Abstract
Background
The purpose of this meta‐analysis was to evaluate the impact of image integration technique on clinical and procedural outcomes in patients undergoing radiofrequency catheter ablation of atrial fibrillation with a three‐dimensional electroanatomic mapping system.
Methods
Randomized controlled trials were identified through a systematic literature search of PubMed and CENTRAL databases from inception to April 2020. The primary outcome was arrhythmia recurrence during the follow‐up period. The secondary outcomes were the difference in total procedural time and fluoroscopy time.
Results
Four studies with a total of 749 patients were included. The pooled result showed no statistically significant difference between the groups with respect to arrhythmia recurrence (RR, 0.75; 95% CI, 0.47‐1.21), fluoroscopy time (MD, −6 minutes; 95% CI, −23.4 to 11.3), and total procedural time (MD, 1.1 minutes; 95% CI, −31.8 to 34.1).
Conclusion
Image integration to guide radiofrequency catheter ablation for patients with atrial fibrillation does not improve clinical and procedural outcomes.
Keywords: atrial fibrillation, catheter ablation, electroanatomic mapping, image integration, pulmonary vein isolation
Meta‐analysis of four randomized controlled trials showed that integration of preprocedural CT or MRI images with real‐time electroanatomic maps to guide pulmonary vein isolation does not improve clinical and procedural outcomes.

1. INTRODUCTION
Pulmonary veins (PV) play a major role in initiating and maintaining atrial fibrillation (AF), especially in the settings of paroxysmal AF. 1 Hence, the electrical isolation of the pulmonary veins is the primary goal of the AF ablation procedures. 2 Circumferential antral pulmonary vein isolation (PVI) is the preferred ablation strategy in most cases. This anatomically based ablation strategy requires detailed information on left atrium (LA) and PV morphology and catheter tip location. Electroanatomic mapping (EAM) systems can accurately determine the location of the catheter within the reconstructed three‐dimensional (3D) geometry of the cardiac chamber. However, the limitations of surrogate geometries for reflecting true cardiac anatomy led to the development of image integration techniques. Ensite NavX Fusion (St. Jude Medical, St. Paul, MN, USA) and CARTO‐Merge (Biosense Webster Inc, Diamond Bar, CA, USA) are two commercially available software modules to integrate 3D computed tomography (CT) and magnetic resonance (MR) images into real‐time electroanatomic maps.
The image integration technique can facilitate complex ablation procedures such as PVI. 3 Additionally, it can potentially improve clinical outcomes, reduce complications, and shorten the duration of fluoroscopy. A few controlled clinical studies have investigated the impact of image integration on clinical outcomes and have reported mixed results. 4 , 5 , 6 , 7 , 8 , 9 , 10 Therefore, we collected randomized controlled trials (RCTs) comparing clinical outcomes of image integrated versus EAM‐only ablation strategies and conducted a meta‐analysis to determine the overall effect of image integration on clinical and procedural outcomes.
2. METHODS
2.1. Literature search
Two authors (AM, AID) independently searched PubMed and Cochrane Central Register of Controlled Trials from inception to April 2020, without any language restriction. We used sensitivity and precision maximizing version of Cochrane Highly Sensitive Search Strategy for identifying randomized trials. Details of search terms and strategy of PubMed search are provided in the Appendix S1. The bibliography of all selected manuscripts and review articles was also manually searched for any additional studies.
The titles and abstracts of initial search results were screened for determining relevant studies. After excluding the non‐relevant results and duplicates, the full texts of the remaining studies were independently assessed by two authors (AM, AID) for inclusion. Any disagreements were resolved by discussion with the third investigator.
