Abstract
Introduction
Despite much research on chronic kidney disease of uncertain etiology (CKDu) in Sri Lanka and the Mesoamerican nephropathy, the etiology and pathogenesis of this disease remains elusive. The pathology has broadly been described as chronic tubulointerstitial nephritis and no specific signature lesions have been identified.
Methods
A scoping review was conducted through MEDLINE and Google Scholar databases for peer-reviewed publications on biopsy studies related to CKDu – Sri Lanka and Mesoamerican nephropathy to develop a comparative and critical analysis of the renal pathology found in these patients.
Results
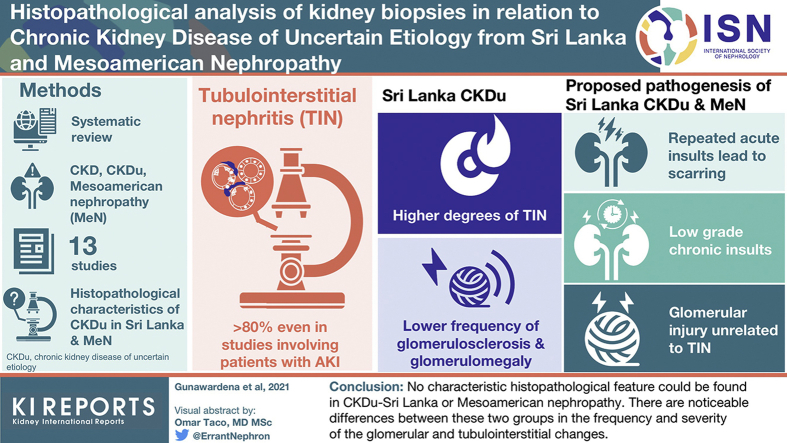
Thirteen studies met the selection criteria. Interstitial fibrosis was the predominant lesion in all the studies. Tubulointerstitial and glomerular abnormalities showed a more variable distribution. No characteristic histopathological feature was reported other than a proximal tubular lysosomal inclusion body which was claimed to indicate a toxic etiology. Three main pathogenetic mechanisms were postulated: repeated acute insults leading to scarring, low-grade chronic insults leading to non-inflammatory fibrosis, and tubulointerstitial damage in combination with glomerular injury. The main limitations in the interpretation and comparative analysis of these studies were the heterogeneity in case selection and biopsy reporting.
Conclusions
Although no characteristic histopathological feature could be found in CKDu–Sri Lanka or Mesoamerican nephropathy, there are noticeable differences between these two groups in the frequency and severity of the glomerular and tubulointerstitial changes which warrant more explorative studies preferably on kidneys in early stages of the disease. Future strategies should ensure that more uniform selection criteria and reporting methods are used.
Key words: chronic kidney disease of uncertain etiology, Mesoamerican nephropathy, renal biopsy, tubulointerstitial nephritis
Graphical abstract
Chronic kidney disease (CKD) continues to show an increasing global burden in terms of mortality and disability-adjusted life years,1 and it is believed that this trend is likely to aggravate over the next few decades due to the expanding aging population.2 There is a high concentration of CKD cases within middle- and lower-income countries,2, 3, 4, 5 of which a considerable proportion do not have a clearly identifiable aetiology.6,7 CKD without an identifiable cause, known as CKDu, has been reported from time to time from different regions around the world. The itai-itai disease in Japan and the Balkan endemic nephropathy were such regional epidemics of CKDu where the etiologies were identified much later. Recent years have seen at least five other regions being affected by CKDu in epidemic proportions.8,9 Of these regions, much attention has been given to Sri Lanka and Central America where, despite research and discussion spanning more than 2 decades, the cause for the nephropathy remains a mystery.
In both regions, the earliest reports on the emergence of the disease have been in the late 1990s and early 2000s. In Sri Lanka, a higher incidence of CKD was noticed in the districts of Anuradhapura and Polonnaruwa of the North Central Province which could not be attributed to common causes such as diabetes, hypertension, glomerulonephritis, snake bites or obstructive uropathies.10, 11, 12, 13 Subsequently, cases were also detected from some parts of North Central, Central, and Uva provinces. The age of onset was noticeably lower than other conventional forms of CKD and there was apparent clustering of the disease among rural paddy farming communities.11,12,14 At around the same time, several countries in Central America also reported high rates of CKD, mostly among individuals involved in agricultural work.15, 16, 17, 18 Here too, the age of onset was relatively young and no identifiable cause could be found. The nephropathy was localized to the Mesoamerican region with a similar disease pattern in all the affected countries and therefore was termed Mesoamerican nephropathy.18 Nicaragua and El Salvador were two of the most affected countries, and workers on sugarcane plantations were found to be at highest risk.15,19,20
Sri Lanka and countries in Central America are severely burdened by the high cost in managing these patients, particularly when they require long-term dialysis.21 In 2017, the incidence of CKDu in Anuradhapura and Polonnaruwa was reported as 0.29% and 0.41%, respectively.12 Recent estimates from El Salvador showed the CKD prevalence as 12.8%,22 whereas in Nicaragua, a follow-up study of healthy young adults showed that almost 10% of males and 3.4% of females developed a marked decline in their kidney functions over a period of 2 years.23 Although numerous hypotheses have been investigated as the cause for this disease, none have found a definite association with any single biological, agrochemical, or hydrogeochemical etiological factor.9,24,25 Similarly, to date, no clinical or histopathological feature has been found to be diagnostic of CKDu in Sri Lanka or the Mesoamerican nephropathy. It is still unclear if the cause for the disease in both these regions is the same or if they are different entities and the lack of an unequivocal etiopathogenesis has been a major hindrance to effective management of these patients. To enhance scientific understanding of the pathological processes and ultrastructural changes related to this debilitating disease, this paper provides a scoping review of all published studies that have conducted histopathological examination of renal biopsy specimens from patients affected by CKDu in Sri Lanka and the Mesoamerican nephropathy.
Methods
Search Strategy
Medline database was searched in June 2020 using the following search terms (ckd OR ckdu OR ckd unknown OR chronic kidney disease OR Mesoamerican nephropathy) AND (pathology OR biopsy) AND (Sri Lanka OR Mesoamerica OR Central America). The search was not time restricted. Only published articles in peer reviewed journals in the English language were considered. At the time of developing this article, the International Prospective Register of Systematic Reviews did not accept applications for registration of scoping reviews.
Study Selection
Abstracts were reviewed for suitability and relevance by two investigators (SG and HW). Both investigators reviewed the full papers and screened the cited references to ensure that all the relevant studies were included. Studies that had not performed light microscopy or did not include histopathological findings were excluded.
Data Extraction
All four authors then extracted data from each study in relation to the year of publication and study period, the aim of the study, the study setting or geographic locations of the study subjects, the number of biopsy specimens analyzed, the staining methods, the histopathological features including the frequency distributions according to histopathological severity, and the main conclusions. Each study was then analyzed for their strengths and limitations in relation to case selection, sample number, study design, and methods used for histopathological analysis. Wherever necessary, data were also extracted from the supplementary files.
Results
Search Results and Studies Selected
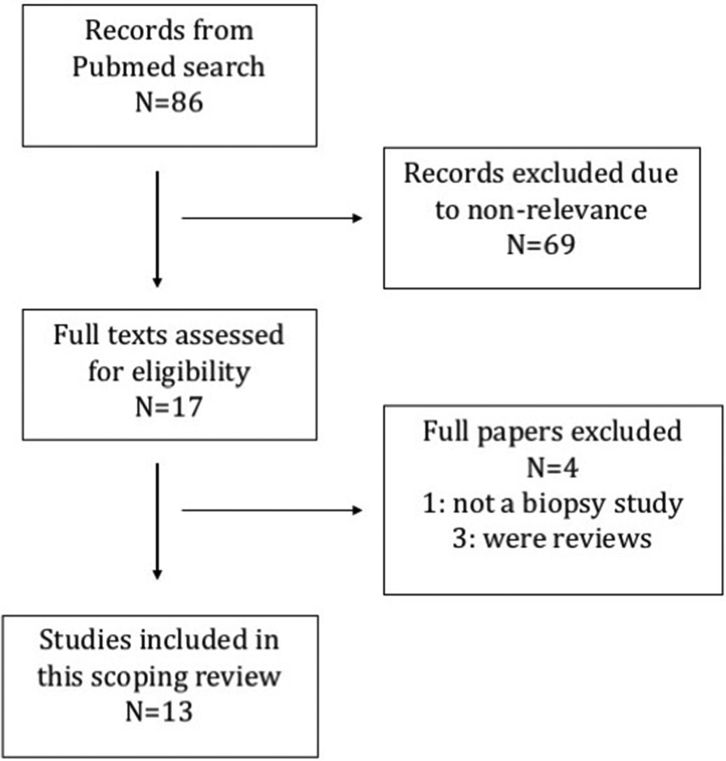
The search resulted in 86 publications of which 17 were selected based on the abstracts. (Figure 1) After reviewing the full papers, 4 were excluded as they did not fall within the selection criteria. Review of the cited references in these full papers did not reveal any further studies. A total of 13 papers were chosen. Eight had studied histopathological features in relation to CKDu in Sri Lanka,26, 27, 28, 29, 30, 31, 32, 33 4 were in relation to Mesoamerican nephropathy,34, 35, 36, 37 and 1 paper included study cohorts from both regions.38
Figure 1.
PRISMA flow diagram.
Characteristics of Selected Studies
Study Design and Sample Population
Characteristics of each of the studies are summarized in Supplementary Table S1. Two studies, both from Sri Lanka, were based on histology of previously collected kidney biopsy samples28,29 whereas others were based on biopsy specimens of newly diagnosed patients selected either through population screening26 or from patients presenting/referred to hospitals.27,30, 31, 32, 33, 34, 35, 36, 37 The most recent study followed an experimental approach to explore a lysosomal lesion in the proximal tubules and included patients from Sri Lanka and El Salvador.38
All the Sri Lankan cohorts were reported to be from endemic areas, although the basis on how endemicity was determined was not clear. Two studies specified the division or town of residency26,29 whereas others mentioned the province or the referring hospital. The majority of patients were from the North Central province, except for one study where the patients were predominantly from the Central province.33 Sample sizes in the Sri Lankan cohorts ranged from 11 to 251. Among the Mesoamerican cohorts, three were from El Salvador34,35,38 and two from Nicaragua,36,37 with sample sizes ranging from 8 to 46 patients. The patients were predominantly from sugar cane plantations except in one study35 which screened nonagricultural communities as well.
