Abstract
Introduction
Cardiac rehabilitation (CR) is a proven therapy for reducing cardiovascular death and hospitalization. Whether CR participation is associated with improved outcomes in patients with chronic kidney disease (CKD) is unknown.
Methods
We obtained data on all adult patients in Calgary, Alberta, Canada with angiographically proven coronary artery disease from 1996 to 2016 referred to CR from The Alberta Provincial Project for Outcome Assessment in Coronary Heart Disease and TotalCardiology Rehabilitation. An estimated glomerular filtration rate (eGFR) <60 ml/min/1.73 m2 or kidney replacement therapy defined CKD. Predictors of CR use were estimated with multinomial logistic regression. The association between starting versus not starting and completion versus noncompletion of CR and clinical outcomes were estimated using multivariable Cox proportional hazards models.
Results
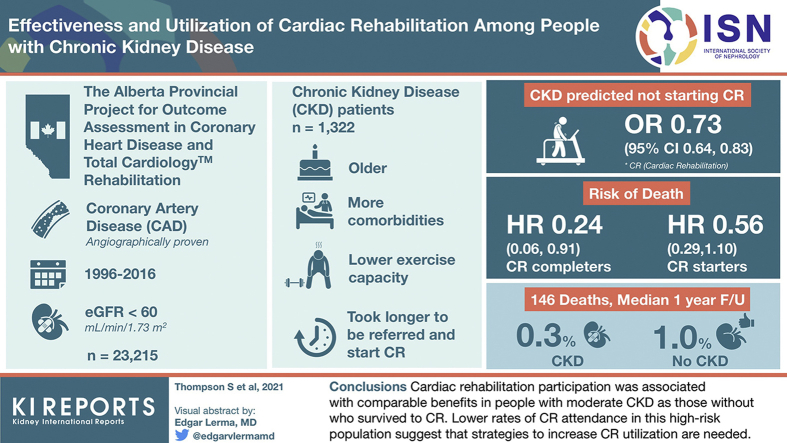
Of 23,215 patients referred to CR, 12,084 were eligible for inclusion. Participants with CKD (N = 1322) were older, had more comorbidity, lower exercise capacity on graded treadmill testing, and took longer to be referred and to start CR than those without CKD. CKD predicted not starting CR: odds ratio 0.73 (95% confidence interval [CI] 0.64–0.83). Over a median 1 year follow-up, there were 146 deaths, 40 (0.3%) from CKD and 106 (1.0%) not from CKD. Similar to those without CKD, the risk of death was lower in CR completers (hazard ratio [HR] 0.24 [95% CI 0.06–0.91) and starters (HR 0.56 [95% CI 0.29– 1.10]) with CKD.
Conclusion
CR participation was associated with comparable benefits in people with moderate CKD as those without who survived to CR. Lower rates of CR attendance in this high-risk population suggest that strategies to increase CR utilization are needed.
Keywords: cardiac events, cardiac rehabilitation, chronic kidney disease, exercise, mortality, observational study
Graphical abstract
Cardiovascular (CV) disease is the leading cause of death in patients with CKD, with a risk of myocardial infarction and CV mortality 4- to 10-fold higher than in the general population.1,2 Despite these risks, patients with CKD are less likely to receive established therapies that have shown to be efficacious in the general population, such as CV medications and revascularization.3, 4, 5 Evidence from randomized trials has established exercise-based cardiac rehabilitation (CR) as a proven therapy for reducing CV mortality and all-cause hospitalization in populations with coronary artery disease (CAD).6,7 However, whether coexisting CKD in patients with CV disease influences the utilization and outcomes associated with CR is not known.
Evaluating the utilization of CR and its relationship with clinical outcomes in people with CKD is important for several reasons. First, CKD is independently associated with a poorer prognosis after a cardiac event,8 even for those with moderate CKD.9, 10, 11 Second, it is important to show whether interventions proven efficacious in randomized trials are effective in people that may have been excluded (i.e., those with a high burden of comorbidity and kidney disease). Finally, many of the known barriers to CR participation, such as multimorbidity and advanced age, are highly prevalent among people with CKD. Therefore, characterizing the pattern of CR utilization in people with CKD could be used to focus recommendations and tailor strategies for increasing CR participation in this high-risk population.
Our primary objective was to evaluate the association between CR and death in people with CKD. Our secondary objectives were to evaluate the associations between CR and CV death, hospitalization, and cardiac events. We also sought to describe the association between patients with CKD and CR utilization.
Methods
This observational study is reported according to the Strengthening the Reporting of Observational Studies in Epidemiology guidelines.12 The institutional review boards at the Universities of Alberta (Pro00073253) and Calgary (REB15-0476) approved this study.
Data Sources and Cohort
We used data from the Alberta Provincial Project for Outcome Assessment in Coronary Heart Disease (APPROACH) and the TotalCardiology Rehabilitation Database. APPROACH prospectively collects data on demographic and clinical characteristics on all patients undergoing coronary angiography in the province of Alberta, Canada.13
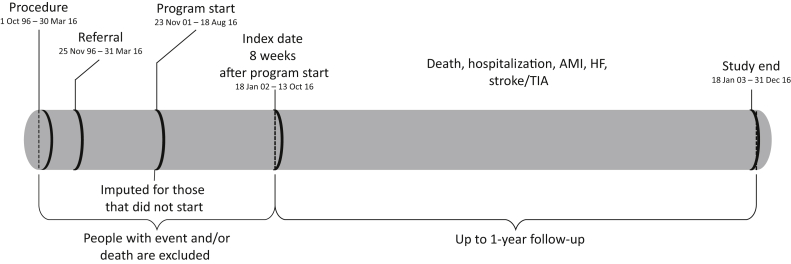
Clinical outcomes, laboratory values, and additional demographic and clinical characteristics were determined by linkage to the Alberta Kidney Disease Network (AKDN) database which incorporates data from Alberta Health (AH; the provincial health ministry) on physician claims, hospitalizations and ambulatory care utilization, vital statistics from Service Alberta, and all clinical laboratories in Alberta. Laboratory data for creatinine was uniformly available from all health regions in Alberta from May 1, 2002 onward. Additional information on the AKDN database is available elsewhere, including the validation of selected data elements and the standardization and calibration of serum creatinine assays.14 The databases were used to assemble a cohort of adults (≥18 years of age) who had angiographically proven CAD and were referred to CR. Eligible participants required ≥1 serum creatinine measurement within 2 years before coronary angiography or in the 1 to 8 weeks after the procedure. We followed participants 8 weeks after the start of CR (index date; January 18, 2002 or later) until death, out-migration, or for 1 year, whichever was earliest (Figure 1). If an individual selected not to participate or was missing an enrollment date (15.8%), we imputed their hypothetical start date of the program with random sampling and replacement from the available wait times (from catheterization to CR start).
Figure 1.
Study timeline. AMI = acute myocardial infarction; HF = heart failure; TIA = transient ischemic attack.
