Abstract
Background
Sickle cell disease (SCD) is commonly encountered in Africa and Middle Eastern countries. The causative mutation in the gene encoding the hemoglobin subunit β (HBB) leads to various genotypic variants of the disease. This results in varied phenotypes, with a spectrum of complications, from benign to fatal. Hemoglobin SS (HBSS) genotype is associated with most of these complications; hence, it is a severe form of SCD. On the other hand, rare genotypes such as hemoglobin SE (HBSE) are considered benign. There is limited literature about the clinical manifestations and characteristics of patients with HBSE. We pooled all available data describing the phenotypic manifestations of HBSE heterozygote worldwide to perform a systematic review.
Methods
We performed a systematic review according to PRISMA guidelines using PubMed, SCOPUS, and Google Scholar databases. Two independent reviewers (FA and IK) evaluated studies for eligibility and extracted data. We synthesized data on demographics, manifestations, and management of HBSE disease. PROSPERO Registration Number: CRD42021229877.
Results
We found 68 HBSE patients reported in the literature. 24 cases were extracted from case reports whereas 44 cases from case series and retrospective studies. Turkey reported the highest number of patients (n = 22). 32 (47%) of the patients were males. The mean age was 20.9 ± 18.26 years. The mean HBS and HBE percentages were 61.1% ± 7.25% and 32.3% ± 5.06%, respectively, whereas the mean hemoglobin was 11.64 ± 1.73 g/dl. Reported manifestations of HBSE disease included acute vaso-occlusive pain crisis (n = 22, 32.3%), splenomegaly (n = 11, 16.1%), hemolytic anemia (n = 10, 14.7%), infections (n = 8. 11.7%), bone infarction (n = 4, 5.8%), gallstones (n = 3, 4.4%), venous thromboembolism (VTE) (n = 2, 2.9%) and stroke (n = 2, 2.9%), and hematuria (n = 2, 2.9%). Death due to HBSE complications was reported in three patients.
Conclusion
HBSE is a rare genotypic variant of SCD. It has been considered a benign form; however, there are multiple reports of severe complications. Severe complications observed in HBSE disease include vaso-occlusive crisis, acute chest syndrome, stroke, bone marrow embolism, and death.
Keywords: Sickle cell disease, SCD, Hemoglobin SE, HBSE, Sickle genotype
Introduction
SCD is a spectrum of hereditary hemoglobinopathies characterized by abnormal hemoglobin S (HbS) polymer. Globally, there are around 3.2 million SCD patients, and 43 million people have sickle cell trait. Out of these, around 176,000 people have fatal complications [1]. The causative mutation in the gene encoding the hemoglobin subunit β (HBB) leads to the formation of various genotypic variants of the disease [2]. This results in a cascade of sickling and unsickling erythrocytes, ultimately leading to hemolysis and/or vaso-occlusion [3]. Common manifestations and complications include but are not limited to hyposthenuria, acute chest syndrome, renal papillary necrosis, painful crisis, pulmonary hypertension, priapism, lower limb ulcerations, osteonecrosis, stroke, and chronic hemolysis [4, 5]. Significant complications such as acute chest syndrome are mainly observed in patients having common genotypes such as HBSS [6].
HBSE, one of the rare genotypes of SCD, has been historically considered to have a benign clinical course in most of cases and is categorized as a mild form of SCD [7, 8]. However, many case reports mention severe vaso-occlusive symptoms in the HBSE genotype, indicating that HBSE might be more severe than thought. Masiello et al. in their concise review described 26 cases of HBSE disease, among whom nine patients had sickling-related complications of varying severity. The review did not report any case with mortality. The authors concluded that HBSE might not be a mild disease, as evident from symptomatic cases [9]. To date, 68 patients with HBSE disease have been reported [7, 9–36]. Many of these had severe complications, and some even died due to the disease manifestations. Management of SCD is expanding, with newly approved disease-modifying drugs such as Voxelotor (1500 mg daily) and Crizanlizumab (5 mg/kg) [37, 38]. Additionally, there are recent trials on gene therapy in SCD with promising effectiveness [39]. Understanding the phenotypic manifestations of less studied SCD genotypes such as HBSE may improve our understanding of the effect of genotype and phenotype interaction on disease severity and suggest targeted therapies. The last review on HBSE was published in 2007; it was a concise review on 26 patients [9]. There have been multiple published cases of HBSE after that, representing variable manifestations of this condition. Many of these reports describe a severe form of SCD, mandating an updated systematic review focused on demographics and manifestations of patients with HBSE disease [10, 15, 24, 28, 30, 33–36]. This review’s main objective is to accumulate all the evidence to date on the demographics, phenotypic manifestations, and complications of the HBSE variant of SCD for its better classification and understanding.
Materials and methods
Literature search
A systematic literature search was performed for articles using PubMed, Google Scholar, and Scopus for any date up to January 10, 2021, and all articles in English were analyzed by two authors individually (FA and IK). The following search term was used: “HBSE” OR “Hemoglobin SE.” The extracted articles were screened initially from the title and abstract, and subsequently, a detailed screening was conducted. The quality of the added cases was assessed by two reviewers independently (FA and IK) using the Joanna Briggs Institute case report appraisal checklist for inclusion in systematic reviews [40]. In case of any dispute among the quality assessment, a third reviewer (MAY) independently analyzed the quality of disputed articles to reach a conclusion.
