Abstract
Background
It is expected that ablation procedures will be increasingly offered to a more aged population affected with persistent AF (persAF); however, the clinical outcomes of ablation in this specific population are not well described. We aimed to analyze the efficacy and safety of CB‐A in this group of patients compared with a younger cohort.
Methods and results
Eighty‐three patients with (persAF) aged ≥75 years (group 1; mean age 78.2 ± 3.1 years) and 166 patients also affected with persAF aged <75 years (group 2; mean age 64.3 ± 6.6 years) were included in the study. The primary outcome was freedom from recurrent sustained (>30 seconds) atrial arrhythmias without anti‐arrhythmic medication after a blanking period of 3 months. At 2 years, clinical success was achieved in 108 out of 249 patients (43.4%). Median follow‐up was 24 months (IQR: 18.4‐25.5 months). Older patients suffered from more recurrences than those in the younger cohort ((53/83 patients, 63.9% vs 88/166 patients, 53.0%; P = .03). Thirty (12.0%) patients suffered a complication, but the incidence of complications was not different between both groups. The most frequent complication was transient phrenic nerve injury.
Conclusions
The global 2 years efficacy of CB‐A PVI in persAF is 43.4%. A lower success rate is achieved in the older patients (36.1%) compared to the younger age group (47.0%). However, the complication rate was not different between age groups.
The mid‐term success rate of pulmonary vein isolation by means of second‐generation cryoballoon in persistent atrial fibrillation is significantly lower in older patients (>75 years) compared with the younger cohort. There is no difference in the complication rate of pulmonary vein isolation by means of second‐generation cryoballoon of between younger and older patients.
1. INTRODUCTION
Isolation of the pulmonary veins (PVI) using the 28mm second‐generation cryoballoon (CB‐A) (Arctic Front Advance TM, Medtronic, Minneapolis, MN, USA), is an effective treatment for patients with symptomatic drug‐refractory atrial fibrillation (AF), with reported success rate of 60%‐70% for patients with paroxysmal AF (PAF) and about 50% in those affected by persistent AF (persAF). 1 There are several publications reporting the outcomes in terms of safety and efficacy of radiofrequency (RF) 2 , 3 ablation and CB‐A 4 , 5 in the elderly population, most of them addressing only patients with PAF or with only a small number of patients with PersAF. In view of the demographical trends of AF, it is expected that ablation procedures will be increasingly offered to a more aged population affected with PersAF. Our present aim was to study the outcomes of CB‐A ablation in a large group of persAF patients aged 75 years or older and compare them with a younger age cohort of <75years.
2. METHODS
2.1. Patient characteristics
The study included patients from two centres (ZNA Heart Centre, Middelheim, Antwerp and HRMC, UZ Brussels, Brussels). A first group of patients with an age of 75 years or more (group 1), with PersAF resistant to medical treatment, who were treated by means of PVI using the CB‐A as the index procedure between October 2012 and December 2018 were included in the analysis. A control group of patients aged less than 75 years (group 2), who were also treated by means of PVI using the CB‐A as the index procedure at the same period, were propensity score‐matched (by gender and left atrial diameter (LAD)) in a ratio of 1:2.
Persistent AF was defined according to current guidelines 6 as AF episodes lasting more than 7 days, including episodes terminated by cardioversion, after 7 days or more.
The Ethical Committees of the two centres approved the study. The ethical principles for medical research were respected, and the privacy of all the participants as well as the confidentiality of their personal information was protected.
2.2. Pre‐procedural management
All patients signed an informed consent for the procedure. A transoesophageal echocardiography (TEE) was performed 24 hours prior to the ablation to rule out the presence of intracavitary thrombi. The left atrium (LA) and pulmonary vein (PV) anatomy were assessed using a computed tomography (CT)‐scan. Non‐direct oral anticoagulants were continued throughout the procedure, and direct oral anticoagulants were discontinued 24 hours in advance.
2.3. Ablation procedure
Our routine PVI protocol using the CB‐A has been previously described. 7 In brief, a loading dose of 100 U/kg of heparin was given prior to transseptal access, thereafter an ACT of >300 seconds was maintained.
After gaining LA access, a 15‐Fr steerable sheath was advanced into the LA cavity (FlexCath Advance®, Medtronic, Minneapolis, MN, USA), and an inner lumen mapping catheter (ILMC) (Achieve®, Medtronic, Minneapolis, MN, USA) was placed in each PV ostium. The 28‐mm CB‐A was positioned at each PV ostium. Once an optimal occlusion was achieved, ablation was started. Our ablation procedure has changed over time, starting from a 4‐minute double‐freeze strategy, and rapidly evolving to a 3‐minute single‐freeze strategy. Since 2014, our ablation protocol consists in a single 3‐minute freeze application; however, if PV isolation is not observed before 60 seconds and a temperature of −40°C is not reached before 60 seconds, cryoapplication was either aborted to attempt a better occlusion or the freeze was continued until the end and a second freeze given attempting for the parameters mentioned above.
