Abstract
Hypothesis:
Localized cooling of the external ear has a protective effect on the susceptibility to cisplatin-induced hearing loss.
Background:
We previously demonstrated significant protection from cisplatin-induced hearing loss using cool water ear canal irrigation. However, the study was limited to a single bolus injection of cisplatin and an acute time period. Here, we examined the application of localized cooling of the ear canal with repeated doses of cisplatin, over an expanded period of time, and using two methods of cooling.
Methods:
Twenty-four guinea pigs (12 male and 12 female) underwent auditory physiological testing (auditory brainstem response and distortion product otoacoustic emissions at 8–32 kHz) and pre/postadministration of cisplatin. Cisplatin (4 mg/kg i.p.) was administered in 3 weekly single injections for a total of 12 mg/kg. While anesthetized, the left ears of the guinea pigs were exposed to either cool water (22°C; ICS Water Caloric Irrigator), a cool ear bar (15°C, cooled by a Peltier device; TNM, Scion NeuroStim), or left uncooled as a sham control. The animals were tested 3 days post each dosage and 1 month post the final dose. At the end of the experiment the animals were euthanized for histological evaluation.
Results:
We found that hearing loss was significantly reduced, and hair cell survival greatly improved, in animals that received cooling treatments compared to cisplatin-only control animals. No significant difference was observed between the two methods of cooling.
Conclusion:
Localized cooling of the ear canal during administration of cisplatin mitigated loss of auditory function and loss of hair cells.
Keywords: Cisplatin, Hypothermia, Ototoxicity
Introduction
Nearly 50% of patients undergoing chemotherapy receive cisplatin as part of their regimen (1,2). A review by Paken et al. (3) reported that ~30–100% of patients receiving cisplatin incur some level of auditory pathology, primarily in the form of high-frequency sensorineural hearing loss (4). The reported prevalence can be influenced by a number of factors including age, dose-schedule, comorbidities, genetics, and how ototoxicity is defined (3,5–9).
A number of otoprotective pharmaceutical agents have been or are currently being considered for clinical application (10). The primary concern for pharmaceutical agents, particularly those administered systemically, is potential decrease in the antitumor efficacy of the cisplatin (11,12). For example, sodium thiosulfate (STS) has recently moved through randomized controlled trials (13,14). Brock et al. (13) observed significantly reduced incidence of hearing loss (as defined by Brock Scale grade 1 criteria of > 40 dBHL at 8 kHz) in children receiving cisplatin plus sodium thiosulfate (33%) for hepatoblastoma compared to a cisplatin-alone group (63%). Freyer et al. (14) examined STS in children receiving cisplatin for several cancer types compared to cisplatin-alone controls. They did not observe a statistically significant difference in threshold shift at individual frequencies, but did report a significantly lower incidence of a hearing loss defined by the American Speech Language and Hearing Association (ASHA) Ototoxicity Criteria (≥20 dB worsening in the threshold at one test frequency or ≥ 10 dB at two adjacent frequencies). Still, STS presents risk for compromised antitumor efficacy of cisplatin and thus requires a delay between administration of cisplatin and STS (15–18). A recent Cochrane Review (19) on otoprotection trials concluded that STS’s effect on antitumor efficacy, survival, and relapse were uncertain. Other groups have attempted to circumvent concerns for antitumor efficacy with administration of drugs via trans-tympanic injection. A randomized controlled trial comparing trans-tympanic injection of STS to a placebo found no clinically significant protection (20). Furthermore, intratympanic injection may be limited by the permeability of the drug, distribution in the cochlea, and clearance (21–23). For example, dexamethasone has been demonstrated to have limited permeability through the round window and has a high rate of elimination (22). Other agents under research for protection against cisplatin-induced ototoxicity include D-methionine, ebselen, N-Acetylcysteine, and others (10). Currently, there are no FDA-approved treatments for the prevention or remediation of cisplatin-induced hearing loss; therefore, novel alternative approaches need to be pursued (24).
The literature is rich and vast on the benefits of hypothermia (body cooling) and brain cooling (localized head cooling) to protect against neural injury and cell death (25–30). For example, Marion et al. (25) found that inducing whole-body hypothermia for 24 hours in patients following severe traumatic brain injury hastened neurological recovery. Therapeutic localized head cooling is a proven neuro-protective technique utilized for newborn infants with perinatal asphyxia and hypoxic-ischemic encephalopathy to improve neurological outcome (28). Furthermore, scalp cooling has been used to mitigate chemotherapy-induced alopecia in breast cancer patients receiving taxane- and/or anthracycline-based chemotherapy (31).
There is also a well-established literature on how temperature can influence auditory physiological measures (32–44). Fernandez et al. (32) first described increased latency and decreased amplitude of cochlear (cochlear microphonic) and auditory neural responses (action potential) with hypothermia (in guinea pigs) and reversibility with subsequent warming. Mild hypothermia down to 36°C (in guinea pigs) has been shown to elevate compound action potential thresholds in response to tone bursts above 24 kHz, which was also fully reversible after restoring cochlear temperature (37). Comparable effects have been demonstrated in humans (45). In addition, hypothermia has been shown to reduce otoacoustic emission amplitude, with recovery upon subsequent warming in humans (42,46) and rodents (43).
Numerous studies have demonstrated that systematic and/or localized hypothermia can protect against noise, ischemic, and iatrogenic damage (47–55). Though hypothermia is not a novel concept, neither hypothermia nor localized cooling has ever been applied as a method for otoprotection from drug-induced hearing loss in vivo until recent work from our group. [One study has examined the effect of hyperthermia on aminoglycoside ototoxicity (56), which reported increased damage related to kanamycin with simultaneous hyperthermia.] We were the first group to show that irrigating the ear canal with cool water could limit cisplatin-induced hearing loss and reduce loss of hair cells (57,58). Here, we present data expanding on this work that includes a repeated dosing design, extended time period for monitoring hearing, and an alternative method of cooling.
METHODS
Subjects
Twenty-four Guinea pigs (Cavia albino) of both sexes (12 male and 12 female) were randomly assigned to control and treatment groups. Animals were housed and cared for in the Center for Comparative Research (CCR) at The XXXX. All procedures were approved by the IACUC committee and adhere to NIH animal care and use guidelines. The Guinea pigs were first exposed to cisplatin at approximately 8 to 10 weeks of age and euthanized 1 month post final dose (~ 15–17 wk of age).
Drug Administration
Cisplatin was administered as weekly 4 mg/kg i.p. bolus injections. One injection was given per week over 3 weeks for a cumulative dose of 12 mg/kg.
Cool OtOProtective Lumen Treatment (COOL)
The left external ear canal only of Guinea pigs (n = 18) was cooled via irrigation with temperature-controlled water or using a static caloric vestibular system (ear bar) before, during, and after cisplatin exposure. This was done for a total of 30 minutes. Animals were anesthetized with 1.5% inhaled isoflurane or 40 mg/kg ketamine, 5 mg/kg xylazine i.p. The animal’s external ear canal was irrigated with temperature-controlled water using a modified ICS NCI-480 Water Caloric Irrigator (GN Otometrics, Taastrup, Denmark) at ~22°C (some variation was observed in water temperature over the 30-min treatment due to system heating). We monitored body temperature with a rectal temperature gauge and maintained at 37°C using a heating pad (Kent Scientific, Torrington, CT). As an alternative to water, we also examined cooling through an ear bar coupled to a Peltier device (Scion NeuroStim, Durham, NC). The temperature was set at ~15°C based on preliminary study which showed that the temperature needed to be reduced approximately 7°C lower than water when using the ear bar to achieve comparable cooling at the level of the round window. An additional group of animals (n = 6) received cisplatin only and underwent sham treatment (anesthetized and body temperature maintained at 37°C, but no ear cooling).
