Abstract
Extracorporeal CO2 removal (ECCO2R) can permit lung protective or noninvasive ventilation strategies in patients with chronic obstructive pulmonary disease (COPD) and acute respiratory distress syndrome (ARDS). With evidence supporting ECCO2R growing, investigating factors which affect CO2 removal is necessary. Multiple factors are known to affect the CO2 removal rate (vCO2) which can complicate the interpretation of changes in vCO2; however, the effect of hematocrit on the vCO2 of artificial lungs has not been investigated. This in vitro study evaluates the relationship between hematocrit level and vCO2 within an ECCO2R device. In vitro gas transfer was measured in bovine blood in accordance with the ISO 7199 standard. Plasma and saline were used to hemodilute the blood to hematocrits between 33% and 8%. The vCO2 significantly decreased as the blood was hemodiluted with saline and plasma by 42% and 32%, respectively, between a hematocrit of 33% and 8%. The hemodilution method did not significantly affect the vCO2. In conclusion, the hematocrit level significantly affects vCO2 and should be taken into account when interpreting changes in the vCO2 of an ECCO2R device.
Keywords: extracorporeal CO2 removal, hematocrit, artificial lung
Patients with moderate acute respiratory distress syndrome and acute exacerbations of chronic obstructive pulmonary disease can benefit from lower tidal volume, lung protective ventilation, or noninvasive ventilation strategies.1–5 These ventilation strategies can prevent ventilator-induced lung injuiy; however, failure is commonly due to hypercapnia and the resulting acidosis and dyspnea.6 Low-flow extracorporeal CO2 removal (ECCO2R) devices have been used in this patient population to remove the excess CO2 while maintaining lung protective or noninvasive ventilation.1,7 ECCO2R devices operate on analogous principles to extracorporeal membrane oxygenation. Because of the primary objective of removing CO2 rather than oxygenation, ECCO2R devices can operate at blood flow rates below 1 L/min.8 The potential benefits of ECCO2R are currently being evaluated in the VENT-AVOID (clinicaltrials.gov: NCT03255057) and REST (clinicaltrials.gov: NCT02654327) clinical trials. ECCO2R device selection and operating parameters dictate the CO2 removal rate (vCO2) but the nuances of CO2 removal can make interpreting changes in vCO2 challenging. Correct interpretation is especially important as vCO2 is the most sensitive indicator of intrabundle thrombosis.9
Numerous factors affect the CO2 transfer rate within native and artificial lungs.10 The vCO2 of a specific artificial lung geometry is a known function of blood flow rate, inlet pCO2, and sweep gas flow rate. The effect of extracorporeal blood flow rate and inlet pCO2 on vCO2 can be taken into account by normalizing the vCO2 to these parameters.11 The effect of sweep gas flow rate on vCO2 and the importance of a sweep gas independent regime have also been well established.12 Diminished vCO2 performance, however, has also been noted in animal studies during periods of anemia.13–15 The relationship between vCO2 and hematocrit (HCT) seen in artificial lungs has been attributed to mechanisms analogous to those within the native lungs. Bidani and Crandall15 used computational modeling to demonstrate that a decrease in HCT from 45% to 15% results in a 50% decrease in vCO2 within the native lungs. A similar vCO2–HCT relationship has been noted in animal studies;13 however, the complexities of animal studies make isolating the artificial lung vCO2–HCT relationship challenging.
In this study, we evaluated the relationship between HCT and the vCO2 of a low-flow ECCO2R device in an in vitro setting. Blood was hemodiluted with saline and plasma to reach the targeted HCT to evaluate any effect plasma proteins may have on vCO2. The in vitro setting allowed the effect of HCT and plasma proteins on vCO2 to be isolated. The results of this study will aid in predicting vCO2 in ECCO2R devices and interpreting changes in vCO2 related to HCT.
