Abstract
Introduction
Chronic kidney disease (CKD) exposes to an increased incidence of fragility fractures. International guidelines recommend performing bone mineral density (BMD) if the results will impact treatment decisions. It remains unknown where bone loss occurs and what would preclude the longitudinal loss in patients with CKD. Here, we aimed to investigate factors influencing BMD and to analyze the longitudinal BMD changes.
Methods
In the NephroTest cohort, we measured BMD at the femoral neck, total hip, lumbar spine, and proximal radius, together with circulating biomarkers and standardized measured glomerular filtration rate (mGFR) by 51Cr-EDTA in a subset of patients with CKD stage 1 to 5 followed during 4.3 ± 2.0 years. A linear mixed model explored the longitudinal bone loss and the relationship of associated factors with BMD changes. A total of 858 patients (mean age 58.9 ± 15.2 years) had at least 1 and 477 had at least 2 BMD measures.
Results
At baseline, cross-sectional analysis showed a significantly lower BMD at femoral neck and total hip and a significant higher serum parathyroid hormone (PTH) along with CKD stages. Baseline age, gender, tobacco, low body mass index (BMI), and high PTH levels were significantly associated with low BMD. Longitudinal analysis during the mean 4.3 years revealed a significant bone loss at the radius only. BMD changes at the femoral neck were associated with BMI, but not CKD stages or basal PTH levels.
Conclusions
CKD is associated with low BMD and high PTH in the cross-sectional analysis. Longitudinal bone loss occurred at the proximal radius after 4.3 years.
Keywords: bone mineral density, chronic kidney disease–mineral bone disorders, fracture, osteoporosis, parathyroid hormone
CKD is a global health concern because of the increasing prevalence and cost. CKD is commonly associated with mineral and bone disorders (MBDs), fragility fractures, and vascular calcifications, which substantially increase the risk of death from cardiovascular disease. Skeletal fractures also represent a burden of increased morbidity, impairing the quality of life and increasing the risk of mortality.1,2 This increased risk of hip fracture progresses in parallel with the decline of GFR from stage 3 to 5,3 suggesting that bone fragility starts from early CKD-MBD and further increases in advanced stages.
The prediction of fractures in patients with CKD has been previously based on clinical risk factors, some of them shared with the general population, such as age, gender, ethnicity, physical inactivity, and BMI in addition to diabetes, cardiovascular diseases, and dementia.4,5 BMD is an established good predictor of the risk of fracture in the general population.6 In patients with CKD, recent cumulative data and meta-analysis of cross-sectional studies, have also demonstrated that low BMD efficiently predicts the risk of fracture.5,7, 8, 9 This led in 2015 to update the 2009 Kidney Disease: Improving Global Outcomes (KDIGO) recommendations in favor to the assessment of BMD in patients with CKD stage 3a–5 if the result would affect treatment decisions.10 BMD is also helpful in identifying patients at high risk of fracture.11 The Fracture Risk Assessment Tool (FRAX) has been validated in CKD populations, where it could either over- or underestimate the fracture risk in frailty patients with CKD. Several prospective studies have found that the relation between FRAX and major osteoporotic fracture is even stronger in patients with CKD compared with those with preserved renal function.3,12 However, the rate of bone loss is not known, making it difficult to determine the population to target and the frequency to monitor BMD measurements.
CKD is also characterized by alteration in several circulating biomarkers of bone metabolism including PTH, 25-hydroxyvitamin D (25OHD), calcitriol (1,25OH2D3), crosslaps, and total and bone-specific alkaline phosphatases (BS-ALP).13, 14, 15 Among them, an increase in serum PTH concentration, mainly secondary hyperparathyroidism, might occur early in the course of CKD15 and is considered as a reliable surrogate marker to predict bone turnover and histological changes.16 PTH concentration is also used to predict increased risk of fracture. In hemodialysis patients, high serum PTH levels are associated with an increased risk of fracture17,18 and histological signs of renal osteodystrophy.19 In early CKD stages, the slight increase in PTH appears to be an appropriate adaptive response to the progressive decline of renal function, this occurring in order to maintain normocalcemia and normophosphatemia as well as bone homeostasis. However, the risk of fracture is also increased with very low serum PTH levels.7,20 Therefore, the impact of serum PTH levels in determining bone loss remains unknown in patients with CKD.
When factors such as clinical, bone, and mineral biomarkers and BMD are analyzed, PTH is constantly found negatively correlated to BMD and bone biomarkers.21 For instance, the highest are the serum PTH levels, the lowest radius BMD are in cross-sectional studies, including dialysis patients, illustrating the importance of cortical bone loss.22, 23, 24 However, there is a lack of longitudinal study in patients with CKD stages 3–5, in whom BMD tends to decline with the renal impairment in parallel to the rise in serum PTH levels.25 Of note, the KDIGO recommendation of assessing BMD is based on data from cross-sectional studies, but not from prospective ones. The characterization of patients to target for the initial evaluation and the monitoring remains unprecise. In addition, evaluation of longitudinal bone loss and the value of baseline serum PTH levels as a predictive factor remain unresolved issues. For these reasons, we here aimed to characterize the patient profiles by analyzing the factors that affect BMD at 4 skeletal sites, including proximal radius, femoral neck, hip, and lumbar spine in patients with CKD stage 1–5 participating to the NephroTest cohort. We further assessed longitudinal changes in BMD related to clinical factors and serum PTH levels.
Materials and Methods
Patients and Study Design
The NephroTest cohort is a prospective hospital-based cohort, enrolling adult subjects older than 18 years with any diagnosis of CKD from stage 1 to 5.26 The cohort has been board-approved. Ambulatory patients were referred in the Department of Nephrology and Physiology for an evaluation of the kidney function and mineral metabolism. They were seen annually with an extensive set of standardized measures to assess CKD progression and complications. Inclusions began in January 2000. Patients were included after providing informed consent. Exclusion criteria were pregnancy, being on dialysis therapy, or with kidney transplants. The patient could stop the follow-up whenever wanted. A subset of 869 patients (Supplementary Figure 1), recruited between 2005 and 2013 underwent an evaluation of bone metabolism including serum biomarkers and BMD measurements as a part of the evaluation. This study was an open cohort; the investigators were not blinded for dual-energy X-ray absorptiometry (DXA) results. The BMD was measured in the same centers using the same device. We described data at baseline from 858 patients for whom BMD was available at least at 1 skeletal site and serum PTH levels. Among the 858 patients, 477 (55.6%) patients had 2 BMD assessments or more with a median duration of 13.2 months (12.0; 20.5) between 2 BMD measurements. For the linear mixed effects models, among the 858 patients, we analyzed 753 patients, including 1166 visits, who had had BMD measures at all skeletal sites (hip, which was presented as the total hip and femoral neck, lumbar spine, and proximal radius) with no BMD missing data at baseline. The mean duration of the follow-up was 4.3 ± 2.0 years.
Clinical and Biomarker Measurements
Data recorded at every visit included demographics (age, sex, and ethnicity), the diagnosis of kidney disease, medical history (cardiovascular diseases), height and weight, and bone-related medications. Determinations of blood and urinary parameters were performed as described as follows. We assessed the mGFR secondly standardized to body surface area, by 51Cr-EDTA renal clearance.27 Blood samples were collected to determine the plasma levels of creatinine, the serum levels of intact PTH, calcium, magnesium, phosphate, ionized calcium, 25OHD, albumin, uric acid, calcitriol, total alkaline phosphatase, BS-ALP, and serum crosslaps.
