Highlights
-
•
The cleaning effects of gas and vapor bubbles in ultrasound fields are compared.
-
•
The cleaning effect is assessed in terms of adhesion strength and wettability.
-
•
The substrates advantageously cleaned with gas or vapor bubbles are identified.
Keywords: Ultrasonic cleaning, Acoustic cavitation, Gas bubbles, Vapor bubbles
Abstract
The dynamic actions of cavitation bubbles in ultrasonic fields can clean surfaces. Gas and vapor cavitation bubbles exhibit different dynamic behaviors in ultrasonic fields, yet little attention has been given to the distinctive cleaning effects of gas and vapor bubbles. We present an experimental investigation of surface cleaning by gas and vapor bubbles in an ultrasonic field. Using high-speed videography, we found that the primary motions of gas and vapor bubbles responsible for surface cleaning differ. Our cleaning tests under different contamination conditions in terms of contaminant adhesion strength and surface wettability reveal that vapor and gas bubbles are more effective at removing contaminants with strong and weak adhesion, respectively, and furthermore that hydrophobic substrates are better cleaned by vapor bubbles. Our study not only provides a better physical understanding of the ultrasonic cleaning process, but also proposes novel techniques to improve ultrasonic cleaning by selectively employing gas and vapor bubbles depending on the characteristics of the surface to be cleaned.
1. Introduction
Ultrasonic cleaning is widely employed in various engineering processes, including semiconductor manufacturing [1], [2], [3], [4], optics cleaning [5], and machinery maintenance [6], [7], and its physical mechanisms have been extensively investigated. In ultrasonic fields with sufficient acoustic pressure, a liquid can stretch in the rarefaction phases of acoustic waves, leading to cavitation. The dynamic motions of the created bubbles in response to the acoustic waves can generate forces which detach contaminants adhering to nearby surfaces, producing cleaning effect. Researchers have attributed cleaning effects to various dynamic actions of cavitation bubbles, such as high-speed jets and shock waves upon bubble collapse [8], [9], [10], [11], [12], [13], microstreaming induced by pulsating bubbles [14], [15], and interfacial collisions of migrating bubbles [16], [17].
The content of the cavitation bubbles can vary with the gas concentration in medium liquid. While cavitation bubbles created in liquids with a sufficient amount of dissolved gases mainly contain those dissolved gases [18], cavitation bubbles generated in degassed liquids mostly consist of the vaporized medium [19], [20]. Although the content of cavitation bubbles is generally a mixture of gas and vapor, cavitation bubbles can be classified as either gas or vapor bubbles depending on the relative composition of the gas and vapor [21]. Cavitation bubbles generated in degassed liquids are often referred to as vapor bubbles [20], [22], otherwise gas bubbles [22], [23].
Gas and vapor bubbles produced by acoustic cavitation differ in terms of the required threshold acoustic pressure, life time, and oscillation strength. Gas dissolved in liquid can serve as seeds for cavitation inception, so gas bubbles can be created at lower acoustic pressures than vapor bubbles [24]. Vapor bubbles pulsating near solid boundaries can collapse in a few periods of acoustic waves, resulting in rapid, vigorous dynamic motions with high-speed liquid jets and shock waves [22], [25], [26]. In contrast, gas bubbles exhibit relatively moderate dynamic motions owing to the gas cushion and thus have a relatively long life time [15], [18], [27], [28], [29].
Given the differences in the dynamic characteristics of gas and vapor bubbles, the cleaning performance of cavitation bubbles is expected to vary with bubble content. However, our current understanding of the difference in cleaning effects of gas and vapor bubbles remains unclear. For instance, a few research groups have suggested techniques to improve the cleaning effect by controlling the dissolved gas content, but the results have been inconsistent. Some groups propose that liquids with a lower gas content can be used to improve cleaning performance [28], [30], [31], [32], while other groups report that large amounts of dissolved gas can be advantageous for improving cleaning efficiency [29], [33]. To advance ultrasonic cleaning technique by selectively exploiting gas or vapor bubbles, it will be necessary to directly compare the cleaning effects of gas and vapor bubbles.
