Abstract
The scenario in which a patient tests positive for human chorionic gonadotropin (hCG) in the absence of pregnancy can pose a diagnostic dilemma for clinicians. The term “phantom hCG” refers to persistently positive hCG levels on diagnostic testing in a nonpregnant patient and such results often lead to a false diagnosis of malignancy and subsequent inappropriate treatment with chemotherapy or hysterectomy. There remains a need for a consistent and rational diagnostic approach to the “phantom hCG.” This article aims to review the different etiologies of positive serum hCG testing in nonpregnant subjects and concludes with a practical, stepwise diagnostic approach to assist clinicians encountering this clinical dilemma.
Keywords: false-positive pregnancy test, gestational trophoblastic disease, heterophile antibody, human chorionic gonadotropin, hyperglycosylated hCG, phantom hCG, pituitary hCG, pregnancy test
Introduction
Human chorionic gonadotropin (hCG) assays have been a staple in both clinical and at-home pregnancy tests since the launch of the first at-home pregnancy tests in the 1970s. 1 hCG is a dimeric glycoprotein hormone comprised of two glycosylated subunits (alpha and beta) that are connected by monovalent hydrophobic and ionic interactions (Figure 1). There are several other dimeric pituitary hormones that share essentially identical alpha-subunits, specifically follicle stimulating hormone (FSH), luteinizing hormone (LH), and thyroid stimulating hormone (TSH).2,3 The unique beta-subunit of hCG is still over 80% homologous to that of the LH beta-subunit. 4 These characteristics are particularly important when considering diagnostic test sensitivity and more importantly, specificity, and the possibility of reagent cross-reactivity and resultant false-positive hormone testing.
Figure 1.
Gonadotropin molecules. Heterodimeric follicle stimulating hormone (FSH), luteinizing hormone (LH), and human chorionic gonadotropin (hCG) share a common alpha-subunit. LH and hCG have very highly homologous beta-subunits, although hCGβ has a unique C-terminal peptide (left; purple tail). Antigenic epitopes in this unique tail are commonly targeted by antibodies used in serum hCG assays. Gray tail-like structures represent glycosylation sites.
Human chorionic gonadotropin plays a vital role during both the establishment and the maintenance of pregnancy.3,4 The human placenta is hemochorial, meaning maternal blood comes into direct contact with fetally derived cells. The placenta is comprised of several types of cells that are all grouped under the term “trophoblast.” The first placental component to arise from the trophectoderm surrounding the human blastocyst is a syncytialized (fused) but invasive trophoblast subtype that has been called the primitive trophoblast (Figure 2).5,6 After the first few days post-conception, fluid-filled spaces called lacunae develop within the primitive trophoblast that subsequently coalesce as the first true placental villi begin to emerge. Placental villi will ultimately consist of an inner, proliferative villous cytotrophoblast layer that fuses to form the outer multinucleated syncytiotrophoblast layer. The syncytiotrophoblast secretes hormones such as hCG and human placental lactogen into the space that has formed from the coalesced lacunae. This space is called the intervillous space because it separates the fetally derived floating (and anchoring, see below) placental villi from each other. The space is filled mostly with glandular secretions during early pregnancy but will become filled with maternal peripheral blood by about 8–10 weeks of gestation. Some placental villi completely cross the intervillous space to come into direct contact with the maternal decidua and are called anchoring villi. A subpopulation of the proliferating villous cytotrophoblast cells will leave the tip of the anchoring villae to invade into the maternal decidua as extravillous trophoblast and remodel the maternal spiral arteries as endovascular trophoblast.
Figure 2.
Early human placentation. hCG is secreted by the primitive trophoblast (early pregnancy; dark purple) and the syncytiotrophoblast (SynT; later pregnancy, dark purple) into the intervillous space, which develops from coalescing lacunae. The intervillous space is initially filled with glandular secretions and serum exudate in early pregnancy, but ultimately with maternal blood from the maternal spiral arteries (MSA) by about 8–10 weeks of gestation. Some placental villi cross the intervillous space and contact the maternal decidua as anchoring villi (right). Extravillous cytotrophoblast (Extravillous T; dark green) leave the tips of these anchoring villi to invade the maternal decidua or to remodel the maternal spiral arteries as endovascular trophoblast (Endovascular T; light green).
During the early post-conception weeks, when implantation is occurring, the primitive trophoblast produces hyperglycosylated hCG (hCG-H). This is the principal form of hCG produced during the first 2 weeks of pregnancy. This hormone appears to promote angiogenesis as well as trophoblast invasion into the uterine wall to form the anchoring villi.7–9 Interestingly, hCG-H has minimal, if any, role in the maintenance of corpus luteum and progesterone production as it only possesses about 1/25th of the biological activity of standard hCG. The ultimate fate of the primitive trophoblast remains unclear, although some feel it may be represented by the scattered trophoblast giant cells seen in the human decidua later in the first trimester of pregnancy and then throughout the remainder of gestation. 10 Regardless of the fate of the primitive trophoblast, the invasive extravillous trophoblast cells also predominately actively secrete hyperglycosylated hCG (hCG-H) throughout the initial 11 weeks of pregnancy, after which its levels will decreases throughout pregnancy. 11 Less than 1% of total hCG in the second and third trimester is hyperglycosylated hCG. 12 As pregnancy progresses, an increasing proportion of the hCG is secreted by the placental syncytiotrophoblast and this form of hCG is not hyperglycosylated. 13
Standard, nonhyperglycosylated hCG promotes and maintains progesterone production in early pregnancy by binding to the luteinizing hormone LH/hCG receptor on ovarian corpus luteal cells. This hormonal activity is absolutely necessary for pregnancy maintenance for the first 4–6 weeks of gestation until the steroidogenic activity of the placenta produces enough progesterone to take over this function. 14 In fact, removal of the corpus luteum during this stage of pregnancy will induce pregnancy failure. By 8–10 weeks of gestation, maternal serum hCG levels typically reach a peak concentration of 100,000 to 200,000 mIU/mL. 7 hCG concentrations then fall to lower but stable serum levels that vary widely, but in general remain above 5000 mIU/mL for the duration of the pregnancy 15 Although the role of hCG in pregnancy is commonly considered to be limited to the promotion of progesterone production by the corpus luteum, research has highlighted the complex function of hCG during a pregnancy and its unique role in placental, uterine, immune, and fetal functionality.
