Abstract
Introduction
Glomerular filtration rate (GFR) is measured from the late plasma disappearance curve of an exogenous tracer, after correction for the early decay—corresponding to the distribution of the tracer—using various equations. These equations display the highest discrepancies in the GFR range above 90 ml/min per 1.73 m2, and their respective performances against a reference, urinary GFR measurement are unclear.
Methods
In patients with mGFR >90 ml/min per 1.73 m2 from 6 different cohorts, we compared GFR obtained from the plasma clearance of iohexol or 51Cr-ethylenediamine tetraacetic acid (EDTA), after correction using Chantler (C), Bröchner-Mortensen (BM), Fleming (F), Jodal-Bröchner-Mortensen (JBM), and Ng (N) equations, with urinary clearance of the same tracers or inulin.
Results
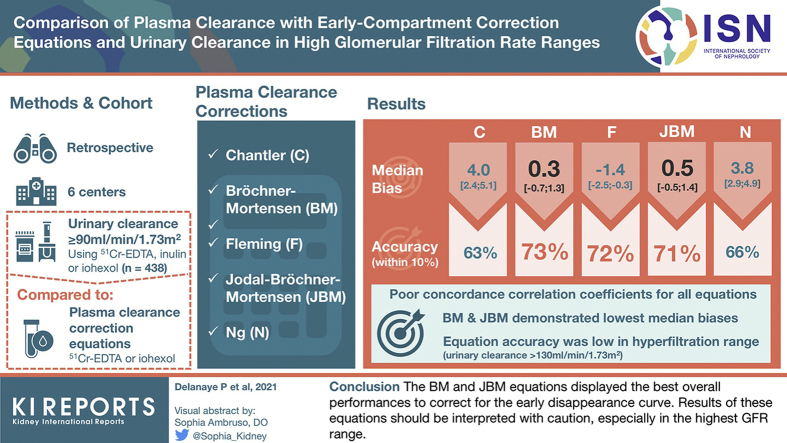
In 438 participants (median age 41 [39–42] years, 43% women), the median urinary clearance was 100.8 (94.7–112.6) ml/min per 1.73 m2. Plasma clearances using the correction equations were 105.7 (96.8–119.2), 102.4 (95.2–112.9), 100.7 (93.6–111.1), 102.6 (95.2–113.4), and 106.0 (98.2–117.6) ml/min per 1.73 m2 for C, BM, F, JBM, and N, respectively. Concordance correlation coefficients between plasma and urinary clearances were poor for all equations. Compared with urinary clearances, BM, F, and JBM displayed the best accuracy within 10% (73%, 72%, and 71%, respectively, vs. 63% and 66% for C and N), whereas BM and JBM had the lowest median biases. Accuracy of all equations was especially low in the hyperfiltration range (urinary clearance >130 ml/min per 1.73 m2).
Conclusion
The BM and JBM equations displayed the best overall performances to correct for the early disappearance curve. Results of these equations should be interpreted with caution, especially in the highest GFR range.
Keywords: 51Cr-EDTA, glomerular filtration rate, inulin, iohexol
Graphical abstract
Measuring glomerular filtration rate (GFR) remains indicated in specific conditions.1,2 Plasma clearance is routinely calculated from the plasma disappearance of an exogenous marker obtained during the renal excretion phase (the second or slow GFR compartment).3,4 Different equations are used to correct the slow compartment for the first or early compartment, which corresponds to the distribution of the marker in the body. Recently, in a large cohort of patients (n=5459) covering the whole GFR range (from 3 to 200 ml/min per 1.73 m2), we showed that all these equations (except the C equation) yielded very concordant results, at least in a GFR range below 90 ml/min per 1.73 m2.5 The goal of the present study was to compare GFR results obtained with the plasma clearances corrected by C,6 BM,7 F,8 JBM,9 and N10 equations (equations provided in Table 1) with urinary clearance in subjects with GFR greater than 90 ml/min per 1.73 m2, that is, in the range of GFR with the highest discrepancies when these different equations are used to correct plasma clearances.5
Table 1.
