Abstract
A 79-year-old woman with a newly detected oval circumscribed lump in subcutaneous location on mammography and ultrasound turned out to be a Schwannoma after ultrasound-guided core needle biopsy. A 72-year-old woman with breast cancer in medical history demonstrated a new axillary mass in follow up, initially regarded as a lymph node metastasis. Core needle biopsy did not lead to a sufficient diagnosis. Pathologic examination after intraoperative sampling revealed a Schwannoma. These 2 case reports illustrate the importance of diagnostic imaging and remind to include Schwannomas in the differential diagnosis of breast and axillary masses.
Keywords: Breast, Axilla, Schwannoma, Mammography, MRI, Ultrasound
Introduction
Schwannomas are benign, slow growing tumors of the peripheral nervous system. They arise from Schwann cells, which are forming the myelin sheath of the neuronal axons [1]. They are more frequent in patients with neurofibromatosis but also occur as independent solitary tumors with the most common locations at the head, neck, and extremities [2–6]. Only 5% are located in the axillary region and intramammary schwannomas account for only 2.6% of all Schwannomas [2,3,7–9]. Here, we present a case of a breast Schwannoma and a Schwannoma in the axillary region, mimicking a lymph node metastasis.
First case
A 79-year-old woman reported about a newly detected painless lump in the left breast a few weeks ago. Besides oophorectomy with a benign result many years ago patient's history was inconspicuous regarding breast and ovarian cancer.
In the clinical examination the lump presented small and slightly movable under the skin near the nipple in the left upper outer quadrant with discrete abutment of the skin. There were no abnormalities of the axillary lymph nodes and in the right breast.
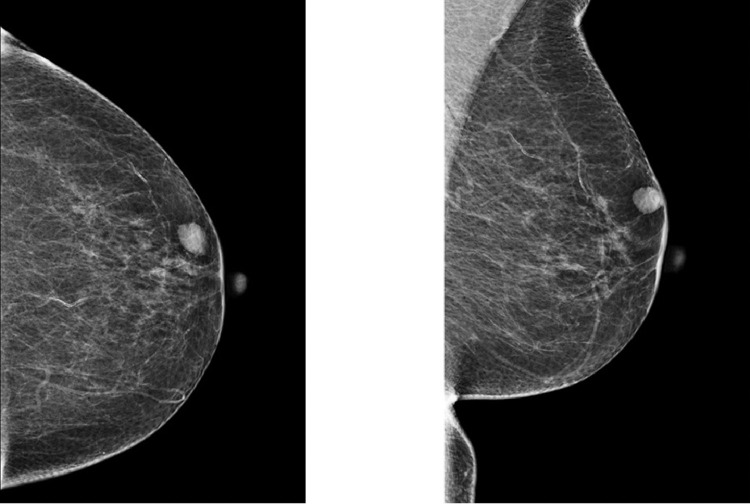
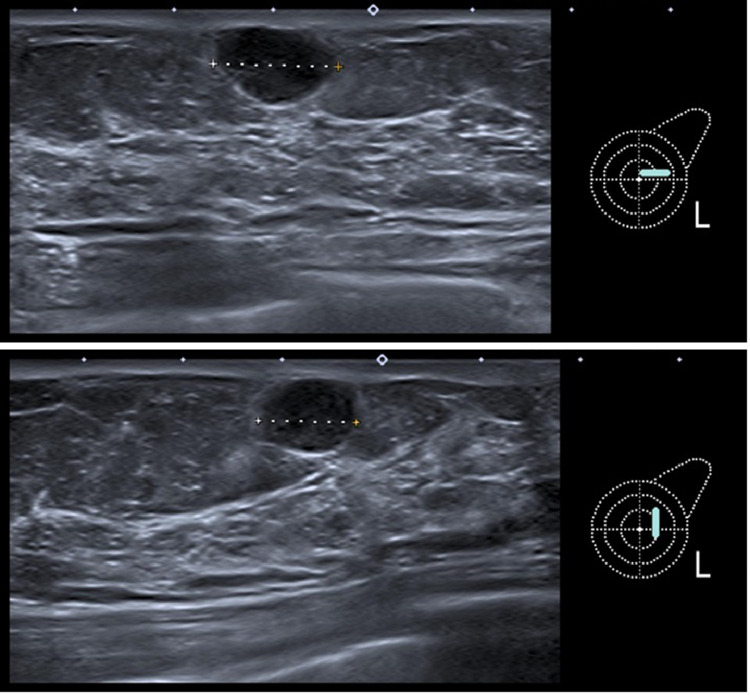
A mammography showed an almost entirely fatty breast, Breast Imaging-Reporting and Data System (BI-RADS) density category A. In location of the palpable lump in the left upper outer quadrant at 2-o'clock position and in 1.6 cm distance from the nipple an oval, circumscribed, equal density, subcutaneous mass without microcalcifications, measuring 13 mm was seen. The remaining mammography was normal. High frequency sonography demonstrated a 10 × 14 mm oval, circumscribed, hypoechoic mass in parallel orientation, without posterior features and in subcutaneous location.