Not all hCG is secreted by placental trophoblast cells. Although much is known about the biochemistry and biological importance of hCG in pregnancy, there are several, often perplexing clinical scenarios in which diagnostic testing indicates that a patient is pregnant but she is not. The balance between synthesis and metabolism of hCG by the placenta and other organs ultimately determines its steady state levels in the body,16,17 and measuring serum and/or urine hCG levels provides insight into pregnancy and nonpregnancy clinical scenarios. 18 This article aims to review situations in which positive serum hCG test results are noted in nonpregnant subjects with a specific focus on what has been called the “phantom hCG.” Phantom hCG is characterized by persistently low hCG levels on diagnostic testing in a nonpregnant patient and can lead to a misdiagnosis of gestational trophoblastic disease (GTD) with subsequent inappropriate treatment with chemotherapy and/or hysterectomy.
Not all hCGs are equal
There are multiple different forms of hCG that vary in structure but still preserve the hCG β-subunit amino acid sequence. 12 Most are produced by specific subpopulations of cells. These forms include (1) intact biologically active heterodimeric hCG (hCG), (2) nicked hCG (hCGn), (3) free beta-subunit of hCG, (4) nicked free beta-subunit of hCG (hCGbn), (5) hCG beta-subunit core fragment (hCGbcf), (6) hyperglycosylated hCG (hCG-H), and (7) Sulfated hCG. Free alpha-subunits of hCG can also be produced and clinically detected. The standards utilized in assays for these forms of hCG are available via the World Health Organization (WHO). 19 The most common and clinically important of these, their origins and their effects are summarized in Table 1 and discussed below. Going forward, the term hCG will refer to the intact, biologically active, heterodimeric, normally glycosylated isomer.
Table 1.
Some forms of clinically relevant hCG and their functions.
| hCG type | Production | Function |
|---|---|---|
| Intact, biologically active heterodimeric, normally glycosylated hCG | Syncytiotrophoblast hydatidiform moles | Pregnancy: • Promotes progesterone production by corpus luteum cells • Promotes angiogenesis in the spiral arteries of the myometrium during pregnancy 20 • Promotes the differentiation of cytotrophoblast cells to syncytiotrophoblast • Promotes quiescence of contractions in the uterine myometrium |
| Hyperglycosylated hCG | Extravillous cytotrophoblast cells2,21 | • Promotes trophoblast invasion • Promotes cytotrophoblast cell growth and placental implantation • Promotes invasion in choriocarcinoma2,22 |
| Free hCG β-subunit | Pregnancy: Implanted blastocysts and trophoblasts
23
Malignancies: choriocarcinoma, nonseminomatous testicular tumors, bladder, cervical, pancreatic, lung, ovarian, endometrial cancers |
Pregnancy: • Thought to play a role in implantation 23 • Maintenance of pregnancy 23 Malignancies: • Promote cell growth & malignant transformation 12 • Blocks apoptosis |
| Pituitary hCG24,25 | Pituitary gonadotrophs during menstrual cycle or after menopause | • Assumed to supplement normal physiologic pituitary LH functions, i.e., follicular growth and progesterone production |
hCG, human chorionic gonadotropin; LH, luteinizing hormone.
hCG is produced by the syncytiotrophoblast. This molecule promotes progesterone production by corpus luteum cells as well as angiogenesis in the spiral arteries of the myometrium during pregnancy. 20 In addition, it both promotes the differentiation of cytotrophoblast cells to syncytiotrophoblast and quiescence of contractions in the uterine myometrium. 9 This form of hCG has also been shown to play an immunological role in pregnancy by promoting the production and release of the cytokine, macrophage inhibitory factor.26–28 Outside of pregnancy, the production of hCG is seen with noninvasive hydatidiform moles, one type of gestational trophoblastic disease (see below).
Hyperglycosylated hCG (hCG-H) is produced by extravillous cytotrophoblast cells and possibly by the primitive trophoblast. It is an autocrine molecule and is the principal form of hCG produced in the first 3 weeks of pregnancy. In pregnancy, hCG-H is involved in implantation, cytotrophoblast cell growth, and induction of early first trimester placental angiogenesis. Outside of pregnancy, hCG-H is produced by invasive moles and choriocarcinoma cells. Some investigators have questioned the true identity of hCG-H but most still believe there exists a form of hCG with the above characteristics
Free hCG β-subunit is a monomeric glycosylated hCG form secreted by trophoblast neoplasms and is the only form of hCG produced by nontrophoblastic malignancies. It promotes tumor growth and malignancy by antagonizing the transforming growth factor beta (TGF-β) receptor in a cellular pathway that promotes apoptosis. This free-hCG β-subunit can be seen in choriocarcinomas as well as nongestational malignancies: bladder, cervical, pancreatic, lung, ovarian, endometrial cancers.2,28 As early as 8 days after conception, free hCG β-subunit is produced by implanted blastocyst and trophoblast cells in pregnancy. 23 It is detectable in maternal serum in all trimesters of pregnancy29,30 It is thought that free hCG β-subunit plays a role in implantation as well as maintenance of pregnancy. 23
Pituitary hCG is characterized by expression of sulfated, rather than the more typical sialylated, oligosaccharides. It is produced by pituitary gonadotropes during the normal menstrual cycle as well as after menopause. Pituitary hCG has been found as the etiology of persistently positive serum hCG in nonpregnant patients with gonadal failure. 31 Its presence should be considered when hCG testing is positive in women with age-related menopause, after bilateral oophorectomy and in young patients with chemotherapy induced menopause.32,33 The effects of pituitary hCG remain unclear, but it is known that hCG and LH bind the same hCG/LH receptor on the corpus luteum to promote progesterone production and are assumed to bind on the same hCG/LH receptor on the granulosa and theca cells. 12 Levels of pituitary hCG have been shown to mirror pituitary LH levels. This appears to result from the close proximity of the hCG β- and LH β-subunit genes on chromosome 19 so that increases in gonadotropin-releasing hormone (GnRH), such as those that accompany the postmenopausal state, stimulate both genes. Since gonadotroph cells decline in number during pregnancy, it is likely that pituitary hCG is produced only at low levels during most pregnancies. 13
Detection of hCG by standard pregnancy tests
Today in the United States, urine and serum qualitative and quantitative hCG tests are all readily available. Urine tests provide “at-home” convenience. Although they are not quantitative, they can be quite sensitive (<15–20 mIU/mL) and can often detect pregnancy even before the missed menstrual period. For urine specimens, a morning urine sample is preferred as the concentration of hCG is typically at its peak in those following standard day-night cycles. 34 There are some instances (see below) when urine pregnancy tests are particularly useful in differentiating the source of an otherwise confusing hCG result.