Study selection
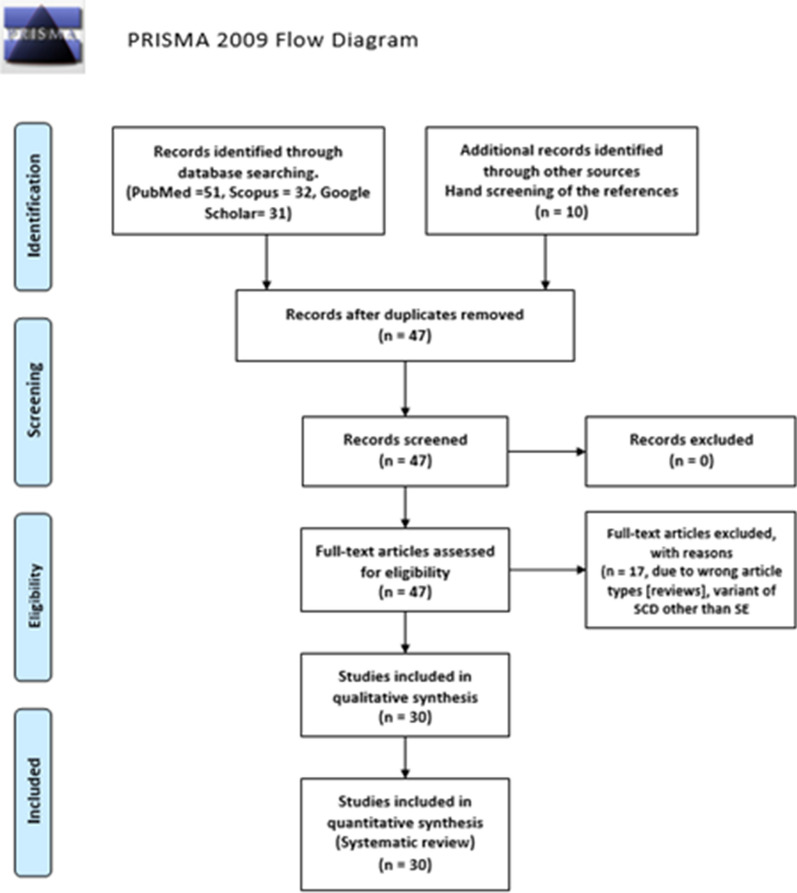
Eligible studies (N = 30) reported the HBSE genetic variant of SCD worldwide and included case reports, case series, and retrospective studies. Data of 68 HBSE patients were extracted and reported from the finalized 30 articles (Fig. 1). Articles that were not original or reported variants of SCD other than HBSE were excluded from the review.
Fig. 1.
Prisma flowchart with details of the article screening process
Data collection
Epidemiological parameters, clinical pictures, including presenting complaints and complications, laboratory profiles, treatments employed, and outcomes, were noted in all the cases where available. Cases were categorized as mild or severe based on the presence or absence of severe sickling complications, which were infections, functional asplenia, stroke, VTE, bone marrow embolism, splenic sequestration, retinopathy, bone infarct, and acute chest syndrome and death. Severe complications included those which were potentially fatal or had end-organ damage. Data were recorded and analyzed in Microsoft Excel 2016 and SPSS 26.
Results
We found 68 cases of HBSE in 30 publications (Table 1). Most cases were extracted from case series and retrospective studies (n = 44), while some were found in case reports (n = 24). The highest number of cases were reported from Turkey (n = 22), Oman (n = 13), USA (n = 11), and India (n = 8) in descending order. Males represented 32 (47%) of our cases, whereas females represented 50% ofcases (n = 34). Gender was not mentioned in two cases. The mean age was 20.9 years (SD = 18.2), ranging from 5 months to a maximum of 70 years (Table 2). Most patients had Asian ethnicity (n = 32, 47%), which included South and Southeast Asians, while African lineage (from at least one parent) was found in 8 cases (11.8%). Arabs represented 20.6% (n = 14) of our total cases.
Table 1.
Reported cases of HBSE to date
| Author | Study type | Patients (N) and nationality | Age (years) | Hgb (g/dL) and Hb electrophoresis (%) | Manifestations/complications | Treatment | Outcomea |
|---|---|---|---|---|---|---|---|
| Sex | |||||||
| Acipayam et al. [10] | RS | 20, Turkey | 21.45 ± 15 y | Hgb: 12.06 | 3 VOC (1 stroke) | HU = 1 | Alive |
| 8 Males | HBS: 59.5 | ||||||
| 12 Females | HBF: 1.9 | 17 asymptomatic | |||||
| HBE: 34.5 | |||||||
| Aksoy [11] | CR | 1, Turkey | 63 y | Hgb: 8.4 | Splenomegaly | None | Alive |
| Female | HBF: 0.8 | ||||||
| Altay et al. [12] | CR | 1, Georgia | 20 y | Hgb: 12.7 | NA | None | Alive |
| Female | HBS: 64.2 | ||||||
| HBF: 1.3 | |||||||
| HBE + HBA2: 34.6 | |||||||
| Andino et al. [13] | CR | 1, USA | 24 y | Hgb: 9 | NA | None | Alive |
| Female | HBS: 69.8 | ||||||
| HBE: 30.2 | |||||||
| Arbefeville et al. [14] | CR | 1, USA | 12 y | Hgb: NA | VOC causing ischemia and cardiopulmonary collapse | None | Death |
| Male | HBS: 57.4 | ||||||
| HBE: 34.2 | |||||||
| Baciu et al. [15] | CR | 1, USA | 56 y | Hgb: 13.3 | Retinopathy | None | Alive |
| Male | HBS: 64.1 | ||||||
| HBF: 1.2 | |||||||
| HBE: 34.7 | |||||||
| Bird et al. [16] | CR | 1, South Africa | NA | HBE: 28 | NA | None | Alive |
| Eichhorn et al. [17] | CR | 1, Turkey | 22 y | Hgb: 10.8 | VOC, pain crisis | Transfusion | Alive |
| Female | HBS: 60 | Splenomegaly | |||||
| HBE: 40 | Parvovirus b19 | ||||||
| Englestad [18] | CR | 1, USA |
34 y Female |
Hgb: 10 | VOC, pain crisis, chronic pain, hemolytic anemia, pneumonia, acute splenic sequestration, functional asplenia, splenomegaly, retinopathy | Transfusion, splenectomy | Alive |
| HBS: 60 | |||||||
| HBF: 40 | |||||||
| Ganesh et al. [19] | CR | 1, Omani |
23 y Male |