Based on mammography and sonography the mass was classified as BI-RADS 4 from an outside facility. Axillary lymph nodes were normal. The patient was referred to our breast center for ultrasound-guided core needle biopsy.
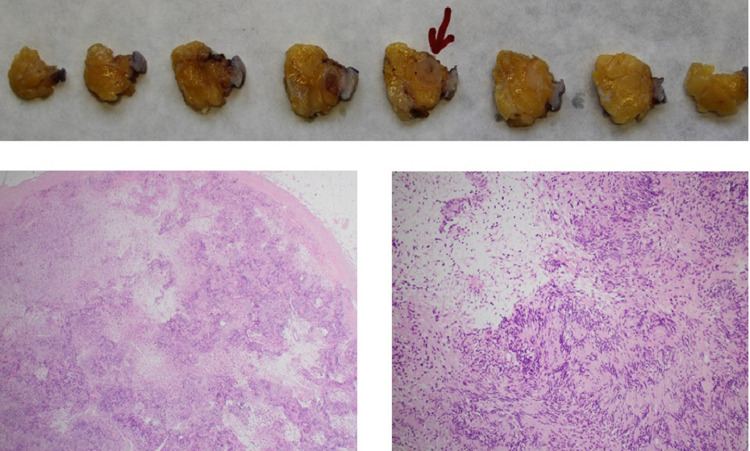
The pathologic examination revealed a spindle cell proliferation arranged in dense (Antoni A tissue) and loosened areas (Antoni B tissue). The mitotic activity was low. The nuclei showed only mild variability in size and shape. The tumor cells exhibited a strong expression of S100. This expression profile as well as lack of atypia or relevant necrosis resulted in the diagnosis of a Schwannoma. A following surgical excision showed a totally removed, encapsulated mass, and above-mentioned pathology was confirmed.
Second case
A 72-year-old woman underwent breast-conserving surgery of the left breast in 2016 because of a triple negative breast carcinoma. A sentinel node removal and adjuvant radiation was refused by the patient at that time. After 4 years of follow-up, new suspicious microcalcifications were found in the left breast and a ductal Carcinoma in situ (DCIS; G3) was detected by vacuum-assisted biopsy in our breast center. Additionally, a superficially located mass in the left axillary region was detected in the physical examination, noted by the patient just a few weeks earlier. Neurologic symptoms or pain were not reported.
Sonography demonstrated a 13 mm nearly round, circumscribed, hypoechoic mass without posterior features, located subcutaneously next to the left axillary artery in the slim patient.
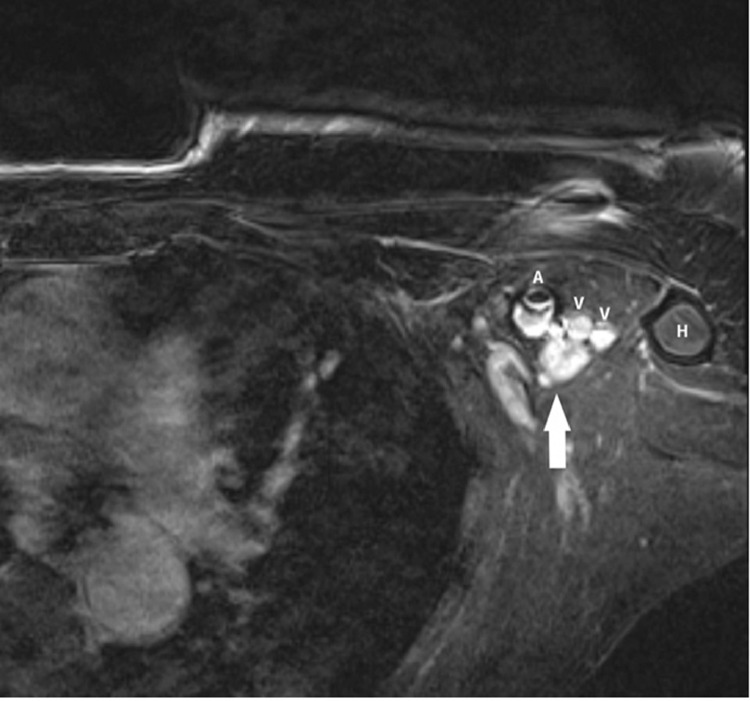
An additional MRI for exclusion of an invasive recurrence in dense breast tissue showed a 11 × 12 mm nearly round, circumscribed mass in the upper left axilla next to the proximal humerus with close contact to the left axillary artery, low signal in T1, a mildly heterogeneous hyperintense signal in T2 and with strong homogeneous contrast enhancement. The mass was assessed as a lymph node metastasis of a cancer of unknown primary. Additional abnormal axillary lymph nodes were not found. An invasive recurrence was excluded.