Thermography
Thermal images were obtained using a smartphone-based infrared-detecting thermal camera (FLIR ONE Pro, Wilsonville, OR) with the Apple iPhone operating system (iOS 13.1.3). The camera has a thermal resolution of 160 × 120 pixels and visual resolution of 1,140×1,080 pixels. It was attached to an Apple iPhone 7S smartphone (Cupertino, CA) and operated with the FLIR Tools application for iOS 1.8.14(97). The camera was mounted (35 mm from animal Cz) on a tripod.
Images were captured just before thermal treatment and then every 10 minutes for a total of 90 minutes (30 min during cooling and 60 min after cooling). Images were transferred to a PC based version of FLIR Tools (v5.12.18031.2002). The temperature range was manually set between 0 and 40°C. The mean temperatures in 4 regions (20×20 mm) were measured at the frontal region (Fz), left ear pinna (A1), right ear pinna (A2), and at the nape of the neck (N). Care was taken not to include the cooling systems directly in the regions of measurement. The temperatures were compared at baseline and 10 and 30 minutes after start of cooling and 10, 30, and 60 minutes post end-of-cooling treatment.
Physiological Experiments
All physiological testing was performed under ketamine and xylazine anesthesia (40 mg/kg ketamine, 5 mg/kg xylazine). Auditory brainstem response (ABR) and distortion product otoacoustic emissions (DPOAE) were measured pre and post cisplatin administration. The animals were tested at 3 days post each dosage and 1 month post the final dose. Physiological testing was completed in a sound-attenuating chamber dedicated for auditory physiological testing using methods previously described (59). In brief, DPOAEs were recorded using two primary tone frequencies, f1 and f2, using an f2/f1 ratio of 1.2, and an intensity level of the second tone, L2, 10 dB lower than L1. Tones were incrementally increased together in 5 dB steps. Input/output functions were obtained by increasing the L1 (and corresponding L2) in 5 dB steps from 20 to 80 dB SPL. The thresholds were defined as the f1 level required to produce DPOAEs of −5 dB SPL. ABR testing was performed at 5 frequencies from 8 to 32 kHz. Acoustic stimuli (5 ms tone pips, 0.5 ms cos2 rise-fall, delivered 30/s alternating polarity) were generated using the National Instruments/LabView-based package. ABR responses were recorded via subdermal needle electrodes (vertex-ventrolateral to pinna) as described. Acoustic stimuli were presented at 5 dB SPL and incremented in 5-dB steps to 80 dB SPL. The thresholds were defined as the lowest intensity of stimulation that yielded a repeatable waveform.
Hair Cell Counts
After the final physiological assessment (at 1 month post final dose) animals were sacrificed for anatomic and histological assessment of hair cells. The animals were deeply anesthetized with isoflurane and injected with 200 mg/kg Fatal Plus. The animals were decapitated and temporal bones promptly retrieved and fixed.
Temporal bones were decalcified in 0.25 M ethylenediaminetetraacetic acid for 5 days at room temperature. The cochlear sensory epithelia were then dissected from the temporal bones, cut into semicircular half-turns, and stained, free-floating, with an antibody against myosin VIIa (Proteus Biosciences) overnight (1:200) and an alexa fluor 488 conjugated phalloidin (ThermoFisher) at 1:200 in phosphate-buffered saline for 1 hour. After washing and mounting to slides using fluorogel with DABCO (Electron Microscopy Sciences), z-stack images of samples were acquired using a Zeiss LSM880 confocal microscope under a ×20 objective. Myo7a and phalloidin labeling showed near complete overlap and effective labeling of all hair cells. As such, outer hair cells, and hair cell absences, were counted based on phalloidin staining due to better image quality. Counts were made manually using the cell counter function in ImageJ, by an experimenter who was blinded to experimental conditions. Cochlear length measurements were made using the segmented line function in imageJ by drawing a line directly over the tunnel of Corti and counts were then binned based on distance from the first detectable hair cells in the apical hook.
Statistical Analysis
One-way analysis of variance (ANOVA) was used to assess the reliability of observed group differences (exposed vs. controls) with treatment as the independent variable; Repeated measure ANOVA were used to examine within-subject effects (pre vs. postcisplatin).
RESULTS
Thermal Imaging
Localized cooling of the left ears of experimental animals was accomplished via cool water lavage or by a Peltier cooled ear bar. Temperature changes across the head and body of the animal were visualized using thermal imaging. Changes in local surface temperature around the targeted ear during and after cooling (n = 6) are shown in Figure 1A and B. Animal core body temperature was maintained at 37°C. Table 1 shows the mean changes in temperature in matched regions at the neck (N), left (A1), and right ear (A2), and toward the nose between the ears (Fz). Changes in temperature were greatest at the left ear, however, small changes were observed across the head area. The method of cooling was not statistically significant in regards to the cooling effect on surface temperature (p > 0.05). Generally, a complete return to baseline temperature in the head was not observed until more than 30 to 60 minutes after cooling.
FIG. 1.
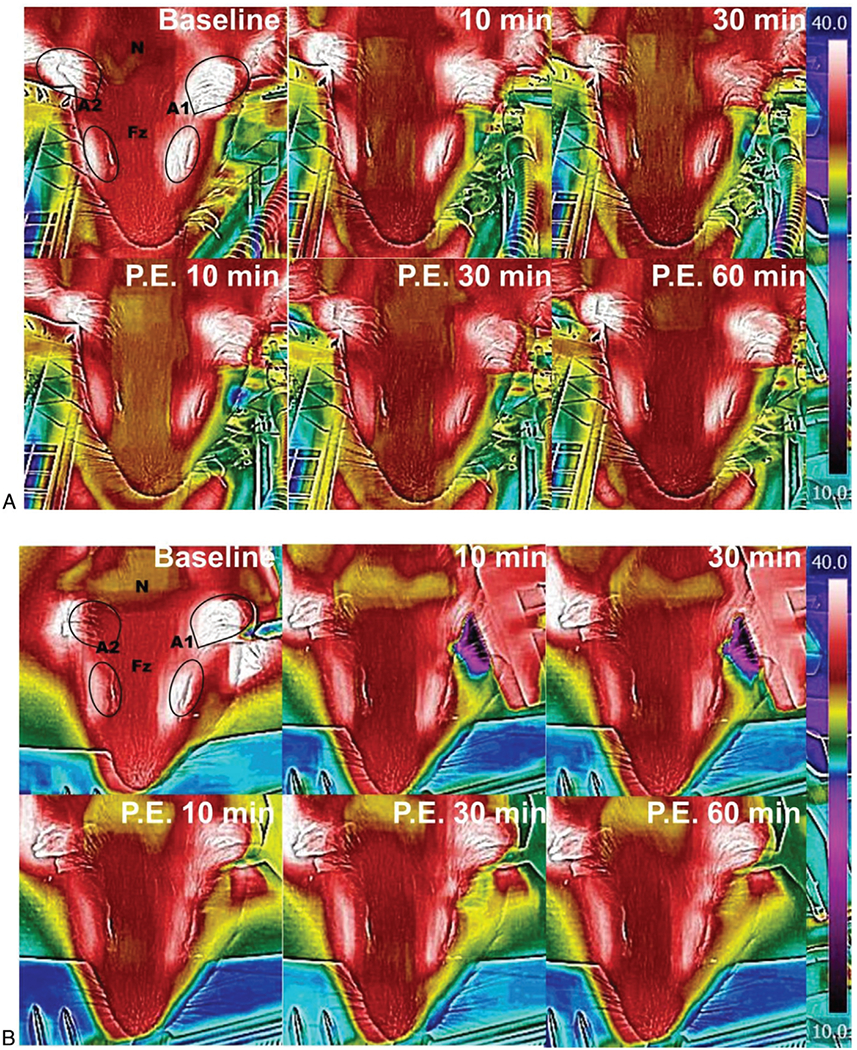
Thermography demonstrates cooling of the targeted ear. Figure 1 shows the thermal distribution on the guinea pig head from time 0 (before cooling) to 60 minutes after discontinuation of cooling with water (A) or ear bar (B). The black lines show the contours of the guinea pig anatomy. The ears are outlined and white in color, the eyes are also white and marked by ovals. The temperature was measured at the frontal portion (Fz) of the skull (at midline between the superior portion of the eye), the anterior portion of the left ear pinna (A1), the anterior portion of the right ear pinna (A2), and nape (N) of the neck. Temperature transference was not limited to the treated ear, but did appear localized to the head, and generally took at least 60 minutes to return to baseline temperature.
