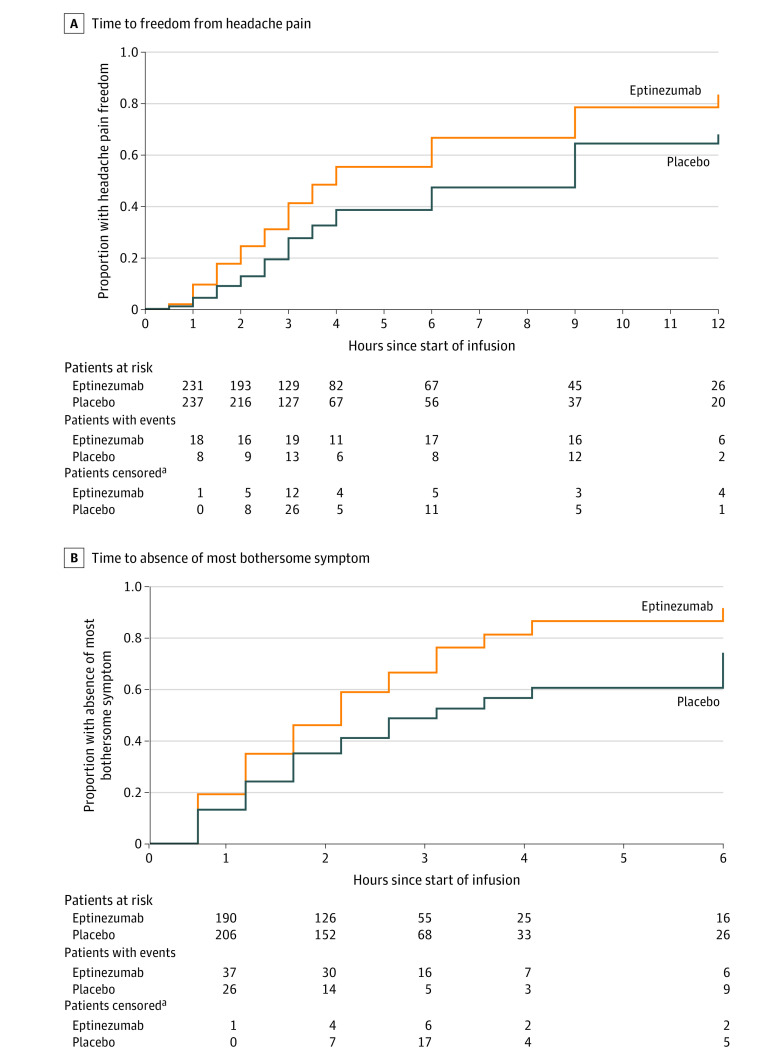
Figure 2. Co-primary End Points: Time to Headache Pain Freedom and Absence of Most Bothersome Symptom in the Full Analysis Set.
For headache pain freedom, the median observation time was 2 hours (interquartile range [IQR], 1-2.5 hours) for the eptinezumab group and 2.5 hours (IQR, 1-3 hours) for the placebo group. For absence of most bothersome symptom, the median observation time was 3 hours (IQR, 2-6 hours) for the eptinezumab group and 3 hours (IQR, 2.5-4 hours) for the placebo group. Median times to headache pain freedom were 4.0 (interquartile range [IQR], 2.5-12.0) hours in the eptinezumab group and 9.0 (IQR, 3.0-48.0) hours in the placebo group; median times to absence of most bothersome symptom were 2.0 (IQR, 1.0-3.5) hours and 3.0 (IQR, 1.5-12.0) hours, respectively.
aAll censoring of patients was due to rescue medication use.