Abstract
Increasingly, land managers have attempted to use extreme prescribed fire as a method to address woody plant encroachment in savanna ecosystems. The effect that these fires have on herbaceous vegetation is poorly understood. We experimentally examined immediate (<24 hr) bud response of two dominant graminoids, a C3 caespitose grass, Nassella leucotricha, and a C4 stoloniferous grass, Hilaria belangeri, following fires of varying energy (J/m2) in a semiarid savanna in the Edwards Plateau ecoregion of Texas. Treatments included high‐ and low‐energy fires determined by contrasting fuel loading and a no burn (control) treatment. Belowground axillary buds were counted and their activities classified to determine immediate effects of fire energy on bud activity, dormancy, and mortality. High‐energy burns resulted in immediate mortality of N. leucotricha and H. belangeri buds (p < .05). Active buds decreased following high‐energy and low‐energy burns for both species (p < .05). In contrast, bud activity, dormancy, and mortality remained constant in the control. In the high‐energy treatment, 100% (n = 24) of N. leucotricha individuals resprouted while only 25% (n = 24) of H. belangeri individuals resprouted (p < .0001) 3 weeks following treatment application. Bud depths differed between species and may account for this divergence, with average bud depths for N. leucotricha 1.3 cm deeper than H. belangeri (p < .0001).
Synthesis and applications: Our results suggest that fire energy directly affects bud activity and mortality through soil heating for these two species. It is imperative to understand how fire energy impacts the bud banks of grasses to better predict grass response to increased use of extreme prescribed fire in land management.
Keywords: bud dormancy, fire management, herbaceous perennial resprouting, plant mortality, vegetative tiller reproduction
We experimentally examined the immediate (<24 hr) belowground bud responses of two dominant graminoids, a C3 caespitose grass, Nassella leucotricha, and a C4 stoloniferous grass, Hilaria belangeri, following fires of varying energy (J/m2) in a semiarid savanna in the Edwards Plateau ecoregion of Texas. Our results suggest that fire energy may directly affect bud activity and mortality for these two species. It is imperative to understand how fire energy impacts the bud banks of grasses to better predict grass response to increased use of extreme prescribed fire in land management.

1. INTRODUCTION
In ecosystems dominated by perennial grasses, aboveground growth and persistence following disturbances are often determined by regrowth from a belowground bud bank (Dalgleish & Hartnett, 2009; Rogers & Hartnett, 2001). This type of growth is overwhelmingly prolific, with some research estimating that more than 99% of all new tiller growth originates from belowground buds (Benson & Hartnett, 2006). As opposed to seeds, these buried buds are associated with a parent plant and thus can remain dormant for a period of time and, once activated, the subsequent outgrowth is supported by the plant's resources (Ott et al., 2019). When disturbance frequency is intermediate, these populations of dormant buds are predicted to play a large role in the regeneration of many perennial grasses following disturbances such as herbivory or fire (Clarke et al., 2013) along with providing population stability in drought conditions (VanderWeide et al., 2014). This regeneration is likely constrained more by the rate of depletion and production of buds (bud bank size) rather than by the amount of resources available to support regeneration via buds (Cruz et al., 2003). Therefore, bud bank size not only determines the growth potential of perennial grasses, but can also directly determine a plant's ability to activate reserves, respond to disturbances, and react to pulses of high resource availability (Busso et al., 1989; Ott et al., 2019; Russell et al., 2015; VanderWeide et al., 2014).
Semiarid savannas developed under fire and grazing regimes that exerted selective pressures on plant community structure and composition (Milchunas & Lauenroth, 1993). In the presence of these aboveground disturbances, regrowth from a belowground bud bank that is insulated by a layer of soil offers a competitive advantage to herbaceous species that regenerate vegetatively from these buds (Dalgleish & Hartnett, 2009; Rogers & Hartnett, 2001; Russell et al., 2015). Fire suppression in turn has led to a marked increase in woody shrub encroachment into formerly herbaceous‐dominated plant communities (Twidwell et al., 2016; Archer et al., 2017).
Low‐energy fires have been utilized by land managers to sustain grass dominance as a substitute for the higher intensity fires that would naturally occur during the dry season. However, once invasion by woody species proceeds beyond a certain threshold, reintroducing low‐energy fire into the system is seldom a viable means to return to a grass‐dominated state (Ansley & Jacoby, 1998). Nevertheless, research has shown that high‐energy fires during drought can result in mortality of mature woody resprouting shrubs (Ansley & Jacoby, 1998; Twidwell et al., 2016).
Immediate changes in bud response to external factors such as disturbances may provide insight into possible long‐term fluctuations and structural shifts in plant community composition. Direct bud mortality due to disturbance may have a greater impact on species that maintain smaller bud banks and, in particular for C3 species, whose buds are short‐lived and recruit tillers from only the current year's buds (Ott & Hartnett, 2012). Increased bud death can also lead to meristem limitations, potentially resulting in a decreased future capacity to respond to external stimuli such as disturbances and changes in nutrient availability, light, and precipitation (Benson et al., 2004; Dalgleish & Hartnett, 2006; Ott et al., 2019). Overall, the bud bank size plays a fundamental role in local plant population structure and persistence and buffers against disturbance (Dalgleish & Hartnett, 2009), so short‐term decreases in bud numbers have the potential to alter population dynamics and community structure.
Prescribed fires, over the long term, maintain stable herbaceous community composition, especially when considering desirable grasses for rangeland managers (Taylor et al., 2012). When composition does change following fire, the primary drivers are legacy effects of pre‐existing variability rather than fire energy (Taylor et al., 2012). Although research has raised concerns that high‐energy fires may lead to long‐term negative effects in grass communities, little research has illustrated short‐term, immediate responses of grass bud banks to high‐energy fires.
The depth of the bud bank below the soil surface contributes to grass survival following disturbances, especially in the case of fire (Choczynska & Johnson, 2009). Soil is an insulator and retards the downward movement of heat into the soil (Clarke et al., 2013; Valettel et al., 1994). Although there are few relevant field studies that directly manipulate fire energy, we expect that high‐energy fires will result in greater heating at the soil surface (see Massman et al., 2010) as well as longer residence times of that heating due to high fuel loads. Studies have shown the impact of higher residence times on seed germination (Dayamba et al., 2010); it may be just as likely to have a significant effect on other plant tissues such as buds. As such, bud position in relation to the soil surface is important and most likely differs among grass growth forms. Stoloniferous and caespitose grasses typically have different bud depths due to their different vegetative growth strategies. Therefore, growth form traits and life‐history strategies likely drive differential effects of fire energy on the bud bank.
This study examined the effects of different levels of fire energy, achieved through two contrasting fuel loading treatments, on bud activity, dormancy, and mortality of two native perennial grass species with contrasting growth forms and photosynthetic pathways in a semiarid savanna during the summer dry season. The objectives of this study were to (a) assess the immediate (<24 hr following treatment) bud responses of a C3 caespitose grass and a C4 stoloniferous grass to different fire energies, (b) evaluate how bud depth may impact these bud responses, and (c) assess the impact of fire energy on initial reemergence of tillers for both species.
2. MATERIALS AND METHODS
2.1. Site description
Research was conducted at the Sonora Texas A&M Agrilife Research Station (SARS), which is on the western edge of the Edwards Plateau ecoregion in Texas (−100.574°, 30.251°). This semiarid, savanna experiences a bimodal precipitation pattern. The average annual precipitation varies from 356 to 889 mm, with the majority falling in the spring and fall. The average annual temperature ranges from 14 to 21°C, with summer temperatures reaching up to 41°C. The western Edwards Plateau historically experienced a fire return interval of 1–12 years, and fires were more common during late winter and late summer when grasses were dormant or dry and lightning strike frequency was high (Stambaugh et al., 2014).
The soils are in the Tarrant soil series (Clayey‐skeletal, smectitic, thermic Lithic Calciustolls; USDA, 2016), which tend to be very shallow and areas of exposed limestone bedrock are common. The dominant vegetation consists of a mosaic of trees and graminoids. The dominant trees in the area are Quercus spp., Juniperus spp., and Prosopis glandulosa Torr. The dominant graminoid species are Hilaria belangeri (Steud.) Nash, Aristida spp., Bouteloua curtipendula (Michx.) Torr., Nassella leucotricha (Trin. & Rupr.) R.W. Phol., and Pleuraphis mutica Buckley.
2.2. Experimental design and fire measurements
Fire treatments were arranged in a randomized design with three treatments (no burn, low fire energy, and high fire energy) replicated 12 times for a total of 36 experimental plots. Each plot was 100 m2 and centered on a mature (10+ years) mesquite shrub (Prosopis glandulosa) ranging from 3 to 5 m in height.
