Abstract
Purpose
Hemodialysis patients with COVID-19 are at increased risk of death. We aimed to describe the characteristics of a cohort of Brazilian hemodialysis patients with COVID-19 and assess their mortality rate and risk factors for death.
Methods
Retrospective cohort study of 741 Brazilian hemodialysis patients with confirmed COVID-19 from Feb–Dec/2020, of 52 dialysis centers of the country. We analyzed comorbid conditions, sociodemographic factors, and dialysis-related parameters. To detect risk factors for mortality in hemodialysis patients, we performed multivariable Cox proportional hazard regression analysis. Survival was analyzed by Kaplan–Meier.
Results
From 9877 hemodialysis patients, 741 were diagnosed with COVID-19. Mean age was 57 ± 16 years, 61% were male, and 51% white. The most frequent symptoms were fever (54.1%), cough (50.9%), and dyspnea (37.2%); 14.2% were asymptomatic. There were 139 deaths (18.8%), with 66% within the disease’s first 15 days. 333 patients (44.9%) required hospitalization, and 211 (28.5%) were admitted to an intensive care unit. The cumulative probability of survival at 90 days of diagnosis was 79% (95% CI 76–82%). In the fully adjusted multivariate model, the risk factors significantly associated with death were diabetes mellitus (HR 1.52, 95% CI 1.05–2.19, P = 0.026), use of a central venous catheter (CVC) (HR 1.79, 95% CI 1.22–2.64, P = 0.003), age (HR 1.03, 95% CI 1.01–1.04, P < 0.001), and origin from the North vs. Southeast region (HR 2.60, 95% CI 1.01–6.68, P = 0.047).
Conclusions
Hemodialysis patients using a CVC as the vascular access, aside from diabetic and elderly ones, should be closely monitored due to their high risk of death in the course of the COVID-19.
Supplementary Information
The online version contains supplementary material available at 10.1007/s11255-021-02920-9.
Keywords: Brazil, Central venous catheter, COVID-19, Hemodialysis, Mortality, Risk factors
Introduction
Access to dialysis treatment is a right of every Brazilian citizen since 1993. Overall, approximately 80% of the patients on maintenance dialysis are financed by the public health system and 20% by private health insurance companies. Data from the Brazilian Dialysis Census estimate that by July, 2019, there were 139,691 patients on a chronic dialysis program [1], ranking the country as third in the number of patients on kidney replacement therapy by dialysis in the world [2].
The first case of the Coronavirus 2 disease (COVID-19) in Brazil was reported on February 26th, 2020 in the city of São Paulo [3]. On February 6th, 2021, there were about 9.4 million cases in Brazil, and the country was the 3rd in the world in number of cases [4].
Kidney failure was identified as a risk factor for a worse prognosis in patients with COVID-19, with mortality rates ranging between 29 and 41%, in different reports [5, 6]. In a nationwide Brazilian study encompassing 207 dialysis centers (37,852 hemodialysis patients) conducted from Feb/2020 to Jun/2020, a COVID-19 diagnosis was reported in 1291 patients, corresponding to an incidence of 341/10,000 patients. The high mortality rate and the case-fatality ratio of these patients of 94.3/10,000 and 27.7%, respectively, were noteworthy, with the highest figures found in the north and northeast regions of the country [7].
The risk factors for mortality in hemodialysis patients diagnosed with COVID-19 described so far are age, non-Hispanic black race, diabetes, coronary heart disease, time on dialysis, presence of fever and cough at diagnosis, need for hospitalization, hypoxemia, mechanical ventilation, use of vasoactive drugs, lymphocytopenia and elevated levels of ferritin, lactate dehydrogenase (DHL) and C-reactive protein (CRP) [5–13].
The present study’s main objective was to analyze the data from the Brazilian COVID-19 Hemodialysis Registry patients, describing their sociodemographic characteristics, clinical aspects, mortality rates, survival probability, and risk factors associated with mortality.
Materials and methods
Study design
This is a retrospective cohort observational study with data collection performed through voluntary filling out of a form available online for dialysis clinics affiliated with the Brazilian Society of Nephrology (BSN). All dialysis clinics in the country were invited to participate in the study utilizing various means of communication. Those whose managers accepted to participate filled out individual patient information and sent it electronically to the research coordinating center. Adult patients (> 18 years) with CKD undergoing kidney replacement therapy for hemodialysis for at least 3 months were selected. The analysis interval included patients diagnosed since the beginning of the country’s pandemic (Feb 26th, 2020) until Dec 12th, 2020. As an inclusion criterion, the patients should have had a diagnosis of COVID-19 by laboratory examination, either by RT-PCR or serology. The Research Ethics Committee of the Federal University of São Paulo approved the study under the registration number 39988220.0.1001.5505.