Cardiac Rehabilitation
TotalCardiology Rehabilitation is a single, centralized CR program located in Calgary, Alberta, Canada. Patients eligible for the CR program must be referred by a physician and have CV disease. Health identifier, demographics, and reason for referral are collected for all referrals. The program is a uniformly delivered 12-week exercise-based program that also includes education, health coaching, and medical management for comprehensive risk factor reduction. The exercise program is delivered as a twice-weekly, 1-hour session supervised by clinical exercise specialists, registered nurses, and physicians.15,16 The typical exercise prescription includes 20 to 60 minutes of steady-state aerobic training at 45% to 85% of heart rate reserve. Resistance training and stretching are offered on alternate days after each aerobic exercise session. Participants are also prescribed 2 to 2 additional hours per week of independent exercise. The education component consists of classes on nutrition, stress management, and smoking cessation plus additional behavioral health counseling with program staff as needed. CR participants complete a baseline assessment that includes a graded exercise test and risk factor assessment. The graded exercise test is typically completed on a treadmill using a Bruce Protocol to determine peak metabolic equivalents (METs). The peak MET value was calculated from treadmill speed and grade during the final stage of the exercise protocol using and established equation.17 The baseline assessment is repeated at the end of the 12-week program and participants are subsequently provided with a home exercise program. In this study, participants were divided into 3 CR exposure groups: 1) those that were referred but did not start the CR program, 2) those that started but that did not complete the 12-week CR program; and 3) those that completed the CR program. Noncompletion was defined as those who started but either self-selected not to complete the program, were medically discharged early, or died. Those that were discharged or died before completing 8 weeks of the program did not contribute any follow-up time and therefore were not included in any analyses.
Covariates from APPROACH
Age, sex, South Asian ethnicity, body mass index, current smoking status, and the number of diagnosed comorbidities (hypertension, hyperlipidemia, diabetes mellitus, heart failure, chronic obstructive pulmonary disease, metastatic cancer, peripheral vascular disease, and cerebrovascular disease) and whether the indication for catheterization was for acute coronary syndrome was collected in the APPROACH registry at the time of the coronary angiogram. Obstructive CAD was defined as ≥50% stenosis on angiogram. Obesity was defined as a body mass index ≥30 kg/m2. The date of the coronary angiogram was used as the index date for program referral and program start.
Covariates from AKDN
Social assistance (registration with a provincial program for those <65 years of age) and postal code were collected from the AH registry in the AKDN database. We determined rural status and income quintile, and geographic coordinates for each 6-digit postal code using the Statistics Canada Postal Code Conversion File (available at www.statcan.ca). Residents in the lowest income quintile were considered participants residing in a low-income neighborhood. Using ArcInfo 10.0 software (Esri), we calculated the shortest distance by road (in 5-km bins) from the postal code of the participant’s residence to the closest TotalCardiology Rehabilitation center.
We captured the single closest measurement of creatinine within the 2 years before angiography to establish baseline CKD category. If a creatinine was not available before angiography, we used the lowest value within 1 to 8 weeks after angiography. The eGFR was estimated using the Chronic Kidney Disease Epidemiology equation.18 Measurements were categorized as follows: ≥60, 45–59, 30–44, 15–29, and <15 ml/min/1.73 m2 or kidney replacement therapy. Albuminuria was captured using the albumin:creatinine ratio (ACR), the protein:creatinine ratio (PCR), and the dipstick. A PCR assessment was used when ACR was not available, and dipstick results were used when PCR was not available. Albuminuria was defined as: ACR ≥3 mg/mmol, PCR ≥15 mg/mmol, and dipstick ≥1+. CKD was defined by an eGFR <60 ml/min/1.73 m2 (including those on kidney replacement therapy).
Three additional comorbidities (alcohol misuse, atrial fibrillation, and chronic depression) were defined using a previously published framework with validated algorithms as applied to Canadian physician claims data, each of which had positive predictive values ≥70% as compared with a criterion standard measure, such as chart review.19 Each participant was classified with respect to the presence or absence of these 3 chronic conditions at the time of the coronary angiogram (look back extended as far as April 1994 where records were available).
Outcomes
The primary outcome was all-cause death. Secondary outcomes were: CV death, first all-cause hospitalization, and hospitalizations for first acute myocardial infarction (AMI) during follow-up,19,20 heart failure, first stroke or transient ischemic attack during follow-up,21 and first CV event (the composite of AMI, heart failure, stroke, and CV death) during follow-up. CV death was defined in an earlier paper using vital statistics.22
Statistical Analyses
We conducted analyses with Stata MP software (version 15.1; StataCorp LLC, College Station, TX) and reported baseline descriptive statistics as counts and percentages, or medians and interquartile limits, as appropriate. Simple associations were tested using the Fisher exact or Kruskal-Wallis tests. We used the Wilcoxon signed-rank test to test for within-group (across time) differences. We used adjusted logistic regression to determine characteristics independently associated with the 3 mutually exclusive CR exposure groups. The model was adjusted for age (categorized as 18–39, 40–64, 65–79, or ≥80 years of age), sex, South Asian status, rural status, low-income neighbourhood, era of catheterization (categorized as 1996–2005, 2006–2010, or 2011–2016), smoking status, obesity, and the 11 comorbidities described above. Missing values for rural status were imputed as urban (1.0%), and a missing indicator variable was used if low-income neighborhood status (1.9%) or obesity (3.7%) were missing. Odds ratios (ORs) and 95% CIs are reported. We used adjusted multinomial logistic regression to show the distribution of these characteristics among 3 mutually exclusive CR exposure groups. Percentages and 95% CIs are reported.
We used adjusted Cox regression to determine whether the clinical outcomes were associated with starting the CR program (combining 2 of the CR exposure groups) versus not starting the CR program and completing the CR program versus starting but not completing the CR program. The models adjusted for the characteristics listed above, and included an interaction term between CKD and starting or completing the program in order to test whether outcomes were modified by the presence of CKD. To mitigate immortal time bias and to account for the period where CR would not be anticipated to yield benefits,23 follow-up time started 8 weeks after the start of the program. The start date for those who did not start the CR program was imputed randomly with replacement using the empirical distribution of time from referral to program starts from the other participants.24 Participants with chronic heart failure at baseline were excluded from the time to heart failure and composite event models. Participants with hospitalizations or cardiac events (AMI, heart failure, or stroke) during the 8 weeks after enrollment were excluded from all analyses. Cause of death was missing in 2.1% of the participants who died during follow-up; these participants were excluded from the CV death models. We determined that the proportional hazard assumption was satisfied by examining plots of the log-negative-log of within-group survivorship probabilities versus log-time. As the median follow time from trials is 1 year, the follow-up time was restricted to 1 year. In a sensitivity analysis, we used a propensity score approach to address treatment selection bias by calculating the propensity to start or complete CR. These propensity scores were then inverted and used as weights in further Cox analyses.25 The threshold P for statistical significance was set at 0.05.