We present an experimental investigation to compare the cleaning effects of gas and vapor bubbles in ultrasonic fields. Using high-speed videography, we experimentally examined the cleaning process by cavitation bubbles, and found clear differences in the motions of gas and vapor bubbles effective for cleaning. Vapor bubbles remove contaminants from the cleaning surface with explosive collapsing motions, while gas bubbles detach contaminants with gentle oscillating motions as they migrate on the surface. We examined the cleaning effects of gas and vapor bubbles on substrates with various contamination conditions in terms of adhesion strength and wettability, and have identified the characteristics of contaminated substrates that can be advantageously cleaned with gas or vapor bubbles. The results demonstrate that vapor and gas bubbles are more effective at removing contaminants with strong and weak adhesion, respectively, and that hydrophobic substrates are better cleaned by vapor bubbles. Our study proposes a novel technique to increase the ultrasonic cleaning effect by selectively using degassed and non-degassed liquids depending on the characteristics of the contaminated surfaces.
2. Experimental methods
2.1. Experimental setup
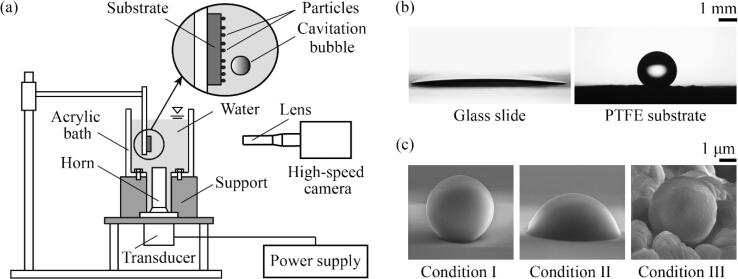
Fig. 1a shows our experimental setup for cleaning tests. We constructed a transparent acrylic bath that was 14 mm, 70 mm, and 30 mm in width, height, and depth (out of plane of Fig. 1a), respectively. A horn-type transducer was used to generate 40 kHz ultrasound waves. An 8 mm diameter cylindrical horn was connected to the transducer and inserted through a hole in the bottom of the bath. The bath was filled with tap water to a volume of 30 mL so that the upper surface of the horn was located 45 mm below the free surface of water. When conducting experiments with vapor bubbles, we used degassed tap water. The tap water was kept in a vacuum chamber at an absolute pressure of 10 kPa for at least 48 h [34]. The concentration of dissolved oxygen of degassed tap water was measured to be 0.9 mg/L, which was reduced from 7.2 mg/L of non-degassed tap water, using an oxygen meter (DO-31P, DKK-TOA). A contaminated substrate was placed 7 mm above the top surface of the horn. The particle removal process was recorded using a high-speed camera (Fastcam Mini AX200, Photron) combined with a long-distance microscope lens (12X Zoom, Navitar) or a microscope lens (10X M Plan APO, Mitutoyo) at a frame rate ranging from 1,000 to 20,000 frames per second. When the contaminated substrate was transparent, a light source illuminated the substrate from the rear, otherwise the substrate was illuminated from the front. The acoustic pressure was measured using a hydrophone (TC4038, Teledyne RESON) placed 7 mm above the top surface of the horn. After cleaning, we evaluated the particle removal efficiency (PRE), defined as η = (C0 − C)/C0 with C0 and C being the number of particles deposited on the substrates before and after cleaning, respectively.
Fig. 1.
(a) Experimental setup. (b) Water droplets on a glass slide and PTFE substrate. (c) Scanning electron microscope images of single particles in contamination conditions I–III.
2.2. Contaminated substrates
To visualize the cleaning process by cavitation bubbles, we prepared glass slides (HSU-1000412, Marienfeld) contaminated with 4 μm diameter polystyrene particles. We used an aqueous polystyrene particle suspension (Micromer-blue, Micromod) with a concentration of 25 mg/mL. When testing surfaces with less contaminants we used the suspension after dilution with a volume of ethanol 10 times the suspension volume. After coating glass slides with the suspension, we baked them on a hot plate at a temperature of 95 °C for 30 min.
For a quantitative analysis of cleaning performance, we produced contaminated substrates using glass slides and home-made polytetrafluoroethylene (PTFE) surfaces. PTFE surfaces were made by pressing PTFE powder (M-18, Daikin Industries) on sand paper at a pressure of 35 MPa for 20 min and then baking in an oven at a temperature of 360 °C for 5 h [35]. While the glass surfaces were smooth and hydrophilic, the PTFE surfaces were rough and hydrophobic. The contact angles of water on the glass and PTFE surfaces were measured to be approximately 20° and 120° with a hysteresis of 25° and 70°, respectively (see Fig. 1b).
