Abstract
The COVID‐19 pandemic significates an enormous number of patients with pneumonia that get complicated with severe acute respiratory distress syndrome (ARDS), some of them with refractory hypercapnia and hypoxemia that need mechanical ventilation (MV). Those patients who are not candidate to extracorporeal membrane oxygenation (ECMO), the extracorporeal removal of CO2 (ECCO2R) can allow ultra protective MV to limit the transpulmonary pressures and avoid ventilatory induced lung injury (VILI).
We report a first case of prolonged ECCO2R support in 38 year male with severe COVID‐19 pneumonia refractory to conventional support. He was admitted tachypneic and oxygen saturation 71% without supplementary oxygen. The patient's clinical condition worsens with severe respiratory failure, increasing the oxygen requirement and initiating MV in the prone position. After 21 days of protective MV, PaCO2 rise to 96.8 mmHg, making it necessary to connect to an ECCO2R system coupled continuous veno‐venous hemodialysis (CVVHD). However, due to the lack of availability of equipment in the context of the pandemic, a pediatric gas exchange membrane adapted to CVVHD allowed to maintain the removal of CO2 until completing 27 days, being finally disconnected from the system without complications and with a satisfactory evolution.
1. INTRODUCTION
Protective mechanical ventilation (MV) involves the use of low tidal volumes (Vt) in order to reduce transpulmonary pressure levels to avoid damage induced by MV. That clinical practice has demonstrated to improve survival in patients with respiratory distress syndrome (ARDS). 1 , 2 However, an undesirable consequence of protective MV is hypoventilation and hypercapnia, 3 acute pulmonary hypertension, diminished myocardial contractility, reduced renal blood flow, and release of endogenous catecholamins. 4 Hypercapnic acidosis associates to increase hospital mortality, prolonged hospital stay, and a reduction in survival with PaCO2 greater than 65 mmHg. 5 Molecular studies had shown a close association between hypercapnia and alveolar cell membrane repair disorders, alveolar clearance, and local immune response. 6
Gattinoni 1986 designed the first device that separated the ventilatory support from oxygen supply, which made possible to optimize lung protection during VM. 7 That was the first published ECCO2R system, which allowed the removal of CO2 from the blood through a gas exchange membrane, without oxygenating the blood in a meaningful way. 2
In the 1990 s, Young et al. was the first to report a system that coupled ECCO2R to continuous renal replacement therapy (CRRT) through an arterio‐venous low flow CO2 removal device. 8 , 9 , 10 In 2013, Forster and colleagues showed a similar device that combined CVVHD associated a ECCO2R using blood flow less than 500 ml/min in patients with severe ARDS. 11
We present the first case of a patient with severe respiratory failure due to COVID‐19 pneumonia in whom CO2 removal therapy is performed using a pediatric oxygenation membrane coupled to HDVVC.
2. CASE PRESENTATION
A 38‐year‐old male with a history of overweight, hypothyroidism and insulin resistance begin with progressive dyspnea and presented to the emergency department for evaluation. Upon admission he was in poor general conditions, febrile, tachypnea, oxygen saturation 71% without supplementary oxygen, blood pressure 108/80 mmHg, heart rate 111 beats/min. Laboratory exams (Table 1) showed paO2 73 mmHg, pCO2 35 mmHg, bicarbonate 22 mmol/L, hemoglobin 120 g/L and creatinine 74,2 umol/L. Respiratory indirect immunofluorescence results negative, a positive polymerase chain reaction for COVID‐19 and thorax computed tomography (CT) was suggestive of COVID‐19 pneumonia (Figure 1). The patient was transferred to the intensive care unit (ICU).
TABLE 1.
