Abstract
Introduction
Atypical hemolytic uremic syndrome (aHUS) is mainly due to complement regulatory gene abnormalities with a dominant pattern but incomplete penetrance. Thus, healthy carriers can be identified in any family of aHUS patients, but it is unpredictable if they will eventually develop aHUS.
Methods
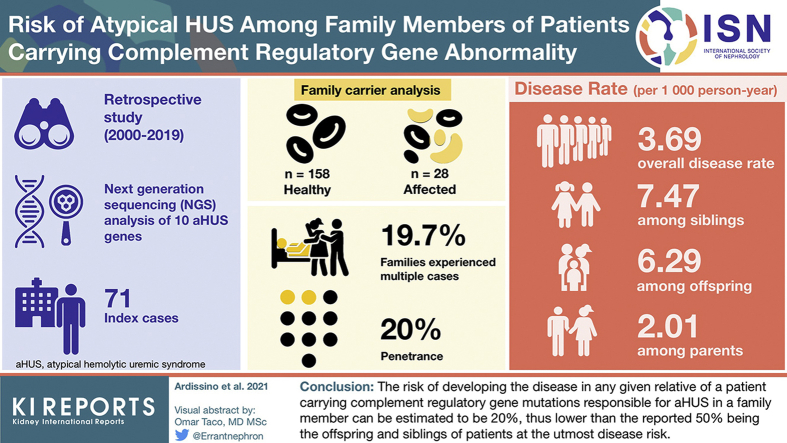
Patients are screened for 10 complement regulatory gene abnormalities and once a genetic alteration is identified, the search is extended to at-risk family members. The present cohort study includes 257 subjects from 71 families: 99 aHUS patients (71 index cases + 28 affected family members) and 158 healthy relatives with a documented complement gene abnormality.
Results
Fourteen families (19.7%) experienced multiple cases. Over a cumulative observation period of 7595 person-years, only 28 family members carrying gene mutations experienced aHUS (overall penetrance of 20%), leading to a disease rate of 3.69 events for 1000 person-years. The disease rate was 7.47 per 1000 person-years among siblings, 6.29 among offspring, 2.01 among parents, 1.84 among carriers of variants of uncertain significance, and 4.43 among carriers of causative variants.
Conclusions
The penetrance of aHUS seems a lot lower than previously reported. Moreover, the disease risk is higher in carriers of causative variants and is not equally distributed among generations: siblings and the offspring of patients have a much greater disease risk than parents. However, risk calculation may depend on variant classification that could change over time.
Keywords: aHUS, complement genes, penetrance
Graphical abstract
aHUS is a rare but severe thrombotic microangiopathy characterized by platelet consumption, hemolysis, and renal damage with a high risk of permanent sequelae and death.1 The disease is often caused by mutations in complement regulatory genes that dysregulate the complement system, ultimately leading to endothelial damage.2,3 Since 2009, eculizumab, a humanized recombinant monoclonal antibody targeted to the complement component 5 (C5), has been successfully used in the treatment of patients with aHUS, with striking positive outcomes.4, 5, 6, 7 The genetic mutations responsible for the disease clearly have a dominant inheritance (heterozygous subjects can exhibit the disease) but with incomplete penetrance; thus, only some of the carriers actually develop aHUS.8,9 Given the genetic origin of the disease, a number of healthy carriers at theoretical risk can be identified in almost any family of aHUS patients. However, it is not clear which, when, or why healthy carriers will eventually manifest the disease.
Any time a case of aHUS related to complement regulatory gene abnormality is diagnosed, the involved family will raise the issue of the risk for relatives to develop the same disease and, so far, the physicians can only provide evasive answers to the anxiety of involved subjects, because a clear prediction and quantification of the risk is not possible. The scanty information available in the literature reports a disease penetrance of 50%, but this clearly clashes with daily experience, where the observed penetrance in relatives of patients is much lower.10, 11, 12
Here, we present a single-center, observational study based on patients referred to our center aimed to provide an estimate of the risk of developing aHUS in family members carrying a complement regulatory gene mutation that has already been responsible for aHUS in the same family.
Methods
Patients: Inclusion and Exclusion Criteria
The present study is an observational, retrospective, cohort study. Patients provided their consent for the genetic screening and for the use of the results for research purposes. The study was approved by our institution’s review board.
All patients with aHUS (platelet consumption, hemolysis, and renal damage in the setting of negative Shiga toxin in the stools and ADAMTS13 >10%) diagnosed at or referred to our center during the past 2 decades (2000–2019) were considered for the analysis. All patients were screened for known genetic causes of aHUS: complement regulatory genes (C3, CFB, CFH, and related genes, CFI, CD46), THBD, and DGKE genes. In addition, anti-CFH autoantibodies and homocysteine were also determined together with specific diagnostic laboratory tests, whenever relevant (Coombs test, T antigen, urine pneumococcal antigen, and HIV serology). Patients who developed aHUS following bone marrow or solid organ transplantation, autoimmune diseases, malignancies, and multiple sclerosis but did not have any complement abnormalities (secondary aHUS) were excluded, as were cases associated with pneumonia or abnormal cobalamin metabolism. Established diagnoses of HELLP syndrome were also excluded.