Sampling
The information was obtained from the medical records of patients in outpatient dialysis clinics, and there was no direct contact with the patients or other collection of material for laboratory examination. Information was collected on the patients’ sociodemographic characteristics (age, gender, and race), previous comorbidities, dialysis, vascular access, medications in continuous use, information about the COVID-19 diagnosis, clinical picture presented by the patient, need for hospitalization, need for admission to an intensive care unit (ICU) and/or intubation, and mortality. The form sent to the clinics for completion is available online (http://censo-sbn.org.br/reglgCovid19). In case of doubts about any of the information sent, an investigator of the Brazilian Society of Nephrology Registry contacted the clinic for data validation.
Statistical analysis
The normality of data distribution was assessed by the Kolmogorov–Smirnov test. Continuous variables were expressed as mean ± standard deviation, and categorical variables were expressed as frequency and percentages. The mortality rate of COVID-19 in hemodialysis patients was calculated as the number of deaths due to COVID-19/total hemodialysis population of the sample and adjusted for 10,000 exposed. A similar approach was used to calculate the incidence rates, but using the number of new cases in the numerator. The case-fatality ratio was calculated by dividing the number of deaths due to COVID-19 by the total number of confirmed sample cases.
The cumulative survival curve was calculated using the Kaplan–Meier method, considering the beginning of the follow-up, the date of the COVID-19 diagnosis and the final date of follow-up, the date of death, or up to 90 days of the diagnosis. If the patient had not completed 90 days of follow-up, he was censored on the last follow-up date. Univariate analysis of death’s risk was performed using Cox proportional hazards regression, with hazard ratios (HR) calculated with 95% confidence intervals (CI). Initially, the following variables were tested for association with mortality: age, gender, obesity (body mass index ≥ 30 kg/m2), patients’ origin by region of the country, comorbidities (previous stroke, chronic liver disease, diabetes mellitus, hypertension, chronic obstructive pulmonary disease (COPD), peripheral arterial obstructive disease, heart failure, previous myocardial infarction, previous kidney transplantation, previous or current neoplasia, and positive HIV serology), current or former smoking, use of renin–angiotensin–aldosterone system (RAAS) inhibitors, dialysis funding, and central venous catheter (CVC) use. Next, variables with P < 0.20 in the univariate analysis were included in multiple Cox proportional hazards models. The comorbidities were treated as the primary interest variable. We subsequently adjusted the findings for other independent variables in a step-to-step fashion.
Results
From 805 dialysis centers invited, 52 agreed to participate. A total of 741 hemodialysis patients with a diagnosis of COVID-19 were studied. The centers were located in 13 out of the 27 states of the union and treated 9897 patients, resulting in an average of 14.3 cases/center and an incidence rate of 749/10,000 patients. The distribution of patients by region is shown in supplementary Figure S1. Patients were predominant from the southeast region, followed by the northeast and south regions. Over time, the distribution of cases is depicted in supplementary Figure S2, with the highest number of patients diagnosed in July and August/2020.
The participants’ general characteristics, whose mean age was 57 ± 16 years, are in Table 1. They were predominantly male (61%) with white skin color (50.9%) and a prevalence of obesity of 17.4%. The most common previously diagnosed comorbidities were hypertension (83.5%), diabetes mellitus (39.5%), and heart failure (17.4%). About 42% of the patients used a RAAS inhibitor, and 4.7% had a prior kidney transplant. The hemodialysis treatment of the vast majority of the patients was funded by the Brazilian Public Health System (SUS) (83.7%) in centers with predominantly private management (78.1%). Approximately, one-quarter of them had a CVC as the vascular access for the procedure. When data from the patients who died were compared with the ones that survived, statistically significant differences were found for age, and prevalence of previous stroke, diabetes mellitus, chronic obstructive pulmonary disease, and central venous catheter use as the vascular access, all higher in the deceased cases.
Table 1.
