Abstract
BACKGROUND:
Identifying clinical characteristics of patients with chronic urticaria (CU) responsive to medication may help guide clinicians select treatment.
OBJECTIVE:
The objective of this study was to investigate patient characteristics and medication use associated with urticaria control.
METHODS:
A retrospective longitudinal chart review of adult patients with CU was conducted at a multisite allergy practice. Inclusion criteria required at least 4 CU office visits to allow for pre- and posttreatment assessment. Control corresponding to medication(s) used was assessed each visit. Univariate analysis followed by multiple logistic regression was performed.
RESULTS:
A total of 221 patients with CU were included; 140 (63%) achieved complete control. The average time to control was 1.4 ± 2.7 years, which required 1–3 classes of medications. Dermatographia odds ratio (OR) = 1.85 (95% CI 1.3–2.7) or other physical urticarias, OR = 1.51 (1–2.4) and neutrophilic infiltrates on skin biopsy were markers of poor control. Thyroid autoantibodies were associated with better control using an H1-antihistamine. Whereas 22% were controlled on a second-generation H1-receptor antagonist plus a leukotriene receptor antagonist (LTRA), an additional 33% were controlled when cyclosporine was added. Use of a first or second H1-antagonist or LTRA was associated with a 3.5–16.9 times higher odds of complete CU control in those with dermatographia. The odds of achieving control for other forms of physical urticaria was greatest when colchicine was added (aOR = 32.6 [12.7–83.2]).
CONCLUSIONS:
Patient-specific CU characteristics associated with medication-disease control may be useful for selecting treatment regimens. A subset of CU patients remains poorly controlled that indicates an unmet need for novel therapeutic agents.
Keywords: Chronic urticaria, Hives, Patient-specific clinical characteristics, Treatment selection, Control, Physical urticaria, Dermatographism, Medications
Chronic urticaria (CU) is defined as urticaria that has been continuously or intermittently present for at least 6 weeks and it affects between 0.5% and 5% of the world’s population, and up to 1% of the US population.1–3 CU has a significant impact on patient quality of life, and the associated morbidity and economic burden associated with this chronic intermittent or persistent disease are substantial.3–8 In fact, the annual economic burden of CU is estimated to be $244 million, the majority of which is due to direct costs related to medications.3 These costs are even more significant when one considers that the treatment of CU may be required over many years.9 CU remains a therapeutic challenge for physicians, because there are no well-structured clinically effective treatment algorithm(s) available, based on the presence or absence of specific patient characteristics.1,10,11 Although the most recent Joint Task Force Practice Parameter (JTFPP) for CU recommends an algorithmic approach (step 1–4) for the treatment of CU, it does not link the step care approach to objective patient-specific characteristics (physical, serologic, or histologic traits).1
A limited number of studies have tried to identify CU phenotypes based on skin histopathology, the presence of serum auto-antibodies, or basophil reactivity in an adult population.12–14 However, none of these characteristics have reliably been able to be used to predict disease severity or response to treatment resulting in control that could help guide the treating physician in the management of these often challenging patients. In fact, there have been very few retrospective or prospective studies that have tried to identify the patient-specific clinical characteristics associated with control of CU in an adult population.15,16 A practical way to identify clinical characteristics of patients with CU is to determine the medication(s) that are associated with their symptom control.
The primary intent of this study was to identify patient-specific characteristics (ie, gender, age, disease duration, prior medications used, serologic or histologic markers, etc.) of patients with CU associated with their response to treatment. We hypothesized that patient-specific clinical characteristics elicit a response to certain class(es) of mediation(s), and are significantly associated with control of disease in an adult population compared with those who do not achieve control of disease symptoms.
METHODS
Study design
A longitudinal chart review of patients 18 years or older evaluated at a tertiary care outpatient allergy clinic from January 1, 1991, to January 1, 2011, was conducted. Patients were identified with the ICD-9 diagnosis codes of 708.0–708.5, 708.8, or 708.9 for CU. Those who had at least 4 or more clinic visits for CU with a complete medical record that would allow for assessment of pre- and posttreatment courses were eligible to be randomly selected for this study. Patients with urticarial vasculitis and acute urticaria were excluded. It was predetermined that a convenience sample of 220 patient charts would be sufficient for identifying relevant clinical characteristics associated with treatment response based on a previous study that assessed demographic, laboratory, and clinical patterns of a cohort of patients with CU.15 The total number of charts reviewed to sequentially obtain 221 charts that met predefined entry criteria was 500. Data were collected for patient demographics, medication use, and treatment response at each clinic visit, serologic testing, skin biopsy histology, and family history. Two reviewers extracted information from charts; approximately 10% of the charts (22/221 charts) reviewed by one reviewer were cross-reviewed by a second reviewer to ensure consistency of data extraction and data entry. If there was >10% discordance between the reviewers, the chart was reviewed and queries were resolved by the Principal Investigator (PI). In this circumstance, the PI reviewed the chart, made a decision that resolved the discrepancy(s), and then met with both reviewers to ensure that there was consensus on this determination. This chart review was approved by the University of Cincinnati Institutional Review Board.
Outcome definitions
Complete control of CU was defined as no hives for at least 30 days on medications and at least one of the following criteria: the first visit when no new medications were added (ie, a step-up in therapy was not required) or the first visit where a step-down in medications was made. Partial control of CU was defined as a decrease in the frequency and/or severity of the urticaria episodes after the initiation or change of medication(s). Remission of CU was defined as no hives for 3 months off all medications.
Statistical analysis
Demographic and laboratory characteristics were analyzed using descriptive statistics. Univariate analyses were performed by Pearson χ-square tests or unpaired Student t-tests to evaluate associations or differences, respectively, between patient baseline and health characteristics and urticaria control status (complete control vs not controlled). Patient characteristics were dichotomized before analysis for ease of interpretation. Patients with partial control of CU were placed in the not controlled category. All factors significant in the univariate analysis at α = 0.05 were included in an initial multiple logistic regression model. From this model, patient characteristics were identified by backward selection of P-values. The characteristic judged to have the smallest influence, as defined by the P-value, was removed and the process repeated on the remaining characteristics, until a final model was determined that could not be improved in a single-step fashion. The P-value threshold for the remaining predictors was <.05 or was determined by minimization of the Akaike information criterion. Statistical analyses were performed using SAS version 9.3 (SAS Institute, Cary, NC).
