Abstract
Yes-associated protein-1 (YAP1) is an important effector of the Hippo pathway and has crosstalk with other cancer signaling pathways. It induces an immunosuppressive tumor microenvironment by activating pathways in several cellular components. However, the mechanisms by which it drives immune infiltration in pancreatic cancer remain poorly understood. We analyzed the expression of YAP1 as well as its prognostic value and correlations with immune infiltrates in various cancers, with a focus on pancreatic cancer. In particular, using the Oncomine database and Gene Expression Profiling Interactive Analysis (GEPIA) database, we found that YAP1 is differentially expressed between tumor tissues and control tissues in a number of cancers and in particular, is elevated in pancreatic cancer. Using the Kaplan–Meier plotter, GEPIA, and Long-term Outcome and Gene Expression Profiling database of pan-cancers (LOGpc), we further established the prognostic value of YAP1. We found that YAP1 expression was significantly related to outcomes in multiple types of cancer based on data from The Cancer Genome Atlas, particularly in pancreatic cancer. Correlations between YAP1 and immune cell infiltration and immune cell marker expression were examined using Tumor Immune Estimation Resource and GEPIA. High expression levels of YAP1 were significantly associated with a variety of immune markers and immune cell subsets in pancreatic cancer. These results suggest that YAP1 is correlated with patient outcomes and tumor immune cell infiltration in multiple cancer types and is a valuable prognostic biomarker in pancreatic cancer.
Keywords: pancreatic cancer, Hippo pathway, prognosis, Yes-associated protein-1, tumor infiltration
Introduction
Pancreatic cancer is the fifth most common cause of cancer-related deaths in developed countries, accounting for 260,000 deaths worldwide every year; its 5-years survival rate is extremely low (5%) (Johansson et al., 2016). Because the early detection of pancreatic tumors is relatively difficult, only as few as 20% of patients are eligible for possible radical surgery; in addition, chemotherapy and radiotherapy do not substantially improve overall survival (OS) (Martinez-Bosch et al., 2018). However, since immunotherapy was declared a breakthrough approach in 2013, the effectiveness of immune checkpoint inhibition has been demonstrated in various solid tumors, and it may be beneficial in pancreatic cancer (Gan et al., 2020). Clinical studies of immunotherapy for pancreatic cancer are ongoing (Zhang and Choi, 2015; Martinez-Bosch et al., 2018), and the characterization of immunophenotypes and identification of novel immune-related therapeutic targets in pancreatic cancer are urgent research goals (Luedke et al., 2012).
Yes-associated protein-1 (YAP1) is a transcriptional coactivator and is a pivotal factor in the Hippo/YAP signaling pathway, promoting tumor formation and development (Zanconato et al., 2019; Kuenzi and Ideker, 2020). YAP1 expression is significantly correlated with the expression levels of several proto-oncogenes, such as KRAS, Wnt/β-catenin, and CTGF (Nguyen and Yi, 2019; Pobbati and Hong, 2020). The expression of YAP1 is elevated in a number of cancer types, such as liver, lung, colorectal, ovarian, and prostate cancers. (Sharma et al., 2017; Yamauchi and Moroishi, 2019). Dephosphorylated YAP1 accumulates in the nucleus due to the inactivation of Hippo signaling, thereby affecting cell proliferation, invasion, epithelial-mesenchymal transition, stemness, and metabolic reprogramming (Zhang et al., 2017; Flinn et al., 2019; Liu et al., 2019; Meng et al., 2020). YAP1 in cancer cells also confers resistance to certain drugs (Zhou et al., 2018; Yao et al., 2019).
Studies of YAP1 in tumor immunity are in the early stages. Early studies have shown that the excessive activation of YAP and TAZ inhibits tumor growth via TEAD-mediated transcription (Gebhardt and Harvey, 2016; Moroishi et al., 2016). Another study has indicated that YAP1 regulates innate immunity by interacting with IRF3 (Du et al., 2018). There is evidence for important roles of YAP in the regulation of the tumor immune checkpoint PD-L1/PD-1 pathway in malignant pleural mesothelioma and non-small cell lung cancer (Miao et al., 2017; Hsu et al., 2018). These previous findings indicate that YAP1 may be a valuable prognostic biomarker (Kim et al., 2018). Although pancreatic cancer is relatively immune-resistant due to tissue fibrosis in the tumor microenvironment and a lack of TILs, B and T cell-specific immune responses are still produced under exposure to tumor cell antigens (Zhang and Choi, 2015; Ibrahim and Wang, 2016). Therefore, the mechanisms by which YAP1 functions in the immune microenvironment in pancreatic cancer deserve further study.
In this study, we systematically investigated YAP1 expression and its relationship with prognosis based on LOGpc, Oncomine, GEPIA, and K-M plotter analyses. Next, we analyzed the correlations between YAP1 and tumor-infiltrating immune cells in the microenvironments of several tumors using TIMER. Our results suggest that YAP1 expression is related to pancreatic cancer and the immune microenvironment.
Materials and Methods
Oncomine Database Analysis
Pan-cancer gene expression array data from the ONCOMINE database (www.oncomine.org), including expression data for 715 genes in 86,733 samples, were obtained. The Student’s t-test was used to compare the mRNA expression levels of YAP1 between cancer specimens and normal specimens. The cut-off p-value was 0.01, and the threshold fold change was 2.0.
Prognostic Value of YAP1 Expression
The relationship between the expression of YAP1 and prognosis was evaluated using two databases, the Long-term Outcome and Gene Expression Profiling database of pan-cancers (LOGpc) (http://bioinfo.henu.edu.cn/DatabaseList.jsp) and Kaplan–Meier plotter (KM plotter; http://kmplot.com/analysis/) (Győrffy et al., 2013). LOGpc included 209 expression datasets and 13 survival analyses of 27 distinct malignancies involving 31,310 patients. Kaplan–Meier plotter is an online database of microarray gene expression data and survival information derived from European Genome-Phenome Archive, Gene Expression Omnibus, and TCGA, including data for 21 different types of cancers and a large number of samples for different cancers cohorts (Györffy et al., 2010).
TIMER Database Analysis
The TIMER (https://cistrome.shinyapps.io/timer/) database was used to explore immune cell infiltration in various cancers. Data for tumor immune infiltration [B cells, CD4+ T cells, CD8+ T cells, neutrophils, macrophages, and dendritic cells (DCs)] determined by statistical methods and validated by pathological examinations are included. Specific immune cell subsets were used to explore the relationship between YAP1 expression and the degree of infiltration. A KM survival analysis was performed to explore the relationship between survival and gene expression or immune cell infiltration. Finally, associations between YAP1 expression and the expression of markers of specific infiltrating immune cell subsets were evaluated.
GEPIA Database Analysis
Gene Expression Profiling Interactive Analysis (GEPIA) uses standard processing pipelines to analyze RNA sequencing expression data for 8,587 normal samples and 9,736 tumors from GTEx and TCGA projects. The relationships between YAP1 expression levels and patient prognosis in several cancers and the link between YAP1 expression and immune cell infiltration, with a focus on tumor markers, were evaluated.
Statistical Analysis
Data were analyzed with a log-rank test, and the HR, fold-change, and p-values were obtained. Spearman's correlation analysis was used to measure the degree of relationship between specific variables, where correlation coefficients r indicate the strength of the relationship as follows: very weak, 0.00–0.19; weak, 0.20–0.39; moderate, 0.40–0.59; strong, 0.60–0.79; very strong, 0.80–1.0. p < 0.05 was the threshold for significance.