For each case identified, an accurate family history was collected, and the pedigree of the family was built with the aim of identifying any potential previous cases of aHUS, in order to distinguish between sporadic and familial cases. Familial aHUS was defined by the presence of affected patients in at least 2 members of the same family, with diagnoses at least 6 months apart. Obligate carriers, subjects who must carry the gene abnormality, based on the analysis of the family history and pedigree, were also considered in the final analysis. At-risk family members were defined as family members of index patients carrying the same complement gene abnormality.
Finally, patients with a de novo mutation in complement regulatory genes or those without any identified genetic abnormality (idiopathic) were also excluded, as were those without any family member carrying the same mutation (either because the test was not performed or because all tested family members were negative).
Once identified, carriers of complement regulatory gene abnormalities were provided with genetic counseling and a written document explaining, in details, the clinical role of the abnormality, the signs and symptoms of aHUS, the actions needed to rule in or out the disease in case of any suspicion, as well as the most appropriate management in case the disease was triggered. All carriers are regularly followed up to enable early identification of those who may, eventually, develop the disease.
Laboratory Investigations
Genomic DNA was extracted from total whole peripheral blood, on an automated QIAsymphonySP platform (Qiagen-GmbH, Hilden, Germany). Nucleotide variations were detected by Next Generation Sequencing using a HaloPlex custom panel (Agilent Technologies, Santa Clara, CA, USA) designed to target complement regulatory genes known to be involved in aHUS (C3, CFB, CFH, CFHR1, CFHR3, CFHR5, CFI, CD46), THBD, and DGKE according to KDIGO guidelines.13 Single libraries were then pooled and run on a MiSeq platform (Illumina Inc, San Diego, CA, USA) with MiSeq reagent kit V2, to obtain a minimum 100X coverage. Bioinformatic analysis of NGS data for the detection of germline single-nucleotide variants and small insertion-deletions and filtering were performed with SureCall application. SAMTools was used to recalibrate the base call quality scores, perform local realignment, index the reads for improved performance, and then identify variants. The annotation of the identified variations was performed with ANNOVAR tool. Identified variants were classified according to ACMG guidelines.14 In detail, ExAC frequency (https://gnomad.broadinstitute.org/), aHUS database (https://www.complement-db.org/),15 bioinformatic tools such as Varsome (https://varsome.com/) and Franklin (https://franklin.genoox.com/), and literature reports were considered. Furthermore, a variant was pathogenic if clear evidence of pathogenicity was found: reported as such in aHUS database or proved in functional studies. Those with discordant information were indicated as variant of unknown significance. Variants with minor allele frequency <0.1% were considered relevant for the pathogenesis of aHUS. Sanger sequencing was used to confirm all clinically significant variants identified in index cases, and to evaluate their segregation in family members willing to be tested. Macro deletions and large genomic rearrangements (LGRs), such as CFH/CFH-related hybrid genes, were identified by multiplex ligation-dependent probe amplification (MRC Holland, Amsterdam).
The data analysis was restricted to complement gene abnormalities; thus, cases associated with THBD or DGKE (characterized by a recessive pattern of inheritance) mutations were not included, as well as those with CFH-related genes that were all considered as noncausative.
Statistical Analysis
Given the genetic nature of the condition and that we reconstructed the clinical history for all family components, the disease-free cumulative observation period (COP) was computed considering carriers at risk of developing aHUS since birth. The described approach increased the COP from the original, prospective follow-up of 589 person-years (mean 3.2 years) to 7595 person-years (mean 40.1 years). We calculated incidence rates of aHUS (per 1000 person-years) and 95% confidence intervals (CIs), stratified by demographic and genetic variables. In calculating person-years, calendar year and age were treated as a time-dependent variable. Statistical analysis was performed with Stata, version 16 (StataCorp, College Station, TX).
