Abstract
Mutations in Janus kinase 2 (JAK2) are implicated in the pathogenesis of Philadelphia-chromosome negative myeloproliferative neoplasms, including primary myelofibrosis, polycythemia vera, and essential thrombocythemia. Gandotinib (LY2784544), a potent inhibitor of JAK2 activity, shows increased potency for the JAK2V6l7F mutation. The study had a standard 3 + 3 dose-escalation design to define the maximum-tolerated dose. Primary objectives were to determine safety, tolerability, and recommended oral daily dose of gandotinib for patients with JAK2V617F-positive myelofibrosis, essential thrombocythemia, or polycythemia vera. Secondary objectives included estimating pharmacokinetic parameters and documenting evidence of efficacy by measuring clinical improvement. Thirty-eight patients were enrolled and treated (31 myelofibrosis, 6 polycythemia vera, 1 essential thrombocythemia). The maximum-tolerated dose of gandotinib was 120 mg daily, based on dose-limiting toxicities of blood creatinine increase or hyperuricemia at higher doses. Maximum plasma concentration was reached 4 h after single and multiple doses, and mean half-life on day 1 was approximately 6 h. Most common treatment-emergent adverse events were diarrhea (55.3%) and nausea (42.1%), a majority of which were of grade 1 severity. Best response of clinical improvement was achieved by 29% of myelofibrosis patients. A ≥ 50% palpable spleen length reduction was observed at any time during therapy in 20/32 evaluable patients. Additionally, ≥ 50% reduction in the Total Symptom Myeloproliferative Neoplasm Symptom Assessment Form Score was seen in 11/21 (52%) and 6/14 patients (43%) receiving ≥ 120 mg at 12 and 24 weeks respectively. Gandotinib demonstrated an acceptable safety and tolerability profile, and findings at the maximum-tolerated dose of 120 mg supported further clinical testing. Clinicaltrials.gov identifier: NCT01134120.
Keywords: Myeloproliferative, Neoplasm, JAK-2, Gandotinib, Dosage
1. Introduction
The classic chronic myeloproliferative neoplasms (MPNs) are a group of hematologic malignancies characterized by the clonal proliferation of one or more myeloid lineages and include myelofibrosis occurring either de novo as primary myelofibrosis (PMF), or as a transformation from other classic MPNs, polycythemia vera (PV) or essential thrombocythemia (ET) [1]. Signs and symptoms of myelofibrosis include anemia, splenomegaly, bone marrow fibrosis, fatigue, weakness, night sweats, and weight loss [2–4], among others.
The mutation in the pseudokinase domain of Janus kinase 2 (JAK2); i.e., JAK2V617F is present in many patients with PMF, ET, and PV [5–7] and results in constitutive activation of JAK2. It is found in most patients with PV (≥ 95%) and in approximately two thirds of patients with ET and PMF [8–11]. Since wild-type JAK2 plays a central role in multiple stages of hematopoiesis, it would be desirable to treat JAK2V617F-positive cases by preferentially inhibiting JAK2V617F while minimizing inhibition of wild-type JAK2. Janus kinase (JAK) inhibitors can modulate JAK signal transducer and activator of transcription (STAT) signaling in MPNs [12,13], and they offer clinical benefits to patients with MPNs [14–18]. In addition to JAK2 mutations, in view of growing evidence of interaction of inflammatory and coagulation pathways and the purported reduction of inflammatory response by JAK2 inhibitors, we selected, among other markers, the changes of plasma levels of C4B binding protein (C4BP) after gandotinib therapy. C4b protein has a known role in complement activation and also binds protein C [19]. Protein C is the principal negative regulator of coagulation factors Va and Villa. C4BP is an important binding partner to vitamin K-dependent protein S in circulation. The high-affinity binding of protein S to C4BP serves the purpose of localizing complement regulatory activity close to the phospholipid membranes [20,21], affecting the regulation of blood coagulation [22]. Therefore, we assessed if C4BP levels correlate with the occurrence of thrombotic events during the study, and performed ad hoc analysis to compare C4BP levels with different parameters of clinical response.
Gandotinib (LY2784544) is a potent inhibitor of JAK2V617F [23]. Inhibition of the constitutive activity of the mutant JAK2 could have a significant impact on the course of disease, disease complication rates, survival, and quality of life for patients with BCR-ABL1-negative classic MPN.
This phase 1 study evaluated the safety, tolerability, and pharmacokinetic parameters of gandotinib, and explored the potential efficacy of this study drug in patients with non-chronic myelogenous leukemia MPN harboring the JAK2V617F mutation.
2. Materials and methods
2.1. Study population
Patients had a diagnosis of PV, ET, or myelofibrosis, as defined by the World Health Organization diagnostic criteria for MPNs [24]; detailed inclusion/exclusion criteria are described in the supplement.
