Abstract
Background: Peru was one of the countries with the highest COVID-19 mortality worldwide during the first stage of the pandemic. It is then relevant to evaluate the risk factors for mortality in patients hospitalized for COVID-19 in three hospitals in Peru in 2020, from March to May, 2020.
Methods: We carried out a retrospective cohort study. The population consisted of patients from three Peruvian hospitals hospitalized for a diagnosis of COVID-19 during the March-May 2020 period. Independent sociodemographic variables, medical history, symptoms, vital functions, laboratory parameters and medical treatment were evaluated. In-hospital mortality was assessed as the outcome. We performed Cox regression models (crude and adjusted) to evaluate risk factors for in-hospital mortality. Hazard ratios (HR) with their respective 95% confidence intervals (95% CI) were calculated.
Results: We analyzed 493 hospitalized adults; 72.8% (n=359) were male and the mean age was 63.3 ± 14.4 years. COVID-19 symptoms appeared on average 7.9 ± 4.0 days before admission to the hospital, and the mean oxygen saturation on admission was 82.6 ± 13.8. While 67.6% (n=333) required intensive care unit admission, only 3.3% (n=16) were admitted to this unit, and 60.2% (n=297) of the sample died. In the adjusted regression analysis, it was found that being 60 years old or older (HR=1.57; 95% CI: 1.14-2.15), having two or more comorbidities (HR=1.53; 95% CI: 1.10-2.14), oxygen saturation between 85-80% (HR=2.52; 95% CI: 1.58-4.02), less than 80% (HR=4.59; 95% CI: 3.01-7.00), and being in the middle (HR=1.65; 95% CI: 1.15-2.39) and higher tertile (HR=2.18; 95% CI: 1.51-3.15) of the neutrophil-to-lymphocyte ratio, increased the risk of mortality.
Conclusions: The risk factors found agree with what has been described in the literature and allow the identification of vulnerable groups in whom monitoring and early identification of symptoms should be prioritized in order to reduce mortality.
Keywords: SARS-CoV-2, COVID-19, Mortality, Adults, Latin America.
Introduction
COVID-19 is a disease characterized by severe pneumonia, and was first registered in December 2019 in Wuhan, China. 1 This disease has generated great impact worldwide, especially in Latin America. 2 According to the data reported by the World Health Organization (WHO), the number of COVID-19 cases at the end of December 2020 exceeded 80 million around the world, while fatal cases amount to more than 1.7 million. 2 However, in regions with emerging economies or with limited access to health services such as Latin America, this disease has had great social impact. 3
It is known that approximately 80% of COVID-19 cases present a mild to moderate course; however, the remaining 20% present a severe to critical course, requiring hospital care and leading to a high risk of death. 4 Thus, factors affecting the prognosis of this disease have been related to the severity of the clinical presentation and analytical markers. The analytical factors include a high neutrophil-to-lymphocyte ratio (NLR), platelet-to-lymphocyte ratio and lymphocyte-to-monocyte ratio, 5 leukopenia, elevated creatinine and lactate dehydrogenase (LDH) levels and prothrombin time. 6 On the other hand, demographic characteristics and medical history have been described to increase the risk of mortality due to COVID-19 and include advanced age, male sex, the presence of comorbidities, such as chronic obstructive pulmonary disease, hypertension, type 2 diabetes mellitus or coronary heart disease. 6, 7
Although risk factors for the disease have been described in different populations around the world, there is little scientific evidence in the Latin American population and especially in Peru, which is one of the countries with the highest mortality from COVID-19 worldwide. 8, 9 In addition, deficiencies in the Peruvian health system, including a scarcity of intensive care unit (ICU) beds and mechanical ventilators, as well as low compliance with government measures to combat the spread of the pandemic, could increase mortality by COVID-19. 8, 10 Likewise, the massive use of therapies without scientific evidence in the hospital setting, as well as the high frequency of self-medication in the Peruvian population can also aggravate the severity and mortality by COVID-19. 11
Despite the government having taken measures to improve access to health services in Peru, the implementation of these strategies was slow during the first months of the pandemic. The impact of the flaws of the health system could have caused a higher mortality in the population. Taking this into account, it is relevant to explore the sociodemographic and clinical characteristics of the population hospitalized for COVID-19 in Peru and the incidence of mortality at the first stage of the pandemic. Therefore, the objective of this study was to evaluate and describe the risk factors for mortality from COVID-19 in patients from three hospitals in Peru hospitalized during the period from March to May 2020.
