Abstract
Coronary artery disease (CAD) is highly prevalent in chronic kidney disease (CKD). CKD modifies the effects of traditional risk factors on atherosclerosis, with CKD-specific mechanisms, such as inflammation and altered mineral metabolism, playing a dominant pathophysiological role as kidney function declines. Traditional risk models and cardiovascular screening tests perform relatively poorly in the CKD population, and medical treatments including lipid-lowering therapies have reduced efficacy. Clinical presentation of cardiac ischemia in CKD is atypical, whereas invasive therapies are associated with higher rates of complications than in with patients with normal or near normal kidney function. The main focus of the present review is on the invasive approach to management of CAD in late-stage CKD, with an in-depth discussion of the findings of the International Study of Comparative Health Effectiveness With Medical and Invasive Approaches (ISCHEMIA)-CKD trial, and their implications for therapeutic approach and future research in this area. We also briefly discuss the existing evidence in the epidemiology, pathogenesis, diagnosis, and medical management of CAD in late-stage CKD, end-stage kidney disease (ESKD), and kidney transplant recipients. We enumerate the evidence gap left by the frequent exclusion of patients with CKD from randomized controlled trials and highlight the priority areas for future research in the CKD population.
Keywords: atherosclerosis, chronic kidney disease, coronary artery bypass graft, coronary artery disease, percutaneous coronary intervention, revascularization
Cardiovascular disease is the leading cause of mortality in patients with CKD.1 Patients with stages G3 to G4 CKD (estimated glomerular filtration rate [eGFR] 15–59 ml/min per 1.73 m2) have 2 to 3 times higher mortality compared with patients without CKD, with the probability of developing CAD increasing linearly as the glomerular filtration rate drops below 60 ml/min per 1.73 m2.2 Patients maintained on dialysis incur the greatest risk of experiencing major adverse cardiovascular events,3 and although kidney transplantation is the best strategy to reduce this risk, cardiovascular disease remains the greatest cause of death for kidney transplant recipients.4 Although atherosclerosis in early CKD is driven by standard risk factors compounded by albuminuria, nonstandard CKD-related risk factors (e.g., inflammation, oxidative stress, and metabolic bone disease, and vascular calcification) play a major role as glomerular filtration rate declines.3,1,3,5,6
Adding to the complexity, the clinical presentation of cardiac ischemia in the CKD population is often atypical. Compared with approximately 70% of patients with normal or near normal kidney function, only 40% of patients with stages G3 to G5 CKD presenting with myocardial infarction (MI) have typical angina symptoms.7 The atypical presentation of cardiac ischemia in patients with CKD thus warrants special effort to identify anginal equivalent symptoms, such as dyspnea or fatigue.1 Diminished exercise tolerance, especially in patients with ESKD, may further limit presentation of classical angina. Indeed, patients with CKD are more likely to have MI as an initial manifestation of CAD,8 and most present with non–ST-segment elevation MI (STEMI).9 The higher frequency of presentation with non-STEMI compared with STEMI in patients with CKD may reflect left ventricular hypertrophy and subendocardial ischemia, the burden of atherosclerosis and degree of calcification, and a lower likelihood of ruptured fibrous cap as opposed to plaque erosion as the substrate for acute coronary syndromes.1
In this review, we provide an overview of cardiovascular risk stratification and diagnostic approach to screening for CAD in late-stage CKD and in candidates for kidney transplantation. We discuss the conservative treatment with optimal medical therapy (OMT) alone or OMT in combination with invasive management, including cardiac catheterization using ultralow contrast volumes and zero-contrast percutaneous coronary intervention (PCI) to minimize the risk of contrast-induced nephropathy (CIN). We discuss the findings of the ISCHEMIA-CKD trial and make recommendations for future research in studying invasive versus conservative approach for management of CAD in advanced CKD.
Cardiovascular Risk Stratification and Screening for CAD in CKD
Cardiovascular Risk Stratification
Predictive models in the general population (e.g., Framingham equation) have poor discrimination (i.e., the ability to separate those who experience a cardiac event from those who do not) in CKD.10 Predicted risks based on these models systematically fall below the actual observed risk.11 This systemic underestimation of cardiovascular risk is nonuniform and is driven by events competing with death, together with significantly higher cardiac event rates in CKD; thus, refitting the equations and assigning different weighted coefficients to traditional risk factors do not adequately improve risk stratification in CKD.11 Although the addition of eGFR and albuminuria can improve calibration (i.e., the measure of how closely predicted outcomes agree with actual outcomes) and risk discrimination of the predictive models,12 current clinical guidelines do not formally incorporate these readily available kidney-specific variables.13
Addition of biomarkers (e.g., vascular calcification, troponin I or T, C-reactive protein) may improve performance of the risk prediction models in early stage CKD; nonetheless, these risk assessment methods function poorly in ESKD.14 Dialysis modifies the effects of standard risk factors, although the increased rates of heart failure and sudden death in the dialysis population are not captured by the standard risk methods.1 Thus, new cardiovascular risk models need to be developed and validated in ESKD. Finally, the Framingham equation underestimates cardiovascular risk in kidney transplant recipients, and modified equations have not been adequately validated in this population.15
Screening for CAD in CKD
Regular screening for CAD in asymptomatic patients with CKD is not recommended because there is no evidence supporting efficacy of coronary revascularization in reducing death or MI in this group of patients.16 In contrast, screening for CAD in symptomatic and asymptomatic, high-risk kidney transplant candidates is currently recommended but remains controversial. Although evidence from randomized controlled trials on the impact of this approach on clinical outcomes is lacking,17 the perioperative safety of kidney transplantation in patients with high risk for CAD remains a rationale for screening and revascularization.