Plasma creatinine was measured by using a modified kinetic Jaffe colorimetric method on a Bayer RA-XT then a Konelab 20 analyzer (ThermoFisher Scientific, Waltham, MA) from the study start to 2008 and an enzymatic assay (KoneLab; Thermo Fisher Scientific) thereafter. Serum PTH concentrations were measured by second-generation 2-site radio-immunometric assay (Allegro-Intact PTH; Nichols Institute Diagnostics, San Clemente, CA), and by chemoluminescent assay (from January 2004 onward, Elecsys Roche [Indianapolis, IN] [normal values 10–65 pg/ml]). Total calcium and magnesium concentrations were measured by atomic absorption spectrophotometric method in plasma (Siemens, Munich, Germany). Normal values range from 2.12 to 2.52 for serum calcium and from 0.75 to 1.03 for magnesium. Phosphate concentration was measured by colorimetry in plasma (phosphomolybdate assay [normal values 0.81–1.58 mmol/l]) and ionized calcium by a specific electrode in serum (normal values 1.15–1.30 mmol/l). Plasma 25OHD concentration was measured by a radioimmunologic method (DiaSorin, Saluggia, Italy) that recognized both 25OHD2 and D3 with similar affinity. Plasma albumin was measured using colorimetry. Uric acid concentrations were measured by enzymatic methods (normal values 200–420 μmol/l). Serum 1,25OH2D was measured using a radioimmunologic method (Immunodiagnostic Systems, Tyne & Wear, UK, www.idsplc.com) with reference values 20 to 60 pg/ml. We measured total alkaline phosphatase concentration by an enzymatic method (Siemens, [normal values 50–136 UI/l]) and BS-ALP by a radioimmunometric assay (Tandem-R, Ostase, Hybritech Europe SA, Brussels, Belgium, reference range, 4.0–25.0 ng/ml). Serum crosslaps were measured using an immunoradiometric assay (Osteometer Biotech S/A, Herlev, Denmark). Reference values were less than 658 pg/ml (5.1 nmol/l) for premenopausal women and men and less than 580 pg/ml (4.5 nmol/l) for menopausal women. The BS-ALP and the crosslaps were measured in a subset of 597 patients: 96 with 1–2 CKD stages, 147 with 3a CKD stages, 177 with 3b stages and in 177 patients with 4–5 CKD stages. We also measured proteinuria in the urine by colorimetry (pyrogallol red with molybdate). Urinary albumin was measured using solid-phase fluorescent immunoassays and expressed as urinary albumin-creatinine ratio.
BMD Measurements
BMD was measured by DXA and performed per protocol once a year, using either a Hologic QDR4500A Scanner (Hologic, Bedford, MA) in one center or an iDXA device (GE Medical Systems Lunar, Madison, WI) in a second center, for the whole duration of the study operated with the same technician and with only 1 referent physician for analysis per center. BMD was measured at the femoral neck, at the hip, at the lumbar spine, and at the proximal radius. We assessed the areal BMD (g/cm2), Z-scores and T-scores using the National Health and Nutrition Examination Survey reference population. We also used the World Health Organization definitions of BMD categories: normal bone density (T-score ≥ −1), low bone mass (T-score between −1.0 and −2.5), and osteoporosis (T-score ≤ −2.5). Results are presented in accordance with the guidelines issued by the International Society for Clinical Densitometry. Stability of the measurements was checked every day, using a phantom for calibration. The mean coefficient of variation for measurement of the patients was as follows: lumbar spine, 0.41%; total hip, 0.53%; femoral neck 1.36%; and radius 1.22%. Cross-calibration equations to standardized BMD measures have been used in the description of the population,28, 29, 30, 31 except from the proximal radius, stratified by center. The mean least significant change for measurement of the patients was as follows: lumbar spine, 1.14%; total hip, 1.47%; femoral neck 3.77%; and radius 3.38%.
Statistical Analysis
Data were presented as mean (± SD) and median and interquartile range when needed. We performed Bartlett’s test to compare variances between each CKD stage. In case of homoscedasticity, an analysis of variance was conducted following by a Tukey’s test as a posteriori multiple comparison when applicable (respectively, if heteroscedasticity, a Kruskal-Wallis test has been performed, following by a Steel-Dwass-Critchlow-Fligner test when applicable).
Four linear mixed models were performed as the BMD was measured at all skeletal sites (femoral neck, hip total, lumbar spine, and proximal radius). The linear mixed model requires neither the same number of BMD measurements per patient, nor that these measurements have been taken at the same time points for all patients. This also allows accounting for patients with only 1 BMD measurement at baseline to reduce selection bias.32 Baseline PTH was considered as a continuous parameter. The covariables included in our models were those known as to be involved in BMD: age, BMI (coded as “<25 kg/m2” vs. “≥25 kg/m2”), gender (including the menopausal status for women), ethnicity (coded as “black origin” or not), tobacco use (coded as “never smoking,” “former smoker,” or “current smoker”). The other variables included as adjustment variables were mGFR expressed as CKD stage classes (1–2, 3a, 3b, and 4–5), PTH (pg/ml), treatments that interfere with bone (corticosteroid or bisphosphonates) and those that interfere with PTH metabolism (diuretic drugs, lithium, or calcitriol). All these factors were those collected at baseline, meaning at the first BMD examination. Time has been calculated as time span between the considered BMD examination and the first BMD examination. As fixed effects, we entered ethnicity, tobacco use, bone treatment, and PTH treatment. Considering the potential effect of PTH on BMD in different groups, we introduced this factor and its interaction terms with age and gender. Time has been introduced in the model with interaction terms with PTH, BMI, and mGFR (i.e., CKD stages). As random effects, we had intercepts for subjects as well as by-subject random slopes for the effect of time. Random effects allow accounting for correlation between measurements of a same patient and also for variability of each patient around mean trajectory. We also entered the factor center to have all the data collected and grouped from the 2 relevant centers. We supposed a linear evolution of BMD measurements over time since a quadratic form of time did not fit the data better. Visual inspection of residual plots did not reveal any obvious deviation from homoscedasticity or normality. The lmerTest package provides P values for fixed effects in type III analysis of variance and summary tables via Satterthwaite’s degrees of freedom method. As a sub-analysis, to visualize the BMD evolution along time according to baseline PTH classes, we divided the values of PTH in low (≤65 pg/ml), normal (65–129 pg/ml), or high PTH (>129 pg/ml). All tests were 2-sided and significance level fixed at 0.05. As statistical software, we used STATA/IC 16.0 (StataCorp., College Station, TX) and R version 3.5.1 (2018-07-02, The R Foundation for Statistical Computing, Vienna, Austria) to estimate linear mixed models33 (lme4 package 1.1–21).
To compare our results with previous studies, we also modeled BMD change over time (difference between first and last measurement divided by follow-up time) using a linear model. This analysis was thus restricted to patients with at least 2 BMD measurements.
Results
Patient Characteristics at Baseline
The 858 patients were issued from the NephroTest cohort that has been described previously (Supplementary Figure 1). The mean age was 58.9 ± 15.2 years, 67% were men, 70.8% postmenopausal women, and the mGFR was 43.0 ± 19.5 ml/min (Table 1). Patients from sub-Saharan Africa or the French West Indies (15.8%) were also included. The mean BMI was 26.8 ± 5.3 kg/m2.
Table 1.
