Abstract
Background
Clinical characteristics and outcomes of coronavirus disease 2019 (COVID‐19) patients have been varied internationally but have not been studied in an Australian cohort.
Aim
To describe characteristics and outcomes of approximately the first 200 documented COVID‐19 cases during the first outbreak in the Gold Coast.
Methods
Retrospective observational cohort study of COVID‐19 patients managed by Gold Coast Hospital and Health Service (GCHHS). Demographics, clinical characteristics and outcomes data were collected.
Results
One hundred and ninety‐seven patients were included (mean age 45 years); 52.3% were female and 9.1% were healthcare workers. Most were overseas travellers (53.8%), contacts of a local confirmed case (25.4%) or cruise ship passengers (17.3%). The commonest comorbidities were hypertension (14.2%) and asthma (11.2%). The commonest symptoms were cough (74.1%), fever (58.9%), sore throat (48.7%), headache (48.7%) and rhinorrhoea (46.2%). Sixty‐three patients were hospitalised and the rest admitted to a ‘virtual ward’. Of 63 hospitalised patients, 5 (7.9%) required intensive care unit (ICU) admission and 3 (4.8%) required intubation. No patients died. Due to low numbers of accurate exposure dates, the incubation period could not be reliably calculated for a significant proportion of the cohort. Average duration of symptoms was 14 days, time from first symptom to hospitalisation was 5.3 days and time from first symptom to ICU admission was 11.6 days. The majority (88%) experienced mild disease and achieved complete symptom resolution (97%). Nasopharyngeal swab polymerase chain reaction was the main diagnostic method (99%). Twenty‐four patients received anti‐viral pharmacotherapy, with 87.5% getting hydroxychloroquine.
Conclusions
The present study provides characteristics and outcomes of the first 197 patients with COVID‐19 in the Gold Coast.
Keywords: COVID‐19, Australia, demography, hospitalisation, intensive care
Introduction
In December 2019, an outbreak of SARS‐like atypical pneumonia in Wuhan, China, resulted in the discovery of the novel virus severe acute respiratory syndrome coronavirus‐2 (SARS‐CoV2). 1 , 2 The new virus spread, leading to a worldwide pandemic of coronavirus disease 2019 (COVID‐19), the clinical syndrome caused by the virus. The first Australian case was diagnosed on 25 January 2020. As of 15 August 2020, there have been 23 035 total cases in Australia, with 379 deaths. 3 Elderly patients and those with co‐morbidities including diabetes, hypertension and asthma are at increased risk for poor outcomes. 4 Clinical characteristics and outcomes have been varied in previously published cohorts internationally, 5 , 6 , 7 but have not been studied in an Australian cohort to date.
The aim of the present study was to assess and better understand the demographics, baseline clinical characteristics and clinical course of approximately the first 200 confirmed cases of COVID‐19 at the Gold Coast Hospital and Health Service.
Methods
Setting and participants
This study was conducted at Gold Coast Hospital and Health Service (GCHHS), the main academic health service serving the Gold Coast region, Queensland. The population of the region serviced by Gold Coast Health is estimated to be greater than 560 000 people. 8 , 9 In 2013, it was estimated that 1.3% of the population are indigenous. 9 , 10 This study was conducted with an endorsement from the GCHHS human research ethics committee (LNR/2020/QGC/63668) with a waiver of consent. This study aimed to include approximately the first 200 consecutive patients who were confirmed to have COVID‐19 on the Gold Coast.
