Abstract
Hematopoietic stem cell transplantation (HCT) is a curative treatment for patients with myelofibrosis (MF); however, many HCT-eligible patients decline this potentially life-saving procedure. The reasons behind this decision are not clear. We sought to survey patients with MF to understand their perspective on HCT. A 63-question survey was posted on myeloproliferative neoplasm patient advocacy websites. A total of 129 patients with MF responded to the survey. Among these patients, 49 (41%) were referred for HCT, and 41(32%) attended the transplantation consult. Of the patients who attended the transplantation consult, 24 (59%) did not plan on going on to HCT, and 16 (41%) intended to proceed with HCT. Reasons for the decision to not undergo transplantation included the desire to not be ill, desire to not spend time in the hospital, and concerns about overall quality of life. Specifically, concerns related to financial impact and the risk of graft-versus-host disease (GVHD) were expressed. Patients who decided to proceed with HCT felt that this would extend their survival and allow them to be around family for longer. This is the first survey to investigate patient perceptions regarding HCT for MF. Less than one-half of the patients were referred for HCT, and of those, less than one-half planned on proceeding with the transplantation, suggesting that many patients do not receive this life-saving procedure. Further exploration of the basis of patients’ reluctance to proceed with HCT is warranted.
Keywords: Myelofibrosis, Bone marrow transplantation, Survey, Patient perspective, Quality of life
INTRODUCTION
Hematopoietic stem cell transplantation (HCT) is a potentially curative treatment for patients with primary myelofibrosis (PMF), post-polycythemia vera MF (PPV-MF) and post-essential thrombocytosis MF (PET-MF) [1–10]. HCT is generally reserved for younger patients with good performance status [11 ] and has been shown to provide a survival benefit in properly selected patients [8]. The optimal timing of HCT is not entirely clear but is dependent on risk/benefit considerations. The risk may be determined using the Dynamic International Prognostic Scoring System (DIPSS) [12], a prognostic score derived from age, percentage of blasts, total WBC count, hemoglobin, and constitutional symptoms. Intermediate-2 risk disease is generally when HCT is considered, but it may be considered earlier in patients with intermediate-1 risk disease and high-risk features based on karyotype or genetic mutations, such as ASXL1 [11,13]. Treatment-related mortality ranges from 25% to 40% [13], and disease relapse occurs in 15% to 20% of patients [13]. Survival at 5 years ranges from 38% to 74% [14,15]. Although HCT is a curative treatment, many patients decline therapy due to the risks of morbidity and mortality.
The reasons behind declining bone marrow HCT have not been fully explored. The purpose of this study was to survey patients with myeloproliferative neoplasms (MPNs) in an attempt to understand their views on bone marrow transplantation.
METHODS
A 63-question survey was developed by MPN and transplantation specialists. The Mayo Clinic Institutional Review Board approved the study protocol and materials before the survey was disseminated. The survey was promoted online via multiple MPN related websites, including the MPN Forum (http://www.mpnforum.com), MPN Research Foundation (www.mpnresearchfoundation.org/), and MPN Voice (www.mpnvoice.org.uk/), along with their respective Facebook pages. The survey was posted for all patients with MPN; however, only patients with MF/PET-MF/PPV-MF were included in the analysis.
The survey consisted of multiple questions about type of disease, duration of disease, previous therapies, transfusion requirements, and referral for HCT. Depending on the answer to the latter question, additional questions asked about the reason for referral or nonreferral, whether they proceeded with the consult, whether they planned to proceed with HCT and the reasoning behind their decisions. The questions pertaining to reasons behind the decisions made were framed on a Likert scale of 1 to 5 (1, not important; 2, slightly important; 3, moderately important; 4, important; 5, very important).
The data for the survey were collected in a REDCap database for analysis and stored in a secure location. All responses were anonymous, with no personal identifying information. Respondents were asked to complete the survey only once and were required to provide a modified consent before accessing the survey. They did not receive compensation for survey completion.
Descriptive statistics were used to summarize patient survey responses and to identify differences between those who planned on proceeding with HCT and those who did not. Fisher’s exact test was used for categorical variables, and the Wilcoxon Mann-Whitney test was used for continuous variables. A P value <.05 was considered significant. Statistical analyses were performed using SAS version 9.4 (SAS Institute, Cary, NC).