Results
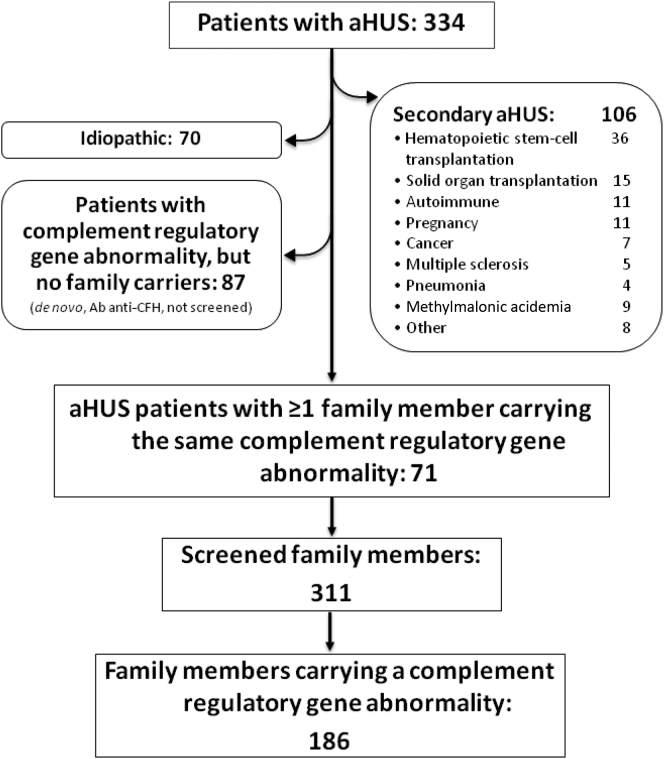
A total of 334 patients with aHUS were diagnosed at or referred to our center over the past 2 decades. The flowchart showing criteria for patient selection is shown in Figure 1. After excluding secondary cases (n = 106), a complement regulatory gene abnormality was identified in 158 subjects (47.3%). In the remaining 70 patients (20.9%), aHUS was related neither to specific conditions nor to documented complement abnormalities (idiopathic). In a stepwise model, we further excluded patients for whom the index case was the only identified carrier in the family, either because no one else in the family had been tested (n = 85) or no one else was found positive (de novo mutation, n = 2). By means of the described exclusion process, 71 families were identified with at least 1 carrier of complement regulatory gene abnormality besides the index case (14.1% [n = 10] C3; 1.4% [n = 1] CFB; and 38% [n = 27] involving CFH gene and related large genomic rearrangement (LGR; 9.9% [n = 7] CFI; 22.5% [n = 16] CD46, and 14.1% [n = 10] with variants involving multiple genes Supplementary Table S1). The screening of the 71 identified families for the specific gene abnormality identified in the respective index case led to the analysis of 311 at-risk healthy family members (Table 1), of which 186 (60%), including obligate carriers, tested positive (median age at the time of the analysis: 45.0 years; interquartile range: 22.0–58.3). Of the 186 family members, 28 subjects had developed the disease (median age at presentation: 27.0 years; interquartile range: 5.5–48.0). Considering the total number of affected subjects (71 index cases and 28 affected relatives, n = 99), 63 identified carriers were parents of aHUS patients, 59 siblings, 38 offspring, and 26 were other-degree relatives.
Figure 1.
Process for patient selection. Schematic representation of the stepwise selection that led to the identification of the 71 aHUS families. Ab anti-CFH, anti–complement factor H autoantibody; aHUS, atypical hemolytic uremic syndrome.
Table 1.
Family members of the 99 patients with aHUS divided in at-risk subjects, tested and found positive to complement gene abnormalities
| Relatives | At risk, n | Tested, n (%) | Positive, n (% of tested) |
|---|---|---|---|
| Parents | 144 | 116 (80.5) | 63 (54.3) |
| Siblings | 103 | 79 (76.7) | 59 (74.6) |
| Offspring | 46 | 45 (97.8) | 38 (84.4) |
| Other | —a | 71 | 26 (36.6) |
| Total | 311 | 186 (59.8) |
aHUS, atypical hemolytic uremic syndrome.
Undeterminable.
Table 2 reports the estimated disease rate in relatives of the index cases carrying the same complement gene abnormality by gender, age, class, relationship with index case, gene abnormality, disease recurrence in the family, and presence of triggers in the index case. The overall disease rate was 3.69 per 1000 person-years. Once stratified by age, the highest disease rates were found in carriers before age 10 years (6.24 per 1000 person-years) and in adults aged 60 years and older (9.17 per 1000 person-years). Furthermore, the 63 parents of index cases showed a lower risk of developing the disease (2.01 per 1000 person-years) than the offspring (6.29) and siblings, who had the utmost risk (7.47 per 1000 person-years). The other-degree relatives (uncles/aunts and cousins of index cases), although carriers of the same gene abnormality, exhibited the lowest disease rate (0.75 per 1000 person-years). No correlation was observed between the age at presentation of index cases and that of their relatives carrying the same gene abnormality. The screening of family members led to the identification of as many as 92 carriers of CFH gene variants and related LGR, 31 of CFI, 32 of CD46, 25 of C3, 3 of CFB, and 3 of multiple gene variants (Supplementary Table S1). Compared with the overall rate, carriers of C3 gene variants exhibited a higher risk (6.44 per 1000 person-years), whereas those carrying CFI and CD46 gene variants seem to be relatively less affected from the possibility of developing the disease. The genetic analysis identified 33 pathogenic (P)/likely pathogenic (LP) variants and 26 variants of unknown significance (Supplementary Table S1). As expected, the carriers of P/LP variants had a significantly higher incidence rate (4.43 vs. 1.84 per 1000 person-years) than those carrying variants of unknown significance. Fourteen of the 71 analyzed families (19.7%) experienced multiple cases (familial aHUS), including 7 families with more than 3 cases (up to a maximum of 6 cases in a single family). The rate of aHUS among carriers in families with multiple cases (n >2) was the highest among those estimated in the present study, and almost 4 times the overall incidence rate (15.8 per 1000 persons-years). Finally, we explored the possible role of triggers of aHUS in index cases for estimating the risk of recurrence among family members carrying the same complement abnormality. When the disease was associated, in the index case, with severe illness or important conditions (n = 27/71) such as myocardial infarction, inflammatory bowel disease, bloody diarrhea, delivery, or abortion, the risk of recurrence in relatives was lower compared with that observed in relatives of patients in whom no trigger was identified, with an event rate of 0.45 versus 5.03 per 1000 person-years, respectively.