Methods
In vitro Gas Exchange
Bovine blood was collected from a local slaughterhouse and was heparinized (30 IU/ml), filtered (40 μm; Pall Biomedical, Inc., Fajardo, PR), and treated with gentamicin (0.1 mg/ml). Blood was diluted to HCT levels of 8, 15, 25, and 33 ± 2% with either isotonic saline (0.9% [w/v] Aqueous, Isotonic Saline; Fisher Scientific, Hampton, NH) or plasma. Plasma was obtained by centrifuging the whole bovine blood (2,819g for 15 minutes) and removing the plasma layer.
Gas exchange testing followed the ISO7199 standard16 with the exception of the intentional alterations in HCT levels. The test circuit (Figure 1) included the Modular Extracorporeal Lung Assist System (ModELAS) artificial pump-lung (0.65 m2 hollow fiber membrane bundle),11 two 6 L blood reservoir bags, and an Affinity oxygenator (Medtronic, Minneapolis, MN). The reservoir bags were submerged in a water bath to maintain a blood temperature of 37 ± 1°C. The Affinity oxygenator was used to condition the blood to venous blood gas tensions (sO2 = 65 ± 5%, pCO2 = 45 ± 5 mmHg). Once the blood reached venous conditions, tubing clamps were manipulated so that blood was drawn from the venous conditioned reservoir, through the device, and returned to the second reservoir. Samples for blood gas measurements were taken immediately before and after the ModELAS. A RapidLab 405 blood gas analyzer was used to measure gas tensions (Siemens, Deerfield, IL). The CO2 removal rate, hemoglobin concentration, and plasma protein concentration were measured at each HCT level in triplicate.
Figure 1.
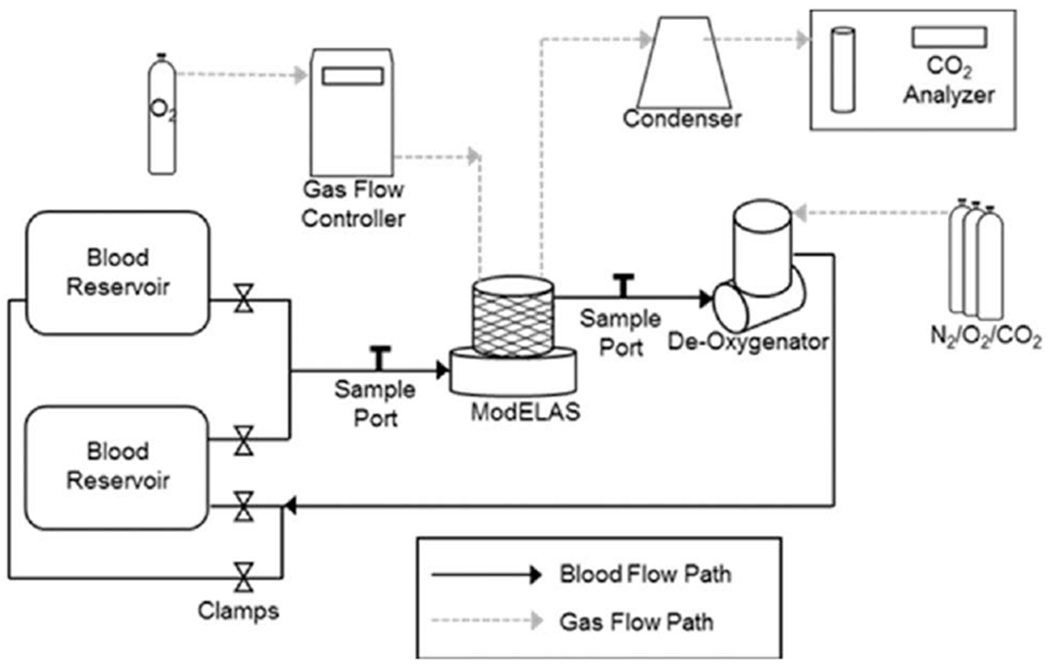
Schematic depicting the blood and gas flow pathways of the in vitro gas exchange loop.
