Abstract
Introduction
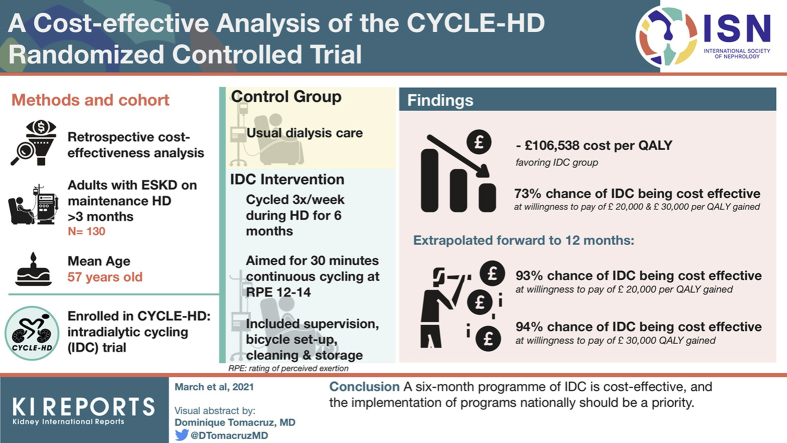
No formal cost-effectiveness analysis has been performed for programs of cycling exercise during dialysis (intradialytic cycling [IDC]). The objective of this analysis is to determine the effect of a 6-month program of IDC on health care costs.
Methods
This is a retrospective formal cost-effectiveness analysis of adult participants with end-stage kidney disease undertaking in-center maintenance hemodialysis enrolled in the CYCLE-HD trial. Data on hospital utilization, primary care consultations, and prescribed medications were extracted from medical records for the 6 months before, during, and after a 6-month program of thrice-weekly IDC. The cost-effectiveness analysis was conducted from a health care service perspective and included the cost of implementing the IDC intervention. The base-case analyses included a 6-month “within trial” analysis and a 12-month “within and posttrial” analysis considering health care utilization and quality of life (QoL) outcomes.
Results
Data from the base-case within trial analysis, based on 109 participants (n = 56 control subjects and n = 53 IDC subjects) showed a reduction in health care utilization costs between groups, favoring the IDC group, and a 73% chance of IDC being cost-effective compared with control subjects at a willingness to pay of £20,000 and £30,000 per quality-adjusted life year (QALY) gained. When QoL data points were extrapolated forward to 12 months, the probability of IDC being cost-effective was 93% and 94% at £20,000 and £30,000 per QALY gained. Sensitivity analysis broadly confirms these findings.
Conclusion
A 6-month program of IDC is cost-effective and the implementation of these programs nationally should be a priority.
Keywords: cost-effectiveness, exercise, hemodialysis, intradialytic exercise, rehabilitation
Graphical abstract
Chronic kidney disease (CKD) is expensive; the cost to the United Kingdom National Health Service (NHS) is estimated to be in excess of £1.4 billion annually, approximately 1.3% of the entire NHS budget.1 In the United States, the Medicare expenditure total is in excess of $120 billion for chronic and end-stage kidney disease (ESKD) combined.2 Health care costs are highest in those individuals with ESKD requiring renal replacement therapy,3 and particularly in those receiving hemodialysis (compared with transplant and peritoneal dialysis).3,4 People receiving hemodialysis have higher rates of hospitalization coupled with a longer duration of stay compared with the general population, individuals with other chronic conditions, and those with less severe stages of CKD.4, 5, 6 This disproportionate use of health care services is expected to grow and puts providers under increased demand as the worldwide prevalence of ESKD requiring dialysis is expected to double by 2030.7
Despite evidence that they may improve patient outcomes8 and being recently recommended in clinical guidelines within the United Kingdom,9 formal exercise programs are not routinely offered to patients with ESKD who are receiving hemodialysis in the same way that they are for other chronic disease populations.10 At the present time, structured exercise programs are most commonly delivered using a specially adapted cycle ergometer during in-center hemodialysis (IDC), with growing evidence that they confer clinical benefits to patients.11 To date, however, there are no data to support the cost effectiveness of these programs.12,13 This remains a major barrier to adoption and may explain the slow implementation of IDC programs into clinical practice across both the United Kingdom and internationally.14 Although no formal cost-effectiveness analysis has been performed for IDC, it has been associated with reductions in hospital admission, duration of stay, and prescribed medications.15,16 Therefore, the aim of this study was to perform a formal cost-effectiveness evaluation of the 6-month structured exercise program, which formed the intervention in the CYCLE-HD trial (ISRCTN11299707).17
Methods
Trial Population
Adult patients with ESKD undertaking maintenance hemodialysis for >3 months were eligible for inclusion. The trial was given ethical approval by the NHS Research Ethics Committee East Midlands (Northampton, UK; REC ref: 14/EM/1190). The trial was conducted according to the Declaration of Helsinki, and all participants provided written informed consent.
Trial Design
This is a cost-effectiveness analysis conducted alongside the CYCLE-HD trial (ISRCTN11299707).17 CYCLE-HD was a prospective, cluster randomized, open-label, blinded endpoint trial. Detailed descriptions of the CYCLE-HD trial design, including specific inclusion and exclusion criteria, randomization, and data collection procedures, have been previously published.17 Briefly, for randomization, days in which the patients were dialyzed at each of the 3 included dialysis units were randomized by an independent statistician to either continue on standard therapy (control group) or standard dialysis therapy plus the intervention of IDC. This meant there were 6 clusters in total (2 clusters at each of the 3 units). Participants were screened against the inclusion and exclusion criteria of the study by members of the study team.
Blinding
Recruitment and delivery of the intervention (IDC) was not performed blind to trial group allocation. Collection of the health care utilization data was undertaken blind to trial group allocation.