Functional Testing and Noninvasive Imaging
In the non-CKD population, functional stress testing and noninvasive coronary imaging are used to assess ischemia and atherosclerosis burden, to evaluate prognosis, and to risk-stratify patients for coronary revascularization and optimization of medical therapy. Diagnosing CAD in patients with CKD may be more challenging. Exercise testing and pharmacologic perfusion imaging have reduced accuracy for detecting CAD in CKD, with higher rates of false-negative and false-positive tests.9,18 Exercise testing is limited by frequently low functional capacity in patients with CKD19 and baseline electrocardiographic abnormalities (e.g., left ventricular hypertrophy) that may affect interpretation of ST-segment changes. In addition, most of the current evidence is from studies in transplant candidates. Patients with ESKD, who are deemed unsuitable candidates for kidney transplantation, typically have lower functional capacity, more comorbidities, and higher burden of CAD; the prognostic value of cardiovascular risk stratification in this larger population of patients with ESKD is unknown.1
Given the high pretest probability of CAD and the moderate sensitivity of noninvasive tests, these tests may have a low negative predictive value, that is, they may not exclude functionally significant or anatomically high-risk disease. Coronary artery calcium score or computed tomography angiography (CTA) has some potential advantages over functional imaging in the CKD population. In a comparison of coronary artery calcium score, CTA, exercise, or pharmacologic stress single-photon emission computed tomography in which stenosis >50% was detected by quantitative coronary angiography in 138 kidney transplant candidates, coronary artery calcium score and single-photon emission computed tomography had modest specificity (67% and 53%, respectively) and sensitivity (77% and 82%, respectively), whereas CTA had a high sensitivity (93%) but low specificity (63%).18 Risk of acute kidney injury (AKI) should be considered with CTA, particularly in late-stage CKD,20 including the diminished use of CTA in the CKD population with accelerated coronary calcification (predominantly medial vascular calcification), which can confound the assessment of occlusive atherosclerotic CAD.21
Assessment of myocardial perfusion with positron emission tomography (PET) using various tracers allows for quantification of rest and stress myocardial blood flow to compute coronary flow reserve (CFR = stress myocardial blood flow/rest myocardial blood flow) in addition to semiquantitative analysis of ischemia and scar.22 In the non-CKD population, sensitivity of flurpiridaz PET for detection of CAD with ≥50% stenosis on angiography was higher than single-photon emission computed tomography (71.9% vs. 53.7%), with improved image quality, diagnostic certainty, and lower radiation exposure23; nonetheless, this comparison has not been performed in the CKD population. Compared with patients with preserved kidney function, PET-CFR is lower in early stage CKD, without further decrement in stage 5 or dialysis-dependent ESKD.24 In late-stage CKD, PET-CFR below the median value of 1.5 was associated with a 2.1-fold increase in the adjusted risk of cardiac death.22 Incorporation of PET-CFR in cardiac death risk assessment models resulted in a net reclassification improvement, with 8% upward and 12% downward reclassification of patients into more accurate risk categories.22 PET-CFR was also independently associated with all-cause and cardiovascular mortality in ESKD, and addition of PET-CFR resulted in risk reclassification in 27% of patients.25
Markers of Myocardial Injury
Cardiac troponins are frequently elevated in advanced CKD. The mechanisms for elevated troponin levels are not fully understood; nevertheless, troponin T and I elevations are associated with increased all-cause and cardiovascular mortality in CKD.26,27 Severe CAD is more common among patients with ESKD and elevated troponin T.28 Elevation may also indicate subclinical myocardial damage, for example, transient myocardial stunning during hemodialysis.29 Although the sensitivity of high-sensitivity troponin I in the diagnosis of MI is not modified by kidney function, its specificity progressively decreases from 93%–95% in patients with preserved renal function to 40%–41% in ESKD.27 Dynamic changes in troponin levels compared with the baseline levels may increase the specificity for diagnosing MI in ESKD.1
Pretransplant Screening for CAD
Deceased donor kidney transplantation is an elective surgery performed under emergent situations.1 Screening of transplant candidates for CAD is performed to guide selection of appropriate candidates, inform transplant options, maintain eligibility during wait-listing, minimize and inform the risk of peritransplant events, and optimize post-transplant survival. Cardiovascular events after transplantation may compromise long-term survival and allograft function.1 Nonetheless, whether treatment guided by screening prevents early post-transplant cardiovascular events and improves long-term outcomes is not known.
Evaluating Patients for CAD Pretransplantation
Patients with signs or symptoms suggestive of CAD should be tested.30 Among asymptomatic patients, screening for subclinical CAD is recommended by the US guidelines and has been integrated in clinical transplant practice despite limited evidence that screening reduces the risk of CAD events.17
Transplant guidelines recommend screening based on the presence of cardiovascular risk factors, using noninvasive screening tests at the time of activation to the wait-list and periodically during wait-listing, with the objective of identifying patients with subclinical CAD who are candidates for revascularization or medical therapy.30 It is possible that screening may, paradoxically, cause harm by unnecessarily subjecting patients to invasive procedures and delaying/excluding them from transplantation.31 There are several issues regarding the current screening paradigm. First, cardiovascular mortality in CKD may be secondary to arrhythmia rather than MI. Second, noninvasive screening tests lack sensitivity and specificity to identify asymptomatic patients with clinically significant CAD warranting revascularization.32 Last, evidence that revascularization would improve outcomes is lacking.17 The American Heart Association/American College of Cardiology scientific statement recommends that initial screening before wait-list activation “may be considered in transplant candidates with no active disease but with multiple risk factors for CAD” (class IIB, level of evidence: C).17 As described earlier, noninvasive testing for CAD has modest sensitivity and specificity in ESKD. Current guidelines recommend exercise or pharmacologic stress echocardiogram or nuclear scintigraphy. There are limited data on the role of CTA in dialysis patients undergoing screening before renal transplantation.33 Given the absence of contemporary data to support revascularization of screen-detected CAD before transplantation to improve transplant outcomes, the 2020 Kidney Disease: Improving Global Outcomes guidelines do not recommend revascularization in asymptomatic candidates.34
Screening Candidates for Deceased Versus Living Donor Transplantation
The risks of perioperative delayed graft function and death are lower among living compared with deceased donor transplantation patients. Nonetheless, the consequences of adverse perioperative events are more troublesome in living donor than in the deceased donor recipients—losing a living donor kidney may have substantial emotional impact.1 In the US health care system, it may lead to increased regulatory scrutiny and penalties for transplant programs. Consequently, there may be an even lower threshold to screen and intervene in asymptomatic living donor candidates despite the relative absence of evidence that this approach is beneficial.1 Given these differences from deceased donor transplantation, development of an evidence-based screening strategy for living donor candidates is warranted.
Frequency of Screening for CAD
In addition to screening before acceptance into the transplant waiting list, the current standard of care involves screening asymptomatic patients at variable intervals after wait-listing until transplantation (class IIB, level of evidence: C).17 Some transplant programs have adopted a strategy of deferred screening in which only patients who have accrued significant waiting time and are expected to receive a deceased donor offer in the near future are screened.1 Until new evidence becomes available, the benefit of periodically screening asymptomatic patients during wait-listing remains uncertain. The Canadian-Australasian Randomised Trial of Screening Kidney Transplant Recipients for Coronary Artery Disease trial (NCT03674307) will test the hypothesis that a conservative strategy of no screening is noninferior to a strategy of mandated and repeated screening among asymptomatic patients wait-listed for kidney transplantation, with symptomatic patients in either arm being investigated and managed as per the standard practice in each center.
Conservative and Invasive Management of CAD in Late-Stage CKD
OMT
Medical therapy is paramount for treatment of CAD. There are, however, specific challenges to effective medical therapy in the CKD population because the relative contribution of atherosclerosis to cardiovascular events in late-stage CKD, and especially ESKD, is low.35 Accordingly, the benefit of statins diminishes as eGFR declines, with no evidence of benefit among patients on dialysis.35 Proprotein convertase subtilisin/kexin type 9 inhibition reduces the composite end point of cardiovascular death, MI, stroke, hospitalization for unstable angina, or coronary revascularization in patients with stage G2 CKD (60–90 ml/min per 1.73 m2), with a numerical trend for benefit in late-stage CKD.36 Overall, patients with late-stage CKD or ESKD are under-represented in clinical trials; as such, the current evidence base to support recommendations is limited.35
Invasive Management and Revascularization
The efficacy of OMT alone or in combination with revascularization (PCI or coronary artery bypass grafting [CABG]) in symptomatic patients with CKD or ESKD remains unclear. Although primary PCI is indicated in patients with CKD and STEMI, there is conflicting evidence for early invasive strategy in non-STEMI. Observational studies have revealed survival benefit with early invasive strategy37; however, no survival benefit from early intervention was observed in CKD stages G3 to G5 in a meta-analysis of randomized controlled trials of non-STEMI.38 Similarly, patients with late-stage CKD or ESKD are under-represented in clinical trials of stable CAD—including Clinical Outcomes Utilizing Revascularization and Aggressive Drug Evaluation37 and Bypass Angioplasty Revascularization Investigation in Type 2 Diabetes39—which revealed no benefit for routine intervention versus OMT.