The ILMC was positioned at the proximal site of each PV to record the vein potentials (PVPs) prior to every application, and the time to isolation was systematically recorded.
2.4. Phrenic nerve monitoring
The right phrenic nerve (PN) was monitored during the ablation of the right superior and the right inferior pulmonary veins. The right PN was stimulated during ablation of the right‐sided PVs using a 10‐poles catheter which was positioned in the right subclavian vein or the superior vena cava. If PN injury was suspected, a double‐stop technique was employed to abort the freeze.
2.5. Post‐ablation management
Subcutaneous low molecular weight heparin was given to every patient after the ablation. Oral anticoagulation therapy (OAT), either warfarin (target INR between 2.0 and 3.0) or a novel oral anticoagulant (NOAC) was initiated or re‐started the day after the procedure. Anti‐arrhythmic drug treatment was re‐started in every patient after ablation and stopped after 3 months. OAT was continued unless the CHA2DS2 — VASc score was of low embolic risk (score of 0). Beta‐blocking agents were continued.
Patients were discharged from the hospital 24 hours after the procedure if a stable clinical situation was observed. After the ablation, all patients were under continuous ECG monitoring until their discharge. To exclude a pericardial effusion, all patients underwent a transthoracic echocardiogram (TTE) after the ablation.
Echocardiographic data including, left ventricular ejection fraction (EF) left ventricular diastolic function (A‐wave, E‐wave, E’‐wave), left atrial diameter (LAD) and the tricuspid annular plane systolic excursion (TAPSE) were obtained from the TTE performed the day after ablation. In those patients presenting in AF, diastolic function data was obtained by calculating the average velocity values of at least 10 consecutive cycles, as recommended in current guidelines. 8
2.6. Follow‐up
Follow‐up (FU) was carried out at 3 months and then every 6 months, and it consisted of a physical examination, ECG, and a clinical questionnaire. In case of symptoms, unscheduled visits were performed. Holter‐recordings (1–7‐day) and/or event loop recording, if necessary repetitive, were only used for arrhythmia documentation in case of recurrent palpitations. Only when the Holter or event‐recording revealed AF/AT as the causative rhythm of the palpitations this was classified as a recurrence.
In those cases that the patient was not followed at our institution, the referring cardiologist was contacted to obtain follow‐up information.
Primary endpoint for the analysis was clinical success, defined as freedom of any documented sustained (>30 seconds) atrial arrhythmias without anti‐arrhythmic drugs after a 3‐month blanking period after a single procedure.
2.7. Statistical analysis
Quantitative variables are expressed as mean ± SD or median or percentile 25 and 75 as required. Categorical variables are expressed as numbers and percentages. A Student's t˗test or the Mann–Whitney U test were used to compare continuous variables, and a χ2 test or the Fischer's exact test were used to compare categorical variables. A Cox proportional‐hazards model was used to calculate hazard ratios (HR). For the multivariate analysis, variables with a P‐value greater than 0.10 were removed from the model. Event‐free survival rates were estimated by the method of Kaplan–Meier and compared by the two‐stage hazard rate comparison method 9 and the log‐rank test. Analyses were performed with SPSS Statistics for Windows (IBM SPSS Version 25.0. Armonk, NY: IBM Corp.) and R statistical software (A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. URL https://www.R‐project.org/).
3. RESULTS
3.1. Baseline characteristics
A total of 249 patients were included in the analysis, 83 in group 1 (≥75 years) and, 166 in group 2 (<75 years). The baseline characteristics are resumed in Table 1. The oldest patient included in group 1 was 86 years old; the mean age in this group was 78.2 ± 3.1 years. In group 2, the mean age was 64.3 ± 6.6 years.
TABLE 1.