TABLE 1.
Mean change in temperature during and post cooling relative to baseline
| Left Ear |
Right Ear |
Nape |
Fz |
|||||
|---|---|---|---|---|---|---|---|---|
| Time | Difference (SE) | 95% CI | Difference (SE) | 95% CI | Difference (SE) | 95% CI | Difference (SE) | 95% CI |
| 10 min | 5.84 (0.54) | 4.1–7.5 | 2.06 (0.92) | −0.8–4.9 | 1.98 (0.53) | 0.3–3.6 | 2.51 (0.94) | −0.5–5.5 |
| 30 min | 6.50 (0.65) | 4.4–8.5 | 3.14 (0.88) | 0.3–5.9 | 2.58 (0.51) | 0.9–4.2 | 2.95 (0.94) | 0.05–5.9 |
| Post cooling | ||||||||
| 10 min | 4.32 (0.96) | 1.3–7.4 | 1.90 (0.84) | −0.7–4.5 | 2.20 (0.88) | −0.6–5.0 | 2.30 (1.13) | −1.2–6.0 |
| 30 min | 3.68 (1.13) | 0.4–7.3 | 2.00 (0.90) | −0.8–4.8 | 1.94 (0.82) | −0.6–4.5 | 2.05 (1.23) | −1.9–6.0 |
| 60 min | 1.24 (0.62) | 0.8–1.6 | 0.467 (0.30) | −0.4–1.3 | 0.33 (0.49) | −1.2–1.9 | 0.13 (0.34) | −0.9–1.2 |
Bold denotes statistical significance, p < 0.05.
Physiological Testing
To determine the effects of local cooling on cisplatin-induced hearing loss, ABRs and DPOAEs were recorded. ABR threshold shift 3 days post each weekly dose of cisplatin for sham controls (red circles), cool-water treatment (dark blue squares), and cool ear bar (light blue diamonds) treatment are shown in Figure 2A. The results demonstrated no significant main effect on threshold shift after the first dose of cisplatin (solid lines) at any frequency, 8 kHz (F = 0.41; p > 0.05), 12 kHz (F = 0.19; p > 0.05), 20 kHz (F = 0.24; p > 0.05), 24 kHz (F = 3.32; p > 0.05), or 32 kHz (F = 1.77; p > 0.05). After the second weekly dose (dashed line) a statistically significant main effect of a threshold shift was observed at 24 (F = 8.34; p = 0.003) and 32 kHz (F = 16.29; p < 0.001), but no significant main effect on threshold shift was observed at 8 kHz (F = 0.55; p > 0.05), 12 kHz (F = 1.39; p > 0.05), or 20 kHz (F = 2.35; p > 0.05). After a third weekly dose (dotted line), a statistically significant main effect of threshold shift was observed at 12 kHz (F = 3.93; p = 0.037), 20 kHz (F = 3.50; p = 0.05), and 24 kHz (F = 3.53; p = 0.04), but no significant main effect of threshold shift was observed at 8 kHz (F = 1.98; p > 0.05) or 32 kHz (F = 1.85; p > 0.05). In general, both the cool-water and cool ear bar groups showed significantly lower threshold shift compared to control animals, but no significant difference was observed for threshold shift between the two cooling methods. Similar findings with DPOAE threshold shifts were observed (Fig. 2B). After the first dose of cisplatin (solid line), no significant main effect on threshold shift was observed at any frequency tested, 8 kHz (F = 0.73; p > 0.05), 12 kHz (F = 0.45; p > 0.05), 20 kHz (F = 0.29; p > 0.05), 24 kHz (F = 0.79; p > 0.05), or 32 kHz (F = 1.35; p > 0.05). After the second weekly dose of cisplatin (dashed line), no significant main effect on threshold shift was observed at 8 kHz (F = 0.59; p > 0.05), 12 kHz (F = 1.87; p > 0.05), 20 kHz (F = 2.65; p > 0.05), or 32 kHz (F = 1.63; p > 0.05), but a significant main effect threshold shift was observed at 24 kHz Bold denotes statistical significance, p < 0.05. (F = 4.95; p = 0.01). After the third dose (dotted line), a significant main effect of threshold shift was observed at 8 kHz (F = 6.62; p = 0.007), 12 kHz (F = 4.27; p = 0.03), 20 kHz (F = 6.06; p = 0.01), and 24 kHz (F = 6.99; p = 0.006), but not 32 kHz (F = 2.29; p > 0.05). In summary, we observed minimal shift in ABR and DPOAEs after one dose of cisplatin for any condition. After the second dose, significant threshold shift was observed in sham controls, while cooled groups showed minimal shift. After a third dose, all groups show high-frequency threshold shift, but this shift was significantly greater for the sham control compared to cooled ears, and no significant difference was observed between cooling methods. Pairwise analyses can be found in Supplemental Table 1, http://links.lww.com/MAO/B107.
FIG. 2.
