Abstract
The central amygdala (CeA) is a critical regulator of emotional behavior that has been implicated in psychiatric illnesses, including anxiety disorders and addiction. The CeA corticotropin releasing factor receptor 1 (CRF1) system has been implicated in alcohol use disorder (AUD) and mood disorders, and has been shown to regulate anxiety-like behavior and alcohol consumption in rodents. However, the effects of CRF signaling within the CRF receptor 1-containing (CRF1+) population of the CeA remain unclear, and the effects of ethanol and CRF1 manipulations in female rodents have not been assessed. Here, we characterized inhibitory control and CRF1 signaling in male and female CRF1-GFP reporter mice. Male and female CRF1+ CeA neurons exhibited similar baseline GABAergic signaling and excitability and were comparably sensitive to CRF-induced increases in presynaptic GABA release. CRF1 antagonism reduced GABA release onto CRF1-containing neurons comparably in both males and females. Acute ethanol application reduced GABA release onto CRF1+ neurons from males, but female CRF1+ neurons were insensitive to ethanol. Exogenous CRF increased the firing rate of CRF1-containing neurons to a greater extent in male cells versus female cells, and CRF1 antagonism reduced firing in females but not males. Together, these findings indicate a critical sex-specific role for the CRF system in regulating inhibitory control and excitability of CRF1-containing neurons in the central amygdala. Sex differences in sensitivity of CRF/CRF1 signaling provide useful context for the sex differences in psychiatric illness reported in human patients, particularly AUD.
Keywords: CRF, female, GABA, CeA, R121919, Astressin 2B
INTRODUCTION
Decades of scientific effort have identified the amygdala as a brain region critical in the expression of emotional behavior. A major function of the amygdala is to assign emotional significance to salient environmental stimuli (Tye, 2018), making this region a site of dysregulation in psychiatric disorders with affective components, including anxiety and alcohol use disorder. The central amygdala (CeA) is a predominantly GABAergic nucleus that is one of the major output regions of the amygdala and has been shown to functionally regulate both alcohol self-administration behavior (Hyytia and Koob, 1995; Roberts et al., 1996) and anxiety-related and fear learning behaviors (Maren and Quirk, 2004; Tye et al., 2011; Ventura-Silva et al., 2013). These findings suggest that neurons within the CeA that are sensitive to the effects of stress and/or drugs of abuse may play a major role in the physiological function of the amygdala as well as the development of psychiatric illness.
Corticotropin releasing factor (CRF) is a peptide expressed throughout the limbic system that has been shown to regulate behaviors associated with emotionally salient stimuli, including stress responsivity, anxiety/fear, reward and aversion (Agoglia and Herman, 2018). CRF exerts these effects through two receptors, CRF1 and CRF2, that are expressed both pre- and postsynaptically in many key limbic regions, including the CeA (Van Pett et al., 2000). Within the CeA, CRF/CRF1 signaling has been shown to regulate GABAergic activity: GABA and CRF colocalize within the CeA (Day et al., 1999; Partridge et al., 2016), and exogenous CRF enhances GABA release in a CRF1-dependent manner (Nie et al., 2004; Roberto et al., 2010; Kang-Park et al., 2015). The use of CRF and CRF1 reporter mice has enabled the characterization of distinct cell populations within the amygdala and allowed investigation of drug and stress effects on this circuitry. CRF neurons of the CeA have been shown to regulate anxiety-like behavior (Pomrenze et al., 2019b) and are required for the expression of fear learning (Pomrenze et al., 2019a). In the lateral CeA, CRF1-containing (CRF1+) neurons exhibit long-term potentiation following fear conditioning and are required for the acquisition of fear learning behavior (Sanford et al., 2017), and CRF+ neurons regulate the expression of conditioned flight (Fadok et al., 2017). In the medial CeA, CRF1+ neurons exhibit GABAA receptor-mediated tonic inhibition, receive local inhibitory control from CRF1-lacking neurons (CRF1-), and project to downstream regions of the extended amygdala, notably the bed nucleus of the stria terminalis (Herman et al., 2013a). Chronic exposure to ethanol dysregulates this population by disrupting tonic inhibitory control of CRF1+ neurons, leading to increased CRF1+ output to downstream regions (Herman et al., 2016). Thus, the CRF1+ cell population of the CeA is an important common target for the effects of both stress and drug exposure.
Despite significant progress in dissecting CRF1 circuitry within the CeA, important limitations remain an obstacle to understanding the complex role of this circuitry in both normal and diseased states. Although CRF1+ neurons have been identified as important targets for the effects of stress and drugs of abuse such as ethanol, the function of CRF/CRF1 signaling within this population has not yet been explored. The complex local architecture of the CeA and the multifaceted role of CRF1+ neurons within this region make determination of the effects of CRF/CRF1 signaling in this specific population crucial. Additionally, work in the CRF1+ population thus far has been conducted exclusively in male mice, leaving potential sex differences an understudied area. This lack of knowledge regarding the female CRF1 system has particular importance for the study of alcohol use disorder, as recent findings in human patients and preclinical animal models have pointed to important differences in the response of the CRF signaling system to alcohol in males and females (Agoglia et al., 2020). The present studies utilized a BAC transgenic CRF1 reporter mouse line and a combined immunohistochemical and electrophysiological approach to address two main goals: 1) Investigation of sex differences in baseline GABAergic synaptic transmission, effects of CRF1 activation and inhibition, and effects of ethanol in medial CeA CRF1+ neurons from male and female mice and 2) Characterization and investigation of sex differences in the effects of CRF1 activation and inhibition on neuronal firing in medial CeA CRF1+ neurons from male and female mice.
MATERIALS AND METHODS
Animals
All experiments were conducted in male and female adult mice (3–8 months old, 19–30 g) from a BAC transgenic line expressing green fluorescent protein (GFP) under the control of the CRF1 promotor (Justice et al., 2008). The CRF1 receptor has been shown to be active in GFP+ neurons in this mouse line (Justice et al., 2008; Jiang et al., 2018), and the cellular effects of CRF on GFP+ neurons can be prevented through the application of CRF receptor antagonists (Ramot et al., 2017; Jiang et al., 2018). Mice were bred in our colony at the University of North Carolina at Chapel Hill and maintained in a temperature and humidity-controlled vivarium with ad libitum access to food and water. All procedures were carried out in accordance with the NIH Guide for the Care and Use of Laboratory Animals and approved by the University of North Carolina at Chapel Hill Institutional Animal Care and Use Committee.
Immunohistochemistry
CRF1:GFP mice (n=6/sex) were deeply anesthetized with pentobarbital (150 mg/kg, i.p.) and transcardially perfused with 1X phosphate-buffered saline (PBS, Thermo-Fisher Scientific) followed by 4% paraformaldehyde. After 24hr of postfixing in paraformaldehyde, brains were transferred to sterile 30% sucrose in PBS for 24–48 hours at 4°C or until brains sank. Brains were sliced into 35 μM sections using a cryostat (Leica CM3050S, Leica Biosystems) and stored at 4°C in PBS containing 0.01% sodium azide.
For GFP immunoreactivity, sections were washed in PBS with gentle agitation for 10 minutes at room temperature (RT) and then blocked for 1 hour at RT in blocking solution [0.3% triton X-100 (Thermo-Fisher), 1mg/ml bovine serum albumin (BSA; Sigma) and 5% normal goat serum (NGS; Sigma)]. Primary chicken anti-GFP antibody (Abcam; ab13970, RRID:AB 300798; 1:2000) was incubated at 4°C for 48hr with gentle agitation in 0.5% tween-20 (Thermo-Fisher) and 5% NGS. Sections were washed 3X in PBS with gentle agitation at RT for 10 minutes followed by 1 hour incubation in Alexa Fluor 488 goat anti-chicken secondary antibody (Thermo Fisher; A-11039, RRID:AB 142924, 1:700) in PBS shielded from light. Sections were then washed for 10 minutes at RT 3X and mounted on slides in Vectashield (Vector labs; H1500, RRID:AB 2336788).
A total of 10 sections from male mice and 9 sections from female mice were imaged for quantification of GFP+ cell counts in bilateral CeA. Slices were imaged on a Leica SP8 laser scanning confocal microscope (Leica Biosystems). All microscope settings were kept the same within experiments during image acquisition. Analysis was performed manually at four anterior-posterior levels (equidistant sections located −1.00 through −1.70 mm from bregma) using ImageJ software (National Institute of Health).
Electrophysiological Recording
Subjects (n=31 male/36 female) were rapidly decapitated and brains removed to a beaker containing ice-cold sucrose solution containing (in mM): sucrose 206.0; KCl 2.5; CaCl2 0.5; MgCl2 7.0; NaH2PO4 1.2; NaHCO3 26; glucose 5.0; HEPES 5. A vibrating microtome (Leica VT1000S, Leica Microsystems) was used to slice 300 μm coronal sections which were next incubated in oxygenated (95% O2/5% CO2) artificial cerebrospinal fluid (aCSF) containing (in mM): NaCl 120; KCl 2.5; EGTA 5; CaCl2 2.0 MgCl2 1.0; NaH2PO4 1.2; NaHCO3 26; Glucose 1.75; HEPES 5 for 30 min at 37 °C, followed by 30 min at room temperature (RT, 21–22 °C) for equilibration. Patch pipettes (4–6 MΏ; King Precision Glass Inc.) filled with internal solution containing (in mM) potassium chloride (KCl) 145; EGTA 5; MgCl2 5; HEPES 10; Na-ATP 2; Na-GTP 0.2 were used to acquire all recordings. Data were acquired with a Multiclamp 700B amplifier (Molecular Devices), low pass filtered at 2–5 kHz, digitized (Digidata 1440A; Molecular Devices), and stored on a computer using pClamp 10 software (Molecular Devices). Series resistance was continuously monitored with hyperpolarizing and depolarizing 10 mV pulses; neurons with access resistance >15 MΩ were excluded from the data set. CRF1 receptor-containing medial CeA neurons were identified by GFP expression using fluorescent optics and brief (<2 s) episcopic illumination in slices from CRF1:GFP reporter mice.
Whole-cell (voltage clamp and current clamp) and juxtacellular (cell-attached) recordings were made from labeled and unlabeled CeA neurons. Electrophysiological properties of neurons were determined during voltage-clamp recording by pClamp 10 Clampex software using a 10 mV pulse delivered after breaking into the cell (Vhold= −60mV). A step protocol of hyperpolarizing to depolarizing current injections in whole-cell current-clamp recordings was used to determine the cell type of CeA neurons by spiking characteristics. Isolation of GABAA spontaneous inhibitory postsynaptic currents (sIPSCs) was achieved through selective pharmacological blockade with the glutamate receptor blockers 6,7-dinitroquinoxaline-2,3-dione (DNQX, 20μM) and DL-2-amino-5-phosphonovalerate (AP-5, 50 μM) and the GABAB receptor antagonist CGP55845A (1μM). Juxtacellular recordings were made by forming a seal on the target cell membrane and leaving the membrane intact with no negative pressure or holding conditions. Cells were recorded for a stable baseline period prior to experimental conditions and only cells that displayed stable regular firing were included in experimental analyses. Recordings were made absent pharmacological blockade except where noted
Drugs
Ethanol for acute application to slice was prepared using 95% ethyl alcohol (Pharmco Products Inc.) in aCSF. DNQX (20μM), AP-5 (50μM), CGP55845A (1μM) and CRF peptide (200nM) were purchased from Tocris Bioscience. The CRF1 antagonist R121919 (1μM) was supplied by Neurocrine Biosciences, Inc. (San Diego, CA.) All stock solutions were prepared in ultrapure water and diluted in aCSF on the day of recording, with the exception of AP-5, which was prepared in DMSO and diluted 1:1000 in aCSF. DNQX, AP-5, and CGP55845A were superfused in the bath solution continuously throughout all sIPSC recordings. Cell-attached firing was recorded in the presence of aCSF only, as previous work suggests that DNQX, AP-5 and CGP55845A application do not significantly alter the discharge rate of CeA CRF1+ neurons (Herman et al., 2013a). Ethanol (5–7min total exposure time), CRF (10–12 min total exposure time) and R121919 (10–12 min total exposure time) were administered via a y-tube positioned for focal application to CeA neurons.