Baseline characteristics
|
Group 1 (≥75 yrs) |
Group 2 (<75 yrs) |
Total | P‐value | |
|---|---|---|---|---|
| Age (years) | 78.2 ± 3.1 | 64.3 ± 6.6 | 68.9 ± 8.7 | <.01 |
| Female (n, %) | 34 (41.0%) | 65 (39.2%) | 99 (39.8%) | .78 |
| Valvular heart disease (n, %) | 9 (10.8%) | 12 (7.2%) | 21 (8.4%) | .33 |
| Heart failure (n, %) | 31 (37.4%) | 43 (25.9%) | 74 (29.7%) | .06 |
| Hypertension (n, %) | 61 (73.5%) | 92 (55.4%) | 153 (61.5%) | <.01 |
| Diabetes (n, %) | 12 (14.5%) | 23 (13.9%) | 35 (14.1%) | .90 |
| Vascular/coronary artery disease (n, %) | 29 (34.9%) | 47 (28.3%) | 76 (30.5%) | .28 |
| Stroke/TIA (n, %) | 8 (9.6%) | 17 (10.2%) | 25 (10.0%) | .88 |
| Previous ablation (n, %) | ||||
| Other | 2 (2.4%) | 1 (0.6%) | 3 (1.2%) | .04 |
| Flutter | 1 (1.2%) | 0 (0.0%) | 1 (0.4%) | |
| Avnrt | 6 (7.2%) | 3 (1.8%) | 9 (3.6%) | |
| AAD class Ic (n, %) | 23 (27.7%) | 56 (33.7%) | 79 (31.7%) | .34 |
| AAD class II (n, %) | 60 (72.3%) | 122 (73.5%) | 182 (73.1%) | .75 |
| AAD class III (n, %) | 27 (32.5%) | 54 (32.5%) | 81 (32.5%) | 1.00 |
| ECVs (n, %) | ||||
| ≥ 3 | 16 (19.3%) | 15 (9.0%) | 31 (12.5%) | .04 |
| 2 | 21 (25.3%) | 35 (21.1%) | 56 (22.5%) | |
| 1 | 30 (36.1%) | 87 (52.4%) | 117 (47.0%) | |
| 0 | 16 (19.3%) | 29 (17.5%) | 45 (18.1%) | |
| CHA2DS2Vasc Score (n, %) | ||||
| ≥ 4 | 57 (68.6%) | 27 (31.4%) | 84 (33.7%) | <.01 |
| 3 | 17 (20.5%) | 44 (26.5%) | 61 (24.5%) | |
| 2 | 9 (10.8%) | 39 (23.5%) | 48 (19.3%) | |
| 1 | 0 (0.0%) | 41 (24.7%) | 41 (16.5%) | |
| 0 | 0 (0.0%) | 15 (9.0%) | 15 (6.0%) | |
| 4 Separate PVs (n, %) | 64 (77.1%) | 131 (78.9%) | 195 (78.3%) | .74 |
| Right middle PV (n, %) | 10 (12.1%) | 13 (7.8%) | 23 (9.2%) | .28 |
| Left common PV (n, %) | 9 (10.8%) | 26 (15.7%) | 35 (14.1%) | .30 |
| AF at the beginning of the procedure (n, %) | 52 (62.7%) | 120 (72.3%) | 172 (69.1%) | .12 |
| Height (cm) | 169.9 ± 8.8 | 173.4 ± 10.1 | 172.1 ± 9.8 | .01 |
| Weight (kgs) | 80.7 ± 16.8 | 85.6 ± 15.6 | 83.9 ± 16.1 | .03 |
| Body mass index (kg/m2) | 27.8 ± 5.0 | 28.5 ± 5.0 | 28.3 ± 5.0 | .31 |
| Left atrial diameter (mm) | 45.8 ± 7.8 | 45.6 ± 7.0 | 45.7 ± 7.28 | .86 |
| E‐wave (cm/s) | 84.0 ± 25.0 | 85.0 ± 19.0 | 85.0 ± 21.0 | .66 |
| E`‐wave (cm/s) | 7.0 ± 2.0 | 7.0 ± 2.0 | 7.0 ± 2.0 | .38 |
| A‐wave (cm/s) | 53.0 ± 22.0 | 50.0 ± 18.0 | 51.0 ± 19.0 | .49 |
| Tapse (mm) | 20.6 ± 5.0 | 20.5 ± 5.6 | 20.5 ± 5.4 | .91 |
| Ejection fraction (%) | 53.2 ± 9.4 | 54.4 ± 9.0 | 54.0 ± 9.2 | .32 |
| Time from the first AF episode (months) a | 45.7 ± 46.2 | 52.4 ± 61.1 | 50.0 ± 56.1 | .58 |
Abbreviations: Avnrt, Atrioventricular node reentrant tachycardia; AADs, Anti‐Arrhythmic drugs; AF, Atrial fibrillation; ECVs, Electrical cardioversions; PV, Pulmonary vein; TAPSE, Tricuspid annular plane systolic excursion; TIA, Transitory ischemic accident.
Refers to the time passed from the first‐registered AF episode (either paroxysmal or persistent) till the date of the ablation procedure.
Arterial hypertension (AHT) was significantly more prevalent in the older vs younger patients (73.5% vs 55.4%; P <.01). The prevalence of valvular heart disease (VHD), heart failure (HF), coronary artery disease (CAD) and diabetes mellitus (DM) was not significantly different between the two groups. Although height (169.9 ± 8.8 vs 173.4 ± 10.1 cm; P =.01) and weight (80.7 ± 16.8 vs 85.6 ± 15.6 Kg; P =.03) were significantly higher in the younger cohort, body mass index (BMI) was similar. As expected, patients in group 1 had a higher CHA2DS2VASC score than the younger group (P <.01). The number of electrical cardioversions (ECV) was significantly different between groups. Older patients underwent more ECV prior to the ablation than patients in the youngest cohort; furthermore, in the latter group, patients underwent more frequently only 1 ECV before the ablation procedure.
3.2. Procedure characteristics
All PVs were isolated in all patients at the end of the procedure. No touch‐up ablations were required. The incidence of AF at the beginning of the procedure was similar in both groups (Group 1:62.3% vs group 2:72.3% P =.12). The mean procedural (79.1 ± 30.3 min vs 73.6 ± 24.8 min) (P =.13) and fluoroscopy times (15.8 ± 11.2 min vs 15.2 ± 9.1 min) were not different between groups (P =.63). Procedural characteristics are presented in Table 2.