Baseline characteristics of Brazilian COVID-19 hemodialysis patients (N = 741)
| All | Fatal course | P value | ||
|---|---|---|---|---|
| No N = 602 |
Yes N = 139 |
|||
| Age, years | 57 ± 16 | 55 ± 16 | 64 ± 15 | < 0.001 |
| Male gender | 452 (61.0) | 364 (60.9) | 88 (63.3) | 0.528 |
| Obesity (body mass index ≥ 30 kg/m2) | 129 (17.4) | 105 (17.6) | 24 (17.3) | 0.958 |
| Comorbidities | ||||
| Previous stroke | 26 (3.5) | 16 (2.7) | 10 (7.2) | 0.009 |
| Chronic liver disease | 15 (2.0) | 13 (2.2) | 2 (1.4) | 0.587 |
| Diabetes mellitus | 293 (39.5) | 216 (35.9) | 77 (55.4) | < 0.001 |
| Hypertension | 619 (83.5) | 498 (82.7) | 121 (87.1) | 0.215 |
| Chronic obstructive pulmonary disease | 27 (3.6) | 17 (2.8) | 10 (7.2) | 0.013 |
| Peripheral arterial obstructive disease | 53 (7.2) | 42(7.0) | 11 (7.9) | 0.699 |
| Heart failure | 129 (17.4) | 100 (16.6) | 29 (20.9) | 0.233 |
| Previous myocardial infarction | 41 (5.5) | 31 (5.1) | 10 (7.2) | 0.342 |
| Previous kidney transplantation | 35 (4.7) | 28 (5.1) | 7 (7.4) | 0.880 |
| Previous or current neoplasia | 27 (3.6) | 21 (3.5) | 6 (4.3) | 0.639 |
| Positive HIV serology | 4 (0.5) | 3 (0.5) | 1 (0.7) | 0.748 |
| Current smoking | 14 (1.9) | 13 (2.2) | 1 (0.7) | 0.261 |
| Former smoking | 49 (6.6) | 38 (6.3) | 11 (7.9) | 0.493 |
| Use of RAAS inhibitors | 310 (46.1) | 250 (45.4) | 60 (49.6) | 0.400 |
| Dialysis aspects | ||||
| Central venous catheter | 186 (25.1) | 133 (22.1) | 53 (38.1) | < 0.001 |
| Funding by the Public Health System | 620 (83.7) | 511 (84.9) | 109 (79.0) | 0.090 |
| Private management | 579 (78.1) | 464 (77.1) | 115 (82.7) | 0.262 |
Values are mean ± SD or n (%)
RAAS renin–angiotensin–aldosterone system
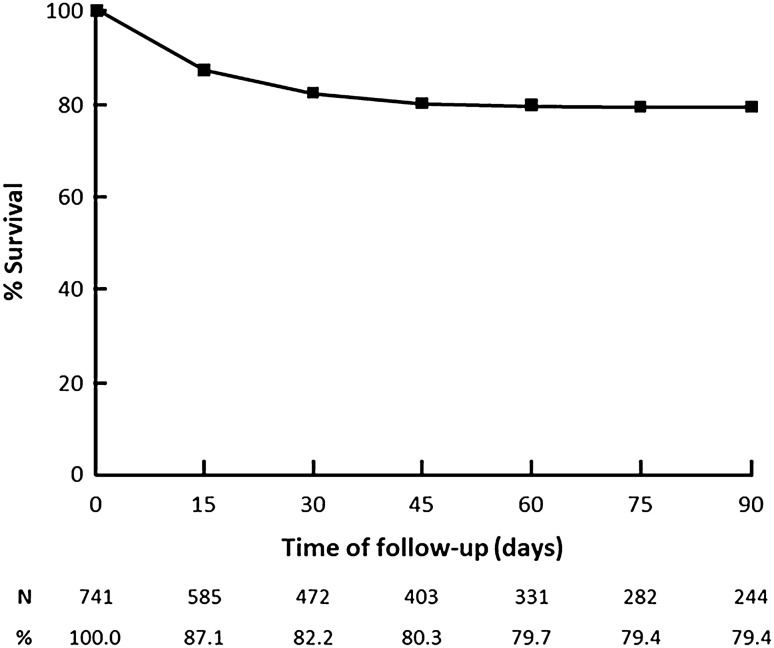
Table 2 shows data regarding the patients’ clinical presentation, diagnostic tests, and outcomes. The most frequent clinical findings were fever (54.1%), coughing (50.9%), and dyspnea (37.2%), with 14.2% of patients asymptomatic. The COVID-19 diagnosis was most commonly based on RT-PCR (68.7%) in nasal-pharyngeal swab samples. Of the 333 (44.9%) patients requiring hospitalization, 211 (63.4%) demanded intensive care, and 146 (43.8%) mechanical ventilation. Within a median follow-up of 74 days, 139 deaths were observed, corresponding to a mortality rate of 113/10,000 hemodialysis patients and a case-fatality ratio of 18.8%. The Kaplan–Meier curve showed that most deaths (92/139) occurred within the first 15 days of hospitalization. The probabilities of cumulative survival after 30, 60, and 90 days of diagnosis were 82% (95% CI 79–85%), 80% (95% CI 77–83%), and 79% (95% CI 76–82%), respectively (Fig. 1).