Results
Assessment of YAP Expression in Cancer and Normal Tissues
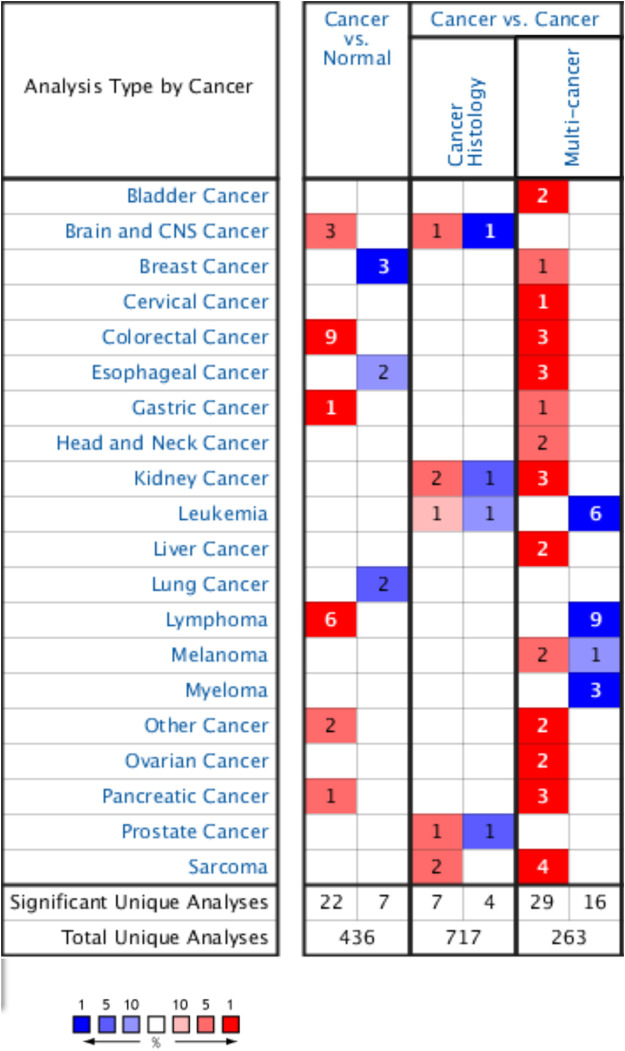
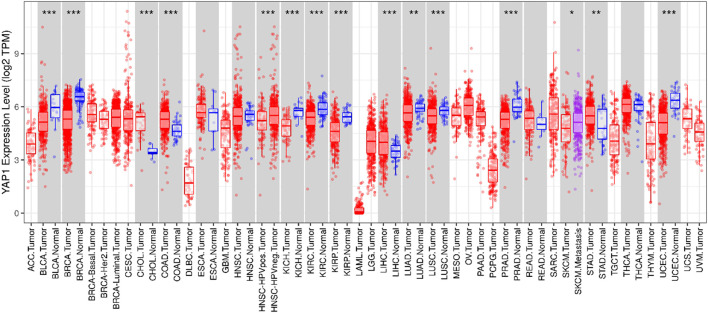
The Oncomine database was used to compare YAP1 expression in pan-tumors and corresponding normal tissues. The expression levels of YAP1 were higher in tumor tissues than in normal control tissues for pancreatic, gastric, colorectal, and brain cancers and lymphoma. In breast, esophageal, and lung cancer tissues, YAP1 expression was lower than that in normal tissue controls (Figure 1). Table 1 summarizes the detailed findings for specific tumor types. Using the TCGA and TIMER databases, YAP1 expression was significantly lower in bladder urothelial carcinoma (BLCA), breast invasive carcinoma (BRCA), kidney chromophobe (KICH), kidney renal clear cell carcinoma (KIRC), kidney renal papillary cell carcinoma (KIRP), liver hepatocellular carcinoma (LIHC), lung adenocarcinoma (LUAD), lung squamous cell carcinoma (LUSC), prostate adenocarcinoma (PRAD), and uterine corpus endometrial carcinoma (UCEC) than in adjacent normal tissues. However, YAP1 expression was significantly higher in cholangial carcinoma (CHOL), colon adenocarcinoma (COAD), and stomach adenocarcinoma (STAD) than in adjacent normal tissues. Differences between YAP1 expression in tumors and normal tissues are summarized in Figure 2. Using GEPIA databases, the expression of YAP1 was significantly higher in tumor tissues than in normal controls in CHOL, Lymphoid Neoplasm Diffuse Large B-cell Lymphoma (DLBC), Glioblastoma multiforme (GBM), Pancreatic adenocarcinoma (PAAD), STAD, and Thymoma (THYM). In contrast, the expression of YAP1 was significantly lower in tumor tissues than in normal control tissues in Adrenocortical carcinoma (ACC), BLCA, Pheochromocytoma and Paraganglioma (PCPG), uterine corpus endometrial carcinoma (UCEC), and Uterine Carcinosarcoma (UCS). Differences between the expression of YAP1 in tumors and normal adjacent tissue samples are shown in Figure 3.
FIGURE 1.
Transcript levels of YAP1 in different cancers based on the ONCOMINE database.
TABLE 1.
Differences in YAP1 expression between cancer tissues and normal tissues based on the Oncomine database.
| Cancer | Cancer type | p-value | Fold change | Rank (%) | Sample | References |
|---|---|---|---|---|---|---|
| Brain and CNS | Glioblastoma vs. Normal | 4.43E−12 | 18.906 | 2 | 230 | Murat brain |
| Glioblastoma vs. Normal | 3.08E−14 | 2.265 | 3 | 579 | Sun Brain | |
| Glioblastoma vs. Normal | 4.43E−6 | 2.152 | 5 | 703 | Bredel brain 2 | |
| Breast | Ductal breast carcinoma vs. Normal | 1.31E−11 | −2.796 | 1 | 110 | Richardson breast 2 |
| Mucinous breast carcinoma vs. Normal | 2.23E−5 | −2.740 | 3 | 429 | TCGA breast | |
| Invasive breast carcinoma stroma vs. Normal | 6.32E−25 | −8.170 | 4 | 667 | Finak breast | |
| Colorectal | Colon adenoma vs. Normal | 1.24E−9 | 3.268 | 1 | 72 | Skrzypczak colorectal 2 |
| Colon adenoma epithelia vs. Normal | 2.35E−7 | 2.633 | 2 | 344 | Skrzypczak colorectal 2 | |
| Colon carcinoma vs. Normal | 1.64E−6 | 2.120 | 10 | 1783 | Skrzypczak colorectal 2 | |
| Cecum adenocarcinoma vs. Normal | 8.18E−9 | 2.238 | 1 | 144 | Kaiser colon | |
| Rectal adenocarcinoma vs. Normal | 6.69E−6 | 2.014 | 1 | 155 | Kaiser colon | |
| Colon adenocarcinoma vs. Normal | 2.89E−12 | 2.234 | 1 | 173 | Kaiser colon | |
| Rectosigmoid adenocarcinoma vs. Normal | 5.25E−6 | 2.158 | 3 | 394 | Kaiser colon | |
| Rectal adenoma vs. Normal | 2.19E−8 | 2.152 | 2 | 196 | Sabates–Bellver Colon | |
| Colorectal carcinoma vs. Normal | 9.10E−14 | 2.129 | 2 | 278 | Hong Colorectal | |
| Esophageal | Barrett's esophagus vs. Normal | 4.04E−10 | −3.347 | 6 | 967 | Kim esophagus |
| Esophageal adenocarcinoma vs. Normal | 1.26E−11 | −2.459 | 9 | 1,575 | Kim esophagus | |
| Gastric | Gastric intestinal type adenocarcinoma vs. Normal | 2.22E−12 | 2.204 | 1 | 168 | DErrico gastric |
| Lung | Small cell lung carcinoma vs. Normal | 1.01E−5 | −3.733 | 3 | 200 | Bhattacharjee Lung |
| Lung carcinoid tumor vs. Normal | 2.68E−8 | −8.584 | 5 | 373 | Bhattacharjee lung | |
| Lymphoma | Follicular lymphoma vs. Normal | 1.16E−33 | 14.570 | 1 | 47 | Compagno lymphoma |
| Diffuse large B-Cell lymphoma vs. Normal | 6.15E−23 | 10.179 | 2 | 215 | Compagno lymphoma | |
| Germinal center B-cell-like diffuse large B-Cell lymphoma vs. Normal | 1.02E−6 | 3.857 | 2 | 385 | Compagno lymphoma | |
| Activated B-cell-like diffuse large B-Cell lymphoma vs. Normal | 2.37E−9 | 6.351 | 5 | 799 | Compagno lymphoma | |
| Unspecified peripheral T-Cell lymphoma vs. Normal | 3.31E−14 | 10.931 | 2 | 357 | Piccaluga lymphoma | |
| Angioimmunoblastic T-Cell lymphoma vs. Normal | 3.67E−5 | 20.196 | 8 | 1,563 | Piccaluga lymphoma | |
| Others | Teratoma, NOS vs. Normal | 7.73E−9 | 2.771 | 3 | 420 | Korkola seminoma |
| Yolk sac tumor, NOS vs. Normal | 5.18E−6 | 3.674 | 4 | 649 | Korkola seminoma | |
| Pancreatic | Pancreatic ductal adenocarcinoma vs. Normal | 9.82E−11 | 2.180 | 3 | 587 | Badea pancreas |
FIGURE 2.