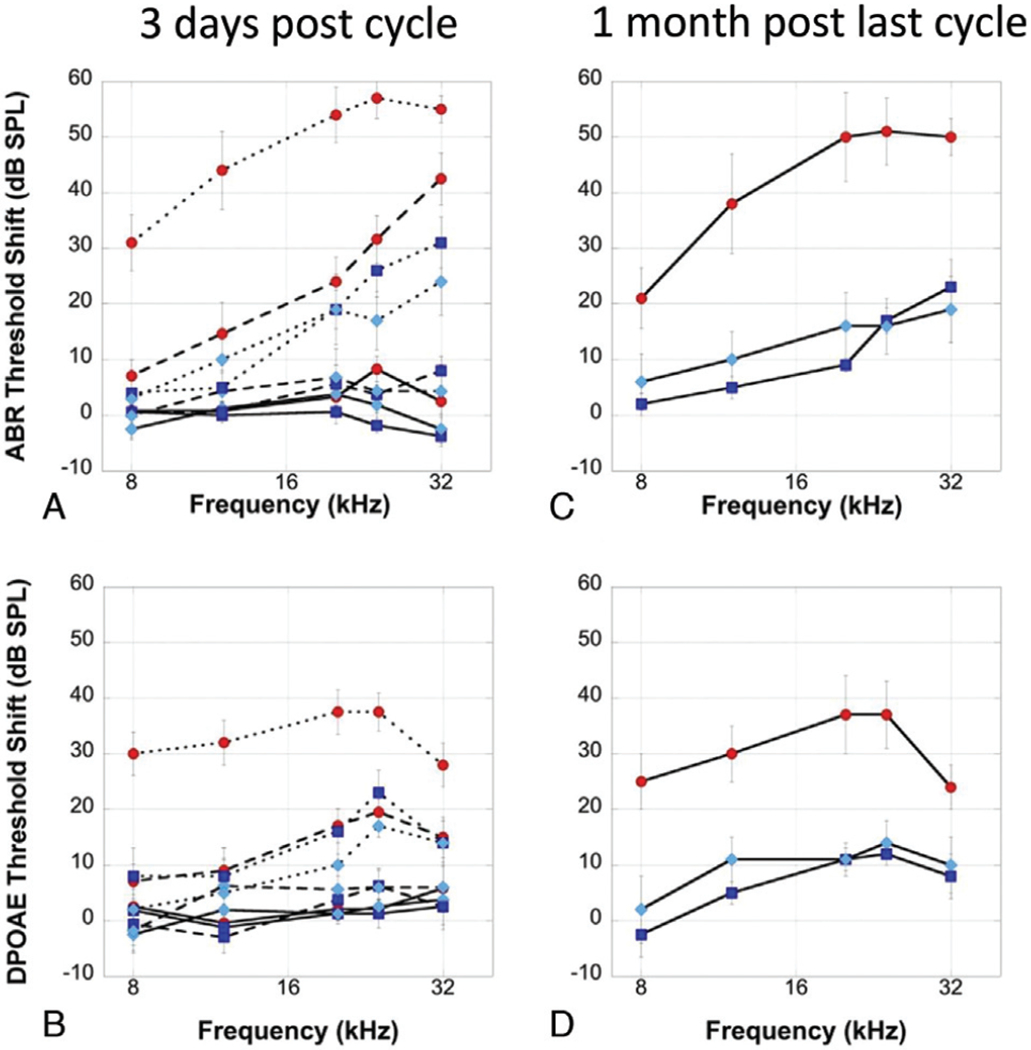
ABR and DPOAE threshold shift across treatments. Guinea pigs (n = 24) were exposed to 3 doses of cisplatin (each of 4 mg/kg i.p.). ABR (A, C) and DPOAE (B, D) were measured 3 days post each cycle (A, B) and 1 month post the final cycle (C, D). After the first dose (solid line) minimal ABR (A) or DPOAE (B) threshold shift was observed for cisplatin-only controls (red circles) or animals with ears treated with either cool water (dark blue squares) or cool ear bar (light blue diamond). After the second dosage (dashed lines) a significant ABR (A) and DPOAE (B) threshold shift was observed for the cisplatin-only animals (red circles), but minimal shift for the cool treated ears (dark blue squares and light blue diamonds). After the third dose (dotted line) a high-frequency shift in ABR (A) and DPOAE (B) threshold was observed for all groups, but smaller in the cooled groups. One month after the final cisplatin treatment threshold shift was significantly lower for ABR (C) and DPOAE (D) for animals undergoing cool treatments (dark blue square = cool water and light blue diamond = cool ear bar) compared to cisplatin only (red circles). No significant differences were observed between the two cooling methods. Error bars show standard error of the mean (SEM). ABR indicates auditory brainstem response; DPOAE, distortion product otoacoustic emissions.
We also examined ABR threshold shift 1 month post the final dose of cisplatin (Fig. 2C). Some minimal recovery was observed in the animals undergoing ear cooling. A statistically significant overall difference was observed at each test frequency (p < 0.05), 8 kHz (F = 4.47; p = 0.02), 12 kHz (F = 8.14; p = 0.003), 20 kHz (F = 12.03; p < 0.001), 24 kHz (F = 13.80; p < 0.001), and 32 kHz (F = 6.66; p = 0.007). Pairwise comparisons revealed significantly lower threshold shift for the cooled groups at all frequencies tested, including 8 and 32 kHz. No significant difference was observed between cooling methods (Table 2). Comparable protection was observed for DPOAE thresholds (Fig. 2D). We observed a statistically significant main effect difference in DPOAE threshold shift at each test frequency, 8 kHz (F = 5.36; p = 0.01), 12 kHz (F = 5.60; p = 0.01), 20 kHz (F = 7.82; p = 0.004), 24 kHz (F = 9.41; p = 0.002), except 32 kHz (F = 1.75; p > 0.05).
TABLE 2.
Pairwise comparison of ABR & DPOAE threshold shift, 1 month post final dose
| Cis Only Vs Water |
Cis Only Vs Ear Bar |
Water Vs. Ear Bar |
||||
|---|---|---|---|---|---|---|
| ABR | Difference (SE) | 95% CI | Difference (SE) | 95% CI | Difference (SE) | 95% CI |
| 8 kHz | 19.1 (6.5) | 5.4–32.8 | 14.8 (6.5) | 1.05–28.4 | −4.3 (5.7) | −16.4–7.6 |
| 12 kHz | 33.6 (8.6) | 15.4–51.8 | 28.0 (8.6) | 9.9–46.2 | −5.6 (7.5) | −21.5–10.3 |
| 20 kHz | 41.3 (8.6) | 23.0–59.5 | 33.75 (8.6) | 15.5–52.0 | −7.5 (7.6) | −23.5–8.50 |
| 24 kHz | 34.1 (7.3) | 18.7–49.5 | 35.4 (7.3) | 19.9–50.8 | 1.2 (6.4) | −12.3–14.8 |
| 32 kHz | 25.9 (8.5) | 7.9–43.8 | 29.6 (8.5) | 11.7–47.6 | 3.7 (7.5) | −11.9–19.5 |
| DPOAE | ||||||
| 8 kHz | 28.0 (8.6) | 9.7–46.2 | 21.12 | 2.8–39.3 | −6.8 (7.6) | −22.8–9.1 |
| 12 kHz | 24.0 (7.1) | 8.8–39.1 | 16.5 (7.1) | 1.3–31.6 | −7.5 (6.3) | −20.7–5.7 |
| 20 kHz | 23.1 (6.5) | 9.4–36.7 | 23.1 (6.5) | 9.4–36.7 | 0.0 (5.7) | −11.9–11.9 |
| 24 kHz | 25.1 (6.1) | 12.1–38.0 | 22.6 (6.1) | 9.6–35.5 | −2.5 (5.4) | −13.8–8.8 |
| 32 kHz | 13.3 (7.4) | −2.3–28.8 | 11.4 (7.4) | −4.1–26.9 | −1.8 (6.4) | −15.5–11.7 |
ABR indicates auditory brainstem response; DPOAE, distortion product otoacoustic emissions.