Table 2.
Clinical presentation, diagnosis, and outcomes in Brazilian COVID-19 hemodialysis patients (N = 741)
| Clinical findings | |
| Fever | 401 (54.1) |
| Cough | 377 (50.9) |
| Dyspnea | 276 (37.2) |
| Fatigue and malaise | 203 (27.4) |
| Myalgia | 182 (24.6) |
| Gastrointestinal symptoms | 121 (16.3) |
| Sensorium perturbations | 28 (3.8) |
| No signs or symptoms | 105 (14.2) |
| COVID-19 diagnosis | |
| RT-PCR | 509 (68.7) |
| Serological test | 180 (24.3) |
| RT-PCR + serological test | 52 (7.0) |
| Outcomes | |
| Hospitalization | 333 (44.9) |
| Intensive care unit | 211 (28.5) |
| Need of mechanical ventilation | 146 (19.7) |
| Death | 139 (18.8) |
Values are n (%)
RAAS renin–angiotensin–aldosterone system
Fig. 1.
Cumulative probabilities of survival of hemodialysis patients after COVID-19 diagnosis
In the univariate analysis using the Cox proportional hazards regression models (supplementary Table S1), the following previous comorbidities showed a high probability of direct association (P < 0.20) with a fatal course: prior stroke, diabetes mellitus, hypertension, COPD, and myocardial infarction. Other independent variables with a high probability of direct association with death were the use of a CVC as the vascular access for hemodialysis, age, and origin from the Northeast and North regions compared to the Southeast one. Public funding tended to be inversely associated with death. In the multivariate analysis (Table 3), the inclusion of all comorbidities with a high probability of association with death (Model 1) resulted that only diabetes mellitus (HR: 1.97, 95% CI: 1.40–2.78, P < 0.001) and COPD (HR: 2.19, 95% CI: 1.12–4.29, P = 0.021) were independently associated with mortality. Diabetes mellitus (HR: 1.73, 95% CI: 1.21–2.46, P = 0.02), and COPD (HR: 2.22, 95% CI: 1.14–4.33, P = 0.019) remained as independently associated with mortality even after adjustment by the use of a CVC (Model 2), which, by itself, emerged as independently associated with death (HR: 1.70, 95% CI: 1.19–2.43, P = 0.003). After age adjustment (Model 3), the only previous comorbidity that persisted associated with mortality was diabetes mellitus (HR: 1.51, 95% CI: 1.05–2.17, P = 0.028) aside of CVC use (HR: 1.66, 95% CI: 1.15–2.39, P = 0.007) and age (HR: 1.03, 95% CI: 1.01–1.04, P < 0.001). Finally, in the fully adjusted Model 4, four variables were found to be independently associated with mortality: diabetes mellitus (HR: 1.52, 95% CI: 1.05–2.19, P = 0.026), the use of CVC (HR: 1.79, 95% CI: 1.22–2.64, P = 0.003), age (HR: 1.03, 95% CI: 1.01–1.04, P < 0.001), and origin from the North region in comparison to the Southeast one (HR: 2.60, 95% CI: 1.01–6.68, P = 0.047). No interaction was found between either venous catheter and diabetes or venous catheter and age with death.
Table 3.