2.2. Study design
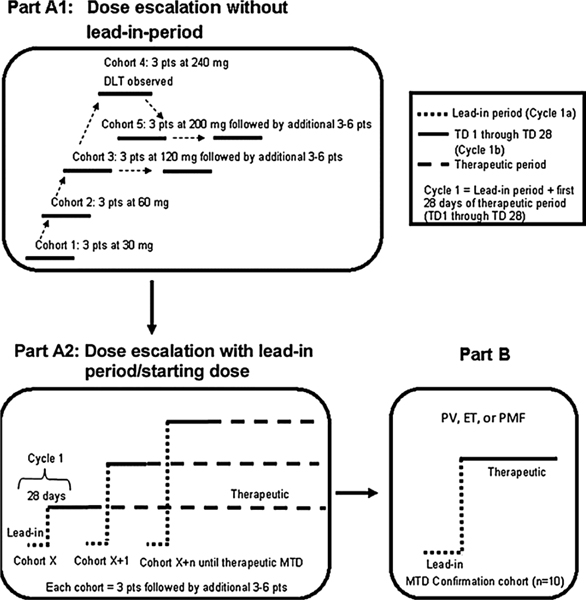
This study was performed in accordance with the principles of good clinical practice to ensure compliance with appropriate ethical and quality standards. This was a multicenter, nonrandomized, open-label, phase 1 study of gandotinib that included a dose-escalation part followed by a maximum tolerated dose (MTD) dose-confirmation part (Fig. 1).
Fig. 1.
Study design. Part A1. Dose-escalation without lead-in period. Part A2. Dose-escalation with lead-in period. Starting dose for lead-in was 120 mg. After the lead-in period doses of 200 and 300 mg were tested. Part B. Dose-confirmation portion of the study; For Part B, a dose of 120 mg was used. Abbreviations: DLT = dose-limiting toxicity; ET = essential thrombocythemia; MTD = maximum tolerated dose; PMF = primary myelofibrosis; pts = patients; PV = polycythemia vera; TD = therapeutic day.
A 3 + 3 dose-escalation paradigm was used (further details of the study design are provided in the supplement). To evaluate the safety of a dose level, all subjects in a cohort must have received 1 cycle (28 days) of therapy. Part A1 was used to define the MTD of gandotinib at a fixed daily dose. As the predicted efficacious human exposure target was not reached at 120 mg (Supplementary Table S1 and Supplementary Fig. S1A), the study was amended after identification of dose-limiting toxicity (DLT) chemistry changes suggesting potential tumor lysis and renal function impairment at doses ≥120 mg. The amendment used a lead-in period where patients received 120 mg daily for 14–28 days prior to increasing to higher doses in an attempt to avoid the previously observed chemistry changes. In part Al, cycles 1 and beyond consisted of 28 days. In part A2, which tested the lead-in strategy, cycle 1 could be 42–56 days, depending on the length of the lead-in period (i.e., a 14 or 28 day lead in plus 28 day DLT period for the higher dose). Dose escalation was based primarily on safety with attention to signs of toxicity including tumor lysis syndrome [25,26]. If evidence of tumor lysis syndrome was observed, the patient was to be managed and re-evaluated appropriately. To prevent and manage potential tumor lysis syndrome, allopurinol could be administered based on MPN subtype and investigator discretion.
Patient-reported quality-of-life measures were assessed by the Myeloproliferative Neoplasm Symptom Assessment Form (MPN-SAF) [27] and the European Organization for Research and Treatment of Cancer (EORTC) 30-item Core Quality of Life Questionnaire (QLQ-C30) [28]. Patient-reported outcomes (PRO) data were collected at baseline, approximately every other cycle for the first year of therapy, quarterly after year 1, and at the post-therapy follow-up visit.
To explore safety and tolerability, the putative recommended phase 2 daily dose was explored during the dose-confirmation portion of the study (part B). Patients in part B were treated at doses no greater than the defined MTD from part A2. Part B was intended to target an enrollment of 10 patients.
2.3. Statistical methods
The analysis for this study was primarily descriptive and details are provided in the supplement. Data analysis was provided by received dose groups and for all study patients combined wherever appropriate. For continuous variables, summary statistics included number of patients, mean, median, standard deviation, minimum, and maximum. Categorical endpoints were summarized using frequency and percentages. Missing data were not imputed.
3. Results
3.1. Patient disposition
Of the 47 patients that entered the study, 38 were enrolled and received at least 1 dose of the study treatment. Of the 38 treated patients, 36 had discontinued treatment and 2 patients remained on treatment at the time of the database lock for this report (Supplementary Fig. S2). Across all cohorts, the most common reasons for discontinuation from study treatment were physician decision (n = 17), adverse events (n = 11), patient decision (n = 4), and progressive disease (n = 3). The most frequent adverse event that resulted in treatment discontinuation was renal failure (n = 4).
3.2. Patient demographics and baseline characteristics
The majority of patients were Caucasian (97.4%) and non-Hispanic (89.5%). Of 38 patients, 21 (55.3%) were male. The median body mass index (BMI) was 26 kg/m2 (range: 18–41), and the mean and standard deviation of the baseline characteristic was 26.6 ± 5.24 kg/m2 which makes the overall population BMI relatively consistent. The majority of patients had myelofibrosis followed by PV and ET (Table 1). The clinic-pathologic diagnosis in all patients was confirmed according to 2008 World Health Organization criteria [24]. Thirty-three patients (86.8%) reported receiving prior systemic therapies before enrollment into the study. The most commonly received prior therapy was hydroxyurea. On study, the most frequendy used concomitant drugs were allopurinol (27 patients [71.1%]) and aspirin (18 patients [47.4%]). Median time from diagnosis to study therapy was 438.5 days (range: 21–5498); the median regimen number of prior systematic treatments was 2 (range: 1–8).