Two grass species, N. leucotricha and H. belangeri, were selected for this study due to their relative abundance at the site and for their contrasting phenological and growth form characteristics. Within each 10 × 10 m plot, two 1‐m2 subplots were demarcated with steel posts (Figure 1). One of these subplots was created around a patch of N. leucotricha, and the other around a patch of H. belangeri, and both served as a reference group for tiller collections described in the next section. Due to H. belangeri's stoloniferous growth form, we defined an individual as a single‐rooted node from which tillers arose. Within each of these subplots, two individuals of the focal species were marked and monitored for regrowth 3 weeks following fire application.
FIGURE 1.
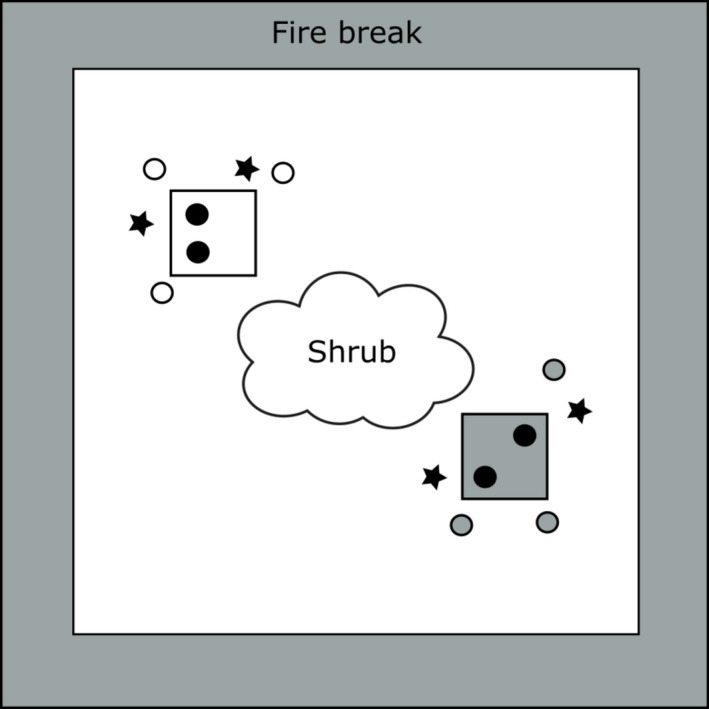
Visual representations of the main methodologies conducted in our experimental plots. All plots were 100 m2 and centered on a mature mesquite shrub, with a 1.8 m fire‐break around the periphery. The two smaller squares are an example of where subplots were created, one subplot per species in each plot. The black circles inside the subplots represent permanently marked individuals evaluated for regrowth 3 weeks following treatment application; circles outside subplots represent random individuals chosen for tiller collections and bud assessments. Black stars represent individuals chosen for bud bank depth measurements, 2 individuals per species in each plot
The last time our study site was burned was in August of 2000 with a high‐energy prescribed burn. Historically, our site was moderately grazed by sheep and goats. One growing season prior to our burns, the pasture containing our study site was rested and all domesticated grazing ceased throughout the study. However, our study site was still subject to herbivory from wildlife including Odocoileus virginianus, Axis axis, Lepus californicus, Sylvilagus floridanus, and a wide variety of invertebrates.
In early spring 2018, the entire pasture, with exception of our experimental plots, was burned to reduce surrounding fuel loads in preparation for our experimental fires. Each plot had a surrounding 1.8 m mineral soil fire‐break installed using heavy machinery.
There are critical fireline intensity thresholds required to induce mortality of woody species (Twidwell et al., 2009, 2013, 2016). Therefore, to manipulate the amount of heat produced by our fires, we added fuels in a way to match critical fireline intensity thresholds achieved in Twidwell et al. (2013). Prior to fuel application, we conducted a series of trials to determine the amount of fuel required to produce flame lengths similar to those observed in this previous research. Fireline intensity (kW/m), an estimate of heat flux along flame fronts, is defined by Byram as the product of fuel consumption, heat of combustion, and rate of spread (Byram, 1959). We could not replicate fire intensities because of the ignition method (see below), but we could replicate fuel loading and, thus, fire energy, which is attendant on fuel consumption (Kremens et al., 2012).
To provide a continuous fuel load across each burn plot, we spread a target amount of 60 kg of hay (approximately 0.6 kg/m2 at ambient moisture content) evenly across each 10 × 10 m low‐ and high‐energy plot. In addition to the hay and to produce flame lengths at the lower end of fires in Twidwell et al. (2013), we spread a target amount of 200 kg of previously harvested and dried juniper branches in a circular area ≤7 m in diameter (approximately 5.3 kg/m2 at ambient moisture content) on half of the plots. We centered the juniper fuels on the focal mesquite shrub to concentrate energy release in the plot interior. Subplots in these high‐energy plots were set up within this circle to ensure proper heat dosage. We determined fuel moistures (on a dry mass basis) for each fuel class from collections made at the time fuels were weighed prior to spreading them on plots and on burn days. We measured volumetric soil moisture at three locations in each plot immediately prior to ignition using an EXTECH MO750 Soil Moisture Meter. We report average wind speed and relative humidity in the 10 min leading up to fire ignition based on data from a portable weather station that we moved among plots.
Each plot was ignited with a ring fire method using two drip torches. Fine fuels were consumed almost completely on plots and were not re‐sampled after fire. However, there was sometimes woody material remaining on juniper addition plots which we collected and weighed to determine consumption from prefire loading.
Fire radiated energy (FRE, kJ/m2) and residence times (s) of fires at each subplot were estimated using 1 Hz imagery from a longwave infrared camera. Although we estimated FRE, we refer to it as fire energy above and hereafter for simplicity and in a relative sense with the understanding that FRE is a fraction (~20%) of total fire energy (Kremens et al., 2012). We used a FLIR SC660 and its internal calibration to produce sequences of radiometric (effective) pixel temperatures. We used the low‐temperature range (high gain) setting (up to 500°C) for the purpose of accurately monitoring mesquite stem temperatures. A boom lift was used to elevate the camera to an oblique perspective upwind of the plot. Oblique imagery was orthorectified using GDAL based on GPS positions of posts at the corners of each 10 × 10 m plot which were identified by use of aluminum targets with low emissivity that appear black in the infrared. Each orthorectified scene was re‐gridded to 1‐m2 pixels and the pixels which corresponded to each subplot were identified based on subplot locations, again determined by GPS. All GPS positions were corrected using data from a base station. Python scripting was used to run the GDAL orthorectification and re‐gridding and to calculate radiated power, radiated energy (kJ/m2), and residence times.
Radiometric pixel temperatures were converted to fire radiated power through the Stefan–Boltzmann equation and the blackbody assumption (O'Brien et al., 2016). FRE is the time integral of fire radiated power over the period described by the full‐width at one‐half of the maximum of radiated power (i.e., the width from before to after the maximum). We used a 450°C radiometric temperature threshold to indicate flame presence in the 1‐m2 pixel corresponding to each subplot and then estimated residence time by adding up the time steps during which temperature was greater than 450°C. We used 450°C because it was close to but below both the saturation and the Draper point (525°C) and roughly coincided with visible flaming and our expectations about residence times for hay‐only fires, expectations based on characteristics of fires in other fuel beds dominated by fine fuels (e.g., Bova & Dickinson, 2008; Butler et al., 2016). We were not able to estimate fireline intensities because, once flames converge, there is no spread in ring fires and fireline intensity loses its meaning.
2.3. Sampling
Tillers were harvested 24 hr before and after fires from three randomly determined individuals of each grass species inside each large plot (Figure 1). Tillers were collected less than 3.5 m from the central shrub to ensure the selection of individuals within the additional fuel loading area in the high‐energy treatment and ensure consistent sampling in the low‐energy and control treatments. These tillers were collected from individuals in similar phenological stages as the permanently marked individuals using the classification system of Moore et al. (1991). All collected tillers were from current year growth and all vegetative. Plants visibly damaged by herbivores, insects, or pathogens were excluded.
In each large plot, two tillers were harvested from each individual plant using a trowel to keep above and belowground structures intact. The buds associated with these tillers were counted and their activity classified as either active, dormant, or dead using the Tetrazolium and Evans Blue staining procedures established by Busso et al. (1989).
The day before the fire treatments were applied, bud depth was measured. Two random individuals of each species from each large plot were selected (Figure 1). Individuals were chosen based on similar size and phenological stage as our permanently marked individuals. A hole dug at the base of each individual grass exposed the deepest buds. We only wanted to examine belowground buds, so extra precaution was taken to only collect data from multitiller individuals (5+ tillers) of H. belangeri which, from pre‐experiment trials, usually indicated a deeper rooting individual and deeper buds.