Short-term procedural risks of both PCI and CABG are higher in patients with CKD compared with those without CKD. For instance, both PCI and CABG are associated with higher risk of AKI in CKD, with higher risk for CABG versus PCI.40 There are limited randomized data to support PCI or CABG in patients with late-stage CKD or ESKD. A meta-analysis of randomized trials suggested benefits of CABG versus PCI in reducing MI and repeat revascularization but not mortality in CKD (stages G3–G5).41 A propensity-matched observational study of CKD (stages G3–G5) suggested higher short-term risks of mortality, stroke, and repeat revascularization with CABG versus PCI, but higher long-term risks of MI and repeat vascularization with PCI versus CABG.42 Limited existing data suggest that dual antiplatelet therapy beyond 6 months after PCI may be associated with excessive bleeding and no clear benefit in reducing ischemic events in late-stage CKD43 and ESKD.44 There are no prospective or randomized data to guide combined antiplatelet and anticoagulation therapy in the CKD/ESKD population with atrial fibrillation undergoing PCI.
Minimizing CIN: UltraLow Contrast Angiography and Zero-Contrast PCI
A major challenge for invasive assessment and treatment of CAD in late-stage CKD is to preserve the remaining kidney function. CIN rarely results in irreversible loss of kidney function; however, radiocontrast exposure is associated with postprocedural morbidity and mortality.45 Preservation of the residual kidney function is also important for patients with ESKD on dialysis, especially for patients who continue to have urine output. Several agents and devices have been proposed to reduce the risk of AKI during angiography,46 PCI,47 and CABG48; nonetheless, these strategies are either ineffective46 or lack supportive data from large randomized trials.
Intravenous hydration during cardiac catheterization as guided by left ventricular end-diastolic pressure (LVEDP) reduces the risk of CIN.49 The LVEDP-guided hydration protocol was tested in the CKD population with a mean glomerular filtration rate of 48 ml/min per 1.73 m2.49 In advanced CKD (glomerular filtration rate <30 ml/min per 1.73 m2), LVEDP is often high; therefore, i.v. hydration is best performed during rather than before the procedure to avoid precipitating acute pulmonary edema. Using low contrast volume may also reduce the risk of CIN. A strategy for ultralow contrast angiography has been developed, in which, in addition to LVEDP-guided intraprocedural hydration, the contrast volume is limited to a maximum guided by a contrast volume-to-eGFR ratio <1.50 Contrast volume/eGFR is a validated measure of systemic exposure to radiocontrast, with contrast volume/eGFR >1 exponentially increasing the risk of CIN in late-stage CKD.51 During ultralow contrast angiography, injection of saline to induce repolarization changes on electrocardiogram monitoring or advancement of a workhorse coronary guidewire (rather than test contrast injections) is used to confirm catheter engagement, and meticulous techniques are used to minimize the contrast administered in a limited number of angiographic projections (Figure 1), both in the native CAD50 and graft conduits.52 When angiographically ambiguous lesions are present, adjunctive tests, such as intravascular imaging and coronary physiology, are used to further assess the lesion severity without using additional contrast (Figure 1). In a single-center, nonrandomized, propensity-matched observational cohort, ultralow contrast angiography reduced the risk of CIN and need for renal replacement therapy (RRT) compared with standard angiography in late-stage CKD during a 24-month follow-up period.53
Figure 1.
Ultralow contrast angiography. (a) The LVEDP guides the intraprocedural i.v. hydration. Prehydration is avoided owing to usually high filling pressures that predispose to pulmonary edema in late-stage chronic kidney disease. (b) Intracoronary injection of saline, which induces repolarization changes on electrocardiogram monitoring, is used to confirm catheter engagement to coronary arteries and replaces the test contrast injections. (c–e) One angiographic projection is used to image the RCA and 2 projections are used to image the LCA system. Each view is taken by using approximately 3 ml of contrast (total = 9 ml). The lesions suspected of being significantly flow limiting (arrows) are further evaluated by intracoronary physiological assessment through the placement of a pressure wire without using additional contrast. In this case, the instantaneous wave-free flow reserve was 1.0 in the RCA, 1.0 in the circumflex artery, and 0.87 in the left anterior descending artery (ischemia threshold is ≤0.89), thus indicating that only the lesion in the left anterior descending artery would require revascularization. AP, anteroposterior; LAO, left anterior oblique; LCA, left coronary artery; LVEDP, left ventricular end-diastolic pressure; RCA, right coronary artery.
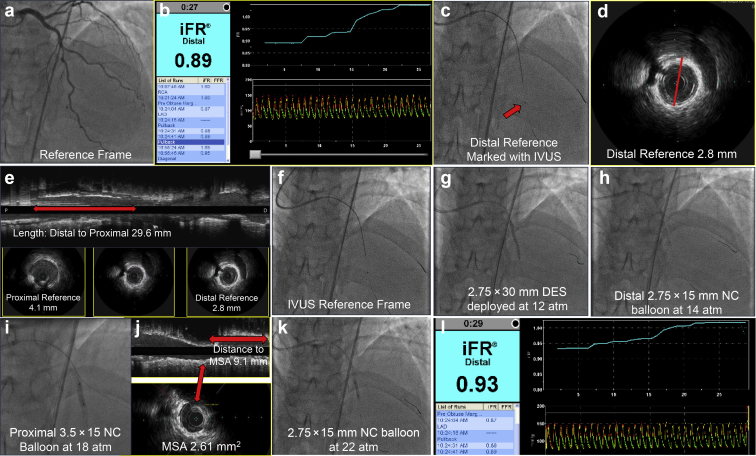
Zero-contrast PCI is a strategy for staged PCI without using contrast in late-stage CKD after a previously performed ultralow contrast angiography.50 Staging the procedure allows for reduction in the total contrast exposure, recovery of kidney function, and discussion of the risks and benefits of zero-contrast PCI with the patient. During the procedure, previous ultralow contrast angiography is used as a roadmap for guiding catheter engagement and placement of guidewires to generate a metallic silhouette of the target vessel and its branches (Figure 2). The procedure is then guided by intravascular physiology and imaging (ultrasound50 or optical coherence tomography with saline flush54). Prespecified criteria indicating procedural complications or suboptimal results are devised to guide the use of contrast to perform angiography during the zero-contrast procedure, if indicated.50
Figure 2.
Zero-contrast percutaneous coronary intervention. (a) A reference angiographic view from a previously performed ultralow contrast angiography is used as a road map. (b) Intracoronary physiology (iFR = 0.89) suggests significant flow limitation by the lesion. The pullback reveals a focal increase in flow at the level of the target lesion. (c) An IVUS catheter is advanced, and the transducer (arrow) is placed at the distal reference segment beyond the target lesion. A dry cine is recorded to mark the landing zone of the stent. (d) The external lamina-based vessel diameter on IVUS at the distal reference segment is measured (2.8 mm) to guide the selection of the stent diameter. (e) The diameters of the distal and proximal references and the length of the lesion on the longitudinal view on IVUS are measured to guide stent length and postdilatation. (f, g) Using the IVUS-fluoro reference frame, a 2.75 × 30 mm DES is placed and deployed in the artery. (h, i) Distal and proximal segments are postdilated by NC balloons. (j) IVUS is repeated to measure MSA and the distance from the distal edge of the stent to the frame with the lowest stent diameter to guide postdilatation. (k) Targeted postdilatation at high inflation pressure is performed. (l) Repeat physiology evaluation reveals increase in iFR to 0.93 (well above the ischemic threshold) and resolution of the focal flow limitation on the pullback. DES, drug-eluting stent; iFR, instantaneous wave-free flow reserve; IVUS, intravascular ultrasound; MSA, minimal stent area; NC, noncompliant.