Table 1.
Patient demographics and baseline characteristics.
| Characteristic | Total (N = 38) |
|---|---|
| Age (years), mean (SD) | 66.3 (10.1) |
| Thrombotic events in last 12 months, n (%) | 3 (7.9) |
| RBC Transfusions in last 2 months, n (%) | 8 (21.1) |
| Phlebotomies, n (%) | 2 (5.3) |
| JAK2V617F burden, mean%, (SD) | 61.5 (32.0) |
| Spleen sizea | 13.0 (7.3) |
| Total protein S (%), mean (SD) | 90.7 (24.2) |
| Free protein S (%), mean (SD) | 65.7 (23.2) |
| C4B binding protein, mean (SD) | 101.7 (28.4) |
| Leukocyte alkaline phosphatase, mean (SD) | 112.8 (29.2) |
| Bone marrow biopsy: total cell count, mean (SD) | 318.1 (170.4) |
| ECOG Performance Status, n (%) | |
| 0 | 3 (7.9) |
| 1 | 30 (78.9) |
| 2 | 3 (7.9) |
| Bone Marrow Fibrosis, n (%) | |
| Grade 0 | 1 (2.6) |
| Grade 1 | 6 (15.8) |
| Grade 2 | 14 (36.8) |
| Grade 3 | 10 (26.3) |
| No bone marrow fibrosisb | 7 (18.4) |
| Basis of Initial Pathological Diagnosis | |
| PMF | 31 (81.6) |
| PV | 6 (15.8) |
| ET | 1 (2.6) |
| Spleen Size, Frequency (%) | |
| ≤ 5 cm | 6 (15.8) |
| > 5, ≤ 10 cm | 6c (15.8) |
| > 10 cm | 22 (57.9) |
| Missing | 4 (10.5) |
This study did not use imaging, and this was a measurement from the costal margin.
All patients with either PV or ET have no value for bone marrow fibrosis.
1 (100.0%) in cohort L (patients withdrawn in the leading phase – never assigned to a cohort).
Abbreviations: ECOG = Eastern Cooperative Oncology Group; ET = essential thrombocythemia; n = number of patients; PMF = primary myelofibrosis; PV = polycythemia vera; RBC = red blood cell; SD = standard deviation.
3.3. Dose-limiting toxicity and maximum tolerated dose
This study sequentially tested daily oral administration of gandotinib at 30, 60, 120, 240, and then an intermediate dose of 200 mg. Table 2 summarizes the observed DLTs by received dose during cycle 1. No DLTs were seen at the first 3 tested doses. At 240 mg, one DLT was observed, and subsequently at 200 mg, 3 DLTs were observed. The protocol was subsequently amended to include a lead-in period at 120 mg before escalation to a higher dose so as to facilitate reaching the targeted predicted efficacious exposure level. In total, 9 patients had a DLT. The most commonly reported DLTs were increased blood creatinine (5 patients), and hyperuricemia (2 patients). The MTD was determined to be 120 mg as this was the highest study dose level at which < 33% of patients experienced a DLT in cycle 1. In addition, the use of a 120-mg lead-in dose did not facilitate a transition to higher doses with acceptable tolerability.
Table 2.
Summary of dose-limiting toxicities by received dose during cycle 1.a
| Cohort | 1 | 2 | 3 | 4 | 5 | 6 | 7 | Lb | Total | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Dose (mg) | 30 | 60 | 120 | 240 | 200 | 120 (Cycle la) | 200 (Cycle 1b) | 120 (Cycle 1a) | 300 (Cycle 1b) | 120 | |
| Population | N = 3 | N = 3 | N = 10 | N = 3 | N = 3 | N = 8 | N = 7 | N = 6 | N = 5 | N = 1 | N = 37 |
| Patients with DLT, n (%) | 0 | 0 | 0 | 1 (33.3) | 3 (100) | 3 (37.5) | 3 (42.9) | 0 | 2 (40.0) | 0 | 9 (24.3) |
| Blood creatinine increased | 0 | 0 | 0 | 0 | 1 (33.3) | 3 (37.5) | 3 (42.9) | 0 | 1 (20.0) | 0 | 5 (13.5) |
| Hyperuricemia | 0 | 0 | 0 | 1 (33.3) | 1 (33.3) | 0 | 0 | 0 | 0 | 0 | 2 (5.4) |
| Hyperkalemia | 0 | 0 | 0 | 0 | 1 (33.3) | 0 | 0 | 0 | 0 | 0 | 1 (2.7) |
| Renal failure acute | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 (20.0) | 0 | 1 (2.7) |
Per protocol, dose-limiting toxicities were those events that occurred within the first cycle of treatment in dose-escalation stage (Part A).
Cohort L = Patients withdrawn in the lead-in phase – never assigned to a cohort.