Clinical parameters
|
Parameters Laboratory |
Basal | ECCO2R | 24 h post ECCO2R | |||
|---|---|---|---|---|---|---|
| 24 h | 7 days | 14 days | 27 days | |||
| Hemoglobin (gr/L) | 119 | 75 | 81 | 76 | 68 | 8,2 |
| Leukocytes (109/L) | 8.8 | 13.7 | 15.2 | 14.2 | 16.3 | 13870 |
| Platelets (109/L) | 398 | 321 | 176 | 166 | 393 | 408000 |
| CRP (nmol/L) | 722.8 | 761.9 | 1160 | 1583 | 393.3 | 310.4 |
| Ureic nitrogen (mmol/L) | 5.0 | 5.7 | 7.0 | 6.4 | 21.5 | 55 |
| Creatinine (umol/L) | 53.0 | 23.5 | 23.5 | 35.36 | 79.5 | 0.97 |
| Sodium (mmol/L) | 133.0 | 136.4 | 134.0 | 141.0 | 143.0 | 141 |
| Potassium (mmol/L) | 4.3 | 4.8 | 4.7 | 3.7 | 4.6 | 4.8 |
| Ventilatories |
3.0 |
2.5 |
2.5 |
3.0 |
4.0 |
4.0 |
| Vt (ml/kg ideal) | ||||||
| RR (breaths/min) | 60 | 24 | 26 | 24 | 30 | 30 |
| PEEP (cmH2O) | 4 | 4 | 4 | 4 | 4 | 4 |
| Plateau pressure (cmH2O) | 18 | 18 | 19 | 19 | 18 | 18 |
| Distension pressure (cmH2O) | 13 | 14 | 15 | 15 | 14 | 14 |
| Compliance (ml/cmH2O) | 16 | 16 | 15 | 15 | 16 | 16 |
| PaO2/FiO2 | 81.4 | 157.4 | 178.2 | 207.1 | 233.0 | 234.2 |
| Blood gases |
7.21 |
7.37 |
7.37 |
7.40 |
7.40 |
7.39 |
| pH | ||||||
| PaO2 (mmHg) | 94.4 | 78,7 | 77.0 | 93.2 | 94.3 | 82.0 |
| PaCO2 (mmHg) | 96.8 | 54.4 | 50.7 | 49.4 | 42.0 | 45.0 |
| Bicarbonate (mmol/L) | 37.5 | 30.9 | 29.2 | 30.2 | 25.7 | 26.7 |
| Lactate (mmol/L) | 0.7 | 0.6 | 0.7 | ‐ | ‐ | ‐ |
| ECCO2R |
500 |
400 |
300 |
320 |
400 |
‐ |
| Blood flow (ml/min) | ||||||
| Oxygen flow (L/min) | 7 | 7 | 7 | 6 | 4 | ‐ |
| CO2 removal (ml/min) | ‐ | ‐ | 61 | 57 | ‐ | ‐ |
| Hemodynamics |
89 |
77 |
78 |
124 |
74 |
91 |
| Mean arterial pressure (mmHg) | ||||||
| HR (heartbeat/min) | 88 | 106 | 119 | 83 | 101 | 98 |
| Norepinephrine dose (ug/kg/min) | 0.01 | 0.10 | 0.00 | 0.00 | 0.00 | 0.00 |
Abbreviations: CRP, C‐reactive protein; ECCO2R, extracorporeal CO2 removal; FiO2, inspired oxygen fraction; HR, heart rate; PaCO2, partial pressure of carbon dioxide; PaO2, partial pressure of oxygen; PEEP, positive end‐expiratory pressure; RR, respiratory rate; TV: tidal volume.
This article is being made freely available through PubMed Central as part of the COVID-19 public health emergency response. It can be used for unrestricted research re-use and analysis in any form or by any means with acknowledgement of the original source, for the duration of the public health emergency.
FIGURE 1.