Different equations to correct for the absence of the early compartment
| Reference | Markers Used for the Development | Equation |
|---|---|---|
| Chantler6 | 51Cr-EDTA | GFR = 0.87 × C2 |
| Bröchner-Mortensen7 | 51Cr-EDTA | GFR = 0.990778 × C2 – 0.001218 C22 |
| Fleming8 | 99mTc-DTPA | GFR = C2/(1 + 0.0017 × C2) |
| Jodal-Bröchner-Mortensen9 | 51Cr-EDTA | GFR = C2/[1 + (0.00185 × BSA–0.3) × C2] |
| Ng10 | Iohexol | GFR = C2/(1 + 0.0012 × C2) |
BSA, body surface area; GFR, glomerular filtration rate.
C2 is the GFR result of the second compartment calculated with the slope-intercept method.
From 6 different cohorts, we collected results of measured GFR by urinary clearances over 90 ml/min per 1.73 m2 (inulin, iohexol, or 51Cr-EDTA) and compared results with plasma clearance (iohexol or 51Cr-EDTA) corrected with the different correction equations. Comparison of urinary and plasma clearances concerned 51Cr-EDTA/51Cr-EDTA in 2 cohorts, inulin/iohexol in 3 cohorts, and iohexol/iohexol in one.
Methods
We collected data on urinary clearances for 438 subjects, all with a GFR result ≥90 ml/min per 1.73 m2. Six different centers have collaborated on this project using various markers for plasma and urinary clearances, respectively: Paris (Bichat hospital), France (n = 315; 51Cr-EDTA/51Cr-EDTA); Créteil, France (n = 62; iohexol/iohexol); Lyon, France (n = 35; iohexol/inulin); Malmö, Sweden (n = 15; iohexol/inulin); Paris (Pompidou hospital), France (n = 7; 51Cr-EDTA/51Cr-EDTA); and Kingston, Canada (n = 4; iohexol/inulin). All centers are experts in GFR measurement and the data used here are from larger cohorts previously described.5,11, 12, 13, 14, 15, 16 Retrospective use of anonymous data was approved by the respective ethics committees.
All measurements were performed in the morning in fasting (or after a light protein-free breakfast) and resting conditions. All subjects were adults (≥18 years). Plasma clearances were calculated from concentrations obtained at 3 different time-points (120±15, 180±15 and 240±15 minutes after injection). Only adequate fit results were retained for further analysis (i.e., R2 ≥ 0.975). The “slow” GFR was calculated as the total dose of iohexol or 51Cr-EDTA injected, divided by the area under the curve (AUC) of the late concentration-time curve, calculated from the 1-compartment slope-intercept method. We then applied 5 different correction equations to compensate for the absence of the early compartment: C6, BM7, F8, JBM9, and N10. Indexing GFR before or after the correction for the early compartment is debatable, even if the impact on the results is relevant only in the extreme body mass index (BMI) range.17,18 Because body surface area indexation is considered in some models,9 we decided to index all results before applying the correction. Urinary clearances were performed using local protocols with the following urine collections: Paris-Bichat: six 30-minute clearance periods; Créteil: six 30-minute clearance periods; Lyon: three to four 30-minute clearance periods; Malmö: two 60-minute clearance periods; Paris-Pompidou: six 30-minute clearance periods; and Kingston: three 60-minute clearance periods. All urinary clearances were measured after a bolus injection of the reference marker, except in Lyon, Kingston and Malmö where inulin was continuously infused.11,13 The plasma clearance could be calculated from the same unique procedure when the same marker is considered (and injected in bolus). When a different marker was used in urinary and plasma clearances, both markers were injected at the same moment during the same procedure (see details in Dubourg et al.,11 Sterner et al., 13 and White et al.15).