2.2. Eligibility criteria
RCTs with at least 6 months of follow‐up were included in this study. Participants aged 18 years or older with symptomatic drug‐refractory paroxysmal or persistent AF undergoing radiofrequency catheter ablation (RFCA) with a 3D EAM system were considered. RFCA was defined as the isolation of pulmonary veins using radiofrequency energy with or without substrate modification. The image integration technique was the intervention of interest compared with the EAM‐only technique. Image integration referred to the process of aligning the preprocedural cardiac CT or MR images with the real‐time electroanatomic maps created by EAM systems. Studies evaluating other than CT/MR images for integration (intracardiac echocardiography images) or evaluating CT overlay technique (superimposing CT image on real‐time fluoroscopy image) were excluded. The primary outcome was the recurrence of arrhythmia during the follow‐up period. Arrhythmia recurrence was defined as an episode of atrial fibrillation or atrial tachycardia lasting at least 30 seconds that persisted after the blanking period. The secondary outcomes were the difference in total procedural time, fluoroscopy time, and complication rate.
2.3. Data extraction
Two reviewers (AM, AID) independently extracted data from all eligible studies. Disagreements were resolved by discussion with the third investigator. Data collected included study characteristics such as the author's name, year of publication, study size, study population characteristics, follow‐up duration, and primary and secondary outcome measures.
2.4. Quality assessment
The risk of bias in individual studies was assessed by the Revised Cochrane risk‐of‐bias tool for randomized trials (RoB 2). 11 The revised version of the tool included five domains such as randomization process, deviations from intended interventions, missing outcome data; measurement of the outcome and selection of the reported result. Each study was graded as “low risk of bias” if the study was had low risk of bias in all fields, “some concerns” if it raised some concerns in at least one field and “high risk of bias” if the study had high risk of bias in at least one field or some concerns in multiple fields. The results may be found enclosed.
2.5. Data analysis
We conducted the meta‐analysis using the Review Manager ver. 5.3 (Cochrane Collaboration). The dichotomous variables were assessed using a risk ratio (RR) with a 95% confidence interval (CI). Mean difference (MD) with 95% CI was used as the effect measure for continuous outcomes. Heterogeneity was assessed using the I2 statistic. The heterogeneity was considered to be substantial if I 2 was greater than 50%, so we used the random‐effects model to estimate the intervention effect. Prespecified sensitivity analyses were performed by excluding one study each time to identify potential sources of heterogeneity. We did not create a funnel plot to investigate possible reporting biases considering that we pooled less than 10 studies.
3. RESULTS
3.1. Study selection
A total of 2340 studies were identified at the initial search. After the review process, 17 full‐text articles were assessed for eligibility. Ultimately, four studies with 749 participants were included in this meta‐analysis. The PRISMA study flow diagram is illustrated in Figure 1.
FIGURE 1.
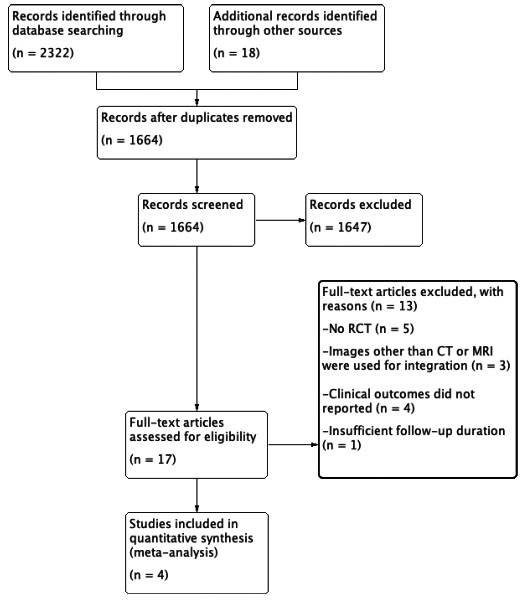
Study flow diagram
3.2. Study characteristics
All included studies were randomized controlled trials published in English between 2008 and 2010. Sample sizes ranged from 77 to 299. The CARTO‐Merge image integration system was used in all of the studies included. Kistler et al performed circumferential PVI followed by linear ablation and complex fractioned electrograms ablation if AF persisted after PVI, and cavotricuspid isthmus (CTI) ablation if the patient had previously documented atrial flutter. Caponi et al performed circumferential PVI plus roofline ablation and mitral isthmus ablation in all patients. Della Bella et al performed circumferential PVI plus mitral isthmus ablation. Tang et al performed PVI plus CTI ablation if the patient had typical atrial flutter. In all studies, RF energy was delivered through an irrigated tip catheter. Studies investigated the arrhythmia recurrence with Holter recordings that were carried out at certain intervals after the blanking period. Study characteristics are provided in Table 1.