Serum hCG tests are also useful in clinical medicine as they are positive earlier in pregnancy than a urine pregnancy test. 35 In addition, their quantitative nature is essential for following hCG patterns in early pregnancy, particularly when the pregnancy is of unknown location. Standard commercial pregnancy tests typically detect total hCG (which includes normal intact hCG, hyperglycosylated hCG, and free hCG beta-subunit). For oncological purposes, it is often useful to be able to detect and differentiate among several hCG isoforms including intact and free beta-subunits and specific tests are available for these purposes.
The identification of specific antigenic regions on each of the hCG subunits and development of antibodies with known epitope specificity that recognize these subunits allowed the development of assays for specific forms and subtypes of hCG. 35 Commercial serum hCG assay are commonly immunometric assays. They utilize a variety of isoform-specific antibody binding sites to detect hCG. Most commercial hCG assays utilize a combination of antibody binding sites to employ the “sandwich principle” (Figure 3). In these assays, a monoclonal anti-hCG antibody is fixed to a solid phase and serves as the capture antibody. The capture antibody typically binds to a specific target site on the hCG isoform to immobilize it and a separate antibody (monoclonal or polyclonal) binds to a distant site on the same molecule. This antibody serves as a tracer antibody and is labeled with either an enzyme, dye, or radioactive substrate. 17 This forms a “immobilized antibody-hCG-tracer antibody” complex. The amount of tracer is directly proportional to the level of hCG. At-home urine hCG pregnancy tests commonly utilize lateral flow mechanisms 36 that leverage capillary action to transport a liquid sample across specific zones of interaction (pads) that house molecules that have a reliable interaction with the analyte of interest. In the case of at-home urine hCG pregnancy tests, the sample is applied to an absorbent sample pad and then flows to the conjugate release pad which contains antibodies against hCG complexed with a detectable particle (color or fluorescent). hCG (if present) plus antibody labeled with the detector molecule are then pulled to the detection zone that houses an anti-analyte antibody. The latter antibody captures the hCG-containing labeled complex in the test line. A control pad housing anti-IgG antibodies is included distal to the detection zone to document adequate liquid capillary flow across the entire device.
Figure 3.

Commercial testing for hCG. A schematic illustration of the “sandwich” principle in commercial hCG assays (left). A capture antibody is fixed in a solid phase and immobilizes hCG by binding to one or more hCG subunit antigens. One or both of these subunits are then bound at a distant site by a tracer antibody. The amount of detected tracer is proportional to the amount of hCG. Heterophile antibodies (right) bind both capture and tracer antibodies in the absence of hCG causing a false-positive assay result.
The structural similarities of hCG to other naturally occurring hormones such as LH, FSH, and TSH significantly impact the specificity of hCG assays. About 80–85% of the hCG beta-subunit is homologous to LH beta-subunit. The LH beta-subunit contains 121 amino acids, whereas hCG beta-subunit contains 145 amino acids (see Figure 1).21,37 The 24 amino acid difference between these two hormones is unique to hCG and is referred to as the C-terminal peptide (CTP). Antibodies that specifically recognize antigen in the CTP of hCG are used in many commercial serum hCG assays.
Positive hCG results outside of pregnancy
The detection of hCG in urine or serum is routine in the confirmation and follow-up of early pregnancy. That said, there are several instances when hCG can be detected using standard tests even though the tested subject is not pregnant. Such occurrences may be due to the presence of unusual forms or sources of hCG. When positive hCG test results present outside of pregnancy, clinicians look toward abnormal and lesser known causes of a positive hCG detection test and often refer to oncologists, sometimes unnecessarily. There are several etiologies for persistently positive low hCG levels outside of pregnancy. 38
Nonmalignant:
Heterophilic antibodies (phantom hCG)
Pituitary hCG
Familial hCG syndrome
Exogenous hCG39,40 (e.g. unsubstantiated use for weight loss, to stimulate endogenous androgen production in athletes, hCG trigger injections for infertility treatments)
Munchausen’s syndrome
Premalignant and malignant:
Active gestational trophoblastic disease (molar pregnancy, invasive mole, choriocarcinoma, placenta site trophoblastic tumor)
Quiescent gestational trophoblastic disease
Nontrophoblast malignancy
Phantom hCG syndrome
The term “phantom hCG” is often incorrectly used to encompass all conditions in which hCG is detected in a nonpregnant patient. The term actually refers to the specific occurrence of a falsely positive immunoassay for hCG due to the presence of cross-reactive antibodies. Patients with a phantom hCG will commonly present with a persistent, low positive quantitative hCG test result despite the fact that no true hCG or trophoblastic tissue exists. The most common of the cross-reactive substances that result in a “phantom hCG” are heterophilic antibodies (see Figure 3) that have been generated against poorly defined antigens and that have typically weak nonspecific interactions with hCG or other reagents in the immunoassay. Humans also produce human against animal antibodies (HAAA) directed against antigens used in vaccinations and other therapies. The antigenic epitopes detected by these antibodies are often specific and the interactions strong. One example of such antibodies are human anti-mouse antibodies (HAMA), 41 and the binding of human antibodies to mouse IgG in hCG assays is the most common cause of a phantom hCG. 42 Individuals with heterophilic antibodies, HAAA, or HAMA can have falsely positive hCG results in both immunometric assays and radioimmunoassays for hCG. 38
As stated above, most commercial hCG assays utilize a capture antibody [animal-derived monoclonal immunoglobulin (IgG)] and a second polyclonal antibody coupled with a signal molecule. This second antibody serves as a tracer. Heterophilic antibodies can bind IgG at sites common to humans and animals and then link the capture and tracer antibodies, causing a falsely positive pregnancy test in the absence of hCG within the serum sample.
It is important to recognize that the presence of cross-reactive antibodies, including heterophilic antibodies, can cause persistently elevated serum hCG tests despite absence of pregnancy to avoid unwarranted attribution to malignancy-related disease and resultant unnecessary surgery and/or chemotherapy. 42
To evaluate the possibility that a phantom hCG is causing false-positive hCG testing, several methods can be employed in accordance to guidance from the American College of Obstetricians and Gynecologists: 43
The clinician should request a quantitative serum hCG which should be run in conjunction with a urine hCG (qualitative or quantitative). 43 The urine sample should be tested utilizing an immunoassay that detects either normal intact hCG (hCG) or an immunoassay that detects all hCG-related molecules: hCG, hCG free beta–subunit, and beta-core fragment.42,44 A positive quantitative serum hCG coupled with either a negative urine hCG or no detectable hCG derivative (free beta-subunit, and beta-core fragment) in the urine is supportive of the diagnosis of phantom hCG. Because heterophilic antibodies are large glycoproteins that are filtered by the glomerulus, patients with phantom hCG will have a negative urine hCG test due to heterophile antibodies despite a positive serum hCG test. The presence of cross-reactivity in the serum hCG immunoassay is confirmed if the serum value is ⩾50 mIU/mL and the urinary value is negative. 43 hCG will be detected in both urine and serum samples in pregnancy and trophoblastic disease.