Data are expressed as mean ± standard deviation (SD) when distribution was normal and as median with interquartile range (IQR) when not. Normality was assessed by the Shapiro-Wilk test. We calculated Lin’s concordance correlation coefficient between plasma clearance corrected for the different equations and urinary clearance.19 Bias (systematic difference) was calculated as the median difference between plasma clearance corrected for the different equations and urinary clearance. We considered plasma clearances as unbiased when the bias was not different from zero (when the 95% confidence interval included zero). Imprecision (random error) was calculated as the IQR of the bias. Results were also plotted using Bland and Altman analysis (with bias as the mean difference between plasma clearance corrected for the different equations and urinary clearances).20 We calculated accuracies (as the percentage of relative difference) within 20%, 10%, and 5% between urinary and plasma methods and we compared them using the McNemar test.21 Imprecision was compared between equations according to the Pitman test for comparing variances of correlated samples. Because we considered 5 equations, we proposed to use P < 0.05/6 = 0.008 to claim statistical significance according to Bonferroni correction for multiple testing.
Results
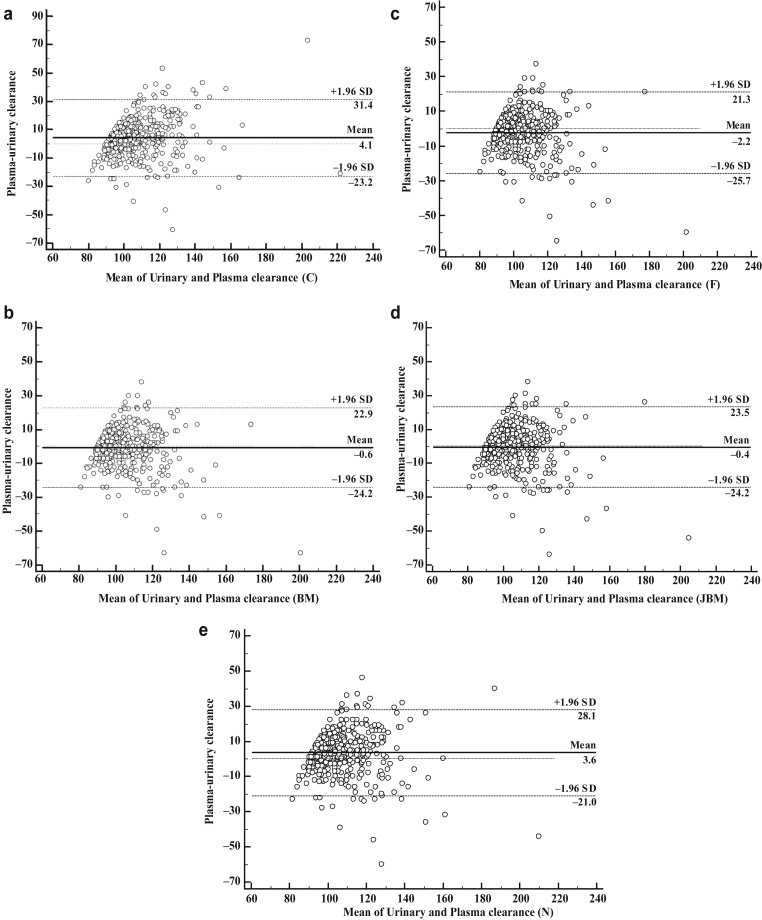
Among the 438 participants, median age was 41 (32–49) years and 42.9% were women. Mean height and median body weight were 171±9 cm and 73.5 (64–83) kg, respectively. Mean BMI and mean body surface area were 25 (22–28) and 1.88±0.22 m2, respectively. Median mGFR measured by urinary clearance was 100.8 (94.7–112.6) ml/min per 1.73 m2. Median plasma clearances with C, BM, F, JBM, and N corrections were 105.7 (96.8–119.2), 102.4 (95.2–112.9), 100.7 (93.6–111.1), 102.6 (95.2–113.4), and 106.0 (98.2–117.6) ml/min per 1.73 m2, respectively. Bland and Altman plots are shown in Figure 1. Lin concordance correlation coefficient, bias, and precision are reported in Table 2 in the whole population. Concordance correlation coefficients showed poor agreement between plasma clearance and urinary clearance for all 5 equations. Regarding bias, the BM and JBM equations showed no bias with the urinary clearance, whereas F underestimated and C and N overestimated urinary clearances. Imprecision (variance of the bias) was higher for C compared with the other equations (P < 0.0001). Imprecision was also higher for N compared with BM, F, and JBM (P < 0.0001). Accuracy within 5% was poorer for C compared with BM, F, JBM, and N (P = 0.0001, P = 0.0003, P < 0.0001, and P = 0.0351, respectively), and for N compared to BM, F, and JBM (P = 0.0167, 0.0070, and 0.0164, respectively). Correcting for multiple testing, only the difference between C-BM, C-F, C-JBM, and F-N remained