Table 2.
Incidence rate of aHUS in family members carrying the same complement regulatory gene abnormality of index cases by selected variables
| Variable | Carriers, n | aHUS Cases Among Carriers, n | COP, person-years | Rate, per 1000 person-years (95% CI) |
|---|---|---|---|---|
| All | 186 | 28 | 7595 | 3.69 (2.45–5.33) |
| Gender | ||||
| Female | 94 | 13 | 4013 | 3.24 (1.72–5.54) |
| Male | 92 | 15 | 3582 | 4.19 (2.34–6.91) |
| Age category (yr) | ||||
| 0–9.9 | 23 | 11 | 1764 | 6.24 (3.11–11.2) |
| 10–19.9 | 20 | 1 | 1510 | 0.66 (0.02–3.69) |
| 20–29.9 | 24 | 5 | 1288 | 3.88 (1.26–9.06) |
| 30–39.9 | 16 | 3 | 1084 | 2.77 (0.57–8.09) |
| 40–49.9 | 32 | 2 | 849 | 2.36 (0.29–8.51) |
| 50–59.9 | 27 | 1 | 555 | 1.80 (0.05–10.0) |
| >60 | 44 | 5 | 545 | 9.17 (2.98–21.4) |
| Relationship with index case | ||||
| Parent | 63 | 7 | 3487 | 2.01 (0.81–4.14) |
| Sibling | 59 | 16 | 2143 | 7.47 (4.27–12.1) |
| Offspring | 38 | 4 | 636 | 6.29 (1.71–16.1) |
| Other | 26 | 1 | 1329 | 0.75 (0.02–4.1) |
| Complement abnormality (gene) | ||||
| C3 | 25 | 6 | 931 | 6.44 (2.37–14.0) |
| CFB | 3 | 0 | 118 | 0.00 (0.00–31.3) |
| CFH | 92 | 19 | 3890 | 4.88 (2.94–7.63) |
| CFI | 31 | 1 | 1196 | 0.84 (0.02–4.66) |
| CD46 | 32 | 2 | 1304 | 1.53 (0.19–5.54) |
| Multiple | 3 | 0 | 156 | 0.00 (0.00–23.6) |
| Complement abnormality (classification) | ||||
| VUS | 55 | 4 | 2179 | 1.84 (0.50–4.70) |
| P/LP | 131 | 24 | 5416 | 4.43 (2.84–6.59) |
| Disease recurrence in the family (>2 cases) | ||||
| Yes | 40 | 21 | 1327 | 15.8 (9.80–24.22) |
| No | 146 | 7 | 6268 | 1.12 (0.45–2.30) |
| Severe trigger in the index case | ||||
| Yes | 59 | 1 | 2222 | 0.45 (0.01–2.50) |
| No | 127 | 27 | 5373 | 5.03 (3.32–7.32) |
aHUS, atypical hemolytic uremic syndrome; CI, confidential interval; COP, cumulative observation period; P/LP, pathogenic/likely pathogenic; VUS, variant of unknown significance.
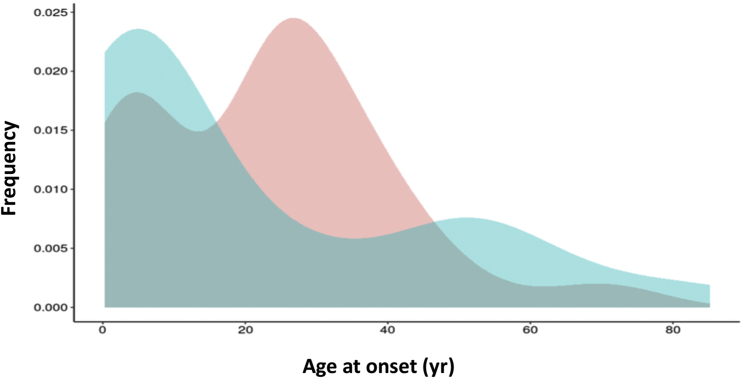
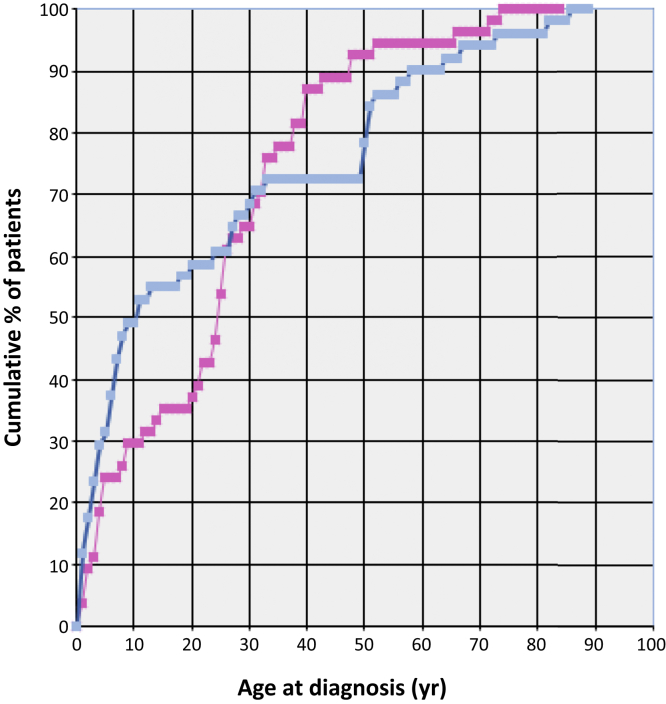
Figure 2 shows the distribution of age at disease onset of affected individuals by gender from which the cumulative relative incidence by age at onset was derived for predicting the life risk based on the age of subjects at the time of analysis. The risk was not different between genders (Table 2) but it was differently distributed during life, with a steeper decrease of the risk in males during the first 2 decades, whereas in females it remained high for an additional decade (Figure 3). However, in both genders, by the age of 30 years only 30% of the risk remained.