RESULTS
Data extracted from the charts of a total of 221 patients with CU were included in this study. An extra chart was inadvertently reviewed because it was not clear to the 2 reviewers that the target number of 220 chart reviews had been achieved and therefore included in the final analysis. There were discrepancies between the reviewers in 10 of the 22 charts (10% of charts) that were cross-reviewed related to interpretation of outcome definitions that were reconciled by the PI after the review of the charts and discussion with the reviewers. In all of these cases, to avoid any bias or inconsistencies, the most stringent definitions of outcomes were applied.
The average number of clinic visits per patient was 11.9 ± 9.4. Table I summarizes the basic demographics of the study cohort. A significant majority of the subjects were female (n = 165 [74.7%]), and their mean age at the time of the first clinic visit was 41.1 ± 11.9 years, which was significantly less than that of male patients. The majority of subjects were Caucasian (n = 157 [83.5%]) who had suffered from CU for an average of 3.2 ± 6.9 years before their first clinic visit. Of the 221 patients included in this study, 140 (63.4%) achieved complete control during any point in time of their longitudinal clinical assessment period. Of these, 88 (62.9%) remained completely controlled at their last clinic visit. Only 27 (12.2%) of the 221 patients met our outcome definition of disease remission that was defined as no hives off medications for 3 months. The percentage of females and males who met the outcome definition of disease control was not significantly different (72.1% vs 69.6%, respectively; P > .05). However, there were significantly more females than males with CU who had associated dermatographia (47.1% vs 14.7%, P < .01) and other physical urticarias (not including dermatographia) (70.1% vs 54.6%, P = .05). More males reported a history of smoking (39.6% vs 23.4%, P = .02), but there were no gender differences between the personal history of thyroid disease, autoimmune disease, or the family history of CU.
TABLE I.
Demographics of the study cohort of adult patients with chronic urticaria
| Characteristics | Total (%)* | Female (%)* | Male (%)* | P-value |
|---|---|---|---|---|
| No. of patients† | 221 | 165 (74.7) | 56 (25.3) | <.01 |
| Mean age at the first clinic visit (y) (mean ± SD) | 42.8 ± 12.2 | 41.1 ± 11.9 | 47.7 ± 11.9 | <.01 |
| Caucasian race | 157 (83.5) | 115 (82.1) | 42 (87.5) | NS |
| Duration of CU before the first clinic visit (y) (mean ± SD) | 3.2 ± 6.9 | 3.1 ± 6.6 | 3.6 ± 7.7 | NS |
| Achieved control during any clinic visit (F: n = 165; M: n = 56) | 140 (63.4) | 101 (72.1) | 39 (69.6) | NS |
| Episodes lasting >48 h (F: n = 89; M: n = 36) | 70 (56) | 50 (56.2) | 20 (55.6) | NS |
| Associated angioedema (F: n = 149; M: n = 50) | 152 (76.4) | 111 (74.5) | 41 (82) | NS |
| Associated dermatographia (F: n = 119; M: n = 34) | 61 (39.9) | 56 (47.1) | 5 (14.7) | <.01 |
| Associated physical urticaria(s)‡ (F: n = 89; M: n = 34) | 120 (66.3) | 96 (70.1) | 24 (54.6) | .05 |
| History of smoking (F: n = 158; M: n = 53) | 58 (27.5) | 37 (23.4) | 21 (39.6) | .02 |
| History of thyroid disease§ (F: n = 158; M: n = 55) | 32 (15) | 27 (17.1) | 5 (9.1) | NS |
| History of autoimmune disease§ (F: n = 155; M: n = 54) | 25 (12) | 16 (10.3) | 9 (16.7) | NS |
| FHx of CU (F: n = 145; M: n = 49) | 32 (16.5) | 25 (17.2) | 7 (14.3) | NS |
| FHx of autoimmune disease (F: n = 138; M: n = 48) | 42 (22.6) | 33 (23.9) | 9 (18.8) | NS |
FHx, family history; NS, not significant at P = .05.
Percent represents total of the column or row.
Numbers represent the total number of male and female patients who reported this information.
Other than dermatographia included cold (n = 12), delayed pressure (n = 2), pressure (n = 60), exercise (n = 33), cholinergic (n = 36), solar (n = 6), vibratory (n = 1), and aquagenic (n = 6).
Based on the patient report; autoimmune disease included the report of endocrine, rheumatologic or hematologic disorders.
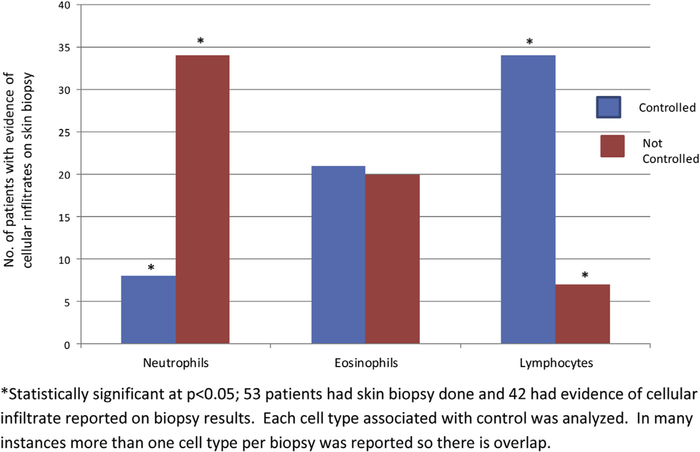
Table II summarizes the laboratory data performed for this patient population. Laboratory tests were ordered either by the referring physician or by the allergist for 191 of the 221 patients. Of note, there was no significant difference in the presence of thyroid auto-antibodies (antithyroperoxidase and/or anti-microsomal antibodies) between males and females. Fifty-three (24%) patients in this cohort had a skin biopsy; for 42 (79.2%) of these, there was an available skin histopathology report that specified the type of cellular infiltrates present (see Figure 1). Thirty-four patients with a predominance of neutrophils on skin biopsy had poorly controlled CU, whereas an additional 34 of the 42 patients with a lymphocyte predominant infiltrate had significantly better CU control. Twenty-one patients with predominant eosinophils were controlled, whereas 20 patients with similar skin histopathology were not.