Methods
Design and population
We carried out a retrospective cohort study. The study population consisted of adults hospitalized during the period from March 18 to May 13, 2020 for the diagnosis of COVID-19 at the Hospital Almanzor Aguinaga Asenjo and Luis Heysen Incháustegui Hospital, located in Chiclayo and the Hospital Clínica EsSalud Chepén, located in Chepén, two cities in Peru. We included patients older than 18 years of age who were hospitalized with a diagnosis confirmed by serological or molecular tests for COVID-19, as well as suspected by a compatible clinical or radiological pattern plus an epidemiological link, despite having a non-reactive serological test for COVID-19. The exclusion criteria were patients under 18 years of age and pregnant women.
Description of the study area
The hospitals included correspond to the Lambayeque social security care network in Peru (EsSalud) and are the reference hospitals with the highest complexity for the management of patients with COVID-19. From the first confirmed case of COVID-19 in Lambayeque in March 2020 until November 30, 2020, there was a total of 18,570 confirmed cases of COVID-19, 5,654 hospitalized cases and 2,579 deaths.
Type of sampling and sample size calculation
The sampling was non-probabilistic and we included all participants hospitalized during the study period who met the inclusion criteria.
Procedures
The information on the participants included in the study was collected by two researchers from the EsSalud virtual medical records registry. The collection of all records was carried out independently by each of the data entry operators, to ensure adequate data collection and reduce erroneous records. Sociodemographic variables, medical history and laboratory markers were collected from hospitalized patients during the period from March 18 to May 13, 2020. Laboratory markers were collected during the first 24 hours of hospitalization. Mortality follow-up was in-hospital and the date of hospital admission was considered as the start of follow-up. The information was tabulated in a Microsoft Excel 2016 document, and quality control of the data was carried out by a researcher of our team.
Bias
This study included participants insured by Peruvian social security, who have socioeconomic characteristics that could differ from the national population; however, this study is one of the first reports carried out in Peru, 12, 53 one of the countries with the highest mortality rates during the first wave of the pandemic. In addition, certain laboratory markers had a significant percentage of missing values, however, we included in the multivariate analysis the most relevant markers for the association of interest.
Variables
Outcome variable: In-hospital mortality
Mortality in hospitalized patients with a confirmed or probable diagnosis of COVID-19 was evaluated as an outcome variable. This was collected according to outcome recorded in the clinical history at the end of the follow-up (June 2, 2020).
Exposure variables
Sociodemographic variables
The demographic characteristics recorded were: age (<50, 50-59, ≥60 years), sex (female, male), comorbidities (obesity, type 2 diabetes mellitus, hypertension, asthma, cancer, chronic kidney disease). Likewise, we generated a variable that grouped these comorbidities into different categories (0, 1, 2 or more).
Symptoms and epidemiological link
The time of disease of the patients (in days), symptoms (respiratory distress, cough, fever, sore throat, diarrhea, headache, nasal congestion, anosmia, ageusia) were included. In addition, contact with a confirmed case of COVID-19 (yes, no) was considered.
Baseline vital functions
Baseline vital function values were collected at admission, including temperature, respiratory rate, heart rate, and oxygen saturation.
Baseline auxiliary exams
The following laboratory values were considered: hemoglobin (g/dL), leukocytes (leukocytosis was defined as a value greater than or equal to 10,000 cells/mm 3), neutrophils (cells/mm 3), lymphocytes (lymphopenia was defined as a value less than 0.8 cells/mm 3), the NLR (categorized into tertiles), platelets (thrombocytopenia was defined as a value less than 150,000 cells/mm 3), creatinine (mg/dL), urea (mg/dL), aspartate transaminase (AST) (U/L), alanine aminotransferase (ALT) (U/L), and LDH (U/L).