YAP1 expression levels in different tumor types based on data from TCGA were determined using TIMER. *p < 0.05, **p < 0.01, ***p < 0.001.
FIGURE 3.
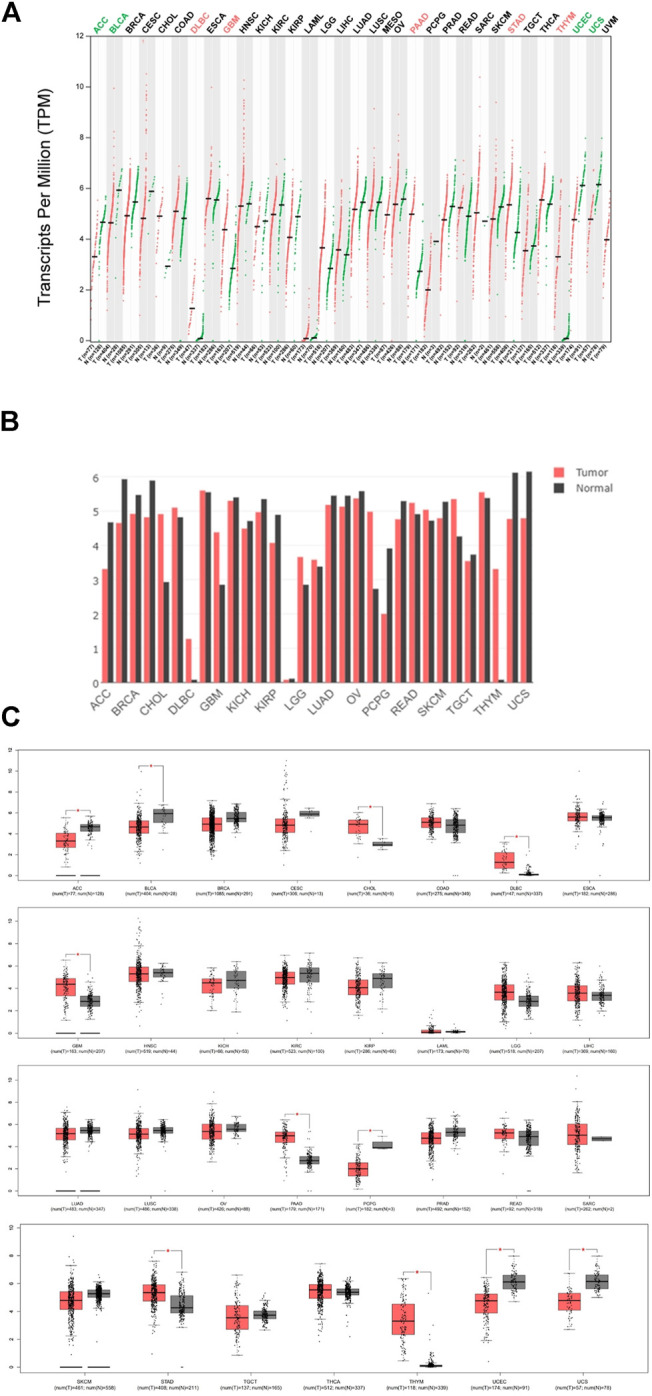
Expression of YAP1 in different cancers (GEPIA). (A) YAP1 expression profile across all tumor samples and paired normal tissues (dot plot). (B) YAP1 expression profile across all tumor samples and paired normal tissues (Bar plot). (C) YAP1 expression profile across all tumor samples and paired normal tissues (box plots).
Correlation Between the Expression of YAP1 and Prognosis
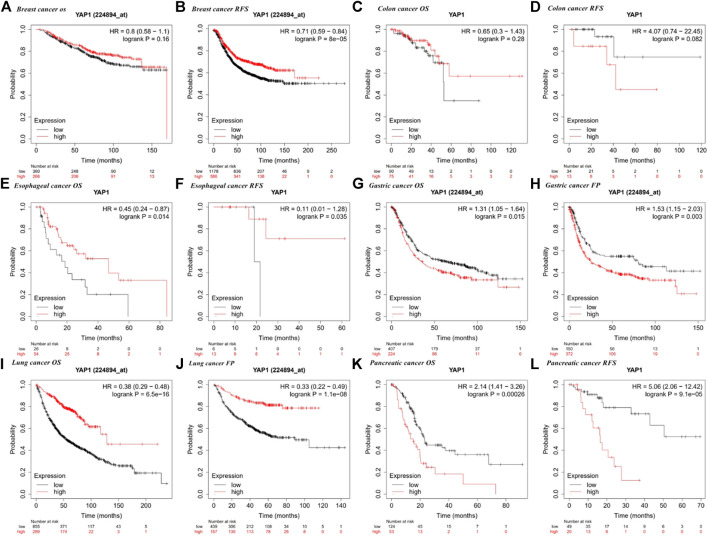
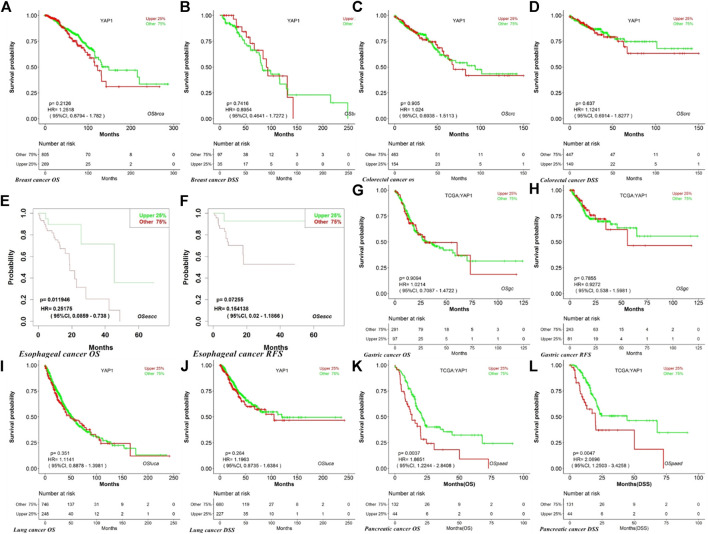
We employed the KM plotter database to explore the effect of YAP1 expression on the survival of patients with cancers showing the most obvious expression differences between tumor tissues and normal tissues (i.e., breast, colorectal, esophageal, gastric, lung, and pancreatic cancers). For multiple cancer types, such as lung, esophageal, gastric, pancreatic, and breast cancer, we detected a significant correlation between prognosis and YAP1 expression (Figure 4). We revealed that a higher YAP1 expression level was significantly related to a poorer prognosis in pancreatic cancer (OS, HR = 2.14, 95% CI = 1.41–3.26, p = 0.00026; relapse free survival (RFS), HR = 5.06, 95% CI = 2.06–12.42, p = 9.1e−0.5) and gastric cancer (OS, HR = 1.31, 95% CI = 1.05–1.64, p = 0.015; first progression (FP), HR = 1.53, 95% CI = 1.15–2.03, p = 0.003) (Figures 4G,H,K,L). Increased YAP1 expression was associated with an improved prognosis in lung cancer (OS, HR = 0.38, 95% CI = 0.29–0.48, p = 6.5e−16; FP, HR = 0.33, 95% CI = 0.22–0.49, p = 1.1e−08) and esophageal cancer (OS, HR = 0.45, 95% CI = 0.24–0.87, p = 0.014; RFS, HR = 0.11, 95% CI = 0.01–1.28, p = 0.035) and with an improved RFS in breast cancer (RFS, HR = 0.71, 95% CI = 0.59–0.84, P = 8e−5) (Figures 4A,B,E,F,I,J). There was no significant correlation between the expression of YAP1 and prognosis in colorectal cancer.
FIGURE 4.
Kaplan–Meier survival curves for high and low YAP1 expression in different types of cancer based on the Kaplan–Meier plotter database. OS, overall survival; DFS, disease-free survival; RFS, relapse-free survival; DSS, disease-specific survival. DMFS, distant metastasis-free survival. FP, first progression.