Figure 2.
Age at presentation of aHUS by gender. Estimate of the distribution of the cumulative relative incidence by age (Gaussian kernel density) of aHUS onset of affected cases by gender. The frequency of cases is shown on the y-axis, and the age at onset is shown on the x-axis. The distribution of age at diagnosis of males is shown in blue, whereas that of females is shown in pink. n = 99, including index cases. aHUS, atypical hemolytic uremic syndrome.
Figure 3.
Cumulative distribution of the relative incidence by age at presentation of aHUS in males and females. The percentage of cases that developed the disease is shown on the y-axis, and the age at disease onset is shown on the x-axis. The distribution of males is shown in blue and that of females in pink. n = 99, including index cases. aHUS, atypical hemolytic uremic syndrome.
Discussion
Over the past 2 decades, studies on thrombotic microangiopathies, and specifically on aHUS, have greatly increased our understanding of these rare, but severe, conditions. In particular, the identification of genetic variants in complement regulatory genes has been defined as one of the mechanisms whereby complement dysregulation leads to the vast majority of aHUS cases.16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27 The better understanding of the pathophysiology of these diseases has provided the rationale for the use of C5 inhibition as the first-line treatment for aHUS with significant improvements in patient care and outcomes.28, 29, 30, 31, 32, 33, 34, 35 The inheritance pattern of aHUS can be considered dominant, and whenever a patient is diagnosed, several family members carrying the same gene abnormality can be identified. Given the incomplete penetrance of the disease, any prediction regarding the risk of healthy family members to develop aHUS remains difficult, being based on little evidence. In the literature, a penetrance of 50% for aHUS is repeatedly reported; however, this clearly seems to overestimate the actual observed penetrance.8,9
We decided to analyze and share our data on the screening of families of aHUS patients in order to provide some reliable clues for predicting the disease risk of any healthy family member carrying a complement regulatory gene abnormality that has already been responsible for aHUS in the same family. Assuming a life expectancy of the general population of 83 years, given the median age at presentation of disease manifestation in carriers of 27.0 years, the median age of included carriers at the time of the analysis of 45.0 years, and the cumulative relative incidence by age at disease onset (Figure 3), the overall risk of developing aHUS during the entire life of family members carrying the same variant responsible for the disease in index cases was estimated to be 20%, much lower than the risk currently proposed by the literature.
Abnormalities in the C3 gene were associated with an increased (>2-fold) risk of disease, whereas CFI and CD46 gene mutations, besides being responsible for a less severe disease, are also less likely to recur in family members. The most interesting and original contribution of the present analysis is that the risk in family members differs according to the relationship with the index case: parents of the index cases have a 3-fold lower risk compared to offspring, whereas siblings of the index cases exhibit the highest risk. Other-degree relatives exhibit the lowest risk (<6 times lower compared with siblings). We speculate that this finding is due to the greater genetic homology between siblings and children of patients and that it represents indirect evidence that a second hit (perhaps introduced in the family by noncarrier partners) is necessary for disease expression, because siblings have the highest homology with index cases (they share the same parents; thus they have an increased possibility of receiving the same genetic conditions received by the index cases themselves). The evaluation of any single carrier for pathogenic complement regulatory gene abnormality requires age to be also taken into consideration: the risk is not equally distributed throughout life and carriers, by the age of 30 years, have lost 70% of the entire risk. On the contrary, younger children will obviously have a higher risk because most of it will still be ahead of them, as clearly shown in Table 2. Finally, in carriers from families with multiple cases, the risk of developing the disease is 4-fold higher than the general risk.