IDC Intervention, Control Group, and Adherence
The IDC group used specially adapted and calibrated exercise bicycles (Letto Series; Motomed, Reck, Germany; cost shown in Supplementary Table S1). The IDC sessions were delivered (which included supervision of participants during exercise and bicycle set-up, cleaning, and storage after use) by the study team at the hemodialysis unit. Participants cycled 3 times a week during dialysis for 6 months, aiming for 30 minutes of continuous cycling at a rating of perceived exertion of 12 to 14. Rating of perceived exertion was recorded at the end of the IDC session to progress training, there was no additional monitoring of participants (over and above the usual clinical observations as part of their treatment). This intervention was chosen as it is the most common form of intradialytic exercise within the United Kingdom11 and can implemented as part of usual clinical practice.18 The bicycles were stored along with other clinical equipment at the unit. The maintenance and repair cost of the bicycles for the duration of the study was covered in the initial purchase cost.
Time Horizon
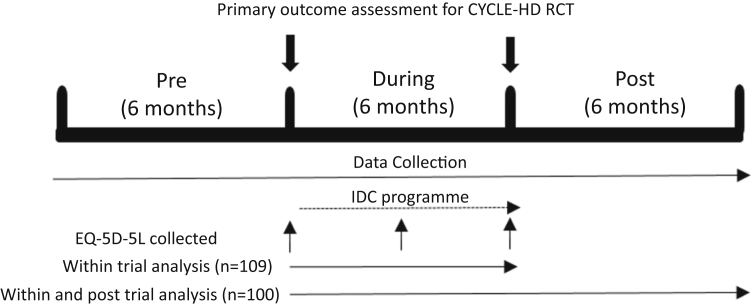
Data on health care utilization were collected from participants’ medical records for 3 distinct 6-month periods: pre-, during, and postenrollment in the CYCLE-HD trial (Figure 1). These time horizons were chosen because this ensures that the 6-month “during” period includes the IDC exercise program for the exercise group and allows direct comparison with the “usual care” only control group (Figure 1).
Figure 1.
Schematic of the time horizon for data collection. Euro QoL-5 dimension-5 level (EQ-5D-5L) data collected as part of the CYCLE-HD randomized controlled trial (RCT). For the “within and posttrial” analysis the EQ-5D-5L data were extrapolated forward by 6 months assuming no change. (a) Base-case “within” analysis. (b) Base-case “within and posttrial” analysis. IDC, intradialytic cycling.
Health-Related QoL and Morbidity Assessment
Health-related QoL (HRQoL) was derived using the Euro QoL-5 dimension-5 level (EQ-5D-5L) questionnaire. The 5 dimensions include mobility, self-care, usual activities, pain/discomfort, and anxiety/depression. Each dimension has 5 levels: no problems, slight problems, moderate problems, severe problems, and extreme problems. This was undertaken at baseline, at 3 months, and at 6 months as part of the CYCLE-HD Trial.17
Sources of Information on Health Care Resources and Unit Costs
Data on associated hospital costs and prescribed medications were collected and cross-referenced from 2 online medical record systems (Integrated Clinical Environment, Sunquest Information Systems, Tucson, AZ; Proton, Clinical Computing Clinical Information Systems, Middlesex, UK). Information on primary care consultations were collected through data extraction forms and cross-referenced (SystmOne; TPP, Horsforth, UK). Intervention costs consisted of physiotherapist time and exercise bikes. Two appropriately trained physiotherapists at whole time (2.0 WTE), and 1 senior, experienced physiotherapist at 10% of their whole time (0.1 WTE) were costed in at current NHS staff salary rates.19 Resources were valued using national tariffs20, 21, 22 to increase generalizability (Supplementary Table S1). All costs were expressed in 2017/2018 UK pounds (£) inflated to this base year where appropriate using the UK Consumer Price Health Index.20
Analysis of Cost-Effectiveness
The cost-effectiveness analysis was conducted from a health care service perspective. A summary of the base-case analysis is provided in Supplementary Table S2. The base-case analysis consisted of 2 elements: a “within trial” analysis (n = 109), i.e. considering outcomes over the 6-month trial period, and a “within and posttrial” analysis (n = 100) where data on health utilization for the 6 months posttrial (i.e., the 6-month trial period plus 6 months posttrial) were included, and the EQ-5D-5L data extrapolated forward (Figure 1). For the analysis, we adopted a bivariate model for estimating incremental costs and effects to acknowledge the joint distribution between them. As this was a cluster randomized controlled trial we adopted a hierarchical modeling approach including random effects to represent the differences in the cluster mean costs and effects from the overall mean for each group.23 Because of the positively skewed distribution of the cost data, a gamma distribution was specified for costs while a normal distribution was specified for effects (QALY). This approach used Markov Chain Monte Carlos simulations to fit the models using WinBUGS software (version 1.4.3; Cambridge Univerity, Cambridge, United Kingdom).24 We then performed 2 distinct sensitivity analyses (SAs). SA1 was a within-trial analysis based on a complete case analysis only imputing for missing primary care costs observations; that is, excluding patients with missing EQ-5D-5L observations and/or lost to follow-up before 6 months (n = 79). SA2 was a within and posttrial analysis (the 6-month trial period plus 6 months posttrial) based on a complete case analysis as described in SA1.