TABLE 2.
Procedural characteristics
|
Group 1 (≥ 75 yrs) |
Group 2 (<75 yrs) |
Total | P | |
|---|---|---|---|---|
| Minimal T° LSPV (°C) | 52.4 ± 5.5 | 52.4 ± 5.5 | 52.4 ± 5.5 | 1.0 |
| Minimal T° RSPV (°C) | 51.8 ± 6.5 | 53.1 ± 6.0 | 52.7 ± 6.2 | .17 |
| Minimal T° LIPV (°C) | 48.5 ± 5.7 | 49.6 ± 6.3 | 49.2 ± 6.1 | .20 |
| Minimal T° RIPV (°C) | 51.2 ± 6.4 | 50.7 ± 6.2 | 50.9 ± 6.2 | .63 |
| T° 60sec LSPV (°C) | 43.7 ± 4.8 | 43.9 ± 4.5 | 43.9 ± 4.6 | .78 |
| T° 60sec RSPV (°C) | 44.3 ± 5.5 | 45.2 ± 5.3 | 44.9 ± 5.4 | .26 |
| T° 60sec LIPV (°C) | 41.7 ± 4.8 | 42.2 ± 4.1 | 42.1 ± 4.4 | .44 |
| T° 60sec RIPV (°C) | 43.2 ± 5.3 | 43.0 ± 4.8 | 43.1 ± 4.9 | .70 |
| Isolation Time LSPV (s) | 46.0 ± 20.5 | 46.5 ± 22.4 | 46.3 ± 21.7 | .90 |
| Isolation Time LIPV (s) | 44.9 ± 26.3 | 45.1 ± 27.3 | 45.0 ± 26.9 | .97 |
| Isolation Time RSPV (s) | 34.7 ± 14.5 | 33.1 ± 14.9 | 33.6 ± 14.7 | .62 |
| Isolation Time RIPV (s) | 33.0 ± 14.0 | 36.2 ± 16.1 | 35.2 ± 15.4 | .38 |
| Isolation T° LSPV (°C) | 35.2 ± 9.5 | 37.5 ± 7.3 | 36.8 ± 8.1 | .14 |
| Isolation T° LIPV (°C) | 33.8 ± 9.2 | 31.8 ± 10.0 | 32.4 ± 9.8 | .34 |
| Isolation T° RSPV (°C) | 33.7 ± 8.5 | 32.9 ± 10.5 | 33.1 ± 9.9 | .69 |
| Isolation T° RIPV (°C) | 29.6 ± 9.9 | 31.6 ± 7.3 | 31.0 ± 8.2 | .28 |
| Freezes LSPV (n, %) | ||||
| 1 | 65 (78.3%) | 140 (84.3%) | 205 (82.3%) | .48 |
| 2 | 17 (20.5%) | 25 (15.1%) | 42 (16.9%) | |
| 3 | 1 (1.2%) | 1 (0.6%) | 2 (0.8%) | |
| Freezes LIPV (n, %) | ||||
| 1 | 75 (90.4%) | 141 (84.9%) | 216 (86.7%) | .23 |
| 2 | 8 (9.6%) | 25 (15.1%) | 33 (13.3%) | |
| Freezes RSPV (n, %) | ||||
| 1 | 74 (89.2%) | 150 (90.4%) | 224 (90.0%) | .23 |
| 2 | 9 (10.8%) | 16 (9.6%) | 25 (10.0%) | |
| Freezes RIPV (n, %) | ||||
| 1 | 72 (86.7%) | 143 (86.1%) | 215 (86.3%) | .77 |
| 2 | 10 (12.0%) | 18 (10.8%) | 28 (11.2%) | |
| 3 | 1 (1.2%) | 5 (3.0%) | 6 (2.4%) | |
| Freezing‐time protocol | ||||
| 180s | 57 (68.7%) | 113 (68.1%) | 170 (68.3%) | |
| 240s | 26 (31.3%) | 53 (31.9%) | 79 (31.7%) | |
| Procedure time (min) | 79.1 ± 30.3 | 73.6 ± 24.6 | 75.4 ± 26.8 | .13 |
| Fluoroscopy time (min) | 15.8 ± 11.2 | 15.2 ± 9.1 | 15.4 ± 9.8 | .63 |
| Complications (n, %) | 13 (15.7%) | 17 (10.2%) | 30 (12.0%) | .22 |
| Phrenic nerve palsy (n, %) | 9 (10.8%) | 10 (6.0%) | 19 (7.6%) | .21 |
| Vascular complications (n, %) | 3 (3.6%) | 7 (4.2%) | 10 (4.0%) | 1.0 |
| Tamponade (n, %) | 1 (1.2%) | 0 (0.0%) | 1 (0.4%) | .33 |
Abbreviations: °C, Celsius degrees; LSPV, Left superior pulmonary vein; LIPV, Left inferior pulmonary vein; min, Minutes; RSPV, Right superior pulmonary vein; RIPV, Right inferior pulmonary vein; sec, Seconds; T°, Temperature.
