Highlights
-
•
Sertoli-Leydig cell tumor and gynandroblastoma are rare ovarian sex cord-stromal tumors that warrant germline DICER1 testing.
-
•
DICER1 mutations are associated with an increased risk of various benign and malignant tumors especially during childhood.
-
•
Childhood genetic testing is controversial but provides earlier screening and diagnosis in DICER1 mutation patients.
Keywords: DICER1 mutation, Gynandroblastoma, Sex cord-stromal tumor
Abstract
Sex cord-stromal tumors (SCSTs) are ovarian tumors that generally present with an adnexal mass and signs/symptoms of hormone excess. Gynandroblastoma is a rare subtype of SCST with a combination of female and male sex cord differentiation. These tumors typically present in premenopausal women and are diagnosed at early stages with benign clinical courses. Here, we present a rare case of recurrent gynandroblastoma in a premenopausal woman with a DICER1 germline mutation. The patient was referred to our clinic for new symptoms of hormonal imbalance with a history of ovarian juvenile granulosa cell tumor (JGCT). Evaluation revealed a 5x5cm complex right adnexal mass and rising inhibin B. Patient underwent total abdominal hysterectomy with right salpingo-oophorectomy, omentectomy and right pelvic and para-aortic lymphadenectomy. Pathology showed a right ovarian gynandroblastoma. Somatic biallelic mutations in the RNase IIIb domain of DICER1 were identified; a 23-gene germline panel confirmed a germline DICER1 pathogenic variant. Cascade testing of her children documented that both daughters inherited the pathogenic variant. Testing for DICER1 mutations has important implications for individual and familial tumor risk assessment given what we know about DICER1 mutation and increased childhood cancer risk.
1. Introduction
Sex cord-stromal ovarian tumors are rare, and malignant SCSTs compose about 8% of ovarian malignancies. SCSTs generally present with an adnexal mass and signs or symptoms of estrogen or androgen excess as these tumors produce steroid hormones. Patients can present with abnormal uterine bleeding, endometrial hyperplasia, infertility, precocious puberty, hirsutism, acne, alopecia, clitoromegaly, or voice deepening (Schultz et al., 2016). SCSTs arise from ovarian stromal cells including granulosa cells, theca cells, sertoli cells, leydig cells and fibroblasts. Gynandroblastoma is a rare subtype of SCST with a combination of female and male sex cord differentiation.
ATK1, FOXL2, and DICER1 gene mutations have been associated with SCSTs (Rosario et al., 2014, Auguste et al., 2015). DICER1 mutations have been associated with Sertoli-Leydig cell tumors (SLCTs) and gynandroblastomas (Heravi-Moussavi et al., 2012 Jan 19, Wang et al., 2018 Aug). In addition to ovarian SCSTs, DICER1 pathogenic variants are also associated with an increased risk of benign and malignant lung, kidney, thyroid, and CNS tumors (Schultz et al., 2018). We present a case of ovarian gynandroblastoma which led to a germline DICER1 mutation diagnosis.
2. Case report
A 24yo G2P2 with history of JGCT of the left ovary was referred to our clinic for new onset hormonal dysfunction. At the age of 15, she was diagnosed with JGCT following a laparoscopic ovarian cystectomy complicated by intraoperative cyst rupture with her local gynecologist. She was referred to gynecologic oncology and underwent left salpingo-oophorectomy, partial bladder resection, and excision of metastatic disease from the abdominal wall peritoneum for Stage IIC JGCT. She then completed 4 cycles of adjuvant chemotherapy with bleomycin, etoposide and cisplatin. She has since recovered from treatment, delivered two children, and had her remaining right fallopian tube removed for sterilization. There are no known genetic syndromes in the patient’s family history but the patient reports her paternal uncles and aunts were diagnosed with testicular cancer, throat cancer and a brain tumor and her paternal grandfather was diagnosed with prostate cancer. She represented to the gynecology oncology clinic with worsening hirsutism, elevated inhibin B, and new onset of irregular menses. Inhibin B levels in remission were stable at < 10, however, it recently increased to 12 and 89. CT scan of the chest, abdomen and pelvis showed a 5x5cm septated, multiloculated right adnexal mass. The patient underwent total abdominal hysterectomy with right salpingo-oophorectomy, omentectomy and right pelvic and para-aortic lymphadenectomy. Operative findings were significant for a cystic mass arising from the right ovary that was ruptured during exploration and right common and lower para-aortic lymphadenopathy. Pathology confirmed recurrent gynandroblastoma of the right ovary and submitted lymph node and omental specimens were without disease.