TABLE 1.
Study characteristics
| First Author | Year | Image | Group | n | Age (y) | Male (%) | PAF (%) | Follow‐up (mo) |
|---|---|---|---|---|---|---|---|---|
| Kistler 5 | 2008 | CT | EAM | 40 | 56 ± 10 | NA | 58 | 14 ± 3 |
| EAM + II | 39 | 56 ± 13 | 62 | 14 ± 3 | ||||
| Tang 9 | 2008 | CT | EAM | 39 | 59 ± 15 | 64 | 100 | 12 ± 3 |
| EAM + II | 42 | 54 ± 13 | 69 | 100 | 12 ± 3 | |||
| D. Bella 10 | 2009 | CT | EAM | 145 | 55 ± 11 | 68 | 69 | 14 ± 11 |
| EAM + II | 145 | 56 ± 10 | 75 | 73 | 14 ± 12 | |||
| Caponi 8 | 2010 | MRI | EAM | 140 | 57 ± 10 | 79 | 67 | 12 |
| EAM + II | 140 | 59 ± 11 | 90 | 66 |
Abbreviations: CT, Computed Tomography; EAM, Electroanatomic Mapping; II, Image Integration; MRI, Magnetic Resonance Imaging; PAF, Paroxysmal Atrial Fibrillation
3.3. Primary outcome
The comparison of the AF/AT recurrence rate after the first ablation procedure was conducted among the four studies included. There was significant heterogeneity among the studies (I 2 = 76%). Therefore, the random‐effects model (Mantel‐Haenszel method) was used. The analysis revealed no significant difference between the EAM‐only and EAM + II groups in terms of AF/AT recurrence (RR, 0.75; 95% CI, 0.47‐1.21; Figure 2).
FIGURE 2.
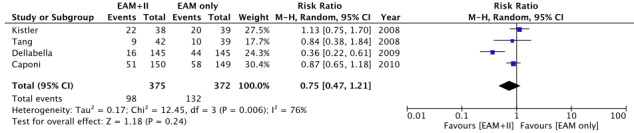
Forest plot of primary outcome: arrhythmia recurrence
3.4. Secondary outcomes
The random‐effects model was used for the assessment of secondary outcomes owing to significant heterogeneity. There was no difference between the two groups with respect to fluoroscopy time (MD, −6 minutes; 95% CI, −23.4 to 11.3; Figure 3) and total procedural time (MD, 1.1 minute; 95% CI, −31.8 to 34.1; Figure 4). Caponi et al did not provide total procedural time. Only Kistler et al provided the definition of procedure duration. There was no difference in the complication rate between the two groups. (RR, 1.09; 95% CI, 0.44‐2.68). Details of complications are provided in Table 2.
FIGURE 3.
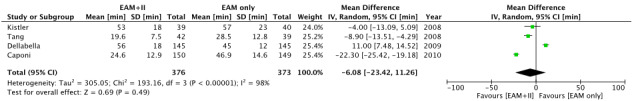
Forest plot of secondary outcome: fluoroscopy time
FIGURE 4.
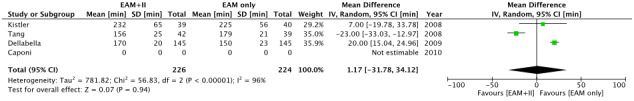
Forest plot of secondary outcome: procedural time
TABLE 2.