Treatment received
The treatment administered to hospitalized patients was included, considering antibiotic therapy (azithromycin, cephalosporins, carbapenems, among others), corticosteroid therapy (methylprednisolone, dexamethasone, hydrocortisone, prednisone), antiparasitic drugs (hydroxychloroquine, ivermectin), anticoagulants (enoxaparin) and antivirals (lopinavir)/ritonavir).
Statistical analysis
The descriptive results of the categorical variables were presented using absolute and relative frequencies, while quantitative variables are shown using central tendency and dispersion measures. The comparison of proportions between the categorical covariates and the outcome was performed using the Chi-square test, while the Student's t test or Mann Whitney U test was used to evaluate differences with numerical covariates.
The Kaplan-Meier method was used to describe the survival function, and the log-rank test was used for the crude comparison of survival functions. A Cox regression analysis (crude and adjusted) was performed to evaluate independent risk factors for mortality in the study sample. The adjusted model included variables the association of which has been described in the literature. 6, 12 Crude and adjusted hazard ratios (HR) were calculated with their respective 95% confidence intervals (95% CI). Compliance with the proportionality of hazards assumption of the Cox model was verified and collinearity relationships were evaluated in the adjusted model. All analyses were conducted with the statistical package STATA v14.0.
Ethical aspects
This study was carried out following the guidelines of the Declaration of Helsinki of 1964 and its subsequent amendments. The virtual medical records were reviewed without affecting the social, psychological and physical integrity of the study participants. We did not request the signing of an informed consent because the data was anonymized and we did not violate the integrity of the participant. In addition, this study was evaluated and approved by the Research Ethics Committee for COVID-19 of EsSalud, Peru (N°42-IETSI-ESSALUD-2020).
Results
Descriptive and bivariate analyses according to mortality in the study sample.
A total of 493 hospitalized adults were analyzed, 72.8% (n=359) of whom were male with a mean age of 63.3 ± 14.4 years. Likewise, 62.5% (n=308) were 60 years of age or older, 25% (n=123) had hypertension, and 16.5% (n=81) were obese. The median length of hospital stay was five days (IQR: 3-9), and symptoms appeared on average 7.9 ± 4.0 days before admission to the hospital, the most common being respiratory distress, followed by cough and fever, while only 3.7% (n=18) reported having had contact with a confirmed case of COVID-19. Likewise, the mean oxygen saturation upon hospital admission was 82.6 ± 13.8; 83.8% (n=413) of the cases were confirmed, but only 3.4% (n=14) of the confirmed cases were diagnosed by a real-time polymerase chain reaction (RT-PCR) test. 61.1% (n=258) of the participants had leukocytosis, and 39.6% (n=167) had lymphopenia. While 67.6% (n=333) required ICU admission, only 3.3% (n=16) were actually admitted to this unit, and 60.2% (n=297) of the sample died. In addition, there were 1.99 deaths per 100 person-days at risk. Table 1 shows the bivariate analysis of the study variables and mortality.
Table 1. Descriptive and bivariate analysis according to in-hospital death in the study sample.