An initial report in 35 patients with eGFR 16 ± 8 ml/min per 1.73 m2 supported the feasibility and safety of this approach, resulting in preserved postprocedural kidney function in all patients without need for RRT.50 A prospective, single-center, propensity-matched comparison with standard angiography alone revealed that combined ultralow contrast angiography and zero-contrast PCI was associated with significant reduction in the rates of RRT within a 12-month follow-up period (hazard ratio [HR] = 0.40, 95% confidence interval [CI] = 0.21–0.75, P = 0.0032).55 Given the frequent presence of severe calcification, atherectomy may be needed during the zero-contrast PCI to modify the fibrocalcific plaques and optimize PCI results.56 The recent advent of intravascular lithotripsy, which is a balloon catheter-based technique, may simplify calcific plaque modification as part of zero-contrast PCI.57 The feasibility of zero-contrast PCI has also been found in complex lesions (such as chronic total occlusions58 and high-risk PCI with hemodynamic support59 or in vein grafts60). Randomized studies are warranted to further establish the role of ultralow contrast angiography and zero-contrast PCI as part of the invasive management strategy for CAD in late-stage CKD.
Conservative Versus Invasive Management of CAD in Late-Stage CKD: The ISCHEMIA-CKD Trial
The ISCHEMIA-CKD trial, undertaken in parallel with the larger ISCHEMIA trial, randomized 777 participants with moderate or severe ischemia on functional testing (site interpreted) and advanced CKD (eGFR <30 ml/min per 1.73 m2 or on dialysis) in a 1:1 fashion to compare a conservative strategy of OMT alone or with cardiac catheterization and revascularization (PCI or CABG, if suitable).61 Key exclusion criteria were left ventricular ejection fraction <35%, heart failure (New York Heart Association classes III–IV), unacceptable level of angina despite OMT, acute coronary syndromes within the previous 2 months, and PCI or CABG during the past 12 months.
In a median follow-up time of 2.5 years, there was no difference in the rates of the composite primary endpoint of all-cause death or nonfatal MI between the initial invasive strategy (36.4%) and conservative strategy (36.7%) (adjusted HR = 1.01, 95% CI = 0.79–1.29, P = 0.95). The composite secondary endpoint of death, nonfatal MI, hospitalization for unstable angina, heart failure, or resuscitated cardiac arrest was not different between the 2 groups (38.5% vs. 39.7%, HR = 1.02, 95% CI = 0.79–1.29, P = 0.93). There were no differences in the individual components of the secondary endpoints between the groups—death, cardiovascular death, unstable angina, or heart failure.61
In contrast to the main ISCHEMIA trial, there were no differences in the rates of procedural or spontaneous MI in ISCHEMIA-CKD. There was a signal for harm with an initial invasive strategy, with higher rates of stroke (HR = 3.76, 95% CI = 1.52–9.32, P = 0.004) and the prespecified safety endpoint of death or new dialysis (HR = 1.48, 95% CI = 1.04–2.11, P = 0.02), which seemed to be driven by a trend for higher risk of new dialysis with initial invasive strategy in the subset of patients (n = 190) who were not on dialysis at trial entry (HR = 1.47, 95% CI = 0.88–2.44, P = 0.13). Contrary to the main ISCHEMIA trial, the degree of ischemia on stress testing significantly correlated with adverse outcomes, a finding that is consistent with previous observational studies,1 which may suggest that, in contrast to non-CKD population, assessing for inducible ischemia may have prognostic value in CKD. Comparison of the design and main findings and of the ISCHEMIA-CKD and ISCHEMIA trials is summarized in Table 1.
Table 1.
Comparison of ISCHEMIA-CKD and ISCHEMIA trial
| Details of the Trial | ISCHEMIA-CKD | ISCHEMIA |
|---|---|---|
| Major inclusion criteria | Moderate or severe ischemia End-stage renal disease on dialysis or eGFR <30 ml/min per 1.73 m2 |
Moderate or severe ischemia ≥50% stenosis in a major epicardial vessel (stress imaging participants) ≥70% stenosis in a proximal or midvessel (ETT participants) |
| Major exclusion criteria | Left ventricular ejection fraction <35% NYHA classes III–IV heart failure Unacceptable level of angina despite maximal medical therapy ACS within the previous 2 mo PCI or CABG within the previous 12 months |
≥50% stenosis in unprotected left main Left ventricular ejection fraction <35% NYHA classes III–IV heart failure Unacceptable level of angina despite maximal medical therapy ACS within the previous 2 mo PCI or CABG within the previous 12 months |
| Number of participants | 777: invasive 388, conservative 389 | 5179: invasive 2588, conservative 2591 |
| Qualifying stress test | Site determined | Core laboratory adjudicated |
| Stress test modality | Invasive: stress imaging 81%, ETT 19% Conservative: stress imaging 82%, ETT 18% |
Invasive: stress imaging 75%, ETT 25% Conservative: stress imaging 76%, ETT 24% |
| Baseline inducible ischemia | Severe: invasive 36%, conservative 39% Moderate: invasive 64%, conservative 61% |
Severe: invasive 53%, conservative 55% Moderate: invasive 34%, conservative 32% Mild/none: invasive 12%, conservative 12% Uninterpretable: invasive 1%, conservative 1% |
| Baseline coronary anatomy by CTA | Not performed | 1 Vessel: invasive 24%, conservative 22% 2 Vessel: invasive 29%, conservative 34% ≥3 Vessel: invasive 47%, conservative 44% |
| Cardiac catheterization | Invasive 85%, conservative 22% | Invasive 96%, conservative 28% |
| Revascularization | Invasive 50%, conservative 12% | Invasive 80%, conservative 23% |
| Reasons for no catheterization in the invasive arm | Patient preference 6%, physician preference 1%, intercurrent illness 4%, death 2%, other 2% | — |
| Reasons for no revascularization in the invasive arm | Nonobstructive CAD 75%, unsuitable anatomy 14%, patient preference 3%, intended PCI/CABG 4%, other 3% | — |
| Reasons for catheterization in the conservative arm | — | Suspected/confirmed event 13.8%, medical therapy failure 3.9%, protocol violation 8.1% |
| Reasons for revascularization in the conservative arm | — | Primary event 16% |
| Composite primary endpoint | Death or MI (adjusted HR = 1.01, 95% CI = 0.79–1.29, P = 0.95) | CV death, MI, or hospitalization for UA, HF, or resuscitated cardiac arrest Adjusted HR = 0.93 (95% CI = 0.80–1.08), P = 0.34 |
| Prespecified subgroups | No heterogeneity of treatment effect with diabetes, severity of angina, dialysis, or moderate ischemia; nonsignificant trend favoring invasive arm in severe ischemia | No heterogeneity of treatment effect with diabetes, severity of baseline ischemia, severity of CAD, or proximal LAD involvement |
| Major secondary endpoint | CV death, MI, or hospitalization for UA, HF, or resuscitated cardiac arrest (adjusted HR = 1.01, 95% CI = 0.79–1.29, P = 0.93) | CV death or MI (adjusted HR = 0.90, 95% CI = 0.77–1.06, P = 0.21) |
| All-cause death | Adjusted HR = 1.02, 95% CI = 0.76–1.35, P = 0.91) | Adjusted HR = 1.05, 95% CI = 0.83–1.32, P = 0.67 |
| Myocardial infarction | Adjusted HR = 0.84, 95% CI = 0.57–1.25, P = 0.39) | Adjusted HR = 0.92, 95% CI = 0.76–1.11, P = 0.38 |
| Impact of baseline ischemia on outcomes | Yes | No |
| Impact of severity of CAD on outcomes | No | Yes |
ACS, acute coronary syndromes; CABG, coronary artery bypass grafting; CAD, coronary artery disease; CI, confidence interval; CKD, chronic kidney disease; CTA, computed tomography angiography; CV, cardiovascular; eGFR, estimated glomerular filtration rate; ETT, exercise tolerance test; HF, heart failure; HR, hazard ratio; ISCHEMIA, International Study of Comparative Health Effectiveness With Medical and Invasive Approaches; LAD, left anterior descending; MI, myocardial infarction; NYHA, New York Heart Association; PCI, percutaneous coronary intervention; UA, unstable angina.
