Abstract
The relationship between COVID-19 severity and viral load is unknown. Our objective was to assess the association between viral load and disease severity in COVID-19. In this single center observational study of adults with laboratory confirmed SARS-CoV-2, the first positive in-hospital nasopharyngeal swab was used to calculate the log10 copies/ml [log10 copy number (CN)] of SARS-CoV-2. Four categories based on level of care and modified sequential organ failure assessment score (mSOFA) at time of swab were determined. Median log10CN was compared between different levels of care and mSOFA quartiles. Median log10CN was compared in patients who did and did not receive influenza vaccine, and the correlation between log10CN and D-dimer was examined. We found that of 396 patients, 54.3% were male, and 25% had no major comorbidity. Hospital mortality was 15.7%. Median mSOFA was 2 (IQR 0–3). Median log10CN was 5.5 (IQR 3.3–8.0). Median log10CN was highest in non-intubated ICU patients [6.4 (IQR 4.4–8.1)] and lowest in intubated ICU patients [3.6 (IQR 2.6–6.9)] (p value < 0.01). In adjusted analyses, this difference remained significant [mean difference 1.16 (95% CI 0.18–2.14)]. There was no significant difference in log10CN between other groups in the remaining pairwise comparisons. There was no association between median log10CN and mSOFA in either unadjusted or adjusted analyses or between median log10CN in patients with and without influenza immunization. There was no correlation between log10CN and D-dimer. We conclude, in our cohort, we did not find a clear association between viral load and disease severity in COVID-19 patients. Though viral load was higher in non-intubated ICU patients than in intubated ICU patients there were no other significant differences in viral load by disease severity.
Supplementary Information
The online version contains supplementary material available at 10.1007/s11739-021-02786-w.
Keywords: COVID-19, Coronavirus, SARS-CoV-2, Viral load, RT-PCR, Organ dysfunction score
Introduction
COVID-19, the illness caused by the severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2), was first reported in December 2019 [1] and was declared a pandemic by the World Health Organization in March 2020. Although the majority of those infected will not become severely ill, in those requiring hospitalization COVID-19 is characterized by pneumonia and hypoxemia. This can further lead to Acute Respiratory Distress Syndrome (ARDS) and respiratory failure requiring invasive mechanical ventilation. Early data from small studies based in China suggested that patients with higher baseline viral loads were more likely to have severe COVID-19 disease [2–4]. However, subsequent studies have reported contrasting results, resulting in a lack of clarity on the relationship between viral load and disease severity in this illness [5–7].
Early indicators of disease severity could help inform which treatments, such as antivirals, could be effective. Such indicators would also be helpful in determining the likelihood a patient will require escalation of the level of care [e.g. admission to an intensive care unit (ICU)]. In addition, as increasing viral load is associated with an increased transmissibility of COVID-19 [8], if viral load were associated with disease severity, this could help guide policies on infection control. Based on the finding of higher viral load in intubated patients in one study [9], the Centers for Disease Control (CDC) modified their recommendation to consider disease severity before removing isolation precautions at day 10 of a patient’s illness [10]. However, currently the relationship between viral load and disease severity in COVID-19 is not well-established.
Physicians and researchers have compared COVID-19 to influenza [11–13] possibly because of the general public's better understanding of the flu. In addition, there is similarity in structure between coronavirus and influenza [14, 15]. Due to the similarity in structure and cross reactivity in immunity between coronavirus and influenza, influenza vaccination providing some protection against COVID-19 has been suggested [16].
To help address the knowledge gap of the relationship between viral load and COVID-19 disease severity, we undertook the following study to compare the viral load at hospital presentation in patients with COVID-19 of different disease severities. We hypothesized that the viral load will be higher in more severe COVID-19 disease and also compared viral load in patients with and without influenza vaccination.
Methods
Study design
This is a single center observational cohort study of adult (≥ 18 years) COVID-19 patients who presented either in the ED or were directly admitted to the Medical/Surgical Unit or ICU, with their first positive SARS-CoV-2 nasopharyngeal swab conducted at our institution. Patients admitted from March 1 until April 30, 2020, were included as this period corresponds to the beginning of the initial peak of COVID-19 cases in Boston. The Institutional Review Board at Beth Israel Deaconess Medical Center approved this study.