Hair Cell Counts
Cooling by either static caloric ear bar or by lavage with water significantly reduced hair cell loss in the cochleae of cisplatin-treated Guinea pigs. Representative confocal image for apical, mid, and basal regions shows large numbers of intact hair cells in animals that received no cisplatin (Fig. 3, A–C) or cisplatin plus cooling (Fig. 3, G–L), but significant loss of hair cells, particularly in the middle and basal regions, in animals receiving cisplatin only (Fig. 3, D–F). The mean numbers of percent missing outer hair cells (OHCs) based on distance from the cochlear apex showed greater hair cell loss in the middle and basal regions of cisplatin-only animals (Fig. 4, red) compared to an animal that received cisplatin and ear cooling (Fig. 4, dark blue = water and light blue = ear bar). The cisplatin only group revealed up to an 80% loss of OHCs in basal regions of the cochlea; this was reduced to below 40% for both cooling methods. No significant main difference in number of missing OHCs was observed at < 6 mm from the apex (p > 0.05), < 2 mm (F = 1.01; p > 0.05), 2–4 mm (F = 0.63; p > 0.05), 4–6 mm (F = 2.76; p > 0.05). Significant main effects were observed at regions > 6 mm from the apex (p < 0.05), 6–8 mm (F = 6.23; p = 0.01), 8–10 mm (F = 5.32; p = 0.02), 10–12 mm (F = 4.36; p = 0.04), 12–14 mm (F = 7.54; p = 0.008), 14–16 mm (F = 7.47; p = 0.008), >16 mm (F = 5.02; p = 0.02). Pairwise comparison (Table 3) revealed significant reductions in the percent of missing OHCs with both cooling treatments, but no statistically significant difference was observed when comparing the two methods of cooling.
FIG. 3.
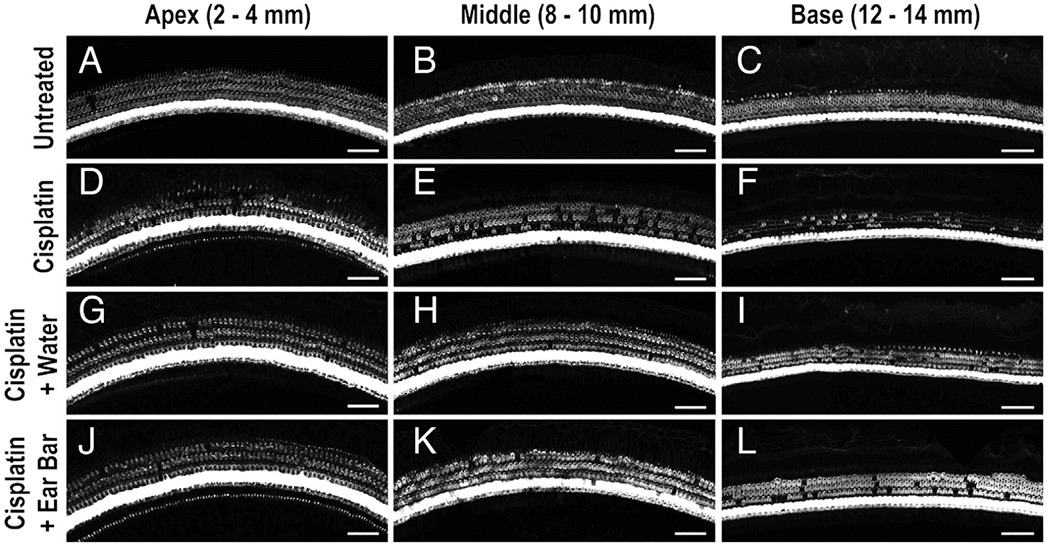
Hair cell confocal images. Representative confocal images are shown for apical (A, D, G, J) middle (B, E, H, K), and basal regions (C, F, I, L) of guinea pig cochleae for untreated, no-cisplatin control (A–C), cisplatin-only control (D–F), and cisplatin plus cooling (G–L). Less hair cell loss was observed for the animals undergoing ear cooling.
FIG. 4.
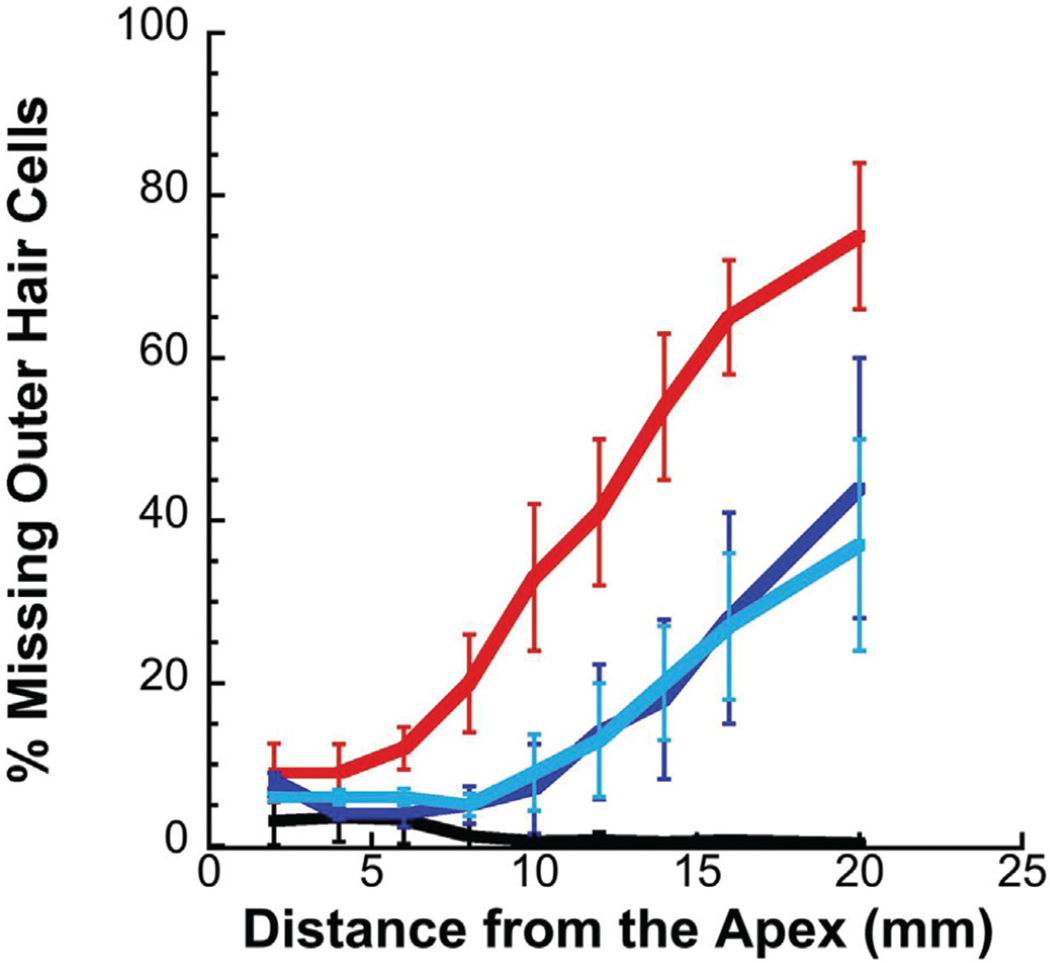
Percent missing outer hair cells (OHC). The percent of missing OHCs was lower in animals undergoing ear cooling (dark blue = water; light blue = ear bar) compared to cisplatin only (red). Age-matched controls animals are represented by the black line. Error bars show standard error of the mean (SEM).
TABLE 3.