The pump speed was adjusted to achieve 500 ml/min of blood flow, typical for low-flow ECCO2R devices,17,18 through the ModELAS. Blood flow rate was measured using an ultrasonic flow probe (Transonic Systems Inc., Ithaca, NY). The pure oxygen sweep gas flow rate (QSG) was set to 14 L/min using a gas flow controller (Fathom Technologies, Georgetown, TX). From preliminary experiments, this sweep gas flow rate is within the sweep gas independent regime. CO2 concentration (FCO2) was measured in the exhaust sweep gas using a WMA-4 gas analyzer (PP Systems, Amesbury, MA). The CO2 removal rate was calculated and normalized () to an inlet pCO2 of 45 mm Hg according to Equation 1.
| (1) |
An additional 4 ml blood sample was drawn from the device inlet to measure HCT and plasma protein each time a CO2 removal measurement was taken. HCT was measured using a capillary tube which was spun down for 3 minutes in a microcentrifuge (International Equipment Co., IEC MB, Needham Hts, MA). The remaining blood was centrifuged for 15 minutes at 0.8g, and the supernatant plasma was removed and centrifuged again at 7.2g for 10 minutes. The plasma protein concentration was measured using a refractometer (TS 400, Reichert, Depew, NY) from this purified plasma.
Statistical Analysis
Data are reported as the mean ± standard deviation. Statistical analysis was completed in SPSS (IBM, Armonk, NY). A Friedman’s analysis of variance was conducted to evaluate the effect of dilution method and HCT on measured vCO2, plasma protein concentration, pH, and calculated bicarbonate concentration. Pairwise comparisons were made using Wilcoxon tests. Statistical significance was determined at p < 0.05.