Bud depth was recorded as the distance between the mineral soil surface and the base of each tiller (approximately at the beginning of the root system). Because our study site had low productivity and few trees, there was very little organic matter or duff on site. However, in the few cases where there was senesced plant material, it was swept away to access the mineral soil.
The area of differentiation between the tiller and root was used as an indicator of where the deepest buds would be located on each tiller. The buds of these species are small and often require a microscope to view. Additionally, since we only examined buds associated with tillers, these buds are often covered by the leaf sheath and are not easily identified in the field. However, previous tiller‐collection and bud‐counting trials revealed that, for both species, the buds begin to grow on the base of the tiller, right above the differentiation between tiller and root. Therefore, the area at the base of the tiller, where the root system begins, was used as a quick indicator of where the deepest buds were located.
2.4. Statistical analyses
Immediate bud response data were analyzed using analysis of variance (ANOVA) tests (Table 1). Data were analyzed by species using analysis of variance (MIXED procedure of SAS, Littell et al., 2006) in order to quantify bud bank response immediately before and after prescribed burns. The model included sampling period (pre‐ and postfire), burn treatment, and their interactions as fixed effects with plots as a random effect. Active, dormant, and dead buds were used as response variables, and the experimental unit was plot. Model assumptions for normality were tested using Shapiro–Wilk tests with the UNIVARIATE procedure of SAS and the normality hypothesis was not rejected for any of the data. Each experimental plot was analyzed for differences in bud responses before and after treatment application. Mean separations were determined with tests of pairwise comparisons using the Tukey–Kramer method following significant F tests on main effects or interactions. Statistical significance was declared at p < .05 for all tests.
TABLE 1.
ANOVA table for total, active, dormant, and dead buds for N. leucotricha and H. belangeri
| N. leucotricha | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Factors | Total | Active | Dormant | Dead | ||||||||
| Effect | df | F value | Pr > F | df | F value | Pr > F | df | F value | Pr > F | df | F value | Pr > F |
| Period | 28 | 10.61 | 0.0029 | 28 | 11.91 | 0.0018 | 28 | 0.41 | 0.5268 | 28 | 3.77 | 0.0624 |
| Energy | 28 | 0.67 | 0.5186 | 28 | 3.86 | 0.0332 | 28 | 0.73 | 0.4931 | 28 | 2.38 | 0.1109 |
| Period * Energy | 28 | 0.82 | 0.4494 | 28 | 6.26 | 0.0057 | 28 | 0.66 | 0.5236 | 28 | 8.87 | 0.001 |
| H. belangeri | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Factors | Total | Active | Dormant | Dead | ||||||||
| Effect | df | F value | Pr > F | df | F value | Pr > F | df | F value | Pr > F | df | F value | Pr > F |
| Period | 29 | 34.53 | <0.0001 | 29 | 69.24 | <0.0001 | 29 | 0.81 | 0.376 | 29 | 23.63 | <0.0001 |
| Energy | 29 | 0.2 | 0.8171 | 29 | 6.64 | 0.0042 | 29 | 1.79 | 0.1849 | 29 | 10.2 | 0.0004 |
| Period * Energy | 29 | 8.99 | 0.0009 | 29 | 18.4 | <0.0001 | 29 | 0.35 | 0.7081 | 29 | 11.13 | 0.0003 |
Effects consisted of sampling period (Period: pre‐ and postfire), fire energy (Energy), and their interaction (Period * Energy) as fixed effects. The analyses were done by species, with total, active, dormant, and dead buds as response variables.
Although we had 36 experimental plots, following treatment application infrared imaging revealed that some of our high‐energy subplots did not reach expected radiative energy output because they were outside the fuel addition area. Therefore, four subplots for both N. leucotricha and H. belangeri were removed from the analyses.
Bud depth data were analyzed using a Mann–Whitney U test to compare N. leucotricha to H. belangeri. Treatment effects on reemergence of our species were analyzed using Fisher's exact test (for H. belangeri) and a chi‐square test (for N. leucotricha). Statistical significance was set at p < .05.
3. RESULTS
3.1. Fire weather, fuels, and fire characteristics
Burns were completed over 5 days from 30 July to 4 August 2018. Wind speed, relative humidity, and soil and fuel moisture are summarized in Table 2. Existing grass and added hay and juniper fuel loadings on a dry basis, both prefire and consumed, are provided in Table 3. Average FRE and residence times for subplots are summarized in Table 4. Because the infrared imagery was saturated at a radiometric temperature of 500°C, subplot FRE values in Table 4 are underestimates. This is particularly the case for high‐energy fires (hay plus juniper) while low‐energy fires (hay only) were minimally saturated. Regardless, fire radiated energy in juniper addition plots was substantially greater than energy than in hay‐only plots (Table 4) primarily because residence times were 50 times longer than on low‐energy plots. Because of the underestimate on high‐energy plots, fire energy can be thought of as a relative index. Residence times were little affected by saturation given our use of a 450°C threshold to indicate visible flame presence.
TABLE 2.
Fire weather and burn day fuel moisture at the plot level for low‐ and high‐energy treatments
| Treatment | Wind (km/hr) | RH (%) | Soil moisture (%) | Moisture (%) | ||||
|---|---|---|---|---|---|---|---|---|
| Grass | Hay | Juniper | ||||||
| Foliage | 10 hr | 100 hr | ||||||
| Low‐energy | 8 (4–11) | 27 (20–35) | 13 (11–15) | 9 (5–17) | 4 (<1–10) | NA | NA | NA |
| High‐energy | 9 (4–16) | 28 (23–31) | 13 (9–15) | 7 (5–9) | 4 (<1–8) | 4 (1–5) | 5 (3–8) | 7 (5–7) |
Average wind and RH and their ranges (in parentheses) are for all plots in each treatment. Fuel moistures are on a dry mass basis and are averages (and ranges) of mean burn day values for a partial set of plots. Volumetric soil moistures are averages (and ranges) of mean burn day values for all plots. Plots including grass subplots described in this paper (N = 12 per treatment) are a subset of total plots in the larger study (N = 24) for which we report weather and fuel data here.
TABLE 3.
Fuel bed heights and fuel additions and consumed loading at the plot level for low‐ and high‐energy treatments
| Treatment | Fuel bed height (m) | Native herbaceous fuel loading (kg/m2) | Loading (kg/m2) | |||
|---|---|---|---|---|---|---|
| Prefire | Consumed | |||||
| Hay | Juniper | Hay | Juniper | |||
| Low‐energy | 0.21 (0.14–0.29) | 0.10 (0.03) | 0.54 (0.07) | NA | 0.54 (0.07) a | NA |
| High‐energy | 0.92 (0.55–1.49) | 0.09 (0.03) | 0.59 (0.14) | 5.18 (0.81) | 0.59 (0.14) a | 5.05 (0.82) |
Hay was added to both low‐ and high‐energy plots to achieve continuous burns over the 10 × 10 m plot area while juniper was only added to high‐energy plots. For high‐energy fires, dried juniper was spread in a circular area (averaged 6.8 m diameter, range 5.8–8.1 m) centered on the focal mesquite shrub in ½ of the plots. Juniper was a mix of foliage and 1, 10, and 100‐hr size‐class woody material. All loadings (mean with standard deviation in parentheses) are on a dry mass basis and were determined for the measured areas over which fuels were spread. Plots including grass subplots described in this paper (N = 12 per treatment) are a subset of total plots in the larger study (N = 24 per treatment) for which we report weather and fuel data here.
Hay consumption was nearly complete for all plots, and postfire loading was not measured.
TABLE 4.
Fire radiated energy and residence times for 1‐m2 pixels corresponding to subplot locations in the low‐ and high‐energy treatment plots
| Treatment | Number of subplots | Energy (kJ/m2) a | Residence time (s) b |
|---|---|---|---|
| Low‐energy | 22 | 423 ± 46 | 23 ± 3 |
| High‐energy | 14 | 23,434 ± 2,523 | 1,216 ± 133 |
Reported are averages (and standard deviation) for the subplots in hay‐only plots (low‐energy) or plots to which both hay and juniper fuels were added (high‐energy). Some subplots in the high‐energy plots were outside of the juniper fuel addition area and are excluded here and from analyses (see Methods).
Energy of grass mini‐plot (1 m2), time integrated over the period in which fire radiated energy was greater than or equal to ½ of the maximum (full‐width at ½ maximum) radiation.
Residence time at grass plot calculated as the time steps for which the 1‐m2 pixel radiometric temperature was greater than a threshold of 450°C.