Results
Characteristics of Study Participants
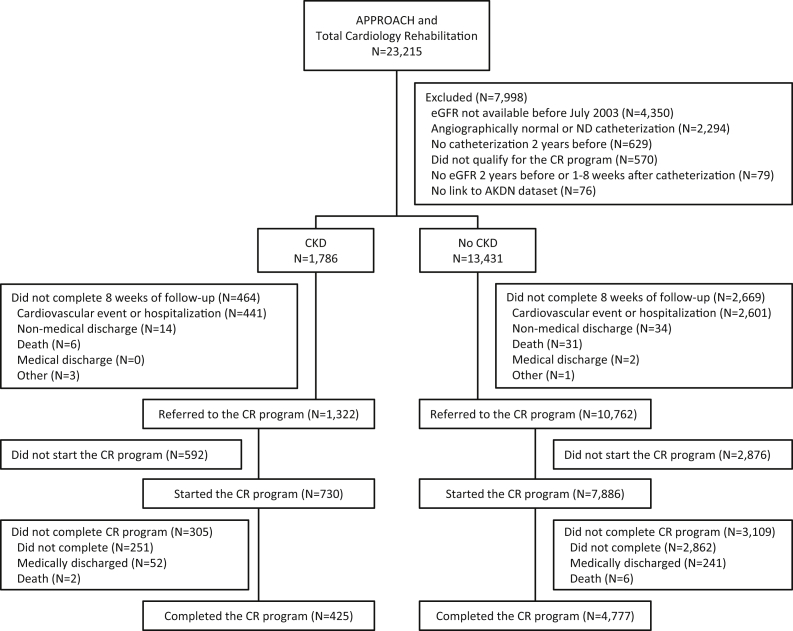
Participant flow is shown in Figure 2. From October 1, 1996 to March 30, 2016, a total of 23,215 patients were identified in the APPROACH registry and the TotalCardiology Rehabilitation database. Patients were excluded because they did not have an eGFR measurement (39.1%, of which 98% underwent angiogram before laboratory data on serum creatinine was available), they had a nonfatal cardiovascular event or hospitalization in the first 8 weeks of the program (27.3%), their coronary anatomy was not determined or it was angiographically normal (20.6%), there was no catheterization in the 2 years prior (5.7%), other (did not meet CR eligibility or died; 5.9%), or they could not be linked to the AKDN dataset (<1.0%). Of the those with (N = 1786) and without CKD (N = 13,431), the primary reason for exclusion from the analysis was a cardiovascular event or hospitalization, which was proportionally similar between groups. Of the final study population of 12,084 participants, 1322 (10.9%) had CKD.
Figure 2.
Participant flow diagram. AKDN = Alberta Kidney Disease Network; APPROACH = Alberta Provincial Project for Outcomes Assessment in Coronary Heart Disease; CKD = chronic kidney disease; CR = cardiac rehabilitation; eGFR = estimated glomerular filtration rate; ND = non-diagnostic; RRT = renal replacement therapy.
During follow-up, there were 146 deaths (1.2%; including 60 from CV causes); 1186 (9.8%) had ≥1 hospitalization; and 567 (5.1%) had ≥1 nonfatal CV event: 100 had a myocardial infarction, 222 had an episode of heart failure (886 had existing heart failure), and 325 had a stroke or a transient ischemic attack.
Demographic and clinical characteristics by CKD status and by the 3 CR exposure groups are summarized in Table 1. In the overall population and within each CR exposure group, participants with CKD were more likely to be older, female, and to take more time to be referred to and to start the program. Within each CR exposure group, most participants with CKD had an eGFR of 45 to 59 ml/min/1.73 m2. Participants with CKD also had more comorbidity compared with those with normal kidney function but were less likely to be smokers, misuse alcohol, or to be referred to CR for acute coronary syndrome.
Table 1.
Demographic and clinical characteristics by CKD and CR status
| Characteristic | Referred to CR but did not start |
Started CR but did not complete |
Completed CR |
|||
|---|---|---|---|---|---|---|
| No CKD | CKD | No CKD | CKD | No CKD | CKD | |
| N | 2876 (82.9) | 592 (17.1) | 3109 (91.1) | 305 (8.9) | 4777 (91.8) | 425 (8.2) |
| Age, yr | 63 [55–71]a | 74 [68–80]a | 59 [52–66]a | 71 [64–77]a | 59 [53–67]a | 71 [64–77]a |
| Female sex | 630 (21.9) | 187 (31.6)a | 595 (19.1)a | 83 (27.2)a | 791 (16.6)a | 94 (22.1)a |
| South Asian | 257 (8.9) | 40 (6.8) | 264 (8.5)a | 15 (4.9)a | 375 (7.9)a | 21 (4.9)a |
| Distance to CR center | 10 [5–30] | 10 [5–30] | 15 [10–25] | 15 [10–25] | 15 [10–25]a | 10 [10–15]a |
| Rural | 187 (6.6) | 36 (6.1) | 129 (4.2) | 11 (3.6) | 132 (2.8) | 10 (2.4) |
| Social assistance | 106 (3.7) | 17 (2.9) | 78 (2.5) | 11 (3.6) | 63 (1.3) | 4 (0.9) |
| Low-income neighborhood | 732 (25.5) | 137 (23.1) | 629 (20.2) | 49 (16.1) | 756 (15.8)a | 51 (12.0)a |
| Era | ||||||
| 1996–2005 | 440 (15.3)a | 99 (16.7)a | 172 (5.5) | 16 (5.2) | 913 (19.1)a | 86 (20.2)a |
| 2006–2010 | 1433 (49.8)a | 251 (42.4)a | 660 (21.2) | 76 (24.9) | 2033 (42.6)a | 204 (48.0)a |
| 2011–2016 | 1003 (34.9)a | 242 (40.9)a | 2277 (73.2) | 213 (69.8) | 1831 (38.3)a | 135 (31.8)a |
| Smoker | 1046 (36.4)a | 106 (17.9)a | 1035 (33.3)a | 50 (16.4)a | 1166 (24.4)a | 45 (10.6)a |
| Days from catheterization to program referral | 6 [2–32]a | 18 [5–62]a | 4 [2–9]a | 7 [3–23]a | 5 [3–15]a | 9 [3–47]a |
| Referral due to ACS | 1966 (68.4)a | 341 (57.6)a | 2370 (76.2)a | 192 (63.0)a | 3612 (75.6)a | 272 (64.0)a |
| Days from catheterization to program start | — | — | 54 [34–97]a | 77 [42–138]a | 74 [39–111]a | 94 [62–147]a |
| Days from catheterization to follow-up | — | — | 120 [92–165]a | 133 [95–181]a | 130 [95–168]a | 150 [118–203]a |
| CAD severity | ||||||
| 1-vessel | 1011 (35.2)a | 129 (21.8)a | 1222 (39.3)a | 83 (27.2)a | 1860 (38.9)a | 132 (31.1)a |
| 2-vessel | 874 (30.4)a | 171 (28.9)a | 1001 (32.2)a | 94 (30.8)a | 1472 (30.8)a | 120 (28.2)a |