Baseline demographic and biochemical characteristics according CKD stages
| Baseline characteristics CKD stages mGFR |
Total n = 858 | 1 - 2 |
3a |
3b |
4 - 5 |
P value |
|---|---|---|---|---|---|---|
| ≥ 60 ml/min |
45-60 ml/min |
30-45 mL/min |
< 30 mL/min |
|||
| n = 156 | n = 203 | n = 262 | n = 237 | |||
| Age (yr) | 58.9±15.2 | 50.8±15.7 | 59.8±13.2 | 61.1±14.8 | 61.1±15.1 | 0.061 |
| Weight (kg) | 75.5±16.5 | 74.6±17.1 | 74.5±15.1 | 75.4±16.8 | 77.1±18.1 | 0.339 |
| Height (cm) | 167.6±9.2 | 168.7±8.4 | 168.2±8.8 | 166.9±9.7 | 167.0±9.6 | 0.134 |
| Intact PTH (pg/ml)a | 57.0(38.0;90.0) | 37.0(26.0;48.2) | 48.0(34.5;66.5) | 62.0(43.0;89.8) | 101.0(68.0;164.0) | < 0.001 |
| ionized calcium (mmol/l)a | 1.23(1.20;1.27) | 1.23(1.20;1.26) | 1.23(1.21;1.27) | 1.23(1.21;1.27) | 1.24(1.20;1.27) | 0.87 |
| serum calcium (mmol/l)a | 2.27(2.20;2.34) | 2.27(2.21;2.34) | 2.28(2.22;2.35) | 2.27(2.21;2.34) | 2.25(2.17;2.32) | < 0.01 |
| corrected calcium (mmol/l)a | 2.29(2.23;2.37) | 2.27(2.21;2.33) | 2.28(2.24;2.37) | 2.31(2.24;2.37) | 2.29(2.21;2.37) | < 0.05 |
| serum magnesium (mmol/l)a | 0.79(0.73;0.85) | 0.77(0.73;0.82) | 0.78(0.73;0.84) | 0.79(0.74;0.84) | 0.81(0.74;0.88) | < 0.05 |
| serum phosphate (mmol/l) | 1.05(0.93;1.17) | 1.02(0.90;1.13) | 0.99(0.89;1.09) | 1.03(0.93;1.14) | 1.15(1.03;1.34) | < 0.001a |
| 25(OH)-vitamin D (ng/ml) | 21.9±12.2 | 20.9±11.5 | 22.8±11.2 | 22.7±13.1 | 21.0±12.2 | 0.066b |
| Calcitriol (pg/ml)a | 20.0(12.5;28.8) | 43.2(31.0;55.2) | 36.2(27.5;45.7) | 31.0(22.9;40.4) | 21.7(15.0;30.0) | < 0.001 |
| BS-ALP (ng/ml)a | 13.6(10.1;18.4) | 13.4(10.2;17.8) | 13.7(10.2;17.5) | 13.7(10.1;19.4) | 13.3(10.0;19.2) | 0.824 |
| uric acid (μmol/l) | 405.7±99.8 | 354.8±83.3 | 399.8±91.8 | 423.4±97.5 | 424.4±106.9 | < 0.001b |
| serum crosslaps (nmol/l)a | 516.1(258.1;774.2) | 258.1(258.1;387.1) | 387.1(258.1;645.2) | 516.1(258.1;645.2) | 774.2(387.1;1064.5) | < 0.001 |
CKD, chronic kidney disease; mGFR, measured glomerular filtration rate; PTH, parathyroid hormone; BS-ALP, bone specific alkaline phosphatases.
Results are expressed as mean±standard deviation. An ANOVA was conducted following by a Tukey’s test as a posteriori multiple comparisons when p-value <0.05
Median (25th through 75th percentile) compared by a Kruskall-Wallis' test following by a Steel-Dwass-Critchlow-Fligner’s test. as a posteriori multiple comparisons when P value <0.05.
Analysis of variance test with log transformation of the variable.
Among them, 156 (18.2%) had an mGFR more than 60 ml/min per 1.73 m2 (1.7% stage 1 and 16.4% stage 2). In 657 subjects (76.6%), mGFR was between 15 and 60 ml/min per 1.73 m2, including 23.7% stage 3a, 30.5% 3b, and 22.4% stage 4, respectively. Finally, 45 patients had an mGFR less than 15 ml/min per 1.73 m2 (5.2% stage 5 CKD). No patients on dialysis therapy or transplanted were included.
The most common comorbidity was hypertension (91%), followed by diabetes mellitus (25.2%) and cardiovascular diseases (16.1%). Most represented nephropathies were vascular nephropathy (18.2%), primary glomerulonephritis (12%), tubulointerstitial nephropathy (9%), diabetic nephropathy (8.5%), polycystic kidney disease (5.9%), or otherwise not determined (53.6%). The history of drugs interfering with bone metabolism found a large use of diuretics (46.2%, including 24.5% loop diuretics and 22.1% thiazide diuretics), 28.2% were receiving phosphate-calcium regulatory medications, 14.7% were under vitamin D supplementation, 9.9% were under calcitriol therapy, but only 7.2% currently having corticosteroids and 2.1% bisphosphonates.
Cross-sectional Analysis of Biochemical and BMD Indices
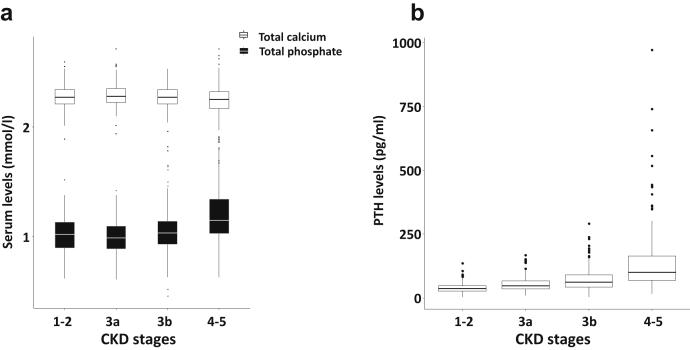
Baseline biochemical characteristics are shown in Table 1. We found a mild but significant decrease in concentrations of serum total calcium (P < 0.01, Figure 1a) and a significant increase of phosphate (P < 0.001, Figure 1a) along with a significant increase of PTH throughout CKD stages (P < 0.001, Figure 1b). Serum PTH concentrations were above the normal range (65 pg/ml) in 370 patients (43.1%) of which 4 were associated with a hypercalcemia suggesting a primary hyperparathyroidism. A borderline significant difference in circulating 25OHD concentrations was found according to CKD severity. Mean concentrations at each CKD stage revealed vitamin D insufficiency. Concentrations of 25OHD were ≤10 ng/ml (25 nmol/l) in 17.1% patients, between 10 and 20 ng/ml (25–50 nmol/l) in 33.3% patients, between 20 and 30 ng/ml (50–75 nmol/l) in 27.4% patients and above 30 ng/ml (75 nmol/l) in 22.2% patients. Calcitriol levels decreased significantly with kidney failure (P < 0.001). Serum crosslaps levels are significantly higher in CKD stages 4–5 (P < 0.001), whereas BS-ALP remained normal at any stage. Associations of mGFR with other markers of mineral and bone metabolism in the whole cohort have been previously detailed.15
Figure 1.
Cross-sectional analysis of biochemical parameters according to chronic kidney disease (CKD) stages. (a) Baseline serum total calcium and total phosphate concentrations. (b) Baseline intact parathyroid hormone (PTH) concentrations.