TABLE II.
Laboratory data available for the study cohort
| Characteristics | Total (%)* | Female (%)* | Male (%)* | P-value |
|---|---|---|---|---|
| Labs ordered (F: n = 165; M: n = 56) | 191 (86.4) | 144 (87.3) | 47 (83.9) | NS |
| Erythrocyte sedimentation rate > 20 (F: n = 133; M: n = 48) | 48 (26.5) | 39 (29.3) | 9 (18.8) | NS |
| Presence of antithyroperoxidase and/or antimicrosomal antibodies (F: n = 89; M: n = 31) | 34 (28.3) | 25 (28.1) | 9 (29) | NS |
| Biopsy performed (F: n = 165; M: n = 56) | 53 (24) | 42 (25.5) | 11 (19.6) | NS |
NS, not significant.
Percent represents total of the column or row.
FIGURE 1.
Skin histopathology associated with chronic urticaria (CU) and disease control.
The average time to disease control for the study population was 1.4 ± 2.7 years (6.1 ± 4.8 clinic visits). On average, 2.1 ± 1.2 (min:max = 1:6) classes of medications were required to achieve complete control. Table III summarizes the univariate analysis of the factors associated with better CU control. There was no significant difference between the age, gender, duration of disease, smoking history, history of associated angioedema, autoimmune, infectious, malignant or thyroid disease, presence of thyroid auto-antibodies, or family history of CU or other autoimmune diseases between the completely controlled and uncontrolled groups. Among patients classified as controlled or not controlled, the presence of dermatographia (odds ratio [OR] = 1.85; 95% CI [1.3–2.7]) or other physical urticarias (excluding dermatographia) (OR = 1.51 [1–2.4]) was significantly associated with poorer CU control.
TABLE III.
Unadjusted OR for the association between complete control of chronic urticaria and other covariates
| Characteristics* | Control (%)† | Not controlled (%)† | P-value | OR (95% CI) |
|---|---|---|---|---|
| No. of patients (n = 221) | 140 (63.3) | 81 (36.7) | - | - |
| Age (y) (mean ± SD) | 44.3 ± 14.2 | 40.9 ± 11.7 | NS | NS |
| Duration of disease (y) (mean ± SD) | 3.8 ± 5.7 | 4.9 ± 4.4 | NS | NS |
| Associated dermatographia (control: n = 91; not controlled: n = 61) | 26 (28.6) | 33 (54.1) | <.01 | 1.85 (1.3–2.7) |
| Associated physical urticaria(s)‡ (control: n = 114; not controlled: n = 68) | 70 (61.4) | 51 (75) | .05 | 1.51 (1–2.4) |
NS, not significant at P ≤ .05; OR, odds ratio.
Gender, smoking, associated angioedema, history of thyroid or autoimmune disease, presence of thyroid auto-antibodies, family history of CU or autoimmune disease were not significantly associated with disease control.
Numbers represent the number of patients controlled or not controlled who had information related to dermatographia or physical urticaria included cold (n = 12), delayed pressure (n = 2), pressure (n = 60), exercise (n = 33), cholinergic (n = 36), solar (n = 6), vibratory (n = 1), and aquagenic (n = 6).
Numbers of patients who had dermatographia or physical urticaria and who were actually completely controlled or not controlled and percent represents the total of the column or row.
Other than dermatographia.
Table IV summarizes the medication classes associated with complete control of CU during any point in time of their longitudinal clinical assessment period. Overall, the best overall control of CU using step 1–3 therapies was more likely to be achieved with combination therapy using a second-generation H1-receptor antagonist (including either cetirizine 10 mg, fexofenadine 180 mg, loratadine 10 mg, levocetirizine 5 mg, or desloratadine 5 mg) in combination with a leukotriene receptor antagonist (LTRA) (n = 16/72 patients controlled, 22.2%), followed by the use of a second-generation H1-receptor antagonist in combination with a H2-receptor antagonist (n = 26/188, 13.8%). There was no difference in the percentage of patients who achieved complete control of CU with 2 classes of medications compared with those treated with 3 different classes of medications (Table IV). The addition of either an anti-inflammatory agent (ie, hydroxychloroquine, sulfasalazine, colchicine), an immunosuppressant agent (ie, cyclosporine), or biologic (ie, Omalizumab) was associated with an increased percentage of patients who achieved complete control of CU. As shown in Table IV, 33% of patients achieved complete disease control when either cyclosporine or omalizumab was added to the existing medication regimen, followed by 25%, 17.8%, and 14.6% of patients who achieved complete control with the addition of sulfasalazine, colchicine, and hydroxychloroquine, respectively, to their baseline medication regimen.
TABLE IV.