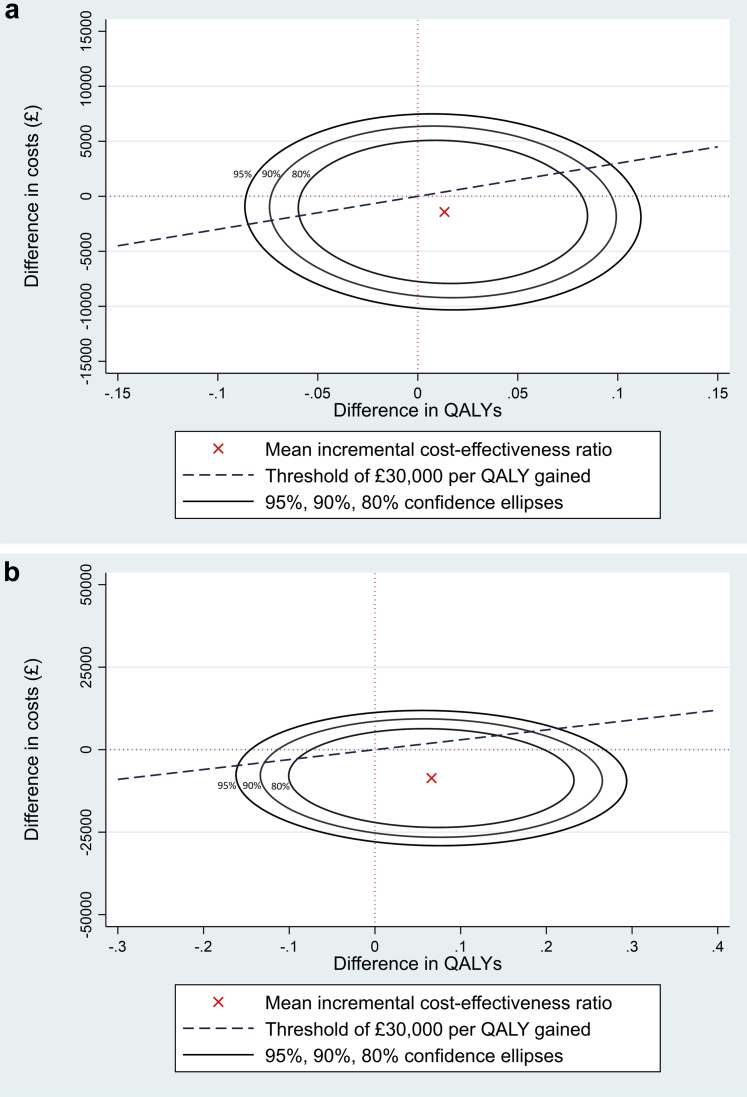
Results were expressed in terms of cost per QALY gained (i.e., the cost-effectiveness ratio), which was estimated for the IDC group compared with the control group. QALYs were calculated from EQ-5D-5L data, using standard utility weights25 collected from participants at baseline and at 3 and 6 months. Area under the curve methods were used to calculate the QALYs accrued by each patient during the intervention period based on EQ-5D-5L data collected at baseline and at 3 and 6 months. For the posttrial analysis, EQ-5D-5L scores recorded at 6 months (end of trial period) were carried forward to 6 months posttrial, and 0- to 12-month QALYs estimated using area under the curve methods. All data are presented as mean difference (95% confidence intervals [CIs] or standard deviation [SD]) or median and interquartile range (IQR), or mean (95% credible intervals [95% CrIs]), and number. Incremental cost-effectiveness ratio measures were estimated for both the within trial and within and posttrial analyses based on 50,000 Markov Chain Monte Carlos samples for each of the 10 imputed datasets and summarised in cost-effectiveness planes (Figures 2a and 2b). For the cost-effectiveness planes, we show confidence ellipses showing the area containing 95%, 90%, and 80% of the Markov Chain Monte Carlos–sampled incremental cost-effectiveness ratio, together with the mean incremental cost-effectiveness ratio from the base-case analyses.
Figure 2.
The cost-effectiveness plane shows the probability of intradialytic cycling being cost-effective at a willingness to pay threshold of £30,000 per quality-adjusted life year (QALY; in UK£) for the base-case “within” and “within and posttrial” analyses. The incremental cost-effectiveness ratio (red dot) estimated from 50,000 Markov Chain Monte Carlo samples for each of the 10 imputed data sets are presented in the form of cost-effectiveness ellipses on the cost-effectiveness planes. It can be observed that although the incremental cost-effectiveness ratios span all 4 quadrants, the largest proportion is in the southeast quadrant indicating intradialytic cycling is likely to be less costly and more effective. The incremental cost-effectiveness ratios estimated lie below the willingness to pay threshold line of £30,000 per QALY gained in (a) 73% and (b) 94%. The 3 confidence ellipses are labeled as 95% for the outer, 90% for the middle, and 80% for the inner. The confidence ellipses provide a visual display of the uncertainty around the point estimate of the incremental cost-effectiveness ratio (ICER).
Missing Data and Imputation
In the base-case analysis, multiple imputation was used to replace missing primary care costs, with observations based on baseline variables and stratified by intervention group. Missing EQ-5D-5L observations were replaced using an index rather than domain imputation.26 The base-case "within trial" analysis imputed missing observations for primary care costs and EQ-5D-5L. The "within and posttrial" analysis imputing missing observations for primary care costs, and EQ-5D-5L (excluding patients lost to follow-up). There were 2 types of missingness in the data: (i) missing health costs and EQ-5D-5L observations for participants who completed the study protocol and (ii) missing observations due to participants being lost to follow-up (Supplementary Table S3). The base-case analyses (both within trial and within and posttrial) imputed missing observations for primary care costs and EQ-5D-5L for patients who completed the study protocol but excluded patients lost to follow-up (e.g., those who moved away from the hemodialysis center, those who dropped out, etc.). Standard practice for accounting for missing data was followed,27 with multiple imputation by chained equations using predictive mean matching (to deal with the nonnormality of the data) fitted to replace item nonresponse (i.e., where individual observations for participants were missing).28 In addition, because of the nonnormal distribution of the cost data, bootstrapped percentile 95% confidence intervals using 500 replications were calculated for each of the 10 imputed datasets and the results were pooled. Missing data imputation was performed with Stata software (version 15; StataCorp LP, College Station, TX).
Results
The study recruited from March 2015 until April 2018. A total of 155 participants were consented to the study, with 130 completing primary outcome assessment at baseline and 101 participants completing the final primary outcome assessment (IDC group, n = 51; control group, n = 50). Baseline demographics of the study participants are shown in Supplementary Table S4. There were no observable differences in baseline demographics between participants completing the study protocol versus those lost to follow-up (and therefore omitted from the 2-element base-case analysis; Supplementary Table S4).
Hospital Admissions
Hospital admissions for both the IDC group and the control group for 6 months pretrial, during, and posttrial are shown in Table 1. Total admissions in the 2 groups were comparable 6 months pretrial but fell in both groups for the during and posttrial periods (Table 1). This was more pronounced in the IDC group (Table 1). Hospital duration of stay for both groups is shown in Supplementary Table S5.
Table 1.