3.2. Nassella leucotricha dynamics
Since only the period main effect was significant (Table 1), changes in total bud numbers were considered across all energy treatments. There was an immediate significant reduction in total buds for N. leucotricha between pre‐ and post‐treatment sampling times; total bud numbers across our treatments decreased by 17% (decrease of 0.37 ± 0.14 buds tiller−1; t 28 = −2.53, p = .003; Figure 2; Table 5).
FIGURE 2.

Difference in mean buds tiller−1 for N. leucotricha between pre‐ and post‐treatment values within each plot. Measurements were taken 24 hr before and after low‐ and high‐energy fire treatment application. Negative values indicate an average decrease in the number of buds tiller−1 from pre‐ to post‐treatment measurements within plots. Bars with an asterisk (*) indicate significant differences between pre‐ and post‐treatment values within treatment. For total buds, since only the period effect was significant, the treatments were combined and the overall change from pre‐ to post‐treatment was graphed. Pairwise comparisons were only performed when main or interaction effects were significant. Error bars indicate one standard error
TABLE 5.
Immediate (<24 hr) fire energy effects on the number of total, active, dormant, and dead belowground buds belonging to N. leucotricha and H. belangeri tillers
| N. leucotricha | ||||||
|---|---|---|---|---|---|---|
| Bud classification | Control | Low | High | |||
| Pre | Post | Pre | Post | Pre | Post | |
| Total | 2.3 ± 0.13 | 2.1 ± 0.13 | 2.2 ± 0.13a | 1.9 ± 0.13ab | 2.3 ± 0.16a | 1.7 ± 0.16b |
| Active | 0.9 ± 0.13ab | 1.0 ± 0.13ab | 1.2 ± 0.13a | 0.7 ± 0.13b | 1.0 ± 0.16ab | 0.2 ± 0.16c |
| Dormant | 1.2 ± 0.13a | 0.99 ± 0.13a | 1.0 ± 0.13a | 1.1 ± 0.13a | 1.3 ± 0.16a | 1.2 ± 0.16a |
| Dead | 0.18 ± 0.057a | 0.056 ± 0.057a | 0.083 ± 0.057a | 0.13 ± 0.059a | 0.083 ± 0.069a | 0.42 ± 0.069b |
| H. belangeri | ||||||
|---|---|---|---|---|---|---|
| Bud classification | Control | Low | High | |||
| Pre | Post | Pre | Post | Pre | Post | |
| Total | 2.8 ± 0.16a | 2.8 ± 0.16a | 3.3 ± 0.16b | 2.2 ± 0.16c | 3.3 ± 0.19b | 2.0 ± 0.19c |
| Active | 1.6 ± 0.16a | 1.6 ± 0.16a | 1.8 ± 0.16a | 0.6 ± 0.16b | 1.8 ± 0.19a | 0.1 ± 0.19b |
| Dormant | 1.2 ± 0.16a | 1.2 ± 0.16a | 1.5 ± 0.16a | 1.5 ± 0.16a | 1.6 ± 0.19a | 1.3 ± 0.19a |
| Dead | 0.014 ± 0.067a | 0.014 ± 0.067a | 0.014 ± 0.067a | 0.18 ± 0.067a | 0 ± 0.081a | 0.69 ± 0.0.081c |
Pre‐ and post‐treatment values are given. All numbers represent bud means which are given in buds tiller−1. Means within bud classification are similar when followed by a common letter (p > .05). Pairwise comparisons were only performed when main or interaction effects were significant.
There was a significant effect of fire energy (Table 1) on the number of active buds for N. leucotricha. When comparing pre‐ and post‐treatment values, active bud numbers in the high‐energy fire treatment decreased by 84% (decrease of 0.79 ± 0.22 buds tiller−1; t 28 = −3.68, p = .001; Figure 2; Table 5) while active bud numbers in the low‐energy treatment decreased by 42% (decrease of 0.49 ± 0.18 buds tiller−1; t 28 = −2.72, p = .01; Figure 2; Table 5). Overall, our fire treatments significantly decreased active buds while the control treatment was unaffected. Twenty‐four hours following fire treatments, the total number of active buds for N. leucotricha differed between the low‐energy and high‐energy treatments. The low‐energy treatment had 0.52 ± 0.24 more buds tiller−1 than the high‐energy treatment post‐treatment (t 28 = −2.41, p = .02).
There was no significant difference in dormant buds for N. leucotricha between pre‐ and post‐treatment sampling periods for any of our treatments (Table 1; Figure 2; Table 5).
There was a significant difference in dead buds between pre‐ and post‐treatment sampling periods for only the high‐energy treatment (Table 1). When comparing pre‐ and post‐treatment values, dead bud numbers in the high‐energy treatments increased by 424% (increase of 0.33 ± 0.08 buds tiller−1; t 28 = 3.9, p < .001; Figure 2; Table 5).
3.3. Hilaria belangeri dynamics
There was a significant difference in total buds for H. belangeri between pre‐ and post‐ treatment sampling times for both the low‐ and high‐energy treatments but not the control (Table 1). When comparing pre‐ and post‐treatment values, total bud numbers in the high‐energy treatment decreased by 38% (decrease of 1.27 ± 0.27 buds tiller−1; t 29 = −4.71, p < .001) while total buds in the low‐energy treatment decreased by 34% (decrease of 1.14 ± 0.22 buds tiller−1; t 29 = −5.17, p < .001) following treatment application. Overall, fire treatments led to a significant decrease in the total number of H. belangeri buds (Figure 3; Table 5). However, the total number of buds post‐treatment was similar between the low‐energy and high‐energy treatments.
FIGURE 3.
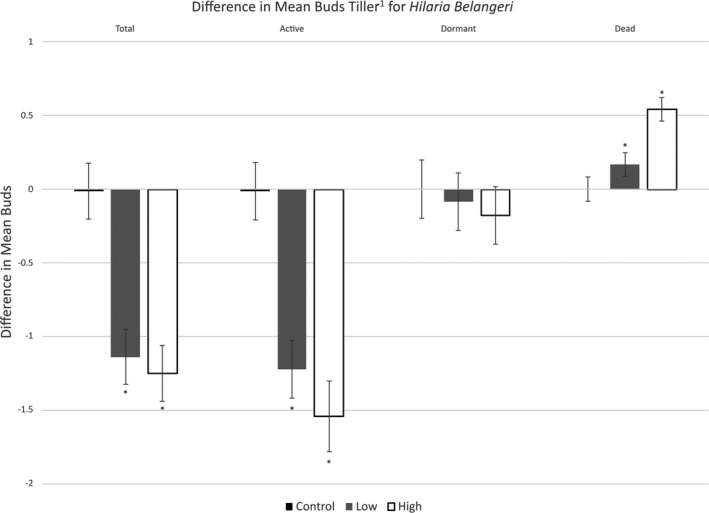
Difference in mean buds tiller−1 for H. belangeri between pre‐ and post‐treatment values. Measurements were taken 24 hr before and 24 hr after low‐ and high‐energy fire treatment application. Negative values indicate a decrease in the number of buds tiller−1 from pre‐ to post‐treatment measurements. Bars with an asterisk (*) indicate significant differences between pre‐ and post‐treatment values within treatment. Error bars indicate one standard error
There was a significant difference in active buds for H. belangeri between pre‐ and post‐ treatment sampling periods for both the low‐ and high‐energy treatments but not the control (Table 1). When comparing pre‐ and post‐treatment values, active bud numbers in the high‐energy treatment decreased by 95% (decrease of 1.67 ± 0.23 buds tiller−1; t 29 = −7.30, p < .001) while active buds in the low‐energy treatment decreased by 69% (decrease of 1.22 ± 0.19 buds tiller−1; t 29 = −6.55, p < .001) following treatment. Overall, our fire treatments led to a decrease in the number of active H. belangeri buds (Figure 3; Table 5). However, the number of active buds post‐treatment was similar between the control, low‐energy, and high‐energy treatments.
There was no statistically significant difference in dormant buds between pre‐ and post‐treatment for any of the fire treatments (Table 1; Figure 3; Table 5).
There was a significant difference in dead buds for H. belangeri between pre‐ and post‐treatment for only the high‐energy treatments (Table 1). When comparing pre‐ and post‐treatment values, the number of dead buds in the high‐energy treatment increased by 0.69 ± 0.12 buds tiller−1; t 29 = 5.98, p < .001).
3.4. Bud depths
Mean bud depth differed between N. leucotricha and H. belangeri (Mann–Whitney U = 2,720, n 1 = n 2 = 72, p < .001 two‐tailed). On average, bud depth was 1.8 ± 0.06 cm for N. leucotricha and 0.5 ± 0.04 cm for H. belangeri.