Medications on which complete control of chronic urticaria was achieved at any clinic visit
| Step 1–3 therapies per guidelines | No. of patients with complete control (no. of patients taking the medication at any clinic visit, %) (n = 85) |
| 1st gen H1-antagonist + 2nd gen H1-antagonist | 10 (147, 6.8%) |
| 1st gen H1-antagonist + H2-antagonists | 4 (107, 3.7%) |
| 2nd gen H1-antagonist + H2-antagonists | 26 (188, 13.8%) |
| LTRA | 18 (137, 13.1%) |
| 1st gen H1-antagonist + LTRA | 1 (72, 1.4%) |
| 2nd gen H1-antagonist + LTRA | 16 (72, 22.2%) |
| 1st gen H1-antagonist + 2nd gen H1-antagonist + LTRA | 1 (80, 1.3%) |
| 1st gen H1-antagonist + doxepin | 3 (51, 5.9%) |
| 2nd gen H1-antagonist + doxepin | 3 (73, 4.1%) |
| 1st gen H1-antagonist + 2nd gen H1-antagonist + doxepin | 3 (70, 4.3%) |
| 1st gen H1-antagonist + 2nd gen H1-antagonist + doxepin + LTRA | - |
| Step 4 therapies per guidelines* | No. of patients with complete control (no. of patients taking the medication at any clinic visit, %) (n = 67†) |
| Prednisone‡ | 33 (178, 18.5%) |
| Cyclosporine 200 mg a day | 10 (30, 33.3%) |
| Hydroxychloroquine 200 mg twice a day | 31 (89, 14.6%) |
| Sulfasalazine 500 mg twice a day | 4 (16, 25%) |
| Dapsone 25–50 mg a day | 5 (23, 21.7%) |
| Colchicine 0.6 mg twice a day | 16 (90, 17.8%) |
| Omalizumab 300 mg once a month | 1 (3, 33.3%) |
| Tacrolimus 1000 mg twice a day | 0 (2, 0%) |
gen, generation; LTRA, leukotriene receptor antagonist.
1st gen H1-antagonists include diphenhydramine or hydroxyzine 25–50 mg orally up to 4 times daily; 2nd gen H1-antagonists include cetirizine 10 mg, fexofenadine 180 mg, loratadine 10 mg, levocetirizine 5 mg, or desloratadine 5 mg once daily; and H2-antagonists include ranitidine 150 mg orally twice daily.
Used in combination with other therapies listed on the top half of the table; patients were maximized on step 1–3 therapies before treatment with step 4 therapies.
Excluding prednisone (12 patients controlled on a particular step 4 therapy were switched to a different step 4 therapy due to side effects or cost and continued to be controlled on the new therapy).
Prednisone was used in conjunction with any of the step 4 therapies listed in the table. No patients were on 2 step 4 therapies at the same time (except if prednisone was added for a flare).
Table V summarizes the results of the multivariate analysis for the association of complete CU control with specific medication classes recommended in steps 1 through 3 of the JTFPP guidelines and other significant covariates. Patients taking a second-generation H1-antagonist who were dermatographic (aOR = 16.5 [95% CI: 9.1–29.7]), had physical urticaria (excluding dermatographism) (aOR = 1.4 [1–1.9]), or thyroid auto-antibodies (aOR = 3.4 [2.3–4.9]) exhibited better CU control. Similarly, those patients with CU associated with dermatographia (aOR = 16.9 [8.2–34.7]) or the presence of thyroid auto-antibodies (aOR = 2.9 [1.9–4.4]) taking a first-generation H1-antagonist were more likely to be completely controlled. Patients with an erythrocyte sedimentation rate > 20 had poorer control of their hives while taking a first-generation H1-antagonist (aOR = 0.3 [0.18–0.54]) or an LTRA (aOR = 0.2 [0.12–0.32]). Caucasians compared with non-Caucasians were more likely to have significantly better control of hives when treated with an LTRA (aOR = 4.3 [2.4–7.5]) or doxepin (aOR = 6.8 [5.9–7.9]). The use of an LTRA was also associated with better hive control in patients with dermatographia compared with those without (aOR = 3.5 [1.9–6.2]). However, doxepin was unlikely to achieve control in those patients with physical urticarias other than dermatographia (aOR = 0.04 [0.02–0.07]). Sex, age, and duration of CU were not significant factors predictive of CU control for patients taking any particular class of medications listed in Table V.
TABLE V.
Adjusted odds ratios and 95% CI for the association between medication-specific complete control of chronic urticaria in relation to the use of H1-, H2-receptor antagonists or leukotriene receptor antagonist
| Covariates | Second-generation antihistamine* | First-generation antihistamine† | LTRA | H2 antagonist‡ | Doxepin |
|---|---|---|---|---|---|
| Race: Caucasian | - | - | 4.3 [2.4–7.5] | - | 6.8 [5.9–7.9] |
| Age (y, median) | |||||
| <43 y | |||||
| ≥43 y | NA | - | NA | - | NA |
| Duration of CU before the 1st clinic visit | NA | NA | NA | NA | NA |
| Associated dermatographia | 16.5 [9.1–29.7] | 16.9 [8.2–34.7] | 3.5 [1.9–6.2] | - | - |
| Associated physical urticarias§ | 1.4 [1–1.9] | - | - | 6.1 [3.1–12] | 0.04 [0.02–0.07] |
| Erythrocyte sedimentation rate > 20 | - | 0.3 [0.18–0.54] | 0.2 [0.12–0.32] | - | - |
| Presence of antithyroperoxidase and/or antimicrosomal antibodies | 3.4 [2.3–4.9] | 2.9 [1.9–4.4] | - | 4.2 [1.6–11] | - |
CU, chronic urticaria; LTRA, Leukotriene receptor antagonist; NA, no association (ie, OR = 1); (-) not significant at P = .1.
Cetirizine 10 mg, fexofenadine 180 mg, loratadine 10 mg, levocetirizine 5 mg, or desloratadine 5 mg once daily.
Diphenhydramine or hydroxyzine 25–50 mg orally up to 4 times daily.
Ranitidine 150 mg orally twice daily.
Excluding dermatographia included cold (n = 12), delayed pressure (n = 2), pressure (n = 60), exercise (n = 33), cholinergic (n = 36), solar (n = 6), vibratory (n = 1), and aquagenic (n = 6). Associated angioedema, history of smoking, personal history of thyroid or autoimmune diseases, or family history of CU or autoimmune disease, and specific cellular infiltrates on skin histopathology were all included in the multivariate models but not significantly associated with better disease control with any of the medication classes at P = .1.