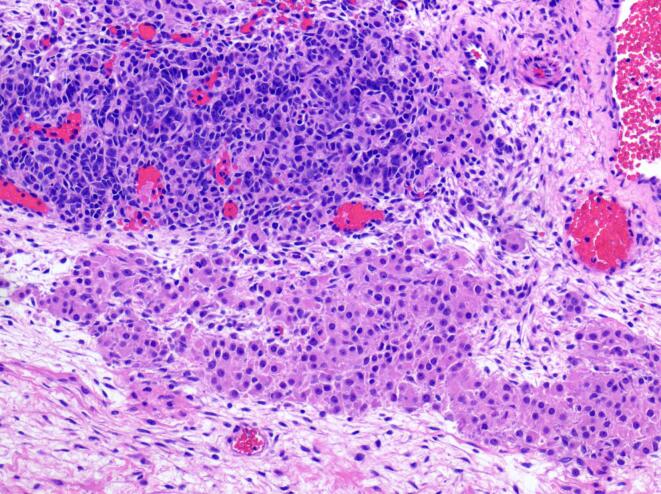
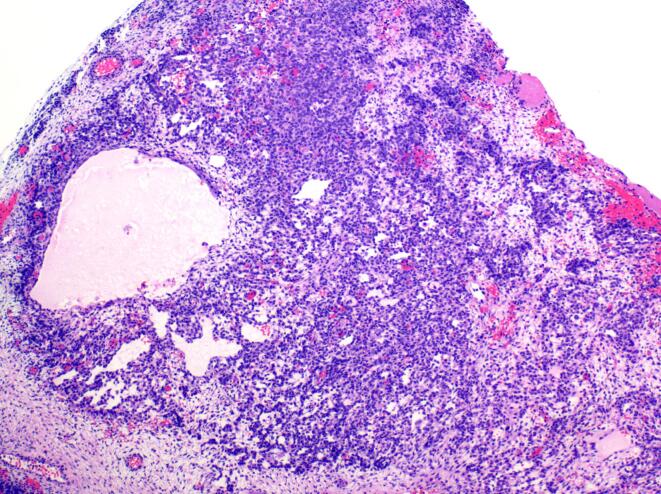
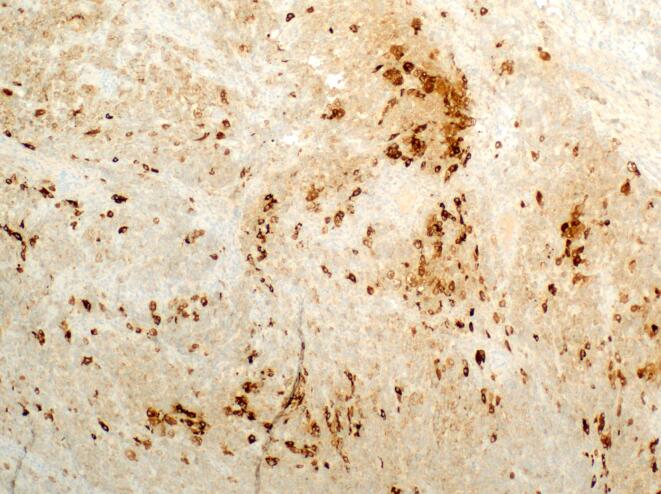
Portions of the tumor were composed of vague cords and nodules of tightly packed Sertoli cells admixed with large, eosinophilic leydig cells, consistent with intermediately differentiated SLCT (Fig. 1). In addition, there were areas of loosely cohesive cells forming variably sized cysts consistent with JGCT (Fig. 2). The tumor was positive for inhibin and calretinin, while reticulin staining was lost in the variably cystic areas histologically consistent with JGCT (Fig. 3). Review of the pathology slides from her surgery in 2012 revealed similar findings suggesting the original tumor was actually a gynandroblastoma.
Fig. 1.
Sertoli-Leydig cell tumor component: The top half of the image depicts cords of deep purple sertoli cells. A large nest of leydig cells is present in the bottom half of the image. (10x).
Fig. 2.
Juvenile granulosa cell tumor component: Loosely cohesive granulosa cells forming microcysts comprised the juvenile granulosa cell tumor component of this neoplasm. (4x).
Fig. 3.
Inhibin staining of gynandroblastoma: Immunohistochemical stain for inhibin demonstrating weak to strong cytoplasmic positivity in the tumor cells, supporting the designation of a sex-cord stromal neoplasm. (4x).
Given gynandroblastoma diagnosis and now recurrence, DICER1 mutation testing was performed on the tumor. Biallelic loss of function and missense mutations in the RNase IIIb domain of DICER1 were noted. After genetic counseling, germline testing with a 23-gene panel was performed. Results showed DICER1 gene mutation (p.E830; c.2488G > T) and an NBN variant of unknown significance (p.N256S). This missense mutation results from a G to T substitution in coding exon 15, which changes the amino acid to a stop codon and causes loss of protein function. Given these results, the patient’s two children were referred for pediatric genetic counseling. Germline testing was performed, revealing that both children carry the DICER1 mutation.