ISCHEMIA-CKD provides the much-needed randomized data for the management of stable CAD in the high-risk yet understudied patients with late-stage CKD. Nonetheless, important points need to be considered in interpreting the data and integrating them into clinical practice.
First, of the 330 patients (80%) in the initial invasive strategy group who had had coronary angiography, only 50% underwent revascularization (85% PCI, 15% CABG) despite the presence of moderate or severe ischemia on the exercise tolerance test or stress imaging.61 Of 134 patients who had had angiography but no PCI, 75% had nonobstructive disease. The reason for no catheterization in 7% of patients was patient or physician preference despite clinical indication, with the reasons for no revascularization in 21% of cases being patient preference, unsuitable anatomy, or intended PCI or CABG. Although these rates are significantly lower than in real-world practice, they may reflect the risk aversion of patients or operators to avoid CIN and the need for RRT, or may reflect the complexity of the CAD, that is, they may indicate the so-called concept of renalism (hesitation to perform indicated procedures owing to the risk of instigating AKI and the need for RRT).62 The high rates of false positivity in ischemia quantification also indicate the aforementioned limitations of electrocardiogram stress testing and stress imaging in late-stage CKD and ESKD, which were not evaluated by a core laboratory during the trial. Importantly, CTA was not undertaken in the ISCHEMIA-CKD trial partly owing to the reduced specificity resulting from calcium-induced blooming artifact that is highly prevalent in this population. The most sensitive and accurate screening modality for assessment of myocardial ischemia in the CKD population remains unknown. Although stress cardiac magnetic resonance imaging is relatively contraindicated in late-stage CKD owing to the risk of nephrogenic systemic fibrosis from gadolinium,63 and because of the low accuracy of single-photon emission computed tomography imaging and stress electrocardiogram, PET myocardial perfusion imaging may be the preferred testing modality to improve risk stratification in CKD. Nonetheless, the relatively high cost and limited availability of PET myocardial perfusion imaging may restrict its use for ischemia quantification in real-world practice.
Second, approximately 50% of patients enrolled in each arm of ISCHEMIA-CKD were on dialysis, with patients in CKD stages G4 and G5 comprising 42% and 8% of the participants, respectively. Dialysis has a major impact on CAD and cardiovascular mortality that are distinct from late-stage CKD, and these may confound the effects of both medical therapy and revascularization (e.g., reduced efficacy of statins in patients on dialysis compared with those in the earlier stages of CKD35,64 and the increasing frequency of nonatherosclerotic disease processes, such as heart failure and arrhythmias, the longer the patients are maintained on dialysis65). Sudden death is common in patients on dialysis possibly because the shifts in fluid and electrolytes and drug concentrations may trigger arrhythmias, especially if the myocardium is not normal (e.g., with left ventricular hypertrophy).1 Dialysis-related factors, such as type and frequency of dialysis and dialysate composition, may affect cardiovascular events.1 Intradialytic hypotension and myocardial stunning are hemodialysis-specific syndromes associated with mortality and are unique to patients on dialysis.66,67
Moreover, although the heterogeneity analysis in ISCHEMIA-CKD trial did not reveal a difference in the primary endpoint based on dialysis status, patients in the invasive arm were on dialysis for a median of 1 year longer compared with those in the conservative arm, with the time on dialysis (or dialysis vintage) found to correlate with mortality. Thus, this confounding effect would not be accounted for by the subgroup analysis based on dialysis status only. Randomized studies need to evaluate the impact of invasive versus conservative management of CAD in separate groups of patients with late-stage CKD or ESKD on incident dialysis or adjust for the accumulated time on prevalent dialysis.
Third, there was a heightened risk of AKI in the invasive arm (7.5% vs. 5.4%); 2.1% of patients were on dialysis within a month of the procedure in the invasive group, and the dialysis rates remained higher during follow-up compared with those of the conservative group. Efforts were made to minimize the risk of AKI with revascularization (PCI or CABG) by using LVEDP-guided hydration and reducing the contrast volume in angiography and PCI. Analysis of the contrast volume used per procedure would determine the degree of adherence to contrast volume minimization, especially whether contrast volume remained within the suggested limit of contrast volume/eGFR <1.50 Unfortunately, contrast volume was not systematically recorded in ISCHEMIA-CKD.
Although ultralow contrast/zero-contrast PCI was recommended in the trial, the technical report was published in early 2016.50 At the time of its publication, approximately 300 patients were already enrolled in ISCHEMIA-CKD.68 Because most nondialysis patients enrolled were in stage G4 (GFR 15–30 ml/min per 1.73 m2), AKI rates after angiography or PCI, although relatively low compared with real-world practice, were higher than would be expected if the core principles of ultralow contrast angiography and zero-contrast PCI were universally adhered to. Future analyses of the trial data set may help determine whether intravascular ultrasound was used to guide PCI,50 if adjunctive atheroablation was used to optimize procedural results on the often severely calcific plaques in advanced CKD,56 and what proportion of flow-limiting lesions based on physiology were treated.
Conclusions and Areas for Future Research
Additional research is required in several areas, including epidemiology, pathophysiology, clinical presentation, risk prediction, and management of CAD, in late-stage CKD, ESKD, and kidney transplant candidates. Future trials evaluating management strategies for stable CAD should be tailored to the population of patients in different stages of CKD; in particular, the ESKD population should be studied in separate, dedicate trials.
A suggested design for future randomized studies evaluating the clinical impact of OMT plus revascularization versus OMT alone for stable CAD in late-stage CKD and ESKD is as follows:
OMT Plus Revascularization Versus OMT in Late-Stage CKD (G4–G5)
Patients with suspected angina—classic angina or angina-equivalent symptoms (e.g., dyspnea [New York Heart Association classes II–III] with left ventricular ejection factor >35%)—will be screened with PET myocardial perfusion imaging.
Patients with moderate or severe ischemia on semiquantitative analysis and/or a reduction in the global PET-CFR <1.5 will be included.
Ultralow contrast angiography will be performed to assess the significant left main CAD—those with significant left main or nonobstructive CAD (by core laboratory assessment) will be excluded.
Participants will be randomized to OMT plus revascularization (if indicated) versus OMT in a 1:1 fashion to assess for the effect on the composite endpoint of death or MI.
Ultralow contrast/zero-contrast PCI will be carried out to minimize the risk of AKI and procedure-related dialysis, with the principles of reducing AKI in CABG adopted.
OMT Plus Revascularization Versus OMT in Patients With ESKD on Incident Dialysis
Patients with suspected angina—classic angina or angina-equivalent symptoms (e.g., dyspnea [New York Heart Association classes II–III] with left ventricular ejection factor >35%), a history of intradialytic hypotension and myocardial stunning, and those with chronic nondynamically elevated troponin will be screened with PET myocardial perfusion imagin.
Patients with moderate or severe ischemia on semiquantitative imaging and/or with a reduction in the global CFR <1.5 will be included.
CTA will be performed to assess the significant left main CAD—those with significant left main or nonobstructive CAD (by core laboratory assessment) will be excluded.
Participants will be randomized to OMT plus revascularization (if indicated) versus OMT in a 1:1 fashion to assess the differences in the composite endpoint of death or MI.