Multivariate Cox regression analysis for association with mortality in Brazilian COVID-19 hemodialysis patients
| Model 1 | Model 2 | Model 3 | Model 4 | |||||
|---|---|---|---|---|---|---|---|---|
| HR (95% CI) | P | HR (95% CI) | P | HR (95% CI) | P | HR (95% CI) | P | |
| Comorbidities | ||||||||
| Stroke | 1.91 (0.99–3.70) | 0.054 | 1.63 (0.81–3.27) | 0.171 | 1.42 (0.71–2.86) | 0.325 | 1.37 (0.67–2.82) | 0.394 |
| Diabetes mellitus | 1.97 (1.40–2.78) | < 0.001 | 1.73 (1.21–2.46) | 0.002 | 1.51 (1.05–2.17) | 0.028 | 1.52 (1.05–2.19) | 0.026 |
| Hypertension | 1.28 (0.78–2.11) | 0.330 | 1.35 (0.80–2.29) | 0.262 | 1.31 (0.76–2.25) | 0.337 | 1.34 (0.77–2.34) | 0.305 |
| COPD | 2.19 (1.12–4.25) | 0.021 | 2.22 (1.14–4.33) | 0.019 | 1.70 (0.86–3.33) | 0.126 | 1.59 (0.77–3.26) | 0.21 |
| MI | 0.98 (0.70–1.36) | 0.895 | 0.96 (0.69–1.34) | 0.813 | 1.02 (0.72–1.45) | 0.906 | 1.02 (0.71–1.46) | 0.913 |
| Use of CVC | 1.70 (1.19–2.43) | 0.003 | 1.66 (1.15–2.39) | 0.007 | 1.79 (1.22–2.64) | 0.003 | ||
| Age, years | 1.03 (1.01–1.04) | < 0.001 | 1.03 (1.01–1.04) | < 0.001 | ||||
| Patient’s origin | ||||||||
| Southeast | Reference | |||||||
| South | 0.93 (0.56–1.55) | 0.783 | ||||||
| Middle west | 1.20 (0.63–2.29) | 0.581 | ||||||
| Northeast | 0.88 (0.52–1.47) | 0.621 | ||||||
| North | 2.60 (1.01–6.68) | 0.047 | ||||||
| Public funding | 0.97 (0.61–1.54) | 0.889 | ||||||
COPD chronic obstructive pulmonary disease; MI myocardial infarction; CVC central venous catheter
Discussion
In this large multicenter study in Brazil, we report that age, diabetes mellitus, CVC, and geographic region were associated with mortality in maintenance HD patients with COVID-19. Some logistic features of in-center hemodialysis treatment, such as the need to stay together 3 or more times per week for 4 h or so in relative proximity and the use of collective means of transportation when traveling to the dialysis center, may favor the spread of the disease. In addition, kidney failure patients undergoing hemodialysis are recognized as a group risk, especially susceptible to severe disease forms worldwide [7, 14–17]. In this regard, our group had already reported the alarming incidence, mortality, and fatality rates in a broad survey of Brazilian dialysis centers by July 2020 [7].
For the present study, we resorted to an online nationwide survey collecting variables of each case of COVID-19 in several dialysis centers in the country. By December 11th, 2020, we had received information of 741 confirmed cases of Brazilian COVID-19 hemodialysis patients derived from 52 centers (9877 HD patients), located in 13 out of the 27 states of the union, from all geographic regions, representing one of the largest samples of COVID-19 hemodialysis patients reported so far. Most of the patients belonged to the Southeast region, which also concentrates the highest number of hemodialysis patients in the country [18].
Compared to our July 2020’s report [7], the present study estimated incidence rate (per each 10,000 exposed) showed a twofold increase (329 vs. 749, respectively), the mortality rate (per each 10,000 exposed) was ~ 20% higher (94 vs. 113, respectively), and the case-fatality ratio, ~ 35% lower (28% vs. 19%, respectively). The peak of contagious took place 1–2 months after the July 2020’s report, in July and August 2020. When the update numbers are compared with the ones from the general Brazilian population by the first week of December, the rates of incidence and mortality of hemodialysis patients were 2.3 times and 13.5 times higher, respectively, and the case-fatality ratio was 7.2 times higher.
The hemodialysis sampling general characteristics regarding age, gender, and obesity are comparable to the whole Brazilian dialysis population and so was the frequency of using a CVC as the hemodialysis vascular access (around 25%). In addition, more than 80% of the dialysis procedures were funded by the public health care system, and the predominant management of the centers was private, accompanying the national trend of the kidney failure treatment model of the country [2, 19].
The clinical presentation was consonant to previous studies in the general population and dialysis centers [11–13]. Fever and cough were the most prevalent findings, followed by dyspnea, fatigue/malaise, and myalgia. Interestingly, 14.6% of our patients were asymptomatic. Consistent with some previous studies with dialysis patients [11, 20], the clinical course of the COVID-19 in our cohort denotes the severity of the disease. The percent of patients demanding hospitalization approached 50% of the cases; close to 30% required intensive care; ~ 20% received tracheal intubation and mechanical ventilation; and 19% had a fatal course. The cumulative patients’ survival rate on the 90th day of follow-up was 79%. Remarkably, 78% of the fatalities took place in the first 20 days of the disease. In the literature, we could not find a previous study with a survival curve of a large number of COVID-19 hemodialysis patients, but early death was a common finding [8–13, 20].
Data from the fully adjusted Cox proportional hazard regression model confirmed diabetes mellitus as an independent risk factor for mortality, increasing the risk of death by 52%. In the general population, many studies have been establishing diabetes mellitus as a risk factor for mortality [17, 21]. However, data in hemodialysis patients are conflicting. Some small sample studies have failed to confirm such association [8, 10, 20], perhaps due to underpowering. Concurring with our finding, though, a recent adequately powered study pointed out that diabetes mellitus poses a significant risk for COVID-19 hemodialysis patients’ prognosis [6].