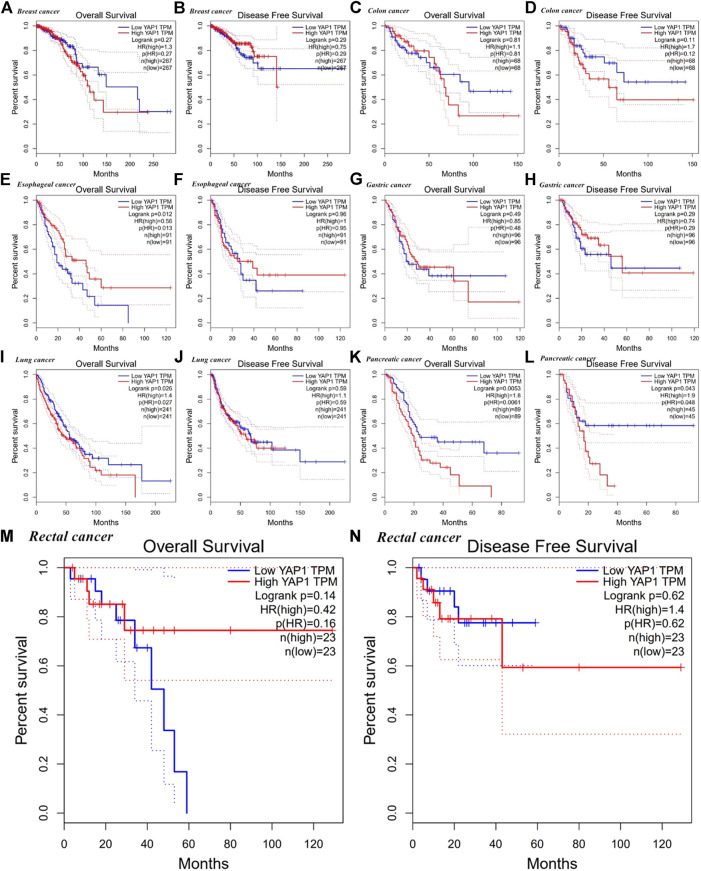
Using the GEPIA database, we found that high YAP1 expression levels were associated with a poorer prognosis based on OS and disease-free survival (DFS) in pancreatic cancer (OS, HR = 1.8, p = 0.0053; DFS HR = 1.9, p = 0.043) but not in gastric cancer (OS, HR = 0.85, p = 0.49; DFS, HR = 0.74, p = 0.29) (Figure 5K,L,G,H). High expression of YAP1 was associated with a better OS in esophageal cancer (OS, HR = 0.56, p = 0.012) (Figure 5E). However, high mRNA levels of YAP1 were significantly correlated with a reduced OS in lung cancer (OS, HR = 1.4, p = 0.026) (Figure 5I). The expression of YAP1 was not significantly correlated with OS and DFS in breast, colorectal, and gastric cancers (Figures 5A–D,G,H).
FIGURE 5.
Kaplan–Meier survival curves for high and low YAP1 expression in different types of cancer based on GEPIA. OS, overall survival; DFS, disease-free survival; RFS, relapse-free survival; DSS, disease-specific survival. DMFS, distant metastasis-free survival.
We next used LOGpc to explore the link between YAP1 expression and outcomes for patients with breast, colorectal, esophageal, gastric, lung, and pancreatic cancers. TCGC database showed that higher YAP1 expression is linked to a poorer prognosis in pancreatic cancer (OS, HR = 1.87, 95% CI = 1.22–2.84, p = 0.0037; disease-specific survival (DSS) HR = 2.07, 95% CI = 1.25–3.43, p = 0.0047) (Figure 6). However, YAP1 expression was not significantly related to prognosis in breast, lung, gastric, esophageal, and colorectal cancer (Figures 6A–N). Our findings suggested that YAP1 has prognostic value in several types of cancers, especially pancreatic cancer, and the YAP1 expression patterns and prognostic value differed among cancer types.
FIGURE 6.
Kaplan–Meier survival curves for high and low YAP1 expression in different types of cancer based on the LOGpc TCCG database. OS, overall survival; DFS, disease-free survival; RFS, relapse-free survival; DSS, disease-specific survival. DMFS, distant metastasis-free survival.
Association Between YAP1 Expression and Immune Cell Infiltration
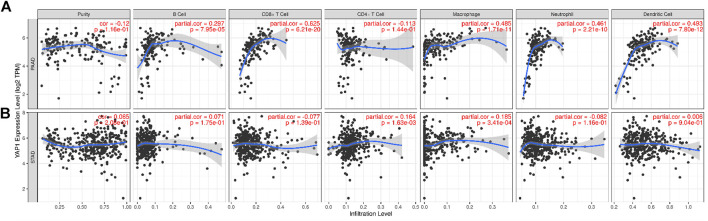
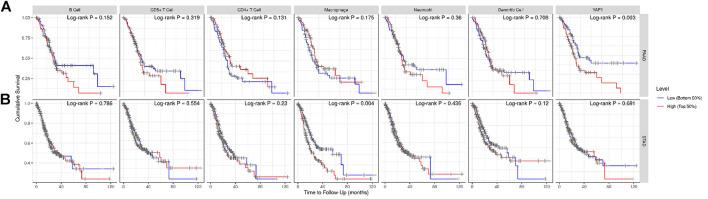
Presence of tumor-infiltrating lymphocytes is an independent predictor of survival in cancers. Therefore, we used the TIMER database to explore the relationship between YAP1 expression and the degree of immune cell infiltration in 39 tumor types (Supplementary Figure S1). Our findings suggested that YAP1 expression is significantly correlated with the tumor purity in 13 cancer types, B cell infiltration in 17 cancer types, CD4+ T cell infiltration in 25 cancer types, CD8+ T cell infiltration in 26 cancer types, macrophage infiltration in 27 cancer types, neutrophil infiltration in 26 cancer types, and DC infiltration in 24 cancer types. In PAAD, the expression of YAP1 was significantly related to levels of B cells (R = 0.297, p = 7.95e−05), CD8+ T cells (R = 0.625, p = 6.21e−20), macrophages (R = 0.485, p = 1.71e−11), neutrophils (R = 0.461 p = 2.21e−10), and DCs (R = 0.493, p = 7.80e−12), whereas there were no correlations with CD4+ T cells and tumor purity (Figure 7A). We did not detect significant associations between YAP1 levels and tumor purity or B cell, CD8+ T cell, neutrophil, and DC infiltration in STAD (Figure 7B). By generating KM plots using the TIMER database, we further explored the correlation between YAP1 expression and immune cell infiltration in PAAD and STAD. We found that mRNA expression of YAP1 was significantly correlated with prognosis in PAAD (p = 0.003) (Figure 8A) and with prognosis and macrophage infiltration in STAD (p = 0.004) (Figure 8B). These results indicate that YAP1 plays an important role in the regulation of immune cell infiltration in pancreatic cancer, with a particularly strong role in the infiltration of macrophages, CD8+ T cells, neutrophils, and DCs.
FIGURE 7.
Correlation between YAP1 expression and immune cell infiltration in pancreatic adenocarcinoma (PAAD) and stomach adenocarcinoma (STAD).
FIGURE 8.
Kaplan–Meier plots of immune infiltration and YAP1 expression levels in pancreatic adenocarcinoma (PAAD) and stomach adenocarcinoma (STAD).