Rerun the hCG assay using serial dilutions of the serum. In the absence of an interferent, the measured concentration of a sample should fall in a parallel or linear fashion as the sample is progressively diluted. A percent recovery outside of 80–120% is considered a nonlinear response and suggests the presence of antibodies. 45 Strong affinity of the interfering antibodies to the assay needs to be considered, however, as a potential confounder.
Attempt to deplete any heterophilic antibodies in the sample and re-run the assay. If the test becomes negative after depletion of the heterophilic antibodies, cross-reactivity is likely.
Among these approaches, the most sensitive discriminatory method would be a negative urine hCG result concurrent with a positive serum hCG result from the same patient. However, it is important to note that a false negative urine test may occur secondary to dilution if, for instance, the subject is overly hydrated 22
Detection of pituitary hCG
Detection of pituitary hCG, particularly at levels seen in postmenopausal women, is a fairly common cause of positive hCG tests in a woman who is not pregnant. Ovarian estrogen production is markedly reduced in the menopausal female. In response, estrogen-associated negative feedback on GnRH production is reduced or absent and GnRH, LH, and FSH levels rise. The alpha-subunit of hCG is similar to that of LH and FSH, and some tests for hCG may become positive due to cross-reactivity of the detection reagent with the alpha-subunits of other heterodimeric pituitary hormones. The LH beta-subunit gene lays between multiple hCG beta-subunit genes. In some women, postmenopausal elevations in GnRH will promote hCG beta-subunit gene activity along with LH gene activity to an extent that elevations in actual hCG beta-subunits can be detected in serum and urine. The presence of pituitary hCG may be suspected in the menopausal female when FSH 46 levels (typically FSH levels > 30 mIU/mL) as well as hCG levels are elevated. Distinction of a pituitary source of a positive hCG in a nonpregnant woman can be determined by exposing the subject to estrogen, often in the form of combined estrogen/progesterone oral contraceptive pills (OCPs). A trial of combined OCPs for a minimum of at least 2–3 weeks has been suggested in a few case reports.47,48 Estrogen exposure will provide the missing negative feedback on the hypothalamic-pituitary axis that is seen in postmenopausal women and if the detected hCG was of pituitary origin, its levels should be suppressed.
Familial hCG syndrome is a rare genetic condition; the USA hCG Reference Service identified 10 families with this disorder and calculated the approximate US incidence to be 1 in 60,000 families. 49 Using these 10 families, investigators determined that inheritance appears to be mostly autosomal dominant and that there is no gender preference in disease manifestations. 49 Affected patients produce hCG moieties that contain multiple CTP modifications that lead to persistently elevated hCG assay results. 50 The hCG present in familial hCG syndrome is thought to lack the beta-subunit C-terminus or have a mutated CTP region. Such hCG molecules with modified CTPs usually have gone undetected because a majority of hCG assays (11 out of 12) cannot detect hCG molecules that are missing the B subunit CTP region. 51 However, some assays, particularly the Siemens Immulite assay, utilize detection antibodies with antigen recognition sites in the beta-subunit core structure (hCGβcf), a part of the molecule that is not affected by a missing or mutated CTP region. 52 Such tests will therefore screen positive for elevated hCG levels. It is thought that the hCG molecules in affected subjects are not active, which could account for reported normal fertility in subjects affected by familial hCG syndrome. 49 In fact, all females with familial hCG syndrome have reported normal menstrual periods and fecundity. Despite its benign phenotype, the syndrome is still an important consideration when presented with persistently elevated hCG levels outside of pregnancy. 38
Exogenous hCG
Another diagnostic consideration for falsely positive hCG testing in a nonpregnant individual is iatrogenic administration of hCG. 38 Administration of hCG-containing products by injection, orally, nasally, and/or sublingually, has been inappropriately used to attain weight loss. 53 Such products have also been used by athletes or body builders to stimulate endogenous androgen production. Even low dose exogenous hCG administration can lead to positive urine or serum hCG testing in nonpregnant patients. In clinical medicine, hCG injections are administered to trigger ovulation during fertility treatment. It is important to include questions about self-administration of hCG when obtaining a medical history on a nonpregnant patient with a positive hCG result. In general, if the source of hCG is exogenous and iatrogenic and use has been discontinued, levels will typically decrease with a 24-to 48-hour half life. 54
Munchausen syndrome
Munchausen syndrome is a psychological disorder characterized by intentional falsification of clinical signs, symptoms, and testing in order to be considered ill. 38 Although rare, Munchausen syndrome has been diagnosed in cases in which a patient presents with laboratory results that are unexpected or uncharacteristic. For instance, a woman could present as pregnant but serum labs reveal an overwhelming percentage of “pure” hCG in comparison to a mixture of free subunits and other hCG variants. In cases where Munchausen’s syndrome was diagnosed, serial serum and urine samples were collected. These serial hCG levels were inconsistent in that they oscillated from near zero to an increased value that then declined again. In addition, blood hCG constituents included abnormally low levels of several forms of hCG, including hCG free β-subunit, hyperglycosylated hCG, and nicked hCG (all <0.1% of total hCG), which is consistent with patterns that would be seen with injection of recombinant, intact hCG dimers (available in products such as Ovidrel by EMD Serono Inc., Rockland, MA, USA). Given that these cases involved two nurses and a physician, it was inferred that the subjects had been self-administering from readily available clinic stock. The two nurses eventually admitted self-administration and were referred to psychiatry. The physician continued to deny use of Ovidrel and the case was never solved. 38
Premalignant and malignant conditions
Active gestational trophoblastic disease
Gestational trophoblastic disease (GTD) is an umbrella category that consists of premalignant and malignant gestational trophoblastic neoplasia (GTN). GTN encompasses placental site trophoblastic tumors, epithelioid trophoblastic tumors, invasive moles, and choriocarcinomas. 55 Premalignant GTD can be subcategorized into complete and partial molar pregnancies and all result from aberrant fertilization. Diagnosis is made based on gross and histopathologic appearance of the placenta and its chromosome content. Complete moles have no identifiable embryonic or fetal tissue and typically have a diploid 46, XX karyotype that arises from fertilization of an empty ovum (without chromosomes) by a haploid sperm that subsequently replicates itself. Partial molar pregnancies, on the other hand, are characterized by morphologically variable chorionic villi, focal trophoblastic hyperplasia, and identifiable fetal or embryonic tissue. Partial moles arise from fertilization of a normal ovum by two spermatozoa, and thus a triploid karyotype. 56 Hydatidiform moles account for almost 80% of GTD. GTD can produce hCG molecules in a variety of forms: free β-subunits, nicked free β-subunits, c-terminal peptides, beta cores, and hyperglycosylated forms. 57 Treatment options for GTD differ based on several factors: (1) an assigned World Health Organization (WHO) prognostic score, (2) patient desire for fertility preservation, and (3) predicted tumor response to chemotherapy. Serum hCG levels are obtained throughout diagnosis and treatment of GTD and are critical to characterizing treatment response.
