Abstract
Background:
Young people who use drugs have a rising hepatitis C (HCV) incidence in the United States, but they may face barriers to testing and treatment adoption due to stigma.
Methods:
We conducted a cross-sectional study of New York City residents aged 18–29 years who reported non-medical prescription opioid and/or heroin use in the past 30 days. Participants were recruited from the community between 2014–2016 via respondent-driven sampling. Participants completed an in-person structured survey that included questions about HCV-testing and treatment and received HCV antibody testing.
Results:
There were 539 respondents: 353 people who inject drugs (PWID) and 186 non-PWID. For PWID, median age was 25 years, 65% were male, and 73% non-Hispanic White. For non-PWID, median age was 23 years, 73% were male, and 39% non-Hispanic White. 20% of PWID and 54% of non-PWID had never been tested for HCV (p<0.001). Years since first injection (aOR 1.16, CI: 1.02–1.32, p=0.02) and history of substance use treatment (aOR 3.17, CI: 1.53–6.61, p=0.02) were associated with prior testing among PWID. The seroprevalence of HCV among PWID was 25%, adjusted for sampling weights. Of the 75 who were aware of their HCV-positive status, 53% had received HCV-related medical care, and 28% had initiated treatment.
Conclusions:
HCV prevalence among young PWID is high and many have never been tested. Injection experience and treatment engagement is associated with testing. Interventions to increase testing earlier in injection careers, and to improve linkage to HCV treatment, will be critical for young PWID.
Keywords: hepatitis C, opioid-related disorders, seroepidemiologic studies, prevalence, health care utilization
Background:
Fueled by the opioid crisis, the incidence of hepatitis C virus (HCV) infection in people who use drugs is rising.1 In national surveillance data, the highest incidence of acute HCV infection is in individuals aged 20 to 29.2 This incidence is driven by initiation of injection drug use in this age group: 20% of individuals become HCV-infected within two years of starting to inject drugs, and half within five years.3 People who use drugs but do not report a history of injection are also at higher risk for hepatitis C than the general population, possibly due to sexual risk or because of unreported injection drug use.4,5 Several jurisdictions, including New York City, have seen a new peak of HCV prevalence in this younger age group.6,7 If HCV elimination efforts are to be effective, engaging and treating this newly infected and highly marginalized population will be critical.8,9
In certain respects, the current population of young people who are newly infected with HCV face a very different reality than previous cohorts. HCV treatment with all-oral direct acting antivirals, only available since 2014, is simple, well-tolerated, and proven to be highly effective in both clinical trials and real-world studies of this population.10 Providers are increasingly aware of the opioid epidemic and its association with HCV. Medicaid expansion and other provisions under the Affordable Care Act provide health insurance and treatment access to many people who inject drugs (PWID).11
Despite this progress, many barriers to HCV testing and treatment remain. People who use drugs face immense stigma within the healthcare system, and because of this, may not access care or disclose drug use behaviors.12 Due to the same stigma, providers may not ask about drug use, or they may be reluctant to offer treatment to this population.13,14 Young people may not perceive HCV as being severe. Knowledge about HCV and its complications is limited among young PWID.15 Various studies have described the infection as being normalized among PWID, seen as inevitable, and even as a part of identity for people who inject.16,17 Medical providers, who know that the health effects of HCV are often decades away, may also diminish the urgency of testing or treatment, especially when HCV co-exists with more immediate risks such as HIV or overdose.16,17 Some insurance plans may restrict reimbursement for treatment if patients are currently using drugs, further limiting access.18
Given the factors described above, young people’s trajectory of HCV care may be changing. There are few studies in the current era of HCV treatment which focus on the critical population of young people who use opioids. The goal of this research is to describe the patterns of HCV testing and treatment among young people who use opioids in New York City.
Methods:
Design and study population:
We analyzed data from a cross-sectional structured interview study conducted from 2014 to 2016 in New York City. Participants were between the ages of 18 and 29 years old, lived in one of the five boroughs of New York City, had used either heroin or prescription opioids non-medically in the past 30 days, and spoke English. Opioid use was assessed by a combination of self-report and point-of-care urine drug screening. Detailed information about study methodology has been previously published.19,20 The study protocol was approved by the institutional review board.
Sampling:
Participants were recruited using respondent-driven sampling (RDS), a form of chain-referral sampling which facilitates recruitment of hard-to-reach populations by using the participants’ own social networks to drive recruitment.21,22 Twenty participants were initially recruited directly by study investigators as seeds, directly from the community or as referrals from clinical providers and other research studies. Each was then invited to refer up to three eligible peers. This process was repeated until the desired sample size was reached. Participants were offered a $60USD incentive to participate in the study and additional incentives for recruitment of eligible peers. The a priori intended sample size was 600 participants based on the power needed to establish prevalence estimates for heroin use, injection drug use and HCV antibody status. By the end of the study, 539 participants had been enrolled and met eligibility criteria. All participants provided informed consent to participate.