Criteria for Case Selection
Majority of the Sri Lankan CKD patients were selected based on their residency within endemic areas and the absence of a clinically detectable cause. Wijetunge et al.29 selected only the biopsy specimens that had primary interstitial renal disease on histology. Similarly, Anand et al.33 used the presence or absence of primary tubulointerstitial kidney disease on biopsy to divide patients into CKDu and non-CKDu groups. Case selection from population screening in Sri Lanka was initially based on positive dipstick proteinuria whereas, with the exception of one study,37 in all the Mesoamerican biopsy studies proteinuria was specified as an exclusion criterion. Wijkström et al.36 highlighted this different approach to proteinuria as a limitation when comparing studies between the two regions.
There were two studies30,37 which selected acutely ill patients from Sri Lanka and Nicaragua, respectively. The latter had leukocyturia and leukocytosis in their inclusion criteria and excluded patients who were older than 39 years of age, whereas the Sri Lankan study had no age restriction.
In some Sri Lankan studies, immunofluorescence was used to exclude immune complex–mediated glomerular diseases when diagnosing cases with CKDu and immunofluorescence-positive biopsy specimens were excluded from further analysis.28,29 Anand et al.33 placed patients with immunofluorescence positivity within the non-CKDu group. In contrast, the Mesoamerican studies did not use immunofluorescence positivity as a criterion for exclusion.
Methods Used for Histopathological Reporting
An overview of the methods used for reporting the histopathological changes and the key findings in each study are given in Table 1 along with the main conclusions. Most studies described their histological findings under glomerular, tubulointerstitial, and vascular compartments using a semiquantitative scoring method. The percentage values given in the Banff classification39 was used by some27,32,35,36 whereas others adopted different percentage values for scoring. Among the three studies that used a nonquantitative approach,26,31,37 Selvarajah et al.31 reported only the presence or absence of lesions whereas Fischer et al.37 provided summarized reports of the individual biopsies. Immunofluorescence features were described mainly in the Mesoamerican studies whereas electron microscopic analysis was conducted in two Sri Lankan32,38 and four Mesoamerican cohorts.34,36,37,38
Table 1.
Comparison of Histopathological Findings of the Studiesa
| Study (Country of origin; N) | Mean age (SD), years M : F ratio |
Histological Analysis | Glomeruli | Tubules | Interstitium | Vessels | Immunofluorescence | Electron Microscopy | Main Conclusions |
|---|---|---|---|---|---|---|---|---|---|
| Athuraliya et al.26 2011 | 45.05b (14.79) | No scoring or grading method used. | Normal to severe GS. GS was proportionate to IF and TA. | TA distribution included varying levels ranging from mild to severe. | IF was mild to severe. | Not mentioned. | Negative | Not done | Primary lesion was tubulointerstitial disease. |
| (SL; 26) | 1.5:1b | Tubulitis not mentioned. | II showed active inflammation accompanying fibrotic lesions. | ||||||
| Nanayakkara et al.27 2012 | 45 (10.5) | % scoring of glomeruli. | Mean GS = 37.1% ± 4 | TA distribution was not quantified. | IF distribution: | Fibrous intimal thickening distribution: | Negative | Not done | Primary lesion was tubulointerstitial nephritis which is unlikely to be from tubular inflammation. |
| ci0 - 7.0% | |||||||||
| (SL; 57) | 2.8:1 | IF, II, and vessels graded using Banff. | Collapsed glomeruli scorea was | Tubulitis was not seen. | ci1 - 31.6 % ci2 - 35.1 % ci3 - 26.3% |
cv0 - 50.0% cv1 - 40.5% cv2 - 9.5% |
Glomerular lesions are due to ischemia and progressive chronic loss of nephrons. | ||
| 17.6 ± 3.7%. | |||||||||
| Glomerular enlargement was 36.8%. | |||||||||
| Perihilar FSGS was 3.5%. | II distribution: | AH distribution: | |||||||
| i0 - 40.4% | |||||||||
| No proliferative changes. | i1 - 36.8% | ah0 - 41.5% | |||||||
| i2 - 21.0% | ah1 - 39.6% | ||||||||
| i3 - 1.8% | ah2 - 18.9% | ||||||||
| IF more prominent than II. | |||||||||
| Wijetunge et al.28 2013 | 36.8 (14) | GS as %. | Mean GS: | TA distribution: | IF distribution: | Hypertensive changes seen in 14.2% but none in early stages | Negative | Not done | Earliest detectable pathology is IF even with normal GFR. |
| (SL; 211) | 2.6:1 | SQ grading for IF, II, and TA (not Banff). | Cat. 1 = 27.7% | Absent – 19.9% | <10% - 35.8% | II is more likely a factor in disease progression rather than initiation. | |||
| Vascular changes were not graded. | Cat. 2 = 35.2% | <10% - 26.1% | 10-50% - 39.2% |
Pathogenesis: Either; a) many episodic exposures to toxins causing acute TIN & healing by IF or b) chronic low grade exposure to toxin causing progressive IF or c) both |
|||||
| Cases grouped into 7 categories based on biopsy findings. | Cat. 6 = 77.7% | 10% to 50% - 49.3% | >50% - 25% | ||||||
| GS absent at 35.8%. | >50%-17.1% | II distribution: | |||||||
| Wide-spread tubulitis - 2 cases. | Absent - 44.1% | ||||||||
| <10% - 19.1% | |||||||||
| 10-50% - 22.1% | |||||||||
| >50% - 16.7% | |||||||||
| IF more prominent than II. | |||||||||
| Wijetunge et al.29 2015 | 37.3 (12.5) | GS as %. | GS absent - 29.9% GS ≤50% - 53.4% |
TA distribution: Absent - 13.9% <10% - 20.3% 10-50%- 37.1% >50% - 28.7% |
IF distribution:<10% - 25.1% 10-50% - 43.8% >50% - 31.1% II distribution: Absent - 29.5% <10% - 16.7% 10-50% - 27.9% >50% - 25.9% |
Hypertension associated vascular changes - 14.3% | Negative | Not done | A significant proportion in all clinical stages are asymptomatic. |
| (SL; 251) | 3.3:1 | SQ grading for IF, II, and TA (not Banff). | There was significant correlation between the advancing histopathological parameters (IF, II, TA, and GS) and the mean GFR | ||||||
| Vascular changes were not graded. |
Pathogenesis: a) Noninflammatory process →IF and TA; b) Vicious cycle of IF → ischemia → further IF |
||||||||
| Pathology compared with clinical staging. | |||||||||
| Badurdeen et al.30 2016 | 44 (9) | SQ scoring not Banff. | GS absent - 22.6% GS <30% - 50% PGF - 41.9%. |
TA distribution: Absent – 8.1% <30% - 35.5% 30-60% - 51.6% >60% - 4.8% |
IF distribution: Absent – 12.9% <10% – 35.5% 10-50%– 46.8% >50% – 4.8% |
Not mentioned | Negative | Not done | Pathology in acute symptomatic CKDu is significant II and wide-spread tubulitis in the background of IF and TA. |
| (SL; 46) |
13.75:1 |
AI and CI calculated. |
Pathogenesis: Multiple acute episodes of interstitial nephritis progressing to residual scarring. |
||||||
|
Tubulitis: |
II distribution: | ||||||||
| Absent – 19.4% | Absent - 3.3% |
||||||||
| <30% - 33.9% |
<10% - 43.5% |
||||||||
| 30-60% -24.2% |
10-50% -35.5% |
||||||||
| >60% - 22.6% | >50% - 17.7% | ||||||||
| Selvarajah et al.31 2016 | 46.21 (11.64) | No scoring or grading system. | Mean GS = 42.2% ± 29.19 | TA present in 70.4%. | IF present in 71.2% | AH - 12.8% | Negative | Not done | Pathological changes supersede the clinical markers. |
| (SL; 125) | 2.8:1 |
GS as %. |
GS absent - 5.2% |
II present in 76.0% (lymphocytic infiltrate - 74.4% neutrophilic infiltrate - 1.6%) |
Progression of CKDu mainly due to II. |
||||
| Others present or absent. |
GS >50% - 48% |
||||||||
| PGF - 16% |
|||||||||
| Mesangial hypercellularity - 10.4% | |||||||||
| Wijkström et al.32 2018 | 48 (11) | SQ scoring similar to Banff. | Mean GS = 43% ± 20 | TA distribution: | IF distribution: | Intimal thickening: | Negative | No immune complexes. | Sri Lankan CKDu showed a more mixed morphological pattern than MeN, which may represent different stages of same disease or different diseases. |
| (SL;11) |
All male |
All had GS |
<6% – 0% |
<6% – 0 |
Mild - 20% |
Segmental podocytic foot process effacement – 18%. |
Glomerular ischemia may not be due to arterial disease alone. |
||
| (Large blood vessels present in only 10 cases) |
GS >50% - 45% |
6-25%% - 91% |
6-25%% - 55% |
Moderate - 30% |
Podocytic cytoplasmic inclusions – 82% |
||||
| Glomerular hypertrophy - 100% |