HBS: 66 | None | Alive | |
| HBF: 0.5 | |||||||
| HBE: 33.5 | |||||||
| George et al. [20] | CR | NA | NA | NA | Avascular necrosis | NA | NA |
| Gupta et al. [21] | CR | 1, Pakistani |
28 y Female |
Hgb: 12.9 | NA | None | Alive |
| HBS: 60 | |||||||
| HBF: 4 | |||||||
| HBE: 36 | |||||||
| Gürkan [22] | CR | 1, USA |
1 y Male |
Hgb: 10 | VOC, pain crisis | None | Alive |
| HBS: 58.9 | |||||||
| HBF: 5.2 | |||||||
| HBE + HBA2: 37.5 | |||||||
| Hardy et al. [23] | CR | 2, Saudi Arabia | 1 y | Hgb: 11.9 | 1 VOC | None | Alive |
| Male | HBS: 70 | ||||||
| 6 y | HBE: 30 | ||||||
| Female | |||||||
| Italia et al. [24] | RS | 4, India | 20.75 y | Hgb: 11.05 | 3 VOC, 1 HA, 1 splenomegaly, 1 chronic pain, pneumonia, ACS | None | Alive |
| 1 Male | HBS: 58.55 | ||||||
| 3 Females | HBF: 3.6 | ||||||
| HBE: 26.2 | |||||||
| Ibrahim K et al. [25] | CR | 1, Qatar | 17 y | Hgb: 13.3 | HU, transfusion | Alive | |
| Male | HBS: 67.1 | ||||||
| HBF: 2.2 | |||||||
| HBE: 28.7 | |||||||
| Knox-Macaulay et al. [7] | RS | 12, Oman | 17.8 y | Hgb: 12 | 1 ACS | None | Alive |
| 8 Males | HBS: 63.5 | 2 VOC | |||||
| 4 Females | HBF: 2.3 | 1 frontal bossing | |||||
| HBE: 32.7 | 1 recurrent UTIs | ||||||
| Masiello et al. [9] | CR | 1, USA |
1 y Male |
Hgb: 10 | VOC | None | Alive |
| HBS: 58.9 | |||||||
| HBF: 5.2 | |||||||
| HBE + HBA2: 37.5 | |||||||
| Mishra et al. [26] | CR | 2, India | 7 y | Hgb: 9.3 | Splenomegaly | None | Alive |
| Male | HBS: 58.05 | HA | |||||
| 20 y | HBF: 2.8 | ||||||
| Female | HBE + HBA2 = 35.4 | ||||||
| Mukhopadhyay et al. [27] | CR | 1, India |
26 y Male |
Hgb: NA | NA | None | Alive |
| HBS: 58.1 | |||||||
| HBF:4.3 | |||||||
| HBE: 33.2 | |||||||
| Pajak et al. [28] | CR | 1, USA |
55 y Female |
Hgb: 11.7 | HA, VOC, splenomegaly, functional asplenia | Splenectomy | Alive |
| HBS: 68.6 | |||||||
| HBF: 1.4 | |||||||
| HBE: 26.3 | |||||||
| Ramahi et al. [29] | CR | 1, USA |
28 y Female |
Hgb: NA | Postpartum endometritis | Transfusion | Alive |
| HBS: 65.3 | |||||||
| HBF: 3.5 | |||||||
| HBE: 31.2 | |||||||
| Rayburg et al. [30] | CR | 1, USA | 7 y | Hgb: 11.4 | VOC, HA, bone marrow infection with parvovirus b19, bone marrow embolism, PE, Splenomegaly asymptomatic | None | Death |
| Female | HBS: 55 | ||||||
| HBF: 1.3 | |||||||
| HBE: 31 | |||||||
| Rey et al. [31] | RS | 4, Haiti | 4.1 y | Hgb: 11.4 | None | Alive | |
| 2 Males | HBS: 61.5 | ||||||
| 2 Females | HBE + HBA2: 30 | ||||||
| Schroeder et al. [32] | CR | 1, USA | 22 y | Hgb: 14.6 | Hematuria | None | Alive |
| Male | HBS: 60 | Splenomegaly | |||||
| HBE + HBA2: 32 | |||||||
| Smith et al. [33] | CR | 1, USA | 52 y | Hgb: 13.4 | VOC, HA, Gallstones | Transfusion | Death |
| Female | HBS: 25 | ||||||
| HBE: 8 | |||||||
| Tamminga et al. [34] | CR | 1, Netherlands | 7 y | Hgb: 6.8 | Rhabdomyolysis | None | Alive |
| Male | HBS: 65 | ||||||
| HBF: 1.4 | |||||||
| HBE: 34 | |||||||
| Tay et al. [35] | CR | 1, Bangladesh |
66 y Female |
Hgb: 9.3 | HA, VOC, ACS, AVN | Transfusion, HU | Alive |
| HBS: 61.2 | |||||||
| HBF: 7.2 | |||||||
| Thornburg et al. [36] | CR | 1, USA |
2 Male |
Hgb: 10.2 | NA | NA | Alive |
| HBS: 68 | |||||||
| HBE: 32 | |||||||
| Vishwanathan et al. [47] | CR | 1, India |
18 y Male |
Hgb: 9 | VOC | NA | Alive |
| HBS: 68 | HA | ||||||
| HBF: 2.1 | Splenomegaly | ||||||
| HBE: 29.9 |
Hgb hemoglobin, HU hydroxyurea, SCT stem cell transplant, VOC vaso-occlusive crisis, HA hemolytic anemia, ACS acute chest syndrome, AVN AVASCULAR necrosis, UTI urinary tract infections, PE pulmonary embolism, RS retrospective study, CR case report, NA not available
aOutcome at the time of the case report/series
Table 2.