Total number of in-patient hospital admissions by category, time period, and group
| Speciality | Control group |
Intradialytic cycling group |
||||
|---|---|---|---|---|---|---|
| 6 months pretrial, n = 65 | 6-month trial period, n = 56 | 6 months posttrial, n = 49 | 6 months pretrial, n = 65 | 6-month trial period, n = 53 | 6 months posttrial, n = 51 | |
| Infection | 14 | 11 | 3 | 8 | 10 | 4 |
| Cardiovasculara | 1 | 3 | 7 | 9 | 8 | 5 |
| Musculoskeletal | 1 | 1 | 0 | 5 | 2 | 1 |
| Gastrointestinal | 7 | 3 | 4 | 7 | 2 | 4 |
| Renalb | 33 | 16 | 27 | 25 | 8 | 7 |
| Miscellaneous medical (nonrenal) | 9 | 10 | 5 | 10 | 3 | 3 |
| Miscellaneous surgical (nonrenal) | 7 | 4 | 3 | 4 | 3 | 4 |
| Other | 3 | 6 | 2 | 6 | 6 | 5 |
| Total | 75 | 54 | 51 | 74 | 42 | 33 |
Cardiovascular admissions were for arrhythmia, arterial stenosis, blood pressure complications, cardiac imaging, chest pain, mesenteric ischemia, miscellaneous (admitted to a cardiology ward), myocardial infarction, peripheral vascular disease, stroke, and venous thromboembolism.
Most renal admissions were for dialysis adequacy, fluid overload, or vascular access.
Costs
Primary care, medication, hospital-associated, and total costs are presented by intervention group at 6 months pretrial, during, and posttrial (Table 2). A reduction in the costs was observed for all 3 cost categories and for the total cost in the 6-month trial period comparing the IDC and control groups (mean cost difference per participant: primary care −£43 [95% CI −£107 to £17]; medication −£189 [95% CI −£786 to £378]; and hospital −£1808 [95% CI −£5788 to £2364]). This reduction was more pronounced in the 6-month posttrial cost period (mean cost difference per participant: primary care −£148 [95% CI −£303 to −£43]; medication −£408 [95% CI −£1055 to £214]; and hospital −£4066 [95% CI −£8063 to £58]).
Table 2.
Summary of observed total costs and costs by category, time period, and group
| Costs (UK£) |
|||||||
|---|---|---|---|---|---|---|---|
| Control group |
IDC group |
IDC group vs. control group |
|||||
| n | Mean (SD) | Median (IQR) | n | Mean (SD) | Median (IQR) | Mean cost difference (95% CIa) | |
| 6 months pretrial | |||||||
| Primary care costs | 59 | 148.71 (127.47) | 110.35 (45.25–228.00) | 55 | 173.89 (214.76) | 139.30 (58.50–204.80) | 25.19 (−34.34 to 95.73) |
| Medication costs | 65 | 2288.51 (1466.57) | 2117.87 (1355–10,2762.28) | 65 | 2218.73 (1376.24) | 1889.58 (1286.09–2992.86) | −69.78 (−559.34 to 404.19) |
| Hospital costs | 65 | 9016.96 (15,530.21) | 3970. 66 (926.89–9429.53) | 65 | 9594.43 (11689.18) | 5239.84 (1546.28–12,492.86) | 577.47 (−4314.25 to 5117.96) |
| Total costs | 59 | 11,017.70 (13,993.76) | 6457.54 (3640.09–12,298.51) | 55 | 12,098.36 (11,687.86) | 8440.37 (4275.35–15,710.95) | 1080.66 (−3723.99 to 5532.77) |
| 6-month trial period | |||||||
| Primary care costs | 51 | 158.09 (167.44) | 114.00 (48.50–185.80) | 44 | 114.94 (137.36) | 94.95 (16.13–140.43) | −43.15 (−106.99 to 17.33) |
| Medication costs | 56 | 2412.48 (1598.78) | 2155.53 (1466.92–2882.38) | 53 | 2223.09 (1491.46) | 1885.54 (1115.30–2960.11) | −189.39 (−786.47 to 378.41) |
| Hospital costs | 56 | 8171.67 (11,499.09) | 2500.01 (725.93–10,694.96) | 53 | 6363.83 (10,475.52) | 2300.74 (830.24–5870.96) | −1807.84 (−5787.50 to 2363.64) |
| Total | 51 | 9630.18 (10,157.12) | 4934.24 (2827.77–12,233.30) | 44 | 8454.034 (9080.27) | 5619.20 (2858.52–9574.24) | −1176.14 (−5137.72 to 2861.21) |
| 6 months posttrial | |||||||
| Primary care costs | 44 | 254.87 (432.02) | 148.65 (96.50–251.35) | 43 | 106.83 (120.29) | 77.80 (21.00–157.50) | −148.04 (−302.60 to −43.10) |
| Medication costs | 49 | 2546.56 (1747.33) | 2311.27 (1539.71–3015.07) | 51 | 2138.56 (1459.61) | 1789.58 (1180.99–2865.03) | −407.99 (−1055.17 to 213.84) |
| Hospital costs | 49 | 9888.67 (11,790.19) | 4509.67 (1057.50–14793.60) | 51 | 5822.68 (9441.34) | 2073.99 (742.00–8059.65) | −4065.98 (−8063.06 to 58.29) |
| Total costs | 44 | 11,906.60 (11,542.80) | 7763.20 (3428.68–16027.79) | 43 | 8444.59 (10,470.97) | 3826.27 (2654.72–10,372.63) | −3462.01 (−7865.58 to 1120.83) |
IDC, intradialytic cycling; IQR, interquartile ratio; SD, standard deviation.
Based on 5000 bootstrapped samples.
HRQoL
HRQoL increased in the IDC group and fell in the control group during the CYCLE-HD trial. This is indicated by higher EQ-5D-5L utility scores at 3 months (mean difference 0.0291 [95% CI −0.0817 to 0.1400]) and at 6 months (mean difference 0.1075 [95% CI 0.0021–0.2135]) in the IDC group compared with the control group (Supplementary Table S6).