3.5. Initial reemergence
Three weeks after the prescribed fire treatments, grasses in the high‐energy plots were beginning to resprout (Figure 4). However, all H. belangeri individuals except those in the high‐energy treatment showed regrowth. In the high‐energy treatment, 75% of the marked individuals failed to resprout (p < .001). Most of the individuals that failed to resprout were not completely consumed by the fire, though some were. Those that were not completely consumed still had intact belowground structures but few retained any aboveground tillers, live or dead. All marked N. leucotricha individuals resprouted in the low‐energy and high‐energy treatments following the fires.
FIGURE 4.

Picture of high‐energy plot immediately after treatment application (a), the same high‐energy plot 3 weeks post‐treatment (b), and a bud on a Nassella leucotricha tiller from a high‐energy plot post‐treatment (c)
4. DISCUSSION
4.1. Immediate effects of fire energy
The use of high‐energy fires as a management tool provides insight into the evaluation of the immediate effect of fire energy on grass bud bank dynamics. Although there are studies capturing the immediate bud bank dynamics of grasses following low‐ and moderate‐energy fires (Russell et al., 2013; Russell et al., 2015, 2019; Russell & Vermeire, 2015), this is the first look at the effect of high‐energy fires on bud responses. While soil has been shown to insulate belowground plant tissue from fire (Volland & Dell, 1981; Young, 1983), studies have shown a potential for soil heating to lethal temperatures, especially at shallower depths ( Balatsos, 1994; Campbell, 2016; Choczynska & Johnson, 2009; Kobziar et al., 2019; Peter, 1992). Increasing fuel consumption leads to increased fire energy (Kremens et al., 2012), increased soil surface heat fluxes and temperatures (Choczynska & Johnson, 2009), and greater soil heating (Bradstock & Auld, 1995). Therefore, high‐energy fires have a greater potential to impact grass bud metabolic activity and survival than low‐energy fires.
In this study, only high‐energy fires had a significant effect on immediate bud mortality for both species examined, with immediate increases in dead buds. The lack of significant bud mortality in our low‐energy plots corresponds to the results in Russell et al. (2015) even though their fuel loads were substantially lower than ours (0.15 vs. 0.6 kg/m2, respectively; Table 3). The fuel loads in our low‐energy treatment were lower, but closer, in approximation, to the 0.8 kg/m2 loadings that Haile (2011) found to result in a 50% probability of mortality for two different Great Plains grasses after heating trials. We can speculate that the relatively dry soils in our study reduced soil heating (Busse et al., 2013) and, in turn, reduced impacts on bud banks. The increase we saw in dead buds in our high‐energy plots is not necessarily indicative of the entirety of bud mortality. Some buds were likely consumed by fire and therefore were not captured in the count.
The increase in dead buds was much greater for H. belangeri than N. leucotricha. Even with this significant fire‐induced bud mortality, the absolute amount of bud death was relatively small. In the high‐energy treatment, approximately 24% of N. leucotricha buds and 34% of H. belangeri buds were dead following treatments. Even though these values seem relatively small, they may have differentially impacted our focal species and resulted in a difference in tiller reemergence following treatment. Therefore, high‐energy fires induce immediate bud mortality in these species, but the differences in the magnitude are likely mediated by growth form and photosynthetic pathway.
Fire energy also had a significant effect on the number of active buds for both N. leucotricha and H. belangeri. Both high‐ and low‐energy fires led to a decrease in active buds, but high‐energy fires led to a greater decrease for N. leucotricha. In contrast, H. belangeri experienced a similar decrease in active buds in both high‐ and low‐energy treatments, suggesting that fire energy was not as important for H. belangeri with regard to changes in bud activity.
Interestingly, dormant buds remained constant between pre‐ and post‐treatment sampling times for both species and across all treatments. Because we saw an increase in bud mortality and a decrease in active buds in our high‐energy treatment while dormant bud numbers remained constant, it is likely that the majority of the bud mortality we saw was from bud death in active buds. This lack of change in bud dormancy may be a product of the timing of our burns and the combination of heat and drought. Newly formed axillary buds often cycle through temporary transition stages of temporary growth or dormancy until developmental or environmental cues signal buds to undergo growth or fully become dormant (Devitt & Stafstrom, 1995; Shimizu‐Sato & Mori, 2001). When axillary buds are in these temporary transitions, environmental cues such as heat or drought can induce quiescence. This is likely what was seen in Russell et al. (2015), with one of their focal C4 species, Bouteloua gracilis, and may be why both of our species did not see an increase in bud activation and instead experienced little change in dormancy. This temporary bud strategy is particularly advantageous in areas, such as our semiarid site, that evolved under summer fire regimes and this temporary dormancy of buds allows plants to survive heat stress from increased summer fire intensities (Higgins, 1984; Umbanhowar, 1996).
With high‐energy fires during drought increasingly being applied to remove invasive shrubs, this study serves to assuage some fears in relation to extreme fires. High‐energy fires may cause immediate bud death, but it was not a large proportion of the available bud bank for either species. In addition, in the case of N. leucotricha, many dormant buds survived, and new tillers were produced a few weeks following treatment. With increased bud mortality, meristematic limitations can occur. Because the overall bud bank size plays a prominent role in plant population dynamics by buffering against disturbance (Benson et al., 2004; Dalgleish & Hartnett, 2009), a decrease in size can result in a decreased capacity to not only recover from disturbance but to also take advantage of the increased nutrient availability and light that often occurs following disturbances such as fire (Benson et al., 2004; Dalgleish & Hartnett, 2006). The fact that we see many N. leucotricha buds survive following these high‐energy fires may indicate that the local population of this grass is likely to persist and remain unchanged in the long‐term.
4.2. Growth form
Although we saw a direct effect of fire energy on bud mortality, the ratio of dead buds to active and dormant buds postfire was higher for H. belangeri than N. leucotricha. This result may be an effect of the relationship between fire energy and residence times and differential growth forms that influence bud depth within the soil profile. Nassella leucotricha had deeper buds, on average, than H. belangeri. Fuel consumption, fire energy, and residence times were substantially higher for high‐energy than low‐energy subplots (Tables 3 and 4).
This is important because soil is considered an effective insulator (Valettel et al., 1994). Choczynska and Johnson (2009) found that most of the temperature increase from fire in soils occurred in the first 1 cm of soil and dropped off steeply below 1 cm. They also found that lethal temperatures for 3 grass species in their study did not occur below the top 2 cm of soil, even with surface temperatures of 700°C sustained for 660 s. Since N. leucotricha buds were located at 1.8 cm below the soil surface on average, with many below the critical 2 cm depth described by Choczynska and Johnson (2009), while H. belangeri buds were located 0.5 cm below the soil surface on average (most within the top 0.55 cm), many more H. belangeri buds were likely within the lethal soil‐heating zone for high‐energy fires. Therefore, higher fuel consumption that results in greater fire energy and residence times is more important for grasses with shallower bud banks because soil heating is increased, and a larger portion of buds will fall within the lethal soil‐heating zone.
Hilaria belangeri's shallow bud depths are consistent with other stoloniferous species, particularly Bouteloua dactyloides, which was found to have growing points primarily at ground level or just below (Branson, 1953). Bunchgrasses have a range of growing points, with growing points extending above the soil to below the surface (Branson, 1953; Edmond & Hoveland, 1972), with one study estimating the mean depth of grass growth points in a tallgrass prairie being 3.2 ± 2.1 cm below the soil surface (Benson et al., 2004). Other studies showed patterns regarding bud distribution in the soil profile, bud death, and disturbance intensity (Klimešová & Klimeš, 2007; Vesk et al., 2004), suggesting bud depth may be an important determinant of grass survival following fires.
Previous studies found that bunchgrasses with caespitose growth forms are more susceptible to fire damage than other growth forms (Engle et al., 1998; Wright, 1971). In general, litter accumulates in the crown of caespitose grasses which increases fuel load at its center, leading to greater heat duration and dosage (Engle et al., 1998; Wright, 1971). This potentially increases bunchgrass susceptibility to fire by increasing bud mortality. This was not observed in our study. Instead, the stoloniferous species H. belangeri was more susceptible to fire damage than the caespitose species N. leucotricha.
This result is consistent with Russell et al. (2015) in which they found that H. comata did not sustain immediate bud mortality despite its bunchgrass form. Russell et al. (2015) reasoned that H. comata's coarse stems and dense plant crown prevented heat transfer to the buds and subsequent mortality. Size may also be a contributing factor in this study. Wright and Klemmedson (1965) suggest that, for some species, the size of the plant is important in determining the effect of fire on bunchgrasses, especially during the latter part of the summer. With our site being semiarid, our N. leucotricha individuals covered a small basal area (~7‐20 cm in diameter) and likely had lower accumulation of litter resulting in less heat duration at the center of the plant and less bud mortality. Overall, it is difficult to determine if growth form played a large role in bud response because of the number of herbaceous fuels added to the plot, potentially offsetting any effect of the grasses themselves on fire behavior.