Demographics of SCD patients with HBSE genotype compared to classic SCD [46]
| Characteristics | HBSE reported cases, N = 68 |
|---|---|
| Agea (years) | Mean: 20.9 ± 18.26 years |
| HbS (percentage) | Mean: 61.1 ± 7.25 |
| HbE (percentage) | Mean: 32.3 ± 5.06 |
| HbF (percentage) | Mean: 2.2 ± 2.16 |
| Hemoglobin (g/dl) | Mean: 11.64 g/dl ± 1.73 |
| Mean (males): 11.69 | |
| Mean (females): 11.61 | |
| MCV (fl) | Mean: 70.5 ± 6.01 |
| Presentation (diagnosis) | Asymptomatic at presentation: 31 (45%) |
| Presentation at birth: 2 (2.8%) | |
| Discovered in Clinic: 1 (1.4%) | |
| Diagnosed during hospitalization: 8 (11.8%) | |
| Discovered Postmortem: 2 (2.8%) | |
| Asymptomatic cases (no complications found) | N: 35 (51.5%) |
| Treatments given |
Transfusion (exchange or simple): 7 (10.2%) Hydroxyurea: 3 (4.4%) |
| Death due to complications | N: 3 (4.2%) |
aAge at presentation
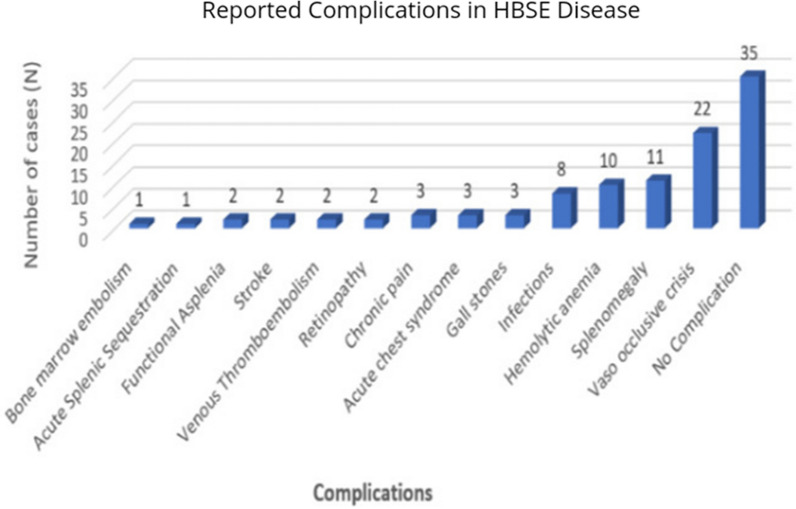
Acute vaso-occlusive pain crisis was the most common complication reported by 22 cases (32.3%), with splenomegaly (n = 11, 16.1%), hemolytic anemia (n = 10, 14.7%), infections (n = 8. 11.8%), bone infarction (n = 4, 5.9%) representing other common complications (Fig. 2). Respiratory manifestations (thoracic pain, cough, bronchitis, dyspnea, or upper respiratory infection) were reported in five cases (7.3%). Gallstones were seen in three cases (4.4%). Venous thromboembolism (VTE) (2.9%) and stroke (2.9%) were both described in two separate cases each. Hematuria was also present in two patients (2.9%).
Fig. 2.
Reported complications in HBSE disease
We did not find any report describing cardiopulmonary complications (pulmonary hypertension, myocardial infarction, cardiomyopathy), which commonly affect SCD patients. Other serious renal complications were not reported in any of the 68 cases in the literature.
Most of the reported patients had mild to moderate disease. However, mortality due to complications was documented in three cases. Out of those three patients, the first patient, a 52-year-old female, had an undiagnosed potentially uncomplicated disease and revealed only postmortem. The disease rapidly deteriorated, leading to venous thromboembolism and cardiac arrest within 26 h of admission. The cause of death was thought to be ischemia due to dehydration that led to a sickling crisis [33]. The second death occurred in a 12-year-old male within 24 h of symptom onset due to sudden cardiopulmonary collapse due to vaso-occlusion leading to ischemia [14]. The third fatality occurred in a 7-year-old female with a history of multiple hospitalizations with pain crises, hemolytic anemia, and Parvovirus B19 infection. Although she had a prolonged and complicated course of her disease, her deterioration before death was acute, secondary to a massive marrow embolism in the pulmonary circulation [30].
Mean hemoglobin (Hgb) was 11.64 mg/dl, slightly higher in males (11.69 mg/dl) than females (11.61 mg/dl). Mean percentages of hemoglobin S (HbS), hemoglobin E (HbE), and fetal hemoglobin (HbF) are given in Table 2. Average MCV was low (70.9 fL), with minimum and maximum reported being 57.9 fl and 82 fl, respectively.