The present analysis, although unique, has several limitations. First, the relatively short cumulative prospective follow-up (less than 600 person-years), which required historical cases to be included, and the observation period was extended backward assuming that the risk begins with the date of birth. We cannot exclude the possibility that some of the relatives, who had aHUS in the past, might have gone unidentified in the retrospective data collection, leading to a possible underestimation of the true rate of recurrence in families. Nevertheless, the severity of aHUS makes this option, although possible, unlikely at least during the last few decades. Another limitation is that some of the potential carriers (almost 20% among the first-degree relatives) have not been screened because they were not interested or not available. Missed healthy carriers surely caused an overestimation of the true rate of recurrence. Moreover, a detailed analysis of the risk should have been performed gene by gene rather than pooling all carriers together; however, the relatively small number of involved subjects did not allow us to break down the population by the 5 involved genes or to perform a detailed statistical analysis for the reported findings. Finally, the genetic picture is incomplete as the study has focused on only 10 complement genes in a disease where rare genetic variants in other complement genes or in genes in other pathways are likely to contribute to the phenotype. Furthermore, variant classification is likely to change over time affecting disease rate estimation. The problem of small numbers particularly affects the risk estimation related to certain genes such as CFB and C3. We strongly encourage other researchers to extend our study and possibly contribute to better define the risk of carriers by means of larger population studies and/or exome analysis.
In conclusion, the risk of developing the disease in any given relative of a patient carrying complement regulatory gene mutations responsible for aHUS in a family member can be estimated to be 20%, thus lower than the reported 50%. This information, which is missing in the current literature, can be very important for patient’s relatives who understandably wonder whether they or their children may also develop this severe life-threatening disease. Although the penetrance is not as high as previously reported, in our opinion the severity of the condition may justify the screening of relatives for the specific mutation responsible for the disease in their family. The test being relatively inexpensive, we tend to screen subjects based on their willingness to be aware of their specific risk (particularly in case of mutations involving C3 or CFH genes in the index case, multiple cases in the same family, young brothers and siblings of the index case, and no clear trigger in the index case). The awareness of the risk can be very important in specific settings (peripartum or in case of severe triggering diseases or major surgeries) where the timing of treatment may be crucial for a better outcome or even for surviving this life-threatening disease.
Disclosure
All the authors declared no competing interests.
Acknowledgments
We are thankful to “Progetto Alice ONLUS. Associazione per la Lotta alla SEU” for their continuous and precious support.
We also want to thank the following physicians (members of the ItalKId-HUS Network) for their dedication to our and their patients and for their precious collaboration and commitment toward our center that has been essential to perform the present analysis: Andrea Airoldi (Novara), Karen Amar (Cernusco SN), Andrea Artoni (Milano), Alice Atzeni (Cagliari), Bruno Basolo (Torino), Teresa Bencivenga (Aversa), Maria Ester Bernardo (Milano), Elena Bezze (Alessandria), Maurizio Brigante (Campobasso), Alessandro Bucalossi (Siena), Valeria Calbi (Milano), Aldo Casani (Massa Carrara), Alessandro Castiglioni (Busto Arsizio), Francesco Catalano (Reggio Calabria), Luigia Costantini (Vercelli), Calogero Cirami (Firenze), Nicola Cassata (Palermo), Giacomo Colussi (Milano), Silvia Consolo (Milano), Ciro Corrado (Palermo), Simone Cortazzi (Asti), Raffaella Cravero (Biella), Olga Credendino (Napoli), Marco D’Amico (Como), Vincenzo De Biase (Verona), Delia Davoli (Modena), Lucia Del Vecchio (Lecco), Chiara De Philippis (Milano), Roberta Fenoglio (Torino), Lucrezia Furian (Padova), Andrea Galassi (Milano), Giovanni Gambaro (Verona), Paolo Giannattasio (Napoli), Fabio Giglio (Milano), Mario Giordano (Bari), Gina Gregorini (Brescia), Cristina Grimoldi (Firenze), Francesco Iannuzzella (Reggio Emilia), Alessandro Inzoli (Cremona), Andrea Mancini ù+(Bari), Maria Cristina Mancuso (Milano), Silvio Maringhini (Palermo), Jacopo Mariotti (Milano), Marco Martini (Arezzo), Alessandra Messuerotti (Torino), Sabrina Milan Manani (Vicenza), Concetta Micalizzi (Genova), Cristina Milocco (Monfalcone), Alessandro Naticchia (Roma), Maria Neunhauserer (Brunico), Francesco Onida (Milano), Fabio Paglialonga (Milano), Giuseppe Palladino (Salerno), Antonello Pani (Cagliari), Werner Passler (Bolzano), Jacopo Peccatori (Milano), Valentina Pellu (Aosta), Federico Pieruzzi (Monza), Lucia Pisano (Desio), Gian Marco Podda (Milano), Vera Polaschi (Milano), Ilaria Possenti (Alessandria), Leonardo Potenza (Modena), Federica Ravera (Milano), Roberto Rona (Monza), Attilio Rovelli (Monza), Patrizia Rovere Querini (Milano), Domenico Russo (Napoli), Rodolfo Russo (Genova), Chiara Salviani (Brescia), Paola Lucia Serbelloni (Vimercate), Giuseppe Seminara (Catania), Giacomo Simonetti (Bellinzona), Danio Somenzi (Reggio Emilia), Tiziana Stellato (Monza), Sara Testa (Milano), Aristide Torre (Nocera I.), Chiara Trenti (Reggio Emilia), Silvia Trisolini (Roma), Simona Verdesca (Milano), Marta Verna (Monza), Giuseppe Visconti (Palermo), and Marco Zecca (Pavia).