We evaluated the effect of ultrasonic cleaning on substrates with three different contamination conditions. In contamination conditions I and II, 4 μm diameter polystyrene particles (C37253, Life technologies) were deposited on glass slides, and they were baked on a hot plate at a temperature of 125 °C for 5 min and at a temperature of 160 °C for 3 h, respectively. In contamination condition III, fluorescent polystyrene particles (FSDG006, Bangs Laboratories, Inc.) were deposited on the PTFE surface and kept at room temperature for 1 h. Since some clustered particles adhered weakly to the surface, the contaminated surface was gently rinsed with water before use to remove the weakly adhering particles [11].
2.3. Comparison of particle adhesion in contamination conditions I-III
To compare the particle adhesion strength in contamination conditions I-III, we tested particle removal using a water jet. The water jet was discharged through a 2 mm diameter nozzle at an average flow speed of 4 m/s for 1 min. We placed the nozzle a distance of 3 mm from the substrate so that the water jet was ejected perpendicular to the substrate. PREs after water jet discharge were measured to be approximately 43%, 0%, and 44% for the substrates with contamination conditions I, II, and III, respectively. Hence, we assumed that the particle adhesion for contamination conditions I and III was comparable, but significantly weaker than that in contamination condition II. Scanning electron microscope images of single particles in contamination conditions I–III are shown in Fig. 1c. The adhesion area of particles increased with baking time in contamination conditions I and II, which is assumed to have resulted in an increase in adhesion [11], [36].
3. Results and discussion
3.1. Visualization of surface cleaning by gas and vapor bubbles
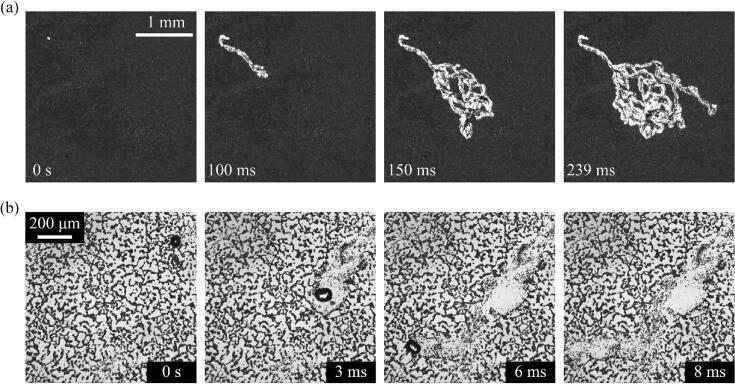
Particle removal by gas and vapor bubbles in an ultrasonic field with an acoustic pressure of 200 kPa were experimentally observed. Fig. 2 shows sequential images of the particle removal by gas bubbles. In the beginning (t = 0 s), the glass slide covered with particles appeared black under backlight illumination. Over time, the surface was cleaned and became locally bright. As shown in Fig. 2a, the cleaned region exhibits random trajectories with a width of ~ 100 μm (see Supplementary Movie 1). As the observation time increased, the trajectories elongated, and a significant portion of the observation area was cleaned after 239 ms. In Fig. 2b, images taken at high magnification show the details of the particle removal process (see Supplementary Movie 2). An oscillating bubble enters the field of view from the right upper corner. As the bubble moves to the left lower corner, the bubble is broken into multiple daughter bubbles. Each daughter bubble oscillates and moves around on the substrate during the observation, and particles were removed in the vicinity of the trajectory of each bubble.
Fig. 2.
Sequential images of particle removal process by gas bubbles taken at (a) low and (b) high magnification.