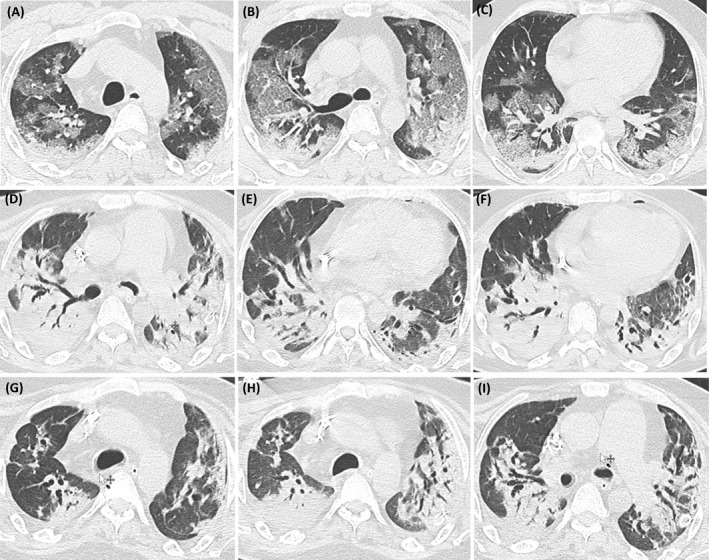
Computed tomography (CT) of the chest during evolution: A, B, C: basal; D, E, F: 10 days of ECCO2R and G, H, I: 48 h of retirement of ECCO2R
Ventilatory support was initiated with high‐flow nasal cannula, prone position ventilation, antibiotics, steroids (1 mg/kg) for 5 days and anticoagulation with low molecular weight heparin for suspected pulmonary thromboembolism. The seventh day in the ICU, he evolves with high oxygen requirements starting invasive MV, analgesia and deep sedation, neuromuscular blockade, and prone position for 10 consecutive days. The 15th day of MV he presented a left pneumothorax that required pleural drainage with adequate lung re‐expansion; however, this event cause a progressive deterioration of the gas exchange. In the following days it was difficult to maintain the protective MV and then he developed severe hypercapnic respiratory insufficiency. After 21 days in MV, it was decided to connect the patient to an ECCO2R system with Braun OMNI® machine through a 23 cm, 14.5 French (Fr) hemodialysis catheter to remove CO2. The CO2 removal membrane was installed pre‐hemodialysis filter to achieve greater efficiency, as described by Terragni. 12 After the initiation of therapy, a progressive reduction of PaCO2 levels observed, which allowed to an ultra protective MV (Table 1). During the entire period of the ECCO2R therapy, it was ensured appropriate systemic anticoagulation with non‐fraction heparin with a target activated partial thromboplastin time (aPTT) of 70–80 seconds.
During the first days of therapy, the patient remained in the original ECCO2R OMNI® Braun system requiring change of the oxygenator membrane and the dialysis filter every 72 h, as suggested by the manufacturer. However, the patient needed prolonged ECCO2R support unfortunately, due to a shortage circuit supply, it was necessary to create an adapted circuit with a pediatric membrane oxygenator connected to a DIAPACT® Braun machine (Figure 2). This new circuit was kept under optimal anticoagulation and the system operated efficiently for 17 consecutive days, without evidence of pediatric membrane oxygenator failure. After 27 days of ECCO2R support, the system was disconnected. During the entire period of ECCO2R support there was no evidence of hemorrhagic, hematological, or infectious complications (Figure 3).
FIGURE 2.