Sample collection and sampling methodology
The SARS-CoV-2 positive nasopharyngeal swabs which were obtained from the patients presenting to the hospital were used to calculate viral load [represented as log10 copies/ml i.e. log10CN (Copy Number)]. Nasopharyngeal swabs of the patients were tested for SARS-CoV-2 using Aldatu PANDAA qDx™ SARS-CoV-2 Reagents or the Abbott RealTime SARS-CoV-2 assay on the Abbott m2000 System. Cycle threshold (CT) value is a semi-quantitative value of viral genetic material [17]. It is inversely proportional to viral load and is being used in COVID-19 studies as a surrogate of viral load [2–4]. The Abbott RealTime assay provides a CT calculation called fractional cycle number [18]. We used the ΔΔCT method (ΔCT for the target sample—ΔCT for the reference positive sample) to calculate the copy number of SARS-CoV-2 in each sample. ΔCT for each sample was calculated as CT (SARS-CoV-2) − CT (internal control). The fold change for each sample compared to the positive control (1000 copies) was calculated as 2(−ΔΔCT). The copy number of SARS-CoV-2 for each sample is calculated as 1000 X [2(−ΔΔCT)]. log10 copy number for each sample were used as viral load.
Study patients, data abstraction and handling of missing data
COVID-19 positive patients were identified using International Classification of Disease-10 (ICD-10) codes (B342, B972, B9721, B9729, J1281, U071). From this cohort, only in-house laboratory-confirmed COVID-19 cases were included in this study. In patients with multiple tests, only the first positive swab was used. Laboratory confirmed COVID-19 patients missed by the above method were found by direct daily abstraction of positive CT values by a trained laboratory technician and were manually included in the study population. Existing inpatients who developed COVID-19 symptoms and later tested positive for the virus were labeled as nosocomial COVID-19 and excluded from this study (see table S1 for definitions and calculations). Patients for whom the internal control was not detected during polymerase chain reaction analysis were excluded from the study.
Study data were obtained from the patient’s electronic medical record (EMR) and reviewed by trained research assistants and physicians. This data included patient demographics, highest D-dimer, time of swab and patient location at the time of swab, duration of hospital and ICU admission, intubation status, disposition and self-reported information on patient’s influenza immunization status for the current flu season (beginning from Fall 2019). In addition, vital signs and laboratory findings pertinent to modified sequential organ failure assessment (mSOFA) score (excluding the neurologic subscore) calculation were obtained, if available within 24 h (before or after) of the positive SARS-CoV-2 result. Missing mSOFA subscore variables were imputed as normal. SOFA score calculations were done based on our previous work [19].
Outcomes
To categorize severity of disease, patients were divided into four groups based on their highest level of care in the 12 h following nasopharyngeal swab collection. Categories included: (1) ICU and intubated, (2) ICU and not intubated, (3) Medical/Surgical floor, or (4) ED discharge. Patients who stayed in the ED for more than 24 h were considered admitted and placed in the Medical/Surgical floor category.
SOFA score is a commonly used scoring system for predicting disease severity [20]. The data on neurologic SOFA subscore was not available in EMR especially for the Medical/Surgical and ED patients, and hence the mSOFA score, which excludes the neurologic component and has been used commonly [21–23], was used. In addition, we evaluated the correlation between viral load and D-dimer, which has been shown to be associated with disease severity [24–26]. We excluded ED discharge patients for this analysis as D-dimer was not consistently measured in this population.
Primary outcome: The primary outcome was the difference in viral load by the four pre-defined groups of level of care. Secondary outcomes: Secondary outcomes included the association of viral load with the mSOFA score; the difference in viral load in patients who did and did not receive influenza vaccination; and correlation of viral load with highest D-dimer.
Statistical analysis
Descriptive statistics are reported as means with standard deviations, medians with interquartile ranges, or counts with frequencies, depending on the type and distribution of data. For continuous outcomes, comparison amongst groups were made using Wilcoxon rank-sum tests or Kruskal–Wallis tests, based on the number of groups being compared. If the overall Kruskal–Wallis test had a p value < 0.05, comparisons were made using Dunn’s test for nonparametric pairwise multiple comparisons. The mSOFA was divided into four quartiles for comparison based on the distribution of data (score 0 = quartile 1, score 1 = quartile 2, score 2–3 = quartile 3 and score 4–11 = quartile 4). To adjust for age, sex and having no major comorbidity, multivariate linear regression was used to compare viral load in patients based on their location at the time of swab, and based on their mSOFA scores (as a continuous predictor). The correlation between viral load and highest D-dimer was evaluated using Spearman’s rank correlation coefficient.
To eliminate any discrepancies caused by combining the estimates from two different assays, a sensitivity analysis was performed by limiting the analyses to the 85.9% of patients tested using the Abbott RealTime SARS-CoV-2 test.
All statistical analyses will be performed using Stata version 16 (StataCorp, College Station, TX). A two-sided p value < 0.05 will be considered statistically significant.