Data Analysis
All data analyses and visualizations were completed with Prism v.7.0 (GraphPad). GFP+ cell counts are represented as mean ± SEM and compared via unpaired two-tailed t-test. Membrane characteristics between males and females are represented as mean ± SEM and were compared via two-tailed unpaired t-test. Frequency and amplitude of sIPSCs were analyzed and visually confirmed using a semi-automated threshold-based detection software (Mini Analysis, Synaptosoft Inc.). sIPSC characteristics were determined from baseline and experimental drug application over a 5 min maximum effect period and containing a minimum of 65 events. Cell-attached firing frequency was determined using Clampfit 10.2 (Molecular Devices). Event data were represented as mean ± SEM or mean % change from baseline ± SEM and analyzed for independent significance using a one-sample t-test and compared via unpaired two-tailed t-test for independent samples (with Welch’s correction where appropriate), paired two-tailed t-test for comparisons made within the same recording, and two-way repeated-measures (RM) ANOVA for comparisons made between 2 or more groups (sex X dose). All data sets were subjected to outlier analysis using Grubb’s test; significant outliers were removed where noted in text. α was set at ≤ 0.05 for all comparisons.
RESULTS
Identification, quantification, and inhibitory control of CRF1+ neurons in CeA
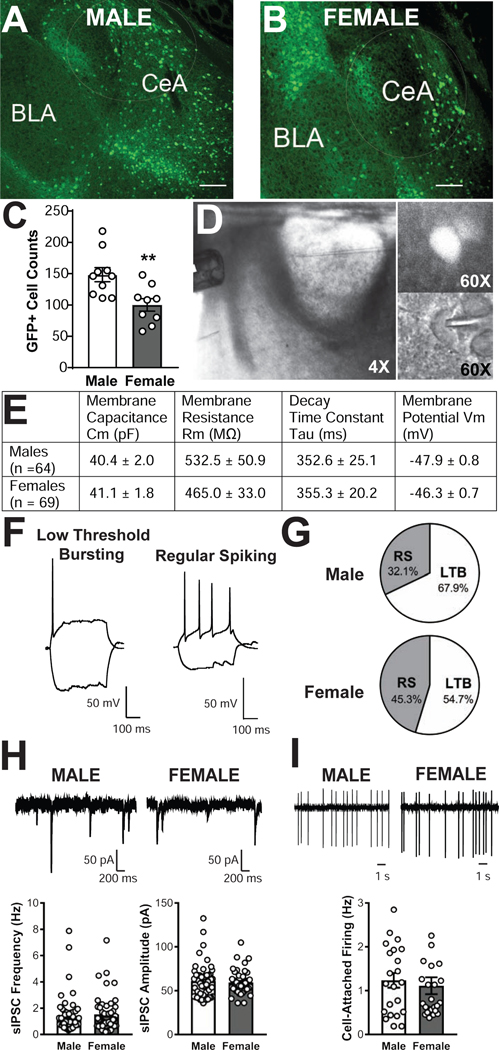
The number of CRF1+ neurons in the male and female CeA was compared using immunohistochemical amplification of the GFP signal. Using an unbiased quantification of CRF1+ neurons in serial sections, we observed that there were significantly more CRF1+ neurons in the male (148 ± 11.1; 10 sections from 6 mice, Figure 1A) as compared to the female (100 ± 10.1; 9 sections from 6 mice, Figure 1B) CeA [t (17) = 3.179, p = .0055; Figure 1C].
Figure 1. Characterization of CRF1+ neurons in the male and female CeA.
Representative 10x photomicrographs of GFP expression in coronal CeA slices from male (A) and female (B) CRF1:GFP mice. Scale bar = 100μM. (C) Quantification of GFP+ cell counts in male and female CeA. ** = p < 0.01 via unpaired t-test. (D) 4x magnification photomicrograph depicting recording configuration of medial central amygdala (CeA) with y-tube and recording pipette (left), with insets depicting a GFP+ neuron in florescent optics (60x magnification, upper right) and in infrared differential interference contrast optics (60x magnification, lower right). (E) Summary of membrane characteristics of male and female CRF1+ CeA neurons. (F) Representative current clamp recordings illustrating low-threshold bursting (left) and regular spiking (right) firing types exhibited by CeA CRF1+ neurons. (G) Representation of each firing type in CeA neurons from male (upper) and female (lower) CeA. (H) Representative voltage-clamp recordings showing baseline sIPSCs from male (upper left) and female (upper right) CRF1+ CeA neurons and quantification of sIPSC frequency (lower left) and amplitude (lower right) of male and female CRF1+ CeA neurons.(I) Representative cell-attached recordings showing baseline firing in male (upper left) and female (upper right) CRF1+ CeA neurons and quantification (lower) of discharge rate.
A representative coronal section of CRF1:GFP mouse brain containing the CeA with y-tube and recording pipette in place is depicted in Figure 1D, left (4x magnification). CRF1+ neurons were visually differentiated from unlabeled neurons as previously described (Herman et al., 2013a; Herman et al., 2016). A representative GFP-labeled CRF1+ neuron is shown in fluorescent optics (Figure 1D, 60x magnification, top right) and in infrared differential interference contrast optics (Figure 1D, 60x magnification, bottom right). Membrane characteristics of male (n = 64 neurons) and female (n = 69 neurons) CRF1+ neurons are shown in Figure 1E; no significant sex differences in membrane capacitance, membrane resistance, time constant tau or resting membrane potential emerged. CRF1+ neurons were cell-typed using whole-cell current injections and firing properties as previously described (Chieng et al., 2006; Herman et al., 2013a; Herman et al., 2016). Consistent with prior reports, both male (n = 64 neurons) and female (n = 69 neurons) CRF1+ CeA neurons exhibited two firing patterns: low-threshold bursting (LTB; Figure 1F, left) and regular spiking (RS; Figure 1F, right). LTB was the most common firing pattern observed in male (68%; Figure 1G, top) and female CRF1+ neurons (55%; Figure 1G, bottom).
We next used whole-cell voltage clamp recordings of GABAA-mediated sIPSCs to assess baseline inhibitory transmission in male and female CRF1+ neurons (Figure 1H, top). No sex difference in baseline sIPSC frequency was observed, t(15) = 1.01, p = 0.326. Average male sIPSC frequency was 1.4 ± 0.2 Hz (n = 52 cells from 30 mice) and average female sIPSC frequency was 1.5 ±0.2 Hz (n = 50 cells from 32 mice), with no significant sex difference [t(100) = 0.34, p = 0.733; Figure 1H, bottom left]. Male sIPSC amplitude averaged 61.0 ± 2.7 pA and female sIPSC amplitude averaged 59.5 ± 1.8 pA, with no significant sex difference [t(89.2) = 0.46, p = 0.643; Figure 1H, bottom right]. We also examined the excitability of male and female CRF1+ CeA neurons via cell-attached recordings of neuronal firing (Figure 1I, top). Cell-attached firing frequency in males was 1.24 ± 0.17 Hz (n = 22 neurons from 15 mice) versus females 0.94 ± 0.12 Hz (n = 24 neurons from 18 mice), with no sex differences in firing observed [t(44) = 0.48, p = 0.633; Figure 61I, bottom.) Neurons that did not fire under basal conditions were observed but were excluded from these experiments.
Sensitivity of male and female CeA neurons to CRF1 activation
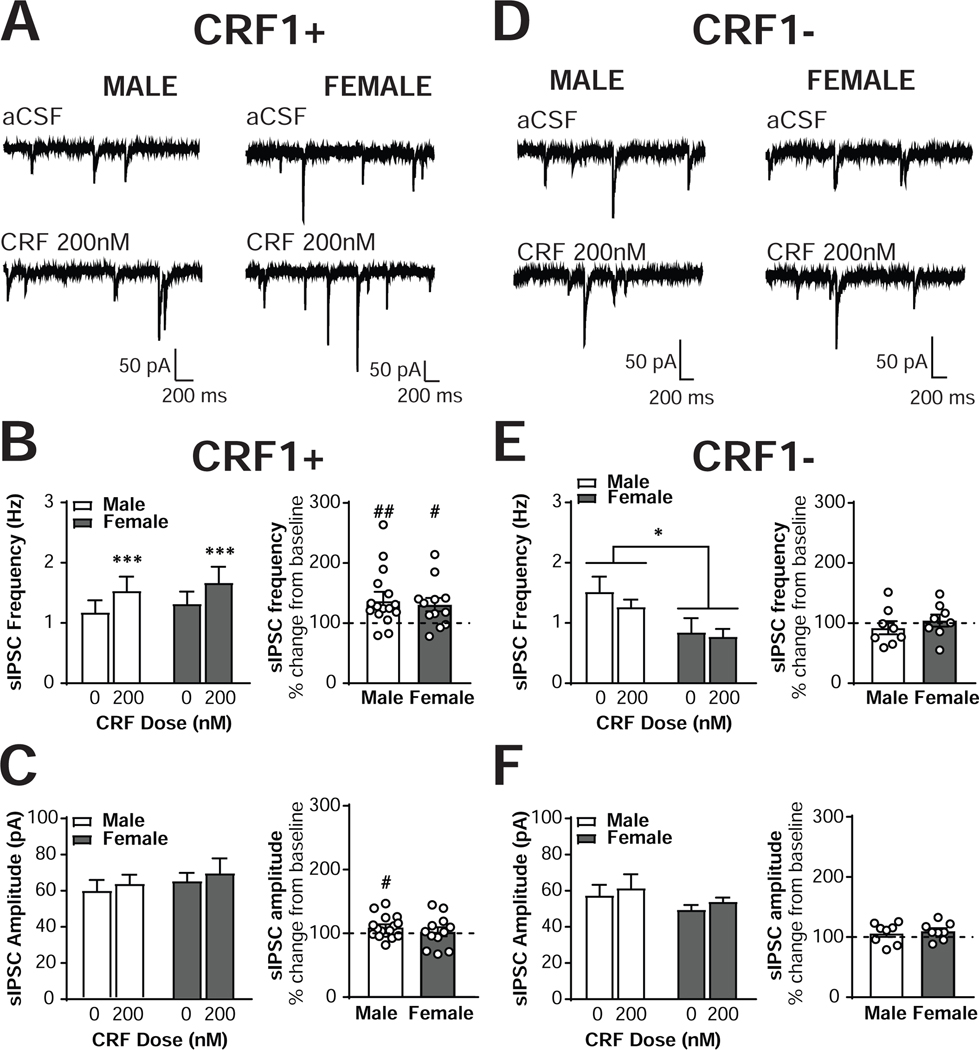
To determine the effects of CRF receptor activation on inhibitory control of CRF1+ CeA neurons, we next assessed the effects of exogenous CRF application (200 nM) on sIPSCs (Figure 2A). CRF1+ neurons from both sexes displayed an increase in sIPSC frequency in response to CRF [main effect of CRF, F(1,26) = 15.8, p = 0.0005], with CRF1+ neurons from male mice (n = 16 cells from 14 mice) increasing from 1.18 ± 0.20 Hz to 1.53 ± 0.24 Hz and female CRF1+ neurons (n = 12 cells from 10 mice) increasing from 1.32 ± 0.20 Hz to 1.65 ± 0.26 Hz (Figure 2B, left). These effects represented a significant percent increase from baseline in both males [137 ±11.9% of baseline frequency; t(15) = 3.11, p = 0.0071] and females [130.8 ±11.1% of baseline frequency; t(11) = 2.78, p = 0.018], and the magnitude of the increase was comparable in both sexes [t(26) = 0.37, p = 0.7132; Figure 2B, right]. No main effect of sex [F(1,26) = 0.20, p = 0.6593] or interaction [F(1,26) = 0.00, p = 0.9743] emerged. The effects of CRF on sIPSC frequency in one male neuron (348% percent of baseline) and one female neuron (434% of baseline) were identified as significant outliers via Grubb’s test and excluded from all analyses. The exclusion of these outliers did not alter the significance or lack thereof of any effects. We did not observe any effect of CRF on sIPSC amplitude [F(1,26) = 2.59, p = 0.1197], indicating that CRF did not alter sIPSC amplitude in either male or female CRF1+ CeA neurons. In male CRF1+ neurons, sIPSC amplitude changed from 56.9 ± 5.32 pA to 60.7 ± 4.49 pA, and from 61.1 ±4.27 pA to 66.2 ±7.28 pA in female neurons; Figure 2C, left). When analyzed as percent change from baseline, male neurons demonstrated a significant increase [109.9 ±4.3% of baseline, t(15) = 2.27, p = 0.0381] whereas female neurons did not [107.9 ±7.2% of baseline, t(11) = 0.63, p = 0.5399; Figure 2C, right]. However, the magnitude of the change in both sexes was not different [t(26) = 0.65, p = 0.5230]. No main effect of sex [F(1,26) = 0.61, p = 0.4412] or interaction [F(1,26) = 0.008, p = 0.9310] emerged. These findings indicate that activation of CRF receptors enhances inhibitory transmission onto CRF1+ neurons in the CeA in both males and females, with no sex difference in the presynaptic release of GABA.