Base-Case Cost Effective Analysis
The base-case within trial analysis shows a mean cost reduction of −£1418 (95% CrI −£8590 to £5834) per participant in health care utilization costs for the IDC group (compared with the control group). In addition, there was a small increment in QALYs (mean difference per participant 0.013 [95% CrI −0.065 to 0.092]) resulting in a cost per QALY of −£106,538 favoring the IDC group. This results in a 73% probability (indicated by the proportion of the ellipses below the willingness to pay threshold line in Figure 2a) of IDC being cost-effective compared with only usual hemodialysis therapy (control group) at the willingness to pay thresholds of £20,000 and £30,000 per QALY gained (Table 3, Figure 2a). The within and posttrial analysis that extrapolates the EQ-5D-5L data forward shows an even larger reduction in health care utilization costs (mean difference per participant −£8603 [95% CrI −£23,362 to £7808]), with an increment in QALYs (mean difference per participant 0.066 [95% CrI −0.117 to 0.248]) in the IDC group compared with the control group. This confers a 93% probability of IDC being cost-effective compared with usual hemodialysis therapy at the willingness to pay threshold of £20,000 per QALY gained, rising to 94% at £30,000 per QALY gained (Table 3, Figure 2b).
Table 3.
Cost-effective analysis results for base-case and sensitivity analyses
| Usual care control group | IDC group | IDC group vs. control group | |
|---|---|---|---|
| Base-case “within trial” analysis,an | 56 | 53 | |
| Mean cost per participant (95% CrI) | £11,097.00 (£6,191.21–£16,002.79) | £9678.30 (£4806.92–£14,459.68) | −£1418.40 (−£8589.76 to £5833.91) |
| Mean QALYs per participant (95% CrI) | 0.336 (0.280–0.391) | 0.349 (0.293–0.405) | 0.013 (−0.065 to 0.092) |
| Cost per QALY gained | −£106,538.48 | ||
| Probability CE @ £20,000 per QALY gained | 0.73 | ||
| Probability CE @ £30,000 per QALY gained | 0.73 | ||
| Base-case “within and posttrial” analysis,bn | 49 | 51 | |
| Mean cost per participant (95% CrI) | £25,334.00 (£13,640.04–£37,027.96) | £16,731.00 (£5714.95–£27,747.05) | −£8603.10 (−£23,361.71 to £7807.69) |
| Mean QALYs per participant (95% CrI) | 0.647 (0.518–0.776) | 0.713 (0.585–0.841) | 0.066 (−0.117 to 0.0.248) |
| Cost per QALY gained | −£118,184.42 | ||
| Probability CE @ £20,000 per QALY gained | 0.93 | ||
| Probability CE @ £30,000 per QALY gained | 0.94 | ||
| SA1,cn | 36 | 43 | |
| Mean cost per participant (95% CrI) | £10,276.00 (£6,645.45–£13,906.55) | £7733.10 (£4573.03–£10,892.22) | −£2541.70 (−£7046.03 to £2463.81) |
| Mean QALYs per participant (95% CrI) | 0.324 (0.249–0.399) | 0.343 (0.271–0.415) | 0.019 (−0.085 to 0.123) |
| Cost per QALY gained | −£120,104.45 | ||
| Probability CE @ £20,000 per QALY gained | 0.90 | ||
| Probability CE @ £30,000 per QALY gained | 0.90 | ||
| SA2,dn | 36 | 43 | |
| Mean cost per participant (95% CrI) | £23,464.00 (£15,000.20–£31,927.80) | £14,140.00 (£6985.24–£21,294.76) | −£9322.90 (−£18,355.11 to −£1601.31) |
| Mean QALYs per participant (95% CrI) | 0.625 (0.485–0.765) | 0.707 (0.572–0.842) | 0.082 (−0.111 to 0.276) |
| Cost per QALY gained | −£101,835.69 | ||
| Probability CE @ £20,000 per QALY gained | 0.98 | ||
| Probability CE @ £30,000 per QALY gained | 0.98 |
CE, cost-effective; CrI, credible interval; EQ-5D-5L, Euro QoL-5 dimension-5 level; GP, general practice; IDC, intradialytic cycling; QALY, quality-adjusted life year; SA, sensitivity analysis.
Six-month within trial analysis imputing for missing GP and EQ-5D-5L observations (excluding participants lost to follow-up during the first 6 months).
Zero to 12-month analysis (including 6 months posttrial) imputing for missing GP and EQ-5D-5L observations, and extrapolating EQ-5D-5L to 12 months (excluding participants lost to follow-up).
“Within trial” cost-utility analysis: 0-6 months within trial analysis imputing for missing GP observations (4 and 7 observations missing for the control group and the IDC group, respectively; excluding participants lost to follow-up during first 6 months and with missing EQ-5D-5L at ≥1 time point).
“Within and posttrial” cost-utility analysis: 0-12 month analysis imputing for missing GP observations (4 and 7 observations missing for the control group and the IDC group, respectively; excluding participants lost to follow-up and with missing EQ-5D-5L at ≥1 time point).
Sensitivity Analysis
The results of SA1 and SA 2 (Table 3) broadly confirm the findings of the base-case analysis. The first analysis (SA1) where we imputed data for missing primary care cost observations (excluding participants with missing EQ-5D-5L observations and/or those lost to follow-up) indicated a mean cost reduction of −£2542 (95% CrI −£7046 to £2464) per participant for the IDC group compared with the control group (Table 3). The increment in QALYs of 0.019 (95% CrI −0.085 to 0.123) resulted in a 90% chance of IDC being cost-effective at £20,000 and £30,000 per QALY gained. SA2 (carrying EQ-5D-5L scores forward and estimating 6-month posttrial QALYs using area under the curve methods) showed an even larger mean reduction of −£9323 (95% CrI −£18,355 to −£1601) per participant, favoring the IDC group (Table 3). The increment in QALYs for SA2 was 0.082 (95% CrI −0.111 to 0.276), increasing the chance of IDC being cost-effective to 98% at willingness to pay thresholds of £20,000 and £30,000 per QALY gained.
