Abstract
Background:
Sentinel lymph node biopsy (SLNB) provides staging information and guides adjuvant therapy in early breast cancer (EBC). Routine SLNB in oncogeriatricians with low-risk EBC remains controversial.
Aims:
To evaluate axillary management in elderly patients diagnosed with oestrogen receptor positive (ER+), clinically lymph node negative (cLN−) EBC, and to assess whether SLNB affects further axillary management or adjuvant chemotherapy (ACTX) decision making.
Methods:
Female patients aged > 65 years, diagnosed with ER+, human epidermal growth factor receptor-2 negative (HER2−), and cLN− breast cancer (BC), who underwent surgery and SLNB were included. Clinicopathological predictors of ACTX and completion axillary lymph node dissection (CALND) were determined. Kaplan-Meier analyses assessed survival outcomes.
Results:
A total of 253 patients were included (median age: 72 years, range: 66-90), all underwent SLNB; 50 (19.8%) had lymphatic metastasis on SLNB (SLNB+). Of these, 19 proceeded to CALND (38.0%), 10 (52.6%) of whom had further axillary disease (ALND+). 20 of the 50 SLNB+ patients received ACTX (40.0%) as did 31 of the 203 SLNB− patients (15.2%) (P < .001). Oncotype DX (ODX) testing was utilized in 82 cases (32.8%). Younger age (P < .001), SLNB+ (P < .001) and ODX score (P = .003) were all associated with ACTX prescription. ODX > 25 (OR: 4.37, 95% CI: 1.38-13.80, P = .012) independently predicted receiving ACTX. Receiving ACTX and proceeding to CALND did not improve disease-free (P = .485 and P = .345) or overall survival (P = .981 and P = .646).
Conclusions:
Routine SNLB may not be necessary in elderly patients diagnosed with ER+, cLN− EBC. Future oncogeriatric practice is likely to see genomic testing guiding ACTX prescription in this group.
Keywords: Breast cancer, oncogeriatrics, surgical oncology, personalized medicine
Introduction
Breast cancer (BC) is the second most common cancer in women worldwide with one-third of BCs occurring in patients above the age of 65. 1 Historically, surgical management of BC involved extensive resection with radical mastectomy and axillary lymph node dissection (ALND) with considerable associated morbidity. 2 However, the majority of women now present with early-stage disease (ie, early BC [EBC]), and surgical practice has evolved such that tumour resection via either breast conserving surgery (BCS) or mastectomy with simultaneous sentinel lymph node biopsy (SLNB) for patients with clinically lymph node negative (cLN−) disease is now performed routinely without compromising oncologic outcome. 3 This paradigm shift towards increasingly conservative surgical approaches to BC has been driven by a desire to minimize morbidity, while maintaining optimal oncologic outcomes. 4 In the case of the axilla, the development of SLNB facilitated accurate minimally invasive nodal staging to inform therapeutic decision-making based on the presence or absence of axillary metastases. 5
The accuracy of SLNB as a staging tool was supported by evidence from the National Surgical Adjuvant Bowel and Breast Project (NSABP) B-32 study which demonstrated that patients with a negative SLNB (SLNB−) could be spared ALND. 6 Further evolution towards conservative axillary management came about following reports from the American College of Surgeons Oncology Group (ACOSOG) Z0011 trial which demonstrated that ALND does not improve survival or local control in BC with less than 3 positive sentinel lymph nodes (LNs).7,8 Furthermore, although traditional clinicopathologic features such as tumour size and axillary nodal disease were the primary drivers of adjuvant therapy decision making, the importance of tumour biology has gained increasing relevance in this regard over the last decade. The NSABP B-14, NSABP B-20, Southwest Oncology Group-8814 (or SWOG-8814), and Trial Assigning Individualized Options for Treatment (TAILORx) studies have demonstrated the potential to deliver rationalized adjuvant chemoendocrine prescription based on recurrence scores calculated from genomic assays5,9-12 This has led to genomic assays, such as Oncotype DX (ODX) Recurrence Score (RS) (Genomic Health Inc., Redwood City, California) and MammaPrint (Agendia Precision Oncology, Amsterdam, The Netherlands), being incorporated as a component of modern BC management in ER+ cases.
Contemporary BC surgical management has been informed by evidence from well-designed, prospective randomized studies; however, most trials have focused their inclusion criteria on younger participants, possibly due to their greater potential survival benefit as well as their overall suitability for inclusion based on their functional status.13-15 This selection bias has led to consistent underrepresentation of elderly patients in clinical trials and a less robust evidence base regarding oncological best practice for these patients. The ‘Choosing Wisely’ campaign was established in 2012 in the United States in an attempt to address this discrepancy in treatment and the associated vulnerability for geriatric patients. 16 Data published from ‘Choosing Wisely’ suggests a more refined approach to oncogeriatric patient management in those with ER+, HER2−, cLN− BC may be beneficial where possible. 17 Although routine SLNB has revolutionized surgical management of the axilla for the vast majority of EBC patients, it is not without associated morbidity, and a shift to an even more conservative approach may now be considered for older patients if SLNB as an axillary staging investigation is not providing information that will inform/change further axillary or systemic treatment. This is likely to be the case considering the fact that the primary driver of adjuvant systemic therapy in contemporary BC management is tumour biology.18,19
Considering the changes in practice in the US following the Society of Surgical Oncology (SSO) Choosing Wisely recommendations, 17 the aims of the present study were to
Evaluate axillary management in an Irish Cohort of elderly patients diagnosed with early stage, ER+ BC;
Identify if SLNB affects further axillary management or adjuvant systemic therapy decision making in elderly patients diagnosed with ER+ BC.
Methods
Patient selection
Local ethical approval was obtained from the Galway University Hospitals Clinical Research Ethics Committee. A single-centre retrospective study was undertaken including all patients above the age of 65 years diagnosed and treated for ER+ EBC in a tertiary referral cancer centre between January 2005 and December 2015. Patients were defined as being elderly if they were ‘aged 66 years or older’ at the time of diagnosis in accordance to the World Health Organization’s definition of ‘elderly’, 20 and not that of the ‘Choosing Wisely’ campaign. All patients included had a diagnosis of tumour stage 1-2, ER+, HER2−, cLN− BC. Patients were categorized based on age into 3 subgroups; 66-70, 71-75, and >76 years. Patients were identified from a prospectively maintained institutional database and information regarding clinical patient, histopathological tumour, treatment characteristics, and survival data were updated from electronic and medical records.