Figure 2. CRF increases sIPSC frequency in male and female CRF1+, but not CRF1-, CeA neurons.
(A) Representative voltage-clamp recordings from male (left) and female (right) CRF1+ CeA neurons during focal application of aCSF (top) or CRF (200nM; bottom). (B) Quantification of sIPSC frequency in male and female CRF1+ CeA neurons following aCSF or CRF (200nM) focal application expressed in Hz (left) and percent of control (right). (C) Quantification of sIPSC amplitude in male and female CRF1+ CeA neurons following aCSF or CRF (200nM) focal application expressed in picoamps (left) and percent of control (right). (D) Representative voltage-clamp recordings from male (left) and female (right) CRF1- CeA neurons during focal application of aCSF (top) or CRF (200nM; bottom). (E) Quantification of sIPSC frequency in male and female CRF1- CeA neurons following aCSF or CRF (200nM) focal application expressed in Hz (left) and percent of control (right). (F) Quantification of sIPSC amplitude in male and female CRF1- CeA neurons following aCSF or CRF (200nM) focal application expressed in picoamps (left) and percent of control (right). * = p < 0.05 by two-way RM ANOVA, *** = p < 0.001 by two-way RM ANOVA, # = p < 0.05 by one-sample t-test, ## = p < 0.01 by one-sample t-test.
We next assessed whether CRF1- neurons would also respond to an acute application of CRF. Male and female CRF1- neurons exhibited comparable membrane characteristics (n= 11 cells from 7 male mice and n= 12 cells from 8 female mice; Supplemental Figure 1A). No sex differences in baseline sIPSC frequency [n= 9 cells from 7 male mice and n= 8 cells from 7 female mice; t(10) = 0.41, p = 0.6942],amplitude [t(10) = 0.76, p = 0.4650] or cell-attached firing [n= 9 cells from 8 male mice and n= 13 cells from 10 female mice; t(20) = 0.74, p = 0.468],were observed in the CRF1- neurons (Supplemental Figure 1B), and no baseline cell type differences in sIPSC frequency [F(1,115) = 0.51, p =0.478],amplitude [F(1,115) = 0.75, p = 0.388], or cell-attached firing [F(1,64) = 0.06, p = 0.814] were noted in either sex (Supplemental Figure 1C). One female CRF1- neuron with a sIPSC frequency of 5.28 Hz was identified as a significant outlier via Grubb’s test and excluded from the analysis; the exclusion of this cell did not alter the significance of any comparison.
Focal application of CRF failed to alter sIPSC frequency in CRF1- neurons [F(1,14) = 1.93, p = 0.1864, Figure 2D], with male (n = 8 cells from 5 mice) frequency at 92.5 ± 11.0% of baseline [one sample t-test : t(7) = 0.69, p = 0.5142] and female (n = 8 cells from 6 mice) frequency at 104.5 ± 10.0% of baseline [one sample t-test: t(7) = 0.67, p = 0.6693; Figure 2E]. The non-significant reduction from baseline was of comparable magnitude in both sexes, t(14) = 0.81, p = 0.4327. A main effect of Sex on sIPSC frequency emerged, F(1,14) = 5.53, p = 0.0339, indicating that sIPSC frequency was higher in male versus female CRF1- neurons regardless of CRF treatment. This sex difference was visually evident but not significant in the larger baseline sIPSC frequency data set (Supplemental Figure 1B). CRF similarly did not alter sIPSC amplitude in CRF1- neurons [F(1,14) = 3.84, p = 0.0703], with male amplitude at 106.4 ± 6.1% of baseline [one sample t-test: t(7) = 1.06, p = 0.3259] and female amplitude at 109.9. ± 5.2% of baseline [one sample t-test: t(7) = 1.98, p = 0.0886; Figure 2F]. The size of these non-significant changes from baseline was comparable in male and female neurons, t(14) = 0.44, p = 0.6678. One male neuron with an increase in sIPSC frequency of 302% in response to CRF was identified as a significant outlier via Grubb’s test and excluded from the analyses. The exclusion of this neuron did not alter the significance or lack thereof for any comparison. Together, these data suggest that activation of CeA CRF1 receptors selectively increases presynaptic release of GABA onto CRF1+, but not CRF1-, neurons. Due to the lack of effect of CRF in the CRF1- population, subsequent sIPSC experiments focused solely on CRF1+ neurons.
Sensitivity of male and female CRF1+ neurons to CRF receptor inhibition
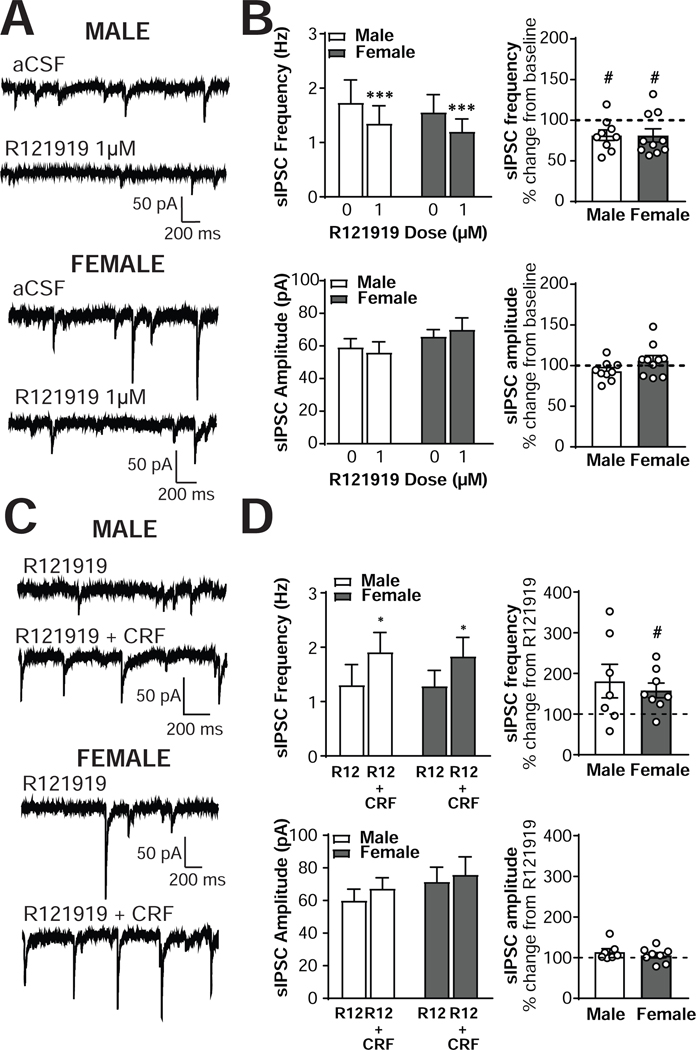
To examine the contributions of CRF1-dependent signaling on inhibitory transmission in CRF1+ neurons, we next assessed the effects of the CRF1-selective antagonist R121919 (1μM) on GABAA receptor sIPSCs (Figure 3A). R121919 produced a significant reduction in sIPSC frequency in both male (n = 9 cells from 9 mice) and female (n = 10 cells from 7 mice) CRF1+ neurons [main effect of R121919, F(1,17) = 15.9, p = 0.001; Figure 3B, top left] with CRF1+ neurons from male mice decreasing from 1.74 ±0.42 Hz to 1.35 ±0.33 Hz and female CRF1+ neurons decreasing from 1.56 ±0.36 Hz to 1.20 ±0.26 Hz. These effects represented a significant percent decrease from baseline in both sexes, with males decreasing to 81.6 ± 6.5 % of baseline [t(8) = 2.83, p = 0.022] and females to 81.3 ± 8.2% of baseline [t (9) = 2.293, p = 0.0475; Figure 3B, top right]. The size of the decrease was of similar magnitude in both sexes, [t(17) = 0.03, p = 0.9791], and no sex differences [F(1,17) = 0.13, p = 0.7217] or interactions [F(1,17) = 0.02, p = 0.8779] emerged. R121919 application did not alter sIPSC amplitude in either sex [F(1,17) = 0.04, p = 0.8532; Figure 3B, bottom left], with male amplitude at 93.4 ±4.0% of baseline [t(8) = 1.63, p = 0.1417] and female amplitude at 106.2 ±6.2% of baseline [t(9) = 1.01, p = 0.3385; Figure 3B, bottom right]. The magnitude of the non-significant changes from baseline was comparable in the two sexes [t(17) = 1.70, p = 0.1082], and no effect of sex [F(1,17) = 1.23, p =0.2061] or interaction [F(1,17) = 1.70, p = 0.2101] emerged. Two male CRF1+ neurons were determined to be significant outliers via Grubb’s test (one with a baseline sIPSC frequency of 8 Hz and one with an increase in sIPSC amplitude of 143% after R121919 application) and were excluded from all analyses. The exclusion of these outliers did not alter the overall significance (or lack thereof) of the effects between or within sexes. Together, these findings indicate that ongoing activity at the CRF1 receptor contributes to baseline GABA release onto CRF1+ CeA neurons in both males and females.
Figure 3. The CRF1 antagonist R121919 alters basal but not CRF-stimulated inhibitory control of male and female CRF1+ CeA neurons.
(A) Representative voltage-clamp recordings from male (top) and female (bottom) CRF1+ CeA neurons during focal application of aCSF (upper) or R121919 (1μM; lower). (B) Quantification of sIPSC frequency in male and female CRF1+ CeA neurons following aCSF or R121919 (1μM) focal application expressed in Hz (upper left) and percent of control (upper right). Quantification of sIPSC amplitude in male and female CRF1+ CeA neurons following aCSF or R121919 (1μM) focal application expressed in picoamps (lower left) and percent of control (lower right). (C) Representative voltage-clamp recordings from male (top) and female (bottom) CRF1+ CeA neurons during focal application of R121919 (1μM; upper) or R121919 (1μM) plus CRF (200nM) co-application (lower). (D) Quantification of sIPSC frequency in male and female CRF1+ CeA neurons following focal application of R121919 (1μM) and R121919 (1μM) plus CRF (200nM) co-application expressed in Hz (upper left) and percent of control (upper right). Quantification of sIPSC amplitude in male and female CRF1+ CeA neurons following focal application of R121919 (1μM) and R121919 (1μM) plus CRF (200nM) co-application expressed in picoamps (lower left) and percent of control (lower right). * = p < 0.05 by two-way RM ANOVA , *** = p < 0.001 by two-way RM ANOVA, # = p < 0.05 by one-sample t-test.