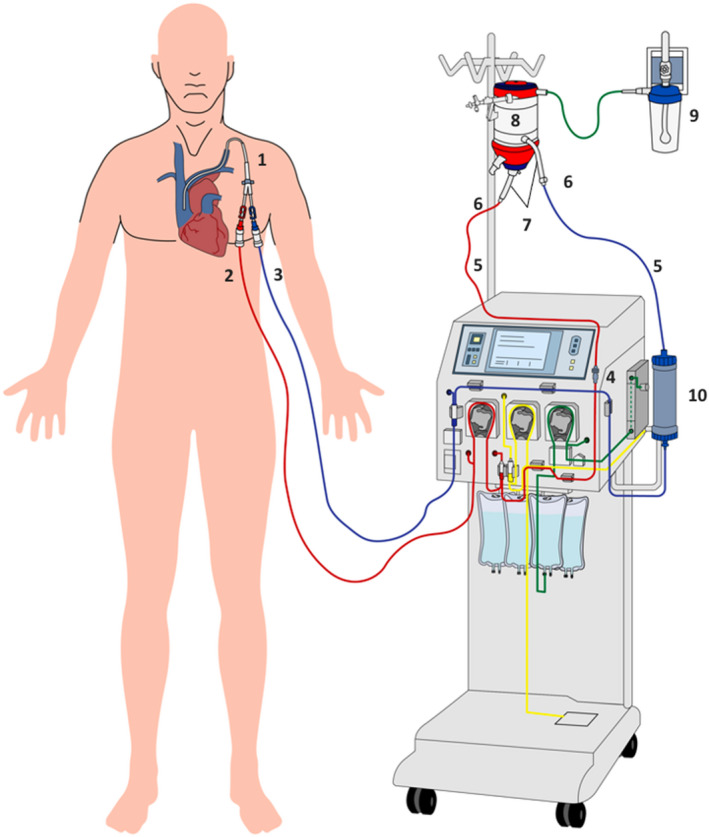
Adapted oxygenation membrane system for removal of CO2: Red line: pre CO2 removal, blue line: post CO2 removal, 1: hemodialysis catheter, 2: arterial lines, 3: venous line, 4: male / male DIN connector, 5: ⅜ to ¼ connector , 6: line ⅜, 7: CO2 exchange membrane, 8: oxygen network, 9: line ⅙, 10: dialyzer [Colour figure can be viewed at wileyonlinelibrary.com]
FIGURE 3.
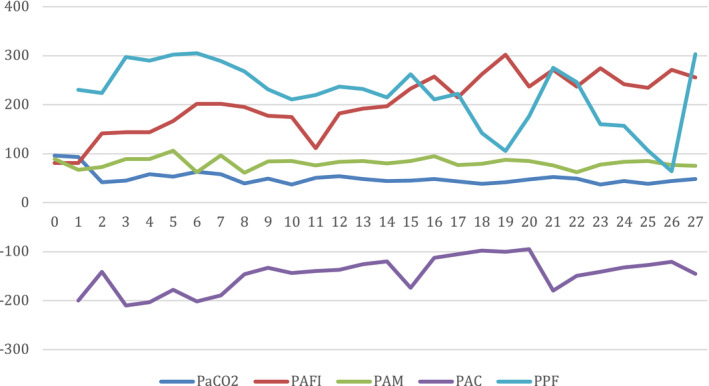
Ventilatory and hemodynamic parameters. The days of treatment with ECCO2R are plotted on the horizontal axis. PaCO2: partial pressure of carbon dioxide, PAFI: PaO2 / FiO2, PAM: mean arterial pressure, PAC: circuit arterial pressure, PPF: pre filter pressure [Colour figure can be viewed at wileyonlinelibrary.com]
The patient continued his evolution in a stable way, he was able to disconnect from the MV without complications and he was discharged from the hospital to his home.
3. DISCUSSION
CO2 extracorporeal removal systems, both the extracorporeal membrane oxygenation (ECMO) and ECCO2R, are useful in multiple clinical scenarios, but in ARDS the ECCO2R should be considered in cases of pH<7.25, PaCO2 >65 mmHg, and PaO2/FiO2 >80. 4 The decision of ECCO2R coupled with CVVHD depends on the need renal support and the availability of trained nursing staff.
The ECCO2R are partial low‐flow respiratory support systems that can be implemented with membranes of different surfaces (0.33 to 1.81 m2) and allows extraction of 25% of the CO2 content in the blood, reducing ventilatory requirements. 4 , 13 The CO2 diffusion capacity is 20 times greater than oxygen, which allows the system to purify CO2 at low flows of blood (Qb <500 ml/min). The main determinant of CO2 diffusive capacity is the blood flow and less important is the flow of oxygen, with a maximum efficacy at 6–8 L/min.