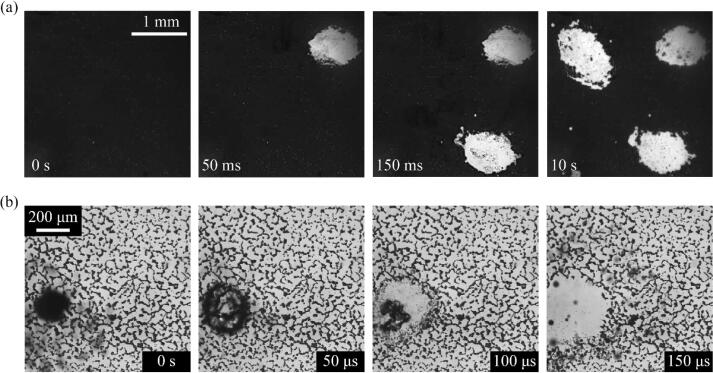
Fig. 3 displays sequential images of particle removal by vapor bubbles in an ultrasonic field with an acoustic pressure of 200 kPa. Here degassed water was used as the cleaning medium. In Fig. 3a, three circular cleaning regions emerged on the dark substrate, which initially had been densely covered with particles (see Supplementary Movie 3). In each cleaned region the particle removal seems to have resulted from a separate cavitation event, because no significant actions occurred in the time interval between cleaning events. The diameter of each cleaned region was approximately 1 mm, which is much larger than the diameter of a resonant air bubble at 40 kHz frequency, 160 μm [37]. Each cleaning event was completed within the time interval of two consecutive images taken at 1,000 fps, which informed us that the cleaning time was less than 1 ms. Fig. 3b shows higher magnification images taken at 20,000 fps (see Supplementary Movie 4). In the beginning (t = 0 s), a large bubble is surrounded by a cloud of numerous small bubbles. The bubbles seem to be floating on the substrate, given that they are out of focus. The bubbles instantaneously collapse close to the substrate, performing particle removal over a large area. Immediately after the collapse, the bubbles are no longer visible.
Fig. 3.
Sequential images of particle removal process by vapor bubbles taken at (a) low and (b) high magnification.
3.2. Analysis of the characteristics of surface cleaning by gas and vapor bubbles
We proceed by analyzing the observed characteristics of surface cleaning by gas bubbles. Once gas bubbles are generated by acoustic cavitation, they likely survive until they escape the liquid. In an ultrasonic field, two gas bubbles oscillating in phase tend to merge because they attract each other due to the secondary Bjerknes force [29], [38], [39]. Since gas diffusion into surrounding water is negligible on the time scale of the period of bubble oscillation, gas bubbles become larger over time via merging. When the diameter of a gas bubble is much larger than the resonance diameter in a given acoustic wave field, the bubble becomes inert and rises to the free surface of the water reservoir by gravity. Meanwhile, the primary Bjerknes force induced by the acoustic waves drives the translational motions of gas bubbles [16], [22], [40]. Accordingly, gas bubbles that adhere to the substrate exhibit random dancing motions on the substrate in response to the acoustic waves. These dancing gas bubbles generate cleaning effects only in their vicinity while moving around on the substrate, because the particle removal forces generated by the oscillating gas bubbles abate sharply with distance from the bubbles [16]. In summary, gas bubbles travel on the substrate for a relatively long time and clean confined areas near their trajectory.
Compared to cleaning by gas bubbles, surface cleaning events by vapor bubbles can be characterized by large cleaning areas and instantaneous, scattered occurrence. In an ultrasonic field with a given acoustic intensity, bubbles containing mostly gases are highly likely to exhibit relatively a stable oscillatory motion than vapor bubbles [18], [26]. A pulsating vapor bubble near a solid boundary can lose sphericity and become asymmetric in several cycles of acoustic waves, leading to a collapse that involves a strong water jet and impact on the boundary [25], [41], [42], [43]. Since particles adhering to the substrate are removed by such an extremely violent bubble collapse, cleaning by vapor bubbles occurs instantaneously, but impacts a distance much greater than the bubble diameter.
Taken together, our experimental observations enabled us to envision the particle contaminant conditions that can be advantageously cleaned by gas and vapor bubbles. Vapor bubbles exhibit more violent motions for a given acoustic pressure, so that strongly attached particles can be advantageously removed by vapor bubbles. In contrast, gas bubbles have a longer life time and can thus encounter more contaminating particles. Therefore, gas bubbles are expected to be more effective for cleaning substrates contaminated with weakly attached particles, because they can clean a relatively larger area.