Table VI summarizes the results of the multivariate analysis for complete control of CU for patients taking step 4 therapy (anti-inflammatory, immunosuppressant, or biologic) and other factors associated with predicting CU control for a specific drug. The use of cyclosporine was associated with significantly better complete control of CU in Caucasians (aOR=5.9 [2.1–16.7]), patients >43 years of age (aOR = 1.3 [1.2–1.4]), and those with hive episodes lasting > 48 hours (aOR = 11.5 [3.6–37]). The presence of physical urticarias (excluding dermatographia) was associated with a significantly poor response to hydroxychloroquine (aOR = 0.3 [0.16–0.49]) treatment. A history of CU occurring for more than 4 months before the first clinic visit and the presence of thyroid auto-antibodies was associated with a poor response to sulfasalazine (aOR = 0.9 [0.8–0.9] and aOR = 0.01 [0.002–0.4], respectively). In contrast, use of dapsone in males (aOR = 3.4 [2.2–5.4]) and those patients with a predominant neutrophilic infiltrate on skin biopsy (aOR = 5.4 [3.2–9.1]) was associated with better CU control. Colchicine was associated with better CU control in Caucasian patients (aOR = 21.1 [6.3–71.2]) with physical urticarias (aOR = 32.6 [12.7–83.2]), but poorer control in those patients with thyroid auto-antibodies (aOR = 0.1 [0.1–0.4]). The sample sizes were too small to analyze for patients controlled on tacrolimus or omalizumab, the latter of which had not been FDA approved at the time of this analysis, and therefore it is not possible to make any reliable statements about their efficacy.
TABLE VI.
Adjusted odds ratios and 95% CI for the association between complete control of chronic urticaria and other covariates in relation to use of step 4 therapy (anti-inflammatory, immunosuppressants or biologics)
| Covariates | Cyclosporine | HCQ | Sulfasalazine | Dapsone | Prednisone | Colchicine |
|---|---|---|---|---|---|---|
| Sex: Male | - | - | - | 3.4 [2.2–5.4] | - | - |
| Race:Caucasian | 5.9 [2.1–16.7] | - | - | - | - | 21.1 [6.3–71.2] |
| Age (y, median) | ||||||
| <43 y | 1.3 [1.2–1.4] | - | - | NA | 1.2 [1–1.5] | - |
| ≥43 y | ||||||
| Duration of CU before the first clinic visit >4 mo (median) | - | NA | 0.9 [0.8–0.9] | - | - | - |
| Episodes lasting >48 h | 11.5 [3.6–37] | - | - | - | - | - |
| Associated dermatographia | - | - | - | - | - | - |
| Associated physical urticarias* | - | 0.3 [0.16–0.49] | - | - | 32.6 [12.7–83.2] | |
| Presence of antithyroperoxidase and/or antimicrosomal antibodies | - | - | 0.01 [0.002–0.04] | - | - | 0.1 [0.1–0.4] |
| Predominance of neutrophils on histology | - | - | - | 5.4 [3.2–9.1] | - | - |
CU, chronic urticaria; HCQ, hydroxychloroquine; NA, no association (ie, OR = 1); (-) not significant at P = .1.
Excluding dermatographia included cold (n = 12), delayed pressure (n = 2), pressure (n = 60), exercise (n = 33), cholinergic (n = 36), solar (n = 6), vibratory (n = 1), and aquagenic (n = 6). Associated angioedema, history of smoking, personal history of thyroid or autoimmune diseases, or family history of CU or autoimmune disease, and cellular infiltrates on skin histopathology of lymphocytes were all included in the models but not significantly associated with better disease control with any drug at = 0.1.
DISCUSSION
The current JTFPP for CU recommends an algorithmic treatment approach beginning with a second-generation H1-antagonist followed by either increasing the H1-antagonist dose 2–4 times the daily recommended dose and/or adding an H2-antagonist or an leukotriene modifying agent if still not controlled.1 Step 3 therapy recommends the use of a more sedating H1-antagonist (ie, hydroxyzine) or a combination of sedating antihistamines (ie, doxepin).1 Finally, if control is not established after step 1 through 3 treatment, then advancement to step 4 therapy is recommended, which includes using either an anti-inflammatory, immunosuppressive, or biologic agent. Short courses of prednisone may be needed during this process to control the hives until an effective treatment regimen can be established.1 However, there is significant variation in the long-term clinical course of different types of CU (ie, idiopathic vs physical), and an individual patient’s response to treatment may also vary significantly.2,17 The current step care treatment approach algorithm would be more useful if there were specific clinical characteristics of patients with CU that could guide physicians as to which therapies were most effective in a spectrum of scenarios. To date, there is still a paucity of clinical “phenotypic” information that can be used in this capacity.15,16 This is the first study that attempts to report potential patient-specific clinical characteristics associated with a favorable or poor response to specific classes of medications used for CU treatment to help fill this current gap.
In our patient cohort, the majority achieved complete control (n = 140/221). In the group of controlled patients, combination therapy with a second-generation H1-antagonist and an LTRA was associated with the highest rate of control compared with all other medication combination options suggested for step 1 or 2 treatments. Although CU associated with physical urticaria was generally more difficult to control, use of a first- or second-generation H1-antagonist or an LTRA was associated with significantly better CU control in patients who had dermatographia (Table V). In most cases, patients had uncontrolled hives for months or years before being seen, which would have affected the time to disease control (Table I). This may be partially explained by the fact that many patients were significantly improved and more comfortable with step 2 therapy over time even though they did not meet the criteria for complete remission. Oftentimes these patients were less interested in advancing to more aggressive therapies (step 3 or 4) if they felt the burden of illness was less than the toxicity of treatment, which likely affected the time to disease control. The finding that patients with CU with dermatographia had a 16 times higher odds of a favorable response to a first- or second-generation H1-antagonist is consistent with previous findings by Kozel et al., who reported that patients with physical urticarias other than pressure, cold, solar, and aquagenic may respond better to an H1-antagonist.10 In addition, a second-generation H1-antagonist was associated with marginally better control of physical urticarias excluding dermatographia (aOR = 1.4) in this study, which is consistent with what has been reported in the literature (Table V).17 Although reports of successful treatment of cold and delayed pressure urticaria with an LTRA have been published, there is little information to support our findings of the association between treatment of dermatographia with an LTRA (aOR = 3.5) and control.18,19 Although leukotrienes are known to cause burning when injected directly into the skin, LTRAs are known to inhibit vascular responses by these mediators, and therefore it is very plausible that they could play a role in attenuating dermatographia in conjunction with H1-antagonists.20 Caucasians were 4–7 times more likely to have a favorable response to an LTRA or doxepin. Although published reports also confirm a favorable response to an LTRA in adult Caucasian CU populations,21–23 there is a scarcity of data supporting our findings for doxepin. This reflects the lack of scientific evidence available to support step 3 treatment that advocates the use of more sedating first-generation or combination antihistamines.