Procedure
Confirmed COVID‐19 cases were identified by the hospital health analytics team over the months of February, March and April 2020. De‐identified clinical and demographic data were then retrospectively collected from the electronic medical record system and recorded onto an electronic data spreadsheet. All documentations for inpatient, outpatient and hospital in the home (HITH) visits are available on integrated electronic Medical Record (ieMR). Public health records are not available on ieMR. Data collected included comorbidities, epidemiologic links, clinical characteristics, diagnostic testing, pharmacotherapy, supportive therapy and health outcomes. Comorbidities were hypertension, asthma, diabetes, ischaemic heart disease, chronic obstructive lung disease, immunosuppression and pregnancy. Comparisons were made between patients who required hospital admission versus those who were solely managed in the home setting (virtual ward). Patients were confirmed to have COVID‐19 if they tested positive for SARS‐CoV2 on the nasopharyngeal polymerase chain reaction (PCR), stool PCR, serology or if they met clinical and epidemiologic criteria. Positive serology would be indicated by SARS‐CoV‐2 IgM test. Clinical and epidemiological criteria would include a person with fever (≥38°C) or history of fever (e.g. night sweats, chills) OR acute respiratory infection (e.g. cough, shortness of breath, sore throat) AND who is a household contact of a confirmed or probable case of COVID‐19, where testing has not been conducted. These criteria were used as per the Communicable Diseases Network Australia National Guidelines for Public Health Units version 2.4.
Isolation clearance criteria information was also collected, which evolved over time. Initially patients were released from isolation on time, symptoms and negative swabs, but this was changed to release based on timeframes and symptoms resolution. Prior to 4 June 2020, confirmed and probable cases who subsequently would be going into a high‐risk setting required the following criteria: at least 10 days after onset of acute illness; afebrile for the previous 48 h; resolution of the acute illness for the previous 24 h; and PCR negative on at least two consecutive respiratory specimens collected at least 24 h apart at least 7 days after symptom onset. For those that had been hospitalised, the timing was based on hospital discharge. As of 4 June, high‐risk workers were cleared by elapsed time and symptoms as well. 11
Results
Out of the first 201 documented COVID19 cases, four were excluded as they were from outside the hospital catchment area, resulting in insufficient clinical data. One hundred and ninety‐seven patients were included in the analysis. The mean age was 45 years (range: 2–85, standard deviation (SD) = 19.15) (Fig. 1). There were 94 (47.7%) male patients and 103 (52.3%) female patients. There were no Indigenous Australian or Torres Strait Islander patients, and none were residents of residential aged care facilities. Four (2%) patients worked in residential aged‐care facilities and there were 18 (9.1%) healthcare workers.
Figure 1.
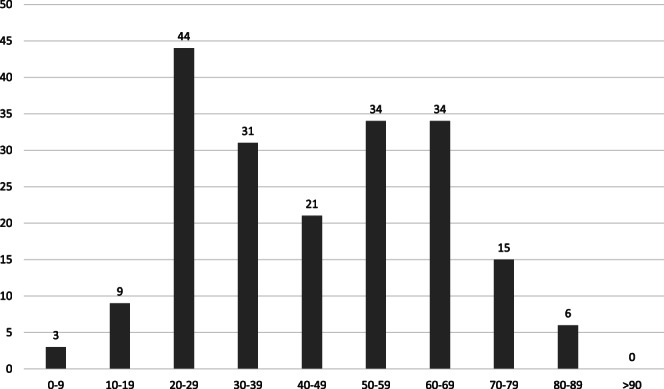
Age distribution in decades for 197 patients with COVID‐19.
Almost all (n = 190, 96.4%) patients had an epidemiological link, with only seven (3.6%) patients having no identified epidemiological association. Epidemiological links included cruise‐ship travel (n = 34, 17.26%), overseas travel (n = 106, 53.8%) and contact with a local confirmed case (n = 50, 25.4%). In the present study, travellers returned to the Gold Coast having travelled to a total of 31 different countries, with the largest cohorts spending time in the United Kingdom (n = 30), the United States (n = 26) and transiting through the United Arab Emirates (n = 15) (Fig. 2).
Figure 2.