Pairwise comparisons of percent (%) missing outer hair cells
| Cis Only Versus Water |
Cis Only Versus Ear Bar |
Water Versus Ear Bar |
||||
|---|---|---|---|---|---|---|
| Distance From Apex | Difference (SE) | 95% CI | Difference (SE) | 95% CI | Difference (SE) | 95% CI |
| 6–8 mm | 14.7 (4.4) | 4.5–24.8 | 14.3 (4.0) | 5.2–23.3 | −0.4 (4.0) | −9.5–8.6 |
| 8–10 mm | 25.7 (8.3) | 6.8–44.6 | 24.0 (7.4) | 7.0–40.7 | −1.9 (7.4) | −18.0–14.9 |
| 10–12 mm | 25.7 (10.0) | 1.0–50 | 28.0 (10.0) | 4.5–50.0 | 1.8 (10.0) | −21.0–25.0 |
| 12–14 mm | 35.1 (10.6) | 11.0–59.2 | 33.4 (9.5) | 11.9–55.0 | −1.7 (9.5) | −23.0–19.7 |
| 14–16 mm | 36.0 (14.0) | 8–64.35 | 37.0 (11.2) | 11.6–62.3 | 1.02 (11.2) | −24.0–26.3 |
| >16 mm | 30.0 (17.3) | 9.65–68.7 | 38.2 (15.4) | 3.2–73.2 | 8.65 (15.4) | −26.4–43.7 |
DISSCUSION
Our findings demonstrated that cooling of the external ear canal with either water or an ear bar can significantly reduce ABR and DPOAE threshold shift due to cisplatin exposure. Our previous work (58) showed nearly complete protection using cool water delivered simultaneous or up to 2 hours before a single high dose (12 mg/kg) bolus injection of cisplatin in an acute time frame (3 d post injection). Our current study, using a repeated cisplatin dosing paradigm (and same cumulative dose) elicited a slightly larger threshold shift overall compared to the more acute dosing. This is consistent with previous descriptions of higher threshold shift with repeated dosing paradigms (60). Despite repeated exposures, cooling-mediated protection was still observed, however not without some evidence of damage in the basal portion of the cochlea. We observed threshold shift at 24 and 32 kHz for all groups, but these shifts were significantly lower for the cooled ears compared with sham-control animals at 1 month postexposure. We also examined protection with a single bolus injection of 12 mg/kg of cisplatin (n = 8; 4 controls, 4 ear bar) and found significantly less threshold shift with ear bar cooling compared to controls (Supplemental Figure 1, http://links.lww.com/MAO/B108).
Thermography revealed surface temperature reduction across the animals’ heads while the body was maintained at 37°C using a heating pad. A smaller change in temperature was observed in head regions removed from the cooled ear (A1 = left ear). In turn, we did find some level of protection with cooling in the contralateral ears of the animals undergoing the cool treatments (Supplemental Figure 2, http://links.lww.com/MAO/B109). This suggests the cooling is not completely localized to the ear, however, with a large skull size (e.g., human) it is plausible the cooling effect will be more localized. Nonetheless, any translation of this approach to humans would involve simultaneous cooling of both external ears to minimize vestibular effects. Thermography also revealed a delay of 30 to 60 minutes or more for the head to return fully to baseline temperatures after cooling. This suggests the possibility that beneficial cooling may continue for some time after removal of the cooling device.
In addition to bolstering previous findings that cool water lavage of the external ear can protect against a more protracted regimen of cisplatin, we also demonstrated that we could achieve comparable protection using an alternative method of cooling; an ear bar attached to a Peltier device. This method of cooling was tidier than a continuous irrigation of water into the ear canal. Further, the device allows us ease in modulating duration of exposure and ramping temperature delivery to reduce risk for reperfusion. The device may also diminish risk for external ear infections compared to water and would not be counter indicated for a perforated eardrum. The device has been previously used for stimulating the brainstem for treatment of migraine (61).
The mechanisms of hypothermia and localized cooling-mediated protection from neural injury are well studied and involve numerous upstream and downstream pathways (30). We suspect numerous pathways may be involved here including modulation of cochlear or hair cell uptake of cisplatin, reduced production of reactive oxygen species, reduced inflammation, and both upstream and downstream cell death pathways. Reduced cochlear uptake is a possibility given what is known regarding the effects of hypothermia on cochlear blood flow (62). Systemic and local hypothermia has demonstrated reduction in blood flow and vascular constriction in vessels of the external ear in rabbit (63). On the contrary, Perlman et al. (64) reported no observable change in vessel diameter of the cochlea with hypothermia in guinea pigs. Rather, they described a decrease in electrical output of the cochlea related to reduced metabolism and suggested reduced blood flow was due to changes in resistance (e.g., viscosity of blood) to blood flow and not changes in blood pressure or vessel diameter. Miller et al. (65,66) reported reduced blood flow as measured with laser Doppler velocimetry with direct cooling at the promontory of the cochlea, and reduced cochlear blood flow in humans undergoing middle ear surgery with cool irrigation (34°C). Warming the ear canal with warm water (44 or 49°C) appears to increase cochlear blood flow during ear canal surgery. Of note, Miettinen et al. (67) reduced cochlear blood flow through local application of epinephrine, but did not find an altered effect on hearing loss or cisplatin levels in the cochlea. Nonetheless, this does not rule out the hypothesis that alterations in blood flow, particularly when coupled with reduced metabolism, could be a contributing factor to our observed protection. Additionally, the potential influence of cooling on the blood–perilymph or intrastrial fluid–blood barriers may be a factor. Konishi et al. (68) demonstrated a reduction in potassium transport in the cochlea and decreased K+ permeability of the endolymph–perilymph barrier with hypothermia (whole-body cooling). Hypothermia has also been shown to reduce glutamate release during experimental ischemia, suggesting a potential role in mitigating glutamate excitotoxicity/synaptopathy (50).
Our study is not without limitations. First, we limited our follow-up to 1 month post the final dose and risk for further damage beyond this timeline is plausible. Nonetheless, we did not observe any statistically significant progression in hearing loss 3 days post the final dose compared to 1 month post. Indeed, we actually observed some minimal improvement. Second, we did not examine mechanisms or show direct measures of cisplatin levels within the cochlea; however, these experiments are underway.
Our current and previous results suggest that cooling can provide protection from cisplatin-induced ototoxicity when it is undertaken during and up to 2 hours before cisplatin injection. Yet, with additional doses provided and cumulative level of cisplatin maintained over a longer period, hearing loss and cochlear pathology was observed. We suspect that the cooling may be most effective at preventing the initial uptake of cisplatin into the cochlea, or in the cellular responses that occur acutely after injection. The pharmacokinetics of cisplatin reveal a peak concentration in cochlear tissues that is fairly rapid, < 1 hour post administration (15,69–71), however circulating cisplatin may eventually gain entrance and this may explain the damage and high-frequency hearing loss that was observed even in the cool-treated groups. It is plausible that variations of our cooling treatment (e.g., longer duration of cooling) or combination of cooling along with other developing methods of otoprotection may work together to further improve overall protection.
Supplementary Material
Acknowledgments
This work was funded by the University of Mississippi Medical Center, the American Otological Society, the National Institutes of Health (R01DC016365 to BW), and the Office of Naval Research (ONR N00014-18-1-2716).
Footnotes
The authors disclose no conflicts of interest.
Supplemental digital content is available in the text.