Relationships Between YAP1 and Immune Marker Expression
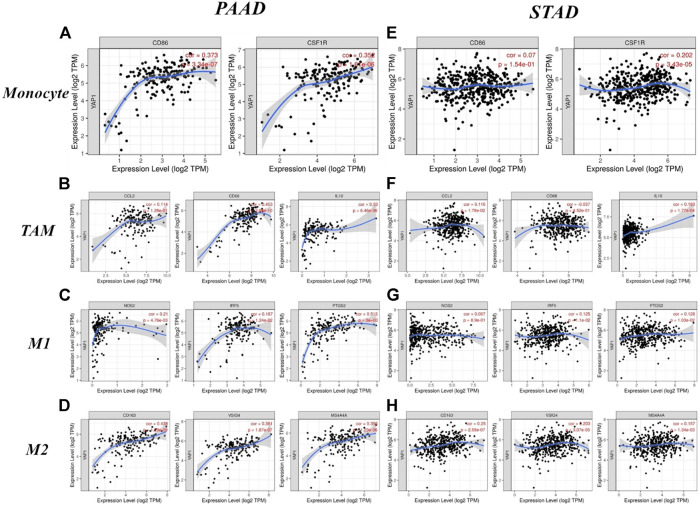
Next, we used the TIMER and GEPIA databases, based on immunological markers sets in PAAD, to explore the relationship between the expression of YAP1 and immune cell infiltration, with STAD as the control group. In addition, we evaluated the relationship between YAP1 expression and several immunological marker subsets, including markers of total T cells, CD8+ T cells, B cells, tumor-associated macrophages (TAMs), monocytes, M1 and M2 macrophages, NK cells, neutrophils, DCs, Tfh cells, Th1 cells, Th2 cells, Tregs, Th17 cells, and exhausted T cells. These results were adjusted based on tumor purity. We detected a significant association between YAP1 expression and markers of total T cells (CD3E and CD2), CD8+ T (CD8A and CD8B), TAMs (CD68 and IL10), monocytes (CD86 and CD115), M1 macrophages (INOS, IRF5, and COX2), M2 macrophages (CD163, VSIG4, and MS4A4A), NK cells (KIR2DL4), neutrophils (CD11b), DCs (HLA-DPB1, HLA-DQB1, HLA-DRA, HLA-DRA, HLA-DRA1, BDCA-4, and CD11C), Tfh (BCL6), Th1 (STAT1 and IFN-γ), Th2 (GATA3, STAT6, and STAT5A), Th17 (STAT3), and Tregs (FOXP3, CCR8, STAT5B, and TGFβ1) in PAAD (Table 2). Conversely, the expression level of YAP1 was associated with only 18 of the markers in STAD (Table 2). Our findings suggest that YAP1 expression had significant correlations with the levels of majority of the markers of TAMs, monocytes, and M1 and M2 macrophages in PAAD (Table 2). Remarkably, YAP1 expression was closely related to levels of CD86 and CD115 (monocyte markers); CD68 and IL10 (TAM markers); COX2 (M1 macrophage marker); and CD163, VSIG4, and MS4A4A (M2 macrophage markers) (p < 0.0001; Figures 9A–D).
TABLE 2.
Correlations between YAP1 and markers of immune cells in TIMER.
| Description | Gene markers | PAAD | STAD | ||||||
| None | Purity | None | Purity | ||||||
| Cor | p | Cor | p | Cor | p | Cor | p | ||
| CD8+T cell | CD8A | 0.254 | ** | 0.211 | * | 0.12 | 0.0143 | 0.114 | 0.0261 |
| CD8B | 0.192 | * | 0.142 | 0.0644 | 0.142 | 3.66e−03 | 0.134 | * | |
| T Cell (general) | CD3D | 0.166 | 0.0266 | 0.109 | 0.158 | −0.01 | 0.842 | −0.003 | 0.957 |
| CD3E | 0.198 | * | 0.141 | 0.0657 | 0.018 | 0.708 | 0.033 | 0.525 | |
| CD2 | 0.207 | * | 0.146 | 0.0538 | 0.078 | 0.114 | 0.091 | 0.0767 | |
| B Cell | CD19 | 0.106 | 0.157 | 0.067 | 0.383 | −0.016 | 0.751 | 0.001 | 0.991 |
| CD79A | 0.133 | 0.0761 | 0.086 | 0.265 | −0.1 | 0.042 | −0.09 | 0.0805 | |
| Monocyte | CD86 | 0.373 | *** | 0.327 | *** | 0.07 | 0.154 | 0.083 | 0.106 |
| CD115 (CSF1R) | 0.352 | *** | 0.297 | *** | 0.202 | 3.43e−05 | 0.209 | *** | |
| TAM | CCL2 | 0.114 | 0.128 | 0.081 | 0.292 | 0.116 | 0.0178 | 0.132 | * |
| CD68 | 0.453 | *** | 0.415 | *** | −0.037 | 0.452 | −0.044 | 0.39 | |
| IL10 | 0.33 | *** | 0.299 | *** | 0.183 | 1.77e−04 | 0.213 | *** | |
| M1 macrophage | INOS (NOS2) | 0.21 | * | 0.004 | * | 0.007 | 0.89 | 0.021 | 0.677 |
| IRF5 | 0.187 | * | 0.172 | 0.0248 | 0.125 | 0.011 | 0.124 | 0.0153 | |
| COX2 (PTGS2) | 0.513 | *** | 0.529 | *** | 0.126 | 0.0103 | 0.131 | 0.0105 | |
| M2 macrophage | CD163 | 0.438 | *** | 0.39 | *** | 0.25 | *** | 0.259 | *** |
| VSIG4 | 0.381 | *** | 0.324 | 1.51e−05 | 0.203 | 3.07e−05 | 0.218 | *** | |
| MS4A4A | 0.356 | *** | 0.303 | 5.51e−05 | 0.157 | 1.34e−03 | 0.163 | * | |
| Neutrophils | CD66b (CEACAM8) | 0.155 | 0.0387 | 0.116 | 0.131 | 0.037 | 0.449 | 0.041 | 0.426 |
| CD11b (ITGAM) | 0.337 | *** | 0.279 | ** | 0.09 | 0.0656 | 0.095 | 0.0644 | |
| CCR7 | 0.089 | 0.235 | 0.045 | 0.558 | 0.067 | 0.172 | 0.093 | 0.0705 | |
| Natural killer cell | KIR2DL1 | 0.124 | 0.0982 | 0.13 | 0.0902 | 0.121 | 0.0139 | 0.108 | 0.035 |
| KIR2DL3 | 0.176 | 0.0182 | 0.17 | 0.026 | 0.082 | 0.0969 | 0.072 | 0.16 | |
| KIR2DL4 | 0.206 | * | 0.22 | * | −0.047 | 0.339 | −0.063 | 0.222 | |
| KIR3DL1 | 0.05 | 0.507 | 0.043 | 0.575 | 0.089 | 0.0697 | 0.071 | 0.167 | |
| KIR3DL2 | 0.136 | 0.0695 | 0.105 | 0.173 | 0.064 | 0.196 | 0.063 | 0.222 | |
| KIR3DL3 | 0.151 | 0.044 | 0.129 | 0.0927 | −0.089 | 0.0686 | −0.108 | 0.0359 | |
| KIR2DS4 | 0.022 | 0.0775 | 0.035 | 0.648 | 0.012 | 0.802 | 0.01 | 8.48e−01 | |
| Dendritic cell | HLA-DPB1 | 0.23 | * | 0.174 | 2.28e−02 | −0.021 | 0.673 | −0.008 | 0.879 |
| HLA-DQB1 | 0.235 | * | 0.191 | 0.0124 | −0.026 | 0.597 | −0.007 | 0.895 | |
| HLA-DRA | 0.367 | *** | 0.323 | *** | 0.005 | 0.925 | 0.021 | 0.688 | |
| HLA-DPA1 | 0.345 | *** | 0.305 | *** | −0.006 | 0.906 | 0.009 | 0.856 | |
| BCDA-1 (CD1C) | 0.139 | 0.0632 | 0.097 | 0.205 | 0.03 | 0.539 | 0.061 | 0.235 | |
| BDCA-4 (NRP1) | 0.604 | *** | 0.582 | *** | 0.341 | *** | 0.355 | *** | |
| CD11c (ITGAX) | 0.224 | * | 0.159 | 0.0373 | 0.113 | 0.0216 | 0.12 | 0.0191 | |
| Th1 | T-bet (TBX21) | 0.117 | 0.119 | 0.079 | 0.302 | 0.096 | 0.0509 | 0.114 | 0.0266 |
| STAT4 | 0.035 | 0.644 | 0.044 | 0.569 | 0.132 | * | 0.154 | * | |
| STAT1 | 0.545 | *** | 0.521 | *** | 0.289 | *** | 0.281 | *** | |
| IFN-γ (IFNG) | 0.196 | * | 0.178 | 1.98e−02 | 0.074 | 0.13 | 0.071 | 0.166 | |
| TNF-α (TNF) | 0.092 | 0.221 | 0.075 | 0.33 | −0.061 | 0.215 | −0.037 | 0.47 | |
| Th2 | GATA3 | 0.258 | ** | 0.248 | * | 0.048 | 0.326 | 0.058 | 0.257 |
| STAT6 | 0.433 | *** | 0.428 | *** | 0.297 | *** | 0.278 | *** | |
| STAT5A | 0.295 | *** | 0.259 | ** | 0.312 | *** | 0.314 | *** | |
| IL13 | −0.04 | 0.595 | −0.023 | 0.763 | 0.034 | 0.485 | 0.05 | 0.332 | |
| Tfh | BCL6 | 0.606 | *** | 0.593 | *** | 0.451 | *** | 0.474 | *** |
| IL21 | 0.064 | 0.396 | 0.059 | 0.44 | 0.021 | 0.67 | 0.019 | 0.717 | |
| Th17 | STAT3 | 0.585 | *** | 0.567 | *** | 0.447 | *** | 0.451 | *** |
| IL17A | 0.1 | 0.184 | 0.078 | 0.311 | −0.075 | 0.128 | −0.059 | 0.249 | |
| Treg | FOXP3 | 0.299 | *** | 0.259 | ** | 0.055 | 0.26 | 0.061 | 0.234 |
| CCR8 | 0.446 | *** | 0.415 | *** | 0.199 | *** | 0.195 | ** | |
| STAT5B | 0.313 | *** | 0.376 | *** | 0.472 | *** | 0.486 | *** | |
| TGFβ (TGFB1) | 0.258 | ** | 0.224 | * | 0.243 | *** | 0.265 | *** | |
| T Cell exhaustion | PD-1 (PDCD1) | 0.162 | 0.0299 | 0.104 | 0.178 | 0.087 | 0.0782 | 0.09 | 0.0785 |
| CTLA4 | 0.212 | * | 0.159 | 0.0382 | 0.146 | * | 0.161 | * | |
| LAG3 | 0.089 | 0.238 | 0.064 | 0.408 | 0.023 | 0.64 | 0.01 | 0.846 | |
| TIM-3 (HAVCR2) | 0.35 | *** | 0.297 | *** | 0.115 | 1.95e−02 | 0.118 | 0.0214 | |
| GZMB | 0.23 | * | 0.187 | 0.0144 | 0.031 | 0.527 | 0.027 | 0.599 | |
Cor, Spearman correlation coefficient R; None, correlation without adjustment. Purity, correlation adjusted by purity. *p < 0 0.01; **p < 0.001; ***p < 0.0001.