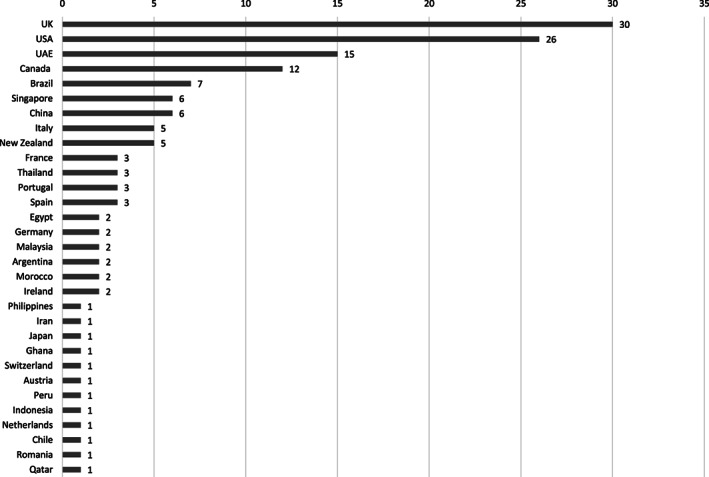
Countries of travel in COVID‐19 patients.
Clinical characteristics
The most common symptoms described were cough (74.1%), followed by subjective or documented fever (58.9%), sore throat (48.7%), headache (48.7%), rhinorrhoea (46.2%), myalgias (34.5%), dyspnoea (26.4%), anosmia (23.9%), diarrhoea (20.3%) and chest tightness (16.2%). Only three (1.5%) patients were truly asymptomatic and were tested due to contact tracing.
The mean duration of symptoms was 13.9 days (range: 1–69 days). The median duration of symptoms was 11 days. The mean time from symptom onset to testing was 4.6 days (median = 3 days, range: 0–33 days).
Twenty‐eight (14.2%) patients had hypertension, 22 (11.2%) had asthma, 13 (6.6%) had diabetes, 8 (4.1%) had ischaemic heart disease, 4 (2%) had chronic obstructive pulmonary disease (COPD), 3 (1.5%) were receiving systemic immunosuppression and 2 (1%) were pregnant.
Diagnosis
The diagnosis was made using PCR from a nasopharyngeal swab in 99% of cases (n = 195). Of these, 95.4% (n = 188) required a single swab for diagnosis, four (2%) required a second swab and three (1.5%) were diagnosed on their third swab. Other diagnostic samples included sputum (0.5%). One patient was diagnosed based on clinical and epidemiological criteria and was later confirmed with serology. Of note, stool PCR was also performed on six patients. While three of these were positive, the diagnosis was made prior to the stool PCR result by a different test, usually NPS PCR.
Sixty‐three (32%) patients were admitted to hospital. The majority (n = 35, 55.6%) of patients were identified as requiring admission at the time of phone notification of a positive result. Other sources of admission included the emergency department (n = 10, 15.9%), fever clinic (n = 7, 11.1%) and home clinic (n = 8, 12.7%). Three (4.8%) patients were admitted from other sources. The mean age of patients admitted to hospital was 53.5 years (range: 9–85, SD = 18.8). In contrast, the mean age of patients admitted to the ‘virtual ward’ was 41.1 years (range: 2–81, SD = 18.0).
The most common reason for admission, in 54 (85.7%) patients, was for medical concerns. Eight (12.7%) patients were admitted for public health reasons (i.e. inability to isolate safely) and one (1.6%) patient was admitted for social reasons.
In those patients where an exposure time could be decided, the mean incubation period was 4.7 days (n = 29, range: 1–23, SD = 4.1). Mean time from first symptom to testing was 4.6 days (n = 185, range: 0–33, SD = 5.4). Mean duration of symptoms was 14 days (n = 191, range: 1–69, SD = 10.3). Mean time from first symptom to hospitalisation was 5.3 days (n = 61, range: 0–37, SD = 5.4). Mean time from first symptom to intensive care unit (ICU) admission was 11.6 days (n = 5, range: 6–16, SD = 3.8).
World Health Organization criteria were used to categorise patients in terms of their disease severity. 12 In our cohort of 197 patients, 3 were asymptomatic, 173 had mild disease and 16 patients had moderate disease. Of the 63 hospitalised patients, 11 (17.4%) required supplemental oxygen, 5 (7.9%) required admission to the ICU and 3 (4.8%) required intubation. There were no deaths.
Table 1 summarises the C‐reactive protein (admission and peak), lymphocyte count (admission and nadir) and EGFR (admission and nadir) of patients who had blood tests performed. As a significant proportion of patients were never hospitalised, only a small proportion had blood tests performed. None of the patients had acute renal failure or required renal replacement therapy (RRT).