Results
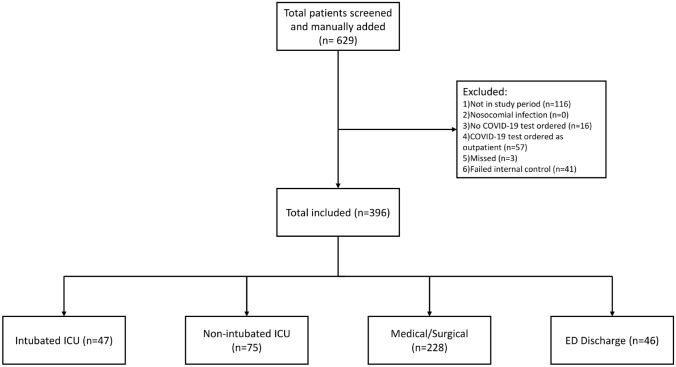
Information on viral load was present in 437 patients. In 41 patients (8.6%), the internal control failed and log10CN values were not able to be calculated with internal control correction as the internal control failed. These 41 patients were excluded from further analyses, leaving a total of 396 patients included in the study (Table 1, Fig. 1).
Table 1.
Patient characteristics
| Total | Intubated ICU | Non-intubated ICU | Medical/surgical | ED discharge | |
|---|---|---|---|---|---|
| N = 396 (100.0%) | N = 47 (11.9%) | N = 75 (18.9%) | N = 228 (57.6%) | N = 46 (11.6%) | |
| Patient demographics | |||||
| Male n (%) | 215 (54.3%) | 28 (59.6%) | 42 (56.0%) | 118 (51.8%) | 27 (58.7%) |
| Age, mean (SD) | 64.8 (17.0) | 59.4 (14.5) | 72.4 (13.2) | 65.7 (17.2) | 53.6 (16.8) |
| Race, n (%) | |||||
| Asian | 18 (4.5%) | 5 (10.6%) | 3 (4.0%) | 7 (3.1%) | 3 (6.5%) |
| African–American | 136 (34.3%) | 8 (17.0%) | 21 (28.0%) | 88 (38.6%) | 19 (41.3%) |
| Other | 45 (11.4%) | 6 (12.8%) | 7 (9.3%) | 25 (11.0%) | 7 (15.2%) |
| Unknown/not reported | 51 (12.9%) | 15 (31.9%) | 8 (10.7%) | 22 (9.6%) | 6 (13.0%) |
| Caucasian | 146 (36.9%) | 13 (27.7%) | 36 (48.0%) | 86 (37.7%) | 11 (23.9%) |
| BMIa, median (IQR) | 29.9 (26.1–34.2) | 31.5 (28.8–37.5) | 28.9 (25.5–32.7) | 29.9 (25.4–34.2) | 34.4 (18.3–38.6) |
| Missing BMI (n %) | 98 (24.8%) | 3 (6.4%) | 12 (16.0%) | 40 (17.5%) | 43 (93.5%) |
| Past medical history, n (%) | |||||
| No major comorbidities | 99 (25.0%) | 17 (36.2%) | 10 (13.3%) | 51 (22.4%) | 21 (45.7%) |
| CADb | 52 (13.1%) | 6 (12.8%) | 8 (10.7%) | 36 (15.8%) | 2 (4.3%) |
| CHFc | 47 (11.9%) | 4 (8.5%) | 14 (18.7%) | 28 (12.3%) | 1 (2.2%) |
| Cancer | 60 (15.2%) | 4 (8.5%) | 14 (18.7%) | 40 (17.5%) | 2 (4.3%) |
| COPDd | 37 (9.3%) | 5 (10.6%) | 13 (17.3%) | 17 (7.5%) | 2 (4.3%) |
| Dementia/Alzheimer’s | 44 (11.1%) | 3 (6.4%) | 11 (14.7%) | 29 (12.7%) | 1 (2.2%) |
| Diabetes | 140 (35.4%) | 16 (34.0%) | 29 (38.7%) | 87 (38.2%) | 8 (17.4%) |
| Renal disease | 75 (18.9%) | 3 (6.4%) | 19 (25.3%) | 52 (22.8%) | 1 (2.2%) |
| Liver disease | 17 (4.3%) | 2 (4.3%) | 3 (4.0%) | 10 (4.4%) | 2 (4.3%) |
| Arrhythmia | 50 (12.6%) | 3 (6.4%) | 14 (18.7%) | 31 (13.6%) | 2 (4.3%) |
| Alcohol use disorder | 23 (5.8%) | 5 (10.6%) | 4 (5.3%) | 12 (5.3%) | 2 (4.3%) |
| Stroke | 32 (8.1%) | 3 (6.4%) | 8 (10.7%) | 20 (8.8%) | 1 (2.2%) |
| Transplant | 4 (1.0%) | 0 (0.0%) | 0 (0.0%) | 4 (1.8%) | 0 (0.0%) |
| HIV/AIDSe | 4 (1.0%) | 0 (0.0%) | 1 (1.3%) | 2 (0.9%) | 1 (2.2%) |
| Asthma | 51 (12.9%) | 3 (6.4%) | 9 (12.0%) | 27 (11.8%) | 12 (26.1%) |
| Morbid obesity (BMI > 40) | 34 (8.7%) | 7 (15.2%) | 4 (5.3%) | 23 (10.2%) | 0 (0.0%) |