Breast cancer diagnosis and work up
All patients underwent triple assessment as part of their EBC workup. Clinical examination was performed by a consultant breast surgeon. Standard radiological assessment consisted of mammography and ultrasound of the breast and ipsilateral axilla. Core biopsy was performed for clinically or radiologically suspicious lesions. All biopsied breast tissue was assessed by a consultant histopathologist with expertise in breast pathology. All breast tissue specimens were analysed in the accredited pathology laboratory at the tertiary referral centre. Staging was performed as per the American Joint Committee on Cancer (AJCC), version 8 Guidelines (2017). 21
Histopathological or immunohistochemical tumour evaluation and staging
ER and progesterone receptor (PgR) status were determined in accordance to American Society of Clinical Oncology/College of American Pathology guidelines and reported using Allred scoring.22-24 HER2 status was analysed using immunohistochemical analyses, 25 and fluorescence in-situ hybridization (or FISH) was requested in the case of equivocal 2+ results. 26 Tumour grade was determined using the Elston Ellis modification of the Scarff-Bloom-Richardson grading system.27,28 Lymphatic invasion was assessed using IHC staining with D2-40 and vascular invasion using CD34.29,30 Perineural invasion was determined using IHC staining with S-100 and a broad-spectrum keratin stain (AE1/AE3). 31 MIB1 antibody testing was used to Ki-67 proliferation. 32
Multidisciplinary care
All cases were discussed at a BC multidisciplinary meeting attended by consultant breast surgeons, histopathologists, radiologist, medical oncologists, and radiation oncologists. Decisions regarding patient specific treatment are determined from clinical, radiological, and pathological factors, as well as each patient’s performance status in line with international guidelines and best practice. Adjuvant prescription of chemoendocrine therapies for some patients diagnosed with ER+, HER2−, cLN− after 2007 were guided by ODX genomic testing following decisions allowing public reimbursement for the assay in oncological practice by the Irish government. 33 ODX testing was carried out in Genomic Health Inc, Redwood City, CA, USA. 34 Neoadjuvant treatment regimens, primary oncological surgeries and adjuvant treatment regimens were carried out in the tertiary referral centre. Patients returned to the tertiary referral centre for examination by a specialized breast surgeon postoperatively and returned for annual clinical and mammographic surveillance after BC treatment.
Patient follow up and clinical outcomes
Individual patient follow-up and survival data was recorded through a prospectively maintained database. All data was cross-referenced with patient electronic and medical records. Patient mortality was confirmed from data obtained from National Registries. The median and mean lengths of follow-up were assessed using the reverse Kaplan-Meier method. 35 Definition of DFS was ‘freedom from invasive disease recurrence, a second primary cancer or death’. Patients with ⩾ 3 LNs positive for disease following SLNB, were considered for CALND, as described in the ASOCOG Z0011 study. 7
Statistical analysis
Details regarding treatment characteristics within each age group were determined and descriptive statistics were used to inform clinicopathological associations with treatment characteristics (ACTX or CALND). Binary logistic regression analysis was used to ascertain patient and tumour characteristics predicting being in receipt of ACTX or CALND expressed in crude odds ratios (OR) with 95% confidence intervals (CIs). Variables with P < .050 in univariable analysis were included in the multivariable logistic regression analysis, which was then used to identify variables that contributed independently to being spared ACTX or CALND. Kaplan-Meier curves and the Log-rank (Mantel-Cox) test were used to associate survival with treatment characteristics. All tests of significance were 2-tailed, with P < .050 indicating statistical significance. Data were analysed using Statistical Package for Social Sciences (SPSS) version 26.
Results
Patient demographics and clinicopathological characteristics
There were 253 patients aged > 65 years diagnosed with ER+ EBC included in this study. The mean age at diagnosis was 73.2 ± 5.5 years (range: 66-90 years, median age: 72). There were 99 patients aged 66-70 (39.1%), 75 aged 71-75 (29.6%), and 79 patients aged >76 years at diagnosis (31.2%). The mean follow-up was 95.0 months (range: 3.0-185.2 months)
All patients were diagnosed with ER+, HER2−, cLN− primary BC, 121 of which were T1 (47.8%) and 132 of whom had T2 disease (52.2%). The majority had grade 2 tumours (80.6%, 204/253) and 203 had invasive ductal carcinoma histological subtype (80.2%). All tumours were ER+ (100.0%), and the mean ER score was 7.8 ± 0.1 (range: 3-8, median: 8). Overall, 214 tumours had positive PgR expression (84.6%), and the mean PgR score was 5.6 ± 2.1 (range: 0-8, median: 7). Clinicopathological data are outlined in Table 1.
Table 1.
Clinicopathological, immunohistochemical, and treatment characteristics for 253 patients aged more than 65 diagnosed with oestrogen receptor positive, clinically lymph node negative breast cancer.
| Clinicopathological, immunohistochemical and treatment characteristics | ||
|---|---|---|
| Age at diagnosis (years) | Mean ± SD (range); median | 73.2 ± 5.5 (66-90); 72 |
| Aged 66-70 | 99 (39.1%) | |
| Aged 71-75 | 75 (29.7%) | |
| Aged > 75 | 79 (31.2%) | |
| Histological tumour type | Invasive ductal carcinoma | 203 (80.2%) |
| Invasive lobular carcinoma | 42 (16.6%) | |
| Other (mucinous, micropapillary, papillary, tubular, tubulolobular, etc) | 8 (3.2%) | |
| Tumour size (mm) | Mean ± SD (range), median | 22.7 ± 10.5 (1-50), 22 |
| Tumour stage | T1 | 121 (47.8%) |
| T2 | 132 (52.2%) | |
| Tumour grade | 1 | 15 (5.9%) |
| 2 | 204 (80.6%) | |
| 3 | 34 (13.4%) | |
| Lymphovascular invasion, n (%) | Present | 43 (17.0%) |
| Absent | 210 (83.0%) | |
| ER score | Mean ± standard deviation (range), median | 7.8 ± 0.1, 8 |
| PgR status PgR score | Positive | 214 (84.6%) |
| Negative | 39 (15.4%) | |
| Mean ± standard deviation (range), median | 5.6 ± 2.1, 7 | |
| Ki67 proliferation index (%) (n = 42) | Mean ± standard deviation (range), median | 13.6 ± 1.7, 10 |
| Primary surgery | Breast conserving surgery | 219 (86.6%) |
| Mastectomy | 34 (13.4%) | |
| SLNB SLNB nodal yield | Underwent SLNB | 253 (100.0%) |
| Did not undergo SLNB | 0.0 (0.0%) | |
| Median ± standard deviation (range) | 3.0 ± 2.5 (1-12) | |
| OncotypeDX score (n = 82, 32.4%) | Score Mean ± standard deviation (range), median | 17.8 ± 9.8 (1-44) |
| Low risk (0-10) | 13 (15.9%) | |
| Intermediate risk (11-25) | 51 (62.2%) | |
| High risk (> 25) | 18 (22.0%) | |
| Adjuvant endocrine therapy | Yes | 253 (100.0%) |
| No | 0.0 (0.0%) | |
| Adjuvant chemotherapy | Yes | 51 (20.2%) |
| No | 202 (79.8%) | |
| Adjuvant radiotherapy | Yes | 173 (68.4%) |
| No | 80 (31.6%) | |
| Completion ALND | Yes | 19 (7.5%) |
| No | 234 (92.5%) | |
Abbreviations: ALND, axillary lymph node dissection; ER, oestrogen receptor; PgR, progesterone receptor; SD, standard deviation; SLNB, sentinel lymph node biopsy.