Thyroid auto-antibodies were found to be present in 28% of patients in this cohort, which is similar to other reports (Table II).24,25 These patients with thyroid auto-antibodies had a 2.9–3.4 times higher odds of achieving control of their CU with a first- or second-generation H1-antagonist (Table V). Najib et al., who studied patients with CU with thyroid auto-antibodies, previously reported that 36% of their patients were controlled with a first- or second-generation H1-antagonist and only a small percentage required the addition of prednisone or cyclosporine for disease control.15
As recommended by the JTFPP urticaria guidelines, when step 1–3 therapy does not control the disease, step 4 therapy using either an anti-inflammatory, immunosuppressive, or biologic agent is recommended.1 The addition of a step 4 anti-inflammatory or immunosuppressive agent was associated with even better overall rates of control. In our study, 33% of patients achieved CU control with the addition of cyclosporine (Table IV). A previous randomized double-blinded placebo-controlled trial demonstrated that although 60% of patients refractory to antihistamines responded to cyclosporine after 4 weeks of therapy, only 27% maintained good response at the end of the 20-week study period.26 Similar response rates of 28% to 39% have been reported by other investigators for corticosteroid-dependent patients with CU treated with cyclosporine.27 A report by Di Gioacchino et al. treated corticosteroid-dependent patients with CU with autologous serum antibodies using cyclosporine and found that after 16 weeks of therapy 36% achieved remission.28 As noted, therapy with cyclosporine was associated with significantly better control in Caucasian patients (Table VI). Although previously mentioned studies have reported treatment benefit with cyclosporine in Caucasians, no studies comparing response rates between other races is available.26–28 Hollander et al. found no significant association between race and response rate to cyclosporine, but did note that a shorter duration of urticaria (mean of 55.2 weeks vs 259.63 weeks) and a positive CU index were associated with a more favorable response29; however, other studies found no difference in cyclosporine response in the presence or absence of an FcER1 αsubunit antibody.30
A significant number of patients with CU were also controlled on sulfasalazine, dapsone and hydroxychloroquine (15% to 25%; Table IV). Similarly for step 1 and 2 therapies and cyclosporine, Caucasian patients were significantly more likely to have better CU control when treated with colchicine (Table VI). Interestingly, use of colchicine was associated with a 32 times better odds of control in patients with all forms of physical urticaria (excluding dermatographia) compared with any other step 4 treatment medication (Table VI). This is in contrast to a double-blinded study that reported colchicine was ineffective in the treatment of delayed pressure urticaria.31 Although the number of patients in our cohort with delayed pressure urticaria as their primary physical trigger was very small, this finding requires confirmation in a larger prospective cohort.
The presence of dermatographia, other physical urticarias, and a neutrophil predominant infiltrate on skin biopsy were markers associated with more difficult to control CU using either step 1–3 or step 4 therapy. This finding for those patients with CU with physical hives could be explained by the fact that they may have more recalcitrant disease due to their inability to avoid physical triggers. Other investigators have also reported a poorer treatment prognosis of CU associated with physical urticaria that supports our findings.32,33 Both neutrophils and eosinophils are commonly found on skin histopathology of CU biopsies.34 In our cohort, patients with a neutrophil predominant infiltrate on skin biopsy overall had significantly poor disease control. These patients did not meet criteria for neutrophilic urticarial dermatosis based on their clinical signs, symptoms and histopathology,35 nor did they have associated rheumatologic diseases. However, those with a neutrophilic infiltrate responded best when treated with dapsone, which provided 5 times greater odds of control compared with any other step 4 agent in this group. These findings are supported by previous case series and reports.36
Although the addition of step 4 therapy provided better CU control compared with step 1–2, 18.5% still required short courses of prednisone due to disease relapse. A recent large study of 750 patients with CU by Asero et al. revealed that a single short course of prednisone induced remission in nearly 50% of the patients.37 Although the effectiveness of corticosteroids in antihistamine-resistant CU is widely accepted, relapse of CU is common during the tapering phase, and systemic side effects related to prolonged use of oral corticosteroids are well documented. Therefore, the long-term use of prednisone is discouraged.1 The inability to achieve sustained remission of hives in a significant percentage of our CU patients using currently available step 4 agents emphasizes an unmet need for novel therapies to treat refractory disease.
The limitations of this study are the retrospective chart review study design, patient recruitment was from a single tertiary referral center, and there were no control groups with placebo to compare the therapeutic responses to various drugs or drug combinations. Because this study was conducted at a single treatment center and patients referred were often more complex and less responsive to conventional therapies, the findings of this study may not be generalizable to other populations with CU. However, this limitation was somewhat mitigated as 5 independent practicing allergists at 3 different clinic locations were involved in the management of this large demographically diverse population with CU. This analysis may have been hindered by not performing statistical analysis on the various intraclass medication dosing iterations used across this population. Therefore, it was not possible to determine individual drug contributions or specific dosing for achieving control with each combination of drugs found to be effective. It is possible that one drug could have been contributing more than another, but in all cases, patients were systemically advanced to additional agents only after previous combinations were deemed ineffective thus making this issue less problematic. Another limitation of this study was the lack of a control group to compare treatment response. This would have been ideal but was not possible for this type of study design. Although it is possible for patients to spontaneously go into remission regardless of the treatment being administered, we believe that this is less likely as patients were seen frequently until it was established that their hives were well controlled. However, future studies addressing phenotypic characteristics predicting treatment response should be multi-centered, placebo controlled and implement consensus treatment algorithms taking into account differences in intraclass agents and dosing regimens.1
In conclusion, specific clinical characteristics were identified based on medication-specific control of disease for some subpopulations of patients with CU. Our findings confirm that the best overall control of CU using step 1–3 therapies is more likely to be achieved with the use of a second-generation H1-antagonist plus an LTRA, and with cyclosporine or omalizumab (although a small number of patients were treated with this agent in this study) when the addition of a step 4 therapy is required. Although CU associated with physical urticaria was in general more difficult to control, first- or second-generation H1-antagonists were associated with the highest odds of complete CU control in patients with dermatographia, whereas the addition of colchicine to step 1–3 therapy (Table IV) was associated with the best control for all other types of physical urticarias. Although the majority of the patients in this cohort achieved control and in some cases complete remission, a substantial number of patients remained symptomatic despite aggressive management with step 4 therapies available at the time of this analysis emphasizing a role for additional novel therapeutic agents in the management of CU.