| SOFA, median (IQR) | |||||
| Modified total SOFA score | 2 (0–3) | 6 (4–7) | 2 (1–4) | 1 (0–2) | 0 (0–0) |
| Respiratory SOFA | 0 (0–1) | 3 (2–4) | 1 (0–2) | 0 (0–1) | 0 (0–0) |
| Cardiac SOFA | 0 (0–0) | 0 (0–3) | 0 (0–0) | 0 (0–0) | 0 (0–0) |
| Coagulation SOFA | 0 (0–0) | 0 (0–1) | 0 (0–0) | 0 (0–0) | 0 (0–0) |
| Liver SOFA | 0 (0–0) | 0 (0–0) | 0 (0–0) | 0 (0–0) | 0 (0–0) |
| Renal SOFA | 0 (0–1) | 0 (0–1) | 1 (0–2) | 0 (0–1) | 0 (0–0) |
| Lab values, median (IQR) | |||||
| Viral load (log10CN), median (IQR) | 5.5 (3.3, 8.0) | 3.6 (2.6, 6.9) | 6.4 (4.4, 8.1) | 5.7 (3.2, 8.1) | 4.9 (3.8, 7.4) |
| Highest D-dimer, median (IQR) | 1504.0 (743.0–4416.0) | 4126.5 (2063.0–15,764.0) | 1640.0 (938.0–7134.0) | 1105.0 (672.0–2102.0) | 313.0 (304.0–886.0) |
| Missing D-dimer, n (%) | 79 (20.0%) | 1 (2.1%) | 8 (10.7%) | 27 (11.9%) | 43 (93.5%) |
| Influenza immunization | |||||
| Influenza immunization, n (%) | 152 (56.3%) | 8 (36.4%) | 25 (55.6%) | 101 (61.6%) | 18 (46.2%) |
| Missing influenza immunization, n (%) | 126 (31.8%) | 25 (53.2%) | 30 (40.0%) | 64 (28.1%) | 7 (15.2%) |
| Mortality | |||||
| n (%) | 62 (15.7%) | 14 (29.8%) | 23 (30.7%) | 25 (11.0%) | N/A |
aBody Mass Index
bCoronary artery disease
cCongestive heart failure
dChronic obstructive pulmonary disease
eHuman Immunodeficiency Virus/Acquired Immunodeficiency Syndrome
Fig. 1.
Consort diagram
Descriptive statistics
Of the 396 patients, 215 (54.3%) were male, 146 (36.9%) were Caucasian, and 136 (34.3%) were African–American. No major comorbidity was seen in 99 (25.0%) patients. The median mSOFA score for the population was 2 (IQR 0–3) with median mSOFA of 0 (IQR 0–0) in the ED discharge group and median mSOFA of 6 (IQR 4–7) in the intubated ICU group. Hospital mortality in the cohort was 15.7%. In the 270 patients with influenza vaccination information, 152 (56.3%) received the influenza vaccine.
Viral load by disease severity
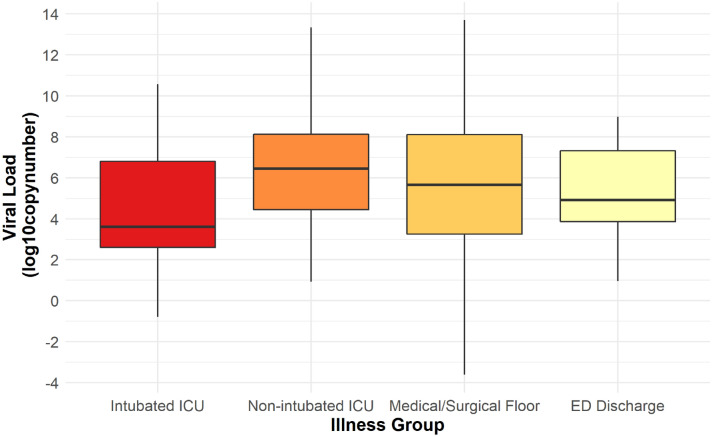
Median viral load was 5.5 (IQR 3.3–8.0). Median viral load was highest in non-intubated ICU patients [6.4 (IQR 4.4–8.1)] and lowest in intubated ICU patients [3.6 (IQR 2.6–6.9)]. This difference was statistically significant. Median viral load was 5.6 (IQR 3.2, 8.1) in Medical/Surgical floor patients and 4.9 (IQR 3.8, 7.4) in ED discharge patients (Fig. 2). There was no statistically significant difference between any of the other groups (p value ≥ 0.05) (Table 2). There was also no statistically significant association between median viral load and quantile of mSOFA score (p value 0.20) (Table 3). No correlation was seen between viral load and highest D-dimer (Spearman’s rho 0.02, p value 0.73).