Table 1.
Laboratory investigations
| Investigations | Number of patients | Average | SD; range |
|---|---|---|---|
| CRP (initial) | 56 | 29.5 | 29.5; 2–254 |
| CRP (peak) | 16 | 150 | 111; 3–360 |
| Lymphocyte count (admission) | 69 | 1.39 | 0.66; 0.27–3.91 |
| Lymphocyte count (nadir) | 55 | 1.23 | 0.63; 0.26–3.07 |
| EGFR (admission) | 67 | 82.3 | 10.9; 47–90 |
| EGFR (nadir) | 48 | 75.6 | 13.6; 44–90 |
CRP, C‐reactive protein; EGFR, estimated glomerular filtration rate; SD, standard deviation.
Sixty patients in total had chest radiographs (Table 2). While most radiographs were normal (n = 41, 68.3%), the main abnormality in the remainder was interstitial infiltrates, seen in 16 (26.6%) patients. Twelve patients underwent computed tomography (CT) scanning of the chest or abdomen. Ten patients had either CT of the chest or CT pulmonary angiogram (CTPA) – nine of these scans demonstrated the presence of radiological features of COVID‐19. Ten patients had both a chest radiograph and a CT of the chest: six patients had both chest X‐ray and chest CT findings consistent with COVID‐19. Three patients had a positive finding on CT of the chest but a normal chest radiograph. One patient had both chest CT and radiograph that were clear, without features of COVID‐19. A CT scan of the abdomen was performed for non‐respiratory reasons for one patient, in which features consistent with COVID‐19 were detected; the diagnosis was subsequently confirmed by respiratory PCR. The chest X‐ray in this case was negative for COVID‐19 features. The visualised lung bases of the final CT of the abdomen, done for trauma indication, was negative for features of COVID‐19, and no chest X‐ray was done in this case.
Table 2.
Radiological investigations
| Chest X‐ray at presentation | No. (%) | |
|---|---|---|
| Total | 60 | |
| No changes | 41 (68.3) | |
| Interstitial infiltrates | 16 (26.6) | |
| Localised consolidation | 1 (1.7) | |
| Multi‐lobar consolidation | 2 (3.3) | |
| Other | 0 (0) | |
| CT scan | No. | Presence of COVID‐19 features |
|---|---|---|
| CT scans performed | 12 | 10 |
| CT of the chest or CTPA | 10 | 9 |
| CT of the abdomen | 2 | 1 |
CT, computed tomography; CTPA, CT pulmonary angiogram.
Twenty‐four patients out of 197 confirmed cases of COVID19 received a form of pharmacological treatment. Twenty‐one patients received hydroxychloroquine (mean duration of therapy 4.4 days), one patient received chloroquine (5 days), two patients received a combination of lopinavir/ritonavir (mean duration of therapy 5 days) and a single patient received oseltamivir (5 days).
One hundred and ninety‐one (97%) patients achieved complete resolution of symptoms. Two (1%) patients experienced serious sequelae and 4 (2%) patients had non‐serious sequelae. Among patients with serious sequelae, one patient had a prolonged admission to ICU complicated by pulmonary emboli, ventilator‐associated pneumonia and critical illness myopathy. The other patient had an ischaemic lower limb due to arterial thrombosis and had residual lung fibrosis/organising pneumonia. On discharge from home clinic follow up, one patient had ongoing anosmia, one patient reported ongoing symptoms of ‘ear congestion’, one patient complained of a post‐viral cough and another patient reported worsening of depression/anxiety.
Clearance of the virus and release from isolation was determined using two criteria (clearance criteria changed during the timeframe of this study): symptom resolution and negative swabs or by time‐frames and symptom resolution (depending on the criteria at the time of management). One hundred and sixty three (82.7%) patients were cleared based on time and symptom resolution while 34 (17.3%) patients were cleared using repeat NPS. Of the patients cleared by NPS, the mean time elapsed from diagnosis to clearance was 28.4 days (range: 8–55 days). On average, 5.1 NPS were required for clearance (range: 2–12 swabs).