Data Collection and Coding:
Participants completed a 951-question, interviewer-administered and computer-assisted survey that lasted between 90–120 minutes. Interviews were conducted at private fixed storefront site in New York City. The interviews were structured, with questions taking the form of multiple-choice or numerical responses, and included questions on substance use behaviors, treatment utilization, and social and medical co-factors including housing, socioeconomic status, mental health, and HCV. The present study analyzes survey data related to HCV testing and treatment, as well as participant demographics and socioeconomic factors.
The survey asked participants about their history of HCV testing, whether they were aware of having HCV infection, and healthcare utilization related to HCV, including history of treatment initiation and completion. Participants reporting having had a previous diagnosis were considered “HCV aware.” Participants who reported any HCV-related medical visit or HCV-related testing (eg RNA testing or liver imaging) were said to have been “linked to care.” Participants were also asked how concerned they were about getting HCV using a 4-point Likert scale, and asked about barriers to HCV testing using a multiple-choice question with a free text option. Additionally, each participant was tested for HCV-antibody status at the same site as the interviews, using the OraQuick HCV Rapid Antibody Test ®. Because this community-based survey could not conduct phlebotomy, only antibody testing was performed. Antibody-positive patients were notified of their results and referred to local medical providers.
Statistical Analysis:
Analyses were stratified based on injection drug use status, because of different HCV risk between these two populations. The injection drug use group (labelled PWID) were those who answered yes to any lifetime history of using drugs by injection. The non-injection group (labelled non-PWID) had used prescription opioids non-medically in the past 30 days, consistent with the study entry criteria, but denied any history of using drugs by injection. We use descriptive statistics to report baseline characteristics, prior receipt of HCV testing, risk perception, and steps along the HCV care cascade. T-tests, Chi-square, and nonparametric tests were used to test for differences between groups depending on the variable. For PWID only, we performed bivariate and multilple logistic regression models to determine factors associated with receipt of HCV testing. Population estimates adjusting for RDS were computed using Gile’s Sequential Sampling estimator weights generated by the RDS Analyst software which was created by the Hard-to-Reach Population Methods Research Group.23,24 The remainder of statistical analyses were conducted using IBM’s SPSS software, version 25.0.25
Results:
Sample characteristics:
There were 539 respondents: 353 (66%) PWID and 186 (34%) non-PWID (with PWID status defined as those who reported having ever injected drugs in their lifetime). The characteristics of both groups are shown in Table 1. Among the 353 PWID, 337 (95%) reported injecting drugs in the past year, 347 (98%) had ever injected heroin, and 208 (59%) had ever injected prescription opioids. Among the 57 PWID not born in the U.S., 47 were born in Eastern Europe, 4 in Latin America, and 2 each in Asia, the Middle East, and Western Europe.
Table 1:
Participant sociodemographic characteristics by injection drug use status
| PWID (n = 353) | Non-PWID (n = 186) | |
|---|---|---|
| Median Age (Range) | 25 (18–29) | 23 (18–29) |
| Gender | ||
| Male | 230 (65%) | 135 (73%) |
| Female | 119 (34%) | 51 (27%) |
| Transgender or non-binary | 4 (1%) | 0 |
| Race/Ethnicity† | ||
| Hispanic | 66 (19%) | 88 (47%) |
| NH-White | 259 (73%) | 73 (39%) |
| NH-Black | 5 (1%) | 17 (9%) |
| NH-Other | 22 (6%) | 6 (3%) |
| Born in US | 295 (84%) | 160 (86%) |
| Currently Homeless‡ | 127 (36%) | 10 (5%) |
| Highest Level of Education | ||
| Did not complete High School | 67 (19%) | 41 (22%) |
| High School graduate or GED | 135 (38%) | 89 (48%) |
| Attended some college | 151 (43%) | 56 (30%) |
| Health Insurance Coverage | 292 (83%) | 151 (81%) |
| Ever in Drug Use Treatment | 301 (85%) | 83 (45%) |
Does not add to 100% due to unreported responses.
“Homeless” was defined as “staying on the street, in a shelter, in a Single Room Occupancy hotel (SRO), temporarily staying with friends or relatives, or living in a car”
Abbreviations: NH = “Non-Hispanic”, US = “United States”, GED = “General Educational Development”
HCV seroprevalence:
All 539 participants received rapid HCV antibody testing in the study. A total of 105 participants (19.5%) had a positive HCV antibody test and all of these were PWID. Hence, the prevalence among the 353 PWID was 29.7%. When adjusted for RDS sampling, the estimated prevalence of HCV antibody positivity was 17.7% for the overall sample and 25.3% among PWID.
HCV Testing and Risk Perception:
Of the 353 PWID, 283 (80%) had been tested for HCV in their lifetime, and 279 of these in the past year. In comparison, 86 of 186 (46%) non-PWID had been tested for HCV in their lifetime, and 84 of these in the past year. PWID were significantly more likely than non-PWID to receive HCV testing in their lifetime and in the past year (p<0.001). PWID were HCV-tested a median of 3 times (IQR 3–5), and non-PWID a median of 2 times (IQR 1–5).Participants who had never been tested reported their perceived barriers to HCV testing (69 PWID and 100 non-PWID). The most frequently identified barrier was “Don’t think you are at risk” (39% of PWID, 65% of non-PWID). 19% of PWID were “Afraid of finding out you have HCV,” but only 3% of non-PWID were worried about this. Other common barriers were participants feeling they “didn’t have time” (14% of PWID, 16% of non-PWID) and who were “afraid of losing their job, housing or insurance if they tested positive” (9% of PWID, 12% of non-PWID).