For clarification of a potential lymph node metastasis an ultrasound-guided biopsy was added with a 14-gauge core needle after local anesthesia. Two specimens were obtained using an automated firing device. During biopsy the patient reported radiating pain in the ipsilateral hand which was self-limiting at the end of the consultation. Bleeding did not occur.
The pathologic examination revealed mature fat tissue including blood vessels with focal scar tissue and chronic inflammation. Lymph node tissues was not described.
Because of the DCIS a breast-conserving surgery with segment resection was performed. Intraoperatively, the mass in the left axilla was very movable and well-circumscripted, localized by palpation. Close contact to the axillar nerve was confirmed and preparation provoked reflex-like movements of the ipsilateral arm and hand. Therefore, sampling was added instead of full excision. Frozen section excluded malignancy.
The final pathologic examination showed a spindle cell proliferation with strong expression of S100, low mitotic activity and nuclei with only mild variability. Areas with nuclear palisading (Verocay bodies) were visible and a Schwannoma was diagnosed.
Discussion
Schwannomas are benign tumors of the peripheral nervous system which arise from Schwann cells and form the myelin sheath of the neuronal axons [1]. Only 2.6% of all Schwannomas are located intramammary and 5% in the axillary region [2,3,[7], [8]–9]. Histologically they demonstrate spindle cells which are arranged in so called Antoni A and B tissue, areas with nuclear palisading (Verocay bodies) and strong expression of S100 [10,11].
In sonography both presented Schwannomas showed typical benign imaging criteria, i.e. round or oval configuration, circumscribed margins, parallel orientation and a homogeneous echo pattern [1,2,4,5,11]. However, Schwannomas with indistinct margins or cystic components have been published and differentiation against malignant lesions or a phyllodes tumor may then be difficult [1,5,6,[11], [12]–13]. We did not observe the frequently reported hyperechoic capsule or spot, representing collagen fibers [14] (Fig. 1, Fig. 2, Fig. 3, Fig. 4. Fig. 5).
Fig. 1.
Left mammography in craniocaudal and mediolateral oblique view shows an almost entirely fatty breast. An oval, circumscribed, equal density, subcutaneous mass without microcalcifications, measuring 13 mm is seen at 2-o'clock position and in 1-6 cm distance from the nipple, corresponding to the lump.
Fig. 2.
Two plane sonography with a 14 MHz ultrasound head demonstrating a 10 × 14 mm oval, circumscribed, hypoechoic mass in parallel orientation, without posterior features and in subcutaneous location, corresponding to the lump.
Fig. 3.
Pathologic examination showing a totally removed encapsulated mass with spindle cell proliferation arranged in dense (Antoni A tissue) and loosened areas (Antoni B tissue). Areas with nuclear palisading are visible (Verocay bodies). The nuclei show only mild variability in size and shape. No atypia or relevant necrosis are seen.
Fig. 4.