Discussion
This study examined the first wave of 197 COVID‐19 cases within the Gold Coast Hospital and Health Service, Queensland, Australia. Our case numbers by date mirror the Australian first peak, with a large increase in numbers seen in March nationally (Fig. 3). 3 , 13 The cohort was unique in that almost all cases had epidemiological links with only seven cases of community transmission. 3
Figure 3.
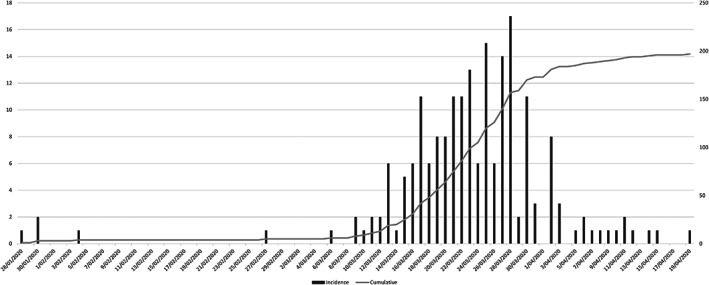
Case numbers by date. ( ), Incidence; (
), Incidence; ( ), cumulative.
), cumulative.
Mortality was lower than other comparable cohorts. 5 , 14 There was an overall low rate of ICU admissions, in contrast to first peak data from other international metropolitan areas. 5 , 6 , 7 Most patients in this study comprised returned travellers and their contacts, were relatively young and had a low comorbidity burden. The age range was similar to Australia‐wide first wave data, although Australian cumulative data now include the subsequent second wave of infections that occurred following the conclusion of our study. 3
Aggressive statewide testing, 15 isolation and public health measures implemented during this time may have contributed to preventing further community transmission. Health systems were not overburdened and some of those with mild disease were hospitalised for a period of observation.
This study also demonstrated that large numbers of patients can be managed effectively in an outpatient setting. Most patients were managed using a virtual ‘home’ clinic and did not require hospital admission. Patients suitable for management in the home setting were monitored through telehealth for evidence of deterioration. Outpatient care was successfully delivered for both young patients with few or no comorbidities, as well as for more comorbid and elderly patients.
>The clinical characteristics and outcomes of patients captured in this study comprise part of the first wave of infections in Australia. Our health service has not yet encountered a ‘second wave’, though, should this come to pass this could be compared with our ‘first wave’ data.
This study had some limitations. As we had accurate exact exposure dates for only a small percentage of the cohort, a reliable incubation period could not be calculated. Systemic thrombosis is a well recorded complication of COVID‐19, with incidence as high as 20–30% of critically ill COVID‐19 patients despite prophylaxis in Dutch and French studies. 16 , 17 The numbers of critically ill patients in this study were inadequate for a reasonable comparison for thrombosis risk; thrombosis was recorded in only one patient in this cohort. Anosmia is a recognised symptom of COVID‐19 illness. 18 Data collection was limited by incomplete documentation of some clinical information due to the retrospective nature of the review of medical records. Anosmia emerged as a recognised symptom during the time period captured in this study 18 and thus in the first few weeks of this COVID‐19 response, these results may underreport the prevalence of this symptom in this cohort.
Conclusion
This study highlights characteristics and outcomes of the first 197 patients with COVID‐19 in Gold Coast, Queensland, Australia. Most of the cases in this cohort had identified epidemiological links, were relatively young and suffered mild disease, managed safely in both inpatient and outpatient settings. A follow‐up study should be performed in the next wave of COVID‐19 in the Gold Coast region for comparison.
Funding: None.
Conflict of interest: None.