3. Discussion
This case of recurrent gynandroblastoma led to identification of a germline DICER1 mutation. To our knowledge, twenty-eight cases of gynandroblastoma have been described in the literature to date with the first case in the 1930s (Meyer, 1931). Recurrent disease is rarer still and the first case was reported in 2007 (Chivukula et al., 2007). Most of these patients had stage 1 disease and were treated with surgical resection. The disease course of gynandroblastoma is uncertain due to its low incidence. Typically, due to symptomatology, these tumors are discovered at early stages and have a clinically benign course, but prognosis is dependent on stage. Initial work-up starts with evaluation of tumor markers (inhibin, estradiol, testosterone and AFP) and pelvic ultrasound. Cross sectional imaging with CT or MRI is typically required. In children and adolescents, fertility sparing surgery with unilateral adnexectomy is preferred if disease is limited to one side, as was done in our patient’s initial presentation. Perimenopausal women and women with undesired future fertility should undergo total hysterectomy and bilateral salpingo-oophorectomy with staging (Schultz et al., 2016).
Recent studies have identified several involved genes in SCSTs. FOXL2 somatic mutation is a well-known cause of adult granulosa cell tumors, (Rosario et al., 2014) and AKT1 mutations have been reported in the majority of JGCTs (Auguste et al., 2015). Somatic or germline mutations in DICER1 have been shown in SLCTs and gynandroblastomas. In a 2011 study, DICER1 mutations in the RNase IIIb domain were found in 29% of nonepithelial ovarian tumors, predominantly in SLCTs (60%), including four tumors with additional germline DICER1 mutations (Heravi-Moussavi et al., 2012 Jan 19). Gynandroblastoma typically consists of adult-type granulosa cells, but seven cases of gynandroblastoma with JGCT have been described in the literature to date. In a 2018 report, three gynandroblastoma cases had a DICER1 mutation, and like our case, all three of these cases had tumors composed of moderately/poorly differentiated SLCT and JGCT (Wang et al., 2018 Aug). The International Ovarian and Testicular Stromal Tumor (OTST) Registry reports the median age of diagnosis for DICER1 ovarian tumors is 16.9 years old. The DICER1 gene is involved with microRNA processing. A mutation results in growth regulation impairment. DICER1 mutations are inherited in an autosomal dominant pattern; therefore, children have a 50% chance of inheritance from an affected parent. DICER1 mutations are associated with an increased risk of pituitary blastoma, thyroid cancer, pleuropulmonary blastoma, cystic nephroma, embryonal rhabdomyosarcoma, ovarian SCSTs, and gastrointestinal and ophthalmologic tumors. DICER1 syndrome was previously known as familial pleuropulmonary blastoma syndrome. Pleuropulmonary blastoma is the most common primary lung malignancy in children. It is a progressive cystic disease that gains higher grade and anaplastic features as it advances (Schultz et al., 2018). Therefore, early detection of a DICER1 mutation is imperative for screening and overall survival of these patients.
At the inaugural International DICER1 Symposium in 2016, consensus guidelines were created based on tumor database analysis, review of the literature, and expert opinion. The guidelines included indications for genetic counseling, genetic testing and screening recommendations for individuals with DICER1 pathogenic variants. Based on these recommendations, the diagnosis of ovarian SLCT or gynandroblastoma are indications for DICER1 germline testing (Schultz et al., 2018). DICER1 mutations are one of the few clinical scenarios where childhood genetic testing is recommended, as the majority of related conditions occur at a young age. Genetic testing in children is controversial, but patient and parent education is key to making this decision. This includes discussion of signs and symptoms of the most common and most severe sequelae of DICER1 pathogenic variants. Recommended screening for DICER1 mutation carriers starts as early as birth with intensive screening in the early childhood for some conditions. Female reproductive tract screening is recommended at age 8–10 years with an abdominal and pelvic ultrasound every 6–12 months until age 40 years. Screening is also recommended in patients with a 50% chance of DICER1 germline pathogenic variant based on family history who decline genetic testing (Schultz et al., 2018). These recommendations are based on expert opinion. Physicians can also counsel patients on signs and symptoms of hormonal excess such as hirsutism, virilization, and menstrual abnormalities (Bailey et al., 2019). Attention to symptomatology led to diagnosis in our case.
In conclusion, gynandroblastoma is a rare hormonally active ovarian SCST. Typically these tumors present in premenopausal women, are diagnosed at early stages and have benign clinical courses. Here, we present a unique case of recurrent gynandroblastoma in a premenopausal woman with a germline DICER1 mutation that has been inherited by her children. Genetic counseling and testing should be offered to all patients diagnosed with SLCT and gynandroblastoma of the ovary. Early identification of this mutation allows for screening and earlier diagnosis of the various tumors that can occur in pathogenic variant carriers (Schultz et al., 2018). More research is needed to better understand the pathogenesis and clinical course of gynandroblastomas. Future studies should focus on understanding the clinical implications of DICER1 pathogenic variants to help reduce morbidity and mortality associated with this condition.