Abbreviations;: DLT = dose-limiting toxicity; N = total population size; n = number of patients.
3.4. Safety
3.4.1. Extent of exposure
For the study population, the median number of cycles completed was 6 (range: 1–44). The median duration of treatment exposure for all 38 patients was 170 days (range 2–1350).
3.4.2. Treatment-emergent adverse events
Of 38 patients who received at least 1 dose of study therapy, 34 (89.5%) reported ≥1 treatment-emergent adverse event (TEAE) that was possibly related to study drug (Supplementary Table S2). The most common TEAEs were diarrhea (21 patients [55.3%]) and nausea (16 patients [42.1%]). The majority of these events were of grade 1 severity.
3.4.3. Serious adverse events and deaths
During the study, 9 patients (23.7%) reported ≥ 1 serious adverse event (SAE) that was possibly related to study therapy (Table 3). Of the 9 patients, 7 had myelofibrosis, while there was 1 each with ET and PV. One patient in cohort 3 died during the study due to bilateral pneumonia which was assessed as not related to the study drug.
Table 3.
Serious adverse events possibly related to study drug treatment.
| 30 mg (N = 3) n% | 60 mg (N = 7) n% | 120 mg (N = 34) n% | 200 mg (N = 15) n% | 240 mg (N = 3) n% | 300 mg (N = 5) n% | Totala (N = 38) | Grade 3 and above, Totala | Grade 4 and above, Totala | |
|---|---|---|---|---|---|---|---|---|---|
| Patients with ≥ 1 SAEb | 0 | 0 | 0 | 4 (26.7) | 1 (33.3) | 1 (20.0) | 9 (23.7) | 6 (15.8) | 2 (5.3) |
| Anemia | 0 | 0 | 0 | 1 (6.7) | 0 | 0 | 1 (2.6) MF | 1 (2.6) | 0 |
| Blood creatinine increased | 0 | 0 | 0 | 3 (20.0) | 1 (33.3) | 0 | 5 (13.2) MF | 1 (2.6) | 0 |
| Hyperuricemia | 0 | 0 | 0 | 1 (6.7) | 0 | 0 | 2 (5.3) MF | 2 (5.3) | 2 (5.3) |
| Hyperkalemia | 0 | 0 | 0 | 0 (0.0) | 0 | 0 | 1 (2.6) MF | 0 | 0 |
| Renal failure, acute | 0 | 0 | 0 | 1 (6.7) | 0 | 1 (20.0) | 4 (10.5) [2 MF, 1 ET, 1 PV] | 2 (5.3) | 0 |
The total column includes events for which the study drug dose that the patients were taking could not be determined;
Three patients reported SAEs while on the study but not receiving treatment. These SAEs were blood creatinine increased, hyperuricemia, hyperkalemia, and renal failure acute.
Abbreviations;: ET = essential thrombocythemia; MF = myelofibrosis; N = total safety population at each received dose or total; n = number of received dose patients with at least one SAE; PV = polycythemia vera; SAE = serious adverse event.
3.4.4. Clinical laboratory evaluation
Using the definitions of Cairo and Bishop [25,26], laboratory tumor lysis syndrome (LTLS) was defined as either a 25% change or level above or below normal for any 2 or more serum values of uric acid (≥ 476 μmol/L), potassium (≥ 6.0 mmol/L), phosphorus (≥ 1.45 mmol/L in adults), and calcium (≤ 1.75 mmol/L) within 3 days before or 7 days after initiation of chemotherapy. Clinical tumor lysis syndrome (CTLS) was defined as the presence of LTLS and any 1 or more of the following criteria, creatinine (≥1.5 times the upper limit of normal), cardiac arrhythmia/sudden death, and seizures. Two patients reported CLTS and LTLS events. Five patients reported 8 LTLS events. Six patients reported 8 CTLS events (6 were grade 2 CTLS events and 2 were grade 3 CTLS events).
3.5. Pharmacokinetics
The maximum plasma concentration (Cmax) was reached, on average, 4 h after single and multiple doses. The mean gandotinib half-life on day 1 across all doses was ~ 6 h, with a coefficient of variation (CV) of 43%. This terminal elimination half-life (t1/2) value was associated with gandotinib being rapidly cleared (mean value of 80.7 L/h for the apparent clearance (CL/F) with a CV of 68) with an extensive distribution into the body (mean value of 682 L for the apparent volume of distribution (Vz/F) with a CV of 58). Supplementary Table S1 represents the pharmacokinetic parameters of interest. Supplementary Fig. S1A represents the individual area under the plasma concentration-time curve from time 0–8 h [AUC(0–8h)] and Supplementary Fig. S1B represents individual Cmax on cycle 1 day 1. A definitive statement on dose proportionality could not be made based on these data due to high variability and available sample size. However, both Cmax and exposures appeared to increase with an increase in dose. The influence of the individual baseline BMI and weight values was assessed on the dose normalized maximum plasma concentration (Cmax) and area under the plasma concentration-time curve (AUC) values and no specific trend was detected from the visual representation.