Other variables that remained as independent factors for mortality in the present study were age, CVC use, and origin from the country's north region. Since the beginning of the SARS-Cov-2 epidemics, age has been recognized as a risk factor for death in the general population [22, 23], a finding confirmed in most studies with hemodialysis patients [6, 13, 14]. As indicated before [24, 25] aging may be associated with a higher burden of comorbidities, and subclinical impairment of organs and systems, some of them critical to recovery from COVID-19, as it seems the case of the respiratory muscles. In addition, the older people may be less immunocompetent [24, 25], a condition that can be aggravated in hemodialysis patients by the superimposing kidney failure associated immunodeficiency [26, 27].
A strong independent risk factor for mortality in COVID-19 observed in our study was the use of a CVC as hemodialysis access, even after adjusting for several variables. Patients portraying such devices had close to 80% higher risk of death. A recent smaller study with 128 hemodialysis patients admitted to ICUs in the U.S. has suggested this association, although without a full multivariate adjustment as in our study [14]. CVC use is known to add substantial morbidity in the general hemodialysis population [28, 29]. The presence of foreign material in regular direct contact with the bloodstream can predispose to infections. Non-rarely, CVC-associated infections in this setting can result in sepsis and endocarditis and become a life-threatening condition. It is possible that microorganisms either previously colonizing CVC or recently adhered to CVC surface more easily disseminate into the bloodstream in the setting of a multisystem inflammation in frail patients such the ones in maintenance dialysis. In addition, the presence of CVC could trigger or worsen the oxidative stress process and inflammation [30]. We wonder if the close monitoring and the early indication for antimicrobial agents in patients with COVID-19 using CVC as vascular access could improve their prognosis. Finally, patients who originated from the north region of the country had more than a twofold increase in death risk after adjustment for confounders. The north is among the country’s less developed areas and had the lowest ratio of dialysis centers per country’s population. The scarcity of well-equipped health care centers/hospitals, which are located far from the dialysis facilities, may have contributed to this finding.
Our study has some limitations. The source of information was the dialysis centers, and as such, data from hospitalized patients were all indirect. Consequently, we did not have access to the laboratory parameters at admission and the treatment offered. Although the study design was retrospective, the time elapsed between the patients’ data obtained, mostly in electronic records, death events, and their report was short, thus minimizing the possibility of bias. Moreover, if a patient’s death occurred, it had to be necessarily informed to the dialysis center. Considering that the CVC was the maintenance access to dialysis and not placed after hospital admission, it is unlikely that indication bias has played a role in our findings. Despite adjustment for several factors associated with mortality, residual bias is inherent to observational studies, and epidemiological associations do not per se represent causal relationships.
In summary, we present data from a nationwide study in Brazil, with one of the largest samples of COVID-19 hemodialysis patients reported so far. High incidence, mortality, and fatality rates were observed. Aging and diabetes were confirmed as risk factors for death. Our findings underscore that patients using CVC as vascular access for hemodialysis are especially susceptible to a dismal prognosis when facing a SARS-Cov-2 infection. If further studies confirm our findings, close monitoring, strict compliance with infection control measures, and evaluation of early interventions are warranted in these patients.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
COVID-19 HD-Brazil Investigators: Adrian M Borborema, M.D., Agostinho Filgueira-Neto, M.D., Ailton C Gallo, M.D., Almir A Nascimento, M.D., Américo L. Cuvello-Neto, M.D., Ana Katarina C Lopes, M.D., Anneliese R Salmeron, M.D., Antonio A Brito, M.D., Carlos A Caniello, M.D., Cristiano V Silva, M.D., Danielle A Bazhuni, M.D., Eduardo A Portioli, M.D., Edvaldo A Costa-Neto, M.D., Eli N Silva, M.D., Eliana A Monteiro, M.D., Evaldo G Terra, M.D., Fabricio S Fonseca, M.D., Fernanda S Polacchini, M.D., Franklin C Barcellos, M.D., Gelzie S Ennes, M.D., Gustavo A Neto, M.D., Henrique Gorla-Neto, M.D., Januário G Roberto, M.D., João D Simões, M.D., José C Guilhen, M.D., José M Obregón, M.D., José R Boselli-Junior., M.D., José R Carvalho, M.D., Luciana K Batista, M.D., Lucíola R Carneiro, M.D., Manif C Jorge, M.D., Marcela M Souto, M.D., Marcelo A Gonçalves, M.D., Marcelo X Carrera, M.D., Marco A Galvão, M.D., Maria E Nardin, M.D., Maria F. Sarro, M.D., Marta A Tormes, M.D., Milene C Gomes, M.D., Nelson Gushi, M.D., Nilo Hoefelmann, M.D., Onesimo Domingos, M.D., Patrícia S Teixeira, M.D., Péricles Sarturi, M.D., Rafael S Humel, M.D., Renan H Emerick, M.D., Roberto Benvenutti, M.D., Roberto Rodrigues, M.D., Rodrigo R Abrita, M.D., Rodrigo T Belila, M.D., Rosilane F Manfrim, M.D., Savina A Bobbio, M.D., Sérgio M Baltar, M.D., Shelle M Cunha, M.D., Simoni P Melo, M.D., Suzana M Melo, M.D.,Tânia L Costa, M.D., Vitor C Vieira, M.D., Wellinton D Silva, M.D.