Different publications have demonstrated the utility of ECCO2R systems in severe ARDS. 14 Among them, the SUPERNOVA study confirmed the benefits of ECCO2R in this group of patients, minimizing the respiratory acidosis and achieving ultra protective MV with the use of membranes of 0.59 to 1.3 m2 and blood flow between 200 to 1,000 ml/min. 15
The clinical use of ECCO2R systems has been described not only in ARDS but also in chronic obstructive pulmonary disease patients, weaning from MV and as a bridge therapy in lung transplant. 16 The ECCO2R allows to reduce the ventilatory demand, decrease Vt to 3–4 ml/kg of ideal weight and to keep plateau pressures less than 25 cmH2O and driving pressures of less than 15 cmH2O allowing ultra protective MV in a safe way, with less risk of hemorrhagic complications related to the vascular access, lower costs, less technical difficulties with less need of trained staff. ECMO system is a more complex technique, requires greater blood flows and bigger vascular cannulas (21 to 31 Fr). 4
As we noted before, the success of the ECCO2R systems depends on vascular access that can achieve blood flow rate up to 500 ml/min and of anticoagulation to maintain aPTT ranges between 70 and 80 seconds. 17 , 18 , 19 The latter is crucial to maintain membrane patency and avoid membrane fouling. In our clinical case, we had two episodes of circuit clotting despite optimal anticoagulation. The possible explanations of this that there are blood‐membrane interaction, activation of the coagulation cascade and a slowdown of the blood in the oxygenator membrane.
The patient in our clinical case had a formal contraindication of ECMO because the respiratory failure and consequent respiratory acidosis (PaCO2 96.8 mmHg and pH 7.21) presented at day 21 of MV, and that was the reason we choose for an ECCO2R strategy as a rescue supportive therapy. 20
The use of devices for CO2 removal as a support strategy in selected critical patients is not new in our environment and has been used for more than a decade. A great number of ECCO2R system currently available requires specialized technology and machines specifically designed for this purpose, which can make this technology less accessible. What is distinctive about the present case is that it was the first patient who was treated with CO2 removal for several weeks, the use of the pediatric oxygenation membrane coupled to HDVVC made it possible to efficiently treat respiratory failure and CO2 retention, maintaining ultra‐protective MV using low Vt for a long period of time.
In this case of severe respiratory failure by COVID‐19, the innovation of the therapy was able to stabilize gas exchange of the patient and lung function until recovery and withdrawal of invasive MV.
4. CONCLUSION
This report presents a clinical case of severe hypercapnic respiratory acidosis in which the use of an oxygenation membrane coupled to CRRT allowed after 27 days to reduce the lung injury associated with MV, stabilizing the patient's gas exchange at a low cost.
ECCO2R associated with HDVVC is a safe and effective therapy in reducing CO2, correcting respiratory acidosis and allowing ultra‐protective MV.
CONFLICT OF INTERESTS
The authors have no financial conflicts of interest to declare.
ACKNOWLEDGEMENTS
The authors thank Luis Toro for his support, who belongs to the Department of Nephrology, and Diego Espinoza, Resident of Radiology, Hospital Clínico Universidad de Chile, for providing us with images for this manuscript.
REFERENCES
- 1. The acute respiratory distress syndrome network . Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. NEJM. 2000;342(5):1301‐1308. [DOI] [PubMed] [Google Scholar]
- 2. Nentwich J, Wichmann D, Kluge S, Lindau S, Mutlak H, John S. Low‐flow CO2 removal in combination with renal replacement therapy effectively reduces ventilation requirements in hypercapnic patients. Ann Intensive Care. 2019;9:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Schmidt M, Jaber S, Zogheib E, Godet T, Capellier G, Combes A. Feasibility and safety of low‐flow extracorporeal CO2 removal managed with a renal replacement platform to enhance lung‐protective ventilation of patients with mild‐to‐moderate ARDS. Crit. Care. 2018;22:122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Staudinger T. Update on extracorporeal carbon dioxide removal: a comprehensive review on principles, indications, efficiency, and complications. Perfusion. 2020;3:1‐10. [DOI] [PubMed] [Google Scholar]