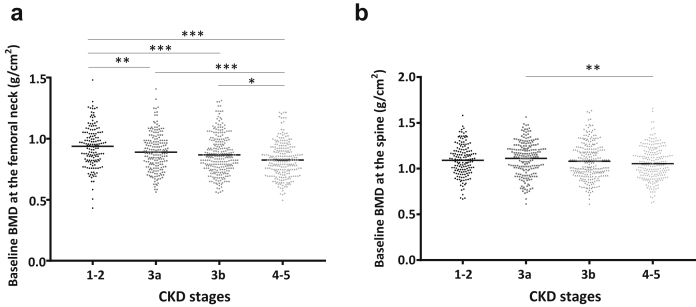
Table 2 shows the prevalence of osteopenia and osteoporosis at all sites and for any CKD stages. Indeed, 46% to 66% had a T-score above −1 SD, whereas T-score was below −2.5 SD in 10% to 14% throughout the CKD stages. Moreover, BMD was significantly lower with advanced CKD stages at the femoral neck (P < 0.0001), total hip (P < 0.001), and at lumbar spine (P < 0.05 Figure 2). At the proximal radius, BMD was significantly lower in late CKD stages (P < 0.05) with the Hologic DXA device, but not with the GE-Lunar device.
Table 2.
Baseline BMD measurements according CKD stages
| Skeletal sites | CKD stages mGFR | Total n = 858 | 1 - 2 |
3a |
3b |
4 - 5 |
P value |
|---|---|---|---|---|---|---|---|
| ≥ 60 ml/min |
45-60 ml/min |
30-45 ml/min |
< 30 ml/min |
||||
| n = 156 | n = 203 | n = 262 | n = 237 | ||||
| Femoral neck | BMD (g/cm2) | 0.87±0.16 | 0.94 ± 0.17 | 0.89 ± 0.15 | 0.87 ± 0.16 | 0.83 ± 0.14 | <0.0001 |
| Z-score | 0.20±1.08 | 0.40±1.16 | 0.30±1.08 | 0.26±1.04 | -0.11±1.00 | <0.0001 | |
| T-scorea | -0.9(-1.7;-0.01) | -0.28(-1.10;0.70) | -0.80(-1.60;0.10) | -1.00(-1.70;-0.06) | -1.28(-1.91;-0.53) | <0.0001 | |
| Total hip | BMD (g/cm2) | 0.97±.017 | 1.02 ± 0.17 | 0.98 ± 0.17 | 0.96 ± 0.17 | 0.93 ± 0.16 | <0.0001 |
| Z-score | 0.39±1.11 | 0.50±1.12 | 0.46±1.08 | 0.46±1.13 | 0.18±1.10 | <0.05 | |
| T-score | -0.25±1.22 | 0.20±1.29 | -0.17±1.20 | -0.29±1.21 | -0.55±1.12 | <0.0001 | |
| Lumbar spine | BMD (g/cm2) | 1.08±0.18 | 1.09 ± 0.17 | 1.11 ± 0.19 | 1.08 ± 0.19 | 1.05 ± 0.18 | <0.05 |
| Z-score | 0.11±1.54 | -0.12±1.42 | 0.36±1.47 | 0.20±1.58 | -0.06±1.59 | <0.01 | |
| T-score | -0.48±1.58 | -0.38±1.50 | -0.25±1.59 | -0.49±1.66 | -0.73±1.48 | <0.05 | |
| Proximal radius | |||||||
| Hologic | BMD (g/cm2) | 0.50 ± 0.12 | 0.51 ± 0.11 | 0.51 ± 0.10 | 0.50 ± 0.13 | 0.50 ± 0.12 | 0.764b |
| Z-score | 0.59 ± 1.42 | 0.48 ± 1.36 | 0.66 ± 1.37 | 0.72 ± 1.46 | 0.44 ± 1.45 | 0.318 | |
| T-score | -0.60 ± 1.52 | -0.46 ± 1.51 | -0.51 ± 1.48 | -0.54 ± 1.60 | -0.77 ± 1.47 | 0.357 | |
| GE-Lunar | BMD (g/cm2) | 0.89 ± 0.12 | 0.91 ± 0.11 | 0.91 ± 0.12 | 0.88 ± 0.12 | 0.85 ± 0.15 | <0.05 |
| Z-score | -0.31 ± 1.08 | -0.49 ± 0.98 | -0.12 ± 0.99 | -0.26 ± 1.11 | -0.35 ± 1.23 | 0.219 | |
| T-score | -0.76 ± 1.13 | -0.57 ± 1.07 | -0.61 ± 1.01 | -0.87 ± 1.15 | -1.03 ± 1.25 | <0.05 | |
| Bone statusc | normal (n, %) | 473 (56%) | 104 (66%) | 122 (61%) | 139 (53%) | 108 (46%) | <0.01d |
| osteopenia (n, %) | 283 (33%) | 37 (24%) | 59 (29%) | 94 (36%) | 93 (40%) | ||
| osteoporosis (n, %) | 96 (11%) | 15 (10%) | 20 (10%) | 28 (11%) | 33 (14%) |
CKD, chronic kidney disease; BMD, bone mineral density; mGFR, measured glomerular filtration rate.
Results are expressed as mean ± SD. An analysis of variance was conducted following by a Tukey’s test as a posteriori multiple comparisons when P value <0.05
Median (25ththrough 75thpercentile) compared by a Kruskall-Wallis test following by a Steel-Dwass-Critchlow-Fligner test as a posteriori multiple comparisons when P value <0.05.
Aanlysis of variance test with log transformation of the variable.
According to World Health Organization definition including T-score at L1-L4 and Total Hip.
Chi-square test.
Figure 2.
Baseline standardized bone mineral density (BMD) throughout chronic kidney disease (CKD) stages. BMD was measured using dual-energy X-ray absorptiometry in 858 patients. Results are presented as baseline BMD at the femoral neck (a) and the spine (b) according to the CKD stages. For each skeletal site, an analysis of variance was conducted following by a Tukey’s test as a posteriori multiple comparisons. ∗P < 0.05, ∗∗ P < 0.01, ∗∗∗P < 0.001.
Association Between Clinical Factors and Serum PTH Levels for BMD at Baseline
The final linear mixed effect models included age, gender, ethnicity, and tobacco use without interaction terms with duration of follow-up and BMI, PTH, and CKD stages with interaction terms with time of follow-up. Head of Table 3 shows the effects of variables at baseline for BMD measured at the femoral neck. After adjustment, there is a strong negative association with age; the higher is the age the lower is the BMD. A significant negative association was also found with gender; compared with men, premenopausal women had a mean BMD lower (by 0.0481 g/cm2; 95% CI −0.0833 to −0.0130) and a further reduction in postmenopausal women (by 0.0849 g/cm2; 95% CI −0.1082 to −0.0617). BMD was negatively impacted in advanced CKD stages (3b to 4–5). In contrast, high BMI and African ethnicity were positively associated with BMD. The adjusted baseline PTH had a negative relationship with BMD at the femoral neck (estimated effect = −0.023, P < 0.01). At the radius site, BMD was negatively associated with age, gender, and tobacco use, whereas ethnicity, CKD stages, and PTH levels had no significant impact (Table 4). Considering baseline BMD at the total hip and at the lumbar spine, we found a positive association with age and high BMI (Supplementary Tables S1 and S2). Spinal BMD was negatively associated with female gender and baseline PTH levels. Altogether, age, gender, BMI, and PTH are significant influencing factors for BMD.
Table 3.