Fig. 2.
Viral load in different disease severity
Table 2.
Comparison of viral load (log10CN) in different disease severity
| (A) Unadjusted pairwise comparison | ||
|---|---|---|
| Illness group | log10CN (median, IQR) | p value (Kruskal–Wallis) |
| Intubated ICU | 3.6 (2.6, 6.9) | 0.01 |
| Non-intubated ICU | 6.4 (4.4, 8.1) | |
| Medical/surgical floor | 5.6 (3.2, 8.1) | |
| ED discharge | 4.9 (3.8, 7.4) | |
| Pairwise comparisons (using Dunn’s test) | |||
|---|---|---|---|
| Intubated ICU | Non-intubated ICU | Medical/surgical floor | |
| Non-intubated ICU | < 0.01 | ||
| Medical/surgical floor | 0.05 | 0.41 | |
| ED discharge | 0.87 | 0.17 | 0.97 |
| (B) Adjusted comparison#, $ | |||
|---|---|---|---|
| Mean difference | p value | 95% Confidence Interval | |
| Non-intubated ICU | 1.16 | 0.02 | (0.18, 2.14) |
| Medical/surgical floor | 0.74 | 0.08 | (− 0.09, 1.57) |
| ED discharge | 0.81 | 0.13 | (− 0.25, 1.88) |
#Using linear regression with Intubated ICU group as the reference
$Covariates used are age, sex and no major comorbidities
Table 3.
Comparison of viral load (log10CN) with modified sofa score
| (A) Unadjusted comparison | ||
|---|---|---|
| mSOFA quartile | log10CN (median, IQR) | p value (Kruskal–Wallis) |
| 1 (m SOFA = 0) | 4.6 (3.0, 7.6) | 0.20 |
| 2 (mSOFA = 1) | 5.7 (3.4, 7.9) | |
| 3 (mSOFA = 2–3) | 5.7 (3.3, 8.2) | |
| 4 (mSOFA = 4–11) | 6.2 (3.4, 8.0) | |
| (B) Adjusted comparison#, $ | |||
|---|---|---|---|
| Mean difference | p value | 95% CI | |
| 1 unit increase in mSOFA | 0.01 | 0.92 | (− 0.12, 0.13) |
No pairwise comparisons performed as overall Kruskal–Wallis is not significant
#Using linear regression
$Covariates used are age, sex and no major comorbidities
Adjusted analyses
In the adjusted model controlling for age, sex, and having no major comorbidities, viral load was significantly higher in non-intubated ICU compared to intubated ICU patients with mean difference of 1.16 (95% CI 0.18–2.14). No difference was observed comparing intubated ICU patients to Medical/Surgical floor patients or to ED discharge patients. After adjusting for the same covariates as above, there was no statistically significant association between viral load and mSOFA score with a mean difference of 0.01 (95% CI − 0.12 to 0.13) per unit increase in mSOFA score (Table 3).
Viral load and influenza immunization
There was no statistically significant difference in median viral load based on influenza immunization status [median log10CN in immunized patients 5.2 (IQR 3.2, 8.0) versus in non-immunized patients 5.2 (IQR 3.3, 8.1); p value 0.78] (Table S2).
Sensitivity analyses
In a sensitivity analysis only including patients whose viral load was obtained using Abbott RealTime SARS-CoV-2 (340 patients), there was no statistically significant difference between viral load in different levels of care in both unadjusted and adjusted analyses. No statistically significant difference was seen comparing viral load to mSOFA in both unadjusted and adjusted analyses, and no differences were seen in viral load based on influenza immunization status. Please see Table S3 for complete details of the sensitivity analysis.