The 4-year estimated event rates in the ISCHEMIA-CKD trial were 41% to 48%, which included approximately equal number of patients with and without ESKD, with the incidence of primary outcomes assumed to be 22% to 24% lower in the invasive strategy group.61 Although the estimated cumulative event rates for death or MI are expected to be higher in the ESKD population compared with the late-stage CKD population (50%–55% vs. 35%–40% in 4 years), the estimated impact of an invasive strategy would be lower in the ESKD population (20% vs. 25%) owing to the higher contribution of nonatherosclerotic events to mortality in the dialysis-dependent ESKD population. Taking these considerations into account and with a study power of 80%, approximately n = 680 and n = 750 participants would be required in the ESKD and late-stage CKD trials, respectively, to detect a difference with the invasive versus conservative approach in a 4-year period, that is, the 2 studies taken together would approximately equal twice the size of the ISCHEMIA-CKD trial (n = 777). Such randomized studies will no doubt require enormous international effort to conduct and substantial grant support from governmental and nongovernmental bodies; nevertheless, we believe that they are necessary in the post-ISCHEMIA era to inform optimal management using the best available therapeutic strategies in these 2 distinct groups of patients. Finally, although the science, technology, and techniques of percutaneous revascularization have substantially improved, the pathology, diagnosis, and management of CAD in CKD and ESKD remain mostly poorly understood. That cardiovascular disease remains the most common cause of death in people in all stages of CKD/ESKD is a clear reason for us to strive to better understand these aspects through further studies at the basic, translational, and clinical levels.
Disclosure
SC reports receiving National Health and Medical Research Council of Australia Funding which is paid to the institution for Canadian-Australasian Randomised Trial of Screening Kidney Transplant Recipients for Coronary Artery Disease Trial and has Advisory Board membership in AstraZeneca and CSL Behring. RM reports receiving institutional research grants from Abbott Laboratories, AstraZeneca, Bayer, Beth Israel Deaconess, Bristol-Myers Squibb, CERC, Chiesi, Concept Medical, CSL Behring, DSI, Medtronic, Novartis Pharmaceuticals, and OrbusNeich; consultant fees from Abbott Laboratories, Boston Scientific, Janssen Scientific Affairs, Medscape/WebMD, Medtelligence (Janssen Scientific Affairs), Roivant Sciences, Sanofi, and Siemens Medical Solutions; consultant fees paid to the institution from Abbott Laboratories and Bristol-Myers Squibb; advisory board funding paid to the institution from Spectranetics/Philips/Volcano Corp.; consultant (RM's spouse) from Abiomed and The Medicines Company; owns equity <1% from Claret Medical and Elixir Medical; has data safety monitoring board membership fees paid to the institution from Watermark Research Partners; and consults (no fee) for Idorsia Pharmaceuticals Ltd. and Regeneron Pharmaceuticals; and is an associate editor for American College of Cardiology, American Medical Association. SB reports receiving grant funding from the National Heart, Lung, and Blood Institute and Abbott Vascular. GMC reports receiving grant support from the National Institute of Diabetes and Digestive and Kidney Diseases, National Institute of Allergy and Infectious Diseases and reports serving as a consultant to Akebia, Ardelyx, AstraZeneca, Baxter, CloudCath, Cricket, DiaMedica, Durect, Gilead, Miromatrix, Outset, Reata, Sanifit, and Vertex, and provides Data Safety Monitoring Board service for Angion, Bayer, National Institute of Diabetes and Digestive and Kidney Diseases, and ReCor. ZAA reports receiving institutional research grants for Columbia University from Abbott, Cardiovascular Systems Inc., and serving as a consultant for Abbott, Abiomed, AstraZeneca, and Shockwave. KKG declared no competing interests.
References
- 1.Sarnak M.J., Amann K., Bangalore S. Chronic kidney disease and coronary artery disease: JACC state-of-the-art review. J Am Coll Cardiol. 2019;74:1823–1838. doi: 10.1016/j.jacc.2019.08.1017. [DOI] [PubMed] [Google Scholar]
- 2.Manjunath G., Tighiouart H., Ibrahim H. Level of kidney function as a risk factor for atherosclerotic cardiovascular outcomes in the community. J Am Coll Cardiol. 2003;41:47–55. doi: 10.1016/s0735-1097(02)02663-3. [DOI] [PubMed] [Google Scholar]
- 3.Cozzolino M., Mangano M., Stucchi A. Cardiovascular disease in dialysis patients. Nephrol Dial Transplant. 2018;33(suppl 3):iii28–iii34. doi: 10.1093/ndt/gfy174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ying T., Shi B., Kelly P.J. Death after kidney transplantation: an analysis by era and time post-transplant. J Am Soc Nephrol. 2020;31:2887–2899. doi: 10.1681/ASN.2020050566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fox C.S., Matsushita K., Woodward M. Associations of kidney disease measures with mortality and end-stage renal disease in individuals with and without diabetes: a meta-analysis. Lancet. 2012;380:1662–1673. doi: 10.1016/S0140-6736(12)61350-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mahmoodi B.K., Matsushita K., Woodward M. Associations of kidney disease measures with mortality and end-stage renal disease in individuals with and without hypertension: a meta-analysis. Lancet. 2012;380:1649–1661. doi: 10.1016/S0140-6736(12)61272-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sosnov J., Lessard D., Goldberg R.J. Differential symptoms of acute myocardial infarction in patients with kidney disease: a community-wide perspective. Am J Kidney Dis. 2006;47:378–384. doi: 10.1053/j.ajkd.2005.11.017. [DOI] [PubMed] [Google Scholar]
- 8.Go A.S., Bansal N., Chandra M. Chronic kidney disease and risk for presenting with acute myocardial infarction versus stable exertional angina in adults with coronary heart disease. J Am Coll Cardiol. 2011;58:1600–1607. doi: 10.1016/j.jacc.2011.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shroff G.R., Li S., Herzog C.A. Trends in discharge claims for acute myocardial infarction among patients on dialysis. J Am Soc Nephrol. 2017;28:1379–1383. doi: 10.1681/ASN.2016050560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Goff D.C., Jr., Lloyd-Jones D.M., Bennett G. 2013 ACC/AHA guideline on the assessment of cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129(Suppl 2):S49–S73. doi: 10.1161/01.cir.0000437741.48606.98. [DOI] [PubMed] [Google Scholar]
- 11.Weiner D.E., Tighiouart H., Elsayed E.F. The Framingham predictive instrument in chronic kidney disease. J Am Coll Cardiol. 2007;50:217–224. doi: 10.1016/j.jacc.2007.03.037. [DOI] [PubMed] [Google Scholar]
- 12.Matsushita K., Coresh J., Sang Y. Estimated glomerular filtration rate and albuminuria for prediction of cardiovascular outcomes: a collaborative meta-analysis of individual participant data. Lancet Diabetes Endocrinol. 2015;3:514–525. doi: 10.1016/S2213-8587(15)00040-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stone N.J., Robinson J.G., Lichtenstein A.H. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;63:2889–2934. doi: 10.1016/j.jacc.2013.11.002. [DOI] [PubMed] [Google Scholar]