3.3. Outcomes
3.3.1. Efficacy
The median follow‐up (FU) period was 24 months (IQR: 18.4‐25.5 months). At the end of FU, 108 out of 249 patients (43.4%) remained free of documented AF after the blanking period. Patients in group 1 suffered more recurrences (53/83 patients, 63.9%) than those in group 2 (88/166 patients, 53.0%), P =.03 (Figure 1).
FIGURE 1.
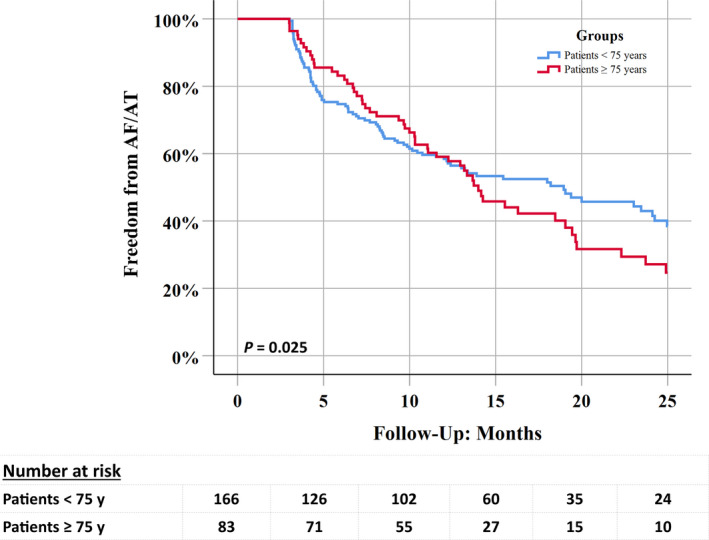
Kaplan‐Meir curve showing freedom from AF/AT recurrences at 24 months
At 1‐year FU, freedom from documented AF was similar in both groups (group 1:49/83 patients, 59.0% vs group 2:97/166 patients, 58.4%; Log Rank = 0.70), however, afterwards, the success rate stabilised in the younger group but continued to decline in the elderly population with a clear separation of survival curves. The recurrence rate between the first and the second year was 38.8% (19/49 patients) in group 1 and 19.6% (19/97 patients) in group 2 (log Rank = 0.01) (Figure 2).
FIGURE 2.

Left: Freedom from AF/AT during the first year of follow‐up. Right: Freedom from AF/AT between the first and the second year of follow‐up
3.3.2. Redo procedures
Seventy‐four of the 141 patients (52.5%) with recurrent AF underwent an open irrigated tip RF ablation as a redo‐procedure. The redo‐ablation procedure consisted of re‐isolation of reconnected veins and mapping and ablation of spontaneous or inducible atrial tachycardias. Patients in group 1 were less likely to undergo a repeat procedure compared to the younger cohort (group 1:17/53 (32.1%), group 2:57/88 (64.8%), P <.01). In 46/74 patients (62.2%) a PV was found to be reconnected (group 1:10/17 (58.8%) vs group 2:36/57 (63.2%) (P =.75)). In the 57 patients of the younger cohort, out of the 221 veins mapped, we found reconnections in 62 (28.1%) (4 LCPVs, 10 LSPVs, 11 LIPVs, 16 RSPVs and 21 RIPVs) of them. This proportion was not significantly different from that found in the 17 patients in the older group, in which, out of 72 veins, 14 (19.4%) (3 LSPVs, 1 LIPV, 2 RSPVs and 8 RIPVs) were reconnected (P =.20).
In addition to PV re‐isolation, a posterior box was performed in 15 (20.3%) patients, a cavo‐tricuspid isthmus (CTI) ablation in 21 (28.4%) patients, a roofline in 10 (13.5%), an anteroseptal line in 13 (17.6%) and a mitral line in 11 (14.9%) patients.
3.3.3. Predictors of recurrences
The univariate analysis revealed that the E‐wave value, the left atrial diameter (LAD) and the number of electrical cardioversions were found to be predictors of recurrence. In the multivariate analysis, however, only LAD (HR 1.05; 95% CI: 1.02‐1.09; P <.01) and E‐wave (HR 2.93; 95% CI: 1.10‐7.80; P =.03) were independent predictors of recurrence (Table 3).
TABLE 3.