RESULTS
Participants
A total of 366 individuals responded to the survey, including 129 with MF, 62 with primary MF, and 67 with PET-MF/PPV-MF. The baseline characteristics are presented in Table 1. The median age was 61 years (range, 31 to 84 years), and 49 (38%) were male. The majority identified themselves as white (n = 125; 97%).
Table 1.
Patient Characteristics
| Characteristic | Value |
|---|---|
| Number of patients | 129 |
| Age, yr, median (range) | 61.0 (31.0–84.0) |
| Sex, n (%) | |
| Male | 49 (38.0) |
| Female | 80 (62.0) |
| Race, n (%) | |
| American Indian or Alaska Native | 1 (.8) |
| Asian | 1 (.8) |
| Black or African American | 2(1.6) |
| White | 125 (96.9) |
| Relationship status, n (%) | |
| Single | 14(10.9) |
| Married/partnered | 95 (73.6) |
| Separated | 1 (−8) |
| Divorced | 14(10.9) |
| Widowed | 5(3.9) |
| Education, n (%) | |
| Missing | 4 |
| High school or less | 7(5.6) |
| Some college | 30 (24.0) |
| Bachelor’s degree | 42 (33.6) |
| Postgraduate degree | 46 (36.8) |
| Currently employed, n (%) | |
| Missing | 3 |
| No | 83 (65.9) |
| Yes | 43(34.1) |
| Employment status, n (%) | |
| Missing | 90 |
| Full time | 32(82.1) |
| Part time | 7(17.9) |
| Health insurance, n (%) | |
| Private insurance with 1 insurance company | 60 (46.5) |
| Private insurance with 2 insurance companies | 3(2.3) |
| Medicare/Medicaid | 45 (34.9) |
| None | 5(3.9) |
| Other | 16(12.4) |
| Annual household income, n (%) | |
| Missing | 4 |
| <$25,000 | 13(10.4) |
| $25,000–$50,000 | 18(14.4) |
| $50,000–$ 100,000 | 42 (33.6) |
| $100,000–$250,000 | 42 (33.6) |
| >$250,000 | 10(8.0) |
| Transplantation specialist, n (%) | |
| Private practice/community practice | 45 (34.9) |
| Academic medical center/teaching program | 76 (58.9) |
| Other | 8(6.2) |
One hundred eighteen (91%) had at least some college, and 46 (37%) had a postgraduate degree. Sixty-three (49%) had private insurance, and 45 (35%) had Medicare/Medicaid. The patients’ disease characteristics are presented in Table 2. Sixty-nine (53%) were on JAK inhibitor therapy, and 19 (14.7%) had received an RBC transfusion within the previous 3 months.
Table 2.
Disease Characteristics (N = 129)
| Characteristic | Value |
|---|---|
| Type of MPN first diagnosed, n (%) | |
| MF | 62 (48.1) |
| PET-MF | 35 (27.1) |
| PPV-MF | 32 (24.8) |
| Age at diagnosis of first MPN, yr, median (range) | 49.0(19.0–77.0) |
| Did your disease transform into a new disease?, n (%) | |
| No | 61 (47.3) |
| Yes | 68 (52.7) |