When respondents who were not known to be HCV positive were asked about their perception of risk, 24% of HCV-negative PWID (n=278) and 46% of HCV-negative non-PWID (n=186) were “Not at all concerned” about getting HCV. 57% of PWID and 86% of non-PWID had “Rarely” or “Never” spoken to their drug-using peers about HCV.
Factors associated with HCV testing among PWID:
In bivariate testing, older age, US-born status, and more years of injection experience were associated with higher odds of receiving HCV testing, as was use of syringe exchange services in the past year and lifetime receipt of substance use disorder treatment. Individuals who had ever injected prescription opioids (in contrast to heroin-only) were also more likely to have received testing (Table 2). In a multiple regression model, more years of injection, and ever having attended substance use treatment and US-born status remained independently associated with higher odds of testing (Table 2).
Table 2:
Factors associated with HCV Testing among PWID (n = 353)
| Unadjusted Results† | Adjusted Regression | |||||
|---|---|---|---|---|---|---|
| Ever HCV Tested | Never HCV Tested | p-value§ | aOR | 95%CI | p-value | |
| Age (years)‡ | 24.7 (0.2) | 23.7 (0.2) | 0.02 | 1.03 | 0.93 – 1.15 | 0.59 |
| Gender | 0.82 | |||||
| Male | 186 (66%) | 45 (64%) | Ref | |||
| Female | 97 (34%) | 25 (36%) | 0.85 | 0.45 – 1.62 | 0.63 | |
| Race/Ethnicity | 0.03 | |||||
| NH Black or other | 20 (7%) | 7 (10%) | Ref | |||
| NH White | 216 (77%) | 43 (61%) | 1.19 | 0.38 – 2.24 | 0.73 | |
| Hispanic | 46 (16%) | 20 (29%) | 0.49 | 0.14 – 1.69 | 0.26 | |
| Currently Homeless¶ | 0.07 | |||||
| No | 171 (61%) | 51 (73%) | Ref | |||
| Yes | 108 (39%) | 19 (27%) | 1.13 | 0.57 – 2.25 | 0.73 | |
| US-born | 0.002 | |||||
| No | 38 (13%) | 20 (29%) | Ref | |||
| Yes | 245 (87%) | 50 (71%) | 2.06 | 1.01 – 4.22 | 0.05 | |
| Years since 1st injection‡ | 3.5 (1–6) | 1 (0–4) | <0.001 | 1.16 | 1.02 – 1.32 | 0.02 |
| Used Syringe Exchange (past year) | <0.001 | |||||
| No | 113 (40%) | 47 (67%) | Ref | |||
| Yes | 170 (60%) | 23 (33%) | 1.89 | 0.92 – 3.72 | 0.08 | |
| Ever Injected PO | <0.001 | |||||
| No | 97 (35%) | 42 (63%) | Ref | |||
| Yes | 183 (65%) | 25 (37%) | 1.32 | 0.67 – 2.60 | 0.42 | |
| Ever in SUD Treatment | <0.001 | |||||
| No | 28 (10%) | 24 (34%) | ||||
| Yes | 255 (90%) | 46 (66%) | 3.17 | 1.53 – 6.61 | 0.02 | |
| Any Health Insurance | 0.62 | |||||
| No | 56 (19%) | 13 (22%) | ||||
| Yes | 236 (81%) | 46 (78%) | ||||
| Highest level of education | 0.38 | |||||
| Did not complete HS | 56 (20%) | 11 (16%) | ||||
| HS graduate or GED | 111 (39%) | 24 (34%) | ||||
| Attended some college | 116 (41%) | 35 (50%) | ||||
Age shown as mean years (SD), years since 1st injection as median (IQR), all others as n (%)
Modeled as a continuous variable with 1-year units.
“Homeless” was defined as “staying on the street, in a shelter, in a Single Room Occupancy hotel (SRO), temporarily staying with friends or relatives, or living in a car”
t-test for Age, Wilcoxon-Rank Sum for years since 1st injection, Chi-square for all others.
Abbreviations: GED = “General Educational Development, NH = “Non-Hispanic”, PO = prescription opioids, SUD = substance use disorder, US = “United States”
Linkage to HCV treatment and care cascade:
105 participants tested HCV antibody-positive (all were PWID), 32 (30%) of whom learned of their HCV antibody status in the study, and 73 of whom reported having previously received a positive HCV test result. Two participants reported a previous positive result, but tested negative in the study. Figure 1 shows the cascade of care from testing through treatment for the PWID subgroup.
FIGURE 1. HCV Care Cascade among Young PWID in New York City.