There are caveats. Particle removal by gas bubbles occurs during their translational motion on the substrate. The shape of a gas bubble on a substrate depends on wettability. A gas bubble has a spherical shape on hydrophilic surfaces which prefer contacting water to gas, but it has a thin film shape on hydrophobic surfaces that prefer contacting gas to water. The translational motions of gas bubbles on hydrophobic surfaces are significantly limited by the contact line pinning force, which scales as σl(cos θa − cos θr), where σ is the surface tension coefficient, l is the contact line perimeter, and θa and θr are the advancing and receding contact angles, respectively. A gas bubble with a given volume has a greater contact line perimeter on a hydrophobic surface and therefore experiences a greater resistance against translational motion. Hence, we conjecture that cleaning by gas bubbles can be severely limited on hydrophobic substrates.
3.3. Assessment of cleaning performance in contamination conditions I-III
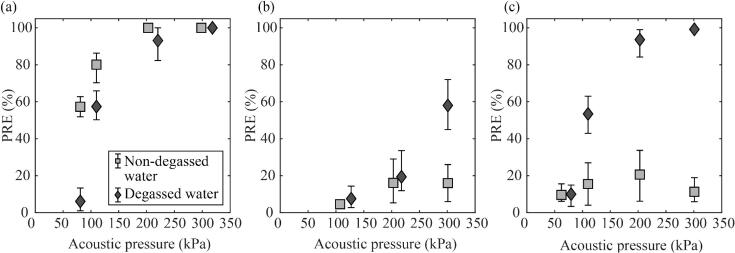
We carried out an assessment of PRE for contamination conditions I-III to verify our expectations about the characteristics of contaminated substrates that could be advantageously cleaned with gas or vapor bubbles. Fig. 4a shows the cleaning results for contamination condition I (weak adhesion on hydrophilic surface) after 10 min. The PRE in both degassed and non-degassed waters increased with the acoustic pressure and became close to 100% at an acoustic pressure greater than 200 kPa. The results suggest that particles weakly attached to a hydrophilic surface can be effectively removed by both gas and vapor bubbles at a sufficient acoustic pressure. Although non-degassed water exhibited a PRE of approximately 50% at an acoustic pressure of 80 kPa, degassed water had a PRE less than 10%. Cavitation inception in degassed water requires a higher acoustic pressure threshold compared to non-degassed water due to the lack of dissolved gas, which serves as seeds for cavitation [24]. Hence, ultrasonic cleaning with degassed water needs a relatively higher acoustic pressure. Ultrasonic waves with an excessively high acoustic pressure may have negative effects, such as surface damage and temperature rise of the cleaning medium [3], [4], [28], [44], so we deduced that surfaces with weakly adhering contaminants can be advantageously cleaned with non-degassed water.
Fig. 4.
Dependence of PRE on the acoustic pressure for contamination conditions (a) I, (b) II, and (c) III.
Fig. 4b shows the results for contamination condition II (strong adhesion on hydrophilic surface). At acoustic pressures less than 150 kPa, both waters exhibited low PREs of less than 10%. Although the PRE increased with acoustic pressure it remained below the upper limit of approximately 20% for non-degassed water and 60% for degassed water. This result suggests that ultrasonic cleaning with gas bubbles will remain at a markedly low level no matter how high the applied acoustic pressure is. This means that gas bubbles are insufficient for detaching the particles in contamination condition II. Such low PRE could potentially be attributed to the gas cushion effect, which leads to the relatively gentle dynamic behavior of gas bubbles [15], [18], [27], [28], [29]. In contrast, vapor bubbles can detach tightly attached particles with vigorous collapsing motions [28], [31], [32], [43]. Although we only employed an acoustic pressure up to 300 kPa due to the limitations of our experimental setup, the population and dynamic strength of vapor bubbles can increase with acoustic pressure, which can further increase the PRE. Consequently, these experiments suggest that substrates contaminated with particles with strong adhesion can be cleaned exclusively by vapor bubbles generated in degassed water.