| How old were you when your disease trans-formed?, yr, median (range) | 59.0 (30.0–80.0) |
| Have you ever received genetic testing for mutations that contribute to your disease? | |
| No | 32 (24.8) |
| Yes | 97 (75.2) |
| If you answered “yes” to the previous question, check all you have been told you are positive for, n (%): | |
| JAK2 | 69 (53.5) |
| TET2 | 2(1.6) |
| CALR | 21 (16.3) |
| MPL | 6(4.7) |
| ASXL1 | 8 (6.2) |
| EXH2 | 3(2.3) |
| IDH1/2 | 1 (.8) |
| Are you currently on a JAK inhibitor?, n (%) | |
| No | 60 (46.5) |
| Yes | 69 (53.5) |
| Please indicate which JAK2 inhibitor you have received, n (%) | |
| Ruxolitinib | 65 (50.4) |
| Momelotinib | 3(2.3) |
| Other JAK2 inhibitor/study drug JAK2 inhibitor | 2(1.6) |
| Hemorrhage, n (%) | |
| No | 121 (93.8) |
| Yes | 8 (6.2) |
| Thrombosis, n (%) | |
| No | 108 (83.7) |
| Yes | 21 (16.3) |
| Have you required a red blood cell transfusion in the last 3 months?, n (%) | |
| No | 110(85.3) |
| Yes | 19(14.7) |
| How many red blood cell transfusions?, median (range) | 4.0(1.0–12.0) |
| Have you required a platelet transfusion in the last 3 months?, n (%) | |
| Missing | 2 |
| No | 124(97.6) |
| Yes | 3(2.4) |
| Have you ever participated in a clinical trial for your MPN?, n (%) | |
| Missing | 2 |
| No | 100(78.7%) |
| Yes | 27 (21.3%) |
| Have you been referred for a bone marrow transplantation?, n (%) | |
| Missing | 9 |
| No | 71 (59.2) |
| Yes | 49 (40.8) |
| If yes, what website did you use (choose all that apply)?, n (%) | |
| 57 (44.2) | |
| MPN Forum | 102(79.1) |
| MPN Research Foundation | 95 (73.6) |
| MPN Connect | 38 (29.5) |
| Other | 32 (24.8) |
Transplantation
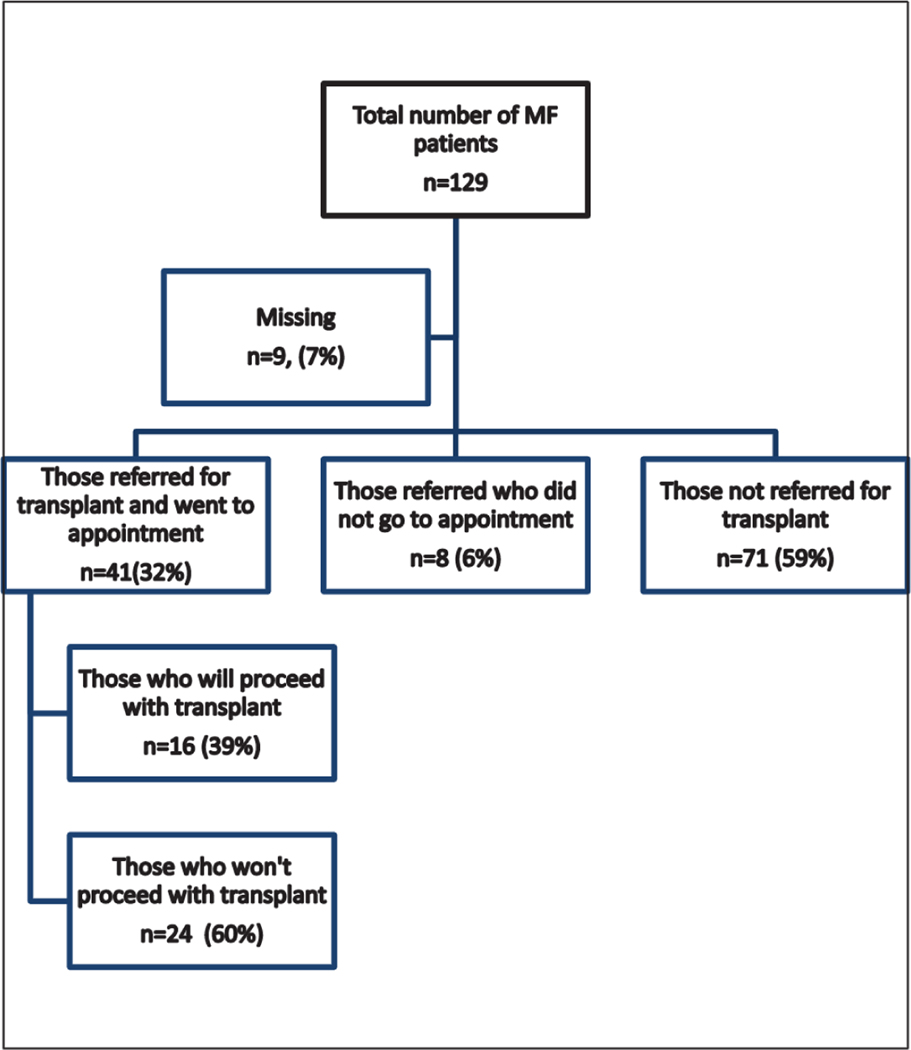
Of the 129 respondents with MF, 49 (41%) were referred for HCT, and 41 (32%) subsequently attended an HCT consult. The reasons that 8 patients chose not to attend the consult are summarized in Supplementary Table 1. Seventy-one patients (59%) were not referred for HCT (summarized in Figure 1). A comparison of the patients referred for HCT and those not referred showed similarities in terms of age, time of diagnosis, and education. Data from the transplantation consult are presented in Table 3. Of those who attended a transplantation consult, 30 (73%) had HLA typing themselves and 21 (55%) had typing of siblings. The majority of the patients felt that they received adequate information to make a decision regarding transplantation (n = 35; 85%), and 11 patients (27%) sought more than 1 opinion.