significant. All other accuracies within 5% were similar. Accuracy within 10% was significantly lower for C compared with BM, F, JBM, and N (P < 0.0001, P = 0.0001, P < 0.0001, and P = 0.0066, respectively), and for N compared with BM, F, and JBM (P < 0.0001, P = 0.0049, and P = 0.0065, respectively). All other accuracies within 10% were similar. Accuracy within 20% was similar for all equations. Analyses according to marker used and patient’s characteristics (subgroup of GFR range, age, gender, and BMI) are shown in Tables 3 and 4. The best accuracy between plasma and urinary clearances was found when 51Cr-EDTA was used in the plasma and urinary clearances. Precision was also better when the same marker was considered in both plasma and urinary clearances (iohexol/iohexol and even more 51Cr-EDTA/51Cr-EDTA) (Table 3). Accuracies observed in most subgroup analyses according to GFR levels, gender, BMI, and age were globally similar to accuracies observed in the whole population (with the same conclusion: the performance of N and C equations was slightly poorer). No difference of accuracy was observed between men and women (all P values >0.008). Accuracies were poorer in subjects with hyperfiltration (GFR > 130 ml/min per 1.73 m2) and in low BMI (<20) (Table 4). In subjects with hyperfiltration, there is a trend for a better accuracy within 5% for the C equation compared with the BM and F equations (P = 0.0391 and 0.0215, respectively) and for the N equation compared with BM and F equations (P = 0.0313 and 0.0156, respectively). In subjects with low BMI, all equations had the same poor performances.
Figure 1.
Bland and Altman analyses between urinary clearances and plasma clearances with different correction equations: (a) C: Chantler, (b) BM: Bröchner-Mortensen, (c) F: Fleming, (d) JBM: Jodal- Bröchner-Mortensen, (e) N: Ng. All results are in milliliters per minute per 1.73 m2. SD, standard deviation.
Table 2.
Performance of plasma clearances with different equations correcting the slow GFR for the early compartment compared to urinary clearances
| Whole Cohort (n=438) | CCC (95% CI) | Median Bias (95% CI) (ml/min per 1.73 m2) |
IQR of the Bias (ml/min per 1.73 m2) | Accuracy Within 5% (%) | Accuracy Within 10% (%) | Accuracy Within 20% (%) |
|---|---|---|---|---|---|---|
| C | 0.6584 (0.6163–0.7182) | 4.0 (2.4–5.1) | 14.4 | 34 | 63 | 91 |
| BM | 0.6704 (0.6163–0.7182) | 0.3 (–0.7 to 1.3) | 12.0 | 43 | 73 | 93 |
| F | 0.6683 (0.6142–0.7162) | –1.4 (–2.5 to –0.3) | 11.9 | 44 | 72 | 94 |
| JBM | 0.6753 (0.6212–0.7229) | 0.5 (–0.5 to 1.4) | 12.3 | 43 | 71 | 92 |
| N | 0.6651 (0.6108–0.7133) | 3.8 (2.9–4.9) | 12.7 | 37 | 66 | 93 |
BM, Bröchner-Mortensen; C, Chantler; CCC, concordance correlation coefficient; CI, confidence interval; IQR, interquartile range; F, Fleming; JBM, Jodal-Bröchner-Mortensen; N, Ng.
Table 3.