| 3-vessel | 757 (26.3)a | 216 (36.5)a | 775 (24.9)a | 101 (33.1)a | 1188 (24.9)a | 134 (31.5)a |
| Left main | 234 (8.1)a | 76 (12.8)a | 111 (3.6)a | 27 (8.9)a | 257 (5.4)a | 39 (9.2)a |
| Exercise capacity (METs) | — | — | 7.0 [5.7–8.7]a | 5.1 [3.8–7.0]a | 7.7 [6.4–9.0]a | 6.4 [5.0–7.7]a |
| eGFR, mL/min/1.73 m2 | 87 [76–97]a | 49 [40–56]a | 91 [80–100]a | 50 [42–55] | 89 [78–98]a | 52 [45–56]a |
| 45–59 | — | 377 (63.7) | — | 206 (67.5) | — | 320 (75.3) |
| 30–44 | — | 145 (24.5) | — | 68 (22.3) | — | 79 (18.6) |
| 15–29 | — | 38 (6.4) | — | 13 (4.3) | — | 13 (3.1) |
| <15 or RRT | — | 32 (5.4) | — | 18 (5.9) | — | 13 (3.1) |
| Comorbidity | ||||||
| Albuminuria | 205 (7.1)a | 126 (21.3)a | 171 (5.5)a | 69 (22.6)a | 196 (4.1)a | 68 (16.0)a |
| Alcohol misuse | 160 (5.6) | 22 (3.7) | 140 (4.5) | 11 (3.6) | 110 (2.3) | 6 (1.4) |
| Atrial fibrillation | 245 (8.5)a | 126 (21.3)a | 163 (5.2)a | 40 (13.1)a | 308 (6.4)a | 52 (12.2)a |
| Metastatic cancer | 142 (4.9)a | 42 (7.1)a | 118 (3.8)a | 21 (6.9)a | 154 (3.2)a | 26 (6.1)a |
| Chronic heart failure | 244 (8.5)a | 145 (24.5)a | 149 (4.8)a | 53 (17.4)a | 238 (5.0)a | 57 (13.4)a |
| COPD | 462 (16.1)a | 151 (25.5)a | 361 (11.6)a | 53 (17.4)a | 477 (10.0)a | 75 (17.6)a |
| Depression | 296 (10.3) | 62 (10.5) | 293 (9.4) | 27 (8.9) | 397 (8.3) | 34 (8.0) |
| Diabetes | 766 (26.6)a | 244 (41.2)a | 708 (22.8)a | 107 (35.1)a | 861 (18.0)a | 110 (25.9)a |
| Hypertension | 1961 (68.2)a | 500 (84.5)a | 1860 (59.8)a | 234 (76.7)a | 2864 (60.0)a | 333 (78.4)a |
| Hyperlipidemia | 2081 (72.4) | 432 (73.0) | 2007 (64.6) | 198 (64.9) | 3312 (69.3) | 295 (69.4) |
| Obesity | 790 (28.2) | 141 (24.9) | 927 (31.3) | 84 (28.2) | 1304 (28.4) | 118 (28.6) |
| PVD | 188 (6.5)a | 60 (10.1)a | 150 (4.8)a | 25 (8.2)a | 179 (3.7)a | 35 (8.2)a |
| Stroke | 148 (5.1)a | 61 (10.3)a | 92 (3.0)a | 29 (9.5)a | 121 (2.5)a | 25 (5.9)a |
ACS, acute coronary syndrome; CAD, coronary artery disease; CKD, chronic kidney disease; CR, cardiac rehabilitation; COPD, chronic obstructive pulmonary disease; eGFR, estimated glomerular filtration rate; MET, metabolic equivalent of task; PVD, peripheral vascular disease; RRT, renal replacement therapy.
Data shown as n (%) or median [interquartile range].
Indicates that Fisher exact or Kruskal-Wallis test was significant (P < 0.05).
Exercise Capacity
For CR completers, median baseline exercise capacity was 6.4 (IQR 5.0–7.7) peak METs for those with CKD and 7.7 (6.4–9.0) METs for those without (P < 0.001). Within each group, median exercise capacity improved from baseline for those with CKD 0.65 (IQR 0.00–1.31) and without CKD 0.70 (IQR 0.60–1.31; P < 0.001). Participants with CKD had a lower overall change in exercise capacity than those without CKD (P = 0.04). In the 395 patients with CKD who completed CR and had data on peak METs, we did not detect a significant association (adjusted for age and sex) between increase in peak METs and lower mortality (HR 0.55 [95% CI 0.21–1.45]).
Characteristics Associated With CR Referral, Attendance, and Completion
Supplementary Table S1 shows the distribution of characteristics by the 3 CR exposure groups. Of those referred to CR, 29% did not start the program, 28% started but did not complete the program, and 43% completed the program. Older age, rural residence, social assistance, later era, smoking, and alcohol misuse were characteristics associated with a higher proportion (>65%) of people not completing the program.
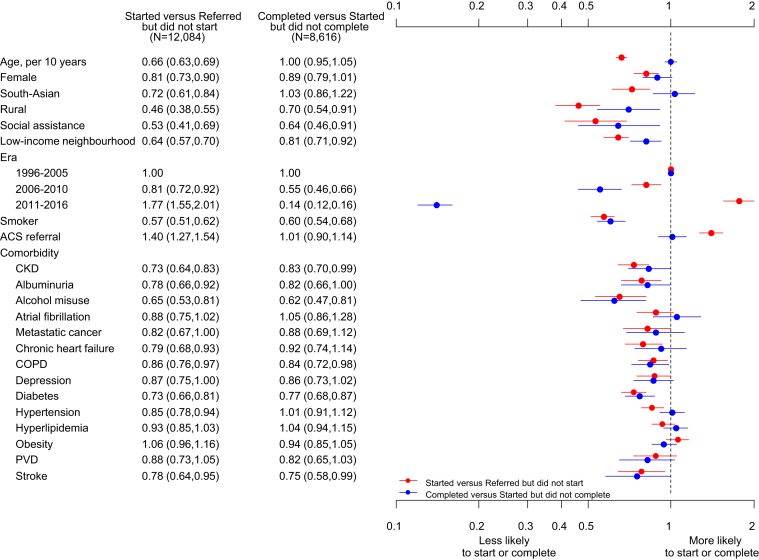
Characteristics independently associated with CR utilization are shown in Figure 3. CKD was independently associated with not starting CR (OR 0.73 [95% CI 0.64–0.83]). Other predictors of not starting CR were older age (OR 0.66 per 10 years [95% CI 0.63–0.69]), female gender (OR 0.81 [95% CI 0.73–0.90]), South Asian ethnicity (OR 0.72 [95% CI 0.61–0.84]), rural residence (OR 0.46 [95% CI 0.38–0.55]), social assistance (OR 0.53 [95% CI 0.41–0.69]), residing in a low-income neighborhood (OR 0.64 [95% CI 0.57–0.70]), current smoking (OR 0.57 [95% CI 0.51–0.62]), albuminuria (OR 0.78 [95% CI 0.66–0.92]), or any comorbid condition with the exception of atrial fibrillation, metastatic cancer, chronic depression, hyperlipidemia, obesity, or peripheral vascular disease. People with a referral for an acute coronary syndrome and those in the most recent era had a higher odds of starting the CR program (OR 1.40 [95% CI 1.27–1.54] and OR 1.77 [95% CI 1.55–2.01], respectively).