4.3. Phenology
Fire season has been shown to directly affect bud activity, dormancy, and mortality for several grass species following moderate‐energy fires (Russell et al., 2015, 2019). Bud bank size and seasonal bud bank dynamics vary among species (Lehtilä, 2000; Zhang & Biswas, 2017) and have been shown to differ among grasses with different photosynthetic pathways (Ott & Hartnett, 2012). Therefore, another possible explanation for higher bud mortality in H. belangeri may be the timing of our burns. Nassella leucotricha is a C3, cool‐season grass while H. belangeri is a C4, warm‐season grass. These different functional groups have different phenological timing of increased bud activity or dormancy (Ott & Hartnett, 2012). Summer fires have been shown to favor C3 over C4 perennial grasses (Engle et al., 1998) because actively growing grasses are more easily damaged by fire than dormant grasses (Briske, 1991).
In Russell et al. (2015), moderate‐intensity summer fires had little impact on immediate bud activity or mortality in a C3 bunchgrass, H. comata. Although our low‐energy fires did not produce a significant increase in bud mortality, there was a decrease in active buds in N. leucotricha. In contrast, the sod‐forming C4 grass, B. gracilis, saw an immediate increase in bud activity following summer fires (Russell et al., 2015). Since total bud numbers did not change, there was likely a shift from dormant buds to active (Russell et al., 2015). We likely did not see this same shift because H. belangeri experienced a significant decrease in active buds with an increase in bud mortality. Given that total decrease in bud numbers, it is likely that active buds in both the low‐ and high‐energy plots experienced greater mortality than dormant buds.
All permanently marked N. leucotricha individuals produced new tillers in the high‐energy treatment. Although not all H. belangeri buds died in the high‐energy fires, very few individuals produce new tillers 3 weeks following fire despite increased precipitation following fires which led to many grass species, including H. belangeri, to resprout in the low‐energy plots. Since C4 grasses become dormant during the fall, early August fire likely induced dormancy earlier than usual due to increased stress from high‐energy fire and low water availability. We expect induced dormancy because bud activity and outgrowth are modulated by environmental conditions (Ott et al., 2019; Shimizu‐Sato & Mori, 2001). In fact, Russell et al. (2019) found that summer fire increased overwintering buds of C4 grasses. This is most likely the reason resprouting did not occur in our high‐energy treatments. However, a longer‐term examination of fire seasonality effects on bud dynamics is warranted for corroboration (Hiers, 2019).
Differences between C3 and C4 grass responses to fire energy manifest over longer time frames since overall bud numbers, overwintering strategies, and seasonal patterns of bud activity all drive long‐term grass responses to fire and have been shown to differ between C3 and C4 grasses (Ott & Hartnett, 2012; Russell et al., 2015). So, although functional group has a large influence on phenological patterns of bud growth and dormancy (Ott & Hartnett, 2012; Russell et al., 2015), and therefore grass response to fire energy, the difference in photosynthetic pathway seems less important for immediate bud response to fire energy than growth form in our study.
Despite indications of immediate loss of vegetative reproduction and potential mortality of individuals, these results may not necessarily translate to loss of biomass of these species over longer time periods. Studies of long‐term grazing have shown that bud numbers for H. belangeri were significantly greater in grazed than ungrazed communities due to increases in plant density (Hendrickson & Briske, 1997). Hendrickson and Briske (1997) demonstrated that long‐term effects of herbivory, and likely other disturbances, are predominantly expressed at the population level rather than at the individual or tiller level. In other semiarid savanna ecosystems, a reduction in neighborhood density of perennial grasses can increase the long‐term survival and productivity of surviving individuals (Zimmerman et al., 2010). In addition, increases in nutrient availability and light can offset direct loss of buds, allowing for increased growth and reproduction in years following fire (Dalgleish & Hartnett, 2008; Russell & Vermeire, 2015; Tomlinson & O'Connor, 2004). These studies point to the likelihood that H. belangeri should recover in the future despite indications of decreased vegetative reproductivity and possible meristem limitations.
Also, given that our study site tends to have patchy herbaceous cover, pockets of grasses would be protected from high‐intensity fires under more natural conditions (i.e., without added fuel). We also expect to see that, due to spatial heterogeneity, stoloniferous and rhizomatous grasses in areas not impacted by high‐intensity fires will colonize areas that were affected. Additionally, recruitment from the seedbank will likely impact the colonization of areas but to what extent is a potential future area of study. Although we saw greater bud death in H. belangeri and little regrowth, long‐term studies indicate that high‐intensity fires do not lead to legacy changes in the herbaceous understory. As such, we expect that H. belangeri will recover in the next few growing seasons (Taylor et al., 2012).
5. CONCLUSIONS
Most grass regrowth occurs primarily via a belowground bank of axillary buds (Latzel et al., 2008; Ott et al., 2019; Vítová et al., 2017). It is therefore necessary to understand bud bank dynamics to predict grass population and community responses to disturbances such as fire (Benson & Hartnett, 2006; Dalgleish & Hartnett, 2009). In this study, we saw a significant increase in bud mortality in both our species in the high‐energy treatment; however, this bud mortality was greater in H. belangeri and monitored individuals failed to resprout 3 weeks following treatment application. Our immediate bud responses are most likely the result of contrasts in the phenology and growth forms of our two grass species, which suggests the need for managers to consider both in predicting grass survival following high‐energy fires during low water availability.
CONFLICT OF INTEREST
None declared.
AUTHOR CONTRIBUTIONS
Quinn A. Hiers: Formal analysis (equal); Methodology (equal); Visualization (lead); Writing‐original draft (lead); Writing‐review & editing (equal). Morgan L. Treadwell: Conceptualization (equal); Formal analysis (equal); Methodology (equal); Validation (equal); Writing‐original draft (supporting); Writing‐review & editing (equal). Matthew B. Dickinson: Conceptualization (equal); Methodology (equal); Writing‐review & editing (equal). Kathleen L. Kavanagh: Conceptualization (equal); Methodology (equal); Writing‐review & editing (equal). Alexandra G. Lodge: Conceptualization (equal); Methodology (equal); Writing‐review & editing (equal). Heath D. Starns: Methodology (equal); Writing‐review & editing (equal). Doug R. Tolleson: Methodology (equal); Writing‐review & editing (equal). Dirac Twidwell: Conceptualization (equal); Writing‐review & editing (equal). Carissa L. Wonkka: Conceptualization (equal); Methodology (equal); Writing‐original draft (supporting); Writing‐review & editing (equal). William E. Rogers: Conceptualization (equal); Funding acquisition (lead); Methodology (equal); Supervision (lead); Writing‐original draft (supporting); Writing‐review & editing (equal).
Supporting information
Fig S1
ACKNOWLEDGMENTS
We would like to thank Dr. Joe O'Brien for lending us his FLIR camera and Dr. Louise Loudermilk for the python code used to analyze the imagery obtained from the FLIR camera. L. Culpepper, A. Labrecque, H. Hannusch, J. Gaster, C. Ferguson, J. Walker, N. Garza, and R. Moen provided technical assistance. This research was funded by the United States Department of Agriculture Bureau of Land Management Joint Fire Sciences Program Project #17‐1‐04‐7. At the time of experimentation, graduate study was supported by a Teaching Assistantship from Texas A&M University, specifically the Sid Kyle Graduate Fellowship.
Hiers QA, Treadwell ML, Dickinson MB, et al. Grass bud responses to fire in a semiarid savanna system. Ecol Evol. 2021;11:6620–6633. 10.1002/ece3.7516
DATA AVAILABILITY STATEMENT
Data are archived in the Dryad Digital Repository (https://doi.org/10.5061/dryad.kprr4xh48).