4. Consent
Informed consent to publish this information was obtained from the patient.
CRediT authorship contribution statement
Ann Marie Mercier: Conceptualization, Data curation, Writing - original draft, Writing - review & editing. Kristin K. Zorn: Data curation, Writing - review & editing. Charles M. Quick: Data curation, Writing - review & editing. Laura B. Huffman: Conceptualization, Data curation, Writing - review & editing.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Schultz K.A., Harris A.K., Schneider D.T. Ovarian Sex Cord-Stromal Tumors. J Oncol Pract. 2016;12(10):940–946. doi: 10.1200/JOP.2016.016261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosario R., Cohen P.A., Shelling A.N. The role of FOXL2 in the pathogenesis of adult ovarian granulosa cell tumors. Gynecol Oncol. 2014;133:382. doi: 10.1016/j.ygyno.2013.12.012. 382-287. [DOI] [PubMed] [Google Scholar]
- Auguste A., Bessière L., Todeschini A.L., Caburet S., Sarnacki S., Prat J., D'angelo E., DeLaGrange P., Ariste O., Lemoine F., Legois B., Sultan C., Zider A., Galmiche L., Kalfa N., Veitia R.A. Molecular analyses of juvenile granulosa cell tumors bearing AKT1 mutations provide insights into tumor biology and therapeutic leads. Hum Mol Genet. 2015;24(23):6687–6698. doi: 10.1093/hmg/ddv373. Epub 2015 Sep 11. PMID: 26362254. [DOI] [PubMed] [Google Scholar]
- Heravi-Moussavi A., Anglesio M.S., Cheng S.W., Senz J., Yang W., Prentice L., Fejes A.P., Chow C., Tone A., Kalloger S.E., Hamel N., Roth A., Ha G., Wan A.N., Maines-Bandiera S., Salamanca C., Pasini B., Clarke B.A., Lee A.F., Lee C.H., Zhao C., Young R.H., Aparicio S.A., Sorensen P.H., Woo M.M., Boyd N., Jones S.J., Hirst M., Marra M.A., Gilks B., Shah S.P., Foulkes W.D., Morin G.B., Huntsman D.G. Recurrent somatic DICER1 mutations in nonepithelial ovarian cancers. N Engl J Med. 2012 Jan 19;366(3):234–242. doi: 10.1056/NEJMoa1102903. Epub 2011 Dec 21 PMID: 22187960. [DOI] [PubMed] [Google Scholar]
- Wang Y., Karnezis A.N., Magrill J., Tessier-Cloutier B., Lum A., Senz J., Gilks C.B., McCluggage W.G., Huntsman D.G., Kommoss F. DICER1 hot-spot mutations in ovarian gynandroblastoma. Histopathology. 2018 Aug;73(2):306–313. doi: 10.1111/his.13630. Epub 2018 Jun 5 PMID: 29660837. [DOI] [PubMed] [Google Scholar]
- Schultz K.A.P., Williams G.M., Kamihara J., Stewart D.R., Harris A.K., Bauer A.J., Turner J., Shah R., Schneider K., Schneider K.W., Carr A.G., Harney L.A., Baldinger S., Frazier A.L., Orbach D., Schneider D.T., Malkin D., Dehner L.P., Messinger Y.H., Hill D.A. DICER1 and Associated Conditions: Identification of At-risk Individuals and Recommended Surveillance Strategies. Clin Cancer Res. 2018;24(10):2251–2261. doi: 10.1158/1078-0432.CCR-17-3089. Epub 2018 Jan 17. PMID: 29343557; PMCID: PMC6260592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer R. The pathology of some special ovarian tumors and their relation to sex characteristics. Am. J. Obstet. Gynecol. 1931;22:697. [Google Scholar]
- Chivukula M., Hunt J., Carter G., Kelley J., Patel M., Kanbour- S.A. Recurrent gynandroblastoma of ovary – a case report: a molecular and immunohistochemical analysis. Int. J. Gynecol. Pathol. 2007;26:30–33. doi: 10.1097/01.pgp.0000225387.48868.39. [DOI] [PubMed] [Google Scholar]
- Bailey K., Jacobs M., Anderson B., Rabah R., Wu Y., Else T., Mody R. DICER1 Mutations in the Era of Expanding Integrative Clinical Sequencing in Pediatric Oncology. JCO Precision Oncology. 2019;3:1–8. doi: 10.1200/PO.18.00172. [DOI] [PMC free article] [PubMed] [Google Scholar]