Figure 3.
Cardiac rehabilitation program attendance and completion in those who started versus those who were referred but did not start, and in those who completed versus those who started but did not complete. ACS = acute coronary syndrome; CKD = chronic kidney disease; COPD = chronic obstructive pulmonary disease; PVD = peripheral vascular disease.
Many of the same demographic and socioeconomic factors that predicted not starting CR were associated with not completing: CKD (OR 0.83 [95% CI 0.70–0.99]), rural residence (OR 0.70 [95% CI 0.54–0.91]), social assistance (OR 0.64 [95% CI 0.46–0.91]), residing in a low-income neighborhood (OR 0.81 [95% CI 0.71–0.92]), most recent era (OR 0.14 [95% CI 0.12–0.16]), current smoking (OR 0.60 [95% CI 0.54–0.68]), alcohol misuse (OR 0.62 [95% CI 0.47–0.81]), chronic obstructive pulmonary disease (OR 0.84 [95% CI 0.72–0.98]), diabetes (OR 0.77 [95% CI 0.68–0.87]), and stroke (OR 0.75 [95% CI 0.58–0.99]).
Clinical Outcomes by CKD Status
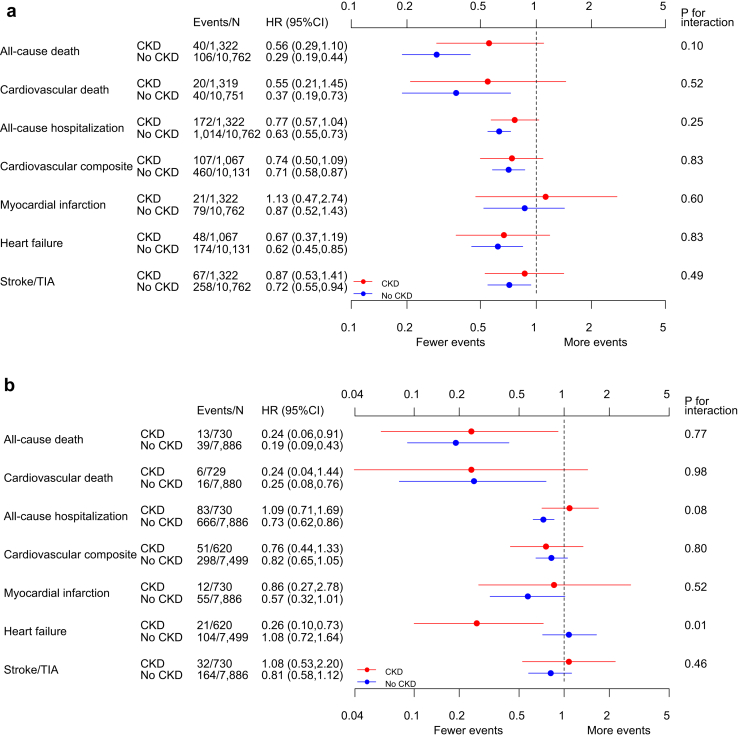
Associations between CR attendance and clinical outcomes did not significantly differ between participants with and without CKD (Figure 4a; P values for interaction ≥ 0.10). For the primary outcome, participants without CKD who started CR had a lower hazard of death (HR 0.29 [95% CI 0.19–0.44]) compared with nonstarters. The HR for death in the CKD group was also consistent with a lower mortality risk associated with starting CR but did not reach statistical significance (HR 0.56 [95% CI 0.29–1.10]). Except for the outcome of myocardial infarction, starting versus not-starting CR was associated with a lower hazard for each of the specified secondary outcomes among those without CKD and a nonsignificant trend toward a lower risk of events in those with CKD.
Figure 4.
(A) Cardiac rehabilitation in chronic kidney disease (CKD) outcomes in those who started but did not complete versus those who were referred but did not start. (B) Cardiac rehabilitation in CKD outcomes in those who completed versus those who started but did not complete. CI = confidence interval; HR = hazard ratio; TIA = transient ischemic attack.
Associations between completion of CR and clinical outcomes did not significantly differ between participants with and without CKD (Figure 4b; P values for interaction ≥ 0.08) except for heart failure. Participants who completed CR had a lower hazard of death than those who did not complete CR in those with or without CKD (CKD: HR 0.24 [95% CI 0.06–0.91]; no CKD: HR 0.19 [95% CI 0.09–0.43]). In the no CKD group, completers had a lower hazard of cardiovascular death (HR 0.25 [95% CI 0.08–0.76]) and all-cause hospitalization (HR 0.73 [95% CI 0.62–0.86]) than noncompleters. Participants with CKD who completed CR had a lower hazard of heart failure than those who did not complete CR in the no CKD group, but not in the no CKD group (CKD: HR 0.26 [95% CI 0.10–0.73]; no CKD: HR 1.08 [95% CI 0.72–1.64]; P = .001 for interaction). The results of the full Cox regression for the hazard of starting and completing CR on the primary outcome of mortality are shown in Supplementary Table 2.
Sensitivity Analysis
Results from the propensity-weighted analysis were similar to the primary analysis (Supplementary Table S3). For example, the HR for death for those who started the CR program with CKD and without CKD was 0.61 (95% CI 0.30–1.26) and 0.19 (95% CI 0.09–0.41), respectively. The HR for CV death for CR starters with CKD and without CKD was 0.66 (95% CI 0.23–1.91) and 0.33 (95% CI 0.17–0.66). Standardized differences from the weighted analysis are shown in Supplementary Table S4.
Discussion
In this observational cohort study we examined the associations between CR and clinical outcomes including death from all causes, CV death, hospitalization, and CV events and whether these associations differed by CKD status. We also examined the relation between CKD and CR utilization. Overall, and like those with and without CKD, there was a lower risk of death in people who started CR versus those who did not, and even lower risk for those with and without CKD who completed CR versus those who did not. There were nonsignificant associations of CR completion with lower CV death and other nonfatal CV events among those with CKD who completed CR. These findings are important because CKD was also a predictor of not starting and not completing CR.