Estimated adjusted effects of variables on mean femoral neck BMD at baseline and BMD change over 4.3 years follow-up (n = 753 patients, 1166 visits)
| Variable | Estimated effect | 95% CI | P value |
|---|---|---|---|
| Mean difference of BMD at baseline | |||
| Age at baseline (y) | -0.0037 | -0.0044to-0.0029 | < 0.0001 |
| Gender | |||
| Men | Ref. | < 0.0001 | |
| Premenopausal women at baseline | -0.0481 | -0.0833to-0.0130 | |
| Postmenopausal women | -0.0849 | -0.1082to-0.0617 | |
| BMI at baseline (kg/m2) | |||
| <25 | Ref. | < 0.0001 | |
| ≥25 | 0.0894 | 0.0703to0.1086 | |
| Ethnicity | |||
| No African origin | Ref. | < 0.0001 | |
| African origin | 0.0746 | 0.0486to0.1008 | |
| Tobacco use at baseline | |||
| Never smoking | Ref. | 0.060 | |
| Former smoker | -0.0036 | -0.0254 to 0.0179 | |
| Current smoker | -0.0341 | -0.0626to-0.0059 | |
| CKD stages at baseline | |||
| 1-2 | Ref. | < 0.01 | |
| 3a | -0.0171 | -0.0458 to 0.0113 | |
| 3b | -0.0313 | -0.0598to-0.0030 | |
| 4-5 | -0.0575 | -0.0894to-0.0257 | |
| log(PTH) at baseline | -0.0230 | -0.0391to-0.0069 | < 0.01 |
| Mean BMD change | |||
| Time (yrs) | -0.0023 | -0.0127 to 0.0082 | 0.677 |
| BMI at baseline (kg/m2) | |||
| <25 | Ref. | <0.05 | |
| ≥25 | 0.0031 | 0.0002to0.0060 | |
| CKD stages at baseline | |||
| 1-2 | Ref. | 0.099 | |
| 3a | -0.0014 | -0.0066 to 0.0037 | |
| 3b | -0.0045 | -0.0096 to 0.0005 | |
| 4-5 | -0.0004 | -0.0060 to 0.0052 | |
| log(PTH) at baseline | -0.0005 | -0.0032 to 0.0022 | 0.704 |
BMD, bone mineral density; BMI, body mass index; CI, confidence interval; CKD, chronic kidney disease; PTH: parathyroid hormone.
The factor center has been included as random effect
Table 4.
Estimated adjusted effects of variables on mean radius BMD at baseline and BMD change over 4.3 years follow-up (n = 753 patients, 1166 visits)
| Variable | Estimated effect | 95% CI | P value |
|---|---|---|---|
| Mean difference of BMD at baseline | |||
| Age at baseline (y) | -0.0017 | -0.0023to-0.0012 | < 0.0001 |
| Gender | |||
| Men | Ref. | < 0.0001 | |
| Premenopausal women at baseline | -0.0941 | -0.1216to-0.0666 | |
| Postmenopausal women | -0.1277 | -0.1459to-0.1095 | |
| BMI at baseline (kg/m2) | |||
| <25 | Ref. | < 0.001 | |
| ≥25 | 0.0270 | 0.0119to0.0422 | |
| Ethnicity | |||
| No African origin | Ref. | 0.204 | |
| African origin | 0.0134 | -0.0071 to 0.0339 | |
| Tobacco use at baseline | |||
| Never smoking | Ref. | 0.088 | |
| Former smoker | -0.0184 | -0.0353to-0.0014 | |
| Current smoker | -0.0002 | -0.0224 to 0.0220 | |
| CKD stages at baseline | |||
| 1-2 | Ref. | 0.467 | |
| 3a | 0.0171 | -0.0056 to 0.0397 | |
| 3b | 0.0053 | -0.0173 to 0.0279 | |
| 4-5 | 0.0059 | -0.0194 to 0.0311 | |
| log(PTH) at baseline | -0.0107 | -0.0234 to 0.0021 | 0.105 |
| Mean BMD change | |||
| Time (y) | -0.0158 | -0.0292to-0.0023 | <0.05 |
| BMI at baseline (kg/m2) | |||
| <25 | Ref. | 0.545 | |
| ≥25 | -0.0011 | -0.0048 to 0.0025 | |
| CKD stages at baseline | |||
| 1-2 | Ref. | 0.951 | |
| 3a | 0.0012 | -0.0054 to 0.0078 | |
| 3b | 0.0007 | -0.0058 to 0.0073 | |
| 4-5 | -0.0002 | -0.0075 to 0.0070 | |
| log(PTH) at baseline | 0.0030 | -0.0004 to 0.0065 | 0.089 |
BMD, bone mineral density; BMI, body mass index; CI, confidence interval; CKD, chronic kidney disease; PTH, parathyroid hormone.
The factor center has been included as random effect.
Analysis of Longitudinal Changes in BMD
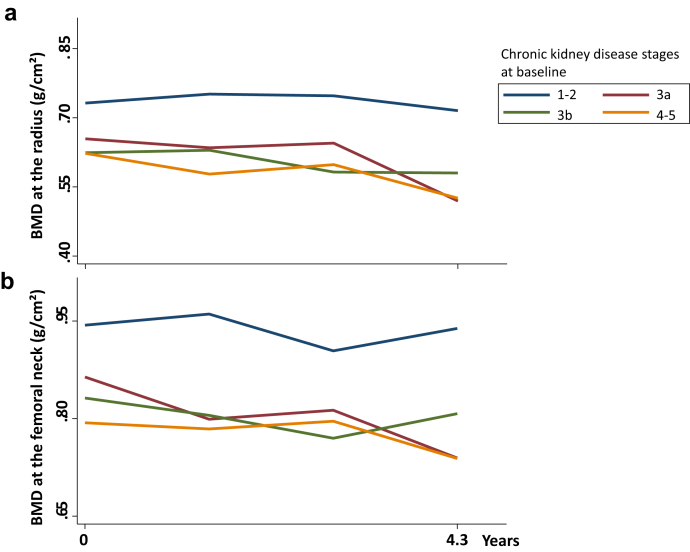
During the mean follow-up of 4.3 years, there was a slight, but significant reduction in mGFR throughout time: 43 ± 19, 38 ± 17, 38 ± 16, 41 ± 18 ml/min per 1.73 m2 from baseline to 1.5, 3.0, and 4.5 years, respectively (P < 0.0001). On average, mGFR decreased by 1.2 mL/min per year. Longitudinal data revealed a significant BMD loss only at the radius throughout time (Table 4). Over follow-up, no significant change in BMD occurred at the femoral neck, total hip, and spine (Table 3, Supplementary Tables S1 and S2), and no factor was associated with the evolution of BMD at the radius, total hip, and spine (Table 4, Figure 3a, Supplementary Tables S1 and S2). Evolution of BMD at the femoral neck is positively impacted in subjects with higher BMI, whereas it is not influenced by CKD stages (Figure 3b).
Figure 3.
Mean changes of bone mineral density (BMD) throughout time according to baseline chronic kidney disease stages at (a) the radius and (b) the femoral neck.
When baseline serum PTH concentrations were considered in the lowest (0–65), normal (1–2 fold: 65–129) or highest (>129 pg/ml) ranges, BMD changes were similar regardless of PTH classes at the radius and femoral neck (Supplementary Table S3, Supplementary Figure S1).
We also performed a linear model as a sensitivity analysis. We included the factors used in the mixed model that could affect the annualized BMD changes (Supplementary Table S4). This showed that no factor at baseline is involved in BMD changes.
Discussion
The results of this prospective study from the NephroTest cohort, a well-characterized population of patients with CKD with mGFR, showed a lower BMD with the progression of CKD. At any skeletal sites, BMD was negatively associated with clinical factors, including age, female gender regardless of menopause, current tobacco use, and with baseline serum PTH levels. In contrast, higher BMI, African ethnicity, and higher mGFR (expressed as CKD stages) are associated with higher BMD. The study provides several new findings. First, T-score and Z-score BMD were above −1 SD at any skeletal sites and at any stage of CKD. This might be related in part to the population that was mostly composed of men at their mid-age. The prevalence of osteoporosis was between 10% and 14% and increased slightly according to the CKD stages. Second, longitudinal BMD loss could be detected only at the proximal radius after 4.3 years of follow-up and independently of baseline CKD stages and PTH classes.