Figure 1.
Diagram of survey participation.
Table 3.
Factors Associated with HCT Consults (N = 41)
| Factor | Value, n (%) |
|---|---|
| How far did you travel to the appointment? | |
| <50 miles | 21 (51.2) |
| 50–100 miles | 8(19.5) |
| 100–300 miles | 8(19.5) |
| >300 miles | 4(9.8) |
| Did your insurance cover the consult? | |
| No | 2(4.9) |
| Yes | 39 (95.1) |
| Did your insurance cover travel expenses? | |
| No | 38 (92.7) |
| Yes | 3(7.3) |
| Did you feel like you received all the information needed to make a decision regarding bone marrow transplantation? | |
| No | 6(14.6) |
| Yes | 35 (85.4) |
| Did you undergo tissue typing or HLA typing (did they draw your blood to help identify a donor)? | |
| No | 11 (26.8) |
| Yes | 30 (73.2) |
| Were siblings HLA-typed? | |
| Missing | 3 |
| No | 17(44.7) |
| Yes | 21 (55.3) |
| Did you seek more than 1 opinion on bone marrow transplantation? | |
| No | 30 (73.2) |
| Yes | 11 (26.8) |
Among the patients who attended a transplantation consult, 24 (59%) did not plan to go to F1CT and 16 (41%) intended to proceed with HCT. The reasons for these decisions are summarized in Table 4. Respondents rated items as either not important (1 on the Likert scale) or important (2 to 5 on the Likert scale, representing slightly important, moderately important, important, and very important). Patients endorsed improved survival and the desire to be around for family as reasons to proceed with F1CT. Reasons for not proceeding with F1CT reported by more than one-half of patients included the desire to not get sick or spend time in the hospital, and the belief that transplantation would worsen quality of life and would not increase life expectancy. The majority of patients were concerned about graft-versus-host disease and dying from the transplantation. Approximately one-half of patients expressed concerns about the financial impact of transplantation on themselves and/or their family.
Table 4.
Reasons for Proceeding or Not Proceeding with HCT
| Reasons | Not important, n | Important, n |
|---|---|---|
| Reasons for proceeding (n = 16) | ||
| Feel my survival is better with transplantation | 0 | 16 |
| Want to be around for my family | 0 | 16 |
| Am afraid of dying | 8 | 8 |
| Feel my current treatment plan is not working | 10 | 6 |
| Have heard positive stories from other transplant patients | 6 | 10 |
| Reasons for not proceeding (n = 24) | ||
| Worried about the financial impact it will have on my family | 13 | 11 |
| Worried about the financial impact it will have on me | 12 | 12 |
| Do not want my family to have to take care of me | 10 | 14 |
| Do not want to get sick | 6 | 18 |
| Would rather enjoy the time I have left | 5 | 19 |
| Do not want to spend a long time in the hospital | 5 | 18 |
| Do not believe it will dramatically improve my life expectancy | 4 | 20 |
| Feel my quality of life will be worse with the transplantation | 3 | 21 |
| Worried about graft-versus-host disease | 2 | 22 |
| Worried about dying from the transplantation | 2 | 22 |
There were no significant differences in age, distance from the transplantation center, or symptom score between patients planning to proceed with HCT and those not proceeding (Table 5).
Table 5.
Variables Associated with Proceeding with or Declining HCT
| Variable | No (N = 24) | Yes(N = 16) | Total (N = 40) | P Value | |
|---|---|---|---|---|---|
| Age, yr, median (range) | 62.0(31.0–78.0) | 61.0 (34.0–67.0) | 62.0 (31.0–78.0) | .2393 | |
| TSS, median (range) | 23.0 (6.0–83.0) | 24.5 (0.0–69.0) | 24.5 (0.0–83.0) | .8251 | |
| Education, n (%) | |||||
| Missing | 1 | 0 | 1 | .3015 | |
| High school or less | 1 (4.3) | 2(12.5) | 3(7.7) | ||
| Some college | 9(39.1) | 2(12.5) | 11 (28.2) | ||
| Bachelor’s degree | 8 (34.8) | 6(37.5) | 14(35.9) | ||
| Postgraduate degree | 5(21.7) | 6(37.5) | 11 (28.2) | ||
| Travel distance | |||||
| <50 miles | 14(58.3) | 6(37.5) | 20 (50.0) | .1570 | |
| 50–100 miles | 5 (20.8) | 3(18.8) | 8 (20.0) | ||
| 100–300 miles | 3(12.5) | 5(31.3) | 8 (20.0) | ||
| >300 miles | 2(8.3) | 2(12.5) | 4(10.0) | ||
| Time since diagnosis, yr, median (range) | 10.0(1.0–29.0) | 6.0(0.0–31.0) | 6.5 (0.0–31.0) | .3833* | |
Wilcoxon rank-sum test.