From a toxicity-versus-exposure relationship perspective, daily administration of dose levels above 120 mg once daily was associated with several cases of renal adverse events. There is a clear exposure difference between the 120-mg dose and higher doses (such as 200 mg). Hence, one plausible reason for the toxicity observed is the increased exposure at doses of 200 mg or above, even if, at the patient level and within a dose level, this trend is less apparent. It is also important to note that the predicted daily human exposure target of 6920 ng*h/mL was not reached at 120 mg.
3.6. Myeloproliferative Neoplasm Symptom Assessment Form (MPN-SAF) and European Organization for Research and Treatment of Cancer 30-item Core Quality of Life Questionnaire (EORTC QLQ-C30)
A total of 38 patients contributed PRO data from 238 visits (35 baseline visits, 182 on-therapy visits, and 21 post-therapy follow-up visits). PRO data was reported for 87% of the visits where PRO data was requested.
For all doses at weeks 16 and 32, no statistically significant deterioration in functioning or QOL was observed from patient-reported EORTC data (cognitive, emotional, physical, role, social and global health status/QOL). A numerical improvement at week 16 was observed in physical and social functioning (Supplementary Table S3).
In the same cohort of 21 patients with a starting dose of ≥120 mg, no statistically significant deterioration or improvement was observed from baseline in EORTC scores either at week 16 or at week 32.
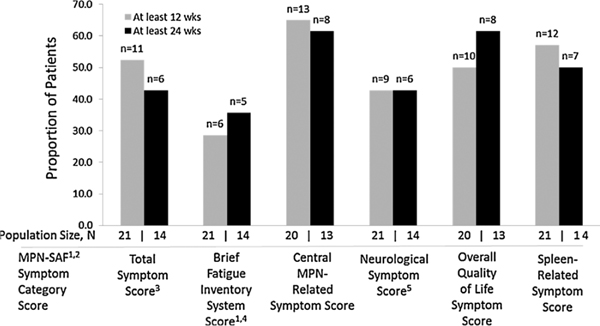
For the MPN-SAF scores, among patients who received a starting dose of ≥120 mg at 12 weeks, 11 of 21 patients had a ≥ 50% reduction in Total Symptom Score. At 24 weeks, 6 of 14 patients had a ≥50% reduction in Total Symptom Score (Fig. 2).
Fig. 2.
Number of patients with a ≥50% reduction in MPN-SAF scores among patients receiving starting dose ≥ 120 mg. References: 1Mesa RA, et al. Leuk Res 2009;33:1199–1203; 2Mesa RA, et al. Cancer 2007;109:68–76; 3Mesa RA, Cortes J. J Hematol Oncol 2013;6:79; 4Mendoza TR, et al. Cancer 1999;85:1186–1196; 5Scherber R, et al. Blood 2011;118:401–408. Abbreviations: MPN-SAF = Myeloproliferative Neoplasm-Symptom Assessment Form; wks = weeks.
3.7. Efficacy
Among 31 patients with myelofibrosis, 9 (29%) patients achieved a best response of clinical improvement. Investigator International Working Group (IWG) responses were seen in the first dose (30 mg) level tested. Efficacy was documented by measuring clinical improvement as defined by the IWG criteria, and the European Leukemia Net response criteria for PV and ET [29,30]. The duration of best response for these 9 patients ranged from 1 to 18 cycles. Across all cohorts, 20 of 32 evaluable patients had a palpable spleen length reduction of ≥ 50% at any time during therapy. Of the 6 patients with PV, 3 (2 in cohort 6 and 1 in cohort 7) had a clinic-hematologic partial response that varied in duration (range: 3–16 cycles). The remaining 3 PV patients had no clinic-hematologic response. One patient with ET had a partial response.
Changes in the JAK2V617F allele mutation burden across the 3 MPN subtypes of patients showed a heterogeneous trend (Supplementary Fig. S3). Across all cohorts, 5 of 36 evaluable patients had an allele burden reduction of ≥50% at any time. Among all the laboratory markers studied, coagulation marker C4BP showed potential for being a biomarker for spleen size reduction. A longitudinal review of the relationship between C4BP and spleen size for different doses of gandotinib, showed an inverse correlative trend for many patients in the myelofibrosis subtype and this trend was further confirmed by plotting the maximum reduction in spleen size from baseline versus C4BP for various doses of gandotinib (Supplementary Fig. S4). The reduction in spleen size corresponded with an increase in C4BP compared at various doses of gandotinib. At the maximum dose of 300 mg, increased C4BP corresponded with a maximum percent change in spleen size from baseline. In the scatter plot of C4BP versus total protein S at baseline, myelofibrosis patients had a positive correlation of 0.7. Over the short follow-up of this study there was no clear trend seen in terms of bone marrow myelofibrosis grading for myelofibrosis patients, however, this potentially could be related to the fact that many patients lacked enough data points.
4. Discussion
In this phase 1 dose-escalation study in patients with JAK2V617F-mutation positive MPN, a recommended phase 2 dose of 120 mg given once daily by mouth with food was identified as the MTD. Treatment with gandotinib at a daily oral dose of 120 mg or lower was associated with an acceptable safety and tolerability profile. Clinical improvements were observed in MPN patients at this dose.