Discussion
This study represents an updated comprehensive systematic review on demographics and manifestations of HBSE genotypic variant of SCD. The genetic basis of HBS lies in glutamine to valine, at position six in the beta-globin gene, whereas that of HBE lies in the substitution of lysine for glutamine at amino acid number 26, in the beta-globin gene [41, 42]. HBSE variant occurs as a result of a combination of HBS and HBE genotypes [28]. Hemoglobin E trait and disease are not uncommon and are second only to the HBS spectrum in global prevalence [43]. However, the combination of two different abnormal mutations makes HBSE a rare occurrence.
The exact incidence of the HBSE genotype remains unknown. A retrospective review of 12 patients in Oman mentioned a point prevalence of HBSE heterozygotes at 0.05%. The authors also concluded that given a very small fraction of HBSE patients developing symptoms, it is a very mild condition, and severe sickling complications are rarely seen [7]. However, in our review, we found a wide spectrum of SCD complications ranging from asymptomatic patients (51.5%) to death (4.4%). Complications included VOC pain crisis (32.3%), splenomegaly (16.1%), hemolysis (14.7%), infections (11.8%), bone infarction (5.9%), cholelithiasis (4.4%), thromboembolism (2.9%), stroke (2.9%), and hematuria (2.9%).
In a review on HBSE disease published in 2007, David Masiello et al. found that among the 26 patients with HBSE disease, nine patients aged 18 or younger were generally well. However, more than half of the patients older than 18 presented with sickling-related complications. They attributed this to the years of accumulating sickling vasculopathy that makes complication rate increase with aging [9]. We analyzed the complications based on age by dividing them into below 18 and above 18 years in cases where ages were specified for the complications (22 children and 24 adults). Overall, the complication rate in adults was higher than the pediatric age group, which is in line with the previous review of 16 patients. The only significant difference in complications based on age was found in the occurrence of hemolytic anemia, which was present in 4.5% (1/22) of cases < 18 years old compared to 37.5% (9/24) of cases > 18 years of age (OR 12.6, P-value = 0.011). The low incidence of hemolytic anemia in children with HBSE can be related to the presence of fetal hemoglobin, which has a protective effect against many of the complications secondary to sickling, including death [8]. Acute vaso-occlusive pain crisis was seen in 31.8% (7/22) children (age < 18 years) compared to 37.5% (9/24) adults. On the other hand, chronic pain was slightly more common in adults; 4.5% (1/22) of pediatric cases compared to 8.3% (2/24) of adult cases. Pain crisis was not considered a severe complication of SCD, but it is a clinically significant symptom for the patient due to the type of sensation involved. Additionally, in patients > 20 years of age, pain frequency has been described to be associated with early mortality [8]. Those patients who presented with gallstones (n = 3), retinopathy (n = 2), functional asplenia (n = 2), and splenic sequestration (n = 1) were adults. The infection rate was comparable in both age groups; 13.6% (3/22) in children and 16.7% (4/24) in adults. Splenomegaly was present in 18.2% (4/22) of pediatric cases compared to 29.2% (7/24) of adults. Bone infarction and acute chest syndrome were present in 4.5% (1/22) of children compared to 8.3% (2/24) of adults. Bone marrow embolism was reported in one pediatric case only. VTE was present in one pediatric and one adult patient. Mortality, however, was seen more in children (two cases) compared to adults (one case).
We found a slight female predominance in the HBSE population (50%) compared to males (47%). It is to be noted that gender was not reported in two patients, which could have increased or decreased the asymmetry between both genders. Our finding, although less disproportionate, is in keeping with a previous review on 27 patients with HBSE disease, where the authors described 73.6% female cases. In their review, Mishra P et al. reported that 40.7% of the total patients were symptomatic [26]. In our review, 35 (51.5%) of the 68 patients had no complications reported, while 48.5% suffered from mild, moderate, or severe SCD related complications. Although high levels of HBF are considered protective against SCD complications, no such correlation was found by Mishra et al. in their review [26, 44]. To analyze the association of HBF with the severity of complications, we divided the complications into mild-moderate and severe. The mean HBF level in those with mild to moderate complications was higher than those with severe complications, including death (2.4% versus 1.9%). However, no statistical significance was found. A better understanding of this patient-to-patient clinical variability in more extensive studies would dramatically improve care and guide the development of novel therapies. Studies of the natural history of these β-hemoglobinopathies have identified fetal hemoglobin levels and concomitant α-thalassemia as important modifiers of disease severity. Fortunately, improved knowledge of the human genome and the development of new genomic tools, such as genome-wide genotyping arrays and next-generation DNA sequencers, offer new opportunities to use genetics to understand better the causes of the many complications observed in β-hemoglobinopathy patients [45]. Correlation of the clinical and hematological features of HbSE cases with their α-globin gene status and β-cluster haplotypes merits additional considerations in future studies to better understand phenotypes and the disease modifiers and probably novel agents.
Turkey is a country with a higher prevalence of hemoglobinopathies such as HBE and HBS with significant population admixture and racial intermarriages. One retrospective study on 20 patients with HBSE disease reported a mean Hgb of 12.06 g/dl, which is slightly higher than what we found in our review (11.64 g/dl). The study reported a mean MCV of 69.9 fl, which on the other hand, is slightly lower than the one in our review, 70.9 fl. 15% of the patients in that study had sickling-related complications compared to 68% in our review [10]. The increased published reports of complications related to HBSE reveals the potential morbidity and mortality related to the genotype. Similar to HBSS, HBSE genotype results in a range of phenotypes and may be fatal. There may be subclinical or long-term subtle complications of HBSE that have not yet been well described. The increasing evidence emphasizes early detection and close follow-up of HBSE patients for better patient management. Additionally, it is imperative to reclassify HBSE disease as a moderate form of SCD rather than mild.