Results
In vitro vCO2 for plasma and saline diluted blood are shown in Figure 2. There was no significant effect of dilution method (plasma dilution or saline dilution) on vCO2 (p = 0.564). The CO2 significantly decreased (p = 0.000) with decreasing HCT in a nearly linear fashion for both saline dilution (R2 = 0.998) and plasma dilution (R2 = 0.984). Decreasing the HCT from 33% to 8% resulted in a 32% and 42% decrease in vCO2 when diluted with plasma and saline, respectively. The plasma protein concentration (Figure 3) decreased significantly after each hemodilution with saline (p = 0.001) and was significantly different between plasma and saline dilution (p < 0.05). Inlet and outlet bicarbonate ion concentrations were not affected by the dilution method (Figure 4; p = 0.248 and p = 0.083, respectively). The inlet pH was not affect by the dilution method (p = 0.083); however, the dilution method substantially affected the device outlet pH (p = 0.001) (Figure 4).
Figure 2.
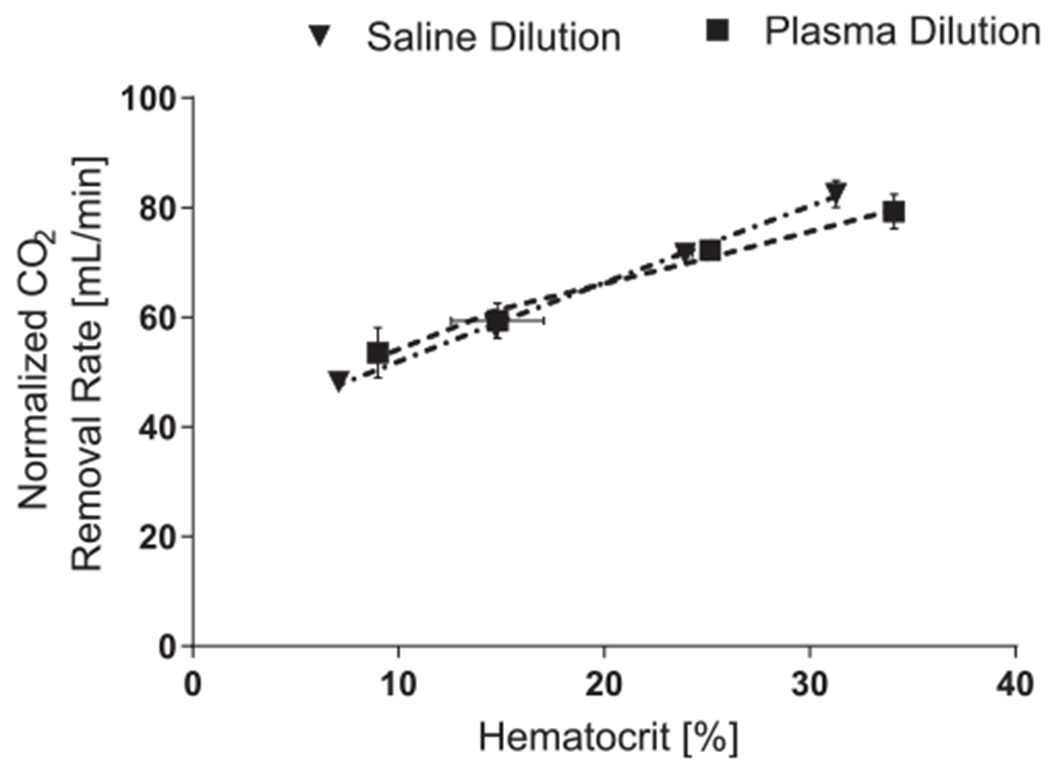
CO2 removal rates measured at hematocrit increments of 8, 15, 25, and 32 ± 2% for blood diluted with saline or plasma. The blood flow rate through the device was held constant at 0.5 L/min.
Figure 3.
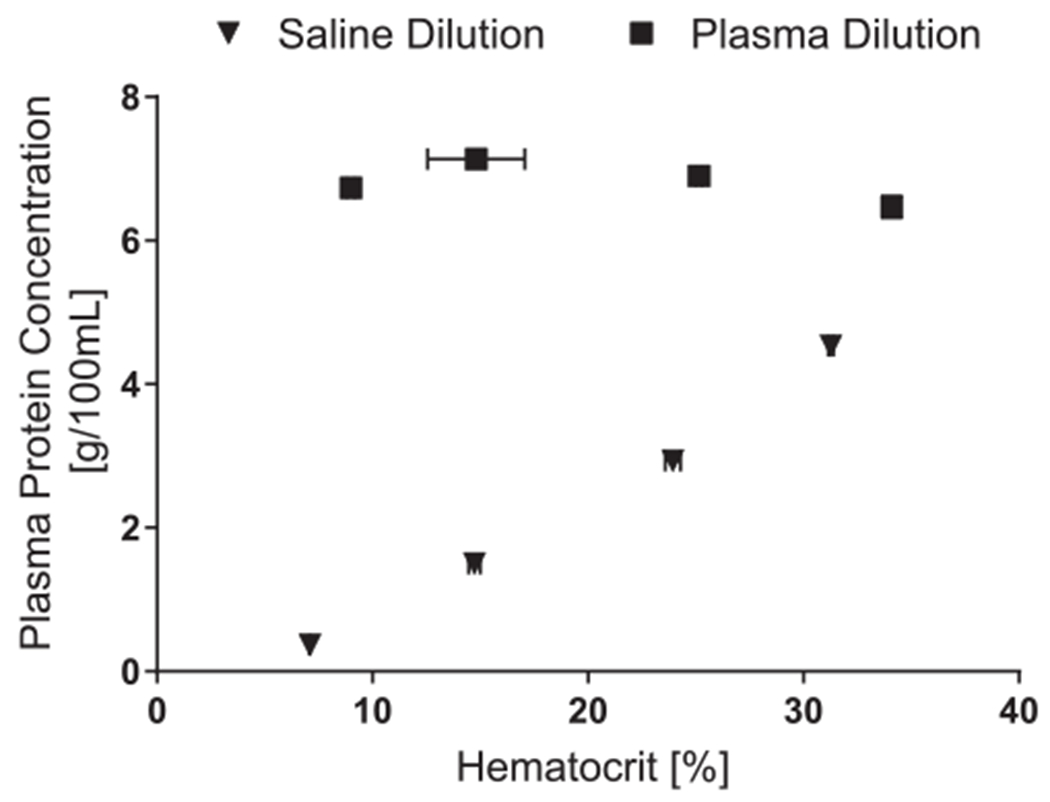
Plasma protein concentration of the circulating blood after hemodilution with saline or plasma. Hemodilution with saline significantly decreased the plasma protein concentration at all hematocrit levels p < 0.05).