What is already known about this topic?
Many patients with chronic urticaria (CU) respond to H1-antagonists, H2-antagonists, and/or leukotriene-modifying agents, but others require alternative agents. Limited information is available regarding patient-specific characteristics associated with response to different combinations of treatment.
What does this article add to our knowledge?
This article provides useful information regarding treatment response to a spectrum of medications and disease-specific characteristics associated with poor, partial, or complete CU control.
How does this study impact current management guidelines?
Results are discussed in the context of the urticaria Joint Task Force Practice Parameter. Although a significant number of patients can be controlled using step 1–4 therapies, there is still an unmet need for novel therapies to control hives.
Acknowledgments
This research was supported by Novartis and Genetech Pharmaceuticals grant CIGE025A-US42T.
Conflicts of interest: J. A. Bernstein has received research support from Novartis, National Institute of Allergy and Infectious Disease (U44 collaborative grant), and Meda; has received consultancy fees from Dyax, Shire/Viropharma, CSL Behring, Salix, and Genentech/Novartis; is on the Boards for American Academy of Allergy, Asthma, & Immunology Hereditary Angioedema Association, and Allergists for Israel; and is employed by the Bernstein Allergy Group/Clinical Research Group and the University of Cincinnati. The rest of the authors declare that they have no relevant conflicts.
Abbreviations used
- CU
Chronic urticaria
- JTFPP
Joint Task Force Practice Parameter
- LTRA
Leukotriene receptor antagonist
- OR
Odds ratio
REFERENCES
- 1.Bernstein JA, Lang DM, Khan DA, Craig T, Dreyfus D, Hsieh F, et al. The diagnosis and management of acute and chronic urticaria: 2014 update. J Allergy Clin Immunol 2014;133:1270–7. [DOI] [PubMed] [Google Scholar]
- 2.Greaves M Chronic urticaria. J Allergy Clinical Immunol 2000;105:664–72. [DOI] [PubMed] [Google Scholar]
- 3.Grob JJ, Gaudy-Marqueste C. Urticaria and quality of life. Clin Rev Allergy Immunol 2006;30:47–51. [DOI] [PubMed] [Google Scholar]
- 4.Delong LK, Culler SD, Saini SS, Beck LA, Chen SC. Annual direct and indirect health care costs of chronic idiopathic uritcaria: a cost analysis of 50 non-immunosuppressed patients. Arch Dermatol 2008;144:35–9. [DOI] [PubMed] [Google Scholar]
- 5.Gaig P, Olona M, Muñoz Lejarazu D, Caballero MT, Domínguez FJ, Echechipia S, et al. Epidemiology of urticaria in Spain. J Investig Allergol Clin Immunol 2004;14:214–20. [PubMed] [Google Scholar]
- 6.Jiamton S, Swad-Ampiraks P, Kulthanan K, Suthipinittharm P. Urticaria and angioedema in Siriraj medical students. J Med Assoc Thai 2003;86:74–81. [PubMed] [Google Scholar]
- 7.Vazquez Nava F, Almeida Arvizu VM, Sánchez Nuncio HR, Villanueva Carreto Mde L, Guidos Fogelbach GA. Prevalence and potential triggering factors of chronic urticaria and angioedema in an urban area of northeastern Mexico. Rev Alerg Mex 2004;51:181–8. [PubMed] [Google Scholar]
- 8.Bickers DR, Lim HW, Margolis D, Weinstock MA, Goodman C, Faulkner E, et al. The burden of skin diseases: 2004 a joint project of the American Academy of Dermatology Association and the Society for Investigative Dermatology. J Am Acad Dermatol 2006;55:490–500. [DOI] [PubMed] [Google Scholar]
- 9.Marrouche N, Grattan C. Update and insights into treatment options for chronic spontaneous urticaria. Expert Rev Clin Immunol 2014;10:397–403. [DOI] [PubMed] [Google Scholar]
- 10.Kozel MM, Sabroe RA. Chronic urticaria: aetiology, management and current and future treatment options. Drugs 2004;64:2515–36. [DOI] [PubMed] [Google Scholar]
- 11.Lang DM. Evidence-based diagnosis and treatment of chronic urticaria/angioedema. Allergy Asthma Proc 2014;35:10–6. [DOI] [PubMed] [Google Scholar]
- 12.Stewart GE 2nd. Histopathology of chronic urticarial. Clin Rev Allergy Immunol 2002;23:195–200. [DOI] [PubMed] [Google Scholar]
- 13.Eckman JA, Hamilton RG, Gober LM, Sterba PM, Saini SS. Basophil phenotypes in chronic idiopathic urticarial in relation to disease activity and autoantibodies. J Invst Dermatol 2008;128:1956–63. [DOI] [PubMed] [Google Scholar]
- 14.Vonakis BM, Saini SS. New concepts in chronic urticarial. Curr Opin Immunol 2008;20:709–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Najib U, Bajwa ZH, Ostro MG, Sheikh J. A retrospective review of clinical presentation, thyroid autoimmunity, laboratory characteristics, and therapies used in patients with chronic idiopathic urticaria. Ann Allergy Asthma Immunol 2009;103:496–501. [DOI] [PubMed] [Google Scholar]
- 16.Caproni M, Volpi W, Giomi B, Cardinali C, Antiga E, Melani L, et al. Chronic idiopathic and chronic autoimmune urticaria: clinical and immunopathological features of 68 subjects. Acta Derm Venereol 2004;84:288–90. [DOI] [PubMed] [Google Scholar]