DISCUSSION
Our survey yielded several interesting findings. First, less than one-half the patients with MF were referred for bone marrow transplantation, and second, less than one-half of the patients who attended a transplantation consultation indicated a plan to proceed with HCT. The finding that less than one-half of the patients were referred for transplantation was surprising, given the median age of respondents of 61 years, with more than one-half at an age appropriate for HCT. However, because we do not have data on DIPSS scores or other clinical variables, it is difficult to determine whether these patients would be appropriate candidates for HCT. When this was evaluated in patients with myelodysplastic syndrome, a similar type of disease and with a similar HCT-appropriate age range, only 11% of patients were referred for transplantation, even though 27% were age <65 years and >40% had disease risk appropriate for transplantation [16]. Both MDS and MF frequently do not have a significant symptom burden but can progress over time. Patients are often reluctant to proceed with transplantation when they are feeling well, not taking into account the fact that these diseases can develop more serious manifestations, possibly necessitating transfusions and potentially transforming into acute leukemia. Future studies should examine barriers to the referral process on the part of community hematology/oncology providers. The 2018 National Cancer Center Network guidelines may help educate physicians and provide guidance to the appropriate time for referral [17].
Another unexpected finding was that less than one-half the patients who attended a transplantation consultation planned on proceeding to HCT. Interestingly, the decision to proceed with transplantation does not appear to be dependent on age, MPN symptom score, duration of disease, educational level, or distance from the transplantation center. Although financial and quality of life concerns are important in this decision, it is difficult to know how patients perceive transplantation and where these perceptions originate. In reviewing the literature, patients’ perception of HCT is not well understood. Different transplantation physicians emphasize different aspects of the transplantation procedure. However, there likely are factors outside of the consultation with the transplantation physician that impact perception. One study involving self-administered questionnaires in patients during the peritransplantation period found that more than one-half of the patients had made a decision about transplantation before their first consultation [18]. Other sources of information may include nontransplantation hematologists and the Internet. Information on social media can have a significant impact on patients’ decision making. A 2009 study showed that 5% of patients participated in an online support group, 7% followed a blog, and 23% were part of a social media group [19]. A Pew Research Center Internet study found that >80% of patients have access to the Internet and use it for medical information, suggesting that the majority of patients receive at least some of their information from Internet sources [20].
Our study has several limitations. The data were acquired through questionnaires posted on patient advocacy websites, and thus these patients likely are better informed and are better self-advocates. We do not have detailed medical data on the patients, and so where they are in the disease spectrum is unknown. Our population was fairly homogenous in terms of ethnicity and education. Furthermore, a team of investigators created responses to questions, and respondents were asked to select all/any applicable responses with an option in many items for an open-ended response. Although optimally we would have asked opened-ended questions to obtain more information, we were limited by the ability of staff to code open responses. Focus groups would have been optimal to generate a comprehensive list of patient responses.
These limitations notwithstanding, our data highlight the need to provide accurate information and education about the benefits of transplantation versus nontransplantation therapy. Information not only on survival, but also on the impact on different domains of quality of life, is critical for these patients.
Supplementary Material
Footnotes
Conflict of interest statement: There are no conflicts of interest to report.
SUPPLEMENTARY DATA
Supplementary data related to this article can be found online at doi:10.1016/j.bbmt.2018.09.033.