Discussion
Our study focuses on the comparison of viral load between different categories of disease severity. We found that the median viral load was higher in non-intubated ICU patients as compared to intubated ICU patients in both unadjusted and adjusted analyses, but there was no difference in viral load between other groups. In addition, there was no association seen between viral load and mSOFA score or between viral load and patient influenza vaccination status. However, when the analyses were restricted to only the 86% of patients whose viral load was obtained using Abbott RealTime SARS-CoV-2, even the difference between intubated ICU patients and non-intubated ICU patients was no longer significant.
Healthcare professionals, researchers and the lay-public have compared COVID-19 and influenza characteristics [11–13, 27]. In influenza, studies have found a positive relationship between viral load and disease severity [28–31] but there is conflicting data regarding this relationship in COVID-19 [2, 3, 5–7]. One explanation for this could be that in severe COVID-19 patients, viral pneumonia progresses to acute respiratory distress syndrome (ARDS) [32], which is the main driver of intubation in COVID-19 [12] and presents relatively later in the disease course. Due to the temporal relationship between viral load in COVID-19 and disease progression wherein viral load decreases with duration of the disease [5, 33, 34], by the time COVID-19 patients are intubated, they may be in the latter part of their disease, which could explain their relatively lower viral load.
Barring the intubated ICU group where the viral load was the lowest, the viral load in this study did appear to increase with increasing severity based on levels of care, but there was no statistically significant difference between these groups (Table 2). In addition to comparing the viral load between four categorical disease severity groups, we analyzed the association of the viral load in COVID-19 with a patient’s mSOFA score. In our study, the viral load was not associated with the mSOFA, further supporting the lack of a clear association between viral load in COVID-19 patients and disease severity.
Van Kampen et al. [9] reported that intubated COVID-19 patients have higher viral loads and prolonged viral shedding and suggested considering disease severity before removing isolation precautions. Based on this finding, the CDC modified their recommendations and suggested incorporating severity status into the decision to remove isolation precautions [10]. Conventional wisdom suggests that isolation precautions are most necessary during the most severe stages of disease. Our data, however, argues that there is a strong need to maintain isolation precautions during handling of COVID-19 patients irrespective of their disease severity; in fact it might be more important in patients who are not intubated.
There is a hypothesis that prior immunization against influenza provides improved immunity against SARS-CoV-2 [16]; however, in our study population both influenza immunized and non-immunized patients had similar viral load (p value 0.78). Any infection, like COVID-19, induces a cascade of events leading to activation of fibrinolytic system and production of D-dimer [35], and it increases with increasing viral load [35]. In our study, we did not find a correlation between D-dimer and viral load.
Our study results taken together did not show a clear relationship between viral load and disease severity. Though not a focus of this manuscript, other inflammatory and cytokine markers like D-dimer, interleukin, interferon-γ, TNF-α etc. have shown promise in being associated with COVID-19 disease and disease severity [24, 36].
Our study has the following limitations. First, this is a retrospective study and our result of higher viral load in non-intubated ICU patients compared to intubated ICU patients was not statistically significant in sensitivity analyses. Second, our study design did not allow us to gather data on onset and duration of COVID-19 symptoms and hence we were unable to account for disease duration prior to testing. Finally, viral load measurement is dependent on the quality and quantity of the specimen obtained by the nasopharyngeal swab; variation in this could have affected our results. However, due to lack of better, relatively non-invasive and simpler techniques to obtain mucus specimens in COVID-19 patients, this is currently the best available method.
Conclusion
In our cohort, we did not find a clear association between viral load and disease severity in COVID-19 patients. Viral load was higher in non-intubated ICU patients than in intubated ICU patients though this difference was not seen in the sensitivity analysis. There was no difference in viral load between intubated ICU patients and patients with other levels of care or between patients with disease severity stratified by mSOFA.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
We would like to thank Annie Cheng for sharing CT correlation data for Abbott and Aldatu assays, and Susan Chin for bioinformatic support. Both are in the Department of Pathology at Beth Israel Deaconess Medical Center, and Francesca Montillo, Department of Emergency Medicine, for the editorial support with the manuscript.
Author contributions
RP, XL, AG, KB, AM, MD prepared the manuscripts; RP, SM, AC, NP, MI, PP collected the data; LB, AG analyzed the data; LB prepared figures; MD, JK, CR, RP conceptualized the study. All authors reviewed the manuscript.
Funding
Dr. Donnino’s effort is supported, in part, by grants from the National Institutes of Health (K24HL127101, R01HL136705, 1R01DK112886, 1R03 AA026093).
Availability of data and materials
Raw data were generated at Beth Israel Deaconess Medical Center. Derived data supporting the findings of this study are available on request.