To determine the role of the CRF1 receptor in the effects of acute CRF application, we next treated CRF1+ neurons with R121919 (1μM) followed by co-application of both CRF (200 nM) and R121919 (1μM; Figure 3C). In both male (n = 7 cells from 7 mice) and female (n = 8 cells from 7 mice) CRF1+ neurons, R121919 pretreatment did not prevent the increase in sIPSC frequency induced by CRF [main effect of CRF; F (1,13) = 5.75, p = 0.0322; Figure 3D, top left]. CRF1+ neurons from male mice displayed an increase in sIPSC frequency from 1.46 ±0.36 Hz to 2.31 ±0.26 Hz, representing a non-significant 181.2 ± 41.3 % increase from R129191 alone [t(6) = 2.00, p = 0.0969], and female CRF1+ neurons increased from 1.23 ± 0.27 Hz to 1.71 ±0.33 Hz, a 158.2 ± 18.1% increase from R121919 alone [t(7) = 3.22, p = 0.0146; Figure 3D, top right]. The magnitude of the increase in sIPSC frequency induced by CRF + R121919 co-application was comparable in the two sexes, t(13) = 0.53, p = 0.6024. R121919 + CRF co-application did not produce any changes in sIPSC amplitude in either sex [F(1,13) = 3.30, p = 0.0922), with male amplitude at 114.3 ± 8.0% of baseline [t(6) = 1.80, p = 0.1224] and female amplitude at 105.8 ± 9.6% of baseline [t(7) = 0.89, p = 0.4050; Figure 3D, bottom]. The magnitude of the non-significant change in sIPSC amplitude induced by CRF + R121919 co-application was comparable in the two sexes, t(13) = 0.83, p = 0.4230. One male neuron with a 299% increase in sIPSC amplitude following R121919 + CRF application and one female neuron with a 256% increase in sIPSC amplitude following R121919 + CRF application were identified as significant outliers via Grubb’s test and were excluded from all analyses. The exclusion of these neurons did not alter the significance of the ANOVA comparisons or the between-group t-test, but did reduce the significance of the one-sample t-test for sIPSC frequency in male neurons from a significant increase (p = 0.050) to a trend (p =0 .0969). Female one-sample significance was unchanged. These findings indicate that the ability of CRF to enhance GABA release onto CRF1+ neurons is intact despite blockade of the CRF1 receptor. The modest CRF-induced increase in sIPSC amplitude observed in male neurons, however, was not evident in the CRF + R121919 co-application experiments, suggesting that blockade of the CRF1 receptor may inhibit the postsynaptic response to CRF in male neurons.
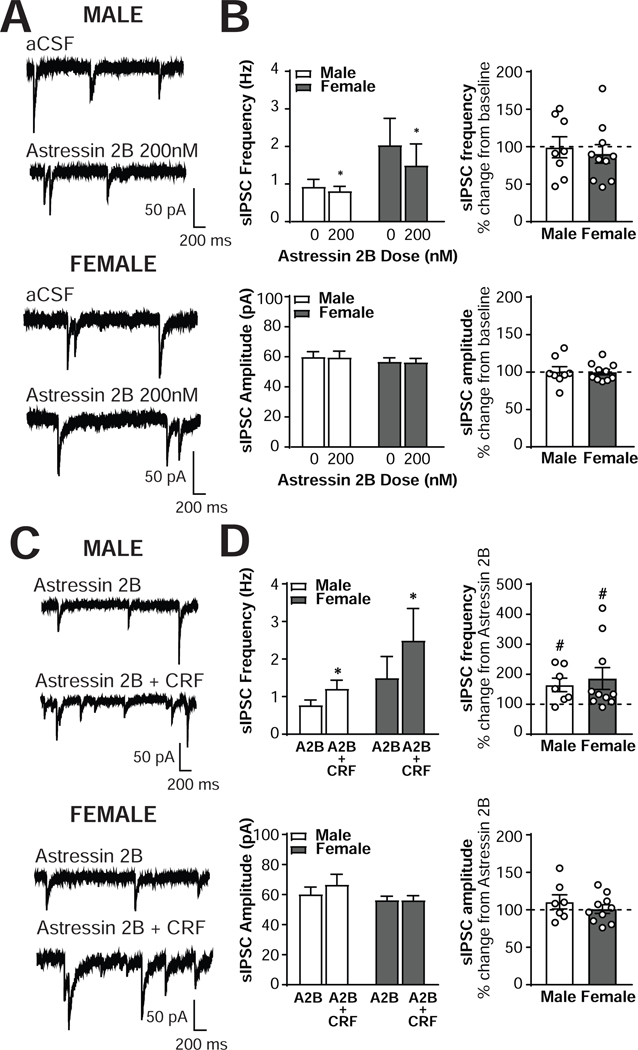
To determine the extent to which signaling at the CRF2 receptor contributes to the inhibitory control of CRF1+ neurons, we treated male (n = 8 cells from 4 mice) and female (n = 10 cells from 5 mice) CRF1+ CeA neurons with the CRF2 receptor antagonist Astressin 2B (200nM; Figure 4A). Astressin 2B application produced a small but significant reduction in sIPSC frequency in male and female neurons [main effect of Astressin 2B, F(1,16) = 5.221, p = 0.036; Figure 4B, top left]. Astressin 2B reduced male sIPSC frequency from 0.93 ±0.19 Hz to 0.82 ±0.12 Hz, a nonsignificant change of 99.2 ±14.0% from baseline [t(7) = 0.06, p = 0.9567; Figure 4B, top right]. In female CRF1+ neurons, Astressin 2B decreased sIPSC frequency from 2.04 ±0.78 Hz to 1.59 ±0.63 Hz, a non-significant decrease of 90.2 ±12.4% of baseline [t(9) = 0.79, p = 0.4506; Figure 4B, top right]. No significant sex differences [F(1,16) =1.52, p =0.2356] or interactions [F(1,16) = 2.15, p = 0.1621] emerged, and the size of the reduction in sIPSC frequency was comparable between the two sexes [t(16) = 0.48, p = 0.6372]. No significant effects of Astressin 2B on sIPSC amplitude emerged [F(1,16) = 0.03, p = 0.8707; Figure 4B bottom left and right), with male amplitude at 100.6 ± 6.5% of baseline [t(7) = 0.10, p = 0.9261] and female amplitude at 99.9 ± 3.7% of baseline [t(9) = 0.02, p = 0.9817]. No main effects of sex [F(1,16) = 0.67, p = 0.4258] or interactions [F(1,16) = 0.00, p = 0.9854] emerged, and the magnitude of the non-significant change in sIPSC amplitude was comparable between the two sexes [t(16) = 0.10, p = 0.9215]. These findings suggest that ongoing activity at the CRF2 receptor may influence baseline GABA release onto CRF1+ neurons.
Figure 4. The CRF2 antagonist Astressin 2B alters basal but not CRF-stimulated inhibitory control of male and female CRF1+ CeA neurons.
(A) Representative voltage-clamp recordings from male (top) and female (bottom) CRF1+ CeA neurons during focal application of aCSF (upper) or Astressin 2B (200nM; lower). (B) Quantification of sIPSC frequency in male and female CRF1+ CeA neurons following aCSF or Astressin 2B (200nM) focal application expressed in Hz (upper left) and percent of control (upper right). Quantification of sIPSC amplitude in male and female CRF1+ CeA neurons following aCSF or Astressin 2B (200nM) focal application expressed in picoamps (lower left) and percent of control (lower right). (C) Representative voltage-clamp recordings from male (top) and female (bottom) CRF1+ CeA neurons during focal application of Astressin 2B (200nM; upper) or Astressin 2B (200nM) plus CRF (200nM) co-application (lower). (D) Quantification of sIPSC frequency in male and female CRF1+ CeA neurons following focal application of Astressin 2B (200nM) and Astressin 2B (200nM) plus CRF (200nM) co-application expressed in Hz (upper left) and percent of control (upper right). Quantification of sIPSC amplitude in male and female CRF1+ CeA neurons following focal application of Astressin 2B (200nM) and Astressin 2B (200nM) plus CRF (200nM) co-application expressed in picoamps (lower left) and percent of control (lower right). * = p < 0.05 by two-way RM ANOVA, # = p < 0.05 by one-sample t-test.
To determine the role of the CRF2 receptor in the effects of acute CRF application, we next treated CRF1+ neurons with Astressin 2B (200 nM) followed by co-application of both CRF (200 nM) and Astressin 2B (200 nM; Figure 4C). In both male (n = 7 cells from 4 mice) and female (n = 10 cells from 5 mice) CRF1+ neurons, Astressin 2B pretreatment did not prevent the increase in sIPSC frequency induced by CRF [main effect of CRF; F (1,15) = 8.20, p = 0.0118; Figure 4D, top left]. CRF1+ neurons from male mice displayed an increase in sIPSC frequency from 0.78 ± 0.14 Hz to 1.21 ± 0.23 Hz, representing a significant 164.3 ± 22.5 % increase from baseline [t(6) = 2.859, p = 0.029], and female CRF1+ neurons increased from 1.50 ± 0.63 Hz to 2.49 ±0.93 Hz, a significant 185.7 ± 36.3% increase from baseline [t(9) = 2.36, p = 0.043; Figure 4D, top right]. The magnitude of the increase from baseline was comparable between the two sexes [t(15) = 0.45, p = 0.6602], and no main effect of sex [F(1,15) = 1.29, p = 0.2577] or interaction [F(1,15) = 1.26, p = 0.2791] emerged. Astressin 2B + CRF co-application did not produce any changes in sIPSC amplitude in either sex [F(1,15) = 1.13, p = 0.3041], with male amplitude at 110.6 ± 9.5% of baseline [t(6) = 1.12, p = 0.3057] and female amplitude at 101.1 ± 5.8% of baseline [t(9) = 0.18, p = 0.8595; Figure 4D, bottom]. The non-significant change from baseline was of comparable magnitude in both sexes [t(15) = 0.91, p = 0.3794], and no main effect of sex [F(1,15) = 1.92, p =0.1863] or interaction [F(1,15) = 1.17, p = 0.2964] emerged. These findings indicate that the ability of CRF to enhance GABA release onto CRF1+ neurons is intact despite blockade of the CRF2 receptor.