Fig. 4c presents the results for contamination condition III (weak adhesion on hydrophobic surface). The PRE values of degassed water are approximately the same as the PRE values for contamination condition I, indicating that the cleaning effect of the vapor bubbles has a negligible dependence on wettability. However, the PRE of the non-degassed water was lower than 20%, even at the highest acoustic pressure, and was lower than the PRE obtained for contamination condition I. Because the particle adhesion forces in contamination condition I and III are similar, this result suggests that gas bubbles are ineffective for removing particles adhering to hydrophobic surfaces. Fig. 5 illustrates the cleaning process by gas bubbles on the PTFE substrate (see Supplementary Movie 5). Here, the bubbles appear bright because we used a reflected light to illuminate the bubbles on the opaque PTFE substrate. In Fig. 5, the translation motion speed is less than 1 mm/s, at least two orders of magnitude less than the typical translation speed of bubbles on the hydrophilic glass slides, so that gas bubbles seem virtually stationary. This observation confirms that, as aforementioned, the translational motion of gas bubbles is limited on hydrophobic surfaces due to the contact line pinning force. As a result, the cleaning area of individual gas bubbles was significantly reduced. It is noteworthy that the PRE in non-degassed water at 300 kPa was lower than that of 200 kPa. Gas bubbles with an excessively large population interfere with the transmission of ultrasound waves to the surface, and the cleaning effect can thus be reduced at high acoustic pressures, which is consistent with the observations in a previous research [29].
Fig. 5.
Motion of gas bubbles on a PTFE substrate.
4. Conclusions
We have presented a comparative study on the cleaning effects of gas and vapor bubbles in ultrasound fields. Our high-speed videography has revealed that while gas bubbles move around on the substrate for a relatively long time and clean confined areas near the trajectory, vapor bubbles clean the surface with their explosive collapse, thus leading to rapid, scattered cleaning events. These observations suggest that vapor and gas bubbles are more effective for cleaning contaminants with strong and weak adhesion, respectively, and that hydrophobic substrates can be cleaned exclusively by vapor bubbles. By assessing the cleaning effects of gas and vapor bubbles on substrates with various contamination conditions in terms of the adhesion strength and wettability, we have identified the characteristics of contaminated substrates that can be advantageously cleaned with gas or vapor bubbles. The results of our study not only provide a better physical understanding of ultrasonic cleaning mechanisms, but also propose a novel ultrasonic cleaning technique, to enhance the cleaning effect by selectively using degassed and non-degassed liquids depending on the characteristics of the surface to be cleaned.
CRediT authorship contribution statement
Ryeol Park: Investigation, Validation, Writing - original draft. Minsu Choi: Investigation, Validation. Eun Hyun Park: Writing - review & editing. Won-Jun Shon: Writing - review & editing, Funding acquisition. Ho-Young Kim: Conceptualization, Writing - review & editing, Funding acquisition. Wonjung Kim: Conceptualization, Supervision, Funding acquisition.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This work was supported by a grant of the Korea Health Industry Development Institute (KHIDI) funded by the Ministry of Health & Welfare (grant no. HI18C0432) and a grant of the National Research Foundation of Korea (NRF) funded by the Ministry of Science and ICT (grant no. 2018-052541), Republic of Korea.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ultsonch.2021.105618.
Contributor Information
Ho-Young Kim, Email: hyk@snu.ac.kr.