Univariate and multivariate analysis
| Variable | P‐value | Hazard Ratio (HR) | 95%CI of HR | |
|---|---|---|---|---|
| Lower | Upper | |||
| Univariate Analysis | ||||
| Age (years) | .24 | 1.01 | 0.99 | 1.03 |
| Gender | .25 | 1.22 | 0.87 | 1.72 |
| Hypertension | .84 | 0.97 | 0.69 | 1.35 |
| Stroke/TIA | .77 | 0.92 | 0.52 | 1.63 |
| Vascular or Coronary disease | .64 | 0.92 | 0.63 | 1.33 |
| Valvular Heart Disease | .48 | 1.23 | 0.70 | 2.18 |
| Heart failure | .67 | 1.08 | 0.75 | 1.55 |
| CHA2DS2Vasc Score ≥ 3 points | .45 | 1.14 | 0.81 | 1.60 |
| CHA2DS2VASC | 1.00 | 1.00 | 0.90 | 1.11 |
| EE’ prime ratio | .28 | 1.02 | 0.98 | 1.07 |
| Ejection Fraction | .68 | 1.00 | 0.98 | 1.02 |
| A – Wave (m/s) | .24 | 0.50 | 0.15 | 1.60 |
| E – Wave (m/s) | <.01 | 3.43 | 1.35 | 8.68 |
| Left Atrial Diameter (mm) | <.01 | 1.05 | 1.02 | 1.07 |
| Number of ECVs | <.01 | 1.30 | 1.09 | 1.56 |
| Body mass index (kg/m2) | .17 | 1.02 | 0.99 | 1.06 |
| Weight (Kg) | .13 | 1.01 | 1.00 | 1.02 |
| Height (cm) | .52 | 1.01 | 0.99 | 1.02 |
| Sig. | Hazard Ratio (HR) | 95%CI of HR | ||
|---|---|---|---|---|
| Lower | Upper | |||
| Multivariate Analysis | ||||
| E – Wave (m/s) | .03 | 2.93 | 1.10 | 7.80 |
| Left Atrial Diameter (mm) | <.01 | 1.05 | 1.02 | 1.09 |
| Number of ECVs | .23 | 1.14 | 0.92 | 1.43 |
Abbreviations: ECVs, Electrical cardioversions; TIA, Transitory ischemic accident.
3.3.4. Complications
Thirty out of 249 (12.0%) patients presented a complication. Complications details are resumed in Table 2. The complication rate was not different between groups (group 1:13/83 (15.7%), group 2:17/166 (10.2%), P =.22). There were no procedure‐related deaths. The most frequent complication consisted of PNP, which was temporary in 17 patients. In 2 patients (1 patient in each group), there was a persistent elevation of the right hemidiaphragm on the chest X‐ray. These last two patients were followed at the department of pneumology without requiring any further therapy.
There were five groin hematomas that did not require further intervention and five femoral pseudoaneurysms of which one required surgical therapy, the others were treated percutaneously with thrombin injection. There was one subacute tamponade that was treated with pericardiocentesis.
Despite the high CHA2DS2VASc score, there were no cerebrovascular accidents.
4. DISCUSSION
The main findings of our study are: (a) Mid‐term success rate of CB‐A PVI in persAF is 43.4%, (b) A significantly lower success rate is achieved in older patients (>75 years) compared with the younger cohort. (c) The complication rate of CB‐PVI is not different between age groups and (d) LAD, and E wave are independent predictors of recurrences of AAE.
4.1. Efficacy
The results of catheter ablation in patients with persAF are still far from optimal. In the STAR‐AF II study 10 freedom from any atrial arrhythmia after 18 months of FU was achieved in 49% of patients in the PVI‐only group and in 37% in those undergoing PVI plus linear ablation. In the SARA study 11 the success rate was around 60% at 12 months. While these trials used point‐by‐point RF ablation to perform PVI, the CRYO4PERSISTENT AF trial 12 evaluated the efficacy and safety of CB‐A in patients with persAF showing comparable results. A common aspect of these trials is that the elderly population was somehow excluded. Data reporting clinical outcomes and complications of CB‐A in elderly patients (>75 years) is scarce in the setting of persAF. Previous studies included exclusively PAF patients or mainly PAF with only a small number of patients with persAF. 4 , 5 , 13
Tscholl et al 5 studied two groups of 40 patients older and younger than 75 years undergoing PVI with CB‐A, after a median FU of 12 (IQR: 6‐24) months, arrhythmia recurrence was similar in both groups (12/40 (30%) vs 10/40 (25%) (P =.62). Unfortunately, the number of patients with persAF was too small to perform a survival analysis. Similarly, in a recent paper by Heeger and coworkers 14 studying CB‐A in a mixed population of PAF and persAF patients older than 75 years showed a similar outcome than the younger population during their 3 years follow‐up. The number of persAF patients was substantial (45 patients in each group), unfortunately the survival of the persAF group was not separately analysed.
In our study, the global 1‐year success rate was 58.6%, which is in line with previously published data. 1 , 11 , 12 An interesting observation is that after 1 year, there was a significant divergence in the survival curves with a stabilization of recurrences in the younger cohort and a steady decline in success in older patients. Notably, follow‐up was similar between groups minimizing the risk for bias. Moreover palpitations, which is the classical symptom raising the alerts for AF recurrences, are less common in older patients. 15 In turn, this atypical presentation of AF in the elderly might predispose this group to have under‐detected episodes of AF.
The reason why the success rate in the older age group continues to decline at a similar speed after year 1 (vs a deceleration in the younger age group) is not entirely clear. AF recurrences after a successful PVI can be attributed either to recovered PV conduction or the presence of extra PV mechanisms of AF initiation and maintenance. PV reconnections are a common finding in repeat AF ablation procedures.