While the majority of the 38 patients enrolled and treated in the study had myelofibrosis (81.6%), 15.8% of patients had a diagnosis of PV and 1 patient (2.6%) had ET. At the time of the final study report, 36 (94.7%) had discontinued study treatment while 2 (5.3%) remained on study treatment. The most common reason for early discontinuation from study treatment was physician decision. Case report forms collected additional explanation details to support this decision. In a majority of cases, the explanations were related to challenges in implementation of the response criteria which did not reflect the need to account for discontinuation due to a lack of initial response/failure to respond or a loss of response. Of the 17 patients who discontinued due to physician decision, 7 did so for a lack/loss of efficacy (verbatim terms from the investigators), 4 due to lost response to medication/lack/loss of response (verbatim terms from the investigators), and 1 each for no evidence of specific response per investigator, suboptimal response to study medication, relapse, patient lost response due to dose reduction, loss of control of the disease sign and symptoms, and not responding to treatment.
Other reasons for discontinuation included adverse events, patient decision, and progressive disease. One patient died during the course of the study due to bilateral pneumonia. Nineteen patients reported at least 1 SAE and 11 patients discontinued the study treatment due to adverse events. The most frequent adverse event that resulted in treatment discontinuation was renal failure. At 120 mg, the most frequently reported treatment-related adverse events were diarrhea and nausea.
Previous studies with other JAK2 inhibitors have also reported common gastrointestinal side effects such as nausea and diarrhea suggesting the possibility there may be a class effect in the gastrointestinal tract or a relationship to a treatment effect on disease specific gastrointestinal involvement [16,18,31,32]. During the study the observed increases in serum creatinine were handled as potential renal toxicities. In the majority of patients with an increase in serum creatinine the increase was small, without progression, and resolved after drug discontinuation. While gandotinib has not been tested against transporters involved in creatinine disposition, the results of studies with INCB039110 suggest that the conclusions drawn from the use of serum creatinine as a marker of renal function should be made with caution, acknowledging the possibility of artifactual increases resulting from modulation of transporters involved in creatinine clearance [33].
Approximately 29% (9/31) of patients with myelofibrosis achieved a best response of clinical improvement. Across cohorts, 20 of 32 evaluable patients had a palpable spleen length reduction of ≥ 50% at any time. We report here a strong inverse correlation between reduction of spleen size and C4BP protein; however, the validity of this observation and its biological significance will need to be elaborated by future laboratory as well as clinical studies. Approximately 52% of patients who received a starting dose of ≥120 mg at 12 weeks (43% at 24 weeks) had a ≥ 50% reduction in Total Symptom Score.
The JAK2V617F allele mutation burden across the MPN subtypes of patients showed a heterogeneous trend, 5 of 36 evaluable patients had an allele burden reduction of ≥ 50% at any time. Previous studies have shown that although patients may experience improvements in splenomegaly and symptoms with JAK2 inhibitors, the JAK2V617F allele burden may not change with treatment [16,18,32]. Consistent with the findings of this study, the relationship between clinical benefit and reduction in the JAK2V617F allele burden has not yet been demonstrated [34]. It is possible that the JAK2V617F allele burden did not change significantly, because the optimal efficacy AUC was not reached. Patients with myelofibrosis had a positive correlation of 0.7 in the scatter plot of C4BP versus total protein S at baseline. C4B binding protein is known to inhibit the classic complement cascade by blocking the formation and promoting the decay of the C3 convertase, C4b, C2a. Protein S is a cofactor for the anticoagulant effects of activated protein C. About 60% of protein S is complexed to C4BP, and therefore the positive correlation is expected between C4BP and protein S. C4B binding protein and protein S were initially assessed in this study to determine a possible correlation between the circulating levels of these proteins and the occurrence of thrombotic events in patients with myelofibrosis. Although we did not see any correlation between C4B binding protein and the very few observed thrombotic events in this study (data not shown) we did identify as part of an ad hoc analysis that baseline levels of C4B binding protein were correlated with better responses, particularly in patients receiving the higher doses of gandotinib (Supplementary Fig. S4). It is unclear at this time what the underlying mechanism for this association is, and whether high C4B binding protein levels at baseline is a good prognostic factor in myelofibrosis, or if it could be a predictive marker for JAK inhibitor treatments in myelofibrosis. Additional studies in larger cohorts are necessary to answer these questions.
Gandotinib pharmacokinetic parameters showed high variability; although a statistical analysis could not conclude dose proportionality, both Cmax and AUC increased with dose. Gandotinib appeared to be eliminated rapidly, with a t1/2 of approximately 6 h.
In conclusion, gandotinib was generally well tolerated in the enrolled MPN patients, and the recommended phase 2 dose of 120 mg daily was associated with clinical improvements.
Supplementary Material
Acknowledgements
This study was funded by Eli Lilly and Company. The authors would like to thank Angela Lorio for editorial assistance.
Footnotes
Appendix A. Supplementary data
Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.leukres.2017.08.010.