26-50% - 9% |
26-50% - 36% |
Mild smooth muscle hyperplasia 40% |
||||||
| Glomerular ischemia - 63.6% |
>50% - 0% |
>50% - 9% |
AH: |
||||||
| Endocapillary proliferation – Absent | Tubulitis - 3 cases |
II distribution: |
Mild - 63.6% | ||||||
| Intratubular granulocytes - 2 cases |
<6% – 18% 6-25%% - 45% 26-50% - 18% >50% - 18% |
Moderate - 27.3% | |||||||
| Anand et al.33 2019 (SL;87) (Histology described only in the PTKD group; N = 43) |
48 (11) 6.2:1 |
SQ scoring not Banff. AI and CI calculated. |
GS >25% - 15% Coexistent glomerular disease - 10% |
TA distribution: <25% - 85% ≥26% - 15% Tubulitis Foci with 5-10 cells/tubular cross section – 30% |
IF distribution: <25% - 85% >=26% - 15% II distribution: >=26% - 30% |
Arteriosclerosis – only mild changes. | IMF used to exclude cases of GN. Coexisting glomerular disease - 4. |
Not done. | Young or middle-aged CKD patients with negative dipstick proteinuria and normal serum albumin were more likely to have CKDu. Diabetes should not be an exclusion criterion for CKDu. |
| Vervaet et al.38 2020 | SL– 48.61 | Experimental exploration for specific lysosomal lesion in the proximal tubules. | GS absent - 38.9%. | IFTA distribution: | IFTA distribution: | Arterial intimal fibrosis in 33.3% | A subset of the proximal tubular granules that were autofluorescent and agyrophylic on silver stain were positive for lysosomal associated membrane protein 1 (LAMP1) and cathepsin B. | Electron dense lysosomal inclusion bodies were identified in proximal tubular epithelium. | A proximal tubular cell (lysosomal) lesion identical to that found in calcineurin inhibitor nephrotoxicity was identified in CINAC in different geographic regions. |
| (SL;18) | 3.5:1 |
SQ scoring not Banff. |
PGF - 27.8%. |
0-5% - 22.2% |
0-5% - 22.2% |
Vascular muscular hypertrophy: |
Pathogenesis: CINAC occurs due to a tubulotoxic mechanism similar to calcineurin inhibitor nephrotoxicity. |
||
| Mild – 44.4% | |||||||||
| Glomerulomegaly - 22.2%. |
6-25% - 38.9% |
6-25% - 38.9% |
Moderate – Severe - 38.9% |
||||||
| 26-50% - 28% |
26-50% - 28% |
AH in 22.2% |
|||||||
| >50 – 11.1% Tubular inflammation - 1 case Tubular luminal neutrophils - 1case Jones silver stain - light brown - black cytoplasmic granules in cortical tubular cells. |
>50 – 11.1% |
||||||||
|
II distribution: 0-5% - 33.3% 6-25% – 33.3% 26-50% - 22.2% >50% - 11.1% |
|||||||||
| Wijkström et al.36 2017 | 33 (8) | SQ scoring similar to Banff. | Mean GS = 38%± 21. | TA distribution: | IF distribution: | Intimal thickening: | Negative | No immune deposits. | Ratio between glomerular and tubulointerstitial damages suggest that glomerular changes cannot be explained by tubulointerstitial damage alone. |
| (NCG;19) | Absent – 6% | ||||||||
| (Histology evaluated in 16 biopsies. Large blood vessels were present in only 15 cases) |
All male. |
All had GS; |
<25%% - 81% |
Absent – 6% |
Mild - 20% |
Mild thickening of GBM- 31.25%. |
Findings compatible with the hypothesis of heat stress. |
||
| 25-50% grade – 44% |
26-50% - 13% >50% - 0 |
<25%% - 50% |
Moderate–7% |
Podocytic foot process effacement – 56.25%. |
|||||
| Glomerular hypertrophy - 100%. |
26-50% - 44% >50% - 0 |
Smooth muscle hyperplasia: |
Inclusion-like vacuoles in podocytic cytoplasm. |
||||||
| Wrinkled GBM and PGF - 94%. |
Few granulocytes in tubules – 2 cases |
Mild - 40% |
. |
||||||
|
II distribution: |
Moderate - 27% |
||||||||
| Absent – 13% |
AH: |
||||||||
| <25%% - 75% |
Mild – 18.75% |
||||||||
| 26-50% - 13% |
Moderate – 12.5% | ||||||||
| >50% - 0 |
|||||||||
| Fischer et al.37 2017 | 26 | Chronic TIN and GS as a %. | Mean GS = 13.37% (0-50) | All showed TIN with a predominantly mononuclear cell infiltrate (confirmed to be T cells and macrophages on IHC). Some had a mild neutrophilic infiltrate with neutrophils casts in tubules. | Mild intimal fibrosis – 27.3% | Focal segmental mesangial staining for IgA - 5 | Nonspecific, mild, focal segmental changes of podocyte effacement, mesangial sclerosis, and changes of chronic ischemic injury – 36.4%. | Renal histopathology in MeN reveals primary interstitial disease with intact glomeruli. | |
| (NCG;11) |
All male. |
GS absent - 54.5% |
Acute TIN – 18.2% |
IgG - negative |
No immune-type electron-dense deposits. |
||||
| Ischemic changes and mild glomerular enlargement – few cases |
Acute and chronic TIN – 36.4% |
IgM (mesangial) - all cases |
|||||||
| Chronic TIN – 45.5% |
|||||||||
|
Chronic TINdistribution: |
|||||||||
| Absent – 18.2% <10% - 9.1% 10-25% - 36.4% 25 - 50% - 27.3%% >50% - 9.1% |
|||||||||
| Wijkström et al.34 2013 | 44.25 | SQ scoring for IF, II, and TA (not Banff). | Mean GS = 51.75% (29-78). | TA distribution: | IF distribution: | Mild intimal thickening -25% | Small amounts of IgG-1 (postulated to be a previous episode of GN). | Segmental foot process effacement - 37.5%. | GS and glomerular ischemia were more advanced than tubulointerstitial changes suggesting possible primary injury to glomeruli in addition to tubulointerstitial damage. |
| (ES; 8) |
All male |
All had GS; majority (62.5%) within 25-50% grade. |
<25% - 50% |
<25% - 50% |
Mild smooth muscle hyperplasia - 87.5% |
Podocyte vacuolations - 75%. |
|||
| Glomerular hypertrophy -100%. |
26-50% - 50% |
26-50% - 50% |
AH - 37.5% |
Electron dense deposits - 12.5%. |
|||||
| Wrinkled GBM & PGF - 87.5%. |
Tubulitis - 1 case |
II distribution: |
|||||||
| No crystals on polarized light |
Absent - 12.5% |
||||||||
| <25% - 50% |
|||||||||
| 26-50% - 37.5% | |||||||||
| Lopez-Marin et al.35 2014 | 45.4 | SQ scoring for IF, II, and TA stated as Banff 97. | GS >25% - 58.7%. | TA distribution: | IF distribution: | Intimal proliferation - 19.6% | One case with IgG deposition (coexistent early membranous nephropathy). | Not done | Pathology is chronic TIN. |
| (ES; 46) |
3.6:1 |
Glomerulomegaly≥10% - 47.8%. |
Absent - 10.9% |
≤5% - 37% |
Tunica media thickening - 52.2% |
Severity increased with CKD stage. |
|||
| <25% - 76.1% |
6-50% - 37 % |
Consistent with a multifactorial etiology. |
|||||||
| ≥25% - 13% |
>50% - 26.1 % II distribution: ≤25% - 89.1% 26-50% - 10.9% |
||||||||
| Vervaet et al.38 2020 | ES – 43.73 | GS absent - 9.1% | IFTA distribution: | IFTA distribution: | Arterial intimal fibrosis in 36.7% | ||||
| (ES; 11) | 4.5:1 |
PGF - 63.6% |
0-5% - 45.4% |
0-5% - 45.4% |
Vascular muscular hypertrophy: | ||||
| Mild - 63.6% | |||||||||
| Glomerulomegaly - 72.7% |
6-25% - 45.4% |
6-25% - 45.4% |
Moderate - severe - 27.3% |
||||||
| 26-50% - 9.1% |
26-50% - 9.1% |
AH in 27.3% |
|||||||
| >50 - 0% Tubular inflammation - 2 cases Tubular luminal neutrophils – 1 case |
>50 - 0% II distribution: 0-5% - 45.4% 6-25% – 45.4% 26-50% - 9.1% >50% - 0% |
||||||||
TA distribution refers to the percentage of Tubular atrophy seen. It is graded as Absent, <10%, 10-50% and >50%.
AH, arteriolar hyalinosis; ah#, arteriolar hyalinosis grading according to BANFF ; AI, activity index; Banff, Banff classification for kidney transplant pathology; Cat., category; CINAC, chronic interstitial nephritis in agricultural communities; CI, chronicity index; ci#, interstitial fibrosis grading according to BANFF ; CKD, chronic kidney disease; CKDu, chronic kidney disease of unknown origin; cv#, vascular fibrous intimal thickening grading according to BANFF; ES, El Salvador; FSGS, focal segmental glomerulosclerosis ; GBM, glomerular basement membrane; GFR, glomerular filtration rate; GN, glomerulonephritis; GS, glomerulosclerosis; i#, interstitial inflammation grading according to BANFF; Ig, immunoglobulin; IF, interstitial fibrosis; IFTA, interstitial fibrosis and tubular atrophy combined; IHC, immunohistochemistry; II, interstitial inflammation; IMF, immunofluorescence; MeN, Mesoamerican nephropathy; NCG, Nicaragua; PGF, periglomerular fibrosis; PTKD, primary tubulointerstitial kidney disease; SL, Sri Lanka; SQ, semiquantitative; TA, tubular atrophy; TIN, tubulointerstitial nephritis.
Total screened population.
Table 2 provides a comparative overview of the reported frequencies of cases showing absence and severe degrees of the main histopathological changes.
Table 2.