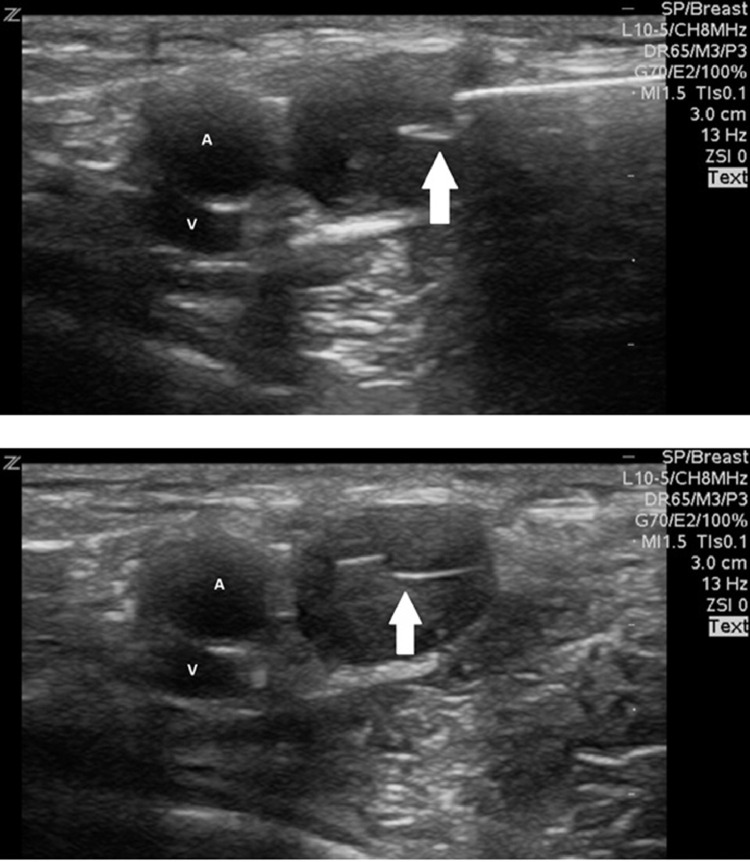
1,5T MRI: T1 with fat suppression after IV contrast injection showing a 11 × 12 mm oval, circumscribed mass (arrow) with strong homogeneous enhancement in the immediate posterior vicinity of the left axillary artery (A) and vein (V), medial of the proximal humerus (H).
Fig. 5.
Ultrasound-guided biopsy with a 14-gauge core needle demonstrating the central position of the core needle (arrow) in the target mass next to the axillary artery (A) and vein (V).
MRI revealed a low signal in T1, a discrete heterogeneous hyperintense signal in T2 and a strong and homogeneous contrast enhancement, typical for a Schwannoma, although, occasionally also observed in some malignant lymph nodes. We did not observe a central low and peripheral high T2 signal (target-sign) or small, central T2 hypointense foci (fascicular sign) [15].
An intramammary subcutaneous location of Schwannomas is common among the reported cases in literature and can lead to the suspicion of a mesenchymal tumor [2,4,10]. However, we followed the external recommendation of an ultrasound-guided core needle biopsy and in spite of a typical histology with lack of atypia a surgical excision was recommended, following the patients wish.
The location and the peripheral nerve stimulation during manipulation could have led to the differential diagnosis of an axillary Schwannoma, especially as a singular axillary lymph node metastasis in the presence of breast cancer is usually initially seen in the inferior lymph node group near the breast tail (level I). Cases of axillary Schwannomas imitating lymphadenopathy have been published, impeding the correct diagnosis, particularly if the patient has a simultaneous breast cancer or another malignancy potentially spreading into axillary lymph nodes, as for example a malignant melanoma [7–9,14,16].
A biopsy close to the vessel-nerve cord bears a higher risk of complications like hemorrhage and nerve injury. In our case, we had no major complications. Despite local anesthetic infiltration to the margin of the mass we observed peripheral nerve stimulation causing bearable pain which led us to interrupt the biopsy. Radiating pain during ultrasound-guided sampling of axillary Schwannomas is described elsewhere [14]. As axillary Schwannomas are part of the brachial plexus, a sufficient local anesthesia is difficult and radiating pain can be expected.
Due to insufficient representative stromal tissue in specimens diagnostic accuracy of core needle biopsy of extremity soft tissue masses is low (45.6%), especially in spindle cell tumors [17,18]. To improve diagnostic accuracy several specimens should be obtained from the target mass, which can be challenging for small masses in a “high risk” location. Sometimes even open biopsy or full extirpation is recommended [7,13–15,17]. Despite the central core needle position in the mass of our second case histology revealed scar tissue only and we could not determine the Schwannoma at the first histopathologic examination although the location was non–operated and non–inflamed. Therefore, intraoperative sampling was required for establishing the final diagnosis.
In summary, breast, and axillary Schwannomas are absolutely rare, but should be considered in the diagnostic assessment of breast and axillary soft tissue masses.
Patient consent
Patient's written informed consent is present.
Footnotes
Competing Interests: None.