Gestational choriocarcinoma, which is distinct from pure nongestational choriocarcinoma,27,58 is a malignancy of the placental trophoblast cell. In females in Europe and North America, gestational choriocarcinoma develops in 1/400,000 pregnant patients and 1/40 patients with hydatidiform moles.59,60
Gestational choriocarcinoma is highly metastatic, with fairly early hematogenous spread. Brain metastasis is the main cause of death and disability in patients with choriocarcinoma. 61 Most gestational choriocarcinoma/low-risk gestational trophoblastic neoplasms are highly responsive to chemotherapy and successfully cured with single-agent protocols of methotrexate (MTX) or actinomycin D (ActD); higher-risk patients often need combination therapy. 62
The development of choriocarcinomas is driven by TGF-β antagonists and hyperglycosylated hCG which promote cell growth and prevent apoptosis of the transformed cells, along with other actions.63,64 Thus, the proportion of total hCG that is represented by hyperglycosylated hCG in relation to total hCG is a useful marker for choriocarcinoma and typically, hyperglycosylated hCG accounts for more than 50% of total hCG.
Quiescent gestational trophoblastic disease (Q-GTD) is another cause of persistently low hCG levels outside of pregnancy. In 2010, the USA hCG Reference Service reported 168 cases over 10 years. Most Q-GTD cases will present after diagnosis and treatment of a gestational trophoblastic neoplasm; however, cases have been reported following otherwise uncomplicated early pregnancy loss. Patients with Q-GTD typically have low positive serum hCG levels (often approximately 100–300 mIU/mL) that can persist with little variation over several months. Because the hCG molecules detected in subjects with quiescent GTD are produced by highly differentiated syncytiotrophoblast and not by cytotrophoblast cells, hyperglycosylated hCG levels will be low (typically <10% of total hCG) and the trophoblast cells do not become invasive. Measurement of hyperglycosylated hCG can help to differentiate patients with Q-GTD from others with persistent positive hCG test results outside of pregnancy. If a test for hyperglycosylated hCG is not available, close monitoring of hCG levels over 3 months with detection of no more than a twofold oscillation in hCG levels and can be suggestive of the condition. 38 Q-GTD typically resolves within 6 months without treatment, although tenacious cases do occur rarely; one was reported to persist for nearly 9 years.
Nontrophoblast malignancies should also be included within a comprehensive differential diagnosis for elevated hCG testing outside of pregnancy. This is validated by recent endorsements by several societies to include hCG analysis in cancer diagnosis protocols.65,66 Pure nongestational choriocarcinoma is a rare type of germ cell tumor that typically presents with clinical symptoms that reflect high hCG levels. This type of choriocarcinoma can present in both males and females. Distinction from its gestational counterpart is important as nongestational forms carry a poorer prognosis. 67 There also have been case reports of women with breast cancer, ovarian dysgerminoma, squamous cell carcinoma of head and neck, 68 brain glioblastoma, 38 renal carcinoma, non-small cell lung cancer, 69 hepatocellular carcinoma, endometrial adenocarcinoma, and duodenal adenocarcinoma 70 who have been found to have positive hCG testing despite not being pregnant. Typically, the diagnostic process for nontrophoblastic malignancies starts by excluding a pituitary source for the hCG, the presence of HAAA/HAMA/heterophilic antibodies, and the diagnosis of quiescent GTD. Unlike most other etiologies for elevated hCG testing outside of pregnancy, the form of hCG secreted by nontrophoblastic cancers consists mainly of free beta-subunits. A negative intact hCG test can be used to rule out pregnancy in patients producing only beta hCG fragments coming from a nontrophoblastic cancer. 71 It is thought that these carcinomatous tissues undergo metaplasia and become similar to poorly differentiated trophoblast cells. There is evidence that production of free β-hCG subunits by nontrophoblastic tumors is associated with poor prognosis. 72 Therefore, the detection of low serum levels of hCG (less than 700 mIU/mL) that comprise mostly of free beta-subunits outside of pregnancy warrants further assessment for the presence of a neoplasm. 38 Consideration should be given to referral for a CT scan of the chest and abdomen as well as magnetic resonance imaging of the brain and pelvis to determine the site of the putative primary malignancy and possible metastases.
Proposed clinical evaluation of hCG detected in the absence of pregnancy
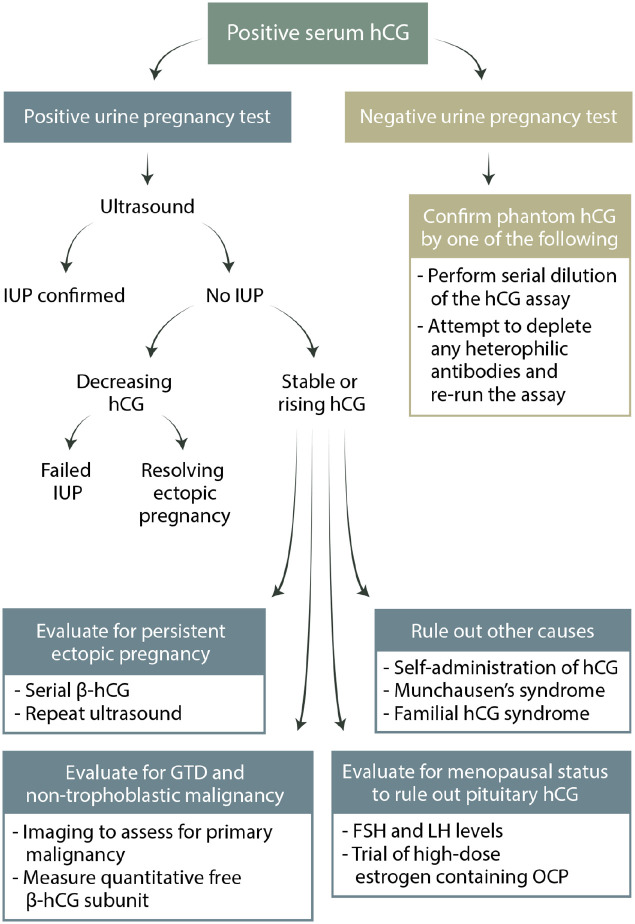
Due to the implications of a missed cancer diagnosis, premature attribution of the detection of hCG outside of pregnancy to gestational trophoblastic disease is not infrequent. This can unfortunately lead to unnecessary treatments and surgical interventions. To reduce the possibility of misdiagnosis of nonpregnant patients with persistently elevated hCG levels, we suggest the following streamlined approach (Figure 4):
Figure 4.