Author contribution
Research idea and study design: JRL, PDMMN, APA, MMN and RS; data acquisition: COVID-19 Hd-Br Investigators; statistical analysis: JRL and RS; supervision and mentorship: JRL, RS. Each author contributed important intellectual content during manuscript drafting or revision and accepts accountability for the overall work by ensuring that questions pertaining to the accuracy or integrity of any portion of the work are appropriately investigated and resolved.
Funding
Brazilian Society of Nephrology.
Availability of the data and material
Available under request to the corresponding author.
Declarations
Conflict of interest
The authors of this study have no conflict of interest.
Ethics approval
This study was approved by the Ethics Committee of the Federal University of São Paulo (# 39988220.0.1001.5505) and was performed in accordance with the ethical standards as laid down in the 1964 Helsinki Declaration.
Footnotes
COVID-19 HD-Brazil Investigators are listed in the Acknowledgment section.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Neves PDMM, Sesso RCC, Thomé FS, Lugon JR, Nascimento MM (2021) Brazilian dialysis inquiry 2019. Braz J Nephrol (ahead of print)
- 2.Sesso R, Lugon J. Global dialysis perspective: Brazil. Kidney 360. 2020;1(3):216–219. doi: 10.34067/KID.0000642019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Melo CML, Silva GAS, Melo ARS, Freitas AC. COVID-19 pandemic outbreak: the Brazilian reality from the first case to the collapse of health services. An Acad Bras Cienc. 2020;92(4):e20200709. doi: 10.1590/0001-3765202020200709. [DOI] [PubMed] [Google Scholar]
- 4.Worldometer—COVID-19 coronavirus pandemic. https://www.worldometers.info/coronavirus/?utm_campaign=homeAdvegas1. Accessed 16 November 2020
- 5.Wu J, Li J, Zhu G, et al. Clinical features of maintenance hemodialysis patients with novel coronavirus-infected pneumonia in Wuhan, China. Clin J Am Soc Nephrol. 2019;15(8):1139–1145. doi: 10.2215/CJN.04160320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jager KJ, Kramer A, Chesnaye NC, et al. Results from the ERA-EDTA Registry indicate a high mortality due to COVID-19 in dialysis patients and kidney transplant recipients across Europe. Kidney Int. 2020;98(6):1540–1548. doi: 10.1016/j.kint.2020.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pio-Abreu A, Nascimento MM, Vieira MA, Neves PDMM, Lugon JR, Sesso R. High mortality of CKD patients on hemodialysis with Covid-19 in Brazil. J Nephrol. 2020;33(5):875–877. doi: 10.1007/s40620-020-00823-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ng JH, Hirsch JS, Wanchoo R, et al. Outcomes of patients with end-stage kidney disease hospitalized with COVID-19. Kidney Int. 2020;98(6):1530–1539. doi: 10.1016/j.kint.2020.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Goicoechea M, Sánchez Cámara LA, Macías N, et al. COVID-19: clinical course and outcomes of 36 hemodialysis patients in Spain. Kidney Int. 2020;98(1):27–34. doi: 10.1016/j.kint.2020.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kular D, Ster IC, Sarnowski A, et al. The characteristics, dynamics, and the risk of death in COVID-19 positive dialysis patients in London, UK. Kidney360. 2020;1(11):1226–1243. doi: 10.34067/KID.0004502020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zou R, Chen F, Chen D, Xu CL, Xiong F. Clinical characteristics, and outcome of hemodialysis patients with COVID-19: a large cohort study in a single Chinese center. Ren Fail. 2020;42(1):950–957. doi: 10.1080/0886022X.2020.1816179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Weiss S, Bhat P, Fernandez MDP, Bhat JG, Coritsidis GN. COVID-19 infection in ESKD: findings from a prospective disease surveillance program at dialysis facilities in New York City and Long Island. J Am Soc Nephrol. 2020;31(11):2517–2521. doi: 10.1681/ASN.2020070932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Valeri AM, Robbins-Juarez SY, Stevens JS, et al. Presentation and outcomes of patients with ESKD and COVID-19. J Am Soc Nephrol. 