Abbreviations: PAAD, pancreatic adenocarcinoma; STAD, stomach adenocarcinoma; TAM, tumor-correlated macrophage; Tfh, follicular helper T cell; Th, T helper cell; Treg, regulatory T cell.
FIGURE 9.
Correlations between YAP1 expression and macrophage polarization in pancreatic adenocarcinoma (PAAD) and stomach adenocarcinoma (STAD). Markers include CD86 and CSF1R for monocytes; CCL2, CD68, and IL10 for tumor-associated macrophages (TAMs); NOS2, IRF5, and PTGS2 for M1 macrophages; and CD163, VSIG4, and MS4A4A for M2 macrophages.
We also explored relationships between YAP1 expression and the expression levels of monocyte, M1 and M2 macrophage, and TAM markers in PAAD and STAD using the GEPIA database. The correlations between PAAD and monocyte and TAM markers were similar to those in the TIMER analysis (Table 3). These findings suggested that YAP1 regulates macrophage polarization in PAAD. Additionally, in PAAD, high YAP1 expression was associated with high levels of DC infiltration, and DC markers, such as HLA-DRA, HLA-DPA1, BDCA-4 and CD11c, were also significantly associated with YAP1 expression. Our findings further indicated a significant correlation between YAP1 and DC infiltration. Furthermore, for Treg cells and exhausted T cells, YAP1 expression was significantly associated with FOXP3, STAT5B, CCR8, CTLA4, TGFβ, TIM-3, and GZMB in PAAD (Table 2). DCs promoted tumor metastasis by reducing CD8+ T cells and increasing Treg cell cytotoxicity (Gebhardt and Harvey, 2016; Ni et al., 2018; Meng et al., 2020). It is not clear whether YAP1 is a key factor in tumor metastasis and DC infiltration. FOXP3 has a crucial role in Treg cells, suppressing the effect of cytotoxic T cells on tumor cells (Kim et al., 2018; He et al., 2019). TIM-3 is a key factor in the regulation of T cell exhaustion. As evidenced by the significant association between YAP1 expression and both FOXP3 and TIM-3, high YAP1 expression contributes to TIM-3-mediated T cell exhaustion. These results confirmed that the expression of YAP1 was significantly related to infiltrating immune cells in PAAD and played a significant role in immune escape in the pancreatic cancer microenvironment.
TABLE 3.
Correlations between YAP1 expression and markers of monocytes and macrophages in GEPIA Description Gene markers PAAD STAD.
| Description | Gene markers | Tumor Cor p | Normal Cor p | Tumor Cor p | Normal Cor p | ||||
|---|---|---|---|---|---|---|---|---|---|
| Monocyte | CD86 | 0.41 | *** | 0.42 | 0.6 | 0.1 | 0.04 | −0.54 | ** |
| CD115 (CSF1R) | 0.39 | *** | 0.2 | 0.92 | 0.29 | 3.3e−09 | −0.17 | 0.31 | |
| TAM | CCL2 | 0.15 | 0.045 | 0.4 | 0.75 | 0.14 | * | 0.2 | 0.23 |
| CD68 | 0.55 | *** | 0.8 | 0.33 | 0.1 | 0.037 | −0.35 | 0.037 | |
| IL10 | 0.37 | *** | 0.8 | 0.33 | 0.27 | *** | −0.35 | 0.039 | |
| M1 macrophage | INOS (NOS2) | 0.25 | *** | 0.4 | 0.75 | 0.032 | 0.52 | −0.12 | 0.47 |
| IRF5 | 0.21 | * | −1 | 0.083 | 0.24 | *** | 0.1 | 0.56 | |
| COX2 (PTGS2) | 0.5 | *** | 0.4 | 0.75 | 0.17 | *** | 0.43 | * | |
| M2 macrophage | CD163 | 0.37 | *** | 0.4 | 0.75 | 0.17 | *** | 0.32 | 0.061 |
| VSIG4 | 0.38 | *** | 0.8 | 0.33 | 0.25 | *** | −0.041 | 0.81 | |
| MS4A4A | 0.39 | *** | 0.8 | 0.33 | 0.2 | *** | −0.001 | 1 | |
PAAD, pancreatic adenocarcinoma; STAD, stomach adenocarcinoma; TAM, Tumor-associated macrophages. Tumor, tumor tissue in TCGA. Normal, normal tissue in TCGA. Cor, Spearman correlation coefficient R, *p < 0.01; **p < 0.001; ***p < 0.0001.
Discussion
YAP1 is a downstream effector of the Hippo signaling pathway (Wang et al., 2016; Ma et al., 2020). It is negatively regulated by upstream factors in the Hippo pathway; when this pathway is activated, it is exported to the cytoplasm and degraded (Wang et al., 2017; Liu et al., 2019). In normal cells, Hippo/YAP is a crucial determinant of organ size (Wang et al., 2017; Elster et al., 2018). YAP1 is overexpressed in many cancers, such as colorectal, lung, liver, ovarian, and prostate cancers, and promotes tumor formation and development (Zhou et al., 2018; Nguyen and Yi, 2019; White et al., 2019; Yang et al., 2020). There is accumulating evidence that YAP1 facilitates the immunosuppressive tumor microenvironment, affecting myeloid-derived suppressor cells, macrophages, and regulatory T-cells (Shibata et al., 2018; Taha et al., 2018; White et al., 2019). However, the underlying mechanisms by which YAP1 contributes to tumor immunity is not clearly established (Hong et al., 2018; Zhang et al., 2018; Wang et al., 2020; Zhang et al., 2020).
In this study, we found that YAP1 expression is associated with prognosis in several types of cancer. In particular, our analyses showed that increased YAP1 expression is associated with a poorer prognosis in PAAD. Furthermore, expression levels of YAP1 were significantly related to levels of immune cell infiltration and diverse immune marker sets in PAAD. Thus, these results suggest that YAP1 contributes to the immune response in PAAD and may be a novel prognostic biomarker.