Sensitivity of male and female CRF1+ neurons to acute ethanol
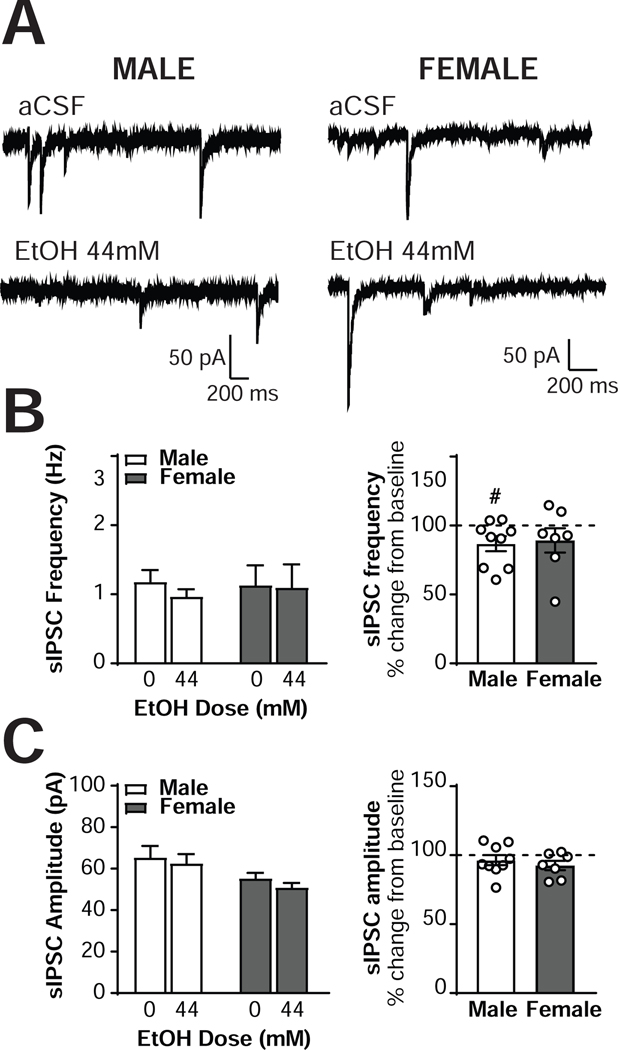
Having established that male and female CRF1+ neurons exhibit similar inhibitory control both at baseline and under the effects of focal CRF application, we next assessed the effects of exogenous ethanol application (44mM; Figure 5A) on sIPSCs in male (n = 9 neurons from 4 mice) and female (n= 7 neurons from 4 mice) CRF1+ neurons. A trend for a main effect of ethanol was evident [F(1,14) = 3.93, p = 0.068], with male sIPSC frequency decreasing from 1.18 ± 0.17 Hz to 0.97 ± 0.10 Hz; Figure 5B, left) and female sIPSC frequency decreasing from 1.13 ± 0.29 Hz to 1.10 ± 0.33 Hz. Analysis of the normalized data indicated that male CRF1+ neurons exhibited a significant 86.8 ± 5.5% decrease from baseline [t(8) = 2.41, p = 0.042], whereas females exhibited a nonsignificant 89.2 ± 8.8% decrease from baseline (p > 0.05; Figure 5B, right). The magnitude of the change from baseline was not significantly different between males and females, t(14) = 0.24, p = 0.8119. No sex differences in sIPSC frequency [F(1,14) = 0.02, p = 0.8967] or interactions of sex and ethanol [F(1,14) = 2.09, p = 0.1701] were evident. A trend for ethanol to decrease sIPSC amplitude in CRF1+ neurons was observed [main effect of ethanol, F(1,14) = 3.43, p = 0.0851]. Male sIPSC amplitude exhibited a nonsignificant decrease of 96.4 ± 3.6% from baseline [t(8) = 0.99, p = 0.3527] and female sIPSC amplitude exhibited a trend towards a significant decrease of 92.5 ± 3.4% from baseline [t(6) = 2.23, p = 0.0675; Figure 5C, left and right]. However, the size of the decrease was small and not different between the two sexes, t(14) = 0.77, p = 0.4553. A trend for a main effect of sex was also observed, F(1,14) = 3.60, p = 0.0786, but no significant interactions were detected [F(1,14) = 0.15, p = 0.7034]. These findings suggest that acute ethanol exposure reduces GABA release onto male CRF1+ neurons, but does not alter GABA release onto female CRF1+ neurons.
Figure 5. Ethanol alters inhibitory control of male but not female CRF1+ neurons.
(A) Representative voltage-clamp recordings from male (left) and female (right) CeA CRF1+ neurons during focal application of aCSF (top) and ethanol (44mM; bottom). (B) Quantification of sIPSC frequency in male and female CRF1+ neurons following aCSF or ethanol (44mM) focal application expressed in Hz (left) and percent of control (right). (C) Quantification of sIPSC amplitude in male and female CRF1+ neurons following focal application of aCSF or ethanol (44mM) expressed in picoamps (left) and percent of control (right). # = p < 0.05 by one-sample t-test.
Sensitivity of male and female CRF1+ and CRF1- neuronal excitability to CRF receptor activation
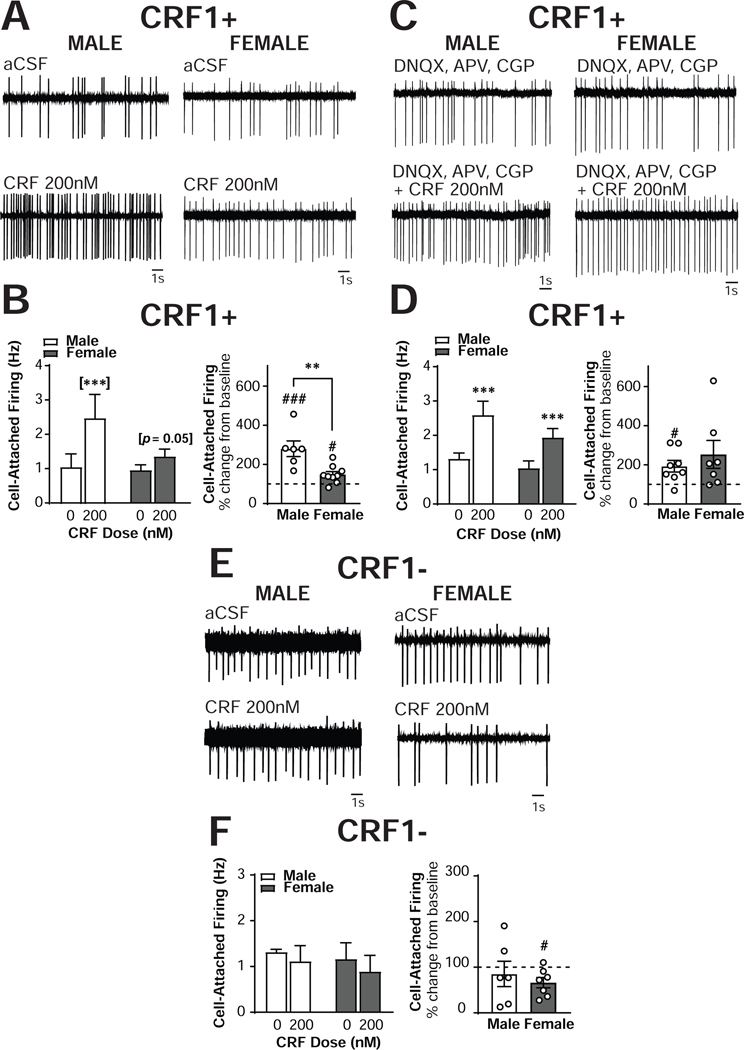
To determine the effects of CRF1 activation and inhibition on the firing rate of CRF1+ CeA neurons with all network transmission intact, spontaneously firing CRF1+ CeA neurons from male (n = 6 cells from 4 mice) and female (n= 9 cells from 8 mice) mice were recorded in the presence of aCSF only (absent pharmacological blockade used in sIPSC recordings; Figure 6A). Consistent with the baseline firing data (Figure 1I), no effect of sex emerged, F(1,13) = 1.75, p = 0.2082. Application of CRF (200nM) revealed a significant Sex X CRF interaction, F(1,13) = 10.24, p = 0.007, in addition to a main effect of CRF, F(1,13) = 32.43, p = 0.0001 (Figure 6B, left). Sidak’s multiple comparison test indicated that CRF significantly increased cell-attached firing in male CRF1+ CeA neurons from 1.07 ±0.38 Hz to 2.50 ±0.69 Hz (p = 0.0001) and exhibited a strong trend towards increased cell-attached firing in female CRF1+ CeA neurons from 0.95 ±0.15 Hz to 1.36 ±0.21 Hz (p = 0.0523) CRF1+ neurons (Figure 6B, left). When the data were transformed into percent of control, a significant sex difference was observed, t(13) = 3.55, p < 0.0036 (Figure 6B, right), indicating that the percent increase in firing elicited by CRF in male neurons was greater than the percent increase in female neurons. The magnitude of the increase in male neurons was 280.2 ±40.0 %, a significant increase from baseline [t(5) = 4.503, p = 0.0064], and in female neurons was a significant 148.7 ±15.0% of baseline [t(8) = 0.3.248, p = 0.0117; Figure 6D, right]. One female neuron was determined to be a significant outlier via Grubb’s test (with a percent increase of 481% following CRF application) and excluded from all analyses. The exclusion of this outlier allowed the between-sex t-test to achieve statistical significance, but did not alter the significance of the within-sex one sample t-tests, the ANOVA main effect, or the interaction. Therefore, although CRF significantly increased the firing rate of CRF1+ neurons from both male and female mice, the magnitude of the effect was significantly greater in male neurons.
Figure 6. Sex differences in CRF regulation of excitability in CRF1+, but not CRF1-, CeA neurons.
(A) Representative cell-attached recording of male (left) and female (right) CRF1+ CeA neurons during focal application of aCSF (top) and CRF (200nm; bottom). (B) Quantification of cell-attached firing frequency in male and female CRF1+ CeA neurons following focal application of aCSF and CRF (200nM) expressed in Hz (left) and percent of control (right). (C) Representative cell-attached recording of male (left) and female (right) CRF1+ CeA neurons during bath superfusion of DNQX, APV and CGP with focal application of aCSF (top) and CRF (200nm; bottom). (D) Quantification of cell-attached firing frequency in male and female CRF1+ CeA neurons following focal application of aCSF and CRF (200nM) in the presence of DNQX, APV and CGP expressed in Hz (left) and percent of control (right). (E) Representative cell-attached recording of male (left) and female (right) CRF11 CeA neurons during focal application of aCSF (top) and CRF (200nm; bottom). (F) Quantification of cell-attached firing frequency in male and female CRF1- CeA neurons following focal application of aCSF and CRF (200nM) expressed in Hz (left) and percent of control (right). ** = p < 0.01 by two-sample t-test, # = p < 0.05 by one-sample t-test, ## = p < 0.01 by one-sample t-test, [***] = p < 0.001 by Sidak’s multiple comparison’s test.
Next, CRF was focally applied to CRF1+ neurons during bath superfusion of the same pharmacological blockade used during sIPSC recordings (APV [NMDA antagonist], DNQX [AMPA antagonist], CGP [GABAB antagonist]) to evaluate the contributions of glutamatergic signaling to the effects of CRF and the sex differences in CRF sensitivity. A main effect of CRF emerged [F(1,13) = 23.76, p = 0.0003], indicating that CRF still enhanced cell-attached firing in CRF1+ neurons from both sexes in the presence of glutamatergic receptor blockade. In male CRF1+ neurons (n = 8 cells from 5 mice), CRF increased firing frequency from 1.32 ± 0.2 Hz to 2.59 ± 0.4 Hz. In female CRF1+ neurons (n = 7 cells from 5 mice), CRF increased firing frequency from 1.04 ± 0.2 Hz to 1.94 ± 0.3 Hz. Importantly, no significant interaction between Sex and CRF appeared in this analysis [F(1,13) = 0.72, p = 0.4103]. When data were analyzed as a percent change from baseline, the magnitude of the increase in cell-attached firing induced by CRF in males (193.4 ± 29.7%) and females (253 ± 71.1%) was comparable [t(13) = 0.82, p = 0.4281]. Males exhibited a significant increase from baseline [t(7) = 3.15, p = 0.0162]whereas females exhibited a trend towards an increase [t(6) = 2.16, p = 0.0743] despite their greater percent change. Therefore, although the effects of CRF on neuronal excitability do not require the NMDA or AMPA receptor, these signaling systems may contribute to sex differences in the effects of CRF in the CRF1+ population.