Code availability
All code for data cleaning and analysis associated with the current submission is available on request.
Declarations
Conflict of interest
The authors declare that there is no conflict of interest.
Statements on human and animal rights
The Institutional Review Board at Beth Israel Deaconess Medical Center approved this study.
Informed consent
Patient consent was waived as there was no patient contact.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Wang P, Anderson N, Pan Y, et al. The SARS-CoV-2 outbreak: diagnosis, infection prevention, and public perception. Clin Chem. 2020 doi: 10.1093/clinchem/hvaa080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yu X, Sun S, Shi Y, et al. SARS-CoV-2 viral load in sputum correlates with risk of COVID-19 progression. Crit Care. 2020;24:170. doi: 10.1186/s13054-020-02893-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liu Y, Yan L-M, Wan L, et al. Viral dynamics in mild and severe cases of COVID-19. Lancet Infect Dis. 2020;20:656–657. doi: 10.1016/S1473-3099(20)30232-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zheng S, Fan J, Yu F, et al. Viral load dynamics and disease severity in patients infected with SARS-CoV-2 in Zhejiang province, China, January-March 2020: retrospective cohort study. BMJ. 2020;369:m1443. doi: 10.1136/bmj.m1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zou L, Ruan F, Huang M, et al. SARS-CoV-2 viral load in upper respiratory specimens of infected patients. N Engl J Med. 2020;382:1177–1179. doi: 10.1056/NEJMc2001737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zacharioudakis IM, Prasad PJ, Zervou FN, et al. Association of SARS-CoV-2 genomic load with COVID-19 patient outcomes. Ann Am Thorac Soc. 2020 doi: 10.1513/AnnalsATS.202008-931RL. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Argyropoulos KV, Serrano A, Hu J, et al. Association of initial viral load in severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) patients with outcome and symptoms. Am J Pathol. 2020;190:1881–1887. doi: 10.1016/j.ajpath.2020.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.He X, Lau EHY, Wu P, et al. Temporal dynamics in viral shedding and transmissibility of COVID-19. Nat Med. 2020;26:672–675. doi: 10.1038/s41591-020-0869-5. [DOI] [PubMed] [Google Scholar]
- 9.van Kampen JJA, van de Vijver DAMC, Fraaij PLA, et al. Shedding of infectious virus in hospitalized patients with coronavirus disease-2019 (COVID-19): duration and key determinants. medRxiv. 2020 doi: 10.1101/2020.06.08.20125310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Duration of Isolation and Precautions for Adults with COVID-19 | CDC. https://www.cdc.gov/coronavirus/2019-ncov/hcp/duration-isolation.html?CDC_AA_refVal=https%3A%2F%2Fwww.cdc.gov%2Fcoronavirus%2F2019-ncov%2Fcommunity%2Fstrategy-discontinue-isolation.html. Accessed 13 Dec 2020
- 11.Gjurašin B, Santini M, Krajinović V, et al. A retrospective comparison between influenza and COVID-19-associated ARDS in a Croatian tertiary care center. Wien Klin Wochenschr. 2020 doi: 10.1007/s00508-020-01759-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Donnino MW, Moskowitz A, Thompson GS, et al. Comparison between patients hospitalized with influenza and COVID-19 at a tertiary care center. J Gen Intern Med. 2021;36:1689–1695. doi: 10.1007/s11606-021-06647-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Is the Coronavirus Worse Than the Flu? - Coronavirus vs. Flu | Health.com. https://www.health.com/condition/infectious-diseases/coronavirus-worse-than-flu. Accessed 24 Dec 2020
- 14.Abdella R, Aggarwal M, Okura T, et al. Structure of a paramyxovirus polymerase complex reveals a unique methyltransferase-CTD conformation. Proc Natl Acad Sci USA. 2020;117:4931–4941. doi: 10.1073/pnas.1919837117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zeng Q, Langereis MA, van Vliet ALW, et al. Structure of coronavirus hemagglutinin-esterase offers insight into corona and influenza virus evolution. Proc Natl Acad Sci USA. 2008;105:9065–9069. doi: 10.1073/pnas.0800502105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Salem ML, El-Hennawy D. The possible beneficial adjuvant effect of influenza vaccine to minimize the severity of COVID-19. Med Hypotheses. 2020;140:109752. doi: 10.1016/j.mehy.2020.109752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/926410/Understanding_Cycle_Threshold__Ct__in_SARS-CoV-2_RT-PCR_.pdf. https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/926410/Understanding_Cycle_Threshold__Ct__in_SARS-CoV-2_RT-PCR_.pdf. Accessed 16 Dec 2020