Details of complications in individual studies
| First author | EAM only | EAM + II |
|---|---|---|
| Kistler 5 | 1 PV stenosis | 2 tamponade |
| Tang 9 | 1 tamponade | 1 tamponade |
| D. Bella 10 | 1 pericardial effusion |
1 tamponade 1 PV stenosis |
| Caponi 8 |
1 tamponade 2 pericardial effusion 1 PV stenosis 1 inguinal hematoma 1 transient ischemic attack |
1 tamponade 2 pericardial effusion 1 pericarditis |
Abbreviations: EAM, Electroanatomic Mapping; II, Image Integration; PV, Pulmonary Vein
3.5. Sensitivity analysis
We calculated the I 2 statistics as 0% for the primary outcome (indicating no heterogeneity) after the study of Della Bella et al was excluded.
4. DISCUSSION
Several observational studies demonstrated the benefit of image integration on AF recurrence compared to the EAM‐only mapping strategy. 4 , 7 However, these results could not be reported in randomized controlled trials. Only one randomized study showed a clear benefit of image integration on clinical outcomes. 10 A meta‐analysis performed by Liu et al 12 combining three randomized and two observational studies reported that the CARTO‐Merge system was associated with an insignificant decrease in AF recurrence.
The key determinant of clinical success is the completeness of electrical isolation regardless of the usage of image integration or not. Complete electrical isolation can be achieved with or without image integration by experienced operators. The fact that the clinics that participated in these studies are highly experienced centers in ablation procedures may have obscured the advantage of image integration on procedural outcomes. The results of two different studies published by Kistler et al support this hypothesis. The image integration technique was associated with lower arrhythmia recurrence and shorter fluoroscopy time in the observational study published in 2006. However, there was no significant difference in the randomized controlled study they published in 2008. The authors noted that the difference between the results of the two studies might be partially related to the completion of the learning curve.
Our meta‐analysis did not show a significant difference, however, most of the studies reported reduced X‐ray and procedural time with image integration. Only Della Bella et al reported increased fluoroscopy and procedural time in the CARTO‐Merge group. 10 We cannot explain why the image integration technique extended fluoroscopy time in only one study although the author of the study linked this result to the time spent on image processing. We observed a significant difference between groups for X‐ray duration (MD, −8.9 minutes; 95% CI, −13.5 to −4.3) after excluding the study by Della Bella et al from the current meta‐analysis. The study by Della Bella et al also differs from others in terms of the result of the primary outcome and it was the only study that reported a significant difference in arrhythmia recurrence between the two groups (RR, 0.36, 95% CI, 0.22‐0.61, P < .05).
A considerable number of patients need repeat procedures owing to recurrences. Therefore, reducing the amount of X‐ray exposure is essential for patients and also operators. Image integration may reduce radiation exposure in the laboratory, but the preprocedural CT scan is a significant source of radiation received by the patient. This unfavorable condition can be eliminated using MRI instead of a CT scan.
There is insufficient evidence that using image integration has an impact on the rate of complications such as bleeding, tamponade, or esophageal injury. Image integration can reduce the risk of PV stenosis as it provides a detailed anatomy of PV ostium. However, the prevalence of PV stenosis is very low with circumferential antral ablation. Hence, a large sample size is required to get a significant difference between groups. Studies included in our meta‐analysis were underpowered to show a significant difference in complication rate.
This study had several limitations. First, two studies had relatively small sample sizes. Second, the definition of arrhythmia recurrence slightly varied among the included studies with regard to the inclusion of atrial tachyarrhythmias other than AF. Third, the duration and interval of Holter recordings and the definition of the blanking period differed among the studies. Lastly, the studies included in this meta‐analysis were published more than 10 years ago. Several novel technologies such as multielectrode high‐density mapping catheters have been available in the last decade and this reduced the importance of image integration.
Image integration to guide radiofrequency catheter ablation for patients with atrial fibrillation did not improve clinical and procedural outcomes. Nevertheless, it can potentially reduce the duration of fluoroscopy and accordingly, the exposure of patients and operators to radiation.
CONFLICT OF INTEREST
The authors declare no conflict of interests for this article.
AUTHOR CONTRIBUTIONS
All authors equally contributed to the study.