Our finding that CR participation was associated with a lower risk of hospital admissions, all-cause and CV mortality, but not myocardial infarction in those without CKD is consistent with findings from a previous meta-analysis of randomized trials.7 In a more recent review, a comparable effect on CV mortality was reported (relative risk 0.74 [95% CI 0.64–0.86]) but the relation between CR participation and all-cause mortality was not significant (relative risk 0.96 [95% CI 0.88–1.04]).6 This difference was attributed to the inclusion of trials conducted in the era of optimized medical management of CV disease. However, the underrepresentation of older, higher-risk populations with major comorbidities remains a recognized limitation of CR trials. It is conceivable that in unselected populations where the delivery of optimized medical therapy is more variable, the opportunity for a reduction in mortality with CR remains. In a meta-analysis of contemporary studies that mainly included observational studies with mixed populations, CR was associated with reduced all-cause mortality (HR 0.37 [95% CI 0.20–0.69]).26 This is highly relevant to our findings, because it is known that people with CKD are less likely to receive guideline recommended therapy.27
There was an association between CR and CV death in attenders with CKD and without CKD. The non–statistically significant association between CR completion and CV death in those with CKD is likely related to the low number of CV events. That said, although the association between higher physical activity and lower mortality in people with CKD has been reported in previous studies, the effect on CV events and CV death is not known.28, 29, 30, 31 In an analysis of the Chronic Renal Insufficiency Cohort Study, a high level of self-reported physical activity (defined as moderate exercise ≥150 minutes/week, vigorous ≥75 minutes/week, or moderate plus vigorous ≥150 minutes/week) was associated with a lower risk of death but not CV events.29 One potential explanation for this finding is that physical activity alone cannot modify the nontraditional risk factors (e.g., positive calcium balance, uremic toxins, abnormal bone mineral metabolism, and anemia) that contribute to the elevated CV risk in CKD. However, from an analysis of the Cardiovascular Health Study, traditional CV risk factors had larger associations with CV death than nontraditional risk factors in those with CKD.28 Importantly, 4 of the 6 main CV risk factors that were examined in that study were modifiable (i.e., smoking, physical inactivity, systolic hypertension, and alcohol consumption), suggesting that multimodal behavioral approaches such as CR may be more effective than physical activity alone in reducing CV events in CKD. Furthermore, in 1 study that was limited to patients with advanced kidney disease requiring dialysis, CR participation after coronary artery bypass graft was associated with a reduced risk for both all-cause and cardiac death, compared with patients who did not participate.32
We observed an association between CR completion and reduction in risk of an episode of heart failure in those with CKD but not those without CKD. Compared with those with normal kidney function, even mildly impaired eGFR is independently associated with incident heart failure in people with or without CAD.33 Although studies evaluating the efficacy of self-management strategies in lifestyle changes in patients with CKD are limited,34 it is plausible that the lower risk associated with heart failure could be attributed to the disease management aspect of CR, i.e., management of volume status through education on sodium reduction and medication adherence.
Dialysis dependence has been identified as a predictor of reduced CR utilization in other studies,35, 36, 37 but to our knowledge this is the first report of this association in a cohort with earlier stages of CKD. Higher comorbidity and older age are 2 factors associated with delayed CR referral and attendance.38,39 Therefore, although it is unclear whether this finding is caused by CKD itself or whether this association is caused by confounding from higher unmeasured morbidity, we have identified a population that could be targeted using established strategies for improving CR attendance.40 These strategies include inpatient referral, appointments for CR within 10 days of hospital discharge, as well as motivational letters or telephone contact.41,42 Notably, from other registry cohort data in CAD populations, the prevalence of CKD was significantly higher than our CR cohort at 20% to 43%.5,43,44 Although we do not have data on those who were not referred to CR, we speculate that this difference is likely because the presence of CKD, and particularly more advanced CKD, may influence decisions to refer to CR.
We are aware of only 1 previous study that examined the relationship between CR participation and death in CKD.32 In that study, Medicare claims were used to identify hemodialysis-dependent patients who had participated in CR post–coronary artery bypass graft. We extend these findings by examining the relation between CR and clinically important endpoints in a cohort including more moderate stage CKD. In addition, compared with previous studies of lifestyle factors in people with CKD that used self-report to measure exposure status, we used data collected on program attendance and completion. To address secular changes in CR eligibility for CAD over the study period, we considered the indication for the angiogram in the analysis. Other strengths of this study include the use of data sources based on validated algorithms to ascertain comorbidities and outcomes. Our study also has limitations that are important to consider in interpreting and generalizing the results, which should be considered as hypothesis generating. First, our findings are likely influenced by treatment selection bias; specifically, the healthy user effect, which would result in an overestimation of the effect of CR. Although we applied 2 approaches to mitigate confounding caused by unmeasured differences in illness severity, health utilization, and behavioral and socioeconomic factors between those who did and did not participate in CR, our adjusted results remain vulnerable to residual confounding from treatment selection bias. Second, although program completion was associated with a greater reduction in mortality than program attendance, we did not have complete data on the number of sessions attended to estimate a dose-response relationship. In attempt to address this limitation, we used data on exercise capacity to verify the exposure among completers. Exercise capacity is an independent predictor of all-cause mortality in people with CAD,45 which also supports the biological plausibility of our findings. Third, to define CKD we used a single measurement of eGFR, a method that may have led to misclassification of exposure. This limitation may have led to an underestimation of risk in the CKD group. In addition, a large proportion of people from earlier years of the study were excluded because they underwent angiography before eGFR measurements were routinely captured in the AKDN database. Fourth, it is unclear how the benefits of CR would be modified in the era of newer therapies that lower CV risk such as the sodium-glucose cotransporter 2 inhibitors. Fifth, as we focused on a selected population that was referred for CR, there were a limited number of events, so some estimates lacked precision. Finally, this cohort was largely composed of those with moderate severity CKD who survived to CR and results may not therefore generalize to later stages of CKD with more frailty and a higher risk of adverse CV outcomes.
In conclusion, in this observational study, we found that CR participation was associated with a lower risk of death in people with CAD, with or without CKD. CKD was also associated with not starting CR despite referral. Given that CV risk factors amenable to lifestyle modification contribute to the burden of CV disease in CKD, our findings suggest that future research and program investments should target the implementation and evaluation of interventions to improve the attendance of eligible patients with CKD in CR programs.
Disclosures
All the authors declared no competing interests.
Acknowledgments
Supported by Heart and Stroke Foundation Mazankowski Leadership Fund. The funders had no role in the design or interpretation of the results. This study is based in part by data provided by Alberta Health and Alberta Health Services. The interpretation and conclusions contained herein are those of the researchers and do not represent the views of the Government of Alberta or Alberta Health Services. Neither the Government of Alberta nor Alberta Health or Alberta Health Services express any opinion in relation to this study. We thank Sophanny Tiv, who provided graphics support, and Daniele Chirico for review.
Footnotes
Table S1. Distribution of characteristics by program attendance, and completion.
Table S2. Full Cox regression results for the primary outcome of mortality.
Table S3. Clinical outcomes associated with attending and completing the program, in those with CKD vs no CKD—an inverse-weight propensity scoring sensitivity analysis.
Table S4. Standardized differences between groups for inverse-weight propensity scoring sensitivity analysis for the time to all-cause death dataset.
Supplementary Material
Table S1. Distribution of characteristics by program attendance, and completion.