Diagnostic algorithm for evaluating a positive serum hCG test. Serial β-hCG refers to the assessment of the pattern of rise (or fall) over time with repeated quantitative hCG measurement. This is typically done every 48 hours and only in early pregnancy.
Conclusion
Positive hCG in the absence of pregnancy is a diagnostic dilemma for clinicians in several fields of medicine. Although not frequently encountered, ready knowledge of the differential diagnosis for these patients is necessary to ensure accurate disease identification and help to guide timely, appropriate, and effective care for these patients. A thorough patient history and physical exam, directed diagnostic imaging and laboratory testing and a consistent streamlined and rational approach to diagnosis are essential to optimize clinical care.
Supplemental Material
Supplemental material, sj-pdf-1-reh-10.1177_26334941211016412 for A rational diagnostic approach to the “phantom hCG” and other clinical scenarios in which a patient is thought to be pregnant but is not by Oluwafunmilayo Oyatogun, Mandeep Sandhu, Stephanie Barata-Kirby, Erin Tuller and Danny J. Schust in Therapeutic Advances in Reproductive Health
Acknowledgments
Oluwafunmilayo Oyatogun and Mandeep Sandhu had equal contributions.
Footnotes
Conflict of interest statement: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Mandeep Sandhu  https://orcid.org/0000-0001-6244-5329
https://orcid.org/0000-0001-6244-5329
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Oluwafunmilayo Oyatogun, Institute for Women’s Health Research and Department of Obstetrics, Gynecology and Women’s Health, University of Missouri, 500 North Keene St Suite 203, Columbia, MO 65201, USA.
Mandeep Sandhu, Institute for Women’s Health Research and Department of Obstetrics, Gynecology and Women’s Health, University of Missouri, Columbia, MO, USA.
Stephanie Barata-Kirby, Institute for Women’s Health Research and Department of Obstetrics, Gynecology and Women’s Health, University of Missouri, Columbia, MO, USA.
Erin Tuller, Institute for Women’s Health Research and Department of Obstetrics, Gynecology and Women’s Health, University of Missouri, Columbia, MO, USA.
Danny J. Schust, Institute for Women’s Health Research and Department of Obstetrics, Gynecology and Women’s Health, University of Missouri, Columbia, MO, USA
References
- 1. Gnoth C, Johnson S. Strips of hope: accuracy of home pregnancy tests and new developments. Geburtshilfe Frauenheilkd 2014; 74: 661–669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Fournier T, Guibourdenche J, Evain-Brion D. Review: hCGs: different sources of production, different glycoforms and functions. Placenta 2015; 36(Suppl. 1): S60–S65. [DOI] [PubMed] [Google Scholar]
- 3. Muyan M, Boime I. Secretion of chorionic gonadotropin from human trophoblasts. Placenta 1997; 18: 237–241. [DOI] [PubMed] [Google Scholar]
- 4. Nwabuobi C, Arlier S, Schatz F, et al. hCG: biological functions and clinical applications. Int J Mol Sci 2017; 18: 2037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Sheridan MA, Yunusov D, Balaraman V, et al. Vulnerability of primitive human placental trophoblast to Zika virus. Proc Natl Acad Sci USA 2017; 114: E1587–E1596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Norwitz ER, Schust DJ, Fisher SJ. Implantation and the survival of early pregnancy. N Engl J Med 2001; 345: 1400–1408. [DOI] [PubMed] [Google Scholar]
- 7. Fournier T. Human chorionic gonadotropin: different glycoforms and biological activity depending on its source of production. Ann Endocrinol 2016; 77: 75–81. [DOI] [PubMed] [Google Scholar]
- 8. Hyperglycosylated hCG is a marker of early human trophoblast invasion. J Clin Endocrinol Metab, https://academic.oup.com/jcem/article/95/10/E240/2835384 (accessed 22 December 2019). [DOI] [PubMed]
- 9. Cole LA. Hyperglycosylated hCG, a review. Placenta 2010; 31: 653–664. [DOI] [PubMed] [Google Scholar]
- 10. James JL, Carter AM, Chamley LW. Human placentation from nidation to 5 weeks of gestation. Part I: what do we know about formative placental development following implantation? Placenta 2012; 33: 327–334. [DOI] [PubMed] [Google Scholar]
- 11. Hay DL, Lopata A. Chorionic gonadotropin secretion by human embryos in vitro. J Clin Endocrinol Metab 1988; 67: 1322–1324. [DOI] [PubMed] [Google Scholar]
- 12. Cole LA. Biological functions of hCG and hCG-related molecules. Reprod Biol Endocrinol 2010; 8: 102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Cole LA. hCG, the wonder of today’s science. Reprod Biol Endocrinol 2012; 10: 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Dufau ML. The luteinizing hormone receptor. Annu Rev Physiol 1998; 60: 461–496. [DOI] [PubMed] [Google Scholar]
- 15. Feldkamp CS, Pfeffer WH. The measurement of human chorionic gonadotropin for pregnancy testing. Henry Ford Hosp Med J 1982; 30: 207–213. [PubMed] [Google Scholar]
- 16. Nisula BC, Blithe DL, Akar A, et al. Metabolic fate of human choriogonadotropin. J Steroid Biochem 1989; 33: 733–737. [DOI] [PubMed] [Google Scholar]
- 17. Cole LA. Immunoassay of human chorionic gonadotropin, its free subunits, and metabolites. Clin Chem 1997; 43: 2233–2243. [PubMed] [Google Scholar]
- 18. Stenman U-H, Tiitinen A, Alfthan H, et al. The classification, functions and clinical use of different isoforms of HCG. Hum Reprod Update 2006; 12: 769–784. [DOI] [PubMed] [Google Scholar]