Nery et al 16 analyzed the relationship between PV reconnection and freedom from AF in a meta‐analysis, including studies using RF ablation, CB‐A, and laser balloon ablation. Among patients with and without AF recurrence, 86% and 59% had at least 1 PV reconnection, respectively. In our cohort, even though a repeat procedure was less frequently performed in older patients, the proportion of patients presenting with a PV reconnection was similar in both groups. Although PV reconnections are also an important factor affecting long‐term ablation outcomes 17 ; however, assuming equal procedural characteristics, it is safe to assume an equal incidence of PV reconnection between groups, in consequence, another variable beyond PV triggers should be the cause of the worst outcomes in the elder group.
Atrial fibrosis is another factor influencing outcomes after PVI. Khurram et al. 18 studied the relationship between the proportion of late gadolinium enhancement (LGE) in the LA and AF recurrence after AF ablation. Regardless of the type of AF, patients with LGE >35% had a higher recurrence rate during the first year after ablation when compared to those with LGE ≤35%. Although, histological studies have shown that age contribution is not likely to be the only explanation for the increased amount of scar observed in AF patients, 19 age positively correlates with atrial fibrosis in studies using magnetic resonance imaging (MRI), 20 and it is a well know predisposing factor for AF progression 21 and structural remodelling of the LA. 22 Even though this might not be so relevant in patients with PAF, it may gain importance in the setting of persAF. Moreover studies have shown that abnormal substrate does not reverse, but in some patients, it progresses even after successful catheter ablation. 23 Therefore, it is reasonable to speculate that at the time of ablation baseline left atrial structural remodelling is already in a more advanced state in the aged population placing these patients at higher risk for AF recurrences than the younger cohort. This is supported by the survival curves of both groups: in the younger cohort the KM curve resembles an expected AF recurrence pattern with early decline and then a plateau while in the older cohort a steady decline without plateau occurs. One can even wonder if elimination of the main AF triggers by PVI had any influence on the survival curve in the older group, supporting a too far advanced baseline state of left atrial structural remodelling.
4.2. Safety
Our results show that CB‐A ablation is a safe procedure in elderly patients and that the occurrence of complications is not significant different between the two groups. This is in line with previous reports in which neither CBA nor RF‐ablation in elderly patients was not associated with an increased number of complications if compared with younger patients. 5 , 13
4.3. Limitations
Our study has several limitations. First, the study is retrospective, which may restrict our ability to draw substantial conclusions. Second, follow‐up was clinical, based on symptoms and Holter monitoring, which were performed if symptoms were present, and therefore, asymptomatic episodes may have occurred unnoticed, and our success rate may have been different. Moreover our definition of success was based on the absence of recurrences, and we did not consider other important points like reduction the AF burden, which especially in the elderly population might have an important impact in quality of life, hospitalizations, and survival. Finally, as the initial procedure was performed by means of CB‐A, a voltage map was not performed.
5. CONCLUSION
The 2‐years success rate of PVI using the CB‐A in persAF is significantly lower in older patients (≥75 years) compared with the younger cohort; however, there is no difference in the complication rate of CB‐PVI between age groups.
CONFLICT OF INTERESTS
Dr Chierchia and Dr Asmundis have received compensation for teaching purposes and proctoring from AF Solutions and Medtronic. Dr de Asmundis is a consultant for Daiichi Sankyo. The other authors have no conflict of interests to declare.
Vermeersch G, Abugattas J‐P, Varnavas V, et al. Efficacy and safety of the second‐generation cryoballoon ablation for the treatment of persistent atrial fibrillation in elderly patients. J Arrhythmia. 2021;37:626–634. 10.1002/joa3.12531
Gaëlle Vermeersch and Juan‐Pablo Abugattas contributed equally as first authors.