Figure 4.
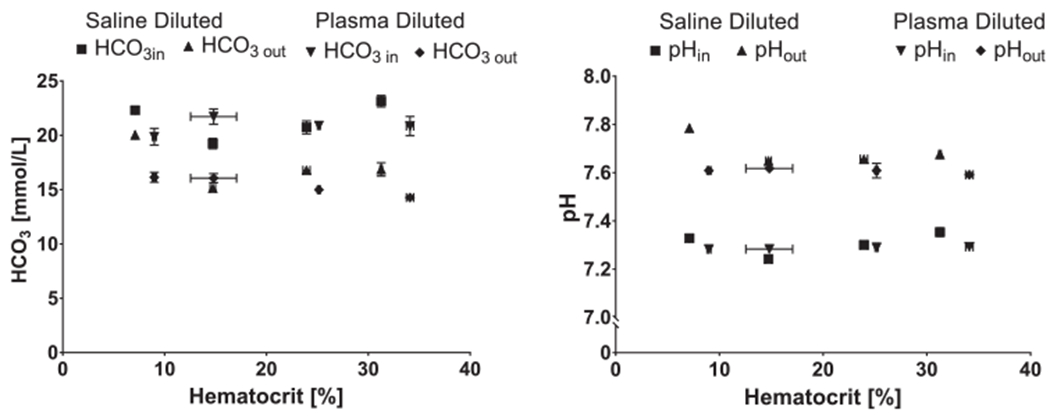
Calculated concentration and measured pH of device inlet and out blood at each hemodilution level. concentration was calculated using the Henderson-Hasselbalch equation19.
Discussion
The evidence supporting the use of ECCO2R to permit noninvasive or lung protective ventilation strategies in patients with moderate acute respiratory distress syndrome and acute exacerbations of chronic obstructive pulmonary disease continues to increase. With this comes a need to identify variables which influence the vCO2 of artificial lungs, especially because a decrease in vCO2 can be indicative of intrabundle thrombosis.9 The vCO2 of an artificial lung can be affected by blood and sweep gas flow rates, and the inlet pCO2 of the blood.11,12 The effect of HCT on CO2 transfer has been documented in computational studies within the native lungs but only observationally noted in artificial lungs during in vivo studies.13 This in vitro study evaluated the vCO2 within an ECCO2R device in blood diluted to multiple HCT levels using plasma and saline. In vitro testing demonstrated that the vCO2 linearly and significantly decreased with decreasing HCT level. CO2 removal rate was not affected by the method of hemodilution.