Author Contributions
GA, SL, LP, GP, BS,VC, DC, GL, FT, SS, MS, LM, EMR, NBG, GM, MS, MC, FC, DC, and ST contributed to the design of the study, to the analysis of the results, and to the writing of the manuscript. All authors read and approved the final version of the manuscript.
Footnotes
Table S1. Complement gene abnormalities identified in index cases as well as in affected and healthy carriers with their frequency, pathogenicity, and classification.
STROBE Statement.
Supplementary Material
Table S1. Complement gene abnormalities identified in index cases as well as in affected and healthy carriers with their frequency, pathogenicity, and classification.
STROBE Statement.
References
- 1.Durkan A.M., Kim S., Craig J., Elliott E. The long-term outcomes of atypical hemolytic uremic syndrome: a national surveillance study. Arch Dis Child. 2016;101:387–391. doi: 10.1136/archdischild-2015-309471. [DOI] [PubMed] [Google Scholar]
- 2.Fakhouri F., Zuber J., Fremeux-Bacchi Haemolytic uremic syndrome. Lancet. 2017;390:681–696. doi: 10.1016/S0140-6736(17)30062-4. [DOI] [PubMed] [Google Scholar]
- 3.Noris M., Remuzzi G. Overview of complement activation and regulation. Semin Nephrol. 2013;33:479–492. doi: 10.1016/j.semnephrol.2013.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fakhouri F., Hourmant M., Campistol J.M. Terminal complement inhibitor eculizumab in adult patients with atypical hemolytic uremic syndrome: a single-arm, open-label trial. Am J Kidney Dis. 2016;68:84–93. doi: 10.1053/j.ajkd.2015.12.034. [DOI] [PubMed] [Google Scholar]
- 5.Fakhouri F., Hourmant M., Campistol J.M. Eculizumab inhibits thrombotic microangiopathy and improves renal function in adult atypical hemolytic uremic syndrome patients: 1 year update. J Am Soc Nephrol. 2014;25:751. [Google Scholar]
- 6.Greenbaum L.A., Fila M., Ardissino G. Eculizumab is a safe and effective treatment in pediatric patients with atypical hemolytic uremic syndrome. Kidney Int. 2016;89:701:11. doi: 10.1016/j.kint.2015.11.026. [DOI] [PubMed] [Google Scholar]
- 7.Fakhouri F., Delmas Y., Provot F. Insights from the use in clinical practice of eculizumab in adult patients with atypical hemolytic uremic syndrome affecting the native kidneys: an analysis of 19 cases. Am J Kidney Dis. 2014;63:40–48. doi: 10.1053/j.ajkd.2013.07.011. [DOI] [PubMed] [Google Scholar]
- 8.De Cordoba S.R. aHUS: a disorder with many risk factors. Blood. 2010;115:158–160. doi: 10.1182/blood-2009-11-252627. [DOI] [PubMed] [Google Scholar]
- 9.Bu F., Borsa N., Ardissino G. Familial atypical hemolytic uremic syndrome: a review of its genetic and clinical aspects. Clin Dev Immunol. 2012;2012:370426. doi: 10.1155/2012/370426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Noris M., Bresin E., Mele C. GeneReviews® [Internet] University of Washington, Seattle; 1993–2021; Seattle, WA: 2007 Nov 16. Genetic atypical hemolytic-uremic syndrome. (updated 2016 Jun 9) [Google Scholar]
- 11.Noris M., Caprioli J., Bresin E. Relative role of genetic complement abnormalities in sporadic and familial aHUS and their impact on clinical phenotype. Clin J Am Soc Nephrol. 2010;5:1844–1859. doi: 10.2215/CJN.02210310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sullivan M., Erlic Z., Hoffmann M.M. Epidemiological approach to identifying genetic predispositions for atypical hemolytic uremic syndrome. Ann Hum Genet. 2010;74:17–26. doi: 10.1111/j.1469-1809.2009.00554.x. [DOI] [PubMed] [Google Scholar]
- 13.Goodship T.H.J., Cook T., Fakhouri F. Atypical hemolytic uremic syndrome and C3 glomerulopathy: conclusions from a "Kidney Disease: Improving Global Outcomes" (KDIGO) Controversies Conference. Kidney Int. 2017;91:539–551. doi: 10.1016/j.kint.2016.10.005. [DOI] [PubMed] [Google Scholar]
- 14.Sue R., Aziz N., Bale S. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med. 2015;17:405–424. doi: 10.1038/gim.2015.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Osborne A.J., Breno M., Ghiringhelli Borsa N. Statistical validation of rare complement variants provides insights into the molecular basis of atypical hemolytic uremic syndrome and C3 glomerulopathy. J Immunol. 2018;1(200):2464–2478. doi: 10.4049/jimmunol.1701695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fremeaux-Bacchi V., Fakhouri F., Garnier A. Genetics and outcome of atypical hemolytic uremic syndrome: a nationwide French series comparing children and adults. Clin J Am Soc Nephrol. 2013;8:554–562. doi: 10.2215/CJN.04760512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sanchez-Corral P., Perez-Caballero D., Huarte O. Structural and functional characterization of factor H mutations associated with atypical hemolytic uremic syndrome. Am J Hum Genet. 2002;71:1285–1295. doi: 10.1086/344515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Atkinson J.P., Liszewwski M.K., Richards A. Hemolytic uremic syndrome: an example of insufficient complement regulation on self-tissue. Ann N Y Acad Sci. 2005;1056:144–152. doi: 10.1196/annals.1352.032. [DOI] [PubMed] [Google Scholar]