Treatment characteristics
In total, 219 patients underwent BCS (86.6%). All patients in this series underwent SLNB as part of their oncological staging, with a median of 3.0 ± 2.3 nodes excised for histopathological analysis. Nineteen patients proceeded to CALND (7.5%, 19/253) and rates were similar for all age groups (P = .973 χ2) (Figure 1). The ODX testing was performed in 82 cases (32.4%, mean score 17.8 ± 8.8, range: 1-44); 13 of which were in the low risk group (ODX < 11, 15.9%), 51 in the intermediate risk group (ODX: 11-25, 62.2%), and 18 in the high-risk group (ODX > 25, 22.0%). All patients received adjuvant EHT (100.0%) and 51 and 173 patients were treated with ACTX and radiotherapy (21.2% and 68.4%, respectively). In patients aged 66-70 years, 31.3% received ACTX (31/99), as did 20.0% aged 71-75 years (15/75), and 6.3% of those aged > 75 years (5/79) (P < .001, χ2). Treatment characteristics based on age groups and nodal status are outlined in Tables 2 and 3.
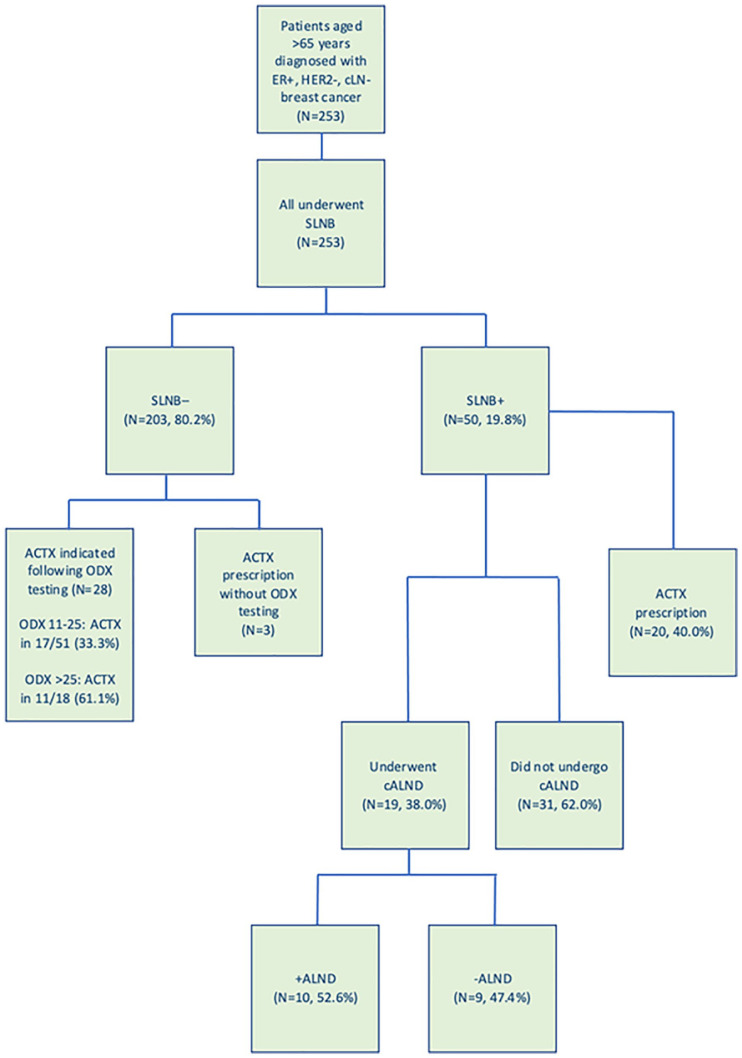
Figure 1.
Flow diagram of the clinical utility of sentinel lymph node biopsy in guiding adjuvant treatment strategies in patients diagnosed with oestrogen receptor positive, clinically node negative breast cancer aged greater than 65 years.
ACTX indicates adjuvant chemotherapy; cALND, completion axillary lymph node dissection; cLN−, clinically lymph node negative; ER+, oestrogen receptor positive; HER2−, human epidermal growth factor receptor-2 negative; ODX, OncotypeDX Recurrence Score testing; SLNB, sentinel lymph node biopsy.
Table 2.
Adjuvant therapy and tumour characteristics for patients with oestrogen receptor positive, clinically node negative breast cancer (N = 253).
| Tumour characteristics | Age 66-70 (n = 99, 39.1%) | Age 71-75 (n = 75, 29.7%) | Age > 75 (n = 79, 31.2%) | Total | P-value |
|---|---|---|---|---|---|
| Grade | .294 χ2 | ||||
| 1 | 7 (7.1%) | 5 (6.6%) | 3 (3.8%) | 15 (5.9%) | |
| 2 | 75 (75.6%) | 59 (78.7%) | 70 (88.6%) | 204 (80.6%) | |
| 3 | 17 (17.3%) | 11 (14.7%) | 6 (7.6%) | 34 (13.4%) | |
| Tumour stage | .093† | ||||
| 1 | 55 (55.6%) | 35 (46.7%) | 31 (39.8%) | 121 (47.8%) | |
| 2 | 44 (44.4%) | 40 (54.3%) | 48 (60.2%) | 132 (52.2%) | |
| Histological subtype | .906 χ2 | ||||
| IDC | 81 (81.8%) | 58 (77.3%) | 64 (81.0%) | 203 (80.2%) | |
| ILC | 15 (15.1%) | 15 (20.0%) | 12 (15.2%) | 42 (16.6%) | |
| Other | 3 (3.0%) | 2 (2.7%) | 3 (6.3%) | 8 (3.2%) | |
| Oncotype DX Group (n = 82) | .573 χ2 | ||||
| Low risk (< 11) | 6 (14.0%) | 2 (10.0%) | 5 (26.3%) | 13 (15.9%) | |
| Intermediate risk (11-25) | 28 (65.1%) | 12 (60.0%) | 11 (57.9%) | 51 (62.2%) | |
| High risk (> 25) | 9 (20.9%) | 6 (30.0%) | 3 (15.8%) | 18 (22.0%) | |
| Surgery | .462† | ||||
| BCS | 84 (38.4%) | 68 (31.1%) | 67 (30.6%) | 219 (86.6%) | |
| Mastectomy | 15 (44.1%) | 7 (20.6%) | 12 (35.3%) | 34 (13.4%) | |
| Adjuvant chemotherapy | <.001* χ2 | ||||
| Underwent treatment | 31 (31.3%) | 15 (20.0%) | 5 (6.3%) | 51 (20.2%) | |
| Did not undergo treatment | 68 (68.7%) | 60 (80.0%) | 74 (93.7%) | 202 (79.8%) | |
| Adjuvant radiotherapy | .184 χ2 | ||||
| Underwent treatment | 70 (70.7%) | 56 (74.7%) | 48 (60.8%) | 173 (68.4%) | |
| Did not undergo treatment | 29 (28.3%) | 19 (25.3%) | 31 (39.2%) | 80 (31.6%) | |
| Completion ALND | .973 χ2 | ||||
| Underwent treatment | 92 (92.9%) | 69 (92.0%) | 73 (92.4%) | 234 (92.5%) | |
| Did not undergo treatment | 7 (7.1%) | 6 (8.0%) | 6 (7.6%) | 19 (7.5%) | |
Abbreviations: ALND, axillary lymph node dissection; BCS, breast conserving surgery; IDC, invasive ductal carcinoma; ILC, invasive lobular carcinoma.