Performance of plasma clearances with different equations correcting the slow GFR for the early compartment compared to urinary clearances in subgroups according to reference markers
| CCC (95% CI) | Median Bias (95% CI) (ml/min per 1.73 m2) |
IQR (ml/min per 1.73 m2) | Accuracy Within 5% (%) | Accuracy Within 10% (%) | Accuracy Within 20% (%) | |
|---|---|---|---|---|---|---|
| 51Cr-EDTA/ 51Cr-EDTA (n = 322) | ||||||
| C | 0.6925 (0.6354–0.7421) | 3.3 (1.9 to 5.0) | 12.9 | 38 | 70 | 94 |
| BM | 0.7245 (0.6684–0.7724) | 0.2 (–1.0 to 1.3) | 10.3 | 48 | 80 | 96 |
| F | 0.7169 (0.6603–0.7655) | –1.5 (–2.6 to –0.4) | 10.3 | 50 | 78 | 97 |
| JBM | 0.7253 (0.6692–0.7732) | –0.02 (–1.2 to 0.8) | 10.6 | 48 | 78 | 96 |
| N | 0.7032 (0.6456–0.7529) | 3.7 (2.6–4.9) | 11.3 | 41 | 73 | 96 |
| Iohexol/inulin (n = 54) | ||||||
| C | 0.6203 (0.4394–0.7529) | –2.7 (–9.0 to 3.0) | 19.0 | 33 | 52 | 81 |
| BM | 0.5724 (0.3917–0.7107) | –7.3 (–14.0 to –3.1) | 19.4 | 35 | 56 | 78 |
| F | 0.5536 (0.3724–0.6941) | –8.9 (–15.4 to –4.8) | 19.3 | 30 | 50 | 78 |
| JBM | 0.5728 (0.3873–0.7137) | –7.2 (–14.2 to –2.5) | 19.8 | 37 | 54 | 78 |
| N | 0.6286 (0.4435–0.7622) | –3.5 (–10.9 to 1.6) | 19.1 | 39 | 54 | 85 |
| Iohexol/iohexol (n = 62) | ||||||
| C | 0.5946 (0.4444–0.7122) | 13.6 (9.1–17.8) | 17.6 | 13 | 35 | 79 |
| BM | 0.6207 (0.4633–0.7401) | 8.7 (5.1–11.6) | 11.3 | 24 | 53 | 90 |
| F | 0.6568 (0.5039–0.7698) | 7.1 (3.7–10.0) | 11.4 | 24 | 58 | 94 |
| JBM | 0.6448 (0.4917–0.7592) | 9.0 (5.6–12.0) | 11.8 | 23 | 52 | 87 |
| N | 0.5884 (0.4336–0.7095) | 12.9 (9.6–16.1) | 13.3 | 15 | 37 | 82 |
BM, Bröchner-Mortensen; C, Chantler; CCC, concordance correlation coefficient; CI, confidence interval; F, Fleming; IQR, interquartile range; JBM, Jodal-Bröchner-Mortensen; N, Ng.
Table 4.
Accuracy (5%/10%) by equation according to different subgroups defined by GFR levels, gender, BMI, and age
| C | BM | F | JBM | N | |
|---|---|---|---|---|---|
| GFR (ml/min per 1.73 m2) | |||||
| 90–130 (n=406) | 34/63 | 46/76 | 47/75 | 46/75 | 38/67 |
| 90–100 (n=207) | 37/67 | 47/79 | 51/80 | 48/77 | 39/68 |
| 100–110 (n=103) | 34/64 | 46/76 | 46/74 | 45/76 | 38/71 |
| 110–120 (n=66) | 32/61 | 44/76 | 41/74 | 44/73 | 39/67 |
| 120–130 (n=30) | 20/43 | 37/52 | 10/53 | 37/57 | 23/53 |
| >130 (n=32) | 28/53 | 6/34 | 3/25 | 13/28 | 25/45 |
| Gender | |||||
| Men (n=250) | 37/66 | 45/75 | 44/73 | 46/73 | 41/68 |
| Women (n=188) | 30/57 | 40/70 | 43/70 | 40/69 | 31/62 |
| BMI (kg/m²) | |||||
| ≤20 (n=31) | 19/42 | 23/55 | 26/48 | 29/55 | 19/52 |
| 20–30 (n=346) | 35/65 | 43/75 | 45/75 | 44/74 | 38/68 |
| ≥30 (n=61) | 34/61 | 49/67 | 46/67 | 46/66 | 36/62 |
| Age (yr) | |||||
| <40 (n=203) | 31/57 | 36/68 | 39/68 | 37/67 | 33/58 |
| ≥40 (n=235) | 37/68 | 48/77 | 48/75 | 49/75 | 40/73 |
BM, Bröchner-Mortensen; BMI, body mass index; C, Chantler; F, Fleming; GFR, glomerular filtration rate; JBM, Jodal-Bröchner-Mortensen; N, Ng.