Wonjung Kim, Email: wonjungkim@sogang.ac.kr.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Kern W. The evolution of silicon wafer cleaning technology. J. Electrochem. Soc. 1990;137(6):1887–1892. [Google Scholar]
- 2.Gale G.W., Busnaina A.A. Removal of particulate contaminants using ultrasonics and megasonics: a review. Particul. Sci. Technol. 1995;13(3–4):197–211. [Google Scholar]
- 3.Kim W., Park K., Oh J., Choi J., Kim H.-Y. Visualization and minimization of disruptive bubble behavior in ultrasonic field. Ultrasonics. 2010;50(8):798–802. doi: 10.1016/j.ultras.2010.04.002. [DOI] [PubMed] [Google Scholar]
- 4.Kim T.-H., Kim H.-Y. Disruptive bubble behaviour leading to microstructure damage in an ultrasonic field. J. Fluid Mech. 2014;750:355–371. [Google Scholar]
- 5.Ding T., Xie Y., Shen Z., Cheng X., Wang Z. Ultrasonic cleaning optimization research for ultrasmooth optical substrate with artificial micron/submicron silica spheres. Opt. Eng. 2014;53 [Google Scholar]
- 6.Bulat T.J. Macrosonics in industry: 3. Ultrasonic cleaning, Ultrasonics. 1974;12:59–68. [Google Scholar]
- 7.Mason T.J. Ultrasonic cleaning: An historical perspective. Ultrason. Sonochem. 2016;29:519–523. doi: 10.1016/j.ultsonch.2015.05.004. [DOI] [PubMed] [Google Scholar]
- 8.Dijkink R., Ohl C.D. Measurement of cavitation induced wall shear stress. Appl. Phys. Lett. 2008;93 [Google Scholar]
- 9.Chahine G.L., Kapahi A., Choi J.-K., Hsiao C.-T. Modeling of surface cleaning by cavitation bubble dynamics and collapse. Ultrason. Sonochem. 2016;29:528–549. doi: 10.1016/j.ultsonch.2015.04.026. [DOI] [PubMed] [Google Scholar]
- 10.Yusof N.S.M., Babgi B., Alghamdi Y., Aksu M., Madhavan J., Ashokkumar M. Physical and chemical effects of acoustic cavitation in selected ultrasonic cleaning applications. Ultrason. Sonochem. 2016;29:568–576. doi: 10.1016/j.ultsonch.2015.06.013. [DOI] [PubMed] [Google Scholar]
- 11.Reuter F., Mettin R. Mechanisms of single bubble cleaning. Ultrason. Sonochem. 2016;29:550–562. doi: 10.1016/j.ultsonch.2015.06.017. [DOI] [PubMed] [Google Scholar]
- 12.Reuter F., Cairós C., Mettin R. Vortex dynamics of collapsing bubbles: Impact on the boundary layer measured by chronoamperometry. Ultrason. Sonochem. 2016;33:170–181. doi: 10.1016/j.ultsonch.2016.04.023. [DOI] [PubMed] [Google Scholar]
- 13.Zeng Q., Gonzalez-Avila S.R., Dijkink R., Koukouvinis P., Gavaises M., Ohl C.-D. Wall shear stress from jetting cavitation bubbles. J. Fluid Mech. 2018;846:341–355. [Google Scholar]
- 14.Lamminen M.O., Walker H.W., Weavers L.K. Mechanisms and factors influencing the ultrasonic cleaning of particle-fouled ceramic membranes. J. Memb. Sci. 2004;237:213–223. [Google Scholar]
- 15.Vyas N., Manmi K., Wang Q., Jadhav A.J., Barigou M., Sammons R.L., Kuehne S.A., Walmsley A.D. Which parameters affect biofilm removal with acoustic cavitation? A review. Ultrasound Med. Biol. 2019;45(5):1044–1055. doi: 10.1016/j.ultrasmedbio.2019.01.002. [DOI] [PubMed] [Google Scholar]
- 16.Kim W., Kim T.-H., Choi J., Kim H.-Y. Mechanism of particle removal by megasonic waves. Appl. Phys. Lett. 2009;94 [Google Scholar]
- 17.Choi J., Kim T.-H., Kim H.-Y., Kim W. Ultrasonic washing of textiles. Ultrason. Sonochem. 2016;29:563–567. doi: 10.1016/j.ultsonch.2015.07.018. [DOI] [PubMed] [Google Scholar]
- 18.Liu L., Yang Y., Liu P., Tan W. The influence of air content in water on ultrasonic cavitation field. Ultrason. Sonochem. 2014;21(2):566–571. doi: 10.1016/j.ultsonch.2013.10.007. [DOI] [PubMed] [Google Scholar]
- 19.Prosperetti A. Vapor bubbles. Annu. Rev. Fluid Mech. 2017;49(1):221–248. [Google Scholar]
- 20.Namura K., Okai S., Kumar S., Nakajima K., Suzuki M. Self-oscillation of locally heated water vapor microbubbles in degassed water. Adv. Mater. Interfaces. 2020;7:2000483. [Google Scholar]
- 21.Plesset M.S., Prosperetti A. Bubble dynamics and cavitation. Annu. Rev. Fluid Mech. 1977;9(1):145–185. [Google Scholar]