- 17.Kavosh E, Khan DA. Second-generation H1-antihistamine in chronic urticaria: a evidence-based review. Am J Clin Dermatol 2011;12:361–76. [DOI] [PubMed] [Google Scholar]
- 18.Hani N, Hartmann K, Casper C, Peters T, Schneider LA, Hunzelmann N, et al. Improvement of cold urticaria by treatment with the leukotriene receptor antagonist montelukast. Acta Derm Venereol 2000;80:229. [DOI] [PubMed] [Google Scholar]
- 19.Berkun Y, Shalit M. Successful treatment of delayed pressure urticaria with montelukast. Allergy 2000;55:203–4. [DOI] [PubMed] [Google Scholar]
- 20.Bernstein JA, Greenberger PA, Patterson R, Glass M, Krell R, Thyrum PT. The effect of the oral leukotriene antagonist, ICI 204,219, on leukotriene D4 and histamine-induced cutaneous vascular reactions in man. J Allergy Clin Immunol 1991;87(Pt 1):93–8. [DOI] [PubMed] [Google Scholar]
- 21.Pacor ML, Lorenzo DI, Corrocher R. Efficacy of leukotriene receptor antagonist in chronic urticaria. A double-blind, placebo-controlled comparison of treatment with montelukast and cetirizine in patients with chronic urticaria with intolerance to food additive and/or acetylsalicylic acid. Clin Exp Allergy 2001;31: 1607–14. [DOI] [PubMed] [Google Scholar]
- 22.Lorenzo GD, D’Alcamo A, Rizzo M, Leto-Barone MS, Bianco CL, Ditta V, et al. Leukotriene receptor antagonist in monotherapy or in combination with antihistamines in the treatment of chronic urticaria: a systematic review. J Asthma Allergy 2009;2:9–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nettis E, Colanardi MC, Paradiso MT, Ferrannini A. Desloratadine in combination with montelukast in the treatment of chronic urticaria: a randomized, double-blind, placebo-controlled study. Clin Exp Allergy 2004;34:1401–7. [DOI] [PubMed] [Google Scholar]
- 24.Doutre MS. Chronic urticaria and thyroid auto-immunity. Clin Rev Allergy Immunol 2006;30:31–7. [DOI] [PubMed] [Google Scholar]
- 25.Wan KS, Wu CS. The essential role of anti-thyroid antibodies in chronic idiopathic urticaria. Endocr Res 2013;38:85–8. [DOI] [PubMed] [Google Scholar]
- 26.Grattan CE, O’Donnell B, Francis D, Niimi N, Barlow RJ, Seed PT, et al. Randomized double-blind study of cyclosporin in chronic “idiopathic” urticaria. Br J Dermatol 2000;143:365–72. [DOI] [PubMed] [Google Scholar]
- 27.Di Leo E, Nettis E, Aloia AM, Moschetta M, Carbonara M, Dammacco F, et al. Cyclosporine-A efficacy in chronic idiopathic urticaria. Int J Immunopathol Pharmacol 2011;24:195–200. [DOI] [PubMed] [Google Scholar]
- 28.Di Gioacchino M, Di Stefano F, Cavallucci E, Verna N, Ramondo S, Paolini F, et al. Treatment of chronic idiopathic urticaria and positive autologous serum skin test with cyclosporine: clinical and immunological evaluation. Allergy Asthma Proc 2003;24:285–90. [PubMed] [Google Scholar]
- 29.Hollander SM, Joo SS, Wender HJ. Factors that predict the success of cyclosporine treatment for chronic urticaria. Ann Allergy Asthma Immunol 2011; 107:523–8. [DOI] [PubMed] [Google Scholar]
- 30.Toubi E, Blant A, Kessel A, Golan TD. Low-dose cyclosporin A in the treatment of severe chronic idiopathic urticaria. Allergy 1997;52:312–6. [DOI] [PubMed] [Google Scholar]
- 31.Lawlor F, Black AK, Ward AM, Morris R, Greaves MW. Delayed pressure urticaria, objective evaluation of a variable disease using a dermographometer and assessment of treatment using colchicine. Br J Dermatol 1989;120:403–8. [DOI] [PubMed] [Google Scholar]
- 32.Gregoriou S, Rigopoulos D, Katsambas A, Katsarou A, Papaioannou D, Gkouvi A, et al. Etiologic aspects and prognostic factors of patients with chronic urticaria: nonrandomized, prospective, descriptive study. J Cutan Med Surg 2009;13:198–203. [DOI] [PubMed] [Google Scholar]
- 33.Metz M, Altrichter S, Ardelean E, Kessler B, Krause K, Magerl M, et al. Anti-immunoglobulin E treatment of patients with recalcitrant physical urticaria. Int Arch Allergy Immunol 2011;154:177–80. [DOI] [PubMed] [Google Scholar]
- 34.Asero R, Cugno M, Tedeschi A. Eosinophils in chronic urticaria: supporting or leading actors? World Allergy Organ J 2009;2:213–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Belani H, Gensler L, Bajpai U, Meinhard E, Graf J, Pincus L, et al. Neutrophilic urticaria with systemic inflammation: a case series. JAMA Dermatol 2013;149: 453–8. [DOI] [PubMed] [Google Scholar]
- 36.Morgan M, Khan DA. Therapeutic alternatives for chronic urticaria: an evidence-based review, part 1. Ann Allergy Asthma Immunol 2008;100:403–12. [DOI] [PubMed] [Google Scholar]
- 37.Asero R, Tedeschi A. Usefulness of a short course of oral prednisone in antihistamine-resistant chronic urticaria: a retrospective analysis. J Investig Allergol Clin Immunol 2010;20:386–90. [PubMed] [Google Scholar]