Discussion
Study Findings
This study found that a 6-month program of IDC reduced health care utilization costs compared with usual hemodialysis therapy only. Health care utilization costs were similar between groups in the 6 months before the CYCLE-HD trial. There was a reduction in costs for the IDC group compared with the control group during the 6-month trial period, which was more pronounced in the 6-month posttrial period. This resulted in a 73% chance of IDC being cost-effective compared to usual hemodialysis therapy at £20,000 and £30,000 per QALY gained. When EQ-5D-5L data were extrapolated to 6 months posttrial, the chance of IDC being cost-effective rose to 93% and 94% at £20,000 and £30,000 per gained QALY. The 2 SAs confirmed the findings of the base-case analysis with the likelihood of cost-effectiveness at 90% and 98%, respectively. In addition, we have shown that every increment in QALYs as a result of a 6-month program of IDC is associated with a −£106,538 reduction in health care costs.
This study shows for the first time that a program of IDC is cost-effective. A previous study assessed the effect of a 6-month program of IDC on hospital admissions and duration of stay.16 The authors reported that during the 6-month program of IDC there was a nonsignificant decrease in hospitalization rate and a significant reduction in duration of stay, which fell from 7.7 to 3.7 days.16 Similar cost benefits have previously been reported after exercise interventions in other chronic disease populations, such as cardiac and pulmonary patients.29, 30, 31 Unfortunately, this previous study in the dialysis population did not have a usual care (control) group and a full economic analysis was not performed. A separate trial demonstrated that a 12-week program of cardiac rehabilitation was cost-effective in the hemodialysis population for up to 36 months after coronary artery bypass graft.32 The cost savings associated with cardiac rehabilitation programs are driven by a reduction in risk of subsequent events and hospitalization33; our data showing a reduction in hospital admissions after the IDC program support this. However, we have shown that the cost-effectiveness of a program of IDC is realized more immediately (after 6 and 12 months) than the previous program of cardiac rehabilitation.21 This may be because hemodialysis participants included in the CYCLE-HD trial were likely a fitter cohort than hemodialysis patients who required a coronary artery bypass graft in the previous report.32 Moreover, the previous study involved a program of exercise taking place outside of hemodialysis,32 which may be less deliverable in clinical practice than program of IDC and result in lower levels of participant adherence.34
Generalizability
Program of IDC may not be feasible for extended periods because of a lack of resources and staffing. However, this study shows that IDC is cost-effective over 6- and 12-month periods, even with equipment and staff costs incorporated into the analysis. These cost savings can be redeployed to cover the resource (staff and equipment) costs that are needed to maintain programs of IDC. We believe that the success of programs of IDC within normal clinical care is dependent on the use of dedicated personnel. The staff costs included in the analysis included 2 physiotherapist assistants to deliver the program as well as a more experienced senior physiotherapist to oversee the governance. We believe that this method of providing a program of IDC (by individuals specifically trained in exercise or rehabilitation delivery) will increase adherence and reduce dropout compared with delivery by nursing staff. Assuming a cost savings per participant of £1418.40 over 6 months and calculating the total cost of running a program over this time, is 2 trained physiotherapists (2.0 WTE) and 1 senior physiotherapist (0.1 WTE) plus the cost of an exercise bike. Calculating the total cost divided by the cost saving indicates that around 21 participants across all hemodialysis shifts (i.e., 3–4 participants per shift) are required for a program to be cost effective. The cost of implementation for a program of IDC will be largest in the setup phase but may be lower through subsequent years (with the effects of IDC on health care utilization being maintained), as has been demonstrated previously for programs of cardiac rehabilitation.35
Within the United Kingdom there are around 24,000 adult patients with ESKD receiving in-center hemodialysis.36 From previous data published by our group,18 approximately 27% of in-center HD patients would be able to take part in a program of IDC; this represents a significant potential cost savings to the health economy. There may also be wider economic implications to society for programs of IDC if participants are more physically fit and are therefore able to work, although this is difficult to ascertain and therefore was not explored in our current analysis. The intricacies of health care delivery differ between countries; however, based on the data we have presented (showing reductions in primary care, hospital, and medication costs) we would expect a program of IDC to result in health care cost savings if implemented in other regions globally. In addition, in other countries such as the United States, hospitalization expenditures are high relative to the cost of maintenance hemodialysis, and in such a setting the cost-effectiveness of IDC might be more apparent.
Strengths and Limitations
The CYCLE-HD trial was based in a single network (Leicester, UK) comprising predominantly patients of white/Caucasian and South Asian ethnicity.36 However, this center contains 3 hemodialysis units (2 operated by the NHS and 1 operated commercially) and therefore is typical of the normal provision of hemodialysis care within the United Kingdom. It is reasonable to conclude that the cost-effectiveness of programs of IDC could be extended to other centers, although this will need to be explored in a formal implementation plan. The average ages of the study participants were 59 and 56 years for the control and IDC groups, respectively, which is lower than the average age of 67 years for people on in-center hemodialysis in the United Kingdom.36 This might influence the implementation of IDC in hemodialysis units that have an older population. For our 6-month within and posttrial analyses we extrapolated the EQ-5D-5L score forward by 6 months assuming no change in QoL. We feel that this estimate is conservative as the QoL within the control group decreased with trial progression (from baseline to 6 months), and this is supported by previous data which have shown that increasing dialysis vintage is associated with decreases in HRQoL.37 In addition, the QoL increased in the IDC group during the trial, which also supports previous data38 showing that programs of exercise are associated with improvements in HRQoL in the dialysis population. Therefore, in the present analysis we believe it is conservative to assume no change in participant QoL when the EQ-5D-5L data are extrapolated forward for the IDC group in the within and posttrial analyses. However, neither of these assumptions are certain and we acknowledge that HRQoL may not have remained the same over the 6 months that it was extrapolated forward. This cost-effectiveness analysis used multiple imputation to minimize the impact of missing data, and it has recently been shown that unbiased results can be obtained even with large proportions of missing data.39 Our analysis assumed that the data were “missing at random”; if this assumption does not hold, then it could bias our results. In the CYCLE-HD trial, the control group was not allocated a nonexercise intervention, therefore simply taking part in a program of exercise may be responsible for some of the associations that we have observed rather than the intervention per se. Lastly, we also acknowledge that many of the cost differences presented in the results are imprecise, with wide confidence intervals.