CO2 transfer decreases with the decrease in HCT within artificial lungs, as demonstrated here, as well as within the native lungs.14,15,20,21 The vCO2–HCT relationship within the native lungs has been attributed to 1) decreased buffering capacity provided by hemoglobin, 2) reduced flux of across the red blood cell (RBC) membrane, and 3) the release of fewer Bohr protons.15 The first two mechanisms are a direct result of fewer RBCs. Carbonic anhydrase (CA) within the RBC plays an integral role in rapidly hydrating CO2 via the catalysis of . Hemoglobin present within the RBC acts as a buffer by binding to H+. In addition, the RBC membrane-bound exchanger rapidly exchanges for Cl−, the chloride shift, to prevent the rate-limiting accumulation of within the RBC. These two mechanisms prevent the accumulation of H+ and within the RBC which shifts equilibrium to the right. Reductions in hemoglobin and RBC concentration diminish the buffering capacity and the surface area available for the chloride shift to occur. Thus, the reaction reaches equilibrium sooner and less CO2 is combined with blood per increase in pCO2. The third mechanism is the classic Haldane effect whereby the uptake of oxygen by hemoglobin in the lungs decreases the affinity of hemoglobin for CO2 and results in the release of H+ and bound CO2. The released H+ drives the equilibrium of the reaction to the left, toward dissolved CO2. Although less than 5% of the total CO2 content within blood is transported bound to hemoglobin, this source accounts for 30% of exhaled CO2.22 Bidani and Crandall15 used a computational model to isolate each of these mechanisms and demonstrated that 50–60% of the decrease in vCO2 because of decreased HCT is attributable to the third mechanism. Thus, even moderate decreases in HCT will result in decreased CO2 removal. These three factors result in anemic blood dissociating with less CO2 per decrease in pCO2. This essentially results in a flattening of the CO2 dissociation curve as the HCT level decreases.14 The above three mechanisms are inherent to properties of the blood, and not of the native lungs. Thus, these mechanisms are likely identical to those influencing the vCO2–HCT relationship within the artificial lung.
The effects of anemia on CO2 exchange within the native lungs is often masked by compensatory mechanisms within the body. In this study, however, a 25% decrease in HCT led to a 13% decrease in vCO2. Normal gas exchange within the body is primarily maintained during periods of anemia by increases in cardiac output and ventilation rate, and changes to the microcirculation and metabolism.20 Within an artificial lung, decreases in HCT will result in increased extracorporeal blood flow rate if the pump speed is held constant and would be analogous to increased cardiac output. In the current study, however, the pump speed was adjusted to maintain an extracorporeal blood flow rate of 500 ml/min as the HCT level was decreased. The other three compensatory mechanisms would not be mimicked within an artificial lung. Hence, even small changes to the HCT level affected the vCO2 of the artificial lung during this study.
The effect of plasma proteins was evaluated in the current study by comparing the vCO2 of blood diluted with plasma versus that diluted with normal saline. Plasma proteins, mainly albumin and phosphates, typically contribute minimally to CO2 transport and CO2 removal. This is becasue of the ability of normal levels of hemoglobin to buffer approximately six times more H+ than plasma proteins are capable of.23 In anemic blood, however, the role of the buffering action of plasma proteins has been postulated to be more significant because of the reduced amount of hemoglobin.14 There was not a significant effect of dilution method (saline versus plasma) on vCO2 in this study, even though there was a significant difference in plasma protein concentration at each HCT level between the two dilution methods. Arazawa et al.24 report a similar in vitro result, whereby a miniature artificial lung only removed 13 ml/min/m2 more CO2 in a phosphate buffered saline+albumin solution than in a phosphate buffered saline solution. The results of the current study, and that of Arazawa et al., demonstrate that the buffering capacity of plasma proteins is not significant enough to accommodate for the reduction, or absence, of HCT within an artificial lung. This is because of the CO2 carrying capacity of plasma being substantially due to the chloride shift, which requires the presence of the RBC, to occur.23 Thus, regardless of the nature of anemia (hemodilution versus diminished RBC production), an equivalent decrease in artificial lung vCO2 should be anticipated as HCT level decreases.
The device was operated at a high sweep gas flow rate to achieve sweep gas independence and thereby isolate the effect of HCT on vCO2. Sweep gas flow rate and CO2 removal are more inter-related than oxygenation and sweep gas flow rate. At low sweep gas flow rates, CO2 begins to accumulate within the sweep gas as it passes through the lumen of the fiber. This causes the CO2 concentration driving gradient between the blood and sweep gas to become diminished and reduces the overall CO2 removal of the device. Thus, to remove the influence of CO2 build-up within the fiber lumen, benchtop ECCO2R experiments are typically operated in a sweep gas independent regime. Clinically, the sweep gas flow rate may be adjusted to achieve the level of CO2 removal the patient requires and even steadily decreased to wean patients off of the device. By implication, a clinician would be lowering sweep gas because the extracorporeal CO2 removal is more than adequate and hence the effect of HCT on extracorporeal CO2 removal would be less relevant. In the present benchtop study, the goal was to isolate the effect of HCT; thus, the device was operated within a sweep gas independent regime.