- 18.Shain EB, Clemens JM. A new method for robust quantitative and qualitative analysis of real-time PCR. Nucleic Acids Res. 2008;36:e91. doi: 10.1093/nar/gkn408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pawar RD, Shih JA, Balaji L, et al. Variation in SOFA (sequential organ failure assessment) score performance in different infectious states. J Intensive Care Med. 2020 doi: 10.1177/0885066620944879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vincent JL, Moreno R, Takala J, et al. The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure. Intensive Care Med. 1996;22:707–710. doi: 10.1007/s001340050156. [DOI] [PubMed] [Google Scholar]
- 21.Gordon AC, Perkins GD, Singer M, et al. Levosimendan for the prevention of acute organ dysfunction in sepsis. N Engl J Med. 2016;375:1638–1648. doi: 10.1056/NEJMoa1609409. [DOI] [PubMed] [Google Scholar]
- 22.Hamano N, Nishi K, Onose A, et al. Efficacy of single-dose intravenous immunoglobulin administration for severe sepsis and septic shock. J Intensive Care. 2013;1:4. doi: 10.1186/2052-0492-1-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Andrews PJD, Avenell A, Noble DW, et al. Randomised trial of glutamine, selenium, or both, to supplement parenteral nutrition for critically ill patients. BMJ. 2011;342:d1542. doi: 10.1136/bmj.d1542. [DOI] [PubMed] [Google Scholar]
- 24.Singhania N, Bansal S, Nimmatoori DP, et al. Current overview on hypercoagulability in COVID-19. Am J Cardiovasc Drugs. 2020;20:393–403. doi: 10.1007/s40256-020-00431-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yao Y, Cao J, Wang Q, et al. D-dimer as a biomarker for disease severity and mortality in COVID-19 patients: a case control study. J Intensive Care. 2020;8:49. doi: 10.1186/s40560-020-00466-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tang N, Bai H, Chen X, et al. Anticoagulant treatment is associated with decreased mortality in severe coronavirus disease 2019 patients with coagulopathy. J Thromb Haemost. 2020;18:1094–1099. doi: 10.1111/jth.14817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.How does the new coronavirus compare with the flu? | Live Science. https://www.livescience.com/new-coronavirus-compare-with-flu.html. Accessed 24 Dec 2020
- 28.Lee N, Chan PKS, Hui DSC, et al. Viral loads and duration of viral shedding in adult patients hospitalized with influenza. J Infect Dis. 2009;200:492–500. doi: 10.1086/600383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lee N, Chan PKS, Choi KW, et al. Factors associated with early hospital discharge of adult influenza patients. Antivir Ther (Lond) 2007;12:501–508. [PubMed] [Google Scholar]
- 30.Hayden FG, Fritz R, Lobo MC, et al. Local and systemic cytokine responses during experimental human influenza A virus infection. Relation to symptom formation and host defense. J Clin Invest. 1998;101:643–649. doi: 10.1172/JCI1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kaiser L, Fritz RS, Straus SE, et al. Symptom pathogenesis during acute influenza: interleukin-6 and other cytokine responses. J Med Virol. 2001;64:262–268. doi: 10.1002/jmv.1045. [DOI] [PubMed] [Google Scholar]
- 32.Gibson PG, Qin L, Puah SH. COVID-19 acute respiratory distress syndrome (ARDS): clinical features and differences from typical pre-COVID-19 ARDS. Med J Aust. 2020;213:54–56.e1. doi: 10.5694/mja2.50674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yu F, Yan L, Wang N, et al. Quantitative detection and viral load analysis of SARS-CoV-2 in infected patients. Clin Infect Dis. 2020;71:793–798. doi: 10.1093/cid/ciaa345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.To KK-W, Tsang OT-Y, Leung W-S, et al. Temporal profiles of viral load in posterior oropharyngeal saliva samples and serum antibody responses during infection by SARS-CoV-2: an observational cohort study. Lancet Infect Dis. 2020;20:565–574. doi: 10.1016/S1473-3099(20)30196-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Srivastava S, Garg I, Bansal A, Kumar B. COVID-19 infection and thrombosis. Clin Chim Acta. 2020;510:344–346. doi: 10.1016/j.cca.2020.07.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Costela-Ruiz VJ, Illescas-Montes R, Puerta-Puerta JM, et al. SARS-CoV-2 infection: the role of cytokines in COVID-19 disease. Cytokine Growth Factor Rev. 2020;54:62–75. doi: 10.1016/j.cytogfr.2020.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Raw data were generated at Beth Israel Deaconess Medical Center. Derived data supporting the findings of this study are available on request.
All code for data cleaning and analysis associated with the current submission is available on request.