2020;31(7):1409–1415. doi: 10.1681/ASN.2020040470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Flythe JE, Assimon MM, Tugman MJ, et al. Characteristics and outcomes of individuals with pre-existing kidney disease and COVID-19 admitted to intensive care units in the United States. Am J Kidney Dis. 2021;77(2):190–203. doi: 10.1053/j.ajkd.2020.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cheng Y, Luo R, Wang K, et al. Kidney disease is associated with in-hospital death of patients with COVID-19. Kidney Int. 2020;97(5):829–838. doi: 10.1016/j.kint.2020.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Petrilli CM, Jones SA, Yang J, et al. Factors associated with hospital admission and critical illness among 5279 people with coronavirus disease 2019 in New York City: prospective cohort study. BMJ. 2020;369:m1966. doi: 10.1136/bmj.m1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Williamson EJ, Walker AJ, Bhaskaran K, et al. Factors associated with COVID-19-related death using OpenSAFELY. Nature. 2020;584(7821):430–436. doi: 10.1038/s41586-020-2521-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lugon JR, Gordan PA, Thomé FS, et al. A web-based platform to collect data from ESRD patients undergoing dialysis: methods and preliminary results from the Brazilian Dialysis Registry. Int J Nephrol. 2018;2018:9894754. doi: 10.1155/2018/9894754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Thomé FS, Sesso RC, Lopes AA, Lugon JR, Martins CT. Brazilian chronic dialysis survey 2017. J Bras Nefrol. 2019;41(2):208–214. doi: 10.1590/2175-8239-jbn-2018-0178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Alberici F, Delbarba E, Manenti C, et al. Management of patients on dialysis and with kidney transplantation during the SARS-CoV-2 (COVID-19) pandemic in Brescia, Italy. Kidney Int Rep. 2020;5(5):580–585. doi: 10.1016/j.ekir.2020.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Barron E, Bakhai C, Kar P, et al. (2020) Associations of type 1 and type 2 diabetes with COVID-19-related mortality in England: a whole-population study. Lancet Diabetes Endocrinol. 2020;8(10):813–822. doi: 10.1016/S2213-8587(20)30272-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Grasselli G, Greco M, Zanella A, et al. Risk factors associated with mortality among patients with COVID-19 in intensive care units in Lombardy, Italy. JAMA Intern Med. 2020;180(10):1345–1355. doi: 10.1001/jamainternmed.2020.3539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Crimmins EM. Age-related vulnerability to coronavirus disease 2019 (COVID-19): biological, contextual, and policy-related factors. Public Policy Aging Rep. 2020;30(4):142–146. doi: 10.1093/ppar/praa023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xu W, Wong G, Hwang YY, Larbi A. The untwining of immunosenescence and aging. Semin Immunopathol. 2020;42(5):559–572. doi: 10.1007/s00281-020-00824-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cohen G, Hörl WH. Immune dysfunction in uremia—an update. Toxins (Basel) 2012;4(11):962–990. doi: 10.3390/toxins4110962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chonchol M. Neutrophil dysfunction and infection risk in end-stage renal disease. Semin Dial. 2006;19(4):291–296. doi: 10.1111/j.1525-139X.2006.00175.x. [DOI] [PubMed] [Google Scholar]
- 28.Raimann JG, Chu FI, Kalloo S, et al. Delayed conversion from central venous catheter to non-catheter hemodialysis access associates with an increased risk of death: a retrospective cohort study based on data from a large dialysis provider. Hemodial Int. 2020;24(3):299–308. doi: 10.1111/hdi.12831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nelveg-Kristensen KE, Laier GH, Heaf JG. Risk of death after first-time blood stream infection in incident dialysis patients with specific consideration on vascular access and comorbidity. BMC Infect Dis. 2018;18(1):688. doi: 10.1186/s12879-018-3594-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liakopoulos V, Roumeliotis S, Gorny X, Dounousi E, Mertens PR. Oxidative stress in hemodialysis patients: a review of the literature. Oxid Med Cell Longev. 2017;2017:3081856. doi: 10.1155/2017/3081856. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Available under request to the corresponding author.