As it is a variant of sickle cell disease, the clinical features and biochemical profile of HBSE patients are comparable to the classic HBSS SCD. However, there are some notable differences. In our review, we found the mean age at presentation with symptoms 20.9 years, whereas the median age of presentation in classic SCD is reported around 36 years in a previous study [46]. Another difference that we appreciated is in mean HBF percentage, which is 2.2% in HBSE patients, and 5% in HBSS patients. Lastly, because management of HBSE disease is not well-established, the use of HU in HBSE patients is considerably low (4.4%) compared to HBSS patients (39%) [46]. A direct comparison of both types of SCD in a similar patient population in extensive studies can validate our findings.
Over the years, many treatment modalities have been developed for SCD, among which hydroxyurea and simple or exchange blood transfusion have an established role in treating or preventing complications. Curative management includes historical treatment such as stem cell transplantation and new and upcoming gene therapy [3]. Some newer management drugs that have recently been approved for the treatment of SCD include Voxelotor and Crizanlizumab [37, 38]. In our review, blood transfusion and hydroxyurea have been used in few patients and improved their conditions [10, 17, 25, 29, 33, 35].
Our review has some limitations inherent when conducting systematic reviews of rare conditions. Firstly, around 30 percent of the data was extracted from case reports. Secondly, missing data from case reports and retrospective studies limited an extensive data analysis. Thirdly, we acknowledge that publication bias may have contributed to the increased number of complications reported in recent years. Many patients with asymptomatic course who do not require medical attention would go unnoticed. The lack of systematic prospective data precludes a proper understanding of the actual rates, incidence, or timeline of the development of major or minor complications. Lastly, as it is challenging to distinguish HBSE from other variants of SCD, such as HBSC on readily available tests, a bias in reporting HBSE as HBSC (or other variants) could be present in the literature, decreasing the total number of reported HBSE cases.
This review opens the door to consider various SCD therapies in managing HBSE patients with severe or recurrent complications. More extensive studies on the HBSE patient population must formulate management guidelines to treat its moderate to severe complications. Hydroxyurea, the mainstay of treatment of sickle cell disease, has a well-established role in HBSS and Hb SB+ [9]. However, due to the rare occurrence of HBSE, there are no trials available for the use of HU in this variant of SCD. In our review, HU was used in 4.4% of patients, with good outcomes. Establishing a definite role of HU in symptomatic patients with HBSE might take years as the incidence of HBSE itself, and then the prevalence of symptoms in this patient population is still too low to analyze the use of HU in HBSE prospectively. Meanwhile, we suggest using HU in HBSE patients with moderate to severe VOC and acute chest syndrome symptoms on a case-to-case basis until more extensive evidence becomes available.
Conclusion
HBSE disease has been considered as a very benign variant of SCD with minimal to no sickling symptoms. However, a growing literature on HBSE and its complications suggests that HBSE considerable number of patients have a moderate form of SCD with a wide range of significant complications, including mortality. More extensive studies with additional data can help categorize and understand HBSE disease and its manifestations better.
Acknowledgements
The Qatar National Library funded the publication of this article.
Authors’ contributions
IK: data collection, literature search, manuscript preparation, critical review and revisions in the manuscript. FA: methodology, data collection, literature search, manuscript preparation, data analysis and interpretation, critical review, and revisions in the manuscript. HC: data collection, literature search, manuscript preparation, data analysis, and interpretation. AS and VD: literature review, critical review, and revisions in the manuscript. MAY: supervision, literature Search, critical review, and revisions in the manuscript. All authors read and approved the final manuscript.
Funding
Open access funding provided by the Qatar National Library.
Availability of data and materials
Data sharing not applicable.
Declarations
Ethics approval and consent to participate
Private information from individuals will not be published. This systematic review also does not involve endangering participant rights. Ethical approval was not required.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Sundd P, Gladwin MT, Novelli EM. Pathophysiology of sickle cell disease. Annu Rev Pathol. 2019;14:263–292. doi: 10.1146/annurev-pathmechdis-012418-012838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kato GJ, Piel FB, Reid CD, Gaston MH, Ohene-Frempong K, Krishnamurti L, Smith WR, Panepinto JA, Weatherall DJ, Costa FF, Vichinsky EP. Sickle cell disease. Nat Rev Dis Primers. 2018;4:18010. doi: 10.1038/nrdp.2018.10. [DOI] [PubMed] [Google Scholar]