- 22.Leighton T. Academic press; 2012. The acoustic bubble. [Google Scholar]
- 23.Strasberg M. Onset of ultrasonic cavitation in tap water. J. Acoust. Soc. Am. 1959;31(2):163–176. [Google Scholar]
- 24.Gondrexon N., Renaudin V., Boldo P., Gonthier Y., Bernis A., Pettier C. Degassing effect and gas liquid transfer in a high frequency sonochemical reactor. Chem. Eng. J. 1997;66:21–26. [Google Scholar]
- 25.Plesset M.S., Chapman R.B. Collapse of an initially spherical vapour cavity in the neighbourhood of a solid boundary. J. Fluid Mech. 1971;47(2):283–290. [Google Scholar]
- 26.Brennen C.E. Oxford University Press; 1995. Cavitation and bubble dynamics. [Google Scholar]
- 27.Rooze J., Rebrov E.V., Schouten J.C., Keurentjes J.T.F. Dissolved gas and ultrasonic cavitation–a review. Ultrason. Sonochem. 2013;20(1):1–11. doi: 10.1016/j.ultsonch.2012.04.013. [DOI] [PubMed] [Google Scholar]
- 28.Fuchs F.J. Ultrasonic cleaning and washing of surfaces. In: Gallego-Juárez J.A., Graff K.F., editors. Power Ultrasonics. Woodhead Publishing; 2015. pp. 577–609. [Google Scholar]
- 29.Yamashita T., Ando K. Low-intensity ultrasound induced cavitation and streaming in oxygen-supersaturated water: Role of cavitation bubbles as physical cleaning agents. Ultrason. Sonochem. 2019;52:268–279. doi: 10.1016/j.ultsonch.2018.11.025. [DOI] [PubMed] [Google Scholar]
- 30.Moholkar V.S., Warmoeskerken M.M., Ohl C.D., Prosperetti A. Mechanism of mass-transfer enhancement in textiles by ultrasound. AICHE J. 2004;50:58–64. [Google Scholar]
- 31.Mattox D.M. Handbook of physical vapor deposition (PVD) processing. William Andrew; 2010. [Google Scholar]
- 32.Tuziuti T. Influence of sonication conditions on the efficiency of ultrasonic cleaning with flowing micrometer-sized air bubbles. Ultrason. Sonochem. 2016;29:604–611. doi: 10.1016/j.ultsonch.2015.09.011. [DOI] [PubMed] [Google Scholar]
- 33.Goh B.C., Goh F., Lim C., Ismail Z., Zhou M.S. Impact of re-gasified water on megasonic cleaning. Solid State Phenom. 2008;134:217–220. [Google Scholar]
- 34.Kim N., Park H., Do H. Evolution of cavitation bubble in tap water by continuous-wave laser focused on a metallic surface. Langmuir. 2019;35(9):3308–3318. doi: 10.1021/acs.langmuir.8b04083. [DOI] [PubMed] [Google Scholar]
- 35.Jiang C., Hou W., Wang Q., Wang T. Facile fabrication of superhydrophobic polytetrafluoroethylene surface by cold pressing and sintering. Appl. Surf. Sci. 2011;257(11):4821–4825. [Google Scholar]
- 36.Tomas J. Adhesion of ultrafine particles—a micromechanical approach. Chem. Eng. Sci. 2007;62(7):1997–2010. [Google Scholar]
- 37.Minnaert M. On musical air-bubbles and the sounds of running water. Philos. Mag. 1933;16:235–248. [Google Scholar]
- 38.Crum L.A. Bjerknes forces on bubbles in a stationary sound field. J. Acoust. Soc. Am. 1975;57(6):1363–1370. [Google Scholar]
- 39.Mettin R., Akhatov I., Parlitz U., Ohl C.D., Lauterborn W. Bjerknes forces between small cavitation bubbles in a strong acoustic field. Phys. Rev. E. 1997;56:2924. [Google Scholar]
- 40.Doinikov A.A. Translational motion of a spherical bubble in an acoustic standing wave of high intensity. Phys. Fluids. 2002;14(4):1420–1425. [Google Scholar]
- 41.Benjamin T.B., Ellis A.T. The collapse of cavitation bubbles and the pressures thereby produced against solid boundaries. Philos. Trans. R. Soc. London, Ser. A. 1966;260:221–240. [Google Scholar]
- 42.Blake J.R., Taib B.B., Doherty G. Transient cavities near boundaries. Part 1. Rigid boundary. J. Fluid Mech. 1986;170:479–497. [Google Scholar]
- 43.Tagawa Y., Peters I.R. Bubble collapse and jet formation in corner geometries. Phys. Rev. Fluids. 2018;3 [Google Scholar]
- 44.Kang B.-K., Kim M.-S., Park J.-G. Effect of dissolved gases in water on acoustic cavitation and bubble growth rate in 0.83 MHz megasonic of interest to wafer cleaning. Ultrason. Sonochem. 2014;21(4):1496–1503. doi: 10.1016/j.ultsonch.2014.01.012. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.