Distribution of Cases Showing Absent/Mild Versus Severe Histopathological Changes in the Selected Studiesa,b
| Study | N | Percentage of Cases Reported as Absent or Grade 0 |
Percentage of Cases Reported as Severe or Grade 3 |
||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| GS | PGF | IF | II | TA | T | CV | Ah | GS | PGF | IF | II | TA | T | CV | Ah | ||
| Sri Lanka | |||||||||||||||||
| Nanayakkara et al.,27 2012 | 57 | na | na | 7 | 40 | na | 100 | 50 | 41.5 | na | na | 26 | 2 | na | 0 | 0 | 0 |
| Wijetunge et al.,28 2013 | 211 | 38 | na | 3 | 44 | 20 | ≥37 | -- 63c-- | na | na | 24 | 16 | 17 | na | na | na | |
| Wijetunge et al.,29 2015 | 251 | 30 | na | 0 | 30 | 14 | na | -- 86c -- | 17 | na | 31 | 26 | 29 | na | na | na | |
| Badurdeen et al.,30 2016 | 46 | 23 | 58 | 13 | 3.3 | 8 | 19 | na | na | 11 | 2 | 5 | 18 | 5 | 23 | na | na |
| Selvarajah et al.,31 2016 | 125 | 5 | 84 | 29 | 24 | 30 | na | na | 87 | 48 | na | na | na | na | na | na | na |
| Wijkström et al.,32 2018 | 11 | 0 | 36 | 0 | 18 | 0 | 73 | 50d | 9 | 45 | na | 9 | 18 | 0 | na | 0d | 0 |
| Vervaet et al.,38 2020 | 18 | 39 | 73 | 22e | 33 | 22e | 94 | 67 | 78 | na | na | 11e | 11 | 11e | na | na | na |
| Nicaragua | |||||||||||||||||
| Wijkström et al.,36 2017 | 16 | 0 | na | 6 | 12 | 6 | 94 | 73g | 67 | 31 | na | 0 | 0 | 0 | 0 | 0 | 0 |
| Fischer et al.,37 2017 | 11 | 54 | na | 18h | na | 18h | na | 73 | na | 0 | na | na | na | na | na | na | na |
| Wijkström et al.,34 2013 | 8 | 0 | na | 0 | 12 | 0 | 88 | 12 | 62 | 50 | na | 12 | 0 | 0 | 0 | 0 | 0 |
| Lopez-Marin et al.,35 2014 | 46 | na | na | 37 | na | 11 | na | na | na | 59f | na | 26 | 0 | 0 | na | na | na |
| El Salvador | |||||||||||||||||
| Vervaet et al.,38 2020 | 11 | 9 | 61 | 45e | 45 | 45e | 82 | 64 | 73 | na | na | 0e | 0 | 0e | na | na | na |
Ah, hyaline arteriolosclerosis; CV, vascular changes; GS, glomerulosclerosis; IF, interstitial fibrosis; II, interstitial inflammation; na, not available; PGF, periglomerular fibrosis; T, tubulitis; TA, tubular atrophy.
The percentages above have been rounded to nearest whole number for better clarity. The studies of Athuraliya et al.26 and Anand et al.33 are not included in this table as they did not contain details on the number of cases under each category of pathological changes.
Described as hypertensive vascular changes.
Reported for 10 biopsies.
Reported as interstitial fibrosis and tubular atrophy combined.
Based on >25% instead of >50%.
Reported for 15 biopsies.
Reported as chronic tubulointerstitial nephritis.
Histopathological Changes in Interstitium and Tubules
Interstitial Fibrosis
All studies reported interstitial fibrosis to be the predominant and earliest pathological change. Even in the studies involving patients presenting with acute symptoms,30,37 interstitial fibrosis was noted in >80% of cases. Patients with severe degrees of interstitial fibrosis were often in advanced clinical stages of CKD.28,29,35 Wijetunge et al.29 showed a correlation between interstitial fibrosis and mean glomerular filtration rate (GFR) (R2 = 0.47) and Selvarajah et al.31 identified interstitial fibrosis as an independent predictor for CKD stage 3 (P = 0.005). In general, severe grades of interstitial fibrosis were rare in the Mesoamerican patients (Table 2), especially in those who were unrelated to sugarcane farming.35
Interstitial Inflammation
The degree of interstitial inflammation was generally less than the degree of interstitial fibrosis except in the studies on patients with acute presentations. Inflammation was found to be either absent or mild in the early stages of the disease.29 However, in advanced stages with higher degrees of fibrosis, higher degrees of interstitial inflammation was also noted. The correlation between mean GFR and interstitial inflammation (R2 = 0.47) was found to be similar to that of mean GFR and interstitial fibrosis.29 Interstitial inflammation was also identified as an independent predictor for CKD stage 3 or more (P = 0.03).31 In contrast to the Sri Lankan cases, none of the Mesoamerican studies reported severe grades of interstitial inflammation (Table 2). Immunohistochemical analysis showed that the infiltrates were comprised predominantly of T lymphocytes and macrophages with fewer numbers of B lymphocytes and plasma cells.37
Tubular Atrophy
The majority of the studies reported the presence of tubular atrophy; even among the acutely presenting patients, it was seen in >90%.30 In general, the severity was predominantly within mild to moderate grades affecting <50% of the cortical area. Severe tubular atrophy was noted in at least three Sri Lankan cohorts,28, 29, 30 whereas none of the Mesoamerican studies reported severe grades (Table 2). A high correlation was noted between tubular atrophy and the degree of renal impairment.29,31 Fischer et al.37 studied tubules under electron microscopy and noted infolding of the plasma membrane, organelle loss, cell detachment from basement membrane, and chromatin loss in nuclei. In some cases, thickening of basement membranes and interstitial deposition of collagen fibrils were also noted. None of the cases showed any electron dense deposits or viral particles.
Tubulitis
Tubulitis was the least reported histopathological lesion and the findings are less detailed. Five of 13 studies did not include tubulitis in their analysis. The basis on which tubulitis was defined was only available in the study by Anand et al.33 within their supplementary data; however, descriptive details were not available. Few cases of tubulitis were reported in both Sri Lankan and Mesoamerican cohorts.28,32,36,38 Nanayakkara et al.27 did not find tubulitis in any of their cases. In contrast, Badurdeen et al.30 found tubulitis in >80% of acute cases of which 22.6% had tubulitis involving more than 60% of the biopsy area. In the Mesoamerican study on acutely ill patients, tubulitis was not mentioned; however, acute tubular cell injury and neutrophil accumulation in the tubular lumen were noted.37
Other Tubular Changes
An intracellular inclusion body in the proximal tubular cells described as an argyrophilic lysosomal lesion detectable on light microscopy using a modified Jones stain was reported by Vervaet et al.38 Through electron microscopy, the lesions were described as single-membrane inclusions lacking cristae, which contained electron-dense aggregates within the matrix. These were seen in a majority of Sri Lankan patients with CKDu and all of the El Salvadorian patients with Mesoamerican nephropathy and were identical to the lysosomal lesions seen in post-transplantation patients on calcineurin inhibitor treatment, patients exposed to nephrotoxic drugs linked to calcineurin inhibition, and rats exposed to cyclosporine. The lesions were rarely seen in healthy controls and patients with other known renal diseases recruited to their study.
Some studies also reported occasional leukocytes associated with tubules, which however, were not attributed to any specific etiological mechanism.32,34,37,38
Pathogenesis of Tubulointerstitial Changes
Nanayakkara et al.27 believed that the interstitial nephritis did not originate from tubular inflammation whereas, Badurdeen et al.30 and Fischer et al.,37 who studied acute patients, believed that acute inflammatory insults were responsible for the initial kidney damage. Wijetunge et al.28 postulated fibrosis as the earliest pathological change based on their findings in asymptomatic patients and along with Selvarajah et al.31 believed that interstitial inflammation only played a role in the progression of the disease.
Wijetunge et al.29 postulated that a nonlethal tubular epithelial injury such as chronic low-grade exposure to a toxin resulted in fibrosis through a noninflammatory process and that the resulting distortion in the vascular architecture triggered a vicious cycle of tubular ischemia, chronic inflammation, and further fibrosis. Vervaet et al.38 suggested that the disease had an agricultural toxin–mediated etiology acting via the calcineurin inhibition pathway.
Histopathological Changes in Glomeruli and Vessels
Glomerulosclerosis
Varying degrees of global glomerulosclerosis were seen with the majority involving <50% of the total glomerular number. In general, higher percentages of Mesoamerican patients had glomerulosclerosis compared to Sri Lankan patients (Table 2) with the exception of one study where the mean age of the cohort was 26 years.37 The severity of glomerulosclerosis was seen to increase progressively across the CKD stages29,31,32,34,36 and a serum creatinine level of >1.2mg/dl was considered an independent histological predictor of glomerulosclerosis >50%.31 Anand et al.33 found a higher degree of glomerulosclerosis with no significant arteriolar changes in the primary tubulointerstitial kidney disease group, where several patients had concomitant diabetes and hypertension.
Other Glomerular Changes
Glomerular enlargement, focal sclerosis, periglomerular fibrosis, glomerular collapse, and wrinkling of glomerular basement membrane were the other common lesions reported. Except for Fischer et al.,37 all the other studies on the Mesoamerican cohorts reported glomerular enlargement or glomerular hypertrophy. Among the Sri Lankan studies, only three studies had analyzed glomerular size.27,32,38 Nanayakkara et al.27 found enlarged glomeruli in 36.8% of their cases and believed it to be due to compensatory hypertrophy of surviving nephrons following global glomerulosclerosis of other nephrons. López-Marin et al.35 found glomerulomegaly of >10% in 47.8% of their cases, the majority of which were in stage 2 CKD. Wijkström et al.32,34,36 found enlarged glomeruli in all their patients in both the Mesoamerican and Sri Lankan cohorts. However, in Vervaet et al.,38 there was a higher percentage of cases without glomerulomegaly in the Sri Lankan cohort (77.8%) than those from El Salvador (27.3%), whereas the latter had more cases with severe glomerulomegaly (36.7%) than the former (5.5%).
With the exception of one study that described mesangial hypercellularity in 10.4% on light microscopy with negative immunoglobulin A (IgA) on immunofluorescence,31 all the studies reported no evidence of mesangial, endocapillary, or extracapillary proliferation. Wijkström et al.34 found one patient with small amounts of IgG-positive mesangial deposits, which was interpreted as a previous episode of glomerulonephritis. López-Marin et al.35 found one patient with diffuse IgG deposits of moderate intensity which was attributed to coexistent early membranous glomerulopathy and also reported nonspecific IgM, and complement deposits in the glomeruli in 30 of 46 patients. Fischer et al.37 found IgM, IgA, and complement deposits in some patients but did not attribute this finding to any specific cause.
On electron microscopy, Wijkström et al.32,34,36 found podocyte cytoplasmic inclusions as vacuoles or lipofuscin-like bodies and segmental podocyte foot process effacement in Sri Lankan and Mesoamerican patients. Fischer et al.37 found podocyte foot process effacement of mild degree in 4 of 11 patients with acute illness.
Vascular Changes
A majority of patients both in the Sri Lankan and Mesoamerican cohorts did not show any vascular changes. When present, they were predominantly of mild to moderate degree and included intimal proliferation, smooth muscle hyperplasia of arteries, and arteriolar hyalinosis. None of the studies reported severe degrees of vascular changes.