References
- 1. Young BE, Ong SWX, Kalimuddin S, Low JG, Tan SY, Loh J et al. Epidemiologic features and clinical course of patients infected with SARS‐CoV‐2 in Singapore. JAMA 2020; 323: 1488–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Zhu N, Zhang D, Wang W, Li X, Yang B, Song J et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med 2020; 382: 727–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Commonwealth of Australia, Department of Health . Coronavirus (COVID‐19) current situation and case numbers. 2020. [cited 2020 Aug 15]. Available from URL: https://www.health.gov.au/news/health-alerts/novel-coronavirus-2019-ncov-health-alert/coronavirus-covid-19-current-situation-and-case-numbers
- 4. Shi Q, Zhang X, Jiang F, Zhang X, Hu N, Bimu C et al. Clinical characteristics and risk factors for mortality of COVID‐19 patients with diabetes in Wuhan, China: a two‐center, retrospective study. Diabetes Care 2020; 43: 1382–91. [DOI] [PubMed] [Google Scholar]
- 5. Richardson S, Hirsch JS, Narasimhan M, Crawford JM, McGinn T, Davidson KW et al. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID‐19 in the New York City area. JAMA 2020; 323: 2052–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Guan WJ, Ni ZY, Hu Y, Liang WH, Ou CQ, He JX et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med 2020; 382: 1708–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Teich VD, Klajner S, de Almeida FAS, Dantas ACB, Laselva CR, Torritesi MG et al. Epidemiologic and clinical features of patients with COVID‐19 in Brazil. Einstein (Sao Paulo) 2020; 18:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Population Australia . Gold Coast Population. 2020. [cited 2020 Dec 24]. Available from URL: http://www.population.net.au/gold-coast-population/
- 9. Strategy and Health Service Planning Department, Gold Coast Hospital . Health Service Plan 2016‐2026. 2016. [cited 2020 Dec 29]. Available from URL: https://www.publications.qld.gov.au/dataset/gold-coast-hospital-and-health-service-plans/resource/2d5a473d-7f45-48bc-bba7-58c919118147
- 10. Gold Coast Hospital and Health Service, and Gold Coast Primary Health Network . Gold Coast Population Health Profile 2015. 2015.
- 11. Communicable Diseases Network Australia . Coronavirus Disease 2019 (COVID‐19). CDNA National Guidelines for Public Health Units. 2020. [cited 2020 Aug 12]. Available from URL: https://www1.health.gov.au/internet/main/publishing.nsf/Content/7A8654A8CB144F5FCA2584F8001F91E2/$File/COVID‐19‐SoNG‐v3.7.pdf
- 12. World Health Organization . Clinical management of COVID‐19: interim guidance, 27 May 2020. Contract No.: WHO/2019‐nCoV/clinical/2020.5. Geneva: World Health Organization; 2020.
- 13. Commonwealth of Australia, Department of Health . Coronavirus (COVID‐19) at a glance infographic collection. 2021. [cited 2021 Jan 21]. Available from URL: https://www.health.gov.au/resources/collections/coronavirus-covid-19-at-a-glance-infographic-collection
- 14. Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID‐19) outbreak in China: summary of a report of 72 314 cases from the Chinese Center for Disease Control and Prevention. JAMA 2020; 323: 1239–42. [DOI] [PubMed] [Google Scholar]
- 15. The State of Queensland . Queensland COVID‐19 statistics. 2021. [cited 2021 Jan 21]. Available from URL: https://www.qld.gov.au/health/conditions/health-alerts/coronavirus-covid-19/current-status/statistics#testbyhhs
- 16. Poissy J, Goutay J, Caplan M, Parmentier E, Duburcq T, Lassalle F et al. Pulmonary embolism in patients with COVID‐19: awareness of an increased prevalence. Circulation 2020; 142: 184–6. [DOI] [PubMed] [Google Scholar]
- 17. Middeldorp S, Coppens M, van Haaps TF, Foppen M, Vlaar AP, Müller MCA et al. Incidence of venous thromboembolism in hospitalized patients with COVID‐19. J Thromb Haemost 2020; 18: 1995–2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Zhang J, Wang X, Jia X, Li J, Hu K, Chen G et al. Risk factors for disease severity, unimprovement, and mortality in COVID‐19 patients in Wuhan, China. Clin Microbiol Infect 2020; 26: 767–72. [DOI] [PMC free article] [PubMed] [Google Scholar]