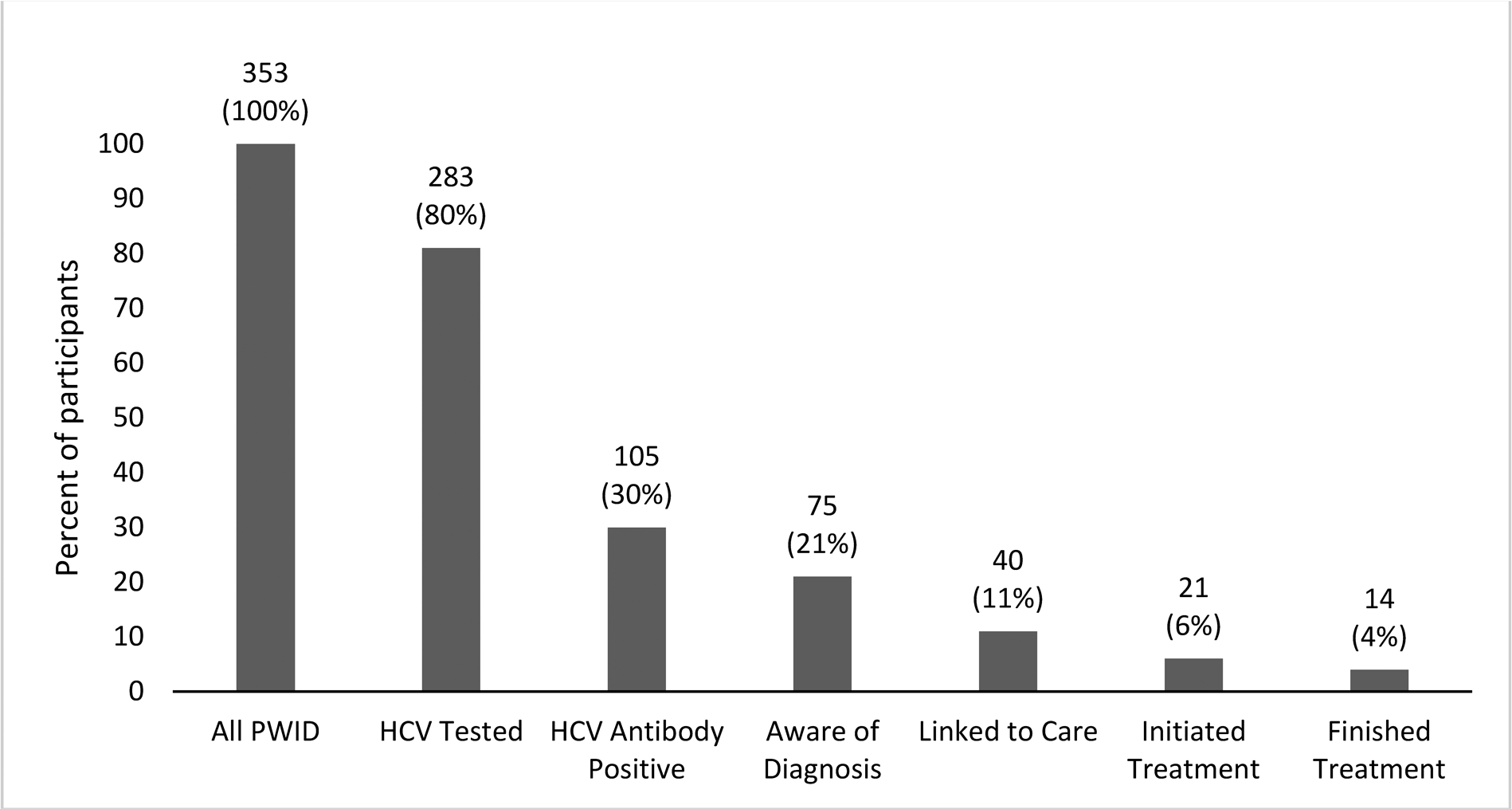
*Data labels are number and percent of participants. HCV = Hepatitis C, PWID = “People Who Inject Drugs”. 2 participants are included in “aware of diagnosis” category because they reported having been diagnosed with HCV despite testing antibody-negative during the survey.