Pathogenesis of Glomerular and Vascular Changes
One hypothesis was that glomerular and vascular changes were secondary to tubulointerstitial disease. This was supported by the fact that tubulointerstitial changes were out of proportion to the glomerular changes.27,28,31,35 Wijetunge et al.28,29 suggested that glomerular ischemia was a consequence of architectural changes secondary to interstitial fibrosis. Nanayakkara et al.27 postulated that the vascular changes were due to long-standing hypertension. Another hypothesis by Wijkström et al.34 was that there was concomitant glomerular injury in addition to the tubulointerstitial injury. They described the glomerulosclerosis as being more advanced than tubulointerstitial changes and stated that the features of glomerular ischemia were unrelated to aging or hypertension. They further suggested that the presence of ultrastructural changes of podocyte could be evidence of primary glomerular injury.
Discussion
This paper reviews published studies involving histopathological analysis of kidney biopsies in relation to CKDu from Sri Lanka and Mesoamerican nephropathy. The studies varied in their approach to case selection and methods of histopathological reporting. Both regions reported high frequencies of global glomerulosclerosis and interstitial fibrosis in kidney biopsy specimens, and all the studies concluded that the histopathological pattern was compatible with tubulointerstitial nephritis as the primary pathology.
Tubulointerstitial Nephritis as the Histopathological Marker of CKDu or Mesoamerican Nephropathy
Many research articles both in Sri Lanka and other countries relate chronic tubulointerstitial nephritis with CKDu.8 Reliance on this histopathological label has led numerous researchers to describe their study subjects as including “biopsy-proven” CKDu patients.40, 41, 42, 43, 44 This is potentially misleading. From a pathological point of view, the term tubulointerstitial nephritis (TIN), also referred to as interstitial nephritis, does not represent any specific disease entity and merely represents inflammation within the tubulointerstitium rather than in the glomerular and vascular compartments.45,46
The term can be traced back to the late 1800s where Councilman describes this condition first reported by Biermer in 1860 in deaths from scarlet fever and diphtheria.47 TIN is the reported pathology in a wide range of infections, obstructive uropathies, analgesic abuse, and adverse drug reactions46,48, 49, 50 in addition to many toxin-mediated nephropathies including the cadmium-induced itai-itai disease in Japan and the Balkan endemic nephropathy now believed to be due to aristolochic acid exposure.
All the studies included in this review demonstrated TIN in varying degrees and have shown a predominance of pathologies within the tubular and interstitial compartments compared to the glomeruli. However, the findings are nonspecific and no single feature of TIN has been identified as a specific or a sensitive marker for the identification of CKDu. Therefore, the mere presence of TIN on a renal biopsy would not be a suitable criterion for a case definition of CKDu.
CKDu in Sri Lanka Versus Mesoamerican Nephropathy
The studies reveal that both CKDu in Sri Lanka and Mesoamerican nephropathy have TIN as the predominant overall histopathological feature. However, as discussed above, this does not necessarily mean that the etiology is the same for both regions. Furthermore, there are noticeable differences between these two groups in the frequency and severity at which these histopathological changes were seen (Table 2).
Firstly, none of the Mesoamerican patients were found to have severe degrees of interstitial inflammation or tubular atrophy (Table 2), and a distinctly higher prevalence of cases with severe chronic tubulointerstitial changes were noted among the Sri Lankan patients. This could be an indicator of either a higher rate of exposure or a more potent causative factor for the tubulointerstitial damage in Sri Lanka. However, the possibility of a selection bias from more cases with advanced disease being in the Sri Lankan cohorts cannot be excluded, particularly as most of the Mesoamerican studies have had much lower sample sizes.
Secondly, there is a higher frequency of glomerulosclerosis and glomerulomegaly in the Mesoamerican cohorts than the Sri Lankan cohorts. Age-related differences are unlikely because, except for Fischer et al.37 who had a very young cohort, the mean ages between the Sri Lankan and Mesoamerican cohorts were very much similar in all the other studies. As proposed by Wijkström et al.,34 it is possible that the higher prevalence of glomerular changes in the Mesoamerican patients are due to an intrinsic or extrinsic condition which causes primary glomerular injury in addition to whatever is causing the TIN.
Although these subtle differences may indicate the possibility of different etiologies or pathogenetic mechanisms for the nephropathies seen in these two regions, they could also reflect the inherent dissimilarities in renal mass due to genetic and early life influences between the Sri Lankan and Mesoamerican patients. Studies have shown that factors such as low birth weight, prematurity, malnutrition, and even racial predisposition are known to influence renal development and thereby susceptibility to renal disease.50, 51, 52, 53, 54, 55, 56 Individuals with low renal mass (nephron numbers) have been found to have higher glomerular volume, which is considered as a surrogate marker for premature glomerulosclerosis.57,58 Glomerular size has been analyzed in only a few Sri Lankan cohorts, but in comparison, glomerulomegaly appears to be more common among the Mesoamerican patients. Whether this is a phenomenon secondary to glomerulosclerosis or whether this is an indicator of lower renal mass in the Mesoamerican populations than in Sri Lankans would be a potentially interesting area of future study.
Pathogenesis of the Renal Lesions
All the studies concluded that chronic TIN is the primary pathology. Two studies showed that the pathological changes were established in early stages of CKD and the histological features preceded the clinical markers.28,31 None of the biopsy studies provided any conclusive evidence towards an etiology for CKDu; however, three main pathogenetic mechanisms were postulated: (1) Several acute insults occurring periodically leading to scarring; (2) Low-grade insult (possibly chronic toxic exposure) leading to progressive fibrosis (possibly noninflammatory related); and (3) Tubulointerstitial damage in combination with a glomerular injury.
The first mechanism above is primarily based on the studies of patients presenting with acute symptoms30,37 where it was postulated that multiple episodes of acute interstitial nephritis led to progressive renal scarring. In a later review, drugs, toxins, and infections have been proposed as potential causes for these acute episodes with subsequent tubulointerstitial scarring occurring through chronic T cell–mediated injury.59 Although there have been studies linking CKDu with hanta virus infection,60 leptospirosis,61 and cyanobacterial toxins,62,63 no causal connection to an acute infective or toxic etiology has been conclusively established.
The second mechanism was mainly based on the observation that the majority of patients with early disease had minimal tubulointerstitial inflammation but had advanced degrees of fibrosis. Nanayakkara et al.,27 who did not find any cases of tubulitis, concluded that tubular inflammation was unlikely to be the cause. Wijetunge et al.28 believed that tubulointerstitial inflammation did not play a role in the initiation of the disease but contributed to the progression of the disease. This theory was supported by Selvarajah et al.31 who believed that treatment strategies in CKDu should target the interstitial inflammatory process. The initial tubular injury was postulated to be a chronic low-grade exposure to a toxin which causes tubular epithelial cell dysfunction leading to apoptosis and fibrosis rather than necrosis and acute inflammation.29 There have been several published studies that have looked at a wide range of low-grade toxic exposures such as pesticides,64 glyphosate,65 heavy metals,66, 67, 68, 69 water hardness, and ionicity.70, 71, 72 Similarly, dehydration and heat stress have been explored mainly in relation to Mesoamerican nephropathy as a mechanism of intermittent subclinical renal injury through rhabdomyolysis, hyperuricemia, and renal ischemia.73,74 However, recent studies and reviews report that the evidence is still inconclusive to support the presence of such environmental toxins, heat stress, or dehydration in affected patients.75, 76, 77, 78
Vervaet et al.38 also proposed a toxic etiological factor which acts via the calcineurin inhibition pathway based on the proximal tubular lysosomal lesions found in the Sri Lankan and Mesoamerican patients. This lesion was also found in one healthy control from Sri Lanka. None of the previous renal biopsy studies reported any similar feature or abnormality in the tubules either on electron microscopy or light microscopy that could be attributed to such lesions. This finding would therefore require validation through further studies, preferably on larger sample numbers.
The third hypothesis was suggested by Wijkström et al.34 who believed that the degree of glomerulosclerosis and glomerular ischemia were disproportionate to the tubulointerstitial and vascular changes mainly in the Mesoamerican patients. They noted that some Sri Lankan CKDu patients also had similar glomerular changes which could not be explained by age- or hypertension-related vascular pathology.36 Their postulation was that the glomerular changes were due to some other concomitant factor unrelated to the TIN. However, they did not suggest any possible cause or mechanism for the glomerular injury.
Study Limitations
For the purposes of this review, we used all available published studies and did not consider the quality of the individual studies, particularly as the number of studies available was low. We identified several aspects that limited the collective interpretation of results especially when comparing Mesoamerican and Sri Lanka studies.
The main limitation noted was the absence of a uniform case definition. Almost all the studies have relied heavily on clinical criteria in selecting or screening for their study participants. As highlighted in numerous publications,12,79, 80, 81 the lack of a proper clinical definition has led to an overestimation of the disease and has possibly resulted in many patients with CKD being labelled as CKDu.82 In Sri Lanka, case definitions of CKDu were continually revised by the World Health Organization and Ministry of Health83, 84, 85 with the most recent update done by the Sri Lanka Society of Nephrology in 2018.86 The majority of patients analyzed in these studies were selected well before these revisions took place; therefore, some of them may not fall within the currently accepted CKDu definitions.
Many studies report their study population to be from endemic areas without specifying the areas or the basis on which endemicity was determined. Although the highest prevalence of CKDu in Sri Lanka has been reported from the North-central province, it is known that even in this region there is clustering of the disease12,87 which creates doubt as to the value of residency in the case selection.
Proteinuria was used as an exclusion criterion in studies of Mesoamerican nephropathy, whereas, in most of the Sri Lankan studies, presence of proteinuria on dipstick was used as a selection criterion. When biopsy specimens with primary tubulointerstitial disease were analyzed by Anand et al.,33 they found that their cases had a strong negative association with proteinuria. Although absence of proteinuria was considered as one of the criteria for selection in Vervaet et al.38 (Supplementary Table S1), it was noted that proteinuria was present in the majority of patients from Sri Lanka. Therefore, it is possible that proteinuria is a variable manifestation of the same disease; in which case, screening for proteinuria may only capture a subset of the affected population. Alternatively, the presence or absence of proteinuria may also indicate different etiologies or pathophysiological mechanisms for CKDu. Either way, this situation raises further doubts on the value of proteinuria as a screening tool.