χ2 denotes chi-square test.
denotes Fisher’s exact test.
denotes statistical significance.
Table 3.
Descriptive statistics illustrating treatment strategies based on nodal status.
| Parameter | Node negative on SLNB (N = 203) | Node positive on SLNB (N = 50) | P-value |
|---|---|---|---|
| Primary surgery | |||
| BCS | 180 | 38 | .063 |
| Mastectomy | 23 | 11 | |
| Adjuvant chemotherapy | |||
| Underwent treatment | 31 | 20 | <.001*† |
| Did not undergo treatment | 172 | 30 | |
| Adjuvant radiotherapy | |||
| Underwent treatment | 140 | 33 | .798 |
| Did not undergo treatment | 73 | 17 | |
| Completion ALND | |||
| Underwent treatment | 0 | 19 | <.001*† |
| Did not undergo treatment | 203 | 31 | |
Abbreviations: ALND, axillary lymph node dissection; BCS, breast conservation surgery; SLNB, sentinel lymph node biopsy.
SLNB; Sentinel lymph node biopsy, BCS; Breast conservation surgery, ALND; axillary lymph node dissection.
denotes Fisher’s exact test.
denotes statistical significance.
Clinical utility of sentinel lymph node biopsy: axillary surgery
Of the 253 patients with cLN− BC, 203 had confirmed node negative disease following SLNB (80.2%) (Figure 1). Fifty patients had histopathological confirmed node positive disease (19.8%), 19 of whom proceeded to CALND (38.0%, 19/50) (P < .001†). Of the 234 patients spared CALND, 13.2% had ⩽ 3 positive nodes (N = 31), while all patients who underwent CALND had ⩾ 3 positive nodes at SLNB (100.0%, 19/19). Following CALND, 10 patients had further positive nodes on ALND (ALND+) (10/19, 52.6%).
Using univariable analyses, patients with PgR− tumours (OR: 5.861, 95% CI: 1.138-30.191, P = .034) and ⩾ 3 positive LNs on SLNB (OR: 16.200, 95% CI: 1.426-184.057, P = .025) were likely to have further positive nodes following CALND. Following multivariable analysis, both PgR− (OR: 5.742, 95% CI: 1.066-30.933, P = .042) and ⩾ 3 positive LNs on SLNB (OR: 15.617, 95% CI: 1.169-208.680, P = .038) independently predicted patients likely to have ALND+ (Table 4). No patient who proceeded to CALND had previously undergone ODX testing (0/19, 0.0%). For all 253 patients, SLNB+ was associated with proceeding to CALND (P < .001†), and there were no other factors predictive of those undergoing CALND (Tables 5 and 6).
Table 4.
Clinicopathological patient factors predictive of those likely to have further positive lymph nodes following completion axillary lymph node dissection.
| Parameter | Univariable |
Multivariable |
||||
|---|---|---|---|---|---|---|
| OR | 95% CI | P-value | OR | 95% CI | P-value | |
| Age < 75 | 1.104 | 0.198-6.156 | .910 | |||
| IDC subtype | 1.035 | 0.118-9.097 | .975 | |||
| Tumour size > 20 mm | 3.055 | 0.336-27.771 | .321 | |||
| Grade 3 | 0.460 | 0.082-2.588 | .378 | |||
| LVI | 0.998 | 0.957-1.040 | .910 | |||
| PgR− | 5.861 | 1.138-30.181 | .034a | 5.742 | 1.066-30.933 | .042* |
| ⩾ 3 +veLN on SLNB | 16.200 | 1.426-184.057 | .025a | 15.617 | 1.169-208.680 | .038* |
Abbreviations: CI, confidence interval; IDC, invasive ductal carcinoma; LVI, lymphovascular invasion; OR, odds ratio; PgR−, progesterone receptor negativity; SLNB, sentinel lymph node biopsy; +veLN, positive lymph nodes.
SLNB; sentinel lymph node biopsy.
denotes statistical significance.
Table 5.
Clinicopathological characteristics and their relationships with adjuvant chemotherapy prescription and completion axillary lymph node dissection (N = 253).
| Clinicopathological characteristics | Received ACTX | Did not receive ACTX | P-value | Underwent cALND | Did not undergo cALND | P-value |
|---|---|---|---|---|---|---|
| Age | .954† | |||||
| < 75 years | 46 | 128 | 7 | 3 | ||
| > 75 years | 5 | 74 | <.001* † | 8 | 3 | |
| Histological subtype | .788 χ2 | |||||
| IDC | 45 | 158 | 9 | 10 | ||
| ILC | 6 | 36 | 1 | 1 | ||
| Other | 0 | 8 | .180 χ2 | 0 | 0 | |
| Tumour stage | .639† | |||||
| 1 | 21 | 100 | 4 | 4 | ||
| 2 | 30 | 102 | .182† | 6 | 7 | |
| Grade | .477 χ2 | |||||
| 1 | 0 | 15 | 0 | 0 | ||
| 2 | 44 | 160 | 7 | 11 | ||
| 3 | 7 | 27 | .257 χ2 | 3 | 0 | |
| PgR status | .079 χ2 | |||||
| PgR+ | 41 | 173 | 6 | 10 | ||
| PgR− | 10 | 29 | .386† | 4 | 1 | |
| Ki67 proliferation index (N = 42) | .659† | |||||
| Ki67 ⩽ 14% | 6 | 26 | 4 | 28 | ||
| Ki67 > 14% | 1 | 9 | .461† | 1 | 9 | |
| Surgery | .308† | |||||
| BCS | 44 | 175 | .551† | 8 | 8 | |
| Mastectomy | 7 | 27 | 2 | 3 | ||
| SLNB | <.001* † | |||||
| Positive SLNB | 20 | 30 | 10 | 9 | ||
| Negative SLNB | 31 | 172 | <.001* † | 0 | 0 | |
| Oncotype DX Group (N = 82) | N/A | |||||
| Low risk (< 11) | 2 | 11 | .003* χ2 | N/A | N/A | |
| Intermediate risk (11-25) | 15 | 36 | ||||
| High risk (> 25) | 11 | 7 | ||||
Abbreviations: ACTX, adjuvant chemotherapy; BCS, breast conserving surgery; cALND, completion axillary lymph node dissection; IDC, invasive ductal carcinoma; ILC, invasive lobular carcinoma; N/A, not applicable; PgR, progesterone receptor; SLNB, sentinel lymph node biopsy.
χ2 denotes chi-square test.
denotes statistical significance.
denotes Fisher’s exact test.