Discussion:
In this cohort of young people who use opioids, we found a high HCV antibody prevalence among PWID and substantial gaps in the HCV testing and treatment cascade that have persisted despite the development of highly effective and well-tolerated direct acting antiviral treatment. New York State, like other jurisdictions, has committed to ending the HCV epidemic by reducing incidence and treating prevalent disease.26 Our findings underscore some of the challenges that public health programs will need to overcome to achieve this goal. The prevalence of HCV antibody estimated from our high-risk sample is higher than the overall prevalence of chronic hepatitis C in New York City (estimated at 1.4% in 2018), and within the range of past reports of seroprevalence in young PWID nationally, where estimates vary from 10% to 53% depending on the cohort.7,27–29
We show that young PWID are more likely to be tested than people who have used opioids but never injected. This expected finding is in line with medical society recommendations about HCV testing at the time of the study, which prioritized testing for PWID. The Centers for Disease Control and Prevention’s 2012 guidelines state that people who use drugs by snorting or sniffing may be at increased risk, whereas the Association for the Study of Liver Disease and Infectious Diseases Society of America’s joint guidelines recommend at least one-time testing for people who use drugs intranasally.30,31 One practical consideration is that individuals may not disclose injection practices in healthcare settings as a means to avoid stigma, preventing clinicians from accurately differentiating these risk groups.12 Additionally, people who misuse prescription opioids are also at increased risk of subsequently injecting drugs.32–34 Data from the current study show that individuals who report injecting drugs reported initiating injection an average of 4 years after starting non-injection opioid misuse.35 While the diagnostic value of screening may be lower in people who have never injected when compared to PWID, this is a critical population to keep engaged in prevention efforts, because of the risk of future transition to injection drug use. Routine screening, informing patients of their HCV status, and normalizing conversations about HCV risk reduction may play an important part in prevention education – similar to approaches taken to prevent HIV.36,37
Among PWID, while most individuals had been tested within the past year, gaps in testing continue to exist. Factors associated with increased likelihood of being tested include greater injection experience and engagement with substance use treatment programs. HCV testing was also associated with syringe service program use, but this association was not significant after adjustment. These associations are not surprising, as increased time and engagement with programs would lead to additional opportunities to be offered testing. Despite relatively high availability of these services in New York City, individuals who have only recently begun injecting may not yet be connected to testing.38 Previously published data from the same population showed an average of 3 years from initiating drug use to entering a substance use treatment program, during which time they are at risk for acquiring HCV and may inadvertently transmit HCV to others.35 Additionally, not all treatment or harm reduction programs offer testing. In 2018, only 10% of New York City syringe service program clients were screened onsite for viral hepatitis, and national data suggest that fewer than half of substance use treatment programs offer HCV testing.39,40 The reasons for low-uptake of HCV testing at these programs are myriad, but potential barriers may include lack of funding, inability to conduct phlebotomy, and the need to train staff.41,42 The low availability of testing in these settings is a missed opportunity for diagnosis of HCV in high-risk populations.
In addition to making testing more available, other approaches can engage individuals who are not yet connected to substance use-related services. The United States Preventive Services task force recently recommended one-time testing in all adults regardless of birth cohort or risk.43 This approach might encourage testing during routine clinical encounters, even when drug use behaviors are not disclosed. The social networks in which people inject can be leveraged to increase the reach of service delivery. PWID who are engaged with services often help educate and connect their peers – these individuals can be empowered to encourage HCV testing.44,45 Public health agencies can extend their reach by implementing contact tracing, in which injection partners of newly infected individuals are offered testing, but these services need to be implemented without fear of criminalization or stigma.9 Educational initiatives may also be effective, but to maximize impact, these would need to successfully reach individuals not currently engaged with programs.
A critical strategy for preventing new HCV infections is reducing community prevalence by treating current PWID, or “treatment as prevention”.46 The care continuum reported in this study shows a higher proportion of individuals who started HCV treatment than other studies of young PWID, but still shows significant gaps in linkage to care and in treatment initiation.47,48 The current study reflects data from early in the direct acting antiviral era; more individuals may have engaged in care since that time due to increasing availability of treatment. Early restrictions by Medicaid programs (including New York) may also have restricted access by limiting reimbursement for people who currently used drugs, but these restrictions have been lifted over time.49 Despite that, the structural factors that inhibit this age group from seeking care are likely to be persistent in the US healthcare system. Low-threshold models that offer care in a destigmatized environment and reduce financial and logistical steps may promote treatment uptake. Our team is studying one such model in a clinical trial recruiting young HCV-infected PWID in New York City that is currently ongoing.50
The association of U.S.-born status with receipt of testing was an unexpected finding of this analysis. Of the non-U.S.-born individuals, the majority had emigrated from former Soviet Union countries in Eastern Europe. Previous research from New York City has shown high levels of stigma, limited engagement with health services, and a low perception of risk related to HCV or HIV among young people who use opioids in the Russian-speaking immigrant community.51,52 Because of low numbers, we were not able to determine associations for different regions of origin. The uptake of HCV testing may be different across immigrant communities, because there is significant variation in the injection risk behaviors of these communities in the United States..53
Strengths of this study include the large sample size, a unique cohort recruited through the community rather than healthcare settings, and detailed information about substance use behaviors. Limitations of the study include the cross-sectional design and the reliance on self-report for information about linkage to care and treatment. Participants were labelled as being aware of their diagnosis if they had been told they were HCV positive before the survey. They were not asked to differentiate between antibody status and HCV-viremia, because this distinction is not always apparent to patients, but it limits our ability to determine which patients are truly chronically infected rather than just seropositive. We also interpreted self-report of any HCV-related medical visit as being linked to care, though in reality there are multiple possible definitions of linkage.
Our study only performed HCV antibody testing using an oral swab, because we did not have capacity for phlebotomy in this community setting. This prevented us from determining the prevalence of HCV viremia. This two-step diagnostic algorithm has been a commonly cited reason for underdiagnosis.54,55 Policies that promote reflex testing, in which laboratories automatically perform an HCV RNA PCR upon result of a positive antibody test, may help close this gap. However, this requires testing via a blood draw, and so is unlikely to change practice in community-based programs that rely on oral swab testing. Other enhanced testing modalities, such as point-of-care RNA testing or RNA testing via dried blood spot may help to increase RNA testing uptake in these settings, but these are not universally available in the United States.56
Our study was conducted in New York City, so generalizability to other areas may be limited, particularly rural populations. The study was conducted early in the era of direct-acting antivirals, and before recent recommendations for universal testing. It is possible that more recent findings would show improved rates of testing because of new recommendations for universal screening, and increased treatment because of the increased uptake of new antiviral treatments. The RDS design was selected because it helped with recruitment of a population that was not connected to healthcare, but the nonrandom sampling may impact effect estimates. In particular, we are cautious in interpreting the results of bivariate and multivariate hypothesis tests, as techniques that account for the effect of RDS have not yet been developed. Finally, the sample size of individuals who had received HCV treatment was small, which limited our ability to investigate factors that promoted or hindered treatment uptake in this study.
