sanderlings Calidris alba increase egg heat input to compensate for low nest attentiveness? PLoS ONE 6, 1-9. ( 10.1371/journal.pone.0016834) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Grant GS. 1982. Avian incubation: egg temperature, nest humidity, and behavioral thermoregulation in a hot environment. Washington, DC: American Ornithologists’ Union. [Google Scholar]
- 76.Tullett SG, Board RG. 1977. Determinants of avian eggshell porosity. J. Zool. 183, 203-211. ( 10.1111/j.1469-7998.1977.tb04182.x) [DOI] [Google Scholar]
- 77.Ricklefs RE, Austin SH, Robinson WD. 2017. The adaptive significance of variation in avian incubation periods. Auk 134, 542-550. ( 10.1642/AUK-16-171.1) [DOI] [Google Scholar]
- 78.Hoyt DF. 1987. A new model of avian embryonic metabolism. J. Exp. Zool. Suppl 1, 127-138. [PubMed] [Google Scholar]
- 79.Sotherland PR, Rahn H. 1987. On the composition of bird eggs. Condor 89, 48-65. ( 10.2307/1368759) [DOI] [Google Scholar]
- 80.Attard MRG, Portugal SJ. 2021. Climate variability and parent nesting strategies influence gas exchange across avian eggshells. FigShare. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Attard MRG, Portugal SJ. 2021. Climate variability and parent nesting strategies influence gas exchange across avian eggshells. FigShare. [DOI] [PMC free article] [PubMed]
Supplementary Materials
Data Availability Statement
Data are publicly available in the FigShare repository, including specimen and species-specific water vapour conductance, life histories and sources used in this study (doi:10.6084/m9.figshare.12490559) [80]. Tables for all PGLS analyses and sources for figure illustrations are available in the electronic supplementary material.