In conclusion, the implementation of IDCs are cost-effective and programs nationally should be a priority. Strategies to reach as many participants as possible (e.g., communities that are harder to engage and commercial vs. noncommercial providers) should be incorporated wherever possible.
Disclosure
All the authors declare no conflict of interest. JOB has received honoraria from Vifor Fresenius Medical Care Renal Pharma and Pharmacosmos UK within the last 3 years. No other authors have received payments from pharmaceutical companies in the past 3 years. This manuscript presents independent research funded by the National Institute of Health Research (NIHR) in the United Kingdom under its Research for Patient Benefit (RfPB) Programme (grant reference no. PB-PG-0317-20005) and supported by Kidney Research UK. The views expressed are those of the authors and not necessarily those of the NHS, the NIHR, or the Department of Health and Social Care. This research has no relationship with industry.
Acknowledgments
We acknowledge the contribution of both the Leicester Kidney Care Appeal and the University of Leicester Research Impact Development Fund in enabling us to obtain preliminary data to support this project. This work was supported by the Stoneygate Trust. An abstract with some of the data in this manuscript was presented at American Society of Nephrology Kidney Week 2019, November 5–10, 2019, Washington, DC (abstract no. 3235623). This work falls under the portfolio of research conducted within the NIHR Leicester Biomedical Research Center.
Footnotes
Table S1. Sources of resource use and unit costs (UK£2017/18).
Table S2. Summary of the base-case cost-effectiveness analysis.
Table S3. Table of missingness in the data.
Table S4. Demographic for CYCLE-HD baseline study participants, participants completing the study protocol (not lost to follow up; all included in base-case cost analysis), and participants lost to follow up (e.g., moved away from hemodialysis center, dropped out, etc.; omitted from base-case cost-analysis).
Table S5. Participant duration of stay for hospital admissions.
Table S6. Summary of QALYs.
Supplementary Material
Table S1. Sources of resource use and unit costs (UK£2017/18).
Table S2. Summary of the base-case cost-effectiveness analysis.
Table S3. Table of missingness in the data.
Table S4. Demographic for CYCLE-HD baseline study participants, participants completing the study protocol (not lost to follow up; all included in base-case cost analysis), and participants lost to follow up (e.g., moved away from hemodialysis center, dropped out, etc.; omitted from base-case cost-analysis).
Table S5. Participant duration of stay for hospital admissions.
Table S6. Summary of QALYs.
References
- 1.Kerr M., Bray B., Medcalf J. Estimating the financial cost of chronic kidney disease to the NHS in England. Nephrol Dial Transplant. 2012;27(suppl 3):iii73–iii80. doi: 10.1093/ndt/gfs269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Saran R., Robinson B., Abbott K.C. US Renal Data System 2019 Annual Data Report: Epidemiology of Kidney Disease in the United States. Am J Kidney Dis. 2020;75(1 suppl 1):A6–A7. doi: 10.1053/j.ajkd.2019.09.003. [DOI] [PubMed] [Google Scholar]
- 3.Eriksson J.K., Neovius M., Jacobson S.H. Healthcare costs in chronic kidney disease and renal replacement therapy: a population-based cohort study in Sweden. BMJ Open. 2016;6 doi: 10.1136/bmjopen-2016-012062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Klarenbach S.W., Tonelli M., Chui B. Economic evaluation of dialysis therapies. Nat Rev Nephrol. 2014;10:644. doi: 10.1038/nrneph.2014.145. [DOI] [PubMed] [Google Scholar]
- 5.Quinn M.P., Cardwell C.R., Rainey A. Patterns of hospitalisation before and following initiation of haemodialysis: a 5 year single centre study. Postgrad Med J. 2011;87:389–393. doi: 10.1136/pgmj.2010.099028. [DOI] [PubMed] [Google Scholar]
- 6.Daratha K.B., Short R.A., Corbett C.F. Risks of subsequent hospitalization and death in patients with kidney disease. Clin J Am Soc Nephrol. 2012;7:409–416. doi: 10.2215/CJN.05070511. [DOI] [PubMed] [Google Scholar]
- 7.Liyanage T., Ninomiya T., Jha V. Worldwide access to treatment for end-stage kidney disease: a systematic review. Lancet. 2015;385:1975–1982. doi: 10.1016/S0140-6736(14)61601-9. [DOI] [PubMed] [Google Scholar]
- 8.Heiwe S., Jacobson S.H. Exercise training in adults with CKD: a systematic review and meta-analysis. Am J Kidney Dis. 2014;64:383–393. doi: 10.1053/j.ajkd.2014.03.020. [DOI] [PubMed] [Google Scholar]
- 9.Ashby D., Borman N., Burton J. Renal association clinical practice guideline on haemodialysis. BMC Nephrol. 2019;20:379. doi: 10.1186/s12882-019-1527-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Anderson L., Oldridge N., Thompson D.R. Exercise-based cardiac rehabilitation for coronary heart disease: Cochrane systematic review and meta-analysis. J Am Coll Cardiol. 2016;67:1–12. doi: 10.1016/j.jacc.2015.10.044. [DOI] [PubMed] [Google Scholar]
- 11.Greenwood S.A., Koufaki P., Rush R. Exercise counselling practices for patients with chronic kidney disease in the UK: a renal multidisciplinary team perspective. Nephron Clinical Pract. 2014;128:67–72. doi: 10.1159/000363453. [DOI] [PubMed] [Google Scholar]
- 12.Graham-Brown M.P.M., Jardine M.J., Burton J.O. Cardiovascular adaptations associated with exercise in patients on hemodialysis. Semin Dial. 2019;32:361–367. doi: 10.1111/sdi.12789. [DOI] [PubMed] [Google Scholar]
- 13.March D.S., Graham-Brown M.P., Young H.M. ‘There is nothing more deceptive than an obvious fact’: more evidence for the prescription of exercise during haemodialysis (intradialytic exercise) is still required. Br J Sports Med. 2017;51:1379. doi: 10.1136/bjsports-2017-097542. [DOI] [PubMed] [Google Scholar]