| Variables | n | % | mean ± SD 1 | In-hospital death | ||
|---|---|---|---|---|---|---|
| Survivor | Non-survivor | P value | ||||
| n=196
(39.8%) |
n=297
(60.2%) |
|||||
| Demographic characteristics | ||||||
| Age | 63.3 ± 14.4 | 56.4 ± 13.4 | 67.9 ± 13.1 | <0.001 | ||
| <50 years | 85 | 17.2 | 58 (68.2) | 27 (31.8) | <0.001 | |
| 50-59 years | 100 | 20.3 | 54 (54.0) | 46 (46.0) | ||
| ≥60 years | 308 | 62.5 | 84 (27.3) | 224 (72.7) | ||
| Sex | 0.638 | |||||
| Female | 134 | 27.2 | 51 (38.1) | 83 (61.9) | ||
| Male | 359 | 72.8 | 145 (40.4) | 214 (59.6) | ||
| Comorbidities | <0.001 | |||||
| 0 | 267 | 54.2 | 115 (43.1) | 152 (56.9) | ||
| 1 | 143 | 29.0 | 63 (44.1) | 80 (55.9) | ||
| 2 or more | 83 | 16.8 | 18 (21.7) | 65 (78.3) | ||
| Obesity | 81 | 16.5 | 28 (34.6) | 53 (65.4) | 0.297 | |
| Type 2 diabetes mellitus | 91 | 18.5 | 31 (34.1) | 60 (65.9) | 0.219 | |
| Hypertension | 123 | 25.0 | 39 (31.7) | 84 (68.3) | 0.035 | |
| Asthma | 14 | 2.8 | 5 (35.7) | 9 (64.3) | 0.754 | |
| Cancer | 11 | 2.2 | 3 (27.3) | 8 (72.7) | 0.539 | |
| Chronic kidney disease | 10 | 2.0 | 2 (20.0) | 8 (80.0) | 0.328 | |
| Symptoms and epidemiological link | ||||||
| Time of disease (n=405) | 7 (5-10) | 7 (6-10) | 7 (5-10) | 0.295 | ||
| Symptoms (n=450) | ||||||
| Breathing distress | 407 | 90.4 | 165 (40.5) | 242 (59.5) | 0.449 | |
| Cough | 357 | 79.3 | 148 (41.5) | 209 (58.5) | 0.770 | |
| Fever | 246 | 54.7 | 104 (42.3) | 142 (57.7) | 0.581 | |
| Sore throat | 48 | 10.7 | 22 (45.8) | 26 (54.2) | 0.482 | |
| Diarrhea | 40 | 8.9 | 21 (52.5) | 19 (47.5) | 0.125 | |
| Headache | 30 | 6.7 | 15 (50.0) | 15 (50.0) | 0.306 | |
| Nasal congestion | 9 | 2.0 | 2 (22.2) | 7 (77.8) | 0.319 | |
| Anosmia | 3 | 0.7 | 2 (66.7) | 1 (33.3) | 0.571 | |
| Ageusia | 2 | 0.4 | 1 (50.0) | 1 (50.0) | 1.000 | |
| Contact with a confirmed case of COVID-19 | ||||||
| Yes | 18 | 3.7 | 7 (38.9) | 11 (61.1) | 0.939 | |
| Baseline vital functions | ||||||
| Temperature (°C) (n=284) | 36.8 (36.6-37.2) | 36.8 (36.6-37.4) | 36.8 (36.5-37.0) | 0.299 | ||
| Fever (≥38 °C) | 30 | 10.6 | 16 (53.3) | 14 (46.7) | 0.476 | |
| Respiratory rate (n=402) | 26 (22-31) | 24 (22-28) | 28 (24-32) | <0.001 | ||
| Tachypnea, ≥22 | 334 | 83.1 | 132 (39.5) | 202 (60.5) | 0.023 | |
| Tachypnea, ≥30 | 136 | 33.8 | 34 (25.0) | 102 (75.0) | <0.001 | |
| Heart rate (n=467) | 91 (82-108) | 88 (80-100) | 95 (84-110) | <0.001 | ||
| Tachycardia, ≥100 | 176 | 37.7 | 49 (27.8) | 127 (72.2) | <0.001 | |
| Tachycardia, ≥120 | 46 | 9.9 | 10 (21.7) | 36 (78.3) | 0.008 | |
| Oxygen saturation, % (n=470) | 88 (78-92) | 90 (87-93) | 82.5 (70-89.5) | <0.001 | ||