In conclusion, we show that gaps in HCV testing remain among young people who use drugs in New York City, though PWID have higher rates of testing than people who do not inject. We also show that longer duration of injection experience and substance use treatment engagement are associated with testing, suggesting a need to improve our capacity to test those who have recently transitioned to injection and PWID who are not yet engaged with programs. Finally, similar to other cohorts, we show gaps in treatment linkage and uptake, suggesting that interventions to promote HCV treatment among this population may enhance HCV elimination efforts.
Acknowledgments:
We would like to acknowledge the study participants for sharing their lived experience. This work was supported by the National Institute on Drug Abuse (R01 DA035146 to PMG, HG and K01 DA048172 to SNK) and the National Institute of Mental Health (T32 MH073553). The contents of this publication are solely the responsibility of the authors and do not necessarily represent the views of the funding agencies or the U.S. government.
Conflict of Interest Statement:
Drs Kapadia and Eckhardt have received grants to their institutions from Gilead Sciences Inc., unrelated to the current study. All other authors have no potential conflicts of interest.
ABBREVIATIONS
- HCV
hepatitis C virus
- HIV
human immunodeficiency virus
- PWID
people who inject drugs
- RDS
respondent driven sampling
- RNA
ribonucleic acid
- U.S.
United States
Contributor Information
Shashi N Kapadia, Division of Infectious Diseases, Weill Cornell Medicine, New York NY.
Caroline Katzman, Department of Medicine, New York University School of Medicine, New York NY.
Chunki Fong, Institute for Implementation Science in Population Health, City University of New York Graduate School of Public Health & Health Policy, New York, NY.
Benjamin J Eckhardt, Department of Medicine, New York University School of Medicine, New York NY.
Honoria Guarino, Institute for Implementation Science in Population Health, City University of New York Graduate School of Public Health & Health Policy, New York, NY.
Pedro Mateu-Gelabert, Department of Community Health and Social Sciences, City University of New York Graduate School of Public Health & Health Policy, New York, NY.
References:
- 1.Zibbell JE, Asher AK, Patel RC, et al. Increases in Acute Hepatitis C Virus Infection Related to a Growing Opioid Epidemic and Associated Injection Drug Use, United States, 2004 to 2014. Am J Public Health. 2018;108(2):175–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Centers for Disease Control and Prevention. Viral Hepatitis Surveillance, 2016. Atlanta, GA: Department of Health and Human Services, Centers for Disease Control and Prevention;2017. [Google Scholar]
- 3.Hagan H, Pouget ER, Des Jarlais DC, Lelutiu-Weinberger C. Meta-regression of hepatitis C virus infection in relation to time since onset of illicit drug injection: the influence of time and place. Am J Epidemiol. 2008;168(10):1099–1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.van den Berg CH, van de Laar TJ, Kok A, Zuure FR, Coutinho RA, Prins M. Never injected, but hepatitis C virus-infected: a study among self-declared never-injecting drug users from the Amsterdam Cohort Studies. J Viral Hepat. 2009;16(8):568–577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hermanstyne KA, Bangsberg DR, Hennessey K, Weinbaum C, Hahn JA. The association between use of non-injection drug implements and hepatitis C virus antibody status in homeless and marginally housed persons in San Francisco. J Public Health (Oxf). 2012;34(3):330–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kim AY, Onofrey S, Church DR. An epidemiologic update on hepatitis C infection in persons living with or at risk of HIV infection. J Infect Dis. 2013;207 Suppl 1:S1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.New York City Deparment of Health and Mental Hygiene. Working Toward a Hep Free NYC - Hepatitis A, B and C in New York City: 2018 Annual Report. New York, NY: 2019. [Google Scholar]
- 8.National Academies of Sciences, Engineering, and Medicine. A national strategy for the elimination of hepatitis B and C. Washington DC: The National Academies Press;2017. [PubMed] [Google Scholar]
- 9.Katzman C, Mateu-Gelabert P, Kapadia SN, Eckhardt BJ. Contact tracing for hepatitis C: The case for novel screening strategies as we strive for viral elimination. Int J Drug Policy. 2019;72:33–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hajarizadeh B, Cunningham EB, Reid H, Law M, Dore GJ, Grebely J. Direct-acting antiviral treatment for hepatitis C among people who use or inject drugs: a systematic review and meta-analysis. Lancet Gastroenterol Hepatol. 2018;3(11):754–767. [DOI] [PubMed] [Google Scholar]