REFERENCES
- 1. Tondo C, Iacopino S, Pieragnoli P, et al. Pulmonary vein isolation cryoablation for patients with persistent and long‐standing persistent atrial fibrillation: Clinical outcomes from the real‐world multicenter observational project. Heart Rhythm. 2018;15:363–8. [DOI] [PubMed] [Google Scholar]
- 2. Corrado A, Patel D, Riedlbauchova L, et al. Efficacy, safety, and outcome of atrial fibrillation ablation in septuagenarians. J Cardiovasc Electrophysiol. 2008;19:807–11. [DOI] [PubMed] [Google Scholar]
- 3. Chen CF, Zhong YG, Jin CL, Gao XF, Liu XH, Xu YZ. Comparing between second‐generation cryoballoon vs open‐irrigated radiofrequency ablation in elderly patients: Acute and long‐term outcomes. Clin Cardiol. 2020;43:500–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Abugattas JP, Iacopino S, Moran D, et al. Efficacy and safety of the second generation cryoballoon ablation for the treatment of paroxysmal atrial fibrillation in patients over 75 years: A comparison with a younger cohort. Europace. 2017;19:1798–803. [DOI] [PubMed] [Google Scholar]
- 5. Tscholl V, Lin T, Lsharaf AK, et al. Cryoballoon ablation in the elderly: one year outcome and safety of the second‐generation 28mm cryoballoon in patients over 75 years old. Europace. 2018;20:772–7. [DOI] [PubMed] [Google Scholar]
- 6. Kirchhof P, Benussi S, Kotecha D, et al. 2016 ESC Guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Eur Heart J. 2016;37:2893–962. [DOI] [PubMed] [Google Scholar]
- 7. Stroker E, De Greef Y, Schwagten B, et al. Over‐the‐needle trans‐septal access using the cryoballoon delivery sheath and dilator in atrial fibrillation ablation. Pacing Clin Electrophysiol. 2019;42:868–73. [DOI] [PubMed] [Google Scholar]
- 8. Nagueh SF, Smiseth OA, Appleton CP, et al. Recommendations for the evaluation of left ventricular diastolic function by echocardiography: an update from the american society of echocardiography and the european association of cardiovascular imaging. European heart journal cardiovascular Imaging. 2016;17:1321–60. [DOI] [PubMed] [Google Scholar]
- 9. Qiu P, Sheng J. A two‐stage procedure for comparing hazard rate functions. J Royal Stat Soc. 2008;70:191–208. [Google Scholar]
- 10. Verma A, Jiang CY, Betts TR, et al. Approaches to catheter ablation for persistent atrial fibrillation. New Eng J Med. 2015;372:1812–22. [DOI] [PubMed] [Google Scholar]
- 11. Mont L, Bisbal F, Hernandez‐Madrid A, et al. Catheter ablation vs. antiarrhythmic drug treatment of persistent atrial fibrillation: a multicentre, randomised, controlled trial (SARA study). Eur Heart J. 2014;35:501–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Boveda S, Metzner A, Nguyen DQ, et al. Single‐procedure outcomes and quality‐of‐life improvement 12 months post‐cryoballoon ablation in persistent atrial fibrillation: results from the multicenter CRYO4PERSISTENT AF trial. JACC Clin Electrophysiol. 2018;4:1440–7. [DOI] [PubMed] [Google Scholar]
- 13. Metzner I, Wissner E, Tilz RR, et al. Ablation of atrial fibrillation in patients >/=75 years: long‐term clinical outcome and safety. Europace. 2016;18:543–9. [DOI] [PubMed] [Google Scholar]
- 14. Heeger CH, Bellmann B, Fink T, et al. Efficacy and safety of cryoballoon ablation in the elderly: a multicenter study. Int J Cardiol. 2019;278:108–13. [DOI] [PubMed] [Google Scholar]
- 15. Brunetti ND, De Gennaro L, Pellegrino PL, Dellegrottaglie G, Antonelli G, Di Biase M. Atrial fibrillation with symptoms other than palpitations: incremental diagnostic sensitivity with at‐home tele‐cardiology assessment for emergency medical service. Eur J Prevent Cardiol. 2012;19:306–13. [DOI] [PubMed] [Google Scholar]
- 16. Nery PB, Belliveau D, Nair GM, et al. Relationship between pulmonary vein reconnection and atrial fibrillation recurrence: a systematic review and meta‐analysis. JACC: Clin Electrophysiol. 2016;2:474–83. [DOI] [PubMed] [Google Scholar]
- 17. Shah S, Barakat AF, Saliba WI, et al. Recurrent atrial fibrillation after initial long‐term ablation success. Circul: Arrhythmia Electrophysiol. 2018;11:e005785. [DOI] [PubMed] [Google Scholar]
- 18. Khurram IM, Habibi M, Gucuk Ipek E, et al. Left atrial LGE and arrhythmia recurrence following pulmonary vein isolation for paroxysmal and persistent AF. JACC Cardiovasc Imaging. 2016;9:142–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Platonov PG, Mitrofanova LB, Orshanskaya V, Ho SY. Structural abnormalities in atrial walls are associated with presence and persistency of atrial fibrillation but not with age. J Am Coll Cardiol. 2011;58:2225–32. [DOI] [PubMed] [Google Scholar]
- 20. Cochet H, Mouries A, Nivet H, et al. Age, atrial fibrillation, and structural heart disease are the main determinants of left atrial fibrosis detected by delayed‐enhanced magnetic resonance imaging in a general cardiology population. J Cardiovasc Electrophysiol. 2015;26:484–92. [DOI] [PubMed] [Google Scholar]
- 21. Blum S, Aeschbacher S, Meyre P, et al. Incidence and predictors of atrial fibrillation progression. J Am Heart Assoc. 2019;8:e012554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Staerk L, Sherer JA, Ko D, Benjamin EJ, Helm RH. Atrial fibrillation: epidemiology, pathophysiology, and clinical outcomes. Circ Res. 2017;120:1501–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Teh AW, Kistler PM, Lee G, et al. Long‐term effects of catheter ablation for lone atrial fibrillation: progressive atrial electroanatomic substrate remodeling despite successful ablation. Heart Rhythm. 2012;9:473–80. [DOI] [PubMed] [Google Scholar]