- 14.Anker S.D., Gillespie I.A., Eckardt K.U. Development and validation of cardiovascular risk scores for haemodialysis patients. Int J Cardiol. 2016;216:68–77. doi: 10.1016/j.ijcard.2016.04.151. [DOI] [PubMed] [Google Scholar]
- 15.Kasiske B.L., Chakkera H.A., Roel J. Explained and unexplained ischemic heart disease risk after renal transplantation. J Am Soc Nephrol. 2000;11:1735–1743. doi: 10.1681/ASN.V1191735. [DOI] [PubMed] [Google Scholar]
- 16.Young L.H., Wackers F.J., Chyun D.A. Cardiac outcomes after screening for asymptomatic coronary artery disease in patients with type 2 diabetes: the DIAD study: a randomized controlled trial. JAMA. 2009;301:1547–1555. doi: 10.1001/jama.2009.476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lentine K.L., Costa S.P., Weir M.R. Cardiac disease evaluation and management among kidney and liver transplantation candidates: a scientific statement from the American Heart Association and the American College of Cardiology Foundation. J Am Coll Cardiol. 2012;60:434–480. doi: 10.1016/j.jacc.2012.05.008. [DOI] [PubMed] [Google Scholar]
- 18.Winther S., Svensson M., Jorgensen H.S. Diagnostic performance of coronary CT angiography and myocardial perfusion imaging in kidney transplantation candidates. JACC Cardiovasc Imaging. 2015;8:553–562. doi: 10.1016/j.jcmg.2014.12.028. [DOI] [PubMed] [Google Scholar]
- 19.Patel R.K., Mark P.B., Johnston N. Prognostic value of cardiovascular screening in potential renal transplant recipients: a single-center prospective observational study. Am J Transplant. 2008;8:1673–1683. doi: 10.1111/j.1600-6143.2008.02281.x. [DOI] [PubMed] [Google Scholar]
- 20.Winther S., Svensson M., Jørgensen H.S. Repeated contrast administration is associated with low risk of postcontrast acute kidney injury and long-term complications in patients with severe chronic kidney disease. Am J Transplant. 2016;16:897–907. doi: 10.1111/ajt.13545. [DOI] [PubMed] [Google Scholar]
- 21.Kruk M., Noll D., Achenbach S. Impact of coronary artery calcium characteristics on accuracy of CT angiography. JACC Cardiovasc Imaging. 2014;7:49–58. doi: 10.1016/j.jcmg.2013.07.013. [DOI] [PubMed] [Google Scholar]
- 22.Murthy V.L., Naya M., Foster C.R. Coronary vascular dysfunction and prognosis in patients with chronic kidney disease. JACC Cardiovasc Imaging. 2012;5:1025–1034. doi: 10.1016/j.jcmg.2012.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Maddahi J., Lazewatsky J., Udelson J.E. Phase-III clinical trial of fluorine-18 Flurpiridaz positron emission tomography for evaluation of coronary artery disease. J Am Coll Cardiol. 2020;76:391–401. doi: 10.1016/j.jacc.2020.05.063. [DOI] [PubMed] [Google Scholar]
- 24.Charytan D.M., Skali H., Shah N.R. Coronary flow reserve is predictive of the risk of cardiovascular death regardless of chronic kidney disease stage. Kidney Int. 2018;93:501–509. doi: 10.1016/j.kint.2017.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shah N.R., Charytan D.M., Murthy V.L. Prognostic value of coronary flow reserve in patients with dialysis-dependent ESRD. J Am Soc Nephrol. 2016;27:1823–1829. doi: 10.1681/ASN.2015030301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Eggers K.M., Lindahl B., Carrero J.J. Cardiac troponins and their prognostic importance in patients with suspected acute coronary syndrome and renal dysfunction. Clin Chem. 2017;63:1409–1417. doi: 10.1373/clinchem.2017.271890. [DOI] [PubMed] [Google Scholar]
- 27.Gunsolus I., Sandoval Y., Smith S.W. Renal dysfunction influences the diagnostic and prognostic performance of high-sensitivity cardiac troponin I. J Am Soc Nephrol. 2018;29:636–643. doi: 10.1681/ASN.2017030341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.deFilippi C., Wasserman S., Rosanio S. Cardiac troponin T and C-reactive protein for predicting prognosis, coronary atherosclerosis, and cardiomyopathy in patients undergoing long-term hemodialysis. JAMA. 2003;290:353–359. doi: 10.1001/jama.290.3.353. [DOI] [PubMed] [Google Scholar]
- 29.Breidthardt T., Burton J.O., Odudu A. Troponin T for the detection of dialysis-induced myocardial stunning in hemodialysis patients. Clin J Am Soc Nephrol. 2012;7:1285–1292. doi: 10.2215/CJN.00460112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bhatti N.K., Karimi Galougahi K., Paz Y. Diagnosis and management of cardiovascular disease in advanced and end-stage renal disease. J Am Heart Assoc. 2016;5 doi: 10.1161/JAHA.116.003648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Friedman S.E., Palac R.T., Zlotnick D.M. A call to action: variability in guidelines for cardiac evaluation before renal transplantation. Clin J Am Soc Nephrol. 2011;6:1185–1191. doi: 10.2215/CJN.09391010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang L.W., Fahim M.A., Hayen A. Cardiac testing for coronary artery disease in potential kidney transplant recipients. Cochrane Database Syst Rev. 2011;2011:CD008691. doi: 10.1002/14651858.CD008691.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mao J., Karthikeyan V., Poopat C. Coronary computed tomography angiography in dialysis patients undergoing pre-renal transplantation cardiac risk stratification. Cardiol J. 2010;17:349–361. [PubMed] [Google Scholar]
- 34.Chadban S.J., Ahn C., Axelrod D.A. KDIGO clinical practice guideline on the evaluation and management of candidates for kidney transplantation. Transplantation. 2020;104(suppl 1):S11–S103. doi: 10.1097/TP.0000000000003136. [DOI] [PubMed] [Google Scholar]
- 35.Wanner C., Tonelli M., Kidney Disease: Improving Global Outcomes Lipid Guideline Development Work Group Members KDIGO Clinical Practice Guideline for Lipid Management in CKD: summary of recommendation statements and clinical approach to the patient. Kidney Int. 2014;85:1303–1309. doi: 10.1038/ki.2014.31. [DOI] [PubMed] [Google Scholar]
- 36.Charytan D.M., Sabatine M.S., Pedersen T.R. Efficacy and safety of evolocumab in chronic kidney disease in the FOURIER trial. J Am Coll Cardiol. 2019;73:2961–2970. doi: 10.1016/j.jacc.2019.03.513. [DOI] [PubMed] [Google Scholar]
- 37.Shaw C., Nitsch D., Lee J. Impact of an Early Invasive Strategy versus Conservative Strategy for Unstable Angina and non-ST Elevation Acute Coronary Syndrome in Patients with Chronic Kidney Disease: a Systematic Review. PLoS One. 2016;11 doi: 10.1371/journal.pone.0153478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Charytan D.M., Wallentin L., Lagerqvist B. Early angiography in patients with chronic kidney disease: a collaborative systematic review. Clin J Am Soc Nephrol. 2009;4:1032–1043. doi: 10.2215/CJN.05551008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.BARI 2D Study Group. Frye R.L., August P. A randomized trial of therapies for type 2 diabetes and coronary artery disease. N Engl J Med. 2009;360:2503–2515. doi: 10.1056/NEJMoa0805796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chang T.I., Leong T.K., Boothroyd D.B. Acute kidney injury after CABG versus PCI: an observational study using 2 cohorts. J Am Coll Cardiol. 2014;64:985–994. doi: 10.1016/j.jacc.2014.04.077. [DOI] [PubMed] [Google Scholar]