Table 6.
Clinicopathological patient factors predictive of those undergoing completion axillary lymph node dissection.
| Parameter | Univariable |
Multivariable |
||||
|---|---|---|---|---|---|---|
| OR | 95% CI | P-value | OR | 95% CI | P-value | |
| Age < 75 | 0.982 | 0.359-2.687 | .972 | |||
| IDC subtype | 1.828 | 0.406-8.229 | .432 | |||
| Tumour size > 20 mm | 1.546 | 0.559-4.275 | .401 | |||
| cT2 | 1.629 | 0.619-4.282 | .323 | |||
| Grade 3 | 1.277 | 0.357-4.570 | .707 | |||
| LVI | 0.997 | 0.971-1.024 | .842 | |||
| PgR− | 2.101 | 0.711-6.211 | .179 | |||
| Ki67 > 14% | 0.778 | 0.077-7.886 | .832 | |||
Abbreviations: CI, confidence interval; cT2, clinical tumour stage 2 disease; IDC, invasive ductal carcinoma; LVI, lymphovascular invasion; OR, odds ratio; PgR−, progesterone receptor negativity.
Completion axillary lymph node dissection and clinical outcomes
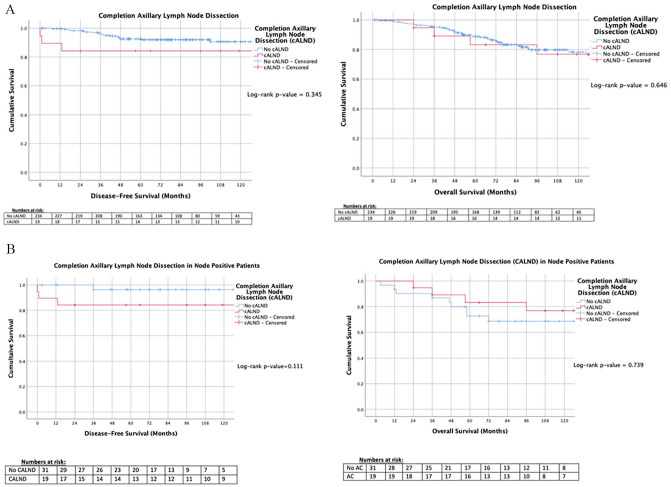
For all 253 patients, DFS and overall survival (OS) was similar for patients undergoing CALND when compared to those who did not (P = .345 and P = .646, respectively, log-rank test ‡) (Figure 2A). In patients with LN+ disease, all patients who did not proceed to CALND had ⩽ 3 LN+ (31/31, range: 0-3 LNs, median: 1 ± 1.3 LNs), while 84.2% of those undergoing CALND did had ⩽ 3 LN+ (16/19, range: 1-7, median: 2 ± 2.3 LNs) (P = .081 χ2). Patients with LN+ disease who were spared CALND had similar LRR, DFS and OS to those who underwent CALND (P = .111 and P = .739, respectively, ‡) (Figure 2B). Twenty-three patients had disease recurrence, of whom, 73.9% had distant recurrence (17/23), while 26.1% had locoregional recurrence (LRR), (6/23). Of those who developed LRR, 50.0% had previously underwent CALND following SLNB (3/6), while the other 3 were SLN− at the time of primary surgery.
Figure 2.
Kaplan-Meier analyses for patients who underwent completion axillary lymph node dissection versus those who did not in the (A) overall cohort, and (B) in those with node positive disease.
AC, indicates adjuvant chemotherapy; cALND, completion axillary lymph node dissection.
Clinical utility of sentinel lymph node biopsy: systemic chemotherapy
Overall, 51 patients were treated with ACTX (21.2%), 20 of these were LN+ and 31 were LN− (39.2%, vs 60.8%). ACTX prescription in 28 patients with LN− disease was guided through ODX testing (90.3%, 28/31). Of those undergoing ODX testing and receiving ACTX, the mean ODX score was 22.1 ± 8.1 (range: 11-44, P = .452 χ2). Figure 2 demonstrates the clinical utility of SLNB in guiding ACTX and CALND.
For the 253 patients included in this series, age at diagnosis, having positive SLNB, and ODX score were all associated with receiving ACTX (P < .001†, P < .001†, and P = .003, respectively, all χ2) (Table 5). Using univariable binary logistic regression analyses, it was demonstrated that age less than 75 years at diagnosis (OR: 5.319, 95% CI: 2.024-13.979, P = .001), having SLNB+ (OR: 3.699, 95% CI: 1.868-7.323, P < .001), and having an ODX score > 25 (OR: 4.345, 95% CI: 1.449-13.026, P = .009) were factors predictive of patients receiving ACTX. On multivariable analyses, ODX score > 25 was the sole predictor of patients receiving ACTX (OR: 4.368, 95% CI: 1.382-13.801, P = .012) (Table 7).
Table 7.
Clinicopathological patient factors predictive of those in receipt of adjuvant chemotherapy.
| Parameter | Univariable |
Multivariable |
||||
|---|---|---|---|---|---|---|
| OR | 95% CI | P-value | OR | 95% CI | P-value | |
| Age < 75 | 5.319 | 2.024-13.979 | .001a | |||
| IDC subtype | 1.709 | 0.677-4.312 | .257 | |||
| Tumour size > 20 mm | 1.809 | 0.579-2.048 | .791 | |||
| cT2 | 1.401 | 0.752-2.609 | .289 | |||
| Grade 3 | 1.611 | 0.676-3.838 | .282 | |||
| LVI | 0.997 | 0.976-1.018 | .753 | |||
| PgR− | 1.455 | 0.657-3.223 | .355 | |||
| Ki67 > 14% | 0.481 | 0.051-4.562 | .524 | |||
| SLNB+ | 3.699 | 1.868-7.323 | <.001a | |||
| ODX score > 25 | 4.345 | 1.449-13.026 | .009a | 4.368 | 1.382-13.801 | .012* |
Abbreviations: CI, confidence interval; cT2, clinical tumour stage 2 disease; IDC, invasive ductal carcinoma; LVI, lymphovascular invasion; ODX, OncotypeDX Recurrence Score testing; OR, odds ratio; PgR−, progesterone receptor negativity; SLNB+, positive sentinel lymph node biopsy.
OR; odds ratio, 95% CI; 95% confidence interval, IDC; invasive ductal carcinoma, cT2; clinical tumour stage 2 disease, LVI; lymphovascular invasion, PgR-; progesterone receptor negativity,
SLNB+; positive sentinel lymph node biopsy, ODX testing.
denotes statistical significance.