We also sought to determine whether CRF would alter the excitability of unlabeled CRF1- CeA neurons (Figure 6E). Focal application of CRF did not alter cell-attached firing in sIPSC neurons from either sex [F(1,11) = 2.05, p = 0.1805; Figure 6F, left]. Male CRF1- neurons (n = 6 cells from 5 mice) had a baseline firing rate of 1.32 ± 0.1 Hz and a CRF-induced firing rate of 1.11 ± 0.3 Hz. Female CRF1- neurons (n = 7 cells from 6 mice) had a baseline firing rate of 1.16 ± 0.4 Hz and a CRF-induced firing rate of 0.9 ± 0.4 Hz. When data were analyzed as a percent change from baseline, no significant differences in effect magnitude emerged [t(11) = 0.67, p = 0.5156; Figure 6F, right]. However, male neurons exhibited no change from baseline [t(5) = 0.52, p = 0.6230] whereas female neurons exhibited a significant decrease from baseline [t(6) = 2.96, p = 0.0252]. Male CRF1- neurons demonstrated an average CRF-induced non-significant reduction in cell-attached firing of 85.4 ± 27.9% and females demonstrated a significant 66.3 ± 11.4% reduction from baseline. One female CRF1- neuron that exhibited a 270% increase in firing rate in response to CRF was identified as a significant outlier via Grubb’s test and was excluded from all analyses. The exclusion of this outlier did not alter any comparisons in the ANOVA or the two-sample t-test, but did allow the one-sample t-test within females to reach significance. Therefore, the ability of CRF to enhance neuronal excitability is restricted to the CRF1+ population. It is possible that CRF may reduce neuronal excitability in CRF1- CeA neurons, particularly in females.
Sensitivity of male and female CRF1+ neuronal excitability to CRF receptor inhibition
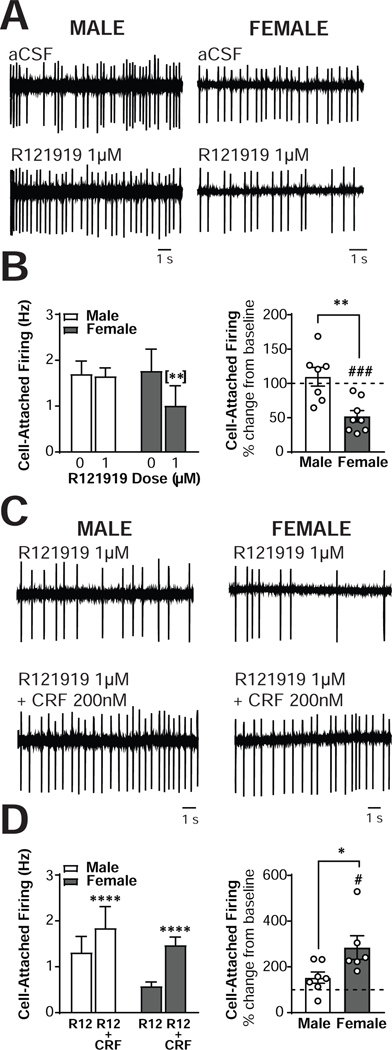
To determine the effects of CRF1+ inhibition on the firing discharge rate of male (n = 7 cells from 5 mice) and female (n = 7 cells from 6 mice) CRF1+ CeA neurons, R121919 (1μM) was superfused during cell-attached firing (Figure 7A). Again, no sex differences emerged [F(1,12) = 0.16, p = 0.7002], consistent with baseline recordings. A significant sex X R121919 interaction emerged, F(1,12) = 7.235, p = 0.0197, in addition to a main effect of R121919, F(1,12) = 9.29, p = 0.0101 (Figure 7B, left). Sidak’s multiple comparison test indicated that R121919 non-significantly reduced cell-attached firing in male CRF1+ CeA neurons from 1.701 ±0.28 Hz to 1.65 ±0.18 Hz (p = 0.96) but significantly reduced cell-attached firing in female CRF1+ CeA neurons from 1.845 ±0.54 Hz to 1.08 ±0.49 Hz (p = 0.0523; Figure 7B, left).When the data were normalized into percent of control, a significant sex difference was observed, t(12) = 3.40, p < 0.0053 (Figure 7B, right), indicating that the percent decrease in firing elicited by R121919 in female neurons was greater than the percent decrease in male neurons. This represented a non-significant 109.6±13.6 % of baseline in male neurons [t(6) = 0.71, p = 0.507) and a significant reduction of 52.9 ±9.6% of baseline in female neurons [t(6) = 4.9, p = 0.0027; Figure 7B, right]. Together, these findings indicate that blockade of the CRF1 receptor reduces the excitability of female but not male CRF1+ neurons.
Figure 7. Sex differences in CRF1 regulation of excitability in CRF1+, but not CRF1-, CeA neurons.
(A) Representative cell-attached recording of male (left) and female (right) CRF1+ CeA neurons during focal application of aCSF (top) and R121919 (1 μm; bottom). (B) Quantification of cell-attached firing frequency in male and female CRF1+ CeA neurons following focal application of aCSF and R121919 (1 μm) expressed in Hz (left) and percent of control (right). (C) Representative cell-attached recording of male (left) and female (right) CRF1+ CeA neurons during focal application of R121919 (1 μm; top) and R121919 + CRF (200 nm; bottom). (D) Quantification of cell-attached firing frequency in male and female CRF1+ CeA neurons following focal application of R121919 (1 μm) and R121919 + CRF (200nM) expressed in Hz (left) and percent of control (right).
Finally, we sought to determine if the CRF effects on neuronal excitability and sex differences in these CRF effects required the CRF1 receptor. CRF1+ neurons were pretreated with R121919 (1μM) followed by co-application of CRF (200nM) + R121919 (1μM) during cell-attached recordings (Figure 7C). Again, no sex differences emerged [F(1,11) = 1.53, p = 0.2426], consistent with baseline recordings. CRF still elicited an increase in cell-attached firing in the presence of R121919 [F(1,11) = 36.9, p < 0.0001; Figure 7D, left]. Male neurons (n = 7 cells from 4 mice) increased from 1.31 ± 0.3 Hz to 1.85 ± 0.5 Hz and female neurons (n = 7 cells from 6 mice) increased from 0.77 ± 0.2 Hz to 1.48 ± 0.1 Hz. Importantly, in the presence of R121919 CRF no longer produced a sex X CRF interaction [F(1,11) = 2.37, p = 0.1520]. When data were analyzed as a percent change from baseline (Figure 7D, right) a significant sex difference emerged [t(11) = 2.42, p = 0.0342]. Whereas in male CRF1+ neurons CRF in the presence of R121919 induced a non-significant 153.0 ± 24.6% increase from baseline, in female CRF1+ neurons CRF + R121919 produced a significant 284.3 ± 51.6% increase from baseline. Thus, blockade of the CRF1 receptor abolished sex differences in the effects of CRF on cell-attached firing that were evident in the absence of CRF1 antagonism.
DISCUSSION
The goals of the present studies were to characterize the effects of CRF receptor activation and inhibition in the CRF1+ neurons of the CeA, and to determine whether sex differences in inhibitory control, excitability, and the effects of ethanol and CRF/CRF1 manipulations were evident in this population. Here, we report significantly greater numbers of CRF1+ neurons in the male versus female CeA. Male and female CRF1+ neurons exhibited similar firing properties, membrane characteristics, baseline sIPSCs and firing discharge rates. Exogenous CRF application significantly increased sIPSC frequency in male and female CRF1+ neurons, but not CRF1- CeA neurons of either sex. Blockade of the CRF1 receptor reduced sIPSC frequency in male and female CRF1+ neurons, but was insufficient to prevent the effects of exogenous CRF on sIPSC frequency in both sexes. Blockade of the CRF2 receptor produced marginal reductions in sIPSC frequency that failed to reach independent significance in both sexes, and this blockade also failed to prevent the effects of CRF on sIPSC frequency. Exogenous CRF increased excitability to a greater extent in male versus female CRF1+ neurons, a sex difference that was prevented by the application of glutamate receptor antagonists and produced reductions in the excitability of female but not male CRF1- neurons. Blockade of the CRF1 receptor reduced excitability in female but not male CRF1+ neurons and abolished the sex differences in CRF effects on excitability observed in this population. Finally, acute ethanol exposure significantly reduced sIPSC frequency in male but not female CRF1+ neurons. These data represent the first characterization of the effects of CRF and CRF1/CRF2 blockade specifically within the CRF1+ population of the CeA, and the first investigation of CRF1+ neurons from female mice. Although no significant sex differences in baseline membrane properties or inhibitory control were observed in either baseline or CRF activated/inhibited synaptic transmission, there were significant sex differences in the postsynaptic effects of acute ethanol and in neuronal excitability response to CRF activation or inhibition. Collectively, these data demonstrate important sex differences in CRF1 expression, ethanol-induced inhibitory control, and CRF-mediated excitability in the CeA. These differences have broader potential mechanistic implications for sex differences in stress and alcohol susceptibility in males and females.
In these studies, exogenous CRF (200 nM) consistently enhanced sIPSC frequency in male and female CRF1+ neurons. This finding suggests that CRF enhances presynaptic GABA release onto CRF1+ neurons in both the male and female CeA. This observation is consistent with prior reports of CRF facilitation of presynaptic GABA release onto CeA neurons independent of cell type (Roberto et al., 2010; Herman et al., 2013b; Kang-Park et al., 2015; Varodayan et al., 2017a). Blockade of the CRF1 receptor also reduced sIPSC frequency in male and female CRF1+ neurons, indicating that the CRF1 receptor plays a role in facilitating basal GABA release onto CRF1+ neurons. Importantly, CRF did not alter GABA release onto CRF1- neurons in either sex. This finding is consistent with prior reports that CRF fails to enhance presynaptic GABA release in CRF1 knockout mice (Nie et al., 2009). The effects of CRF seen in CRF1+ neurons were absent in CRF1- neurons in both males and females, indicating that the postsynaptic CRF1 receptor is required for the enhanced GABA activity induced by exogenous CRF. Prior work has reported that CRF enhances the presynaptic release of GABA onto undifferentiated CeA neurons (Nie et al., 2004; Nie et al., 2009; Roberto et al., 2010), but these studies sampled mixed populations that likely contained CRF1+ neurons among other cell types. The CRF1 receptor is expressed pre- and postsynaptically, and exogenous CRF stimulates CRF1 receptors regardless of cellular localization. The present results suggest the presence of CRF1+ neurons synapsing onto other CRF1+ neurons. Indeed, exogenous CRF also increased the firing discharge rate of CRF1+ neurons, which would be expected to increase GABA release onto downstream neurons, as CeA CRF1+ neurons are predominantly GABAergic (Wolfe et al., 2019). Such seemingly opposing circuit effects instead likely reflect an activating component followed by enhanced inhibitory tone, which has been proposed to play an important role in the fine-tuned regulation of amygdala output (Duvarci and Pare, 2014).
CRF2 is also expressed throughout the amygdala (Chalmers et al., 1995; Primus et al., 1997), and CRF2 inhibition in the CeA has been shown to reduce evoked GABA release onto neurons in that region (Fu and Neugebauer, 2008). Therefore, we sought to determine the extent to which CRF2 activity regulated GABA activity in CRF1+ neurons. Blockade of the CRF2 receptor produced a very small reduction in sIPSC frequency that failed to reach independent significance in either males or females. Therefore, although CRF2 activity may play a role in the presynaptic release of GABA onto CRF1 neurons, the role of the CRF1 receptor appears to be more pronounced. The extent to which CRF1 and CRF2 may co-express within the same cell population in the CeA remains unknown, and like CRF1, the CRF2 receptor can be expressed pre- or postsynaptically on amygdala neurons (Rominger et al., 1998; Lawrence et al., 2002). These factors make the interpretation of CRF2 effects in our CRF1 reporter line more complex, but the increasing availability of sophisticated tools for dissecting discrete cell populations should facilitate further investigation of CRF2 contributions to the effects reported here.