- 11.Abraham AJ, Andrews CM, Grogan CM, et al. The Affordable Care Act Transformation of Substance Use Disorder Treatment. Am J Public Health. 2017;107(1):31–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Biancarelli DL, Biello KB, Childs E, et al. Strategies used by people who inject drugs to avoid stigma in healthcare settings. Drug Alcohol Depend. 2019;198:80–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rogal SS, McCarthy R, Reid A, et al. Primary Care and Hepatology Provider-Perceived Barriers to and Facilitators of Hepatitis C Treatment Candidacy and Adherence. Dig Dis Sci. 2017;62(8):1933–1943. [DOI] [PubMed] [Google Scholar]
- 14.Stephens DB, Young AM, Havens JR. Healthcare contact and treatment uptake following hepatitis C virus screening and counseling among rural Appalachian people who use drugs. Int J Drug Policy. 2017;47:86–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Quinn K, Fong C, Guarino H, Mateu-Gelabert P. Development, validation, and potential applications of the hepatitis C virus injection-risk knowledge scale (HCV-IRKS) among young opioid users in New York City. Drug Alcohol Depend. 2019;194:453–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rhodes T, Treloar C. The social production of hepatitis C risk among injecting drug users: a qualitative synthesis. Addiction. 2008;103(10):1593–1603. [DOI] [PubMed] [Google Scholar]
- 17.Skeer MR, Ladin K, Wilkins LE, Landy DM, Stopka TJ. ‘Hep C’s like the common cold’: understanding barriers along the HCV care continuum among young people who inject drugs. Drug Alcohol Depend. 2018;190:246–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liao JM, Fischer MA. Restrictions of Hepatitis C Treatment for Substance-Using Medicaid Patients: Cost Versus Ethics. Am J Public Health. 2017;107(6):893–899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Guarino H, Mateu-Gelabert P, Teubl J, Goodbody E. Young adults’ opioid use trajectories: From nonmedical prescription opioid use to heroin, drug injection, drug treatment and overdose. Addict Behav. 2018;86:118–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mateu-Gelabert P, Jessell L, Goodbody E, et al. High enhancer, downer, withdrawal helper: Multifunctional nonmedical benzodiazepine use among young adult opioid users in New York City. Int J Drug Policy. 2017;46:17–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Heckathorn DD. Respondent-Driven Sampling: A New Approach to the Study of Hidden Populations. Social Problems. 1997;44(2):174–199. [Google Scholar]
- 22.Abdul-Quader AS, Heckathorn DD, McKnight C, et al. Effectiveness of respondent-driven sampling for recruiting drug users in New York City: findings from a pilot study. J Urban Health. 2006;83(3):459–476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gile K. Improved Inference for Respondent-Driven Sampling Data With Application to HIV Prevalence Estimation. J Am Stat Assoc. 2011;106(93):135–146. [Google Scholar]
- 24.Hard-to-Reach Population Methods Research Group. 2011; http://wiki.stat.ucla.edu/hpmrg/index.php/Hard-to-Reach_Population_Methods_Research_Group. Accessed Sep 2019.
- 25.IBM SPSS Statistics for Windows, Version 25.0 [computer program]. Armonk, NY: IBM Corp.; 2017. [Google Scholar]
- 26.New York State Office of the Governor. Governor Announces Nation’s First State-Level Hepatitis C Elimination Strategy to Increase Access to Medication, Expand Comprehensive Programs and Enhance Treatment Services. 2018; https://www.governor.ny.gov/news/governor-cuomo-announces-statewide-expansion-enhanced-rental-assistance-program-increase-access. Accessed Mar 27, 2018.
- 27.Gicquelais RE, Foxman B, Coyle J, Eisenberg MC. Hepatitis C transmission in young people who inject drugs: Insights using a dynamic model informed by state public health surveillance. Epidemics. 2019;27:86–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Abara WE, Trujillo L, Broz D, et al. Age-Related Differences in Past or Present Hepatitis C Virus Infection Among People Who Inject Drugs: National Human Immunodeficiency Virus Behavioral Surveillance, 8 US Cities, 2015. J Infect Dis. 2019;220(3):377–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Eckhardt B, Winkelstein ER, Shu MA, et al. Risk factors for hepatitis C seropositivity among young people who inject drugs in New York City: Implications for prevention. PLoS One. 2017;12(5):e0177341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.AASLD/IDSA/IAS-USA. Recommendations for testing, managing, and treating hepatitis C. 2020; http://www.hcvguidelines.org. Accessed Jul 10 2020.
- 31.Centers for Disease Control and Prevention. Testing Recommendations for Hepatitis C Virus Infection. 2015; https://www.cdc.gov/hepatitis/hcv/guidelinesc.htm. Accessed Mar 31 2019.