All results are expressed in %.
Discussion
Concordance between the BM, F, JBM, and N equations to correct the slow compartment is very high. Subanalyses, however, revealed that the concordance was less impressive at high GFR levels.5,22 In the current analysis using urinary clearances over 90 ml/min per 1.73 m2 as the reference, we showed that plasma clearance corrected by the BM and JBM equations was unbiased compared with urinary clearances, whereas the F equation underestimated and the C and N equations overestimated urinary clearances. Imprecision was lowest for BM, F and JBM (and imprecision of C higher than N). Also, accuracy was slightly poorer for the N and C equations. Several elements of the study findings warrant further discussion. Overall, the accuracy of corrected plasma clearances compared with urinary clearances was lower than the concordance we previously described between the corrected plasma clearances.5 This is not unexpected. Imprecision in plasma clearances might be due to the analytical imprecision of the measurements of the marker (such an analytical imprecision being theoretically higher in high GFR ranges where the concentrations of the marker are particularly low at the last time point).23 These imprecisions are similar when plasma clearances with different correction equations are compared between themselves but are higher when compared to a totally different methodology, like urinary clearances. When comparing plasma to urinary clearance as the reference, errors in the reference method should be considered as well, notably because of imprecision of urine collections, even in expert centers as those who participated in the current study. This being said, concordance between urinary and plasma clearances in the present analysis is comparable to that reported in previous similar studies with smaller samples.11,22,24,25 The correcting equations have been developed in populations with few data higher than 90 ml/min per 1.73 m2. The lower accuracy of correcting models in the high GFR range was suggested by Fleming.26 However, in the current analysis, we do not confirm an added value of neither the F nor JBM equation compared with the BM equation to correct the slow GFR compartment in high GFR ranges.9 Concordance between plasma and urinary clearances might also be poorer in patients with increased volume of distribution (cirrhosis, edema, etc.) who were not excluded from the analysis.27
Subanalysis according to the reference marker used in plasma and urinary clearances was performed. Imprecision of plasma clearances compared to urinary clearances was lower when iohexol, and even more when 51Cr-EDTA, were used both in plasma and urinary protocols in comparison to iohexol plasma clearances compared to inulin urinary clearances. This once again is not unexpected, as the physiological pattern of the marker can impact the results. Also, we observed a significant bias between urinary and plasma clearances of iohexol. This bias has already been reported in previous studies, but the underlying mechanisms remain not fully elucidated.14,25 The current study, however, was not designed to study the potential superiority of one marker over another. The sample sizes of the marker subgroups were too different to make any valuable conclusion. Other studies comparing reference markers with the same methodology (plasma or urinary clearance) are still necessary to improve the standardization of measured GFR.3,15,28,29 Accuracy of all plasma clearances was also lower in the specific group of patients with low BMI, which could be explained by the fact that these equations were developed in populations with standard weight and height. In the same way, accuracy of all plasma clearances were lower in hyperfiltrating subjects (GFR > 130 ml/min per 1.73 m2). In this specific group, the N and C equations could be slightly better, even if their global performance remains limited.11 The results in these 2 subgroups (low BMI and GFR >130 ml/min per 1.73 m2) must be carefully interpreted because of the limited sample (n=31 and 32, respectively). Another limitation of the current study is that the analysis is limited to adults.
In conclusion, among the equations used to correct plasma clearances for the early compartment, the BM and JBM equations are unbiased. In the very high GFR range, results of these correction equations should be interpreted with caution.
Acknowledgments
We thank all the nurses and colleagues who helped in the GFR procedures or calculations.