REFERENCES
- Ansley, R. J. , & Jacoby, P. W. (1998). Manipulation of fire intensity to achieve mesquite management goals in north Texas. In: Pruden T. L., & Brennan L. A. (Eds.), Fire in ecosystem management: Shifting the paradigm from suppression to prescription. Tall Timbers fire ecology conference proceedings, No. 20. Tall Timbers Research Station, Tallahassee, FL (pp. 195–204). [Google Scholar]
- Archer, S. R. , Andersen, E. M. , Predick, K. I. , Schwinning, S. , Steidl, R. J. , & Woods, S. R. (2017). Woody Plant Encroachment: Causes and Consequences. In: Briske D. (Ed.), Rangeland Systems. Springer Series on Environmental Management. Springer. 10.1007/978-3-319-46709-2_2 [DOI] [Google Scholar]
- Balatsos, P. C. (1994). Pyrogenic heat flow into soils and heat‐induced tissue damage of Agropyron spicatum during simulated fire. University of Idaho. [Google Scholar]
- Benson, E. J. , & Hartnett, D. C. (2006). The role of seed and vegetative reproduction in plant recruitment and demography in tallgrass prairie. Plant Ecology, 187, 163–178. 10.1007/s11258-005-0975-y [DOI] [Google Scholar]
- Benson, E. J. , Hartnett, D. C. , & Kale, H. M. (2004). Belowground bud banks and meristem limitation in tallgrass prairie plant populations. American Journal of Botany, 91(3), 416–421. 10.3732/ajb.91.3.416 [DOI] [PubMed] [Google Scholar]
- Bova, A. S. , & Dickinson, M. B. (2008). Beyond “fire temperatures”: Calibrating thermocouple probes and modeling their response to surface fires in hardwood fuels. Canadian Journal of Forest Research, 38(5), 1008–1020. 10.1139/X07-204 [DOI] [Google Scholar]
- Bradstock, R. A. , & Auld, T. D. (1995). Soil temperatures during experimental bushfires in relation to fire intensity: Consequences for legume germination and fire management in south‐eastern Australia. Journal of Applied Ecology, 76–84. 10.2307/2404417 [DOI] [Google Scholar]
- Branson, F. A. (1953). Two new factors affecting resistance of grasses to grazing. Journal of Range Management, 6(3), 165–171. [Google Scholar]
- Briske, D. D. (1991). Developmental morphology and physiology of grasses. In Heitschmidt R. K., & Stuth J. W. (Eds.), Grazing management: An ecological perspective (pp. 85–108). Timber Press. [Google Scholar]
- Busse, M. D. , Shestak, C. J. , & Hubbert, K. R. (2013). Soil heating during burning of forest slash piles and wood piles. International Journal of Wildland Fire, 22(6), 786–796. 10.1071/WF12179 [DOI] [Google Scholar]
- Busso, C. A. , Mueller, R. J. , & Richards, J. H. (1989). Effects of drought and defoliation on bud viability in two caespitose grasses. Annals of Botany, 63, 477–485. 10.1093/oxfordjournals.aob.a087768 [DOI] [Google Scholar]
- Butler, B. , Teske, C. , Jimenez, D. , O'Brien, J. , Sopko, P. , Wold, C. , Vosburgh, M. , Hornsby, B. , & Loudermilk, E. (2016). Observations of energy transport and rate of spreads from low‐intensity fires in longleaf pine habitat–RxCADRE 2012. International Journal of Wildland Fire, 25(1), 76–89. 10.1071/WF14154 [DOI] [Google Scholar]
- Byram, G. M. (1959). Combustion of forest fuels. In Davis K. P. (Ed.), Forest fire: Control and use. McGraw‐Hill Col. [Google Scholar]
- Campbell, R. (2016). Fires in industrial or manufacturing properties (p. 72). National Fire Protection Association. [Google Scholar]
- Choczynska, J. , & Johnson, E. A. (2009). A soil heat and water transfer model to predict belowground grass rhizome bud death in a grass fire. Journal of Vegetation Science, 20(2), 277–287. 10.1111/j.1654-1103.2009.05757.x [DOI] [Google Scholar]
- Clarke, P. J. , Lawes, M. J. , Midgley, J. J. , Lamont, B. B. , Ojeda, F. , Burrows, G. E. , Enright, N. J. , & Knox, K. J. E. (2013). Resprouting as a key functional trait: How buds, protection, and resources drive persistence after fire. The New Phytologist, 197(1), 19–35. 10.1111/nph.12001 [DOI] [PubMed] [Google Scholar]
- Cruz, A. , Pérez, B. , & Moreno, J. M. (2003). Plant stored reserves do not drive resprouting of the lignotuberous shrub Erica australis. New Phytologist, 157, 251–261. 10.1046/j.1469-8137.2003.00668.x [DOI] [PubMed] [Google Scholar]
- Dalgleish, H. J. , & Hartnett, D. C. (2006). Below‐ground bud banks increase along a precipitation gradient of the North American Great Plains: a test of the meristem limitation hypothesis. New Phytologist, 171, 81–89. 10.1111/j.1469-8137.2006.01739.x [DOI] [PubMed] [Google Scholar]
- Dalgleish, H. J. , & Hartnett, D. C. (2008). The effects of fire frequency and grazing on tallgrass prairie productivity and plant composition are mediated through bud bank demography. In: Van der Valk A. G. (Ed.), Herbaceous Plant Ecology (pp. 47–56). Springer. 10.1007/978-90-481-2798-6_4 [DOI] [Google Scholar]
- Dalgleish, H. J. , & Hartnett, D. C. (2009). The effects of fire frequency and grazing on tallgrass prairie productivity and plant composition are mediated through bud bank demography. Plant Ecology, 201, 411–420. 10.1007/978-90-481-2798-6_4 [DOI] [Google Scholar]
- Dayamba, S. D. , Savadogo, P. , Zida, D. , Sawadogo, L. , Tiveau, D. , & Oden, P. C. (2010). Fire temperature and residence time during dry season burning in a Sudanian savanna‐woodland of West Africa with implication for seed germination. Journal of Forestry Research, 21(4), 445–450. 10.1007/s11676-010-0095-y [DOI] [Google Scholar]
- Devitt, M. L. , & Stafstrom, J. P. (1995). Nucleotide sequence of four ribosomal protein L27 cDNAs from growing axillary buds of pea. Plant Physiology, 107, 1031–1032. 10.1104/pp.107.3.1031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edmond, D. B. , & Hoveland, C. S. (1972). A study of the position of grass growing points in two species under different systems of management. New Zealand Journal of Agricultural Research, 15(1), 7–18. 10.1080/00288233.1972.10421272 [DOI] [Google Scholar]
- Engle, D. M. , Mitchell, R. L. , & Stevens, R. L. (1998). Late growing‐season fire effects in mid‐successional tallgrass prairies. Journal of Range Management, 51, 115–121. 10.2307/4003573 [DOI] [Google Scholar]
- Haile, K. F. (2011). Fuel load and heat effects on northern mixed prairie and four prominent rangeland graminoids. Thesis. Montana State University. [Google Scholar]
- Hendrickson, J. R. , & Briske, D. D. (1997). Axillary bud banks of two semiarid perennial grasses: Occurrence, longevity, and contribution to population persistence. Oecologia, 110(4), 584–591. [DOI] [PubMed] [Google Scholar]
- Hiers, Q. A. (2019). Belowground bud bank dynamics of grasses following extreme fire and drought (Unpublished master's thesis). Texas A&M University. [Google Scholar]
- Higgins, K. F. (1984). Lightning fires in North Dakota grasslands and in pine‐savanna lands of South Dakota and Montana. Journal of Range Management, 37, 100–103. 10.2307/3898892 [DOI] [Google Scholar]
- Klimešová, J. , & Klimeš, L. (2007). Bud banks and their role in vegetative regeneration – A literature review and proposal for simple classification and assessment. Perspectives in Plant Ecology, Evolution and Systematics, 8(3), 115–129. 10.1016/j.ppees.2006.10.00 [DOI] [Google Scholar]
- Kobziar, L. N. , Varner, J. M. , Kreye, J. , Andreu, M. , & Godwin, D. (2019). The consequences of soil heating for prescribed fire use and fire restoration in the South. Final report, Joint Fire Science Program 15‐1‐05‐5. [Google Scholar]
- Kremens, R. L. , Dickinson, M. B. , & Bova, A. S. (2012). Radiant flux density, energy density and fuel consumption in mixed‐oak forest surface fires. International Journal of Wildland Fire, 21(6), 722–730. 10.1071/WF10143 [DOI] [Google Scholar]
- Latzel, V. , Mihulka, S. , & Klimešová, J. (2008). Plant traits and regeneration of urban plant communities after disturbance: Does the bud bank play any role? Applied Vegetation Science, 11(3), 387–394. 10.3170/2008-7-18487 [DOI] [Google Scholar]