We examined the expression levels of YAP1 in multiple tumors and corresponding normal tissues using datasets from Oncomine, TCGA in TIMER, and GEPIA databases. YAP1 was differentially expressed between tumor tissues and normal tissues in multiple cancer types. YAP1 expression was upregulated or downregulated in various cancers (Figures 1–3). The heterogeneity of YAP1 expression among cancer types and databases may be attributed to differences in data collection methods and analytical approaches. Nevertheless, we consistently observed a correlation between higher expression of YAP1 and a poor prognosis in PAAD across these databases.
We selected several cancers with the most obvious expression differences between tumor tissues and normal adjacent tissues (breast, pancreatic, colorectal, esophageal, gastric, and lung cancers) and explored the critical role of YAP1 in patient outcomes. Using the KM plotter, GEPIA, and TCGA databases, we found that high YAP1 expression was significantly related to a poorer prognosis in pancreatic cancer (Figures 4–6). These findings suggest that YAP1 is a novel prognostic biomarker for pancreatic cancer.
Another important aspect of this research was the finding that the expression of YAP1 is significantly correlated with diverse immune cell infiltration levels in multiple cancer types, especially in pancreatic cancer. We detected a strong positive association between the expression level of YAP1 and infiltration level of CD8+ T cells, moderate positive associations between YAP1 expression and the infiltration of macrophages, neutrophils, and DCs, and significant positive associations between the infiltration of B cells and YAP1 expression in PAAD, with no relationships between YAP1 and CD4+T cells and tumor purity (Figure 7A). These results indicate that YAP1 plays an important role in the regulation of immune cell infiltration in pancreatic cancer, with particularly strong effects on CD8+ T cells, macrophages, neutrophils, and DCs infiltration.
Furthermore, to investigate the role of YAP1 in the regulation of tumor immunology in PAAD, we analyzed the relationships between YAP1 expression and marker genes of immune cells. Our results suggested that markers of M1 macrophages (such as NOS2 and IRF5) showed weak associations with YAP1 expression, and PTGS2 showed a moderate relationship with YAP1 expression in PAAD (Tables 2 and 3). M2 macrophage markers (including CD163, MS4A4A, and VSIG4) showed moderate and strong correlations with the expression levels of YAP1 in PAAD (Tables 2 and 3). These findings revealed the potential contribution of YAP1 to TAM polarization. Our findings also suggest that YAP1 may function in the activation of Tregs and induction of T cell exhaustion. Gene markers of Treg and T cell exhaustion, including FOXP3, STAT5B, CCR8, TGFβ, CTLA4, TIM-3, and GZMB, showed moderate or weak correlations with YAP1 expression in PAAD (Table 3). In particular, as a key surface protein in T cell exhaustion, TIM-3 and YAP1 expression levels were closely related in PAAD. Accordingly, YAP1 may suppress T cell-mediated immunity by promoting Treg responses. Moreover, our results demonstrated the relationship between YAP1 expression and T helper cells, such as Th1, Th2, Tfh, and Th17. We found that Th1 markers (STAT1 and IFN-γ), Th2 markers (GATA3, STAT6, and STAT5A), a Tfh marker (BCL6), and a Th17 marker (STAT3) were significantly positively correlated with YAP1 in PAAD. YAP1 may therefore regulate T cell responses in PAAD. Together, these findings suggested that YAP1 is a crucial factor for the recruitment and regulation of infiltrating immune cells in PAAD.
In summary, YAP1 can be a valuable prognostic biomarker as well as a crucial regulator of immune cell infiltration in patients with pancreatic cancer.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Ethics Statement
All the datasets were retrieved from the publishing literature, so it was confirmed that all written informed consent was obtained.
Author Contributions
KS and XZ performed the analysis of the data. KS and XL wrote the manuscript. KS and PL designed the study. All authors read and approved the manuscript.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmolb.2021.625731/full#supplementary-material
Glossary
- YAP1
yes-associated protein-1
- GEPIA
gene expression profiling interactive analysis
- LOGpc
long-term outcome and gene expression profiling database of pan-cancers
- TCGA
the cancer genome atlas
- PC
pancreatic cancer
- RT
chemotherapy and radiotherapy
- EMT
epithelial–mesenchymal transition
- NSCLC
non-small cell lung cancer
- MPM
malignant pleural mesothelioma
- TIMER
tumor immune estimation resource
- ONCOMINE
online cancer microarray database
- KM plotter
Kaplan–Meier plotter
- BLCA
bladder urothelial carcinoma
bladder urothelial carcinoma
- BRCA
breast invasive carcinoma
- KICH
kidney chromophobe
- KIRC
kidney renal clear cell carcinoma
- KIRP
kidney renal papillary cell carcinoma
- LIHC
liver hepatocellular carcinoma
- LUAD
lung adenocarcinoma
- LUSC
lung squamous cell carcinoma
- PRAD
prostate adenocarcinoma
- UCEC
uterine corpus endometrial carcinoma
uterine corpus endometrial carcinoma
- CHOL
cholangial carcinoma
- COAD
colon adenocarcinoma
- STAD
stomach adenocarcinoma
- DLBC
lymphoid neoplasm diffuse large B-cell lymphoma
- GBM
glioblastoma multiforme
- PAAD
pancreatic adenocarcinoma
- THYM
thymoma
- ACC
adrenocortical carcinoma
- BLCA
bladder urothelial carcinoma
bladder urothelial carcinoma
- PCPG
pheochromocytoma and paraganglioma
- UCEC
uterine corpus endometrial carcinoma
uterine corpus endometrial carcinoma
- UCS
uterine carcinosarcoma
- OS
overall survival
- DFS
disease-free survival
- RFS
relapse-free survival
- DSS
disease-specific survival
- DMFS
distant metastasis-free survival
- FP
first progression
- TAM
tumor-associated macrophages
- DCs
dendritic cells
References
- Du X., Wen J., Wang Y., Karmaus P. W. F., Khatamian A., Tan H., et al. (2018). Hippo/Mst Signalling Couples Metabolic State and Immune Function of CD8α+ Dendritic Cells. Nature 558 (7708), 141–145. 10.1038/s41586-018-0177-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elster D., Tollot M., Schlegelmilch K., Ori A., Rosenwald A., Sahai E., et al. (2018). TRPS1 Shapes YAP/TEAD-dependent Transcription in Breast Cancer Cells. Nat. Commun. 9 (1), 3115. 10.1038/s41467-018-05370-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flinn M. A., Jeffery B. E., O’Meara C. C., Link B. A. (2019). Yap Is Required for Scar Formation but Not Myocyte Proliferation during Heart Regeneration in Zebrafish. Cardiovasc. Res. 115 (3), 570–577. 10.1093/cvr/cvy243 [DOI] [PubMed] [Google Scholar]
- Gan L. L., Hii L. W., Wong S. F., Leong C. O., Mai C. W. (2020). Molecular Mechanisms and Potential Therapeutic Reversal of Pancreatic Cancer-Induced Immune Evasion. Cancers (Basel) 12 (7), 1872. 10.3390/cancers12071872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gebhardt T., Harvey K. F. (2016). Hippo Wades into Cancer Immunology. Developmental Cel 39 (6), 635–637. 10.1016/j.devcel.2016.12.010 [DOI] [PubMed] [Google Scholar]
- Györffy B., Lanczky A., Eklund A. C., Denkert C., Budczies J., Li Q., et al. (2010). An Online Survival Analysis Tool to Rapidly Assess the Effect of 22,277 Genes on Breast Cancer Prognosis Using Microarray Data of 1,809 Patients. Breast Cancer Res. Treat. 123 (3), 725–731. 10.1007/s10549-009-0674-9 [DOI] [PubMed] [Google Scholar]