Appendix
PD, MF, LD, FG, CW, EC, MC, and HP are members of the European Kidney Function Consortium
Disclosure
All the authors declared no competing interests.
References
- 1.Agarwal R., Delanaye P. Glomerular filtration rate: when to measure and in which patients? Nephrol Dial Transplant. 2019;34:2001–2007. doi: 10.1093/ndt/gfy363. [DOI] [PubMed] [Google Scholar]
- 2.Delanaye P., Melsom T., Ebert N. Iohexol plasma clearance for measuring glomerular filtration rate in clinical practice and research: a review. Part 2: Why to measure glomerular filtration rate with iohexol? Clin Kidney J. 2016;9:700–704. doi: 10.1093/ckj/sfw071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Delanaye P., Ebert N., Melsom T. Iohexol plasma clearance for measuring glomerular filtration rate in clinical practice and research: a review. Part 1: How to measure glomerular filtration rate with iohexol ? Clin Kidney J. 2016;9:682–699. doi: 10.1093/ckj/sfw070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Soveri I., Berg U.B., Björk J. Measuring GFR: a systematic review. Am J Kidney Dis. 2014;64:411–424. doi: 10.1053/j.ajkd.2014.04.010. [DOI] [PubMed] [Google Scholar]
- 5.Delanaye P., Dubourg L., Flamant M. Comparison of early-compartment correction equations for GFR measurements. Kidney Int Rep. 2020;5:1079–1081. doi: 10.1016/j.ekir.2020.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chantler C., Barratt T.M. Estimation of glomerular filtration rate from plasma clearance of 51-chromium edetic acid. Arch Dis Child. 1972;47:613–617. doi: 10.1136/adc.47.254.613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brochner-Mortensen J. A simple method for the determination of glomerular filtration rate. Scand J Clin Lab Invest. 1972;30:271–274. doi: 10.3109/00365517209084290. [DOI] [PubMed] [Google Scholar]
- 8.Fleming J.S., Zivanovic M.A., Blake G.M. Guidelines for the measurement of glomerular filtration rate using plasma sampling. Nucl Med Commun. 2004;25:759–769. doi: 10.1097/01.mnm.0000136715.71820.4a. [DOI] [PubMed] [Google Scholar]
- 9.Jodal L., Brochner-Mortensen J., Jodal L. Reassessment of a classical single injection 51Cr-EDTA clearance method for determination of renal function in children and adults. Part I: Analytically correct relationship between total and one-pool clearance. Scand J Clin Lab Invest. 2009;69:305–313. doi: 10.1080/00365510802566882. [DOI] [PubMed] [Google Scholar]
- 10.Ng D.K., Schwartz G.J., Jacobson L.P. Universal GFR determination based on two time points during plasma iohexol disappearance. Kidney Int. 2011;80:423–430. doi: 10.1038/ki.2011.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dubourg L., Lemoine S., Joannard B. Comparison of iohexol plasma clearance formulas vs. inulin urinary clearance for measuring glomerular filtration rate. Clin Chem Lab Med. 2020;59:571–579. doi: 10.1515/cclm-2020-0770. [DOI] [PubMed] [Google Scholar]
- 12.Flamant M., Vidal-Petiot E., Metzger M. Performance of GFR estimating equations in African Europeans: basis for a lower race-ethnicity factor than in African Americans. Am J Kidney Dis. 2013;62:182–184. doi: 10.1053/j.ajkd.2013.03.015. [DOI] [PubMed] [Google Scholar]
- 13.Sterner G., Frennby B., Mansson S. Determining “true” glomerular filtration rate in healthy adults using infusion of inulin and comparing it with values obtained using other clearance techniques or prediction equations. Scand J Urol Nephrol. 2008;42:278–285. doi: 10.1080/00365590701701806. [DOI] [PubMed] [Google Scholar]