Here, the cross-sectional analysis showed a lower BMD at the lumbar spine or the total hip in relation to the severity of CKD, as reported in non-CKD individuals of the MrOS study.34,35 These findings might reinforce the KDIGO recommendations of assessing BMD in the CKD population. The consistent findings of normal Z-score at all skeletal sites makes unlikely the contribution of the calcifications of abdominal aorta in lumbar BMD measures, given that the other sites were also normal. Our data also confirmed the progressive increase in serum PTH concentrations along with the severity of CKD; this occurring in order to maintain serum calcium and phosphate within normal ranges. Indeed, BMD at lumbar spine, total hip, and femoral neck was negatively correlated with PTH (Supplementary Table S2), therefore high serum PTH concentrations might mediate low BMD at several skeletal sites despite a differential PTH effect on cortical and trabecular bone as previously reported.22
We also observed a high proportion of patients with CKD with vitamin D insufficiency, whereas KDIGO guidelines recommend maintaining circulating 25OHD levels above 30 ng/ml.10,11 The low serum 25OHD concentrations in most severe patients with CKD could favor and amplify the risk of secondary hyperparathyroidism, resulting in an increased bone resorption and cortical bone loss.36,37 Serum crosslaps, a bone resorption marker, were increased in late stages; this might be related to impaired renal function. In contrast, serum BS-ALP levels, a bone formation biomarker independent of renal impairment, were stable, as reported previously in patients with end-stage CKD to be higher before the initiation of hemodialysis therapy.38, 39, 40 These apparently controversial results might illustrate the differential clearance of these biomarkers; BS-ALP clearance being unaffected by renal impairment.
Here, we did not aim either to identify specifically osteoporosis in CKD-MBD especially in advanced CKD, or identify the type of CKD-MBD, but the longitudinal changes in BMD. A major finding of this study was that the longitudinal bone loss observed in patients with CKD was restricted to the proximal radius, whereas BMD remained stable at the femoral neck, total hip, and lumbar spine. These data invite better definition of the skeletal site and the monitoring schedule of serial BMD measurements in patients with CKD. Here, the lack of longitudinal bone loss at other skeletal sites than the proximal radius after 4.3 years might be due to the male gender, most participants being men in agreement with the initial recruitment design (hypertension and CKD workup). In addition, we cannot exclude that the BMD decline is not linear, as suspected in the Rancho Bernardo study,41 the long-term evaluation would require longer monitoring. Because subtle trabecular and cortical bone changes may occur but cannot be caught by DXA BMD measures, it will be interesting to investigate the changes of BMD and microarchitecture with high-resolution peripheral computed tomography, which may broaden the understanding and differential role of PTH on trabecular and cortical bone.
Baseline high serum PTH levels are negatively associated with low BMD at all skeletal sites in cross-sectional analysis, this confirming several previous data. However, our results show that neither baseline nor changes in serum PTH levels are associated with BMD changes at any skeletal site in patients with CKD despite the reduction of mGFR. Stratification according to 3 PTH classes showed that each PTH group experienced identical bone change over time at any site. One might hypothesize that the deleterious effect of PTH on the skeleton might require a time longer than 4.3 years to be captured or occurs at an early stage of CKD. Indeed, analyzing a cohort of patients with CKD stages 2–3b with a mean age of 70 to 79 years for 11 years, serum PTH was linked to BMD, but no longitudinal bone loss was observed despite an older age.9 A retrospective study based on 374 patients followed for 5 years and selected on BMD availability also failed to show the impact of PTH on BMD.42 Data from the CKD-DOPPS cohort show that although monitoring of CKD-MBD laboratory markers is consistent with the guidelines in many countries, the target levels for many parameters notably vary, and treatment of mineral disorders is generally poor in patients with CKD.43 Indeed, the revised KDIGO recommendations might be weighted when suggesting that treatment should be based on sequential measurements of PTH, but not only on a single elevated serum PTH level.
Here, we could not assess whether baseline serum PTH levels could predict the risk of skeletal fractures because of the lack of data collection. In hemodialysis patients, high PTH is associated with increased risk of fracture,17,18 but this risk is also observed with very low PTH levels.7,20 A large prospective database including 5100 subjects with CKD stages 3–4 showed that PTH was an independent factor predictive of fractures, low PTH having a protective effect.44 Moreover, the reduction of PTH by calcimimetics as in the EVOLVE (EValuation Of Cinacalcet HCl Therapy to Lower CardioVascular Events) trial and using an unadjusted intention-to-treat analysis, showed that cinacalcet did not reduce the rate of clinical fracture. However, when accounting for differences in baseline characteristics of multiple fractures, and/or events prompting discontinuation of study drug, cinacalcet reduced the rate of clinical fracture by 16% to 29%.45 Therefore, these controversial results leave the issue of PTH levels and fracture risk unresolved.
The main strengths of this study are the standardization of the mGFR, the large sample of patients, and the monitoring of the follow-up in the cohort. The study has also some limitations, including that the patients were not naïve of treatment at the inclusion of the study. This might have introduced a bias, although 2% of the patients were receiving bisphosphonates at the inclusion. We have no information if this was changed during the follow-up, and subsequently might have affected DXA results. Also, the lack of exploitable fracture data collected at each visit should be mentioned. Fibroblast growth factor 23 and klotho were not systematically assessed at the beginning of the cohort, the lack of data to achieve a workable outcome. The vitamin D insufficiency in late stages cannot be linked to self-report vitamin D uptake, to explore if there was a lack of vitamin D prescription in these patients. Understanding CKD-MBD and its evolution in nondialysis patients remains partial and noninvasive tools provide limited information in bone metabolism and structure. The need for stronger and more specific biomarkers will help in the global understanding of CKD-MBD.
In conclusion, clinical factors and baseline high serum PTH levels are associated with a low BMD in CKD stages 1–5, then allowing identifying patients with CKD who require BMD measures. Longitudinal analysis shows a bone loss only at the proximal radius, and no predictive value of baseline factors on BMD loss through a period of 4.3 years at any skeletal site, suggesting that monitoring BMD every 4 years could be sufficient. BMD remains stable for 3 classes of PTH, rendering it difficult to establish an optimal PTH value associated with normal BMD in patients with CKD.
Key Findings
In patients with CKD stages 1–5, age, gender, ethnicity, BMI, and serum PTH levels are associated with BMD at all skeletal sites. Longitudinal analysis showed a BMD loss only at the radius after a mean 4.3 years of follow-up. In middle-aged patients with CKD, we recommend BMD measures specifically using clinical profile and serial BMD measurements, including the radius, every 4 years.
Disclosure
PH has received honoraria and/or research funds from Takeda/Shire. PU-T has received honoraria, research funds, and consulting fees from Abbott, Amgen, Novartis/ Genzyme, Reata, Shire, and Fresenius. All the other authors declared no competing interest.
Acknowledgments
Collaborators in the NephroTest Study Group were Emmanuel Letavernier, Pierre Ronco, Hafedh Fessi, Eric Daugas, Caroline du Halgouet, Renaud de La Faille, Christian d’Auzac, Gerard Maruani, Marion Vallet, Cédric Gauci, Jean Philippe Haymann, Eric Thervet, Jean-Jacques Boffa, François Vrtovsnik, Marc Froissart, Bénédicte Stengel, Laurence Nicolet-Barousse, Mélanie Roland, and Christian Jacquot. The data collection work was supported by the NephroTest CKD cohort study grants from French Ministry of Health AOM 09114 and AOM 10245 (M.Fr.); AURA, 2009-152-447G.