- Győrffy B., Surowiak P., Budczies J., Lánczky A. (2013). Online Survival Analysis Software to Assess the Prognostic Value of Biomarkers Using Transcriptomic Data in Non-small-cell Lung Cancer. PLoS One 8 (12), e82241. 10.1371/journal.pone.0082241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- He C., Lv X., Huang C., Angeletti P. C., Hua G., Dong J., et al. (2019). A Human Papillomavirus-independent Cervical Cancer Animal Model Reveals Unconventional Mechanisms of Cervical Carcinogenesis. Cel Rep. 26 (10), 2636–2650.e5. 10.1016/j.celrep.2019.02.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong L., Li X., Zhou D., Geng J., Chen L. (2018). Role of Hippo Signaling in Regulating Immunity. Cell Mol. Immunol. 15 (12), 1003–1009. 10.1038/s41423-018-0007-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu P. C., Yang C. T., Jablons D. M., You L. (2018). The Role of Yes-Associated Protein (YAP) in Regulating Programmed Death-Ligand 1 (PD-L1) in Thoracic Cancer. Biomedicines 6 (4), 114. 10.3390/biomedicines6040114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibrahim A. M., Wang Y. H. (2016). Viro-immune Therapy: A New Strategy for Treatment of Pancreatic Cancer. World J. Gastroenterol. 22 (2), 748–763. 10.3748/wjg.v22.i2.748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson H., Andersson R., Bauden M., Hammes S., Holdenrieder S., Ansari D. (2016). Immune Checkpoint Therapy for Pancreatic Cancer. World J. Gastroenterol. 22 (43), 9457–9476. 10.3748/wjg.v22.i43.9457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim M. H., Kim C. G., Kim S.-K., Shin S. J., Choe E. A., Park S.-H., et al. (2018). YAP-induced PD-L1 Expression Drives Immune Evasion in BRAFi-Resistant Melanoma. Cancer Immunol. Res. 6 (3), 255–266. 10.1158/2326-6066.cir-17-0320 [DOI] [PubMed] [Google Scholar]
- Kuenzi B. M., Ideker T. (2020). A Census of Pathway Maps in Cancer Systems Biology. Nat. Rev. Cancer 20 (4), 233–246. 10.1038/s41568-020-0240-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y., Lu T., Zhang C., Xu J., Xue Z., Busuttil R. W., et al. (2019). Activation of YAP Attenuates Hepatic Damage and Fibrosis in Liver Ischemia-Reperfusion Injury. J. Hepatol. 71 (4), 719–730. 10.1016/j.jhep.2019.05.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luedke E., Cristina Jaime-Ramirez A., Bhave N., Carson W. E., 3rd (2012). Monoclonal Antibody Therapy of Pancreatic Cancer with Cetuximab. J. Immunother. 35 (5), 367–373. 10.1097/cji.0b013e3182562d76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Y. C., Yang Z. S., Ma L. Q., Shu R., Zou C. G., Zhang K. Q. (2020). YAP in Epithelium Senses Gut Barrier Loss to Deploy Defenses against Pathogens. Plos Pathog. 16 (8), e1008766. 10.1371/journal.ppat.1008766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Bosch N., Vinaixa J., Navarro P. (2018). Immune Evasion in Pancreatic Cancer: From Mechanisms to Therapy. Cancers 10 (1), 6. 10.3390/cancers10010006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng K. P., Majedi F. S., Thauland T. J., Butte M. J. (2020). Mechanosensing through YAP Controls T Cell Activation and Metabolism. J. Exp. Med. 217 (8):e20200053. 10.1084/jem.20200053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miao J., Hsu P.-C., Yang Y.-L., Xu Z., Dai Y., Wang Y., et al. (2017). YAP Regulates PD-L1 Expression in Human NSCLC Cells. Oncotarget 8 (70), 114576–114587. 10.18632/oncotarget.23051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moroishi T., Hayashi T., Pan W.-W., Fujita Y., Holt M. V., Qin J., et al. (2016). The Hippo Pathway Kinases LATS1/2 Suppress Cancer Immunity. Cell 167 (6), 1525–1539. 10.1016/j.cell.2016.11.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen C. D. K., Yi C. (2019). YAP/TAZ Signaling and Resistance to Cancer Therapy. Trends Cancer 5 (5), 283–296. 10.1016/j.trecan.2019.02.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ni X., Tao J., Barbi J., Chen Q., Park B. V., Li Z., et al. (2018). YAP Is Essential for Treg-Mediated Suppression of Antitumor Immunity. Cancer Discov. 8 (8), 1026–1043. 10.1158/2159-8290.cd-17-1124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pobbati A. V., Hong W. (2020). A Combat with the YAP/TAZ-TEAD Oncoproteins for Cancer Therapy. Theranostics 10 (8), 3622–3635. 10.7150/thno.40889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma A., Yerra V. G., Kumar A. (2017). Emerging Role of Hippo Signalling in Pancreatic Biology: YAP Re-expression and Plausible Link to Islet Cell Apoptosis and Replication. Biochimie 133, 56–65. 10.1016/j.biochi.2016.12.009 [DOI] [PubMed] [Google Scholar]
- Shibata M., Ham K., Hoque M. O. (2018). A Time for YAP1: Tumorigenesis, Immunosuppression and Targeted Therapy. Int. J. Cancer 143 (9), 2133–2144. 10.1002/ijc.31561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taha Z., Janse van Rensburg H. J., Yang X. (2018). The Hippo Pathway: Immunity and Cancer. Cancers 10 (4), 94. 10.3390/cancers10040094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang G., Lu X., Dey P., Deng P., Wu C. C., Jiang S., et al. (2016). Targeting YAP-dependent MDSC Infiltration Impairs Tumor Progression. Cancer Discov. 6 (1), 80–95. 10.1158/2159-8290.cd-15-0224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S., Xie F., Chu F., Zhang Z., Yang B., Dai T., et al. (2017). YAP Antagonizes Innate Antiviral Immunity and Is Targeted for Lysosomal Degradation through IKKɛ-Mediated Phosphorylation. Nat. Immunol. 18 (7), 733–743. 10.1038/ni.3744 [DOI] [PubMed] [Google Scholar]
- Wang S., Zhou L., Ling L., Meng X., Chu F., Zhang S., et al. (2020). The Crosstalk between Hippo-YAP Pathway and Innate Immunity. Front. Immunol. 11, 323. 10.3389/fimmu.2020.00323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- White S. M., Murakami S., Yi C. (2019). The Complex Entanglement of Hippo-Yap/Taz Signaling in Tumor Immunity. Oncogene 38 (16), 2899–2909. 10.1038/s41388-018-0649-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamauchi T., Moroishi T. (2019). Hippo Pathway in Mammalian Adaptive Immune System. Cells 8 (5):398. 10.3390/cells8050398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang W., Yang S., Zhang F., Cheng F., Wang X., Rao J. (2020). Influence of the Hippo-YAP Signalling Pathway on Tumor Associated Macrophages (TAMs) and its Implications on Cancer Immunosuppressive Microenvironment. Ann. Transl Med. 8 (6), 399. 10.21037/atm.2020.02.11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao L., He J., Li B., Yan M., Wang H., Tan L., et al. (2019). Regulation of YAP by Mammalian Target of Rapamycin Complex 1 in Endothelial Cells Controls Blood Pressure through COX-2/mPGES-1/PGE 2 Cascade. Hypertension 74 (4), 936–946. 10.1161/hypertensionaha.119.12834 [DOI] [PubMed] [Google Scholar]
- Zanconato F., Cordenonsi M., Piccolo S. (2019). YAP and TAZ: a Signalling Hub of the Tumour Microenvironment. Nat. Rev. Cancer 19 (8), 454–464. 10.1038/s41568-019-0168-y [DOI] [PubMed] [Google Scholar]
- Zhang Q., Meng F., Chen S., Plouffe S. W., Wu S., Liu S., et al. (2017). Hippo Signalling Governs Cytosolic Nucleic Acid Sensing through YAP/TAZ-mediated TBK1 Blockade. Nat. Cell Biol. 19 (4), 362–374. 10.1038/ncb3496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q., Zhou R., Xu P. (2020). The Hippo Pathway in Innate Anti-microbial Immunity and Anti-tumor Immunity. Front. Immunol. 11, 1473. 10.3389/fimmu.2020.01473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Choi M. (2015). Immune Therapy in Pancreatic Cancer: Now and the Future? Rev. Recent Clin. Trials 10 (4), 317–325. 10.2174/1574887110666150916142537 [DOI] [PubMed] [Google Scholar]
- Zhang Y., Zhang H., Zhao B. (2018). Hippo Signaling in the Immune System. Trends Biochem. Sci. 43 (2), 77–80. 10.1016/j.tibs.2017.11.009 [DOI] [PubMed] [Google Scholar]
- Zhou Y., Huang T., Zhang J., Cheng A. S. L., Yu J., Kang W., et al. (2018). Emerging Roles of Hippo Signaling in Inflammation and YAP-Driven Tumor Immunity. Cancer Lett. 426, 73–79. 10.1016/j.canlet.2018.04.004 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.