- 14.Stehlé T., El Karoui K., Sakka M. Creatinine clearance after cimetidine administration in a new short procedure: comparison with plasma and renal clearances of iohexol. Clin Kidney J. 2020;13:587–596. doi: 10.1093/ckj/sfz087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.White C.A., Akbari A., Allen C. Simultaneous glomerular filtration rate determination using inulin, iohexol and 99mTc-DTPA demonstrates the need for customized measurement protocols. Kidney Int. 2021;99:957–966. doi: 10.1016/j.kint.2020.06.044. [DOI] [PubMed] [Google Scholar]
- 16.Rouanne M., Gaillard F., Meunier M. Measured glomerular filtration rate (GFR) significantly and rapidly decreases after radical cystectomy for bladder cancer. Sci Rep. 2020;10:16145. doi: 10.1038/s41598-020-73191-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pottel H., Hoste L., De Waele L. Measuring glomerular filtration rate using 51Cr-EDTA: body surface area normalization before or after Bröchner-Mortensen correction? Nucl Med Commun. 2014;35:1150–1155. doi: 10.1097/MNM.0000000000000186. [DOI] [PubMed] [Google Scholar]
- 18.Blake G.M., Barnfield M.C., Burniston M.T. Measuring glomerular filtration rate using chromium-51 EDTA: body surface area normalization before or after Brøchner-Mortensen correction? Nucl Med Commun. 2015;36:295–300. doi: 10.1097/MNM.0000000000000253. [DOI] [PubMed] [Google Scholar]
- 19.Lin L. A concordance correlation coefficient to evaluate reproducibility. Biometrics. 1989;45:255–268. [PubMed] [Google Scholar]
- 20.Bland J.M., Altman D.G. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet. 1986;327:307–310. [PubMed] [Google Scholar]
- 21.Delanaye P., Flamant M., Dubourg L. Single- versus multiple-sample method to measure glomerular filtration rate. Nephrol Dial Transplant. 2018;33:1778–1785. doi: 10.1093/ndt/gfx345. [DOI] [PubMed] [Google Scholar]
- 22.Stehlé T., El Karoui K., Audard V. Comparison of iohexol plasma clearances calculated from 5 early-compartment correction equations with urinary clearance of iohexol. Kidney Int Rep. 2020;5:1842–1843. doi: 10.1016/j.ekir.2020.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cavalier E., Rozet E., Dubois N. Performance of iohexol determination in serum and urine by HPLC: validation, risk and uncertainty assessment. Clin Chim Acta. 2008;396:80–85. doi: 10.1016/j.cca.2008.07.011. [DOI] [PubMed] [Google Scholar]
- 24.Agarwal R. Ambulatory GFR measurement with cold iothalamate in adults with chronic kidney disease. Am J Kidney Dis. 2003;41:752–759. doi: 10.1016/s0272-6386(03)00022-2. [DOI] [PubMed] [Google Scholar]
- 25.Stolz A., Hoizey G., Toupance O. Evaluation of sample bias for measuring plasma iohexol clearance in kidney transplantation. Transplantation. 2010;89:440–445. doi: 10.1097/TP.0b013e3181ca7d1b. [DOI] [PubMed] [Google Scholar]
- 26.Fleming J.S. An improved equation for correcting slope-intercept measurements of glomerular filtration rate for the single exponential approximation. Nucl Med Commun. 2007;28:315–320. doi: 10.1097/MNM.0b013e328014a14a. [DOI] [PubMed] [Google Scholar]
- 27.Skluzacek P.A., Szewc R.G., Nolan C.R., III Prediction of GFR in liver transplant candidates. Am J Kidney Dis. 2003;42:1169–1176. doi: 10.1053/j.ajkd.2003.08.017. [DOI] [PubMed] [Google Scholar]
- 28.Brandstrom E., Grzegorczyk A., Jacobsson L. GFR measurement with iohexol and 51Cr-EDTA. A comparison of the two favoured GFR markers in Europe. Nephrol Dial Transplant. 1998;13:1176–1182. doi: 10.1093/ndt/13.5.1176. [DOI] [PubMed] [Google Scholar]
- 29.Delanaye P., Jouret F., Le Goff C. Concordance between iothalamate and iohexol plasma clearance. Am J Kidney Dis. 2016;68:329–330. doi: 10.1053/j.ajkd.2016.01.007. [DOI] [PubMed] [Google Scholar]