In this study, as the blood was diluted, the viscosity of the blood consequently also decreased. Extracorporeal blood flow rate was maintained at 500 ml/min by reducing the pump speed after each dilution. In an artificial lung, fluid viscosity (ν) affects the fluid boundary layer thickness at the fiber surface by ν1/6. The fluid side boundary layer provides the majority of the diffusional resistance to gas transfer within the artificial lung. Thus, with all else held constant, the gas transfer rate will increase as fluid viscosity decreases within an artificial lung.10 The results described within this article do not make a distinction between increases in vCO2 due to a lower viscosity fluid and diminished vCO2 due to lowered HCT level.
The interpretation of changes in vCO2 is only reliable if all factors influencing vCO2 are accurately accounted for. The results of this in vitro study provide evidence demonstrating that decreases in HCT result in decreased artificial lung CO2 transfer regardless of the nature of the anemia. Changes in the HCT must therefore be taken into account when determining the cause of changes in vCO2 within an artificial lung.
Acknowledgments
This work was supported by the National Institutes of Health (NIH) grants R01HL117637 and R01HL135482 and the McGowan Institute for Regenerative Medicine. Funding for A.G.M. was provided by an NIH training grant (T32 HL076124) for the University of Pittsburgh Cardiovascular Bioengineering Training Program.
Footnotes
Disclosure: W.J.F. chairs the Scientific Advisory Board and is a founder of ALung Technologies, in which he has an equity interest. No other authors have conflicts of interest to report.
References
- 1.Bein T, Weber-Carstens S, Goldmann A, et al. : Lower tidal volume strategy (~3 ml/kg) combined with extracorporeal CO2 removal versus ‘conventional’ protective ventilation (6 ml/kg) in severe ARDS. Intensive Care Med 39: 847–856, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Terragni PP, Del Sorbo L, Mascia L, et al. : Tidal volume lower than 6 ml/kg enhances lung protection: Role of extracorporeal carbon dioxide removal. Anesthesiology 111: 826–835, 2009. [DOI] [PubMed] [Google Scholar]
- 3.Abrams D, Brenner K, Burkart K, et al. : Pilot study of extracorporeal carbon dioxide removal to facilitate extubation and ambulation in exacerbations of chronic obstructive pulmonary disease Ann Am Thoar Soc 10: 307–314, 2013. [DOI] [PubMed] [Google Scholar]
- 4.Bonin F, Sommerwerck U, Lund LW, Teschler H: Avoidance of intubation during acute exacerbation of chronic obstructive pulmonary disease for a lung transplant candidate using extracorporeal carbon dioxide removal with the Hemolung. J Thorac Cardiovasc Surg 145: e43–e44, 2013. [DOI] [PubMed] [Google Scholar]
- 5.Kluge S, Braune SA, Engel M, et al. : Avoiding invasive mechanical ventilation by extracorporeal carbon dioxide removal in patients failing noninvasive ventilation. Intensive Care Med 38: 1632–1639, 2012. [DOI] [PubMed] [Google Scholar]
- 6.Lund LW, Federspiel WJ: Removing extra CO2 in COPD patients. Curr Respir Care Rep 2: 131–138, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zimmermann M, Bein T, Arlt M, et al. : Pumpless extracorporeal interventional lung assist in patients with acute respiratory distress syndrome: A prospective pilot study. Crit Care 13: R10, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Abrams D, Roncon-Albuquerque R Jr, Brodie D: What’s new in extracorporeal carbon dioxide removal for COPD? Intensive Care Med 41: 906–908, 2015. [DOI] [PubMed] [Google Scholar]