REFERENCES
- 1.Galanski M, Jakupec MA, Keppler BK. Update of the preclinical situation of anticancer platinum complexes: Novel design strategies and innovative analytical approaches. Curr Med Chem 2005;12:2075–94. [DOI] [PubMed] [Google Scholar]
- 2.Ghosh S Cisplatin: The first metal based anticancer drug. Bioorg Chem 2019;88:102925. [DOI] [PubMed] [Google Scholar]
- 3.Paken J, Govender CD, Pillay M, Sewram V. A review of cisplatin-associated ototoxicity. Semin Hear 2019;40:108–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Landier W. Ototoxicity and cancer therapy. Cancer 2016; 122:1647–58. [DOI] [PubMed] [Google Scholar]
- 5.Landier W Hearing loss related to ototoxicity in children with cancer. J Pediatr Oncol Nurs 1998;15:195–206. [DOI] [PubMed] [Google Scholar]
- 6.Tserga E, Nandwani T, Edvall NK, et al. The genetic vulnerability to cisplatin ototoxicity: A systematic review. Sci Rep 2019;9: 3455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rademaker-Lakhai JM, Crul M, Zuur L, et al. Relationship between cisplatin administration and the development of ototoxicity. J Clin Oncol 2006;24:918–24. [DOI] [PubMed] [Google Scholar]
- 8.Li Y, Womer RB, Silber JH. Predicting cisplatin ototoxicity in children: The influence of age and the cumulative dose. Eur J Cancer 2004;40:2445–51. [DOI] [PubMed] [Google Scholar]
- 9.Langer T, am Zehnhoff-Dinnesen A, Radtke S, Meitert J, Zolk O. Understanding platinum-induced ototoxicity. Trends Pharmacol Sci 2013;34:458–69. [DOI] [PubMed] [Google Scholar]
- 10.Hazlitt RA, Min J, Zuo J. Progress in the development of preventative drugs for cisplatin-induced hearing loss. J Med Chem 2018;61:5512–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ekborn A, Laurell G, Johnstrom P, Wallin I, Eksborg S, Ehrsson H. D-Methionine and cisplatin ototoxicity in the guinea pig: D-methionine influences cisplatin pharmacokinetics. Hear Res 2002;165: 53–61. [DOI] [PubMed] [Google Scholar]
- 12.Laurell G Pharmacological intervention in the field of ototoxicity. HNO 2019;67:434–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brock PR, Maibach R, Childs M, et al. Sodium thiosulfate for protection from cisplatin-induced hearing loss. N Engl J Med 2018;378:2376–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Freyer DR, Chen L, Krailo MD, et al. Effects of sodium thiosulfate versus observation on development of cisplatin-induced hearing loss in children with cancer (ACCL0431): A multicentre, randomised, controlled, open-label, phase 3 trial. Lancet Oncol 2017; 18:63–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chu YH, Sibrian-Vazquez M, Escobedo JO, et al. Systemic delivery and biodistribution of cisplatin in vivo. Mol Pharm 2016;13:2677–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Muldoon LL, Pagel MA, Kroll RA, et al. Delayed administration of sodium thiosulfate in animal models reduces platinum ototoxicity without reduction of antitumor activity. Clin Cancer Res 2000;6:309–15. [PubMed] [Google Scholar]
- 17.Neuwelt EA, Brummett RE, Remsen LG, et al. In vitro and animal studies of sodium thiosulfate as a potential chemoprotectant against carboplatin-induced ototoxicity. Cancer Res 1996;56:706–9. [PubMed] [Google Scholar]
- 18.Harned TM, Kalous O, Neuwelt A, et al. Sodium thiosulfate administered six hours after cisplatin does not compromise anti-neuroblastoma activity. Clin Cancer Res 2008;14:533–40. [DOI] [PubMed] [Google Scholar]
- 19.van As JW, van den Berg H, van Dalen EC. Medical interventions for the prevention of platinum-induced hearing loss in children with cancer. Cochrane Database Syst Rev 2020;5:CD010885. 1–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rolland V, Meyer F, Guitton MJ, et al. A randomized controlled trial to test the efficacy of trans-tympanic injections of a sodium thiosulfate gel to prevent cisplatin-induced ototoxicity in patients with head and neck cancer. J Otolaryngol Head Neck Surg 2019;48:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Salt AN, Hartsock JJ, Hou J, Piu F. Comparison of the pharmacokinetic properties of triamcinolone and dexamethasone for local therapy of the inner ear. Front Cell Neurosci 2019;13:347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Salt AN, Plontke SK. Pharmacokinetic principles in the inner ear: Influence of drug properties on intratympanic applications. Hear Res 2018;368:28–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Salt AN, Hirose K. Communication pathways to and from the inner ear and their contributions to drug delivery. Hear Res 2018;362:25–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rybak LP, Dhukhwa A, Mukherjea D, Ramkumar V. Local drug delivery for prevention of hearing loss. Front Cell Neurosci 2019;13:300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Marion DW, Penrod LE, Kelsey SF, et al. Treatment of traumatic brain injury with moderate hypothermia. N Engl J Med 1997; 336:540–6. [DOI] [PubMed] [Google Scholar]
- 26.Shankaran S, Laptook AR, Ehrenkranz RA, et al. Whole-body hypothermia for neonates with hypoxic-ischemic encephalopathy. N Engl J Med 2005;353:1574–84. [DOI] [PubMed] [Google Scholar]
- 27.Wagner KR, Zuccarello M. Local brain hypothermia for neuro-protection in stroke treatment and aneurysm repair. Neurol Res 2005;27:238–45. [DOI] [PubMed] [Google Scholar]
- 28.Silveira RC, Procianoy RS. Hypothermia therapy for newborns with hypoxic ischemic encephalopathy. J Pediatr (Rio J) 2015; 91:S78–83. [DOI] [PubMed] [Google Scholar]
- 29.Gunn AJ, Laptook AR, Robertson NJ, et al. Therapeutic hypothermia translates from ancient history in to practice. Pediatr Res 2017;81:202–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sun YJ, Zhang ZY, Fan B, Li GY. Neuroprotection by therapeutic hypothermia. Front Neurosci 2019;13:586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gianotti E, Razzini G, Bini M, et al. Scalp cooling in daily clinical practice for breast cancer patients undergoing curative chemotherapy: A multicenter interventional study. Asia Pac J Oncol Nurs 2019;6:277–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fernandez C, Singh H, Perlman H. Effect of short-term hypothermia on cochlear responses. Acta Otolaryngol 1958;49:189–205. [DOI] [PubMed] [Google Scholar]
- 33.Gulick WL, Cutt RA. The effects of abnormal body temperature upon the ear: Cooling. Ann Otol Rhinol Laryngol 1960;69:35–50. [DOI] [PubMed] [Google Scholar]
- 34.Gcoats AC. Temperature effects on the peripheral auditory apparatus. Science 1965;150:1481–3. [DOI] [PubMed] [Google Scholar]
- 35.Shore SE, Nuttall AL. The effects of cochlear hypothermia on compound action potential tuning. J Acoust Soc Am 1985;77:590–8. [DOI] [PubMed] [Google Scholar]
- 36.Inamura N, Kusakari J, Takasaka T. Effect of hypothermia on the cochlear potentials. Acta Otolaryngol Suppl 1987;435:33–9. [DOI] [PubMed] [Google Scholar]