- 3.Ware RE, de Montalembert M, Tshilolo L, Abboud MR. Sickle cell disease. Lancet. 2017;390:311–323. doi: 10.1016/S0140-6736(17)30193-9. [DOI] [PubMed] [Google Scholar]
- 4.Wali Y, Kini V, Yassin MA. Distribution of sickle cell disease and assessment of risk factors based on transcranial Doppler values in the Gulf region. Hematology. 2020;25:55–62. doi: 10.1080/16078454.2020.1714113. [DOI] [PubMed] [Google Scholar]
- 5.Habara A, Steinberg MH. Minireview: genetic basis of heterogeneity and severity in sickle cell disease. Exp Biol Med. 2016;241:689–696. doi: 10.1177/1535370216636726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Meremikwu MM, Okomo U. Sickle cell disease. BMJ Clin Evid. 2016;2016:2402. [PMC free article] [PubMed] [Google Scholar]
- 7.Knox-Macaulay HH, Ahmed MM, Gravell D, Al-Kindi S, Ganesh A. Sickle cell-haemoglobin E (HbSE) compound heterozygosity: a clinical and haematological study. Int J Lab Hematol. 2007;29:292–301. doi: 10.1111/j.1365-2257.2006.00886.x. [DOI] [PubMed] [Google Scholar]
- 8.Rees DC, Williams TN, Gladwin MT. Sickle-cell disease. Lancet. 2010;376:2018–2031. doi: 10.1016/S0140-6736(10)61029-X. [DOI] [PubMed] [Google Scholar]
- 9.Masiello D, Heeney MM, Adewoye AH, Eung SH, Luo HY, Steinberg MH, Chui DH. Hemoglobin SE disease: a concise review. Am J Hematol. 2007;82:643–649. doi: 10.1002/ajh.20847. [DOI] [PubMed] [Google Scholar]
- 10.Acipayam C, Oktay G, Ilhan G, Çürük MA. Hemoglobin SE disease in Hatay, in the southern part of Turkey. Thal Rep. 2015 doi: 10.4081/thal.2015.4597. [DOI] [Google Scholar]
- 11.Aksoy M. The hemoglobin E syndromes II sickle-cell-hemoglobin E disease. Blood. 1960;15:610–613. doi: 10.1182/blood.V15.5.610.610. [DOI] [PubMed] [Google Scholar]
- 12.Altay C, Niazi GA, Huisman TH. The combination of HB S and HB E in a black female. Hemoglobin. 1976;1:100–102. doi: 10.3109/03630267609031026. [DOI] [PubMed] [Google Scholar]
- 13.Andino L, Risin SA. Pathologic quiz case: a 24-year-old woman with abnormal hemoglobin and thrombocytopenia. Compound heterozygosity for hemoglobin S and E. Arch Pathol Lab Med. 2005;129:257–258. doi: 10.5858/2005-129-257-PQCAYW. [DOI] [PubMed] [Google Scholar]
- 14.Arbefeville EF, Tebbi CK, Chrostowski L, Adams VI. Sudden death after exercise in an adolescent with hemoglobin SE. Am J Forensic Med Pathol. 2011;32:341–343. doi: 10.1097/PAF.0b013e3181d8e390. [DOI] [PubMed] [Google Scholar]
- 15.Baciu P, Yang C, Fantin A, Darnley-Fisch D, Desai U. First reported case of proliferative retinopathy in hemoglobin SE disease. Case Rep Ophthalmol Med. 2014;2014:782923. doi: 10.1155/2014/782923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bird AR, Wood K, Leisegang F, Mathew CG, Ellis P, Hartley PS, Karabus CD. Haemoglobin E variants: a clinical, haematological and biosynthetic study of 4 South African families. Acta Haematol. 1984;72:135–137. doi: 10.1159/000206374. [DOI] [PubMed] [Google Scholar]
- 17.Eichhorn RF, Buurke EJ, Blok P, Berends MJ, Jansen CL. Sickle cell-like crisis and bone marrow necrosis associated with parvovirus B19 infection and heterozygosity for haemoglobins S and E. J Intern Med. 1999;245:103–106. doi: 10.1046/j.1365-2796.1999.0445f.x. [DOI] [PubMed] [Google Scholar]
- 18.Englestad BL. Functional asplenia in hemoglobin SE disease. Clin Nucl Med. 1982;7:100–102. doi: 10.1097/00003072-198203000-00002. [DOI] [PubMed] [Google Scholar]
- 19.Ganesh A, al-Habsi NS, al-Alawi FK, Mitra S, Eriksson A, Venugopalan P. Traumatic hyphaema and sickle cell retinopathy in a patient with sickle cell-haemoglobin E (HbSE) disease. Eye. 2000;14(3):397–400. doi: 10.1038/eye.2000.101. [DOI] [PubMed] [Google Scholar]
- 20.George E, Iqbal QM. Hb ES presenting as avascular necrosis. Southeast Asian J Trop Med Public Health. 1978;9:568–570. [PubMed] [Google Scholar]
- 21.Gupta R, Jarvis M, Yardumian A. Compound heterozygosity for haemoglobin S and haemoglobin E. Br J Haematol. 2000;108:463. doi: 10.1046/j.1365-2141.2000.02035.x. [DOI] [PubMed] [Google Scholar]
- 22.Gürkan E. Vaso-occlusive manifestations in a patient with sickle cell-hemoglobin E (HbSE) disease. Am J Hematol. 2006;81:149. doi: 10.1002/ajh.20488. [DOI] [PubMed] [Google Scholar]
- 23.Hardy MJ, Ragbeer MS. Homozygous HbE and HbSE disease in a Saudi family. Hemoglobin. 1985;9:47–52. doi: 10.3109/03630268508996981. [DOI] [PubMed] [Google Scholar]
- 24.Italia K, Upadhye D, Dabke P, Kangane H, Colaco S, Sawant P, Nadkarni A, Gorakshakar A, Jain D, Italia Y, et al. Clinical and hematological presentation among Indian patients with common hemoglobin variants. Clin Chim Acta. 2014;431:46–51. doi: 10.1016/j.cca.2014.01.028. [DOI] [PubMed] [Google Scholar]