Conflict of interest
Li Li, Celine Pitou, Fabio P. Nunes, Gregory L. Price, Jennifer L. Giles, and Richard Walgren are employees of Eli Lilly and Company. Deborah D’Souza is an employee of inVentiv Health Clinical, LLC. Srdan Verstovsek received research support for conduct of this clinical study. Mohamed Salama received research support for pathology studies. Josef T. Prchal received research support for conduct of this clinical study. Ruben Mesa receives research support from Incyte, Gilead, CTI, Genentech, Lilly, Promedior, NS Pharma and is a consultant for Novartis and Shire.
References
- [1].Tefferi A, Vardiman JW, Classification and diagnosis of myeloproliferative neoplasms: the 2008 World Health Organization criteria and point-of-care diagnostic algorithms, Leukemia 22 (1) (2008) 14–22, 10.1038/sj.leu.2404955. [DOI] [PubMed] [Google Scholar]
- [2].Mesa RA, The evolving treatment paradigm in myelofibrosis, Leuk. Lymphoma 54 (2013) 242–251, 10.3109/10428194.2012.710905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Mesa RA, Niblack J, Wadleigh M, et al. , The burden of fatigue and quality of life in myeloproliferative disorders (MPDs): an international Internet-based survey of 1179 MPD patients, Cancer 109 (2007) 68–76, 10.1002/cncr.22365. [DOI] [PubMed] [Google Scholar]
- [4].Tefferi A, Myelofibrosis with myeloid metaplasia, N. Engl. J. Med 342 (2000) 1255–1265, 10.1056/NEJM200004273421706. [DOI] [PubMed] [Google Scholar]
- [5].Baxter EJ, Scott LM, Campbell PJ, et al. , Acquired mutation of the tyrosine kinase JAK2 in human myeloproliferative disorders, Lancet 365 (2005) 1054–1061, 10.1016/S0140-6736(05)71142-9. [DOI] [PubMed] [Google Scholar]
- [6].James C, Ugo V, Le Couedic JP, et al. , A uniqueclonal JAK2 mutation leading to constitutive signaling causes polycythaemia vera, Nature 434 (2005) 1144–1148, 10.1038/nature03546. [DOI] [PubMed] [Google Scholar]
- [7].Kralovics R, Passamonti F, Buser AS, et al. , A gain-of-function mutation of JAK2 in myeloproliferative disorders, N. Engl. J. Med 352 (17) (2005) 1779–1790, 10.1056/NEJMoa051113. [DOI] [PubMed] [Google Scholar]
- [8].Ries LAG, Melbert D, Krapcho M, et al. (Eds.), SEER Cancer Statistics Review, 1975–2005, National Cancer Institute, Bethesda, MD, 2007. http://seer.cancer.gov/csr/1975_2005. Based on November 2007 SEER data submission. Posted to the SEER web site 2008. [Google Scholar]
- [9].Rollison DE, Howlader N, Smith MT, et al. , Epidemiology of myelodysplastic syndromes and chronic myeloproliferative disorders in the United States, 2001–2004, using data from the NAACCR and SEER programs, Blood 112 (1) (2008) 45–52, 10.1182/blood-2008-01-134858. [DOI] [PubMed] [Google Scholar]
- [10].Vannucchi AM, Antonioli E, Guglielmelli P, et al. , Clinical profile of homozygous JAK2 617VF mutation in patients with polycythemia vera or essential thrombocythemia, Blood 110 (3) (2007) 840–846, 10.1182/blood-2006-12-064287. [DOI] [PubMed] [Google Scholar]
- [11].Barosi G, Bergamaschi G, Marchetti M, et al. , Gruppo Italiano Malattie Ematologiche Maligne delTAdulto (GIMEMA) Italian Registry of Myelofibrosis. JAK2 V617F mutational status predicts progression to large splenomegaly and leukemic transformation in primary myelofibrosis, Blood 110 (12) (2007) 4030–4036, 10.1182/blood-2007-07-099184. [DOI] [PubMed] [Google Scholar]
- [12].Quintas-Cardama A, Vaddi K, Liu P, et al. , Preclinical characterization of the selective JAK1/2 inhibitor INCB018424: therapeutic implications for the treatment of myeloproliferative neoplasms, Blood 115 (2010) 3109–3117, 10.1182/blood-2009-04-214957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Hedvat M, Huszar D, Herrmann A, et al. , The JAK2 inhibitor, AZD1480, potently blocks STAT3 signaling and oncogenesis in solid tumors, Cancer Cell. 16 (2009) 487–497, 10.1016/j.ccr.2009.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Verstovsek S, Hoffman R, Mascarenhas J, et al. , A phase I, open-label, multicenter study of the JAK2 inhibitor AZD1480 in patients with myelofibrosis, Leuk. Res 39 (2015) 157–163, 10.1016/j.leukres.2014.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Tam CS, Verstovsek S, Investigational janus kinase inhibitors, Expert Opin. Investig. Drugs 22 (6) (2013) 687–699, 10.1517/13543784.2013.774373. [DOI] [PubMed] [Google Scholar]