- 14.Thompson S., Clark A., Molzahn A. Increasing the uptake of exercise programs in the dialysis unit: a protocol for a realist synthesis. Syst Rev. 2016;5:67. doi: 10.1186/s13643-016-0224-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Miller B.W., Cress C.L., Johnson M.E. Exercise during hemodialysis decreases the use of antihypertensive medications. Am J Kidney Dis. 2002;39:828–833. doi: 10.1053/ajkd.2002.32004. [DOI] [PubMed] [Google Scholar]
- 16.Parker K., Zhang X., Lewin A. The association between intradialytic exercise and hospital usage among hemodialysis patients. Appl Physiol Nutr Metab. 2014;40:371–378. doi: 10.1139/apnm-2014-0326. [DOI] [PubMed] [Google Scholar]
- 17.Graham-Brown M., March D., Churchward D. Design and methods of CYCLE-HD: improving cardiovascular health in patients with end stage renal disease using a structured programme of exercise: a randomised control trial. BMC Nephrol. 2016;17:69. doi: 10.1186/s12882-016-0294-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Young H.M., Jeurkar S., Churchward D.R. Implementing a theory-based intradialytic exercise programme in practice: a quality improvement project. Clin Kidney J. 2018;11:832–840. doi: 10.1093/ckj/sfy050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.NHS Employers website NHS Terms and Conditions (AfC) pay scales 2018/2019. https://www.nhsemployers.org/pay-pensions-and-reward/agenda-for-change/pay-scales-1819 Available at:
- 20.Personal Social Services Research Unit website Unit costs of health and social care 2017. https://www.pssru.ac.uk/project-pages/unit-costs/unit-costs-2017/ Available at:
- 21.BNF Publications website. https://www.bnf.org/products/bnf-online/ Available at:
- 22.NHS Improvement website Archived reference costs. 2017/18 reference costs. https://improvement.nhs.uk/resources/reference-costs/ Available at:
- 23.Grieve R., Nixon R., Thompson S.G. Bayesian hierarchical models for cost-effectiveness analyses that use data from cluster randomized trials. Med Decis Making. 2010;30:163–175. doi: 10.1177/0272989X09341752. [DOI] [PubMed] [Google Scholar]
- 24.Lunn D., Thomas A., Best N., Spiegelhalter D. WinBUGS: a Bayesian modelling framework: concepts, structure, and extensibility. Stat Comput. 2000;10:325–337. [Google Scholar]
- 25.Dolan P. Modeling valuations for EuroQol health states. Med Care. 1997;35:1095–1108. doi: 10.1097/00005650-199711000-00002. [DOI] [PubMed] [Google Scholar]
- 26.Simons C.L., Rivero-Arias O., Yu L.-M. Multiple imputation to deal with missing EQ-5D-3L data: should we impute individual domains or the actual index? Qual Life Res. 2015;24:805–815. doi: 10.1007/s11136-014-0837-y. [DOI] [PubMed] [Google Scholar]
- 27.Carpenter JR, Kenward MG. Missing Data in Randomised Controlled Trials: A Practical Guide. Birmingham, UK: Health Technology Assessment Methodology Programme.
- 28.White I.R., Royston P., Wood A.M. Multiple imputation using chained equations: issues and guidance for practice. Stat Med. 2011;30:377–399. doi: 10.1002/sim.4067. [DOI] [PubMed] [Google Scholar]
- 29.Davidson P.M., Cockburn J., Newton P.J. Can a heart failure-specific cardiac rehabilitation program decrease hospitalizations and improve outcomes in high-risk patients? Eur J Cardiovasc Prev Rehabil. 2010;17:393–402. doi: 10.1097/HJR.0b013e328334ea56. [DOI] [PubMed] [Google Scholar]
- 30.Hui K.P., Hewitt A.B. A simple pulmonary rehabilitation program improves health outcomes and reduces hospital utilization in patients with COPD. Chest. 2003;124:94–97. doi: 10.1378/chest.124.1.94. [DOI] [PubMed] [Google Scholar]
- 31.Griffiths T.L., Burr M.L., Campbell I.A. Results at 1 year of outpatient multidisciplinary pulmonary rehabilitation: a randomised controlled trial. Lancet. 2000;355:362–368. doi: 10.1016/s0140-6736(99)07042-7. [DOI] [PubMed] [Google Scholar]
- 32.Huang Y., Zhang R., Culler S.D. Costs and effectiveness of cardiac rehabilitation for dialysis patients following coronary bypass. Kidney Int. 2008;74:1079–1084. doi: 10.1038/ki.2008.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shields G.E., Wells A., Doherty P. Cost-effectiveness of cardiac rehabilitation: a systematic review. Heart. 2018;104:1403–1410. doi: 10.1136/heartjnl-2017-312809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Konstantinidou E., Koukouvou G., Kouidi E. Exercise training in patients with end-stage renal disease on hemodialysis: comparison of three rehabilitation programs. J Rehabil Med. 2002;34:40–45. doi: 10.1080/165019702317242695. [DOI] [PubMed] [Google Scholar]
- 35.Lowensteyn I., Coupal L., Zowall H. The cost-effectiveness of exercise training for the primary and secondary prevention of cardiovascular disease. J Cardiopulm Rehabil. 2000;20:147–155. doi: 10.1097/00008483-200005000-00002. [DOI] [PubMed] [Google Scholar]
- 36.UK Renal Registery website UK Renal Registery 22nd annual report - data to 31/12/2018. https://www.renalreg.org/publications-reports/ Available at:
- 37.Pan C.-W., Wu Y., Zhou H.-J. Health-related quality of life and its factors of hemodialysis patients in Suzhou, China. Blood Purif. 2018;45:327–333. doi: 10.1159/000485962. [DOI] [PubMed] [Google Scholar]
- 38.Manfredini F., Mallamaci F., D’Arrigo G. Exercise in patients on dialysis: a multicenter, randomized clinical trial. J Am Soc Nephrol. 2017;28:1259–1268. doi: 10.1681/ASN.2016030378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Madley-Dowd P., Hughes R., Tilling K. The proportion of missing data should not be used to guide decisions on multiple imputation. J Clin Epidemiol. 2019;110:63–73. doi: 10.1016/j.jclinepi.2019.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.