Supporting information
Supplementary Material
Mammadli A, Demirtola AI, Diker E. Impact of image integration on clinical and procedural outcomes of radiofrequency catheter ablation of atrial fibrillation: A meta‐analysis of randomized controlled trials. J Arrhythmia. 2021;37:550–555. 10.1002/joa3.12508
REFERENCES
- 1. Haïssaguerre M, Jaïs P, Shah DC, et al. Spontaneous initiation of atrial fibrillation by ectopic beats originating in the pulmonary veins. N Engl J Med. 1998;339(10):659–66. [DOI] [PubMed] [Google Scholar]
- 2. Calkins H, Kuck KH, Cappato R, et al. 2012 HRS/EHRA/ECAS expert consensus statement on catheter and surgical ablation of atrial fibrillation: recommendations for patient selection. Procedural Techniques, Patient Management and Follow‐up, Definitions, Endpoints, and Research Trial Design EP Eur. 2012;14(4):528–606. [DOI] [PubMed] [Google Scholar]
- 3. Dong J, Calkins H, Solomon SB, et al. Integrated electroanatomic mapping with three‐dimensional computed tomographic images for real‐time guided ablations. Circulation. 2006;113(2):186–94. [DOI] [PubMed] [Google Scholar]
- 4. Kistler PM, Rajappan K, Jahngir M, et al. The impact of CT image integration into an electroanatomic mapping system on clinical outcomes of catheter ablation of atrial fibrillation. J Cardiovasc Electrophysiol. 2006;17(10):1093–101. [DOI] [PubMed] [Google Scholar]
- 5. Kistler PM, Rajappan K, Harris S, et al. The impact of image integration on catheter ablation of atrial fibrillation using electroanatomic mapping: a prospective randomized study. Eur Heart J. 2008;29(24):3029–36. [DOI] [PubMed] [Google Scholar]
- 6. Martinek M, Nesser HJ, Aichinger J, Boehm G, Purerfellner H. Impact of integration of multislice computed tomography imaging into three‐dimensional electroanatomic mapping on clinical outcomes, safety, and efficacy using radiofrequency ablation for atrial fibrillation. PACE ‐ Pacing Clin Electrophysiol. 2007;30(10):1215–23. [DOI] [PubMed] [Google Scholar]
- 7. Bertaglia E, Della BP, Tondo C, et al. Image integration increases the efficacy of paroxysmal atrial fibrillation catheter ablation: results from the CartoMergeTM Italian Registry. EP Eur. 2009;11(8):1004–10. [DOI] [PubMed] [Google Scholar]
- 8. Caponi D, Corleto A, Scaglione M, et al. Ablation of atrial fibrillation: does the addition of three‐dimensional magnetic resonance imaging of the left atrium to electroanatomic mapping improve the clinical outcome?: A randomized comparison of Carto‐Merge vs. Carto‐XP three‐dimensional mapping. EP Eur. 2010;12(8):1098–104. [DOI] [PubMed] [Google Scholar]
- 9. Tang K, Ma J, Zhang S, et al. A randomized prospective comparison of CartoMerge and CartoXP to guide circumferential pulmonary vein isolation for the treatment of paroxysmal atrial fibrillation. Chin Med J. 2008;121(6):508–12. [PubMed] [Google Scholar]
- 10. Della Bella P, Fassini G, Cireddu M, et al. Image integration‐guided catheter ablation of atrial fibrillation: a prospective randomized study. J Cardiovasc Electrophysiol. 2009;20(3):258–65. [DOI] [PubMed] [Google Scholar]
- 11. Sterne JAC, Savović J, Page MJ, et al. RoB 2: a revised tool for assessing risk of bias in randomized trials. BMJ. 2019;366. [DOI] [PubMed] [Google Scholar]
- 12. Liu S‐X, Zhang Y, Zhang X‐W. Impact of image integration on catheter ablation for atrial fibrillation using three‐dimensional electroanatomic mapping: a meta‐analysis. Pacing Clin Electrophysiol. 2012;35(10):1242–7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material