Author Contributions
PUT and MCS initiated and coordinated the research. PEC, AO, MM, and PUT managed and analyzed data. MCS, PEC, OA, PUT, PH, and JB participated in the data interpretation. PH, MF, and BS constituted the NephroTest cohort. PEC, MCS, and PUT wrote the article. PEC and AO contributed equally to the work. All authors contributed substantially to the study and approved the final version of the article.
Footnotes
Figure S1. Flow diagram of the study.
Table S1. Estimated adjusted effects of variables on mean total hip BMD at baseline and BMD change over 4.3 years follow-up (n = 753 patients, 1166 visits).
Table S2. Estimated adjusted effects of variables on mean spine BMD at baseline and BMD change over 4.3 years of follow-up (n = 753 patients, 1166 visits).
Table S3. Estimated adjusted effects of variables on mean femoral neck BMD at baseline including PTH classes and BMD change over 4.3 years follow-up (n = 753 patients, 1166 visits).
Table S4. Annualized BMD changes at the skeletal sites.
Contributor Information
Martine Cohen-Solal, Email: martine.cohen-solal@inserm.fr.
NephroTest Study group:
Emmanuel Letavernier, Pierre Ronco, Hafedh Fessi, Eric Daugas, Caroline du Halgouet, Renaud de La Faille, Christian d’Auzac, Gerard Maruani, Marion Vallet, Cédric Gauci, Jean Philippe Haymann, Eric Thervet, Jean-Jacques Boffa, François Vrtovsnik, Marc Froissart, Bénédicte Stengel, Laurence Nicolet-Barousse, Mélanie Roland, and Christian Jacquot
Supplementary Material
Figure S1. Flow diagram of the study
Table S1. Estimated adjusted effects of variables on mean total hip BMD at baseline and BMD change over 4.3 years follow-up (n = 753 patients, 1166 visits).
Table S2. Estimated adjusted effects of variables on mean spine BMD at baseline and BMD change over 4.3 years of follow-up (n = 753 patients, 1166 visits).
Table S3. Estimated adjusted effects of variables on mean femoral neck BMD at baseline including PTH classes and BMD change over 4.3 years follow-up (n = 753 patients, 1166 visits).
Table S4. Annualized BMD changes at the skeletal sites.
References
- 1.Alem A.M., Sherrard D.J., Gillen D.L. Increased risk of hip fracture among patients with end-stage renal disease. Kidney Int. 2000;58:396–399. doi: 10.1046/j.1523-1755.2000.00178.x. [DOI] [PubMed] [Google Scholar]
- 2.Nickolas T.L., McMahon D.J., Shane E. Relationship between moderate to severe kidney disease and hip fracture in the United States. J Am Soc Nephrol. 2006;17:3223–3232. doi: 10.1681/ASN.2005111194. [DOI] [PubMed] [Google Scholar]
- 3.Naylor K.L., Garg A.X., Zou G. Comparison of fracture risk prediction among individuals with reduced and normal kidney function. Clin J Am Soc Nephrol. 2015;10:646–653. doi: 10.2215/CJN.06040614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Maravic M., Ostertag A., Torres P.U. Incidence and risk factors for hip fractures in dialysis patients. Osteoporos Int. 2014;25:159–165. doi: 10.1007/s00198-013-2435-1. [DOI] [PubMed] [Google Scholar]
- 5.Naylor K.L., Leslie W.D., Hodsman A.B. FRAX predicts fracture risk in kidney transplant recipients. Transplantation. 2014;97:940–945. doi: 10.1097/01.TP.0000438200.84154.1a. [DOI] [PubMed] [Google Scholar]
- 6.Compston J.E., McClung M.R., Leslie W.D. Osteoporosis. Lancet. 2019;393:364–376. doi: 10.1016/S0140-6736(18)32112-3. [DOI] [PubMed] [Google Scholar]
- 7.Iimori S., Mori Y., Akita W. Diagnostic usefulness of bone mineral density and biochemical markers of bone turnover in predicting fracture in CKD stage 5D patients--a single-center cohort study. Nephrol Dial Transplant. 2012;27:345–351. doi: 10.1093/ndt/gfr317. [DOI] [PubMed] [Google Scholar]
- 8.West S.L., Lok C.E., Langsetmo L. Bone mineral density predicts fractures in chronic kidney disease. J Bone Miner Res. 2015;30:913–919. doi: 10.1002/jbmr.2406. [DOI] [PubMed] [Google Scholar]
- 9.Yenchek R.H., Ix J.H., Shlipak M.G. Bone mineral density and fracture risk in older individuals with CKD. Clin J Am Soc Nephrol. 2012;7:1130–1136. doi: 10.2215/CJN.12871211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kidney Disease: Improving Global Outcomes (KDIGO) CKD-MBD Work Group KDIGO clinical practice guideline for the diagnosis, evaluation, prevention, and treatment of Chronic Kidney Disease-Mineral and Bone Disorder (CKD-MBD) Kidney Int Suppl. 2009;113:S1–S130. doi: 10.1038/ki.2009.188. [DOI] [PubMed] [Google Scholar]
- 11.Ketteler M., Block G.A., Evenepoel P. Diagnosis, Evaluation, prevention, and treatment of chronic kidney disease-mineral and bone disorder: synopsis of the Kidney Disease: Improving Global Outcomes. 2017 Clinical Practice Guideline Update. Ann Intern Med. 2018;168:422–430. doi: 10.7326/M17-2640. [DOI] [PubMed] [Google Scholar]
- 12.Whitlock R.H., Leslie W.D., Shaw J. The Fracture Risk Assessment Tool (FRAX(R)) predicts fracture risk in patients with chronic kidney disease. Kidney Int. 2019;95:447–454. doi: 10.1016/j.kint.2018.09.022. [DOI] [PubMed] [Google Scholar]
- 13.Pelletier S., Roth H., Bouchet J.L. [Changes in mineral and bone disorder management in a French cohort of hemodialysis patients between. 2008 and. 2012: The National Bone and Mineral Metabolism observatory (Photo-Graphe 2 and 3)] Nephrol Ther. 2016;12:171–177. doi: 10.1016/j.nephro.2015.11.005. [DOI] [PubMed] [Google Scholar]
- 14.Tentori F., Zepel L., Fuller D.S. The DOPPS practice monitor for US Dialysis Care: PTH levels and management of mineral and bone disorder in US hemodialysis patients. Am J Kidney Dis. 2015;66:536–539. doi: 10.1053/j.ajkd.2015.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Urena-Torres P., Metzger M., Haymann J.P. Association of kidney function, vitamin D deficiency, and circulating markers of mineral and bone disorders in CKD. Am J Kidney Dis. 2011;58:544–553. doi: 10.1053/j.ajkd.2011.04.029. [DOI] [PubMed] [Google Scholar]
- 16.Lehmann G., Ott U., Kaemmerer D. Bone histomorphometry and biochemical markers of bone turnover in patients with chronic kidney disease Stages 3–5. Clin Nephrol. 2008;70:296–305. doi: 10.5414/cnp70296. [DOI] [PubMed] [Google Scholar]
- 17.Fishbane S., Hazzan A.D., Jhaveri K.D. Bone parameters and risk of hip and femur fractures in patients on hemodialysis. Clin J Am Soc Nephrol. 2016;11:1063–1072. doi: 10.2215/CJN.09280915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jadoul M., Albert J.M., Akiba T. Incidence and risk factors for hip or other bone fractures among hemodialysis patients in the Dialysis Outcomes and Practice Patterns Study. Kidney Int. 2006;70:1358–1366. doi: 10.1038/sj.ki.5001754. [DOI] [PubMed] [Google Scholar]