- 37.Brown MC, Smith DI, Nuttall AL. The temperature dependency of neural and hair cell responses evoked by high frequencies. J Acoust Soc Am 1983;73:1662–70. [DOI] [PubMed] [Google Scholar]
- 38.Gummer AW, Klinke R. Influence of temperature on tuning of primary-like units in the guinea pig cochlear nucleus. Hear Res 1983;12:367–80. [DOI] [PubMed] [Google Scholar]
- 39.Santos-Sacchi J The temperature dependence ofelectrical coupling in the organ of Corti. Hear Res 1986;21:205–11. [DOI] [PubMed] [Google Scholar]
- 40.Ohlemiller KK, Siegel JH. The effects of moderate cooling on gross cochlear potentials in the gerbil: Basal and apical differences. Hear Res 1992;63:79–89. [DOI] [PubMed] [Google Scholar]
- 41.Geal-Dor M, Khvoles R, Sohmer H. Cooling induces a decrease in middle ear compliance. J Basic Clin Physiol Pharmacol 1997; 8:127–32. [DOI] [PubMed] [Google Scholar]
- 42.Veuillet E, Gartner M, Champsaur G, Neidecker J, Collet L. Effects of hypothermia on cochlear micromechanical properties in humans. J Neurol Sci 1997;145:69–76. [DOI] [PubMed] [Google Scholar]
- 43.Seifert E, Brand K, van de Flierdt K, Hahn M, Riebandt M, Lamprecht-Dinnesen A. The influence of hypothermia on outer hair cells of the cochlea and its efferents. Br J Audiol 2001;35:87–98. [DOI] [PubMed] [Google Scholar]
- 44.Mietzsch U, Parikh NA, Williams AL, Shankaran S, Lasky RE. Effects of hypoxic-ischemic encephalopathy and whole-body hypothermia on neonatal auditory function: A pilot study. Am J Perinatol 2008;25:435–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hall JW 3rd, Bull JM, Cronau LH. Hypo- and hyperthermia in clinical auditory brain stem response measurement: Two case reports. Ear Hear 1988;9:137–43. [DOI] [PubMed] [Google Scholar]
- 46.Seifert E, Lamprecht-Dinnesen A, Asfour B, Rotering H, Bone HG, Scheld HH. The influence of body temperature on transient evoked otoacoustic emissions. Br J Audiol 1998;32:387–98. [DOI] [PubMed] [Google Scholar]
- 47.Henry KR, Chole RA. Hypothermia protects the cochlea from noise damage. Hear Res 1984;16:225–30. [DOI] [PubMed] [Google Scholar]
- 48.Henry KR. Hyperthermia exacerbates and hypothermia protects from noise-induced threshold elevation of the cochlear nerve envelope response in the C57BL/6J mouse. Hear Res 2003;179:88–96. [DOI] [PubMed] [Google Scholar]
- 49.Watanabe F, Koga K, Hakuba N, Gyo K. Hypothermia prevents hearing loss and progressive hair cell loss after transient cochlear ischemia in gerbils. Neuroscience 2001;102:639–45. [DOI] [PubMed] [Google Scholar]
- 50.Hyodo J, Hakuba N, Koga K, et al. Hypothermia reduces glutamate efflux in perilymph following transient cochlear ischemia. Neuroreport 2001;12:1983–7. [DOI] [PubMed] [Google Scholar]
- 51.Eshraghi AA, Nehme O, Polak M, et al. Cochlear temperature correlates with both temporalis muscle and rectal temperatures. Application for testing the otoprotective effect ofhypothermia. Acta Otolaryngol 2005;125:922–8. [DOI] [PubMed] [Google Scholar]
- 52.Smith LP, Eshraghi AA, Whitley DE, van de Water TR, Balkany TJ. Induction of localized cochlear hypothermia. Acta Otolaryngol 2007;127:228–33. [DOI] [PubMed] [Google Scholar]
- 53.Takeda S, Hakuba N, Yoshida T, et al. Postischemic mild hypothermia alleviates hearing loss because of transient ischemia. Neuroreport 2008;19:1325–8. [DOI] [PubMed] [Google Scholar]
- 54.Hato N, Hyodo J, Takeda S, et al. Local hypothermia in the treatment of idiopathic sudden sensorineural hearing loss. Auris Nasus Larynx 2010;37:626–30. [DOI] [PubMed] [Google Scholar]
- 55.Tamames I, King C, Bas E, Dietrich WD, Telischi F, Rajguru SM. A cool approach to reducing electrode-induced trauma: Localized therapeutic hypothermia conserves residual hearing in cochlear implantation. Hear Res 2016;339:32–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Henry KR, Guess MB, Chole RA. Hyperthermia increases aminoglycoside ototoxicity. Acta Otolaryngol 1983;95:323–7. [DOI] [PubMed] [Google Scholar]
- 57.Spankovich C, Lobarinas E, Le Prell CG. Diametrric effect of “localized” thermal exposure on cisplatin induced ototoxicity. In Association for Research in Otolaryngology Midwinter Meeting. San Diego, CA 2014. [Google Scholar]
- 58.Spankovich C, Lobarinas E, Ding D, Salvi R, Le Prell CG. Assessment of thermal treatment via irrigation of external ear to reduce cisplatin-induced hearing loss. Hear Res 2016;332:55–60. [DOI] [PubMed] [Google Scholar]
- 59.Morgan DS, Arteaga AA, Bosworth NA, et al. Repeated temporary threshold shift and changes in cochlear and neural function. Hear Res 2019;381:107780. [DOI] [PubMed] [Google Scholar]
- 60.Harrison RT, Seiler BM, Bielefeld EC. Ototoxicity of 12 mg/kg cisplatin in the Fischer 344/NHsd rat using multiple dosing strategies. Anticancer Drugs 2016;27:780–6. [DOI] [PubMed] [Google Scholar]
- 61.Wilkinson D, Ade KK, Rogers LL, et al. Preventing episodic migraine with caloric vestibular stimulation: A randomized controlled trial. Headache 2017;57:1065–87. [DOI] [PubMed] [Google Scholar]
- 62.Kimura RS. Animal models of inner ear vascular disturbances. Am J Otolaryngol 1986;7:130–9. [DOI] [PubMed] [Google Scholar]
- 63.Clark ER, Clark EL. Observations on living arterio-venous anastomoses as seen in transparent chambers introduced into the rabbit’s ear. Am J Anat 1934;54:229, 407–467. [Google Scholar]
- 64.Perlman HB, Kimura R, Butler RA. Cochlear blood flow during hypothermia. Ann Otol Rhinol Laryngol 1959;68:803–15. [DOI] [PubMed] [Google Scholar]
- 65.Miller JM, Goodwin PC, Marks NJ. Inner ear blood flow measured with a laser Doppler system. Arch Otolaryngol 1984;110:305–8. [DOI] [PubMed] [Google Scholar]
- 66.Miller JM, Ren TY, Nuttall AL. Studies of inner ear blood flow in animals and human beings. Otolaryngol Head Neck Surg 1995;112:101–13. [DOI] [PubMed] [Google Scholar]
- 67.Miettinen S, Laurell G, Andersson A, Johansson R, Laurikainen E. Blood flow-independent accumulation of cisplatin in the guinea pig cochlea. Acta Otolaryngol 1997;117:55–60. [DOI] [PubMed] [Google Scholar]
- 68.Konishi T, Salt AN, Hamrick PE. Effects of hypothermia on ionic movement in the guinea pig cochlea. Hear Res 1981;4:265–78. [DOI] [PubMed] [Google Scholar]
- 69.Hellberg V, Wallin I, Eriksson S, et al. Cisplatin and oxaliplatin toxicity: importance of cochlear kinetics as a determinant for ototoxicity. J Natl Cancer Inst 2009;101:37–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hellberg V, Wallin I, Ehrsson H, Laurell G. Cochlear pharmacokinetics of cisplatin: An in vivo study in the guinea pig. Laryngoscope 2013;123:3172–7. [DOI] [PubMed] [Google Scholar]
- 71.Laurell G, Andersson A, Engstrom B, Ehrsson H. Distribution of cisplatin in perilymph and cerebrospinal fluid after intravenous administration in the guinea pig. Cancer Chemother Pharmacol 1995;36:83–6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