- 41.Charytan D.M., Desai M., Mathur M. Reduced risk of myocardial infarct and revascularization following coronary artery bypass grafting compared with percutaneous coronary intervention in patients with chronic kidney disease. Kidney Int. 2016;90:411–421. doi: 10.1016/j.kint.2016.03.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bangalore S., Guo Y., Samadashvili Z. Revascularization in patients with multivessel coronary artery disease and chronic kidney disease: everolimus-eluting stents versus coronary artery bypass graft surgery. J Am Coll Cardiol. 2015;66:1209–1220. doi: 10.1016/j.jacc.2015.06.1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gargiulo G., Santucci A., Piccolo R. Impact of chronic kidney disease on 2-year clinical outcomes in patients treated with 6-month or 24-month DAPT duration: an analysis from the PRODIGY trial. Catheter Cardiovasc Interv. 2017;90:E73–E84. doi: 10.1002/ccd.26921. [DOI] [PubMed] [Google Scholar]
- 44.Chen Y.T., Chen H.T., Hsu C.Y. Dual antiplatelet therapy and clinical outcomes after coronary drug-eluting stent implantation in patients on hemodialysis. Clin J Am Soc Nephrol. 2017;12:262–271. doi: 10.2215/CJN.04430416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dangas G., Iakovou I., Nikolsky E. Contrast-induced nephropathy after percutaneous coronary interventions in relation to chronic kidney disease and hemodynamic variables. Am J Cardiol. 2005;95:13–19. doi: 10.1016/j.amjcard.2004.08.056. [DOI] [PubMed] [Google Scholar]
- 46.Weisbord S.D., Gallagher M., Jneid H. Outcomes after angiography with sodium bicarbonate and acetylcysteine. N Engl J Med. 2018;378:603–614. doi: 10.1056/NEJMoa1710933. [DOI] [PubMed] [Google Scholar]
- 47.Desch S., Fuernau G., Poss J. Impact of a novel contrast reduction system on contrast savings in coronary angiography - the DyeVert randomised controlled trial. Int J Cardiol. 2018;257:50–53. doi: 10.1016/j.ijcard.2017.12.107. [DOI] [PubMed] [Google Scholar]
- 48.Luckraz H., Giri R., Wrigley B. The use of the RenalGuard system in cardiac surgery with cardiopulmonary bypass: a first in man prospective, observational, feasibility pilot study. Open Heart. 2017;4 doi: 10.1136/openhrt-2017-000669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Brar S.S., Aharonian V., Mansukhani P. Haemodynamic-guided fluid administration for the prevention of contrast-induced acute kidney injury: the POSEIDON randomised controlled trial. Lancet. 2014;383:1814–1823. doi: 10.1016/S0140-6736(14)60689-9. [DOI] [PubMed] [Google Scholar]
- 50.Ali Z.A., Karimi Galougahi K., Nazif T. Imaging- and physiology-guided percutaneous coronary intervention without contrast administration in advanced renal failure: a feasibility, safety, and outcome study. Eur Heart J. 2016;37:3090–3095. doi: 10.1093/eurheartj/ehw078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nyman U., Björk J., Aspelin P., Marenzi G. Contrast medium dose-to-GFR ratio: a measure of systemic exposure to predict contrast-induced nephropathy after percutaneous coronary intervention. Acta Radiol. 2008;49:658–667. doi: 10.1080/02841850802050762. [DOI] [PubMed] [Google Scholar]
- 52.Rahim H.M., Flattery E., Gkargkoulas F. Ultra-low-contrast angiography in patients with advanced chronic kidney disease and previous coronary artery bypass surgery. Coron Artery Dis. 2019;30:346–351. doi: 10.1097/MCA.0000000000000741. [DOI] [PubMed] [Google Scholar]
- 53.Bhatti N., Rahim H., Chen S. TCT-30 ultra-low contrast coronary angiography in patients with advanced chronic kidney disease: feasibility and outcomes compared with conventional angiography. J Am Coll Cardiol. 2019;74(suppl 13):B30. [Google Scholar]
- 54.Karimi Galougahi K., Zalewski A., Leon M.B. Optical coherence tomography-guided percutaneous coronary intervention in pre-terminal chronic kidney disease with no radio-contrast administration. Eur Heart J. 2016;37:1059. doi: 10.1093/eurheartj/ehv667. [DOI] [PubMed] [Google Scholar]
- 55.Rahim H., Flattery E., Gkargkoulas F. TCT-32 clinical outcomes of imaging- and physiology-guided PCI without contrast administration in advanced renal failure. J Am Coll Cardiol. 2019;74(suppl 13):B32. [Google Scholar]
- 56.Karimi Galougahi K., Mintz G.S., Karmpaliotis D., Ali Z.A. Zero-contrast percutaneous coronary intervention on calcified lesions facilitated by rotational atherectomy. Catheter Cardiovasc Interv. 2017;90:E85–E89. doi: 10.1002/ccd.26999. [DOI] [PubMed] [Google Scholar]
- 57.Karimi Galougahi K., Patel S., Shlofmitz R.A. Calcific plaque modification by acoustic shock waves - intravascular lithotripsy in coronary interventions. Circ Cardiovasc Interv. 2021;14 doi: 10.1161/CIRCINTERVENTIONS.120.009354. [DOI] [PubMed] [Google Scholar]
- 58.Hatem R., Finn M.T., Riley R.F. Zero contrast retrograde chronic total occlusions percutaneous coronary intervention: a case series. Eur Heart J Case Rep. 2018;2:1–5. doi: 10.1093/ehjcr/yty036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Madhavan M.V., Prasad M., Fall K.N. Zero-contrast multivessel revascularization for acute coronary syndrome in a patient with chronic kidney disease. J Am Coll Cardiol Case Reports. 2019;1:774–780. doi: 10.1016/j.jaccas.2019.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Parviz Y., Fall K., Stone G.W. Imaging and physiology to guide venous graft interventions without contrast administration in advanced renal failure. J Invas Cardiol. 2017;29:E163–E165. [PubMed] [Google Scholar]
- 61.Bangalore S., Maron D.J., O’Brien S.M. Management of coronary disease in patients with advanced kidney disease. N Engl J Med. 2020;382:1608–1618. doi: 10.1056/NEJMoa1915925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chertow G.M., Normand S.L., McNeil B.J. “Renalism”: inappropriately low rates of coronary angiography in elderly individuals with renal insufficiency. J Am Soc Nephrol. 2004;15:2462–2468. doi: 10.1097/01.ASN.0000135969.33773.0B. [DOI] [PubMed] [Google Scholar]
- 63.Woolen S.A., Shankar P.R., Gagnier J.J. Risk of nephrogenic systemic fibrosis in patients with stage 4 or 5 chronic kidney disease receiving a group II gadolinium-based contrast agent: a systematic review and meta-analysis. JAMA Intern Med. 2020;180:223–230. doi: 10.1001/jamainternmed.2019.5284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Baigent C., Landray M.J., Reith C. The effects of lowering LDL cholesterol with simvastatin plus ezetimibe in patients with chronic kidney disease (Study of Heart and Renal Protection): a randomised placebo-controlled trial. Lancet. 2011;377:2181–2192. doi: 10.1016/S0140-6736(11)60739-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wanner C., Amann K., Shoji T. The heart and vascular system in dialysis. Lancet. 2016;388:276–284. doi: 10.1016/S0140-6736(16)30508-6. [DOI] [PubMed] [Google Scholar]
- 66.Burton J.O., Jefferies H.J., Selby N.M., McIntyre C.W. Hemodialysis-induced repetitive myocardial injury results in global and segmental reduction in systolic cardiac function. Clin J Am Soc Nephrol. 2009;4:1925–1931. doi: 10.2215/CJN.04470709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Stefánsson B.V., Brunelli S.M., Cabrera C. Intradialytic hypotension and risk of cardiovascular disease. Clin J Am Soc Nephrol. 2014;9:2124–2132. doi: 10.2215/CJN.02680314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bangalore S., Maron D.J., Fleg J.L. International Study of Comparative Health Effectiveness with Medical and Invasive Approaches-Chronic Kidney Disease (ISCHEMIA-CKD): rationale and design. Am Heart J. 2018;205:42–52. doi: 10.1016/j.ahj.2018.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]