- 32.Martins SS, Kim JH, Chen LY, et al. Nonmedical prescription drug use among US young adults by educational attainment. Soc Psychiatry Psychiatr Epidemiol. 2015;50(5):713–724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mateu-Gelabert P, Guarino H, Jessell L, Teper A. Injection and sexual HIV/HCV risk behaviors associated with nonmedical use of prescription opioids among young adults in New York City. J Subst Abuse Treat. 2015;48(1):13–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Syvertsen JL, Paquette CE, Pollini RA. Down in the valley: Trajectories of injection initiation among young injectors in California’s Central Valley. Int J Drug Policy. 2017;44:41–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Guarino H, Mateu-Gelabert P, Teubl J, Goodbody E. Young adults’ opioid use trajectories: From nonmedical prescription opioid use to heroin, drug injection, drug treatment and overdose. Addict Behav. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hall HI, Holtgrave DR, Maulsby C. HIV transmission rates from persons living with HIV who are aware and unaware of their infection. AIDS. 2012;26(7):893–896. [DOI] [PubMed] [Google Scholar]
- 37.Mayer KH, Kippax S, Sohn AH, Bras M. The importance of serostatus awareness in arresting the spread of HIV. J Int AIDS Soc. 2018;21(11):e25217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.amFAR Opioid & Health Indicators Database: New York. 2020; https://opioid.amfar.org/NY. Accessed Sep 21 2020. [Google Scholar]
- 39.New York City Deparment of Health and Mental Hygiene. Epi Data Brief: Syringe Service Programs in New York City. New York, NY: 2019. [Google Scholar]
- 40.Sayas AMS, Honermann B, Blumenthal S, Millett G, Jones A. Despite Infectious Disease Outbreaks Linked To Opioid Crisis, Most Substance Abuse Facilities Don’t Test For HIV Or HCV. Vol 2019: Health Affairs Blog; 2018. [Google Scholar]
- 41.Bini EJ, Kritz S, Brown LS Jr., Robinson J, Alderson D, Rotrosen J. Barriers to providing health services for HIV/AIDS, hepatitis C virus infection and sexually transmitted infections in substance abuse treatment programs in the United States. J Addict Dis. 2011;30(2):98–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Litwin AH, Drolet M, Nwankwo C, et al. Perceived barriers related to testing, management and treatment of HCV infection among physicians prescribing opioid agonist therapy: The C-SCOPE Study. J Viral Hepat. 2019;26(9):1094–1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.US Preventative Services Task Force. Final Recommendation Statement: Hepatitis C Screening. 2019; https://www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/hepatitis-c-screening. Accessed Sep 22 2019.
- 44.Mateu-Gelabert P, Guarino H, Quinn K, et al. Young Drug Users: a Vulnerable Population and an Underutilized Resource in HIV/HCV Prevention. Curr HIV/AIDS Rep. 2018;15(4):324–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Henderson C, Madden A, Kelsall J. ‘Beyond the willing & the waiting’ - The role of peer-based approaches in hepatitis C diagnosis & treatment. Int J Drug Policy. 2017;50:111–115. [DOI] [PubMed] [Google Scholar]
- 46.Martin NK, Vickerman P, Grebely J, et al. Hepatitis C virus treatment for prevention among people who inject drugs: Modeling treatment scale-up in the age of direct-acting antivirals. Hepatology. 2013;58(5):1598–1609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Addish E, Viner K, Trooskin S, et al. Abstract 1632: The Hepatitis C Continuum of Care for People Who Inject Drugs, Philadelphia PA. AASLD The Liver Meeting; 2018; San Francisco, CA. [Google Scholar]
- 48.Morris MD, Mirzazadeh A, Evans JL, et al. Treatment cascade for hepatitis C virus in young adult people who inject drugs in San Francisco: Low number treated. Drug Alcohol Depend. 2019;198:133–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.National Viral Hepatitis Roundtable and the Center for Health Law and Policy Innovation. Hepatitis C: The State of Medicaid Access. 2017; https://stateofhepc.org/. Accessed Jun 1 2020.
- 50.Kapadia SN, Eckhardt BJ, Smith M, et al. HCV Seek, Test and Rapid Treatment (HCV-ST&RT) for young people who use drugs with hepatitis C: Description of model and baseline characteristics. 8th International Conference on Hepatitis Care in Substance Users; 2019; Montreal, Quebec, Canada. [Google Scholar]
- 51.Gunn A, Guarino H. “Not human, dead already”: Perceptions and experiences of drug-related stigma among opioid using young adults of the former Soviet Union living in the US. International Journal of Drug Policy. 2016;38:63–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Guarino H, Marsch L, Deren S, Straussner SLA, Teper A. Opioid Use Trajectories, Injection Drug Use, and HCV Risk Among Yound Adult Immigrants from the Former Soviet Union Living in New York City. J Addict Dis. 2015;34:162–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Melo JS, Mittal ML, Horyniak D, Strathdee S, Werb D. Injection Drug Use Trajectories Among Migrant Populations: A Narrative Review. Subst Use Misuse. 2018;53(9):1558–1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.McGibbon E, Bornschlegel K, Balter S. Half a diagnosis: gap in confirming infection among hepatitis C antibody-positive patients. Am J Med. 2013;126(8):718–722. [DOI] [PubMed] [Google Scholar]
- 55.Spradling PR, Tong X, Rupp LB, et al. Trends in HCV RNA testing among HCV antibody-positive persons in care, 2003–2010. Clin Infect Dis. 2014;59(7):976–981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chevaliez S, Wlassow M, Volant J, et al. Assessing Molecular Point-of-Care Testing and Dried Blood Spot for Hepatitis C Virus Screening in People Who Inject Drugs. Open Forum Infect Dis. 2020;7(6):ofaa196. [DOI] [PMC free article] [PubMed] [Google Scholar]