Table S2. Full Cox regression results for the primary outcome of mortality.
Table S3. Clinical outcomes associated with attending and completing the program, in those with CKD vs no CKD—an inverse-weight propensity scoring sensitivity analysis.
Table S4. Standardized differences between groups for inverse-weight propensity scoring sensitivity analysis for the time to all-cause death dataset.
References
- 1.Wong J.A., Goodman S.G., Yan R.T. Temporal management patterns and outcomes of non-ST elevation acute coronary syndromes in patients with kidney dysfunction. Eur Heart J. 2009;30:549–557. doi: 10.1093/eurheartj/ehp014. [DOI] [PubMed] [Google Scholar]
- 2.Go A.S., Chertow G.M., Fan D., McCulloch C.E., Hsu C. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med. 2004;351:1296–1305. doi: 10.1056/NEJMoa041031. [DOI] [PubMed] [Google Scholar]
- 3.Ezekowitz J., McAlister F.A., Humphries K.H. The association among renal insufficiency, pharmacotherapy, and outcomes in 6,427 patients with heart failure and coronary artery disease. J Am Coll Cardiol. 2004;44:1587–1592. doi: 10.1016/j.jacc.2004.06.072. [DOI] [PubMed] [Google Scholar]
- 4.Shavadia J.S., Southern D.A., James M.T., Welsh R.C., Bainey K.R. Kidney function modifies the selection of treatment strategies and long-term survival in stable ischaemic heart disease: insights from the Alberta Provincial Project for Outcomes Assessment in Coronary Heart Disease (APPROACH) registry. Eur Hear J Qual Care Clin Outcomes. 2018;4:274–282. doi: 10.1093/ehjqcco/qcx042. [DOI] [PubMed] [Google Scholar]
- 5.Fox C.S., Muntner P., Chen A.Y. Use of evidence-based therapies in short-term outcomes of ST-segment elevation myocardial infarction and non-ST-segment elevation myocardial infarction in patients with chronic kidney disease: a report from the National Cardiovascular Data Acute Coronary Treatment and Intervention Outcomes Network registry. Circulation. 2010;121:357–365. doi: 10.1161/CIRCULATIONAHA.109.865352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Anderson L., Oldridge N., Thompson D.R. Exercise-based cardiac rehabilitation for coronary heart disease. J Am Coll Cardiol. 2016;67:1–12. doi: 10.1016/j.jacc.2015.10.044. [DOI] [PubMed] [Google Scholar]
- 7.Heran B.S., Chen J.M., Ebrahim S. Exercise-based cardiac rehabilitation for coronary heart disease. Cochrane Database Syst Rev. 2011;7:CD001800. doi: 10.1002/14651858.CD001800.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gibson C.M., Pinto D.S., Murphy S.A. Association of creatinine and creatinine clearance on presentation in acute myocardial infarction with subsequent mortality. J Am Coll Cardiol. 2003;42:1535–1543. doi: 10.1016/j.jacc.2003.06.001. [DOI] [PubMed] [Google Scholar]
- 9.Anavekar N.S., McMurray J.J.V., Velazquez E.J. Relation between renal dysfunction and cardiovascular outcomes after myocardial infarction. N Engl J Med. 2004;351:1285–1295. doi: 10.1056/NEJMoa041365. [DOI] [PubMed] [Google Scholar]
- 10.Shlipak M.G., Heidenreich P.A., Noguchi H., Chertow G.M., Browner W.S., McClellan M.B. Association of renal insufficiency with treatment and outcomes after myocardial infarction in elderly patients. Ann Intern Med. 2002;137:555–562. doi: 10.7326/0003-4819-137-7-200210010-00006. [DOI] [PubMed] [Google Scholar]
- 11.Wright R.S., Reeder G.S., Herzog C.A. Acute myocardial infarction and renal dysfunction: a high-risk combination. Ann Intern Med. 2002;137:563–570. doi: 10.7326/0003-4819-137-7-200210010-00007. [DOI] [PubMed] [Google Scholar]
- 12.Von Elm E., Altman D.G., Egger M., Pocock S.J., Gøtzsche P.C., Vandenbroucke J.P. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. J Clin Epidemiol. 2008;61:344–349. doi: 10.1016/j.jclinepi.2007.11.008. [DOI] [PubMed] [Google Scholar]
- 13.Ghali W.A., Knudston M.L. Overview of the Alberta Provincial Project for Outcome Assessment in Coronary Heart Disease. On behalf of the APPROACH investigators. Can J Cardiol. 2000;16:1225–1230. [PubMed] [Google Scholar]
- 14.Hemmelgarn B.R., Clement F., Manns B.J. Overview of the Alberta Kidney Disease Network. BMC Nephrol. 2009;10:30. doi: 10.1186/1471-2369-10-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Martin B.J., Arena R., Haykowsky M. Cardiovascular fitness and mortality after contemporary cardiac rehabilitation. Mayo Clin Proc. 2013;88:455–463. doi: 10.1016/j.mayocp.2013.02.013. [DOI] [PubMed] [Google Scholar]
- 16.Laddu D., Ozemek C., Lamb B. Factors associated with cardiorespiratory fitness at completion of cardiac rehabilitation: identification of specific patient features requiring attention. Can J Cardiol. 2018;34:925–932. doi: 10.1016/j.cjca.2018.03.015. [DOI] [PubMed] [Google Scholar]
- 17.Mcconnell T.R., Clark B.A. Prediction of maximal oxygen consumption during handrail-supported treadmill exercise. J Cardiopulm Rehabil. 1987;7:324–331. [Google Scholar]
- 18.Stevens P.E. Evaluation and management of chronic kidney disease: synopsis of the kidney disease: Improving Global Outcomes 2012 Clinical Practice Guideline. Ann Intern Med. 2013;158:825. doi: 10.7326/0003-4819-158-11-201306040-00007. [DOI] [PubMed] [Google Scholar]
- 19.Tonelli M., Wiebe N., Fortin M. Methods for identifying 30 chronic conditions: application to administrative data. BMC Med Inform Decis Mak. 2015;15:31. doi: 10.1186/s12911-015-0155-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Austin P.C., Daly P.A., Tu J.V. A multicenter study of the coding accuracy of hospital discharge administrative data for patients admitted to cardiac care units in Ontario. Am Heart J. 2002;144:290–296. doi: 10.1067/mhj.2002.123839. [DOI] [PubMed] [Google Scholar]
- 21.Kokotailo R.A., Hill M.D. Coding of stroke and stroke risk factors using International Classification of Diseases, revisions 9 and 10. Stroke. 2005;36:1776–1781. doi: 10.1161/01.STR.0000174293.17959.a1. [DOI] [PubMed] [Google Scholar]
- 22.Thompson S., James M., Wiebe N. Cause of death in patients with reduced kidney function. J Am Soc Nephrol. 2015;26:2504–2511. doi: 10.1681/ASN.2014070714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Green D.J., Maiorana A., O’Driscoll G., Taylor R. Effect of exercise training on endothelium-derived nitric oxide function in humans. J Physiol. 2004;561:1–25. doi: 10.1113/jphysiol.2004.068197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lévesque L.E., Hanley J.A., Kezouh A., Suissa S. Problem of immortal time bias in cohort studies: example using statins for preventing progression of diabetes. BMJ. 2010;340:b5087. doi: 10.1136/bmj.b5087. [DOI] [PubMed] [Google Scholar]