- 19. Burns C, Moore M, Sturgeon C, et al. WHO international collaborative study of the proposed 5th [fifth] international standard for chorionic gonadotrophin. Geneva: World Health Organization, 2009. [Google Scholar]
- 20. Zygmunt M, Herr F, Münstedt K, et al. Angiogenesis and vasculogenesis in pregnancy. Eur J Obstet Gynecol Reprod Biol 2003; 110: S10–S18. [DOI] [PubMed] [Google Scholar]
- 21. Cole LA, Dai D, Butler SA, et al. Gestational trophoblastic diseases: 1. Pathophysiology of hyperglycosylated hCG. Gynecol Oncol 2006; 102: 145–150. [DOI] [PubMed] [Google Scholar]
- 22. Betz D, Fane K. Human chorionic gonadotropin (HCG). Treasure Island, FL: StatPearls Publishing, 2019, http://www.ncbi.nlm.nih.gov/books/NBK532950/ (accessed 29 December 2019). [PubMed] [Google Scholar]
- 23. Soni S, Krantz DA, Blitz MJ, et al. Elevated maternal serum-free β-human chorionic gonadotropin (β-hCG) and reduced risk of spontaneous preterm delivery. J Matern Fetal Neonatal Med 2019; 32: 3191–3196. [DOI] [PubMed] [Google Scholar]
- 24. Birken S, Maydelman Y, Gawinowicz MA, et al. Isolation and characterization of human pituitary chorionic gonadotropin. Endocrinology 1996; 137: 1402–1411. [DOI] [PubMed] [Google Scholar]
- 25. Odell WD, Griffin AJ. Pulsatile secretion of chorionic gonadotropin during the normal menstrual cycle. J Clin Endocrinol Metab 1989; 69: 528–532. [DOI] [PubMed] [Google Scholar]
- 26. Akoum A, Metz CN, Morin M. Marked increase in macrophage migration inhibitory factor synthesis and secretion in human endometrial cells in response to human chorionic gonadotropin hormone. J Clin Endocrinol Metab 2005; 90: 2904–2910. [DOI] [PubMed] [Google Scholar]
- 27. Matsuura T, Sugimura M, Iwaki T, et al. Anti-macrophage inhibitory factor antibody inhibits PMSG-hCG-induced follicular growth and ovulation in mice. J Assist Reprod Genet 2002; 19: 591–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Wan H, Versnel MA, Cheung WY, et al. Chorionic gonadotropin can enhance innate immunity by stimulating macrophage function. J Leukoc Biol 2007; 82: 926–933. [DOI] [PubMed] [Google Scholar]
- 29. Wright D, Papadopoulos S, Silva M, et al. Serum free β-human chorionic gonadotropin in the three trimesters of pregnancy: effects of maternal characteristics and medical history: β-hCG in the three trimesters of pregnancy. Ultrasound Obstet Gynecol 2015; 46: 51–59. [DOI] [PubMed] [Google Scholar]
- 30. Sirikunalai P, Wanapirak C, Sirichotiyakul S, et al. Associations between maternal serum free beta human chorionic gonadotropin (β-hCG) levels and adverse pregnancy outcomes. J Obstet Gynaecol 2016; 36: 178–182. [DOI] [PubMed] [Google Scholar]
- 31. Merhi Z, Pollack SE. Pituitary origin of persistently elevated human chorionic gonadotropin in a patient with gonadal failure. Fertil Steril 2013; 99: 293–296. [DOI] [PubMed] [Google Scholar]
- 32. Schmid BC, Reilly A, Oehler MK. Management of nonpregnant women with elevated human chorionic gonadotropin. Case Rep Obstet Gynecol 2013; 2013: 580709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Poniatowski BC, Grimm P, Cohen G. Chemotherapy-induced menopause: a literature review. Cancer Invest 2001; 19: 641–648. [DOI] [PubMed] [Google Scholar]
- 34. Human chorionic gonadotropin (hCG) 510(k)s—guidance for industry and FDA reviewers/staff, https://www.fda.gov/regulatory-information/search-fda-guidance-documents/guidance-over-counter-otc-human-chorionic-gonadotropin-hcg-510ks-guidance-industry-and-fda
- 35. Berger P, Sturgeon C, Bidart JM, et al. The ISOBM TD-7 workshop on hCG and related molecules. Tumour Biol 2002; 23: 1–38. [DOI] [PubMed] [Google Scholar]
- 36. Koczula KM, Gallotta A. Lateral flow assays. Essays Biochem 2016; 60: 111–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Harvey RA, Pursglove HD, Schmid P, et al. Human chorionic gonadotropin free beta-subunit measurement as a marker of placental site trophoblastic tumors. J Reprod Med 2008; 53: 643–648. [PubMed] [Google Scholar]
- 38. Cole LA. Human chorionic gonadotropin (HCG). New York: Elsevier, 2014. [Google Scholar]
- 39. Choi J, Smitz J. Luteinizing hormone and human chorionic gonadotropin: origins of difference. Mol Cell Endocrinol 2014; 383: 203–213. [DOI] [PubMed] [Google Scholar]
- 40. Lijesen GK, Theeuwen I, Assendelft WJ, et al. The effect of human chorionic gonadotropin (HCG) in the treatment of obesity by means of the Simeons therapy: a criteria-based meta-analysis. Br J Clin Pharmacol 1995; 40: 237–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Kricka LJ. Human anti-animal antibody interferences in immunological assays. Clin Chem 1999; 45: 942–956. [PubMed] [Google Scholar]
- 42. Rotmensch S, Cole LA. False diagnosis and needless therapy of presumed malignant disease in women with false-positive human chorionic gonadotropin concentrations. Lancet 2000; 355: 712–715. [DOI] [PubMed] [Google Scholar]
- 43. Avoiding inappropriate clinical decisions based on false-positive human chorionic gonadotropin test results, https://www.acog.org/en/Clinical/ClinicalGuidance/CommitteeOpinion/Articles/2002/11/AvoidingInappropriateClinicalDecisionsBasedonFalse-PositiveHumanChorionicGonadotropinTestResults (accessed 15 August 2020). [DOI] [PubMed]
- 44. Cole LA. Phantom hCG and phantom choriocarcinoma. Gynecol Oncol 1998; 71: 325–329. [DOI] [PubMed] [Google Scholar]
- 45. Jara-Aguirre JC, Baumann NA, Block DR, et al. Human chorionic gonadotropin suspected heterophile interference investigations in immunoassays: a recommended approach. Clin Chem Lab Med 2019; 57: 1192–1196. [DOI] [PubMed] [Google Scholar]