- [16].Verstovsek S, Mesa RA, Gotlib J, et al. , A double blind, placebo-controlled trial of ruxolitinib for myelofibrosis, N. Engl. J. Med 366 (2012) 799–807, 10.1056/NEJMoal110557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Harrison C, Kiladjian JJ, Al-Ali HK, et al. , JAK inhibition with ruxolitinib versus best available therapy for myelofibrosis, N. Engl. J. Med 366 (2012) 787–798, 10.1056/NEJMoa1110556. [DOI] [PubMed] [Google Scholar]
- [18].Verstovsek S, Kantarjian H, Mesa RA, et al. , Safety and efficacy of INCB018424, a JAK1 and JAK2 inhibitor, in myelofibrosis, N. Engl. J. Med 363 (2010) 1117–1127, 10.1056/NEJMoa1002028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Dahlbäck B, Protein S and C4b-binding protein: components involved in the regulation of the protein C anticoagulant system, Thromb. Haemost 66 (1) (1991) 49–61. [PubMed] [Google Scholar]
- [20].Webb JH, Blom AM, Dahlback B, Vitamin K-dependent protein S localizing complement regulator C4b-binding protein to the surface of apoptotic cells, J. Immunol 169 (5) (2002) 2580–2586, 10.4049/jimmunol.169.5.2580. [DOI] [PubMed] [Google Scholar]
- [21].Schwalbe R, Dahlback B, Hillarp A, Nelsestuen G, Assembly of protein S and C4b-binding protein on membranes, J. Biol. Chem 265 (27) (1990) 16074–16081. [PubMed] [Google Scholar]
- [22].Dahlback B, C4b-Binding protein: a forgotten factor in thrombosis and hemostasis, Semin. Thromb. Hemost 37 (4) (2011) 355–361, 10.1055/s-0031-1276584. [DOI] [PubMed] [Google Scholar]
- [23].Ma L, Clayton JR, Walgren RA, et al. , Discovery and characterization of LY2784544, a small-molecule tyrosine kinase inhibitor of JAK2V617F, Blood Cancer J. 3 (2013) el09, 10.1038/bcj.2013.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Swerdlow SH, Campo E, Harris NL, et al. , 4th ed., WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues vol. 2, IARC, Lyon, 2008, pp. 40–50. [Google Scholar]
- [25].Cairo MS, Bishop M, Tumour lysis syndrome: new therapeutic strategies and classification, Br. J. Haematol 127 (2004) 3–11, 10.1111/j.1365-2141.2004.05094.x. [DOI] [PubMed] [Google Scholar]
- [26].Cairo MS, Coiffier B, Reiter A, Younes A, TLS Expert Panel. Recommendations for the evaluation of risk and prophylaxis of tumour lysis syndrome (TLS) in adults and children with malignant diseases: an expert TLS panel consensus, Br. J. Haematol 149 (4) (2010) 578–586, 10.1111/j.1365-2141.2010.08143.x. [DOI] [PubMed] [Google Scholar]
- [27].Mesa RA, Schwager S, Radia D, et al. The myelofibrosis symptom assessment form (MFSAF): an evidence-based brief inventory to measure quality of life and symptomatic response to treatment in myelofibrosis, Leuk. Res 33 (9) (2009) 1199–1203, 10.1016/j.leukres.2009.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Aaronson NK, Ahmedzai S, Bergman B, et al. The European Organization for Research and Treatment of Cancer QLQ-C30: a quality-of-life instrument for use in international clinical trials in oncology, J. Natl. Cancer Inst 85 (5) (1993) 365–376, 10.1093/jnci/85.5.365. [DOI] [PubMed] [Google Scholar]
- [29].Tefferi A, Barosi G, Mesa RA, et al. , International Working Group (IWG) consensus criteria for treatment response in myelofibrosis with myeloid metaplasia, for the IWG for Myelofibrosis Research and Treatment (IWG-MRT), Blood 108 (5) (2006) 1497–1503, 10.1182/blood-2006-03-009746. [DOI] [PubMed] [Google Scholar]
- [30].Barosi G, Birgegard G, Finazzi G, et al. , Response criteria for essential thrombocythemia and polycythemia vera; result of a European LeukemiaNet consensus conference, Blood 113 (20) (2009) 4829–4833, 10.1182/blood-2008-09-176818. [DOI] [PubMed] [Google Scholar]
- [31].Verstovsek S, Tama CS, Wadleigh M, et al. Phase I evaluation of XL019, an oral, potent, and selective JAK2 inhibitor, Leuk. Res 38 (2014) 316–322, 10.1016/j.leukres.2013.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Pardanani A, Gotlib JR, Jamieson C, et al. , Safety and efficacy of TGI 01348, a selective JAK2 inhibitor, in myelofibrosis, J. Clin. Oncol 29 (2011) 789–796, 10.1200/jc0.2010.32.8021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Zhang Y, Warren MS, Zhang X, et al. , Impact on creatinine renal clearance by the interplay of multiple renal transporters: a case study with INCB039110, Drug Metab. Dispos 43 (4) (2015) 485–489, 10.1124/dmd.114.060673. [DOI] [PubMed] [Google Scholar]
- [34].Nicolas CS, Peineau S, Amici M, et al. , The JAK/STAT pathway is involved in synaptic plasticity, Neuron 73 (2012) 374–390, 10.1016/j.neuron.2011.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.