REFERENCES
- 1.Abelsson J, Merup M, Birgegârd G, et al. The outcome of allo-HSCT for 92 patients with myelofibrosis in the Nordic countries. Bone Marrow Transplant. 2012;47:380–386. [DOI] [PubMed] [Google Scholar]
- 2.Alchalby H, Yunus DR, Zabelina T, et al. Risk models predicting survival after reduced-intensity transplantation for myelofibrosis. Br J Haematol. 2012;157:75–85. [DOI] [PubMed] [Google Scholar]
- 3.Ballen KK, Shrestha S, Sobocinski KA, et al. Outcome of transplantation for myelofibrosis. Biol Blood Marrow Transplant. 2010;16:358–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Deeg HJ, Bredeson C, Farnia S, et al. Hematopoietic cell transplantation as curative therapy for patients with myelofibrosis: long-term success in all age groups. Biol Blood Marrow Transplant. 2015;21:1883–1887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gupta V, Malone AK, Hari PN, et al. Reduced-intensity hematopoietic cell transplantation for patients with primary myelofibrosis: a cohort analysis from the Center for International Blood and Marrow Transplant Research. Biol Blood Marrow Transplant. 2014;20:89–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kerbauy DM Gooley TA Sale GE, et al. Hematopoietic cell transplantation as curative therapy for idiopathic myelofibrosis, advanced polycythemia vera, and essential thrombocythemia. Biol Blood Marrow Transplant 2007;13:355–365. [DOI] [PubMed] [Google Scholar]
- 7.Keyzner A, Han S, Shapiro S, et al. Outcome of allogeneic hematopoietic stem cell transplantation for patients with chronic and advanced phase myelofibrosis. Biol Blood Marrow Transplant. 2016;22:2180–2186. [DOI] [PubMed] [Google Scholar]
- 8.Kroger N, Giorgino T, Scott BL, et al. Impact of allogeneic stem cell transplantation on survival of patients less than 65 years of age with primary myelofibrosis. Blood. 2015;125:3347–3350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lussana F, Rambaldi A, Finazzi MC, et al. Allogeneic hematopoietic stem cell transplantation in patients with polycythemia vera or essential thrombocythemia transformed to myelofibrosis or acute myeloid leukemia: a report from the MPN Subcommittee of the Chronic Malignancies Working Party of the European Group for Blood and Marrow Transplantation. Haematologica. 2014;99:916–921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rondelli D, Goldberg JD, Isola L, et al. MPD-RC 101 prospective study of reduced-intensity allogeneic hematopoietic stem cell transplantation in patients with myelofibrosis. Blood. 2014;124:1183–1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kroger NM, Deeg JH, Olavarria E, et al. Indication and management of allogeneic stem cell transplantation in primary myelofibrosis: a consensus process by an EBMT/ELN International Working Group. Leukemia. 2015;29:2126–2133. [DOI] [PubMed] [Google Scholar]
- 12.Passamonti F, Cervantes F, Vannucchi AM, et al. Dynamic International Prognostic Scoring System (DIPSS) predicts progression to acute myeloid leukemia in primary myelofibrosis. Blood. 2010;116:2857–2858. [DOI] [PubMed] [Google Scholar]
- 13.Jain T, Mesa RA Palmer JM. Allogeneic stem cell transplantation in myelofibrosis. Biol Blood Marrow Transplant. 2017;23:1429–1436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kroger N, Holler E, Kobbe G, et al. Allogeneic stem cell transplantation after reduced-intensity conditioning in patients with myelofibrosis: a prospective, multicenter study of the Chronic Leukemia Working Party of the European Group for Blood and Marrow Transplantation. Blood. 2009; 114: 5264–5270. [DOI] [PubMed] [Google Scholar]
- 15.Gupta V, Gotlib J, Radich JP, et al. Janus kinase inhibitors and allogeneic stem cell transplantation for myelofibrosis. Biol Blood Marrow Transplant. 2014;20:1274–1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pease DF, Ross JA Poynter JN, et al. Differences in community and academic practice patterns for newly diagnosed myelodysplastic syndromes (MDS) patients. Cancer Epidemiol. 2015;39:222–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mesa R, Jamieson C, Bhatia R, et al. Myeloproliferative neoplasms, version 2.2017, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Cancer Netw. 2016;14:1572–1611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jacoby LH, Maloy B, Cirenza E, Shelton W, Goggins T, Balint J. The basis of informed consent for BMT patients. Bone Marrow Transplant. 1999;23:711–717. [DOI] [PubMed] [Google Scholar]
- 19.Chou WY, Hunt YM, Beckjord EB, Moser RP, Hesse BW. Social media use in the United States: implications for health communication. J Med Internet Res. 2009;11:e48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pew Research Center. Internet/broadband fact sheet. 2018. Available at: http://www.pewinternet.org/fact-sheet/internet-broadband/. Accessed 2018.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.