To determine the extent to which the CRF1 and CRF2 receptors are required for the effect of CRF to enhance presynaptic GABA release onto CRF1+ neurons, we used a pharmacological blockade strategy consisting of a pretreatment with a CRF1 or a CRF2 antagonist followed by co-application of CRF and the antagonist. CRF was still able to elicit a significant increase in sIPSC frequency in the presence of CRF1 and CRF2 antagonists, respectively, in both male and female CRF1+ neurons. These findings are in contrast to prior reports indicating that the effects of CRF on GABA release in CeA neurons can be blocked by CRF1 antagonists (Nie et al., 2004; Nie et al., 2009). However, there are important methodological considerations that differentiate the present findings from prior work. Previous reports were made only in undifferentiated CeA neurons, and thus were likely performed in a mixed population of CRF1+ and CRF1- neurons. This would tend to enhance the number of CRF-insensitive neurons in the sample, whereas the present findings were selectively conducted in CRF-sensitive (CRF1+) and CRF-insensitive (CRF1-) populations, respectively. The present study also examined spontaneous IPSCs and thus reflects the endogenous activity of CeA neurons with all spontaneous network activity intact, which may be differently sensitive to pharmacological manipulations than the miniature or evoked currents that have previously been described. The doses of the antagonists used here were pharmacologically active in other experiments (reducing sIPSC frequency and, in the case of R121919, reducing firing rate in female neurons), but may have been insufficient to compete with CRF for the CRF1/2 receptor. Doses in prior reports have also generally been high (10μM) and may have impacted additional signaling mechanisms or off-target effects.
Although these methodological differences may explain the disparate findings in the present study as compared with prior reports, the question remains: why does an effect of exogenous CRF on presynaptic release occur selectively in a population that contains a postsynaptic CRF1 receptor? Another potential explanation for this seeming contradiction would be the involvement of a retrograde signaling cascade, such as that mediated by the cannabinoid 1receptor (CB1). CB1 and CRF1 have been shown to colocalize in the amygdala (Gray et al., 2015), and evidence suggests important interactions between the CRF/CRF1 system and CB1/endocannabinoid signaling in the context of stress and addiction (Natividad et al., 2017). CRF enhances the activity of fatty acid amide hydrolase (FAAH), the enzyme that degrades the endogenous CB1 agonist anandamide, in a CRF1-dependent manner (Gray et al., 2015; Natividad et al., 2017). The net effect of this FAAH activity would be a reduction in inhibitory retrograde CB1 transmission, which could facilitate presynaptic GABA release onto postsynaptic CRF1+ neurons. Investigation of CRF-CB1 interactions specifically within the CRF1+ population is an important future direction.
In addition to enhancing GABAergic inputs to CRF1+ neurons, CRF also increased the excitability of CRF1+ neurons in the CeA. In both male and female CRF1+ neurons, exogenous CRF increased cell-attached firing. This increased output of CRF1+ neurons was observed despite increases in GABA release onto these cells, indicating that the net effect of CRF application may be stimulatory on this population. The CRF1 receptor may couple to Gq or Gs in brain, both of which are canonically excitatory in terms of neuronal output via mobilization of intracellular calcium (Valentino et al., 2013). Stimulation of CRF1 activates downstream signaling cascades via both protein kinase C (PKC) and protein kinase A (PKA), which have both been shown to alter GABA signaling in CeA (Bajo et al., 2008; Cruz et al., 2012). Thus, actions of CRF on the postsynaptic CRF1 receptors on CRF1+ neurons may account for this increase in excitability. In support of this proposed mechanism, we find that CRF-induced enhancement of excitability is absent in CRF1- neurons from both sexes. That the excitatory effects of CRF are specific to CeA neurons containing CRF1 could suggest a role of postsynaptic CRF1 receptors in these effects of CRF. In this context, the two effects of CRF (to stimulate presynaptic release of GABA onto CRF1+ neurons and to enhance the firing of CRF1+ neurons) likely represent two dissociable mechanisms that may become dysregulated by chronic stress or drug exposure. The simultaneous enhancement of GABAergic input and excitability may also represent a feedback mechanism that serves to regulate CRF1+ neuron output.
A principal aim of the present work was to identify potential sex differences in inhibitory control and sensitivity to CRF1 manipulations in CRF1+ neurons of the CeA. We observed fewer CRF1+ neurons in the CeA of female mice versus male mice, an anatomical sex difference that may contribute to functional network activity. Although baseline inhibitory transmission and excitability of CRF1+ neurons were comparable between the two sexes under basal conditions, challenging this circuitry with stimulation and inhibition of the CRF1 receptor revealed important sex differences. Male but not female CRF1+ neurons exhibited a significant increase in sIPSC amplitude following exogenous CRF application. Our immunohistochemistry experiments indicated greater numbers of CRF1+ neurons in the male versus female CeA, but these findings cannot determine the relative density of CRF1 receptor expression in the two sexes. Male CRF1+ neurons also exhibited a substantially greater effect of exogenous CRF application on firing than female CRF1+ neurons. This sex difference was absent in cell-attached recordings made in the presence of NMDA/AMPA channel blockers, suggesting that CRF regulation of excitatory transmission may be part of the mechanism underlying the blunted sensitivity of female CRF1+ neurons to CRF effects on excitability. Together, these findings may point to sex differences in the availability or stimulation of postsynaptic CRF1 receptors in male versus female CRF1+ neurons. In support of this proposed mechanism, pretreatment with the CRF1 antagonist R121919 also abolished sex differences in the effect of CRF on CRF1+ neuronal excitability. Further, application of the CRF1 receptor antagonist R121919 reduced firing rates in female but not male CRF1+ neurons. This finding indicates a greater role for the CRF1 receptor in regulating neuronal output under basal conditions in females but not males. This result may also point to sex differences in CRF1 receptor occupancy; higher baseline occupancy of the CRF1 receptor in female versus male CRF1+ neurons may explain the sensitivity to CRF1 blockade observed in females but not males. The lack of reliable antibodies for the CRF1 receptor represents a current barrier to evaluating this hypothesis directly, but here we provide strong evidence for enhanced sensitivity of male CeA CRF1+ neurons to CRF stimulation and greater basal activity of the CRF1 receptor in female CRF1+ CeA neurons.
Although sexually dimorphic responses of the CRF system to stress have been reported in a variety of brain regions (see Pleil & Skelly, 2018 for review), direct investigations of sex differences in CRF signaling within the amygdala have been sparse. The present findings are reminiscent of recordings in serotonin neurons in the dorsal raphe, wherein neurons from female mice exhibited blunted responses to CRF as compared with male neurons (Howerton et al., 2014). In contrast, within the locus coeruleus female neurons have been shown to be more sensitive to the effects of exogenous CRF application (Curtis et al., 2006), possibly via increased signaling via CRF1 and inhibition of receptor internalization in female neurons (Bangasser et al., 2010). Recent functional connectivity data has also demonstrated that central administration of CRF activates different brain circuitry in male versus female rats (Salvatore et al., 2018). Importantly, evidence also suggests that the CRF1 receptor may display significant sex differences in biased GCPR signaling. CRF1 receptors from female mice predominantly bias towards Gq signaling whereas male CRF1 receptors bias towards β–arrestin signaling (Bangasser et al., 2013). These disparate results highlight two important considerations for work in the CRF system: 1) females are dramatically understudied in this arena as compared with males and there is a critical need for more preclinical investigation of sex differences in CRF signaling, and 2) sexually dimorphic responses to CRF/CRF1 manipulations are likely brain region and cell-type specific, making generalizations about the CRF system in either sex as a whole difficult.
The present studies also provide initial indications of sex differences in sensitivity to the effects of ethanol. Here, we report that inhibitory transmission in male but not female CRF1+ neurons is sensitive to the effects of acute alcohol. Exogenous ethanol reduced sIPSC frequency in male but not female CRF1+ neurons, indicating that ethanol reduces GABA release onto male CRF1+ neurons. This finding is consistent with earlier work demonstrating that acute ethanol reduces tonic inhibition of male CRF1- neurons that synapse onto CRF1+ neurons (Herman et al., 2013a), which would be predicted to reduce GABAergic input from CRF1- to CRF1+ neurons. The present findings provide the first evidence that such actions of ethanol may be sex-specific, further emphasizing the need for assessment of alcohol-CRF interactions in the female CeA (Agoglia et al., 2020). Chronic ethanol vapor exposure has been shown to increase the sensitivity of nonspecific CeA neurons to CRF and CRF1 antagonists and increase CRF/CRF1 mRNA in the CeA (Roberto et al., 2010). Chronic ethanol vapor also dysregulates inhibitory control of CeA CRF1+ neurons (Herman et al., 2016), but whether chronic ethanol-induced changes in CRF/CRF1 sensitivity occur in this population remains to be seen. It will be important to assess sex differences in baseline GABAergic transmission and sensitivity to CRF1 activation and inhibition following chronic ethanol exposure, as sex differences in this population may play a functional role in the sex differences in alcohol drinking that have been frequently reported in rodents (Becker and Koob, 2016).
The findings reported here suggest several avenues for further dissection of the CRF1 circuitry in male and female CeA. Future studies to assess sex differences in baseline tonic inhibitory control of CRF1+ neurons and examine the effects of CRF1 activation and inhibition on tonic conductance in CeA CRF1+ cells could provide further knowledge about the regulation of CeA activity and effects on downstream targets of CRF1+ neuron activity, such as the BNST. The present work characterized inhibitory control of CRF1 neurons, but exogenous CRF has also been shown to enhance vesicular glutamate release onto CeA neurons independent of cell population (Varodayan et al., 2017b). CRF/CRF1 signaling may therefore also modulate excitatory-inhibitory balance within the CRF1+ population. Studies to address sex differences in glutamatergic signaling and sensitivity of sEPSCs to CRF1 stimulation and blockade within the CeA would provide additional insight into the mechanisms of CRF/CRF1 effects on neuronal excitability described here. Finally, the central amygdala is a compelling target for studies aimed at dissecting the interaction of stress and drug effects in the context of substance abuse disorders. The ability to separate discrete cell populations within the CeA allows for a better understanding of the ways in which stress and drugs such as ethanol can engage common circuitry in similar or disparate ways (Luthi and Luscher, 2014), and may suggest avenues for better targeted therapies for anxiety and substance use disorders.
Supplementary Material
Supplemental Figure 1: Characterization of CRF1- neurons in the male and female CeA. (A) Summary of membrane characteristics of male and female CRF1- CeA neurons. (B) Quantification of sIPSC frequency (left), amplitude (center) and cell-attached firing (right) of male and female CRF1- CeA neurons. (C) Comparison of sIPSC frequency (left), amplitude (center), and cell-attached firing (right) of CRF1+ and CRF1- neurons from male and female mice.
HIGHLIGHTS.
CRF enhances presynaptic GABA release onto male and female CRF1+ CeA neurons.
CRF1 blockade reduces GABA release onto male and female CRF1+ CeA neurons.
Male but not female CeA CRF1+ neurons are sensitive to effects of acute ethanol.
CRF increases excitability more in male CRF1+ CeA neurons than in female neurons.
CRF1 antagonism reduces the excitability of female but not male CeA CRF1+ neurons.