References
- 1.Das Gupta TK, Brasfield RD, Strong EW, Hajdu SI. Benign solitary Schwannomas (neurilemomas) Cancer. 1969;24(2):355–366. doi: 10.1002/1097-0142(196908)24:2<355::aid-cncr2820240218>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 2.Uchida N, Yokoo H, Kuwano H. Schwannoma of the breast: report of a case. Surg Today. 2005;35(3):238–242. doi: 10.1007/s00595-004-2904-4. [DOI] [PubMed] [Google Scholar]
- 3.Dialani V, Hines N, Wang Y, Slanetz P. Breast schwannoma. Case Rep Med. 2011;2011 doi: 10.1155/2011/930841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Takaaki F, Reina Y, Hiroki M, Soichi T, Takayuki A, Hiroyuki K. A Rare Case of Anterior Chest Wall Schwannoma Masquerading as a Breast Tumor. Int Surg. 2014;99(3):196–199. doi: 10.9738/INTSURG-D-13-00145.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Qing TT, Esther WLC, Sung HC, Ga SH. Schwannoma: an unexpected diagnosis from a breast lump. J Surg Case Rep. 2014;2014(9):rju085. [Google Scholar]
- 6.Sordillo PP, Helson L, Hajdu SI, Magill GB, Kosloff C, Golbey RB. Malignant Schwannoma—clinical characteristics, survival, and response to therapy. Cancer. 1981;47:2503–2509. doi: 10.1002/1097-0142(19810515)47:10<2503::aid-cncr2820471033>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 7.Hager A, Georges AA. Axillary schwannoma, preoperative diagnosis on a tru-cut biopsy: Case report and literature review. Int J Surg Case Rep. 2018;52:49–53. doi: 10.1016/j.ijscr.2018.09.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Huang JH, Samadani U, Zager EL. Brachial plexus region tumors: a review of their history, classification, surgical management and outcomes. Neurosurg. Q. 2003;13:151–161. [Google Scholar]
- 9.Gosk J, Gutkowska O, Urban M, Wnukiewicz W, Reichert P, Ziółkowski P. Results of surgical treatment of schwannomas arising from extremities. Biomed Res. Int. 2015;2015:547926. doi: 10.1155/2015/547926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yasha P, Kush JS, Samir JP, Diane H. Intramammary schwannoma: a palpable breast mass. Radiol Case Rep. 2016;11(3):129–133. doi: 10.1016/j.radcr.2016.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bellezza G, Lombardi T, Panzarola P, Cavaliere A, Giansanti M. Schwannoma of the breast: a case report and review of the literature. Tumori. 2007;93:308–311. [PubMed] [Google Scholar]
- 12.Sordillo PP, Helson L, Hajdu SI, Magill GB, Kosloff C, Golbey RB. Malignant Schwannoma—clinical characteristics, survival, and response to therapy. Cancer. 1981;47:2503–2509. doi: 10.1002/1097-0142(19810515)47:10<2503::aid-cncr2820471033>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 13.Gultekin SH, Cody HS, Hoda SA. Schwannoma of the breast. Southern Medical Journal. 1996;89(2):238–239. doi: 10.1097/00007611-199602000-00018. [DOI] [PubMed] [Google Scholar]
- 14.Lynch A, Peters G. Brachial plexus tumor simulating an axillary metastasis from breast carcinoma. Radiol Case Rep. 2012;7(3):712. doi: 10.2484/rcr.v7i3.712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Harnsberger HR, Glastonbury CM, Michel MA, Koch BL, et al. Diagnostic Imaging Head and Neck, second Edition. 2012;198(1):17[SP]:AU: Please provide at least 6 author names in reference 15..
- 16.Sengul D, Sengul I, Karinoglu U, Apan OC, Oksuz H, Apan A. A rare case of axillary Schwannoma. Ann Ital Chir. 2019;8:S2239253. [PubMed] [Google Scholar]
- 17.Kasraeian S., Allison D.C., Ahlmann E.R., Fedenko A.N., Menendez L.R. A comparison of fine-needle aspiration, core biopsy, and surgical biopsy in the diagnosis of extremity soft tissue masses. Clin. Orthop. Relat. Res. 2010;468:2992–3002. doi: 10.1007/s11999-010-1401-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Powers C, Berardo M, Frable W. Fine needle aspiration biopsy: pitfalls in the diagnosis of spindle-cell lesions. Diagn Cytopathol. 1994;10:232–242. doi: 10.1002/dc.2840100309. [DOI] [PubMed] [Google Scholar]