The presence of diabetes and hypertension was an exclusion criterion in most of the studies.27, 28, 29,31,32,34, 35, 36,38 However, as seen in Anand et al.33, 16% of the patients in the CKDu group who had biopsy features of primary tubulointerstitial disease were also found to have co-existing diabetes. The prevalence of diabetes and hypertension has increased substantially in the community; therefore, the mere presence of either of these illnesses in a patient with CKD does not necessarily mean that the diabetes or hypertension were causative unless the biopsy shows unequivocal evidence of primary glomerular or vascular pathology.
Finally, the criteria used to report the histological changes in the kidney biopsy specimens have not been uniform. Some studies have followed the Banff guideline and others have used different semiquantitative grading systems with different scoring criteria. None of the studies had used any published guidelines for reporting glomerular morphology such as the International Society of Nephrology and the Renal Pathology Society guideline for lupus nephritis88 or the Oxford classification for IgA nephropathy.89 The suitability of Banff classification in describing the pathological changes of CKDu in native kidney biopsy specimens is also debatable as its primary purpose is to diagnose kidney transplant-related pathology. At present, the Banff 2018 reference guide90 has been developed and further revisions are being made online.91 However, even with the use of a standardized guideline, significant interobserver variability and subjectivity is known to occur among pathologists92 which could limit the reliability of the data, particularly in relation to the severity grading of the different histopathological features.
Conclusion
The histopathological changes of CKDu have considerable variation depending on the timing of the renal biopsy in relation to the clinical stage of the disease and in general have been found to precede the clinical manifestations. The biopsy studies of CKDu patients in Sri Lanka and Mesoamerica report chronic TIN as the predominant histopathological picture but do not reveal any specific pathological feature that could differentiate this disease from any of the known causes of TIN. One study has shown evidence of a proximal tubular lesion which appears to be specific for agrochemical toxic exposure; however, this needs further validation with larger population studies.
Three main pathogenetic mechanisms have been postulated through biopsy studies, which include: (1) repeated acute tubulointerstitial injury causing fibrosis; (2) low-grade chronic toxic exposure causing fibrosis through non inflammatory mechanisms; and (3) a combination of TIN with primary glomerular damage.
Further work is needed to determine the etiology and pathogenesis of CKDu in Sri Lanka and Mesoamerica and to identify histopathological markers that would have a higher sensitivity and specificity to the disease affecting these populations. We recommend the following strategies to improve research in this area:
-
1.
Use uniform criteria for case selection and avoid the use of endemic regions as a selection criterion as multiple etiological mechanisms causing TIN could exist in the same region.
-
2.
Proteinuria should not be used as an exclusion criterion unless the levels are extremely high (e.g., >2 g/day) because some cases of biopsy-proven tubulointerstitial disease have shown significant proteinuria.
-
3.
The mere presence of diabetes mellitus and hypertension should not be used as an exclusion criterion unless the biopsy shows unequivocal evidence of diabetic nephropathy or hypertensive nephrosclerosis.
-
4.
Immunofluorescence should always be included in the biopsy evaluation and cases should be separated from CKDu based on the morphological findings rather than on immunofluorescent positivity alone.
-
5.
Biopsy specimens from patients in early stages of CKDu are needed to get a better insight into the pathophysiology of the disease. The use of traditional diagnostic biomarkers, serum creatinine, and urine-albumin would be inappropriate for this purpose as serum creatinine is a delayed biomarker and CKDu is considered a non-albuminuric disease. Therefore, there is a need to identify novel biomarkers to diagnose early disease.
-
6.
Use a uniform method such as the Banff classification when reporting histopathological results with a clear indication of which version is used. The glomerular morphology could be more uniformly described through the use of published guidelines for Systemic Lupus Erythematosus (International Society of Nephrology and the Renal Pathology Society) and IgA (Oxford).
-
7.
Standard criteria should be used when diagnosing glomerulomegaly and its relevance should be discussed in relation to factors such as body mass index, low birth weight, et cetera.
-
8.
More descriptive approaches for biopsy analysis should be used including identification of the type of predominant infiltrate (lymphoid, monocytes, macrophages, neutrophils, or eosinophils) within the interstitial inflammation in both the scarred and nonscarred regions and providing a clear differentiation between acute and chronic tubulointerstitial nephritis.
-
9.
Design more explorative strategies including multicenter retrospective studies and blinded comparative studies with known causes of CKD using modern tools such as predictive modeling techniques, big data analysis, artificial intelligence, and image analysis software.
-
10.
Use other strategies such as autopsied kidneys from sudden and unnatural deaths to obtain larger volumes of kidney tissue, which would also enable researchers to capture asymptomatic individuals who are in early stages of CKD.
Disclosure
All the authors report no conflict of interest in preparing and submitting this manuscript
Acknowledgments
The authors thank Dr. Piumi Dileka and Ms. Indeewarie Keerawelle for assistance with the typesetting and formatting of the manuscript.
Funding
This work was supported through the research grant RPHS/2016/CKDU-03 of the National Research Council of Sri Lanka obtained by the principal and corresponding author.
Authors’ Contributions
Study design and conceptualization: SG; Literature search and data acquisition: SG, MD, HW; Data analysis, interpretation and manuscript preparation: SG, MD, HW, EW.
Each author contributed important intellectual content during manuscript drafting or revision, accepts personal accountability for the author’s own contributions, and agrees to ensure that questions pertaining to the accuracy or integrity of any portion of the work are appropriately investigated and resolved.
Footnotes
Supplementary Material
Table S1. Characteristics of studies
Item S2. PRISMA checklist
References
- 1.James S.L., Abate D., Abate K.H. Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2018;392:1789–1858. doi: 10.1016/S0140-6736(18)32279-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fraser S.D., Roderick P.J. Kidney disease in the global burden of disease study 2017. Nat Rev Nephrol. 2019;15:193. doi: 10.1038/s41581-019-0120-0. [DOI] [PubMed] [Google Scholar]
- 3.Bikbov B., Purcell C.A., Levey A.S. Global, regional, and national burden of chronic kidney disease, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2020;395:709–733. doi: 10.1016/S0140-6736(20)30045-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Xie Y., Bowe B., Mokdad A.H. Analysis of the Global Burden of Disease study highlights the global, regional, and national trends of chronic kidney disease epidemiology from 1990 to 2016. Kidney Int. 2018;94:567–581. doi: 10.1016/j.kint.2018.04.011. [DOI] [PubMed] [Google Scholar]
- 5.Canney M., Birks P., Levin A. Epidemiology of chronic kidney disease-scope of the problem. In: Kimmel P.L., Rosenberg M.E., editors. Chronic Renal Disease. 2nded. Academic Press; Cambridge, Massachusetts: 2020. pp. 75–89. [Google Scholar]
- 6.Jha V., Garcia-Garcia G., Iseki K. Chronic kidney disease: global dimension and perspectives. Lancet. 2013;382:260–272. doi: 10.1016/S0140-6736(13)60687-X. [DOI] [PubMed] [Google Scholar]
- 7.Stalin P., Purty A.J., Abraham G. Distribution and determinants of chronic kidney disease of unknown etiology: a brief overview. Indian J Nephrol. 2020;30:241–244. doi: 10.4103/ijn.IJN_313_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gifford F.J., Gifford R.M., Eddleston M. Endemic nephropathy around the world. Kidney Int Rep. 2017;2:282–292. doi: 10.1016/j.ekir.2016.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Weaver V.M., Fadrowski J.J., Jaar B.G. Global dimensions of chronic kidney disease of unknown etiology (CKDu): a modern era environmental and/or occupational nephropathy? BMC Nephrol. 2015;16:145. doi: 10.1186/s12882-015-0105-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wanigasuriya K.P., Peiris-John R.J., Wickremasinghe R. Chronic renal failure in North Central Province of Sri Lanka: an environmentally induced disease. Trans R Soc Trop Med Hyg. 2007;101:1013–1017. doi: 10.1016/j.trstmh.2007.05.006. [DOI] [PubMed] [Google Scholar]
- 11.Athuraliya T.N., Abeysekera D.T., Amerasinghe P.H. Prevalence of chronic kidney disease in two tertiary care hospitals: high proportion of cases with uncertain aetiology. Ceylon Med J. 2009;54:23–25. doi: 10.4038/cmj.v54i1.471. [DOI] [PubMed] [Google Scholar]
- 12.Ranasinghe A.V., Kumara G.W., Karunarathna R.H. The incidence, prevalence and trends of Chronic Kidney Disease and Chronic Kidney Disease of uncertain aetiology (CKDu) in the North Central Province of Sri Lanka: an analysis of 30,566 patients. BMC Nephrol. 2019;20:338. doi: 10.1186/s12882-019-1501-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.de Alwis A.A., Panawala P.V. A review of the national response to CKDu in Sri Lanka. Sri Lanka J Soc Sci. 2019;42:83–100. [Google Scholar]