- 25.Olmos A., Govindasamy P. A practical guide for using propensity score weighting in R. Practical Assessment, Research & Evaluation. 2015;20:1–7. http://pareonline.net/getvn.asp?v=20&n=13 Available at:
- 26.Rauch B., Davos C.H., Doherty P. The prognostic effect of cardiac rehabilitation in the era of acute revascularisation and statin therapy: a systematic review and meta-analysis of randomized and non-randomized studies – The Cardiac Rehabilitation Outcome Study (CROS) Eur J Prev Cardiol. 2016;23:1914–1939. doi: 10.1177/2047487316671181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Heywood J.T., Fonarow G.C., Costanzo M.R., Mathur V.S., Wigneswaran J.R., Wynne J. High prevalence of renal dysfunction and its impact on outcome in 118,465 patients hospitalized with acute decompensated heart failure: a report from the ADHERE database. J Card Fail. 2007;13:422–430. doi: 10.1016/j.cardfail.2007.03.011. [DOI] [PubMed] [Google Scholar]
- 28.Shlipak M.G., Fried L.F., Cushman M. Cardiovascular mortality risk in chronic kidney disease. JAMA. 2005;293:1737. doi: 10.1001/jama.293.14.1737. [DOI] [PubMed] [Google Scholar]
- 29.Ricardo A.C., Anderson C.A., Yang W. Healthy lifestyle and risk of kidney disease progression, atherosclerotic events, and death in CKD: findings from the Chronic Renal Insufficiency Cohort (CRIC) Study. Am J Kidney Dis. 2015;65:412–424. doi: 10.1053/j.ajkd.2014.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Muntner P., Hamm L.L., Kusek J.W., Chen J., Whelton P.K., He J. The prevalence of nontraditional risk factors for coronary heart disease in patients with chronic kidney disease. Ann Intern Med. 2004;140:9. doi: 10.7326/0003-4819-140-1-200401060-00006. [DOI] [PubMed] [Google Scholar]
- 31.Beddhu S., Baird B.C., Zitterkoph J., Neilson J., Greene T. physical activity and mortality in chronic kidney disease (NHANES III) Clin J Am Soc Nephrol. 2009;4:1901–1906. doi: 10.2215/CJN.01970309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kutner N.G., Zhang R., Huang Y., Herzog C.A. Cardiac rehabilitation and survival of dialysis patients after coronary bypass. J Am Soc Nephrol. 2006;17:1175–1180. doi: 10.1681/ASN.2005101027. [DOI] [PubMed] [Google Scholar]
- 33.Kottgen A., Russell S.D., Loehr L.R. Reduced kidney function as a risk factor for incident heart failure: the Atherosclerosis Risk in Communities (ARIC) study. J Am Soc Nephrol. 2007;18:1307–1315. doi: 10.1681/ASN.2006101159. [DOI] [PubMed] [Google Scholar]
- 34.Donald M., Kahlon B.K., Beanlands H. Self-management interventions for adults with chronic kidney disease: a scoping review. BMJ Open. 2018;8 doi: 10.1136/bmjopen-2017-019814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Aragam K.G., Moscucci M., Smith D.E. Trends and disparities in referral to cardiac rehabilitation after percutaneous coronary intervention. Am Heart J. 2011;161:544–551.e2. doi: 10.1016/j.ahj.2010.11.016. [DOI] [PubMed] [Google Scholar]
- 36.Brown T.M., Hernandez A.F., Bittner V. Predictors of cardiac rehabilitation referral in coronary artery disease patients. Findings from the American Heart Association’s Get With The Guidelines program. J Am Coll Cardiol. 2009;54:515–521. doi: 10.1016/j.jacc.2009.02.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chernomordik F., Sabbag A., Tzur B. Cardiac rehabilitation following an acute coronary syndrome: trends in referral, predictors and mortality outcome in a multicenter national registry between years 2006–2013: report from the Working Group on Cardiac Rehabilitation, the Israeli Heart Society. Eur J Prev Cardiol. 2017;24:123–132. doi: 10.1177/2047487316680692. [DOI] [PubMed] [Google Scholar]
- 38.Marzolini S., Blanchard C., Alter D.A., Grace S.L., Oh P.I. Delays in referral and enrollment are associated with mitigated benefits of cardiac rehabilitation after coronary artery bypass surgery. Circ Cardiovasc Qual Outcomes. 2015;8:608–620. doi: 10.1161/CIRCOUTCOMES.115.001751. [DOI] [PubMed] [Google Scholar]
- 39.Clark A.M., King-Shier K.M., Duncan A. Factors influencing referral to cardiac rehabilitation and secondary prevention programs: a systematic review. Eur J Prev Cardiol. 2013;20:692–700. doi: 10.1177/2047487312447846. [DOI] [PubMed] [Google Scholar]
- 40.Ades P.A., Keteyian S.J., Wright J.S. Increasing cardiac rehabilitation participation from 20% to 70%: a road map from the Million Hearts Cardiac Rehabilitation Collaborative. Mayo Clin Proc. 2017;92:234–242. doi: 10.1016/j.mayocp.2016.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pack Q.R., Mansour M., Barboza J.S. An early appointment to outpatient cardiac rehabilitation at hospital discharge improves attendance at orientation. Circulation. 2013;127:349–355. doi: 10.1161/CIRCULATIONAHA.112.121996. [DOI] [PubMed] [Google Scholar]
- 42.Davies P., Taylor F., Beswick A. Promoting patient uptake and adherence in cardiac rehabilitation. Cochrane Database Syst Rev. 2010;7:CD007131. doi: 10.1002/14651858.CD007131.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lau J.K., Anastasius M.O., Hyun K.K. Evidence-based care in a population with chronic kidney disease and acute coronary syndrome. Findings from the Australian Cooperative National Registry of Acute Coronary Care, Guideline Adherence and Clinical Events (CONCORDANCE) Am Heart J. 2015;170:566–572.e1. doi: 10.1016/j.ahj.2015.06.025. [DOI] [PubMed] [Google Scholar]
- 44.Brown J.R., Solomon R.J., Robey R.B. Chronic kidney disease progression and cardiovascular outcomes following cardiac catheterization-a population-controlled study. J Am Heart Assoc. 2016;5 doi: 10.1161/JAHA.116.003812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Myers J., Prakash M., Froelicher V., Do D., Partington S., Atwood J.E. Exercise capacity and mortality among men referred for exercise testing. N Engl J Med. 2002;346:793–801. doi: 10.1056/NEJMoa011858. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.