- 19.Malluche H.H., Davenport D.L., Cantor T. Bone mineral density and serum biochemical predictors of bone loss in patients with CKD on dialysis. Clin J Am Soc Nephrol. 2014;9:1254–1262. doi: 10.2215/CJN.09470913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Coco M., Rush H. Increased incidence of hip fractures in dialysis patients with low serum parathyroid hormone. Am J Kidney Dis. 2000;36:1115–1121. doi: 10.1053/ajkd.2000.19812. [DOI] [PubMed] [Google Scholar]
- 21.Jorgensen H.S., Winther S., Bottcher M. Bone turnover markers are associated with bone density, but not with fracture in end stage kidney disease:a cross-sectional study. BMC Nephrol. 2017;18:284. doi: 10.1186/s12882-017-0692-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nickolas T.L., Stein E.M., Dworakowski E. Rapid cortical bone loss in patients with chronic kidney disease. J Bone Miner Res. 2013;28:1811–1820. doi: 10.1002/jbmr.1916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Torres P.A.U., Cohen-Solal M. Evaluation of fracture risk in chronic kidney disease. J Nephrol. 2017;30:653–661. doi: 10.1007/s40620-017-0398-6. [DOI] [PubMed] [Google Scholar]
- 24.Urena P., Bernard-Poenaru O., Ostertag A. Bone mineral density, biochemical markers and skeletal fractures in haemodialysis patients. Nephrol Dial Transplant. 2003;18:2325–2331. doi: 10.1093/ndt/gfg403. [DOI] [PubMed] [Google Scholar]
- 25.Bucur R.C., Panjwani D.D., Turner L. Low bone mineral density and fractures in stages 3–5 CKD: an updated systematic review and meta-analysis. Osteoporos Int. 2015;26:449–458. doi: 10.1007/s00198-014-2813-3. [DOI] [PubMed] [Google Scholar]
- 26.Moranne O., Froissart M., Rossert J. Timing of onset of CKD-related metabolic complications. J Am Soc Nephrol. 2009;20:164–171. doi: 10.1681/ASN.2008020159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Froissart M., Rossert J., Jacquot C. Predictive performance of the modification of diet in renal disease and Cockcroft-Gault equations for estimating renal function. J Am Soc Nephrol. 2005;16:763–773. doi: 10.1681/ASN.2004070549. [DOI] [PubMed] [Google Scholar]
- 28.Hanson J. Standardization of femur BMD. J Bone Miner Res. 1997;12:1316–1317. doi: 10.1359/jbmr.1997.12.8.1316. [DOI] [PubMed] [Google Scholar]
- 29.Hui S.L., Gao S., Zhou X.H. Universal standardization of bone density measurements:a method with optimal properties for calibration among several instruments. J Bone Miner Res. 1997;12:1463–1470. doi: 10.1359/jbmr.1997.12.9.1463. [DOI] [PubMed] [Google Scholar]
- 30.Shepherd J.A., Cheng X.G., Lu Y. Universal standardization of forearm bone densitometry. J Bone Miner Res. 2002;17:734–745. doi: 10.1359/jbmr.2002.17.4.734. [DOI] [PubMed] [Google Scholar]
- 31.Shepherd J.A., Lu Y., Wilson K. Cross-calibration and minimum precision standards for dual-energy X-ray absorptiometry: the 2005 ISCD Official Positions. J Clin Densitom. 2006;9:31–36. doi: 10.1016/j.jocd.2006.05.005. [DOI] [PubMed] [Google Scholar]
- 32.Bates D.M., Bolker B., Walker F. Fitting linear mixed-effects models using Ime4. Journal of Statistical Software. 2015;67:1–48. [Google Scholar]
- 33.Thiebaut R., Walker S. When it is better to estimate a slope with only one point. QJM. 2008;101:821–824. doi: 10.1093/qjmed/hcn099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fried L.F., Shlipak M.G., Stehman-Breen C. Kidney function predicts the rate of bone loss in older individuals: the Cardiovascular Health Study. J Gerontol A Biol Sci Med Sci. 2006;61:743–748. doi: 10.1093/gerona/61.7.743. [DOI] [PubMed] [Google Scholar]
- 35.Ishani A., Paudel M., Taylor B.C. Renal function and rate of hip bone loss in older men: the Osteoporotic Fractures in Men Study. Osteoporos Int. 2008;19:1549–1556. doi: 10.1007/s00198-008-0608-0. [DOI] [PubMed] [Google Scholar]
- 36.Metzger M., Houillier P., Gauci C. Relation between circulating levels of 25(OH) vitamin D and parathyroid hormone in chronic kidney disease:quest for a threshold. J Clin Endocrinol Metab. 2013;98:2922–2928. doi: 10.1210/jc.2013-1294. [DOI] [PubMed] [Google Scholar]
- 37.Silva B.C., Costa A.G., Cusano N.E. Catabolic and anabolic actions of parathyroid hormone on the skeleton. J Endocrinol Invest. 2011;34:801–810. doi: 10.3275/7925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Maeno Y., Inaba M., Okuno S. Serum concentrations of cross-linked N-telopeptides of type I collagen:new marker for bone resorption in hemodialysis patients. Clin Chem. 2005;51:2312–2317. doi: 10.1373/clinchem.2005.051524. [DOI] [PubMed] [Google Scholar]
- 39.Salam S., Gallagher O., Gossiel F. Diagnostic accuracy of biomarkers and imaging for bone turnover in renal osteodystrophy. J Am Soc Nephrol. 2018;29:1557–1565. doi: 10.1681/ASN.2017050584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ueda M., Inaba M., Okuno S. Clinical usefulness of the serum N-terminal propeptide of type I collagen as a marker of bone formation in hemodialysis patients. Am J Kidney Dis. 2002;40:802–809. doi: 10.1053/ajkd.2002.35692. [DOI] [PubMed] [Google Scholar]
- 41.Jassal S.K., von Muhlen D., Barrett-Connor E. Measures of renal function, BMD, bone loss, and osteoporotic fracture in older adults: the Rancho Bernardo study. J Bone Miner Res. 2007;22:203–210. doi: 10.1359/jbmr.061014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Prasad B., Ferguson T., Tangri N. Association of bone mineral density with fractures across the spectrum of chronic kidney disease: the Regina CKD-MBD Study. Can J Kidney Health Dis. 2019;6 doi: 10.1177/2054358119870539. 2054358119870539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Liabeuf S., McCullough K., Young E.W. International variation in the management of mineral bone disorder in patients with chronic kidney disease: results from CKDopps. Bone. 2019;129:115058. doi: 10.1016/j.bone.2019.115058. [DOI] [PubMed] [Google Scholar]
- 44.Geng S., Kuang Z., Peissig P.L. Parathyroid hormone independently predicts fracture, vascular events, and death in patients with stage 3 and 4 chronic kidney disease. Osteoporos Int. 2019;30:2019–2025. doi: 10.1007/s00198-019-05033-3. [DOI] [PubMed] [Google Scholar]
- 45.Moe S.M., Abdalla S., Chertow G.M. Effects of Cinacalcet on fracture events in patients receiving hemodialysis: the EVOLVE Trial. J Am Soc Nephrol. 2015;26:1466–1475. doi: 10.1681/ASN.2014040414. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.