- 46. Gronowski AM, Fantz CR, Parvin CA, et al. Use of serum FSH to identify perimenopausal women with pituitary hCG. Clin Chem 2008; 54: 652–656. [DOI] [PubMed] [Google Scholar]
- 47. Kuhadiya ND, Karunakara A, Garg M, et al. A case report of elevated hCG levels in menopause—a clinical dilemma. Endocrinol Metab Int J 2017; 4: 46–48. [Google Scholar]
- 48. Cole LA, Sasaki Y, Muller CY. Normal production of human chorionic gonadotropin in menopause. N Engl J Med 2007; 356: 1184–1186. [DOI] [PubMed] [Google Scholar]
- 49. Cole LA. Familial hCG syndrome. J Reprod Immunol 2012; 93: 52–57. [DOI] [PubMed] [Google Scholar]
- 50. Tan A, Van der Merwe AM, Low X, et al. Familial HCG syndrome: a diagnostic challenge. Gynecol Oncol Rep 2014; 10: 47–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Between-method variation in human chorionic gonadotropin test results. Clin Chem, http://clinchem.aaccjnls.org/content/50/5/874.short (accessed 31 December 2019). [DOI] [PubMed]
- 52. Sturgeon CM, Berger P, Bidart J-M, et al. Differences in recognition of the 1st WHO international reference reagents for hCG-related isoforms by diagnostic immunoassays for human chorionic gonadotropin. Clin Chem 2009; 55: 1484–1491. [DOI] [PubMed] [Google Scholar]
- 53. Simeons ATW. The action of chorionic gonadotrophin in the obese. Lancet 1954; 264: 946–947. [DOI] [PubMed] [Google Scholar]
- 54. Braunstein GD. False-positive serum human chorionic gonadotropin results: causes, characteristics, and recognition. Am J Obstet Gynecol 2002; 187: 217–224. [DOI] [PubMed] [Google Scholar]
- 55. Brown J, Naumann RW, Seckl MJ, et al. 15 years of progress in gestational trophoblastic disease: scoring, standardization, and salvage. Gynecol Oncol 2017; 144: 200–207. [DOI] [PubMed] [Google Scholar]
- 56. Berkowitz RS, Goldstein DP. Clinical practice. Molar pregnancy. N Engl J Med 2009; 360: 1639–1645. [DOI] [PubMed] [Google Scholar]
- 57. Cole LA. hCG, its free subunits and its metabolites. Roles in pregnancy and trophoblastic disease. J Reprod Med 1998; 43: 3–10. [PubMed] [Google Scholar]
- 58. Bishop BN, Edemekong PF. Choriocarcinoma. Treasure Island, FL: StatPearls Publishing, 2020, http://www.ncbi.nlm.nih.gov/books/NBK535434/ (accessed 26 April 2020). [PubMed] [Google Scholar]
- 59. Lurain JR. Gestational trophoblastic disease I: epidemiology, pathology, clinical presentation and diagnosis of gestational trophoblastic disease, and management of hydatidiform mole. Am J Obstet Gynecol 2010; 203: 531–539. [DOI] [PubMed] [Google Scholar]
- 60. Zhang W, Liu B, Wu J, et al. Hemoptysis as primary manifestation in three women with choriocarcinoma with pulmonary metastasis: a case series. J Med Case Rep 2017; 11: 110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Wang D, Shu H, Zhang Q, et al. Brain metastasis of choriocarcinoma presenting as multiple intracranial hematomas. Medicine 2018; 97:e12275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Ngan HYS, Seckl MJ, Berkowitz RS, et al. Update on the diagnosis and management of gestational trophoblastic disease. Int J Gynaecol Obstet 2018; 143(Suppl. 2): 79–85. [DOI] [PubMed] [Google Scholar]
- 63. Cole LA, Butler SA, Khanlian SA, et al. Gestational trophoblastic diseases: 2. Hyperglycosylated hCG as a reliable marker of active neoplasia. Gynecol Oncol 2006; 102: 151–159. [DOI] [PubMed] [Google Scholar]
- 64. Hyperglycosylated hCG in gestational implantation and in choriocarcinoma and testicular germ cell malignancy tumorigenesis. J Reprod Med, https://europepmc.org/article/med/17165440 (accessed 13 January 2020). [PubMed]
- 65. Reisenbichler ES, Hameed O. Non-trophoblastic tumors as other causes of elevated human chorionic gonadotrophin. Lab Med 2010; 41: 183–183. [Google Scholar]
- 66. Stenman U-H, Alfthan H, Hotakainen K. Human chorionic gonadotropin in cancer. Clin Biochem 2004; 37: 549–561. [DOI] [PubMed] [Google Scholar]
- 67. Corakçi A, Ozeren S, Ozkan S, et al. Pure nongestational choriocarcinoma of ovary. Arch Gynecol Obstet 2005; 271: 176–177. [DOI] [PubMed] [Google Scholar]
- 68. Secretion of beta-HCG from squamous cell carcinomas of the head and neck, https://pubmed-ncbi-nlm-nih-gov.proxy.mul.missouri.edu/20620641/ (accessed 15 August 2020). [DOI] [PubMed]
- 69. Vicier C, Tabouret E, Tallet A, et al. BetaHCG secretion by a pulmonary adenocarcinoma. World J Surg Oncol 2013; 11: 228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Kang S, Zaidi AJ, Shokouh-Amiri M, et al. A case report of paraneoplastic syndrome in β-hCG-secreting duodenal adenocarcinoma. J Gastrointest Oncol 2019; 10: 1151–1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. McCash SI, Goldfrank DJ, Pessin MS, et al. Reducing false-positive pregnancy test results in patients with cancer. Obstet Gynecol 2017; 130: 825–829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Marcillac I, Troalen F, Bidart JM, et al. Free human chorionic gonadotropin beta subunit in gonadal and nongonadal neoplasms. Cancer Res 1992; 52: 3901–3907. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-reh-10.1177_26334941211016412 for A rational diagnostic approach to the “phantom hCG” and other clinical scenarios in which a patient is thought to be pregnant but is not by Oluwafunmilayo Oyatogun, Mandeep Sandhu, Stephanie Barata-Kirby, Erin Tuller and Danny J. Schust in Therapeutic Advances in Reproductive Health