Adjuvant chemotherapy and clinical outcomes
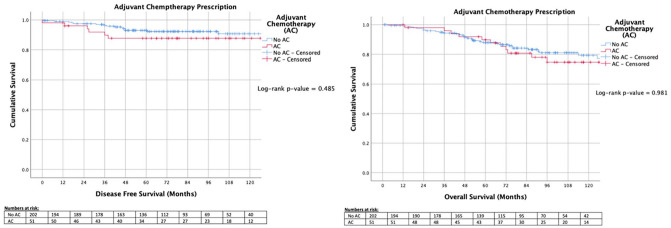
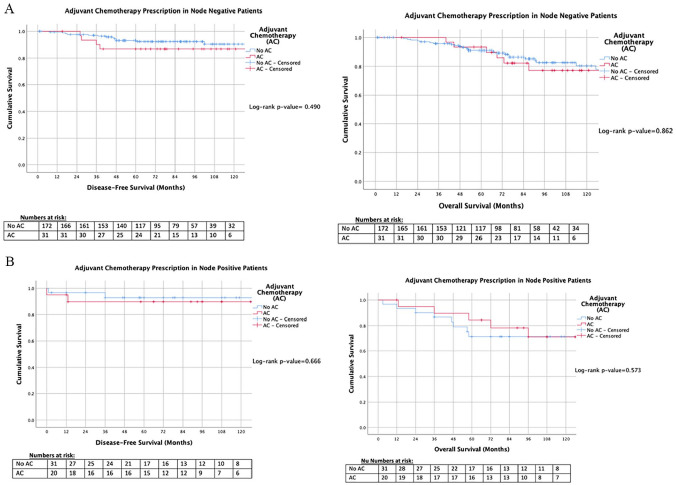
Overall, patients in this series demonstrated a DFS of 90.9% (230/253) and an OS of 80.2% (203/253). DFS and OS were similar for patients irrespective of receiving ACTX (P = .485 and P = .981, respectively, ‡) (Figure 3). For patients with LN− disease, DFS and OS were similar (P = .490 and P = .862, respectively, ‡) (Figure 4A), and for those with LN+ disease, survival outcomes were similar (P = .666 and P = .573, respectively, ‡) (Figure 4B).
Figure 3.
Kaplan-Meier analyses for patients in receipt of adjuvant chemotherapy versus those spared adjuvant chemotherapy.
AC indicates adjuvant chemotherapy.
Figure 4.
Kaplan-Meier analyses for patients who received adjuvant chemotherapy versus those who did not in the (A) node negative cohort, and (B) in those with node positive disease.
AC indicates adjuvant chemotherapy.
Discussion
Traditionally, histopathological features such as tumour size and SLNB+ were predominant factors in guiding decision-making with regard to systemic therapies and the requirement for aggressive locoregional treatment through ALND. However, the majority of elderly patients develop tumours of favourable biology, that is, ER+ve.36,37 In the era of personalized medicine, genomic analysis aims to tailor multimodal therapeutic strategies on the basis of case-specific clinicopathological and molecular properties, instead of standard histopathologic and immunohistochemical assessment.5,9-12,38 There is also a growing recognition that elderly patients are frequently overlooked as participants in oncological research studies, indirectly leaving this cohort vulnerable to over-investigation and overtreatment. Thus, recent studies have proposed ‘de-escalation’ of local therapies and omission of SLNB in elderly patients with EBC, in particular those with favourable prognosis, such as ER+ disease.39,40
The most important finding in this series of elderly patients diagnosed with ER+ EBC is that in spite of SLNB being clinically indicated in all 253 cases, decision making relating to ACTX was more likely to be guided by genomic testing (ODX) than histopathological results of SLNB. Being aged < 75 at diagnosis, having SLNB+, and ODX score > 25 were all factors predictive of ACTX prescription, however, ODX score > 25 was the sole independent predictor following multivariable analysis. These findings are supported by the results of other large volume studies,41,42 and are reflected in the modern multimodal treatment paradigm for early ER+ disease, where clinical decision-making regarding tailoring of chemoendocrine therapeutic strategies is primarily determined by genomic tissue analyses.18,19 Consequently, the estimated risk of distant recurrence, BC-specific mortality and resulting benefit from ACTX is individualized to the patient and their specific tumour biology, with considerations surrounding functional status evaluated by the multidisciplinary team.
Other results of this univariable analysis are somewhat predictable: Patients aged > 75 years at diagnosis were less likely to undergo ACTX than their counterparts, while those with SLNB+ were likely to be prescribed systemic chemotherapy (Table 7). The loss of physical functionality and resultant disability makes elderly patients less likely to tolerate the toxicities associated with ACTX: data from Given and colleagues suggests that up to 60% of patients aged 65 to 74 years report some form physical disability and unsuitability for systemic therapies. 43 These results validate the rationale for prudent use of ACTX in the elderly. Histopathological evaluation of the SLNB has traditionally been one of the catalysts for guiding adjuvant systemic therapies, and therefore it is unsurprising that SLNB+ predicted progression to ACTX. With improvements in clinical staging diagnostic modalities, and the increasing availability and affordability of genomic testing, eliminating the routine requirement for invasive axillary sampling in low-risk, elderly BC patients is mooted in modern practice.44
The primary reason for performing SLNB is to allow for accurate staging and to determine the requirement for ACTX and CALND, however survival outcomes following these interventions after SLNB in elderly patients is less well described. In this series, receipt of ACTX failed to improve DFS or OS for elderly patients with ER+ BC, irrespective of nodal status. The authors acknowledge the possibility of a Type II error due to the small study population and few event rates, as is anticipated with EBC, as well as the increase in competing risks for death in older patients. However, this also highlights the challenges faced in optimizing therapy to maximize life expectancy and the quality of life, while minimizing morbidity for this patient group. Data suggests that negative implications of ACTX prescription often outweigh the perceived benefits in elderly and comorbid patients, 45 with only 15% of patients with cLN− examinations aged > 70 years experienced cancer-related mortality. These statistics demonstrate the difficulty in achieving a survival benefit from prescription of toxic chemotherapy in this group, without risking iatrogenic toxicities or organ failure. 46 Consequently, the authors propose judicious use of ACTX in elderly patients with EBC, with prescription stratified through individualized risk following genomic analysis, obviating the need for SLNB to guide therapeutic decision-making through axillary staging, in this subset of patients.
As previously described, breakthrough trials have reduced indications for surgical resection through CALND in EBC,6-8 and modern studies suggest exemption in elderly patients, given the associated morbidity, minimal survival benefit and oncological control following complete clearance of the axilla.44,47 This recent vogue is further supported by randomized data illustrating no added survival advantage from performing CALND in patients aged > 70 years with clinically occult axillary lymph nodes. 48 Eighty percent of patients in this study had node negative disease, however, for those with node positive disease, proceeding to CALND failed to enhance locoregional control or survival. These results, in tandem with the clinical utility of genomic testing in predicting ACTX prescription, suggest routine SLNB may be unnecessary within this elderly cohort. Focusing on reducing invasive initial diagnostic staging may prove more beneficial in reducing the harmful effects of over-investigation and overtreatment; sonographic axillary staging may be non-inferior to SLNB, with regard to excluding clinically significant metastatic disease in the axilla and requirement for CALND.49,50 Thus, less-invasive staging and management of the axilla may reduce morbidity without any compromise in oncologic outcomes for the elderly, with the further possibility of additional cost-savings over current management strategies.51-53
This study suffers the inherent limitations of being a single centre, retrospective cohort study implying potential selection, ascertainment and confounding bias. Competing risks affecting survival within the elderly cohort as well as the relatively small numbers powering this study may limit conclusions which can be drawn in relation to survival. Furthermore, the failure to derive clinicopathological predictors of patients undergoing CALND is likely explained by a Type II statistical error due to a limited number of participants proceeding to axillary clearance. However, the data reflects real world practice in a multidisciplinary setting and so the results are relevant to clinicians involved in contemporary BC management.