- 9.Kaesler A, Hesselmann F, Zander MO, et al. : Technical indicators to evaluate the degree of large clot formation inside the membrane fiber bundle of an oxygenator in an in vitro setup. Artif Organs 43: 159–166, 2019. [DOI] [PubMed] [Google Scholar]
- 10.Federspiel W, Henchir K: Lung, artificial: Basic principles and current applications. Encyclopedia of Biomaterials and Biomedical Engineering Encycl Biomater Biomed Eng 9: 910–921,2008. [Google Scholar]
- 11.May AG, Jeffries RG, Frankowski BJ, Burgreen GW, Federspiel WJ: Bench validation of a compact low-flow CO2 removal device. Intensive Care Med Exp 6: 34, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Federspiel WJ, Hattler BG: Sweep gas flowrate and CO2 exchange in artificial lungs. Artif Organs 20: 1050–1052, 1996. [DOI] [PubMed] [Google Scholar]
- 13.Wearden PD, Federspiel WJ, Morley SW, et al. : Respiratory dialysis with an active-mixing extracorporeal carbon dioxide removal system in a chronic sheep study. Intensive Care Med 38: 1705–1711, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Barr DP, Peters JP: The carbon dioxide absorption curve and carbon dioxide tension of the blood in severe anemia. J Biol Chem 45: 571–592, 1921. [Google Scholar]
- 15.Bidani A, Crandall ED: Analysis of the effects of hematocrit on pulmonary CO2 transfer. J Appl Physiol Respir Environ Exerc Physiol 53: 413–418, 1982. [DOI] [PubMed] [Google Scholar]
- 16.ANSI/AAMI/ISO 7199:2009: Cardiovascular implants and artificial organs—blood-gas exchangers (oxygenators), 2009.
- 17.Combes A, Fanelli V, Pham T, Ranieri VM; European Society of Intensive Care Medicine Trials Group and the “Strategy of Ultra-Protective lung ventilation with Extracorporeal CO2 Removal for New-Onset moderate to severe ARDS” (SUPERNOVA) Investigators: Feasibility and safety of extracorporeal CO2 removal to enhance protective ventilation in acute respiratory distress syndrome: the SUPERNOVA study Intensive Care Med 45: 592–600, 2019. [DOI] [PubMed] [Google Scholar]
- 18.Burki NK, Mani RK, Herth FJF, et al. : A novel extracorporeal CO(2) removal system: Results of a pilot study of hypercapnic respiratory failure in patients with COPD. Chest 143: 678–686, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Constable PD: Clinical assessment of acid-base status: Comparison of the Henderson-Hasselbalch and strong ion approaches. Vet Clin Pathol 29: 115–128, 2000. [DOI] [PubMed] [Google Scholar]
- 20.Deem S, Alberts MK, Bishop MJ, Bidani A, Swenson ER: CO2 transport in normovolemic anemia: complete compensation and stability of blood CO2 tensions J Appl Physiol 83: 240–246, 1997. [DOI] [PubMed] [Google Scholar]
- 21.Blumgart HL, Altschule MD: Clinical significance of cardiac and respiratory adjustments in chronic anemia. Blood 3: 329–348, 1948. [PubMed] [Google Scholar]
- 22.Arthurs G, Sudhakar M: Carbon dioxide transport Crit Care & Pain 5: 207–210, 2005. [Google Scholar]
- 23.Salathé EP, Fayad R, Schaffer SW: Mathematical analysis of carbon dioxide transport by blood Math Biosci 57: 109–153, 1981. [Google Scholar]
- 24.Arazawa D, Kimmel J, Federspiel W: Kinetics of CO2 exchange with carbonic anhydrase immobilized on fiber membranes in artificial lungs J Mater Sci Mater Med 26: 1–8, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]