- 14.Jayasekara K.B., Dissanayake D.M., Sivakanesan R. Epidemiology of chronic kidney disease, with special emphasis on chronic kidney disease of uncertain etiology, in the north central region of Sri Lanka. J Epidemiol. 2015;25:275–280. doi: 10.2188/jea.JE20140074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Torres C., Aragón A., González M. Decreased kidney function of unknown cause in Nicaragua: a community-based survey. Am J Kidney Dis. 2010;55:485–496. doi: 10.1053/j.ajkd.2009.12.012. [DOI] [PubMed] [Google Scholar]
- 16.Orantes C.M., Herrera R., Almaguer M. Chronic kidney disease and associated risk factors in the Bajo Lempa region of El Salvador: Nefrolempa study, 2009. MEDICC Rev. 2011;13:14–22. doi: 10.37757/MR2011V13.N4.5. [DOI] [PubMed] [Google Scholar]
- 17.Herrera R., Orantes C.M., Almaguer M. Clinical characteristics of chronic kidney disease of nontraditional causes in Salvadoran farming communities. MEDICC rev. 2014;16:39–48. doi: 10.37757/MR2014.V16.N2.7. [DOI] [PubMed] [Google Scholar]
- 18.Correa-Rotter R., Wesseling C., Johnson R.J. CKD of unknown origin in Central America: the case for a Mesoamerican nephropathy. Am J Kidney Dis. 2014;63:506–520. doi: 10.1053/j.ajkd.2013.10.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Peraza S., Wesseling C., Aragon A. Decreased kidney function among agricultural workers in El Salvador. Am J Kidney Dis. 2012;59:531–540. doi: 10.1053/j.ajkd.2011.11.039. [DOI] [PubMed] [Google Scholar]
- 20.Santos U.P., Zanetta D.M., Terra-Filho M. Burnt sugarcane harvesting is associated with acute renal dysfunction. Kidney Int. 2015;87:792–799. doi: 10.1038/ki.2014.306. [DOI] [PubMed] [Google Scholar]
- 21.Couser W.G., Remuzzi G., Mendis S. The contribution of chronic kidney disease to the global burden of major noncommunicable diseases. Kidney Int. 2011;80:1258–1270. doi: 10.1038/ki.2011.368. [DOI] [PubMed] [Google Scholar]
- 22.Orantes-Navarro C.M., Almaguer-López M.M., Alonso-Galbán P. The chronic kidney disease epidemic in El Salvador: a cross-sectional study. MEDICC Rev. 2019;21:29–37. doi: 10.37757/MR2019.V21.N2-3.7. [DOI] [PubMed] [Google Scholar]
- 23.Gonzalez-Quiroz M., Smpokou E.T., Silverwood R.J. Decline in kidney function among apparently healthy young adults at risk of Mesoamerican nephropathy. J Am Soc Nephrol. 2018;29:2200–2212. doi: 10.1681/ASN.2018020151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lunyera J., Mohottige D., Von Isenburg M. CKD of uncertain etiology: a systematic review. Clin J Am Soc Nephrol. 2016;11:379–385. doi: 10.2215/CJN.07500715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pearce N., Caplin B. Let’s take the heat out of the CKDu debate: more evidence is needed. Occup Environ Med. 2019;76:357–359. doi: 10.1136/oemed-2018-105427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Athuraliya N.T., Abeysekera T.D., Amerasinghe P.H. Uncertain etiologies of proteinuric-chronic kidney disease in rural Sri Lanka. Kidney Int. 2011;80:1212–1221. doi: 10.1038/ki.2011.258. [DOI] [PubMed] [Google Scholar]
- 27.Nanayakkara S., Komiya T., Ratnatunga N. Tubulointerstitial damage as the major pathological lesion in endemic chronic kidney disease among farmers in North Central Province of Sri Lanka. Environ Health Prev Med. 2012;17:213–221. doi: 10.1007/s12199-011-0243-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wijetunge S., Ratnatunga N.V., Abeysekera D.T. Retrospective analysis of renal histology in asymptomatic patients with probable chronic kidney disease of unknown aetiology in Sri Lanka. Ceylon Med J. 2013;58:142–147. doi: 10.4038/cmj.v58i4.6304. [DOI] [PubMed] [Google Scholar]
- 29.Wijetunge S., Ratnatunga N.V., Abeysekera T.D. Endemic chronic kidney disease of unknown etiology in Sri Lanka: correlation of pathology with clinical stages. Indian J Nephrol. 2015;25:274–280. doi: 10.4103/0971-4065.145095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Badurdeen Z., Nanayakkara N., Ratnatunga N.V. Chronic kidney disease of uncertain etiology in Sri Lanka is a possible sequel of interstitial nephritis! Clin Nephrol. 2016;86:106–109. doi: 10.5414/CNP86S115. [DOI] [PubMed] [Google Scholar]
- 31.Selvarajah M., Weeratunga P., Sivayoganthan S. Clinicopathological correlates of chronic kidney disease of unknown etiology in Sri Lanka. Indian J Nephrol. 2016;26:357–363. doi: 10.4103/0971-4065.167280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wijkström J., Jayasumana C., Dassanayake R. Morphological and clinical findings in Sri Lankan patients with chronic kidney disease of unknown cause (CKDu): similarities and differences with Mesoamerican Nephropathy. PloS One. 2018;13 doi: 10.1371/journal.pone.0193056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Anand S., Montez-Rath M.E., Adasooriya D. prospective biopsy-based study of CKD of unknown etiology in Sri Lanka. Clin J Am Soc Nephrol. 2019;14:224–232. doi: 10.2215/CJN.07430618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wijkström J., Leiva R., Elinder C.G. Clinical and pathological characterization of Mesoamerican nephropathy: a new kidney disease in Central America. Am J Kidney Dis. 2013;62:908–918. doi: 10.1053/j.ajkd.2013.05.019. [DOI] [PubMed] [Google Scholar]
- 35.López-Marín L., Chávez Y., García X.A. Histopathology of chronic kidney disease of unknown etiology in Salvadoran agricultural communities. MEDICC Rev. 2014;16:49–54. doi: 10.37757/MR2014.V16.N2.8. [DOI] [PubMed] [Google Scholar]
- 36.Wijkström J., González-Quiroz M., Hernandez M. Renal morphology, clinical findings, and progression rate in Mesoamerican nephropathy. Am J Kidney Dis. 2017;69:626–636. doi: 10.1053/j.ajkd.2016.10.036. [DOI] [PubMed] [Google Scholar]
- 37.Fischer R.S.B., Vangala C., Truong L. Early detection of acute tubulointerstitial nephritis in the genesis of Mesoamerican nephropathy. Kidney Int. 2018;93:681–690. doi: 10.1016/j.kint.2017.09.012. [DOI] [PubMed] [Google Scholar]
- 38.Vervaet B.A., Nast C.C., Jayasumana C. Chronic interstitial nephritis in agricultural communities is a toxin-induced proximal tubular nephropathy. Kidney Int. 2020;97:350–369. doi: 10.1016/j.kint.2019.11.009. [DOI] [PubMed] [Google Scholar]
- 39.Solez K., Axelsen R.A., Benediktsson H. International standardization of criteria for the histologic diagnosis of renal allograft rejection: the Banff working classification of kidney transplant pathology. Kidney Int. 1993;44:411–422. doi: 10.1038/ki.1993.259. [DOI] [PubMed] [Google Scholar]
- 40.Ratnayake S., Badurdeen Z., Nanayakkara N. Screening for chronic kidney disease of uncertain aetiology in Sri Lanka: usability of surrogate biomarkers over dipstick proteinuria. BMC Nephrol. 2017;18:199. doi: 10.1186/s12882-017-0610-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Diyabalanage S., Fonseka S., Dasanayake D.M. Environmental exposures of trace elements assessed using keratinized matrices from patients with chronic kidney diseases of uncertain etiology (CKDu) in Sri Lanka. J Trace Elem Med Biol. 2017;39:62–70. doi: 10.1016/j.jtemb.2016.08.003. [DOI] [PubMed] [Google Scholar]
- 42.Fernando W.B., Nanayakkara N., Gunarathne L. Serum and urine fluoride levels in populations of high environmental fluoride exposure with endemic CKDu: a case-control study from Sri Lanka. Environ Geochem Health. 2020;42:1497–1504. doi: 10.1007/s10653-019-00444-x. [DOI] [PubMed] [Google Scholar]
- 43.Badurdeen Z., Hemage R., Fernando B. SAT-176 A pilot study: manifestation of candidate renal biomarkers in patients with chronic kidney disease of uncertain etiology. Kidney Int Rep. 2019;4:S80–S81. doi: 10.1016/j.ekir.2019.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fernando W.B., Hettiarachchi T.W., Sudeshika T. Snap shot view on anemia in chronic kidney disease of uncertain aetiology. Nephrology. 2019;24:1033–1040. doi: 10.1111/nep.13545. [DOI] [PubMed] [Google Scholar]
- 45.Joyce E., Glasner P., Ranganathan S. Tubulointerstitial nephritis: diagnosis, treatment, and monitoring. Pediatr Nephrol. 2017;32:577–587. doi: 10.1007/s00467-016-3394-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hodgkins K.S., Schnaper H.W. Tubulointerstitial injury and the progression of chronic kidney disease. Pediatr Nephrol. 2012;27:901–909. doi: 10.1007/s00467-011-1992-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Councilman W.T. Acute interstitial nephritis. J Exp Med. 1898;3:393–420. doi: 10.1084/jem.3.4-5.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Baker R.J., Pusey C.D. The changing profile of acute tubulointerstitial nephritis. Nephrol Dial Transplant. 2004;19:8–11. doi: 10.1093/ndt/gfg464. [DOI] [PubMed] [Google Scholar]
- 49.Murray T., Goldberg M. Chronic interstitial nephritis: etiologic factors. Ann Intern Med. 1975;82:453–459. doi: 10.7326/0003-4819-82-4-453. [DOI] [PubMed] [Google Scholar]
- 50.Cohen A.H. Chronic interstitial nephritis. In: Fogo A.B., Cohen A.H., Jennette J.C., Bruijn J.A., Colvin R.B., editors. Fundamentals of Renal Pathology. Springer; New York, NY: 2013. pp. 149–152. [Google Scholar]
- 51.Hoy W.E., Rees M., Kile E. A new dimension to the Barker hypothesis: low birthweight and susceptibility to renal disease. Kidney Int. 1999;56:1072–1077. doi: 10.1046/j.1523-1755.1999.00633.x. [DOI] [PubMed] [Google Scholar]
- 52.Lackland D.T., Bendall H.E., Osmond C. Low birth weights contribute to the high rates of early-onset chronic renal failure in the southeastern United States. Arch Intern Med. 2000;160:1472–1476. doi: 10.1001/archinte.160.10.1472. [DOI] [PubMed] [Google Scholar]
- 53.Hsu C.Y., Lin F., Vittinghoff E. Racial differences in the progression from chronic renal insufficiency to end-stage renal disease in the United States. J Am Soc Nephrol. 2003;14:2902–2907. doi: 10.1097/01.asn.0000091586.46532.b4. [DOI] [PubMed] [Google Scholar]