Conclusions
Sentinel lymph node biopsy seems less valuable in guiding axillary surgical management and prescription of ACTX than previously perceived in those aged >65 years with ER+ EBC. Genomic testing offers a personalized and less-invasive means of guiding ACTX prescription in this group, and this may better serve this cohort that are at particular risk of over-investigation and treatment. Moreover, CALND and ACTX prescription indicated through SLNB failed to improve survival outcomes for patients in this series, irrespective of nodal status. These findings suggest the omission of SLNB may not be detrimental to clinical outcomes for elderly patients diagnosed with EBC of favourable tumour biology.
Footnotes
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: MGD received funding from the National Breast Cancer Research Institute, Ireland.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Author Contributions: Study design was performed by AJL. DB and MGD collected data included in this analysis. MGD and EJR performed statistical analysis and drafting of the manuscript. KM and PFM provided corrections. MJK and AJL reviewed manuscript drafts.
ORCID iD: Matthew G Davey  https://orcid.org/0000-0002-9892-9920
https://orcid.org/0000-0002-9892-9920
References
- 1. Tesarova P. Breast cancer in the elderly – should it be treated differently? Rep Pract Oncol Radiother. 2012;18:26-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Rostas JW, Dyess DL. Current operative management of breast cancer: an age of smaller resections and bigger cures. Int J Breast Cancer. 2012;2012:516417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Gherghe M, Bordea C, Blidaru A. Sentinel lymph node biopsy (SLNB) vs. axillary lymph node dissection (ALND) in the current surgical treatment of early stage breast cancer. J Med Life. 2015;8:176-180. [PMC free article] [PubMed] [Google Scholar]
- 4. Vazquez B, Rousseau D, Hurd TC. Surgical management of breast cancer. Semin Oncol. 2007;34:234-240. [DOI] [PubMed] [Google Scholar]
- 5. Sparano JA, Gray RJ, Makower DF, et al. Adjuvant chemotherapy guided by a 21-gene expression assay in breast cancer. N Engl J Med. 2018;379:111-121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Krag DN, Anderson SJ, Julian TB, et al. Technical outcomes of sentinel-lymph-node resection and conventional axillary-lymph-node dissection in patients with clinically node-negative breast cancer: results from the NSABP B-32 randomised phase III trial. Lancet Oncol. 2007;8:881-888. [DOI] [PubMed] [Google Scholar]
- 7. Giuliano AE, Ballman KV, McCall L, et al. Effect of axillary dissection vs no axillary dissection on 10-year overall survival among women with invasive breast cancer and sentinel node metastasis: the ACOSOG Z0011 (alliance) randomized clinical trial. JAMA. 2017;318:918-926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Giuliano AE, Hunt KK, Ballman KV, et al. Axillary dissection vs no axillary dissection in women with invasive breast cancer and sentinel node metastasis: a randomized clinical trial. JAMA. 2011;305:569-575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Albain KS, Barlow WE, Shak S, et al. Prognostic and predictive value of the 21-gene recurrence score assay in postmenopausal women with node-positive, oestrogen-receptor-positive breast cancer on chemotherapy: a retrospective analysis of a randomised trial. Lancet Oncol. 2010;11:55-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Carlson JJ, Roth JA. The impact of the Oncotype Dx breast cancer assay in clinical practice: a systematic review and meta-analysis. Breast Cancer Res Treat. 2013;141:13-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Fisher B, Jeong JH, Bryant J, et al. Treatment of lymph-node-negative, oestrogen-receptor-positive breast cancer: long-term findings from National Surgical Adjuvant Breast and Bowel Project randomised clinical trials. Lancet. 2004;364:858-868. [DOI] [PubMed] [Google Scholar]
- 12. Paik S, Shak S, Tang G, et al. A multigene assay to predict recurrence of tamoxifen-treated, node-negative breast cancer. N Engl J Med. 2004;351:2817-2826. [DOI] [PubMed] [Google Scholar]
- 13. Kemeny MM, Peterson BL, Kornblith AB, et al. Barriers to clinical trial participation by older women with breast cancer. J Clin Oncol. 2003;21:2268-2275. [DOI] [PubMed] [Google Scholar]
- 14. Lewis JH, Kilgore ML, Goldman DP, et al. Participation of patients 65 years of age or older in cancer clinical trials. J Clin Oncol. 2003;21:1383-1389. [DOI] [PubMed] [Google Scholar]
- 15. Early Breast Cancer Trialists’ Collaborative Group (EBCTCG). Long-term outcomes for neoadjuvant versus adjuvant chemotherapy in early breast cancer: meta-analysis of individual patient data from ten randomised trials. Lancet Oncol. 2018;19:27-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Born KB, Levinson W. Choosing Wisely campaigns globally: a shared approach to tackling the problem of overuse in healthcare. J Gen Fam Med. 2018;20:9-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Calderon E, Webb C, Kosiorek HE, et al. Are we choosing wisely in elderly females with breast cancer? Am J Surg. 2019;218:1229-1233. [DOI] [PubMed] [Google Scholar]
- 18. Cardoso F, Kyriakides S, Ohno S, et al. Early breast cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2019;30:1194-1220. [DOI] [PubMed] [Google Scholar]
- 19. Krop I, Ismaila N, Andre F, et al. Use of biomarkers to guide decisions on adjuvant systemic therapy for women with early-stage invasive breast cancer: American Society of Clinical Oncology clinical practice guideline focused update. J Clin Oncol. 2017;35:2838-2847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. World Health Organization. Proposed working definition of an older person in Africa for the MDS Project. https://www.who.int/healthinfo/survey/ageingdefnolder/en/. Published 2010. Accessed August 18, 2020.