ACKNOWLEDGEMENTS
This work was supported by the Bowles Center for Alcohol Studies and NIH grants T32AA007573 (AEA), AA023002 (MH) and AA011605 (MH).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Agoglia AE, Herman MA (2018) The center of the emotional universe: Alcohol, stress, and CRF1 amygdala circuitry. Alcohol 72:61–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agoglia AE, Crofton EJ, Herman MA (2020) Biological intersection of sex, age, and environment in the corticotropin releasing factor (CRF) system and alcohol. Neuropharmacology 170:108045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bajo M, Cruz MT, Siggins GR, Messing R, Roberto M (2008) Protein kinase C epsilon mediation of CRF- and ethanol-induced GABA release in central amygdala. Proc Natl Acad Sci U S A 105:8410–8415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bangasser DA, Curtis A, Reyes BA, Bethea TT, Parastatidis I, Ischiropoulos H, Van Bockstaele EJ, Valentino RJ (2010) Sex differences in corticotropin-releasing factor receptor signaling and trafficking: potential role in female vulnerability to stress-related psychopathology. Mol Psychiatry 15:877, 896–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bangasser DA, Reyes BA, Piel D, Garachh V, Zhang XY, Plona ZM, Van Bockstaele EJ, Beck SG, Valentino RJ (2013) Increased vulnerability of the brain norepinephrine system of females to corticotropin-releasing factor overexpression. Mol Psychiatry 18:166–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker JB, Koob GF (2016) Sex Differences in Animal Models: Focus on Addiction. Pharmacol Rev 68:242–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chalmers DT, Lovenberg TW, De Souza EB (1995) Localization of novel corticotropin-releasing factor receptor (CRF2) mRNA expression to specific subcortical nuclei in rat brain: comparison with CRF1 receptor mRNA expression. J Neurosci 15:6340–6350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chieng BC, Christie MJ, Osborne PB (2006) Characterization of neurons in the rat central nucleus of the amygdala: cellular physiology, morphology, and opioid sensitivity. J Comp Neurol 497:910–927. [DOI] [PubMed] [Google Scholar]
- Cruz MT, Herman MA, Kallupi M, Roberto M (2012) Nociceptin/orphanin FQ blockade of corticotropin-releasing factor-induced gamma-aminobutyric acid release in central amygdala is enhanced after chronic ethanol exposure. Biol Psychiatry 71:666–676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis AL, Bethea T, Valentino RJ (2006) Sexually dimorphic responses of the brain norepinephrine system to stress and corticotropin-releasing factor. Neuropsychopharmacology 31:544–554. [DOI] [PubMed] [Google Scholar]
- Day HE, Curran EJ, Watson SJ Jr., Akil H (1999) Distinct neurochemical populations in the rat central nucleus of the amygdala and bed nucleus of the stria terminalis: evidence for their selective activation by interleukin-1beta. J Comp Neurol 413:113–128. [PubMed] [Google Scholar]
- Duvarci S, Pare D (2014) Amygdala microcircuits controlling learned fear. Neuron 82:966–980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fadok JP, Krabbe S, Markovic M, Courtin J, Xu C, Massi L, Botta P, Bylund K, Muller C, Kovacevic A, Tovote P, Luthi A (2017) A competitive inhibitory circuit for selection of active and passive fear responses. Nature 542:96–100. [DOI] [PubMed] [Google Scholar]
- Fu Y, Neugebauer V (2008) Differential mechanisms of CRF1 and CRF2 receptor functions in the amygdala in pain-related synaptic facilitation and behavior. J Neurosci 28:3861–3876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray JM, Vecchiarelli HA, Morena M, Lee TT, Hermanson DJ, Kim AB, McLaughlin RJ, Hassan KI, Kuhne C, Wotjak CT, Deussing JM, Patel S, Hill MN (2015) Corticotropin-releasing hormone drives anandamide hydrolysis in the amygdala to promote anxiety. J Neurosci 35:3879–3892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herman MA, Contet C, Roberto M (2016) A Functional Switch in Tonic GABA Currents Alters the Output of Central Amygdala Corticotropin Releasing Factor Receptor-1 Neurons Following Chronic Ethanol Exposure. J Neurosci 36:10729–10741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herman MA, Contet C, Justice NJ, Vale W, Roberto M (2013a) Novel subunit-specific tonic GABA currents and differential effects of ethanol in the central amygdala of CRF receptor-1 reporter mice. J Neurosci 33:3284–3298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herman MA, Kallupi M, Luu G, Oleata CS, Heilig M, Koob GF, Ciccocioppo R, Roberto M (2013b) Enhanced GABAergic transmission in the central nucleus of the amygdala of genetically selected Marchigian Sardinian rats: alcohol and CRF effects. Neuropharmacology 67:337–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howerton AR, Roland AV, Fluharty JM, Marshall A, Chen A, Daniels D, Beck SG, Bale TL (2014) Sex differences in corticotropin-releasing factor receptor-1 action within the dorsal raphe nucleus in stress responsivity. Biol Psychiatry 75:873–883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyytia P, Koob GF (1995) GABAA receptor antagonism in the extended amygdala decreases ethanol self-administration in rats. Eur J Pharmacol 283:151–159. [DOI] [PubMed] [Google Scholar]
- Jiang Z, Rajamanickam S, Justice NJ (2018) Local Corticotropin-Releasing Factor Signaling in the Hypothalamic Paraventricular Nucleus. J Neurosci 38:1874–1890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Justice NJ, Yuan ZF, Sawchenko PE, Vale W (2008) Type 1 corticotropin-releasing factor receptor expression reported in BAC transgenic mice: implications for reconciling ligand-receptor mismatch in the central corticotropin-releasing factor system. J Comp Neurol 511:479–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang-Park M, Kieffer BL, Roberts AJ, Siggins GR, Moore SD (2015) Interaction of CRF and kappa opioid systems on GABAergic neurotransmission in the mouse central amygdala. J Pharmacol Exp Ther 355:206–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence AJ, Krstew EV, Dautzenberg FM, Ruhmann A (2002) The highly selective CRF(2) receptor antagonist K41498 binds to presynaptic CRF(2) receptors in rat brain. Br J Pharmacol 136:896–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luthi A, Luscher C (2014) Pathological circuit function underlying addiction and anxiety disorders. Nat Neurosci 17:1635–1643. [DOI] [PubMed] [Google Scholar]
- Maren S, Quirk GJ (2004) Neuronal signalling of fear memory. Nat Rev Neurosci 5:844–852. [DOI] [PubMed] [Google Scholar]
- Natividad LA, Buczynski MW, Herman MA, Kirson D, Oleata CS, Irimia C, Polis I, Ciccocioppo R, Roberto M, Parsons LH (2017) Constitutive Increases in Amygdalar Corticotropin-Releasing Factor and Fatty Acid Amide Hydrolase Drive an Anxious Phenotype. Biol Psychiatry 82:500–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nie Z, Schweitzer P, Roberts AJ, Madamba SG, Moore SD, Siggins GR (2004) Ethanol augments GABAergic transmission in the central amygdala via CRF1 receptors. Science 303:1512–1514. [DOI] [PubMed] [Google Scholar]
- Nie Z, Zorrilla EP, Madamba SG, Rice KC, Roberto M, Siggins GR (2009) Presynaptic CRF1 receptors mediate the ethanol enhancement of GABAergic transmission in the mouse central amygdala. ScientificWorldJournal 9:68–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Partridge JG, Forcelli PA, Luo R, Cashdan JM, Schulkin J, Valentino RJ, Vicini S (2016) Stress increases GABAergic neurotransmission in CRF neurons of the central amygdala and bed nucleus stria terminalis. Neuropharmacology 107:239–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pleil KE, Skelly MJ (2018) CRF modulation of central monoaminergic function: Implications for sex differences in alcohol drinking and anxiety. Alcohol 72:33–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pomrenze MB, Giovanetti SM, Maiya R, Gordon AG, Kreeger LJ, Messing RO (2019a) Dissecting the roles of GABA and neuropeptides from rat central amygdala CRF neurons in anxiety and fear learning. Cell Reports 29:13–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pomrenze MB, Tovar-Diaz J, Blasio A, Maiya R, Giovanetti SM, Lei K, Morikawa H, Hopf FW, Messing RO (2019b) A Corticotropin Releasing Factor Network in the Extended Amygdala for Anxiety. J Neurosci 39:1030–1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Primus RJ, Yevich E, Baltazar C, Gallager DW (1997) Autoradiographic localization of CRF1 and CRF2 binding sites in adult rat brain. Neuropsychopharmacology 17:308–316. [DOI] [PubMed] [Google Scholar]
- Ramot A, Jiang Z, Tian JB, Nahum T, Kuperman Y, Justice N, Chen A (2017) Hypothalamic CRFR1 is essential for HPA axis regulation following chronic stress. Nat Neurosci 20:385–388. [DOI] [PubMed] [Google Scholar]
- Roberto M, Cruz MT, Gilpin NW, Sabino V, Schweitzer P, Bajo M, Cottone P, Madamba SG, Stouffer DG, Zorrilla EP, Koob GF, Siggins GR, Parsons LH (2010) Corticotropin releasing factor-induced amygdala gamma-aminobutyric Acid release plays a key role in alcohol dependence. Biol Psychiatry 67:831–839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts AJ, Cole M, Koob GF (1996) Intra-amygdala muscimol decreases operant ethanol self-administration in dependent rats. Alcohol Clin Exp Res 20:1289–1298. [DOI] [PubMed] [Google Scholar]
- Rominger DH, Rominger CM, Fitzgerald LW, Grzanna R, Largent BL, Zaczek R (1998) Characterization of [125I]sauvagine binding to CRH2 receptors: membrane homogenate and autoradiographic studies. J Pharmacol Exp Ther 286:459–468. [PubMed] [Google Scholar]
- Salvatore M, Wiersielis KR, Luz S, Waxler DE, Bhatnagar S, Bangasser DA (2018) Sex differences in circuits activated by corticotropin releasing factor in rats. Horm Behav 97:145–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanford CA, Soden ME, Baird MA, Miller SM, Schulkin J, Palmiter RD, Clark M, Zweifel LS (2017) A Central Amygdala CRF Circuit Facilitates Learning about Weak Threats. Neuron 93:164–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tye KM (2018) Neural Circuit Motifs in Valence Processing. Neuron 100:436–452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tye KM, Prakash R, Kim SY, Fenno LE, Grosenick L, Zarabi H, Thompson KR, Gradinaru V, Ramakrishnan C, Deisseroth K (2011) Amygdala circuitry mediating reversible and bidirectional control of anxiety. Nature 471:358–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valentino RJ, Van Bockstaele E, Bangasser D (2013) Sex-specific cell signaling: the corticotropin-releasing factor receptor model. Trends Pharmacol Sci 34:437–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Pett K, Viau V, Bittencourt JC, Chan RK, Li HY, Arias C, Prins GS, Perrin M, Vale W, Sawchenko PE (2000) Distribution of mRNAs encoding CRF receptors in brain and pituitary of rat and mouse. J Comp Neurol 428:191–212. [DOI] [PubMed] [Google Scholar]
- Varodayan FP, Logrip ML, Roberto M (2017a) P/Q-type voltage-gated calcium channels mediate the ethanol and CRF sensitivity of central amygdala GABAergic synapses. Neuropharmacology 125:197–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varodayan FP, Correia D, Kirson D, Khom S, Oleata CS, Luu G, Schweitzer P, Roberto M (2017b) CRF modulates glutamate transmission in the central amygdala of naive and ethanol-dependent rats. Neuropharmacology 125:418–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ventura-Silva AP, Melo A, Ferreira AC, Carvalho MM, Campos FL, Sousa N, Pego JM (2013) Excitotoxic lesions in the central nucleus of the amygdala attenuate stress-induced anxiety behavior. Front Behav Neurosci 7:32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfe SA, Sidhu H, Patel RR, Kreifeldt M, D’Ambrosio SR, Contet C, Roberto M (2019) Molecular, Morphological, and Functional Characterization of Corticotropin-Releasing Factor Receptor 1-Expressing Neurons in the Central Nucleus of the Amygdala. eNeuro 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1: Characterization of CRF1- neurons in the male and female CeA. (A) Summary of membrane characteristics of male and female CRF1- CeA neurons. (B) Quantification of sIPSC frequency (left), amplitude (center) and cell-attached firing (right) of male and female CRF1- CeA neurons. (C) Comparison of sIPSC frequency (left), amplitude (center), and cell-attached firing (right) of CRF1+ and CRF1- neurons from male and female mice.