- Lehtilä, K. (2000). Modelling compensatory regrowth with bud dormancy and gradual activation of buds. Evolutionary Ecology, 14(4–6), 315–330. 10.1023/A:1010869605855 [DOI] [Google Scholar]
- Littell, R. C. , Milliken, G. A. , Stroup, W. W. , Wolfinger, R. D. , & Schabenberger, O. (2006). SAS for mixed models (2nd ed.). SAS Institute Inc. [Google Scholar]
- Massman, W. J. , Frank, J. M. , & Mooney, S. J. (2010). Advancing investigation and physical modeling of first‐order fire effects on soils. Fire Ecology, 6(1), 36. 10.4996/fireecology.0601036 [DOI] [Google Scholar]
- Milchunas, D. G. , & Lauenroth, W. K. (1993). Quantitative effects of grazing on vegetation and soils over a global range of environments. Ecological Monographs, 63, 327–366. 10.2307/2937150 [DOI] [Google Scholar]
- Moore, K. J. , Moser, L. E. , Vogel, K. P. , Waller, S. S. , Johnson, B. E. , & Pedersen, J. F. (1991). Describing and quantifying growth stages of perennial forage grasses. Agronomy Journal, 83, 1073–1077. 10.2134/agronj1991.00021962008300060027x [DOI] [Google Scholar]
- O'Brien, J. J. , Loudermilk, E. L. , Hornsby, B. , Hudak, A. T. , Bright, B. C. , Dickinson, M. B. , Hiers, J. K. , Teske, C. , & Ottmar, R. D. (2016). High‐resolution infrared thermography for capturing wildland fire behaviour: RxCADRE 2012. International Journal of Wildland Fire, 25(1), 62–75. 10.1071/WF14165 [DOI] [Google Scholar]
- Ott, J. P. , & Hartnett, D. C. (2012). Contrasting bud bank dynamics of two co‐occurring grasses in tallgrass prairie: Implications for grassland dynamics. Plant Ecology, 213(9), 1437–1448. 10.1007/s11258-012-0102-9 [DOI] [Google Scholar]
- Ott, J. P. , Klimešová, J. , & Hartnett, D. C. (2019). The ecology and significance of below‐ground bud banks in plants. Annals of Botany, 123(7), 1099–1118. 10.1093/aob/mcz051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peter, S. (1992). Heat transfer in soils beneath a spreading fire (p. 479). Dissertation. University of New Brunswick, Fredericton, New Brunswick, Canada. [Google Scholar]
- Rogers, W. E. , & Hartnett, D. C. (2001). Temporal vegetation dynamics and recolonization mechanisms on different‐sized disturbances in tallgrass prairie. American Journal of Botany, 88, 1634–1642. 10.2307/3558408 [DOI] [PubMed] [Google Scholar]
- Russell, M. L. , & Vermeire, L. T. (2015). Fire and nitrogen alter axillary bud number and activity in purple threeawn. Rangeland Ecology & Management, 68(1), 65–70. 10.1016/j.rama.2014.12.009 [DOI] [Google Scholar]
- Russell, M. L. , Vermeire, L. T. , Dufek, N. A. , & Strong, D. J. (2013). Fire, defoliation, and competing species alter Aristida purpurea biomass, tiller, and axillary bud production. Rangeland Ecology and Management., 66, 290–296. [Google Scholar]
- Russell, M. , Vermeire, L. , Ganguli, A. , & Hendrickson, J. (2015). Season of fire manipulates bud bank dynamics in northern mixed‐grass prairie. Plant Ecology, 216, 835–846. 10.1007/s11258-015-0471-y [DOI] [Google Scholar]
- Russell, M. L. , Vermeire, L. T. , Ganguli, A. C. , & Hendrickson, J. R. (2019). Fire return interval and season of fire alter bud banks. Rangeland Ecology & Management, 72(3), 542–550. 10.1016/j.rama.2018.12.003 [DOI] [Google Scholar]
- Shimizu‐Sato, S. , & Mori, H. (2001). Control of outgrowth and dormancy in axillary buds. Plant Physiology, 127(4), 1405–1413. 10.1104/pp.010841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stambaugh, M. C. , Sparks, J. C. , & Abadir, E. R. (2014). Historical pyrogeography of Texas, USA. Ecology, 10(3), 72–89. 10.4996/fireecology.1003072 [DOI] [Google Scholar]
- Taylor, C. A. , Twidwell, D. , Garza, N. E. , Rosser, C. , Hoffman, J. K. , & Brooks, T. D. (2012). Long‐term effects of fire, livestock herbivory removal, and weather variability in Texas semiarid savanna. Rangeland Ecology and Management, 65(1), 21–30. 10.2111/REM-D-10-00124.1 [DOI] [Google Scholar]
- Tomlinson, K. W. , & O'Connor, T. G. (2004). Control of tiller recruitment in bunchgrasses: Uniting physiology and ecology. Functional Ecology, 18(4), 489–496. 10.1111/j.0269-8463.2004.00873.x [DOI] [Google Scholar]
- Twidwell, D. , Fuhlendorf, S. D. , Engle, D. M. , & Taylor, C. A. Jr (2009). Surface fuel sampling strategies: Linking fuel measurements and fire effects. Rangeland Ecology and Management, 62, 223–229. 10.1111/1365-2664.12063 [DOI] [Google Scholar]
- Twidwell, D. , Fuhlendorf, S. D. , Taylor, C. A. Jr , & Rogers, W. E. (2013). Refining thresholds in coupled fire‐vegetation models to improve management of encroaching woody plants in grasslands. Journal of Applied Ecology, 50, 603–613. 10.1111/1365-2664.12674 [DOI] [Google Scholar]
- Twidwell, D. , Rogers, W. E. , Wonkka, C. L. , Taylor, C. A. , & Kreuter, U. P. (2016). Extreme prescribed fire during drought reduces survival and density of woody resprouters. Journal of Applied Ecology, 53, 1585–1596. 10.1111/1365-2664.12674 [DOI] [Google Scholar]
- Umbanhowar, C. E. Jr (1996). Recent fire history of the northern Great Plains. The American Midland Naturalist Journal, 135, 115–121. 10.2307/2426877 [DOI] [Google Scholar]
- USDA (2016). Tarrant series, viewed 17 July 2019. https://soilseries.sc.egov.usda.gov/OSD_Docs/T/TARRANT.html [Google Scholar]
- Valettel, J. , Gomendyl, V. , MarCchal, J. , Houssard, C. , & Gillon, D. (1994). Heat transfer in the soil during very low‐energy experimental fires: The role of duff and soil moisture content. International Journal of Wildland Fire, 4(4), 225–237. [Google Scholar]
- VanderWeide, B. L. , Hartnett, D. C. , & Carter, D. L. (2014). Belowground bud banks of tallgrass prairie are insensitive to multi‐year, growing‐season drought. Ecosphere, 5, 103. 10.1890/ES14-00058.1 [DOI] [Google Scholar]
- Vesk, P. A. , Warton, D. I. , & Westoby, M. (2004). Sprouting by semi‐arid plants: Testing a dichotomy and predictive traits. Oikos, 107(1), 72–89. 10.1111/j.0030-1299.2004.13122.x [DOI] [Google Scholar]
- Vítová, A. , Macek, P. , & Lepš, J. (2017). Disentangling the interplay of generative and vegetative propagation among different functional groups during gap colonization in meadows. Functional Ecology, 31(2), 458–468. 10.1111/1365-2435.12731 [DOI] [Google Scholar]
- Volland, L. A. , & Dell, J. D. (1981). Fire effects on Pacific Northwest forest and range vegetation. R6‐RM‐067. USDA Forest Service Pacific Northwest Region, Range Management and Aviation and Fire Management Report, Portland, Oregon, USA. [Google Scholar]
- Wright, H. A. , & Klemmedson, J. O. (1965). Effect of Fire on Bunchgrasses of the Sagebrush‐Grass Region in Southern Idaho. Ecology, 46, 680–688. 10.2307/1935007 [DOI] [Google Scholar]
- Wright, H. A. (1971). Why squirreltail is more tolerant to burning than needle‐and‐thread. Journal of Range Management, 24, 277–284. 10.2307/3896943 [DOI] [Google Scholar]
- Young, R. P. (1983. ). Fire as a vegetation management tool in rangelands of the Intermountain Region [Includes list of common and scientific names of plant species in the sagebrush and pinyon‐juniper zones, United States; Idaho; Nevada; Utah; Wyoming]. USDA Forest Service General Technical Report INT Intermountain Forest and Range Experiment Station. [Google Scholar]
- Zhang, Y. , & Biswas, A. (2017). The Effects of Forest Fire on Soil Organic Matter and Nutrients in Boreal Forests of North America: A Review. In: Rakshit A., Abhilash P., Singh H., & Ghosh S. (Eds.), Adaptive Soil Management: From Theory to Practices. Springer. 10.1007/978-981-10-3638-5_21 [DOI] [Google Scholar]
- Zimmerman, J. H. , Higgins, S. I. , Grimm, V. , Hoffman, J. , & Linstädter, A. (2010). Grass mortality in semi‐arid savanna: The role of fire, competition and self‐shading. Perspectives in Plant Ecology, Evolution and Systematics, 12, 1–8. 10.1016/j.ppees.2009.09.003 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig S1
Data Availability Statement
Data are archived in the Dryad Digital Repository (https://doi.org/10.5061/dryad.kprr4xh48).