- 21. Amin MB, Edge S, Greene F, et al. AJCC Cancer Staging Manual. 8th ed. Cham, Switzerland: Springer; 2017. [Google Scholar]
- 22. Allred DC. Issues and updates: evaluating estrogen receptor-α, progesterone receptor, and HER2 in breast cancer. Mod Pathol. 2010;23:S52-S59. [DOI] [PubMed] [Google Scholar]
- 23. Hammond MEH, Hayes DF, Dowsett M, et al. American Society of Clinical Oncology/College of American Pathologists guideline recommendations for immunohistochemical testing of estrogen and progesterone receptors in breast cancer. Arch Pathol Lab Med. 2010;134:907-922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Allred DC, Harvey JM, Berardo M, Clark GM. Prognostic and predictive factors in breast cancer by immunohistochemical analysis. Mod Pathol. 1998;11:155-168. [PubMed] [Google Scholar]
- 25. Wolff AC, Hammond ME, Schwartz JN, et al. American Society of Clinical Oncology/College of American Pathologists guideline recommendations for human epidermal growth factor receptor 2 testing in breast cancer. J Clin Oncol. 2007;25:118-145. [DOI] [PubMed] [Google Scholar]
- 26. Press MF, Sauter G, Buyse M, et al. HER2 gene amplification testing by fluorescent in situ hybridization (FISH): comparison of the ASCO-College of American Pathologists guidelines with FISH scores used for enrollment in Breast Cancer International Research Group Clinical Trials. J Clin Oncol. 2016;34:3518-3528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Bloom HJ, Richardson WW. Histological grading and prognosis in breast cancer; a study of 1409 cases of which 359 have been followed for 15 years. Br J Cancer. 1957;11:359-377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Elston CW, Ellis IO. Pathological prognostic factors in breast cancer. I. The value of histological grade in breast cancer: experience from a large study with long-term follow-up. Histopathology. 1991;19:403-410. [DOI] [PubMed] [Google Scholar]
- 29. Chen Z, Xu S, Xu W, et al. Expression of cluster of differentiation 34 and vascular endothelial growth factor in breast cancer, and their prognostic significance. Oncol Lett. 2015;10:723-729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Fohn LE, Rodriguez A, Kelley MC, et al. D2-40 lymphatic marker for detecting lymphatic invasion in thin to intermediate thickness melanomas: association with sentinel lymph node status and prognostic value-a retrospective case study. J Am Acad Dermatol. 2011;64:336-345. [DOI] [PubMed] [Google Scholar]
- 31. Brown IS. Pathology of perineural spread. J Neurol Surg B Skull Base. 2016;77:124-130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Dowsett M, Nielsen TO, A’Hern R, et al. Assessment of Ki67 in breast cancer: recommendations from the International Ki67 in Breast Cancer working group. J Natl Cancer Inst. 2011;103:1656-1664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. McVeigh TP, Kerin MJ. Clinical use of the Oncotype DX genomic test to guide treatment decisions for patients with invasive breast cancer. Breast Cancer (Dove Med Press). 2017;9:393-400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. McVeigh TP, Hughes LM, Miller N, et al. The impact of Oncotype DX testing on breast cancer management and chemotherapy prescribing patterns in a tertiary referral centre. Eur J Cancer. 2014;50:2763-2770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Xue X, Agalliu I, Kim MY, et al. New methods for estimating follow-up rates in cohort studies. BMC Med Res Methodol. 2017;17:155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Pierga JY, Girre V, Laurence V, et al. Characteristics and outcome of 1755 operable breast cancers in women over 70 years of age. Breast. 2004;13:369-375. [DOI] [PubMed] [Google Scholar]
- 37. Davey MG, Brennan M, Ryan ÉJ, et al. Defining clinicopathological and radiological features of breast cancer in women under the age of 35: an epidemiological study. Ir J Med Sci. 2020;189:1195-1202. [DOI] [PubMed] [Google Scholar]
- 38. Bear HD, Wan W, Robidoux A, et al. Using the 21-gene assay from core needle biopsies to choose neoadjuvant therapy for breast cancer: a multicenter trial. J Surg Oncol. 2017;115:917-923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Christian N, Heelan Gladden A, Friedman C, et al. Increasing omission of radiation therapy and sentinel node biopsy in elderly patients with early stage, hormone-positive breast cancer. Breast J. 2020;26:133-138. [DOI] [PubMed] [Google Scholar]
- 40. Wu S-G, Zhang WW, Sun JY, Li FY, Chen YX, He ZY. Omission of postoperative radiotherapy in women aged 65 years or older with tubular carcinoma of the breast after breast-conserving surgery. Front Oncol. 2018;8:190-190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Kizy S, Altman AM, Marmor S, et al. 21-gene recurrence score testing in the older population with estrogen receptor-positive breast cancer. J Geriatr Oncol. 2019;10:322-329. [DOI] [PubMed] [Google Scholar]
- 42. Smith FO, Lee MC, Acs G, et al. Recurrence score across the age spectrum: is there an age discrimination? J Clin Oncol. 2013;31:27. [Google Scholar]
- 43. Given B, Given CW. Older adults and cancer treatment. Cancer. 2008;113:3505-3511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Liang S, Hallet J, Simpson JS, Tricco AC, Scheer AS. Omission of axillary staging in elderly patients with early stage breast cancer impacts regional control but not survival: a systematic review and meta-analysis. J Geriatr Oncol. 2017;8:140-147. [DOI] [PubMed] [Google Scholar]
- 45. Jones EL, Leak A, Muss HB. Adjuvant therapy of breast cancer in women 70 years of age and older: tough decisions, high stakes. Oncology (Williston Park). 2012;26:793-801. [PubMed] [Google Scholar]
- 46. Nurgali K, Jagoe RT, Abalo R. Editorial: adverse effects of cancer chemotherapy: anything new to improve tolerance and reduce sequelae? Front Pharmacol. 2018;9:245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Esposito E, Sollazzo V, Di Micco R, et al. Can axillary node dissection be safely omitted in the elderly? A retrospective study on axillary management of early breast cancer in older women. Int J Surg. 2016;33:S114-S118. [DOI] [PubMed] [Google Scholar]
- 48. Martelli G, Miceli R, Daidone MG, et al. Axillary dissection versus no axillary dissection in elderly patients with breast cancer and no palpable axillary nodes: results after 15 years of follow-up. Ann Surg Oncol. 2011;18:125-133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Cyr AE, Tucker N, Ademuyiwa F, et al. Successful completion of the pilot phase of a randomized controlled trial comparing sentinel lymph node biopsy to no further axillary staging in patients with clinical T1-T2 N0 breast cancer and normal axillary ultrasound. J Am Coll Surg. 2016;223:399-407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Boland MR, Bhatt NR, O’Rahelly M, et al. Axillary ultrasound-guided core biopsy in breast cancer: identifying higher nodal burden and more aggressive clinicopathological characteristics. Ir J Med Sci. 2019;188:425-431. [DOI] [PubMed] [Google Scholar]
- 51. Ahmed M, Usiskin SI, Hall-Craggs MA, Douek M. Is imaging the future of axillary staging in breast cancer? Eur Radiol. 2014;24:288-293. [DOI] [PubMed] [Google Scholar]
- 52. Pandharipande PV, Harisinghani MG, Ozanne EM, et al. Staging MR lymphangiography of the axilla for early breast cancer: cost-effectiveness analysis. Am J Roentgenol. 2008;191:1308-1319. [DOI] [PubMed] [Google Scholar]
- 53. Killelea BK, Long JB, Dang W, et al. Associations between sentinel lymph node biopsy and complications for patients with ductal carcinoma in situ. Ann Surg Oncol. 2018;25:1521-1529. [DOI] [PMC free article] [PubMed] [Google Scholar]