- 54.Luyckx V.A., Brenner B.M. Low birth weight, nephron number, and kidney disease. Kidney Int. 2005;68:S68–S77. doi: 10.1111/j.1523-1755.2005.09712.x. [DOI] [PubMed] [Google Scholar]
- 55.Vikse B.E., Irgens L.M., Leivestad T. Low birth weight increases risk for end-stage renal disease. J Am Soc Nephrol. 2008;19:151–157. doi: 10.1681/ASN.2007020252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.White S.L., Perkovic V., Cass A. Is low birth weight an antecedent of CKD in later life? A systematic review of observational studies. Am J Kidney Dis. 2009;54:248–261. doi: 10.1053/j.ajkd.2008.12.042. [DOI] [PubMed] [Google Scholar]
- 57.Hoy W.E., Hughson M.D., Bertram J.F. Nephron number, hypertension, renal disease, and renal failure. J Am Soc Nephrol. 2005;16:2557–2564. doi: 10.1681/ASN.2005020172. [DOI] [PubMed] [Google Scholar]
- 58.Luyckx V.A., Shukha K., Brenner B.M. Low nephron number and its clinical consequences. Rambam Maimonides Med J. 2011;2:e0061. doi: 10.5041/RMMJ.10061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Haas M. Mesoamerican nephropathy: pathology in search of etiology. Kidney Int. 2018;93:538–540. doi: 10.1016/j.kint.2017.09.025. [DOI] [PubMed] [Google Scholar]
- 60.Gamage C.D., Yoshimatsu K., Sarathkumara Y.D. Serological evidence of hantavirus infection in Girandurukotte, an area endemic for chronic kidney disease of unknown aetiology (CKDu) in Sri Lanka. Int J Infect Dis. 2017;57:77–78. doi: 10.1016/j.ijid.2017.02.004. [DOI] [PubMed] [Google Scholar]
- 61.Riefkohl A., Ramírez-Rubio O., Laws R.L. Leptospira seropositivity as a risk factor for Mesoamerican nephropathy. Int J Occup Environ Health. 2017;23:1–10. doi: 10.1080/10773525.2016.1275462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gunatilake S., Seneff S., Orlando L. Glyphosate’s synergistic toxicity in combination with other factors as a cause of chronic kidney disease of unknown origin. Int J Environ Res Public Health. 2019;16:2734. doi: 10.3390/ijerph16152734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Liyanage M., Maganaarachchi D., Priyadarshika C. Cyanobacteria and cyanotoxins in well waters of the girandurukotte, CKDu endemic area in Sri Lanka; do they drink safe water? J Ecotech Res. 2016;18:17–21. [Google Scholar]
- 64.Peiris-John R.J., Wanigasuriya J.K.P., Wickremasinghe A.R. Exposure to acetylcholinesterase-inhibiting pesticides and chronic renal failure. Ceylon Med J. 2006;51:42–43. doi: 10.4038/cmj.v51i1.1382. [DOI] [PubMed] [Google Scholar]
- 65.Jayasumana C., Gunatilake S., Senanayake P. Glyphosate, hard water and nephrotoxic metals: are they the culprits behind the epidemic of chronic kidney disease of unknown etiology in Sri Lanka? Int J Environ Res Public Health. 2014;11:2125–2147. doi: 10.3390/ijerph110202125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bandara J.M.R.S., Wijewardena H.V.P., Liyanege J. Chronic renal failure in Sri Lanka caused by elevated dietary cadmium: Trojan horse of the green revolution. Toxicol Lett. 2010;198:33–39. doi: 10.1016/j.toxlet.2010.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bandara J.M.R.S., Wijewardena H.V.P., Bandara Y.M.A.Y. Pollution of River Mahaweli and farmlands under irrigation by cadmium from agricultural inputs leading to a chronic renal failure epidemic among farmers in NCP, Sri Lanka. Environ Geochem Health. 2011;33:439–453. doi: 10.1007/s10653-010-9344-4. [DOI] [PubMed] [Google Scholar]
- 68.Wanigasuriya K.P., Peiris-John R.J., Wickremasinghe R. Chronic kidney disease of unknown aetiology in Sri Lanka: is cadmium a likely cause? BMC Nephrol. 2011;12:32. doi: 10.1186/1471-2369-12-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Jayasumana M.A.C.S., Paranagama P.A., Amarasinghe M.D. Possible link of chronic arsenic toxicity with chronic kidney disease of unknown etiology in Sri Lanka. J Nat Sci Res. 2013;3:64–73. [Google Scholar]
- 70.Chandrajith R., Dissanayake C.B., Ariyarathna T. Dose-dependent Na and Ca in fluoride-rich drinking water — another major cause of chronic renal failure in tropical arid regions. Sci Total Environ. 2011;409:671–675. doi: 10.1016/j.scitotenv.2010.10.046. [DOI] [PubMed] [Google Scholar]
- 71.Wasana H.M., Aluthpatabendi D., Kularatne W.M.T.D. Drinking water quality and chronic kidney disease of unknown etiology (CKDu): synergic effects of fluoride, cadmium and hardness of water. Environ Geochem Health. 2016;38:157–168. doi: 10.1007/s10653-015-9699-7. [DOI] [PubMed] [Google Scholar]
- 72.Dharma-Wardana M.W., Amarasiri S.L., Dharmawardene N. Chronic kidney disease of unknown aetiology and ground-water ionicity: study based on Sri Lanka. Environ Geochem Health. 2015;37:221–231. doi: 10.1007/s10653-014-9641-4. [DOI] [PubMed] [Google Scholar]
- 73.Wesseling C., Aragón A., González M. Heat stress, hydration and uric acid: a cross-sectional study in workers of three occupations in a hotspot of Mesoamerican nephropathy in Nicaragua. BMJ Open. 2016;6 doi: 10.1136/bmjopen-2016-011034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.García-Trabanino R., Jarquín E., Wesseling C. Heat stress, dehydration, and kidney function in sugarcane cutters in El Salvador–a cross-shift study of workers at risk of Mesoamerican nephropathy. Environ Res. 2015;142:746–755. doi: 10.1016/j.envres.2015.07.007. [DOI] [PubMed] [Google Scholar]
- 75.González-Quiroz M., Pearce N., Caplin B. What do epidemiological studies tell us about chronic kidney disease of undetermined cause in Meso-America? A systematic review and meta-analysis. Clin Kidney J. 2018;11:496–506. doi: 10.1093/ckj/sfx136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Smpokou E.T., González-Quiroz M., Martins C. Environmental exposures in young adults with declining kidney function in a population at risk of Mesoamerican nephropathy. Occup Environ Med. 2019;76:920–926. doi: 10.1136/oemed-2019-105772. [DOI] [PubMed] [Google Scholar]
- 77.Herath C., Jayasumana C., De Silva P.M.C.S. Kidney diseases in agricultural communities: a case against heat-stress nephropathy. Kidney Int Rep. 2018;3:271–280. doi: 10.1016/j.ekir.2017.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Johnson R.J., Wesseling C., Newman L.S. Chronic kidney disease of unknown cause in agricultural communities. N Engl J Med. 2019;380:1843–1852. doi: 10.1056/NEJMra1813869. [DOI] [PubMed] [Google Scholar]
- 79.Pearce N., Caplin B., Gunawardena N. CKD of unknown cause: a global epidemic? Kidney Int Rep. 2018;4:367–369. doi: 10.1016/j.ekir.2018.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kaur P., Gunawardena N., Kumaresan J. A review of chronic kidney disease of unknown etiology in Sri Lanka, 2001–2015. Indian J Nephrol. 2020;30:245–252. doi: 10.4103/ijn.IJN_359_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lozier M., Turcios-Ruiz R.M., Noonan G. Chronic kidney disease of nontraditional etiology in Central America: a provisional epidemiologic case definition for surveillance and epidemiologic studies. Revista Panamericana de Salud Pública. 2016;40:294–300. [PMC free article] [PubMed] [Google Scholar]
- 82.Redmon J.H., Elledge M.F., Womack D.S. Additional perspectives on chronic kidney disease of unknown aetiology (CKDu) in Sri Lanka–lessons learned from the WHO CKDu population prevalence study. BMC Nephrol. 2014;15:1–10. doi: 10.1186/1471-2369-15-125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Epidemiology Unit, Ministry of Health care and nutrition. Weekly epidemiological report. December 2009: Vol 36. No 49. https://www.epid.gov.lk/web/pdf/wer_2009/vol_36_no_49_english.pdf Available at:
- 84.World Health Organization International Expert Consultation on Chronic Kidney Disease of Unknown Etiology. Colombo, Sri Lanka. 2016:27–29. https://apps.who.int/iris/bitstream/handle/10665/255137/Reportexpertconsultationonckdu.pdf;sequence=1 Available at: Accessed August 16, 2020.
- 85.Kumaresan J., Seneviratne R. Beginning of a journey: unraveling the mystery of chronic kidney disease of unknown aetiology (CKDu) in Sri Lanka. Glob Health. 2017;13:43. doi: 10.1186/s12992-017-0268-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Wijewickrama E.S., Gunawardena N., Jayasinghe S. CKD of unknown etiology (CKDu) in Sri Lanka: a multilevel clinical case definition for surveillance and epidemiological studies. Kidney Int Rep. 2019;4:781–785. doi: 10.1016/j.ekir.2019.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Jayasekara J.M., Dissanayake D.M., Adhikari S.B. Geographical distribution of chronic kidney disease of unknown origin in North Central Region of Sri Lanka. Ceylon Med J. 2013;58:6–10. doi: 10.4038/cmj.v58i1.5356. [DOI] [PubMed] [Google Scholar]
- 88.Bajema I.M., Wilhelmus S., Alpers C.E. Revision of the International Society of Nephrology/Renal Pathology Society classification for lupus nephritis: clarification of definitions, and modified National Institutes of Health activity and chronicity indices. Kidney Int. 2018;93:789–796. doi: 10.1016/j.kint.2017.11.023. [DOI] [PubMed] [Google Scholar]
- 89.Working Group of the International IgA Nephropathy Network and the Renal Pathology Society The Oxford classification of IgA nephropathy: pathology definitions, correlations, and reproducibility. Kidney Int. 2009;76:546–556. doi: 10.1038/ki.2009.168. [DOI] [PubMed] [Google Scholar]
- 90.Roufosse C., Simmonds N., Clahsen-van Groningen M. A 2018 reference guide to the Banff classification of renal allograft pathology. Transplantation. 2018;102:1795–1814. doi: 10.1097/TP.0000000000002366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Banff Foundation for Allograft Pathology. www.banfffoundation.org Available at: Accessed August 16, 2020.
- 92.Furness P.N., Taub N. International variation in the interpretation of renal transplant biopsies: report of the CERTPAP Project. Kidney Int. 2001;60:1998–2012. doi: 10.1046/j.1523-1755.2001.00030.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.