- 25.Khamees I, Yassin M, Rozi W. Acute chest syndrome in sickle cell disease/HBE patient, a case report. Authorea. 2020 doi: 10.22541/au.161158119.94954198/v1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mishra P, Pati HP, Chatterjee T, Dixit A, Choudhary DR, Srinivas MU, Mahapatra M, Choudhry VP. Hb SE disease: a clinico-hematological profile. Ann Hematol. 2005;84:667–670. doi: 10.1007/s00277-005-1044-2. [DOI] [PubMed] [Google Scholar]
- 27.Mukhopadhyay S, Kumar N, Saxena R. Sickle cell-hemoglobin E disease in an Indian family. Indian J Pathol Microbiol. 2001;44:465–466. [PubMed] [Google Scholar]
- 28.Pajak A, Li JC, Liu A, Nazare S, Smith B. Hemoglobin SE disease presenting as a high-altitude massive splenic infarction complicated by hemorrhagic conversion and splenectomy. Cureus. 2020;12:e10321. doi: 10.7759/cureus.10321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ramahi AJ, Lewkow LM, Dombrowski MP, Bottoms SF. Sickle cell E hemoglobinopathy and pregnancy. Obstet Gynecol. 1988;71:493–495. [PubMed] [Google Scholar]
- 30.Rayburg M, Kalinyak KA, Towbin AJ, Baker PB, Joiner CH. Fatal bone marrow embolism in a child with hemoglobin SE disease. Am J Hematol. 2010;85:182–184. doi: 10.1002/ajh.21605. [DOI] [PubMed] [Google Scholar]
- 31.Rey KS, Unger CA, Rao SP, Miller ST. Sickle cell-hemoglobin E disease: clinical findings and implications. J Pediatr. 1991;119:949–951. doi: 10.1016/S0022-3476(05)83053-7. [DOI] [PubMed] [Google Scholar]
- 32.Schroeder WA, Powars D, Reynolds RD, Fisher JI. Hb-E in combination with Hb-S and Hb-C in a black family. Hemoglobin. 1977;1:287–289. doi: 10.3109/03630267709003411. [DOI] [PubMed] [Google Scholar]
- 33.Smith A, Cooper B, Guileyardo J, Mora A., Jr Unrecognized hemoglobin SE disease as microcytosis. Bayl Univ Med Cent Proc. 2016 doi: 10.1080/08998280.2016.11929447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tamminga RY, Doornbos ME, Muskiet FA, Koetse HA. Rhabdomyolysis in a child with hemoglobin SE. Pediatr Hematol Oncol. 2012;29:267–269. doi: 10.3109/08880018.2012.655406. [DOI] [PubMed] [Google Scholar]
- 35.Tay SH, Teng GG, Poon M, Lee VK, Lim AY. A case of hemoglobin SE presenting with sickle cell crisis: case report and histological correlation. Ann Acad Med Singap. 2011;40:552–553. [PubMed] [Google Scholar]
- 36.Thornburg CD, Steinberg MH, Chui DH. Hemoglobin SE disease in Maine, and severe thalassemia in New Hampshire. J Pediatr Hematol Oncol. 2009;31:307. doi: 10.1097/MPH.0b013e31819af9db. [DOI] [PubMed] [Google Scholar]
- 37.Ataga KI, Kutlar A, Kanter J, Liles D, Cancado R, Friedrisch J, Guthrie TH, Knight-Madden J, Alvarez OA, Gordeuk VR, et al. Crizanlizumab for the prevention of pain crises in sickle cell disease. N Engl J Med. 2016;376:429–439. doi: 10.1056/NEJMoa1611770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vichinsky E, Hoppe CC, Ataga KI, Ware RE, Nduba V, El-Beshlawy A, Hassab H, Achebe MM, Alkindi S, Brown RC, et al. A phase 3 randomized trial of voxelotor in sickle cell disease. N Engl J Med. 2019;381:509–519. doi: 10.1056/NEJMoa1903212. [DOI] [PubMed] [Google Scholar]
- 39.Orkin SH, Bauer DE. Emerging genetic therapy for sickle cell disease. Annu Rev Med. 2019;70:257–271. doi: 10.1146/annurev-med-041817-125507. [DOI] [PubMed] [Google Scholar]
- 40.Munn Z, Barker TH, Moola S, Tufanaru C, Stern C, McArthur A, Stephenson M, Aromataris E. Methodological quality of case series studies: an introduction to the JBI critical appraisal tool. JBI Evid Synth. 2020;18(10):2127–2133. doi: 10.11124/JBISRIR-D-19-00099. [DOI] [PubMed] [Google Scholar]
- 41.Matthews DC, Glader B. Erythrocyte disorders in infancy. In: Gleason CA, Devaskar SU, editors. Avery’s diseases of the newborn. 9. Philadelpia: W.B. Saunders; 2012. pp. 1080–1107. [Google Scholar]
- 42.Hurley R. Anemia and red blood cell disorders. In: Walker PF, Barnett ED, editors. Immigrant medicine. Edinburgh: W.B. Saunders; 2007. pp. 611–623. [Google Scholar]
- 43.Weatherall DJ, Clegg JB. Inherited haemoglobin disorders: an increasing global health problem. Bull World Health Organ. 2001;79:704–712. [PMC free article] [PubMed] [Google Scholar]
- 44.Quinn CT. Minireview: clinical severity in sickle cell disease: the challenges of definition and prognostication. Exp Biol Med. 2016;241:679–688. doi: 10.1177/1535370216640385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chang AK, Ginter Summarell CC, Birdie PT, Sheehan VA. Genetic modifiers of severity in sickle cell disease. Clin Hemorheol Microcirc. 2018;68:147–164. doi: 10.3233/CH-189004. [DOI] [PubMed] [Google Scholar]
- 46.Sachdev V, Kato GJ, Gibbs JS, Barst RJ, Machado RF, Nouraie M, Hassell KL, Little JA, Schraufnagel DE, Krishnamurti L, et al. Echocardiographic markers of elevated pulmonary pressure and left ventricular diastolic dysfunction are associated with exercise intolerance in adults and adolescents with homozygous sickle cell anemia in the United States and United Kingdom. Circulation. 2011;124:1452–1460. doi: 10.1161/CIRCULATIONAHA.111.032920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Vishwanathan C, Agarwal MB, Bichile LS, Bhave AB. Double heterozygosity for hemoglobin S and E. Indian Pediatr. 1992;29:895–897. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing not applicable.