- 19.Goicoechea de Jorge E., Harris C.L., Esparza-Gordillo J. Gain-of-function mutations in complement factor B are associated with atypical hemolytic uremic syndrome. Proc Natl Acad Sci U S A. 2007;104:240–245. doi: 10.1073/pnas.0603420103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fremeaux-Bacchi V., Miller E.C., Liszewski M.K. Mutations in complement C3 predispose to development of atypical hemolytic uremic syndrome. Blood. 2008;112:4948–4952. doi: 10.1182/blood-2008-01-133702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rodriguez de Cordoba S., Hidalgo M.S., Pinto S. Genetics of atypical hemolytic uremic syndrome. Semin Thromb Hemost. 2014;40:422–430. doi: 10.1055/s-0034-1375296. [DOI] [PubMed] [Google Scholar]
- 22.Abarrategui-Garrido C., Martinez-Barricarte R., Lopez-Trascasa M. Characterization of complement factor H-related (CFHR1) proteins in plasma reveals novel genetic variations of CFHR1 associated with atypical hemolytic uremic syndrome. Blood. 2009;114:4261–4271. doi: 10.1182/blood-2009-05-223834. [DOI] [PubMed] [Google Scholar]
- 23.Eyler S.J., Meyer N.C., Zhang Y. A novel hybrid CFHR1/CFH gene causes atypical hemolytic uremic syndrome. Pediatr Nephrol. 2013;28:2221–2225. doi: 10.1007/s00467-013-2560-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jozsi M., Strobel S., Dahse H.M. Anti-factor H autoantibodies block C-terminal recognition function of factor H in hemolytic uremic syndrome. Blood. 2007;110:1516–1518. doi: 10.1182/blood-2007-02-071472. [DOI] [PubMed] [Google Scholar]
- 25.Lemaire M., Fremeaux-Bacchi V., Schaefer F. Recessive mutations in DGKE cause atypical hemolytic uremic syndrome. Nat Genet. 2013;45:531–536. doi: 10.1038/ng.2590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Delvaeye M., Noris M., De Vriese A. Thrombomodulin mutations in atypical hemolytic uremic syndrome. N Eng J Med. 2009;361:345–357. doi: 10.1056/NEJMoa0810739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bu F., Maga T., Meyer N.C. Comprehensive genetic analysis of complement and coagulation genes in atypical hemolytic uremic syndrome. J Am Soc Nephrol. 2014;25:55–64. doi: 10.1681/ASN.2013050453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Loirat C., Fakhouri F., Ariceta G. An international consensus approach to the management of atypical hemolytic uremic syndrome in children. Pediatri Nephrol. 2016;31:15–39. doi: 10.1007/s00467-015-3076-8. [DOI] [PubMed] [Google Scholar]
- 29.Cugno M., Gualtierotti R., Possenti I. Complement functional tests for monitoring eculizumab treatment in patients with atypical hemolytic uremic syndrome. J Thromb Haemost. 2014;12:1440:48. doi: 10.1111/jth.12615. [DOI] [PubMed] [Google Scholar]
- 30.Noris M., Galbusera M., Gastoldi S. Dynamics of complement activation in aHUS and how to monitor eculizumab therapy. Blood. 2014;124:171525. doi: 10.1182/blood-2014-02-558296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ardissino G., Testa S., Possenti I. Discontinuation of eculizumab maintenance treatment for atypical hemolytic uremic syndrome: a report of 10 cases. Am J Kidney Dis. 2014;64:633–637. doi: 10.1053/j.ajkd.2014.01.434. [DOI] [PubMed] [Google Scholar]
- 32.Ardissino G., Possenti I., Tel F. Discontinuation of eculizumab treatment in atypical hemolytic syndrome: an update. Am J Kidney Dis. 2015;66:172–173. doi: 10.1053/j.ajkd.2015.04.010. [DOI] [PubMed] [Google Scholar]
- 33.Sheerin N.S., Kavanagh D., Goodship T.H. A national specialized service in England for atypical hemolytic uremic syndrome—the first year’s-experience. QJM. 2016;109:27–33. doi: 10.1093/qjmed/hcv082. [DOI] [PubMed] [Google Scholar]
- 34.Wetzels J.F., van de Kar N.C. Discontinuation of eculizumab maintenance treatment for atypical hemolytic uremic syndrome. Am J Kidney Dis. 2015;65:342. doi: 10.1053/j.ajkd.2014.04.039. [DOI] [PubMed] [Google Scholar]
- 35.Fakhouri F., Fila M., Provot F. Pathogenic variants in complement genes and risk of atypical hemolytic uremic syndrome relapse after eculizumab discontinuation. Clin J Am Soc Nephrol. 2017;12:50–59. doi: 10.2215/CJN.06440616. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.