- 5. Tiruvoipati R, Pilcher D, Buscher H, Botha J, Bailey M. Effects of hypercapnia and hypercapnic acidosis on hospital mortality in mechanically ventilated patients. Crit Care Med. 2017;45(7):e649‐e656. [DOI] [PubMed] [Google Scholar]
- 6. Vadasz I, Hubmayr RD, Nin N, Sporn P, Sznajder J. Hypercapnia: a nonpermissive environment for the lung. Am J Respir Cell Mol Biol. 2012;46(4):417‐421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Gattinoni L, Pesenti A, Mascheroni D, Marcolin R, Fumagalli R, Rossi F. Low‐frequency positive‐pressure ventilation with extracorporeal CO2 removal in severe acute respiratory failure. JAMA. 1986;256(8):881‐886. [PubMed] [Google Scholar]
- 8. Young J, Dorrington K, Blake G, Ryder W. Femoral arteriovenous extracorporeal carbon dioxide elimination using low blood flow. Critical Care Med. 1992;20(6):805‐809. [DOI] [PubMed] [Google Scholar]
- 9. Hermann A, Riss K, Schellongowski P, Bojic A, Wohlfarth P, Robak O. A novel pump‐driven veno‐venous gas exchange system during extracorporeal CO2‐removal. Intensive Care Med. 2015;41(10):1773‐1780. [DOI] [PubMed] [Google Scholar]
- 10. Livigni S, Maio M, Ferretti E, et al. Efficacy and safety of a low‐flow veno‐venous carbon dioxide removal device: results of an experimental study in adult sheep. Crit. Care. 2006;10(5):R151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Forster C, Schriewer J, John S, Eckardt KU, Willam C. Low‐flow CO2 removal integrated into a renal‐replacement circuit can reduce acidosis and decrease vasopressor requirements. Crit. Care. 2013;17:R154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Terragni PP, del Sorbo L, Mascia L, et al. Tidal volume lower than 6 ml/kg enhances lung protection: role of extracorporeal carbon dioxide removal. Anesthesiology. 2009;111(4):826‐835. [DOI] [PubMed] [Google Scholar]
- 13. Peperstraete H, Eloot S, Depuydt P, Somer F, Roosens C, Hoste E. Low flow extracorporeal CO2 removal in ARDS patients: a prospective short‐term crossover pilot study. BMC Anesthesiology. 2017;17:155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Winiszewski H, Aptel F, Belon F, et al. Daily use of extracorporeal CO2 removal in a critical care unit: indications and results. Journal of Intensive Care. 2018;6:36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Combes A, Fanelli V, Pham T, Ranieri VM. Feasibility and safety of extracorporeal CO2 removal to enhance protective ventilation in acute respiratory distress syndrome: the SUPERNOVA study. Intensive Care Med. 2019;45:592‐600. [DOI] [PubMed] [Google Scholar]
- 16. Boyle A, Sklar M, McMamee J, Brodie D, Slutsky A, Brochant L. Extracorporeal carbon dioxide removal for lowering the risk of mechanical ventilation. Lancet Respir Med. 2018;6(11):874‐884. [DOI] [PubMed] [Google Scholar]
- 17. Allardet‐Servent J, Castanier M, Signouret T, Soundaravelou R, Lepidi A, Seghboyan JM. Safety and efficacy of combined extracorporeal CO2 removal and renal replacement therapy in patients with acute respiratory distress syndrome and acute kidney injury. Crit Care Med. 2015;43(12):2570‐2581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Romagnoli S, Ricci Z, Ronco C. Novel extracorporeal therapies for combined renal‐pulmonary dysfunction. Sem Nephrol. 2016;36(1):71‐77. [DOI] [PubMed] [Google Scholar]
- 19. Quintard JM, Barbot O, Thevenot F, et al. Partial extracorporeal carbon dioxide removal using a standard continuous renal replacement therapy device: a preliminary study. ASAIO J. 2014;60(5):564‐569. [DOI] [PubMed] [Google Scholar]
- 20. Shekar K, Badulak J, Peek G, et al. Extracorporeal life support organization coronavirus disease 2019 interim guidelines: a consensus document from an international group of interdisciplinary extracorporeal membrane oxygenation providers. ASAIO J. 2020;66:707‐721. [DOI] [PMC free article] [PubMed] [Google Scholar]