| <96% | 432 | 91.9 | 165 (38.2) | 267 (61.8) | 0.001 | |
| <94% | 403 | 85.7 | 143 (35.5) | 260 (64.5) | <0.001 | |
| <92% | 347 | 73.8 | 111 (32.0) | 236 (68.0) | <0.001 | |
| <90% | 278 | 59.2 | 68 (24.5) | 210 (75.5) | <0.001 | |
| <85% | 181 | 38.5 | 23 (12.7) | 158 (87.3) | <0.001 | |
| <80% | 125 | 26.6 | 11 (8.8) | 114 (91.2) | <0.001 | |
| Case definition and diagnosis | ||||||
| Positive diagnosis (n=413) | 0.547 | |||||
| Rapid serological test positive to IgG | 19 | 4.6 | 7 (36.8) | 12 (63.2) | ||
| Rapid serological test positive to IgM/IgG | 343 | 83.0 | 139 (40.5) | 204 (59.5) | ||
| Rapid serological test positive to IgM | 37 | 9.0 | 15 (40.5) | 22 (59.5) | ||
| RT-PCR | 14 | 3.4 | 3 (21.4) | 11 (78.6) | ||
| Case definition | 0.961 | |||||
| Suspicious | 80 | 16.2 | 32 (40.0) | 48 (60.0) | ||
| Confirmed | 413 | 83.8 | 164 (39.7) | 249 (60.3) | ||
| Baseline auxiliary exams | ||||||
| Hemoglobin, g/dL (n=333) | 13.4 (12.4-14.3) | 13.8 (12.6-14.6) | 13.3 (12.3-14.1) | 0.0198 | ||
| Leukocytes, cells/mm^3 (n=422) | 11.6 (8.3-15.6) | 9.1 (6.9-12.6) | 13.0 (9.9-17.5) | <0.001 | ||
| Leukocytosis (≥10,000 cells/mm^3) | 258 | 61.1 | 77 (29.8) | 181 (70.2) | <0.001 | |
| Neutrophils, cells/mm^3 (n=422) | 9.6 (6.8-14.0) | 7.5 (5.3-11.1) | 111 | <0.001 | ||
| Lymphocytes, cells/mm^3 (n=422) | 0.92 (0.6-1.3) | 1.0 (0.7-1.4) | 0.8 (0.6-1.2) | <0.001 | ||
| Lymphopenia (<0.8 cells/mm^3) | 167 | 39.6 | 51 (30.5) | 116 (69.5) | <0.001 | |
| NLR (n=422) | 11 (6.5-18.2) | 7.7 (4.6-12.7) | 12.9 (8.7-23.3) | <0.001 | ||
| Low tertile | 141 | 33.4 | 4.9 (3.5-6.5) | 92 (65.3) | 49 (34.7) | <0.001 |
| Intermediate tertile | 141 | 33.4 | 11 (9.4-12.6) | 56 (39.7) | 85 (60.3) | |
| High tertile | 140 | 33.2 | 23.5 (18.2-31.7) | 32 (22.9) | 108 (77.1) | |
| Platelets, cells/mm^3 (n=422) | 286.1 ± 124.2 | 295.1 ± 125.2 | 279.3 ± 123.2 | 0.196 | ||
| Thrombocytopenia (<150,000 cells/mm^3) | 47 | 11.1 | 17 (36.2) | 30 (63.8) | 0.340 | |
| Creatinine, mg/dL (n=350) | 0.74 (0.59-0.92) | 0.71 (0.57-0.84) | 0.75 (0.60-0.97) | 0.025 | ||
| Urea, mg/dL (n=189) | 39.2 (28.0-53.0) | 36.5 (27.2-45.8) | 46.6 (30.3-71.0) | <0.001 | ||
| AST, U/L (n=195) | 41.0 (30.0-58.9) | 40 (27-57) | 42.2 (32.0-58.9) | 0.262 | ||
| ALT, U/L (n=203) | 42 (26-73) | 50.3 (24.4-81.0) | 39.9 (26.7-61.0) | 0.259 | ||
| LDH, U/L (n=166) | 403 (302-530) | 302 (225-367) | 484 (409-697) | <0.001 | ||
| LDH ≥245 U/L | 146 | 88.0 | 50 (34.3) | 96 (65.8) | <0.001 | |
| LDH ≥450 U/L | 69 | 41.6 | 4 (5.8) | 65 (94.2) | <0.001 | |
| Time | ||||||
| Hospital stay length, days | 5 (3-9) | 8 (5-11) | 3 (2-6) | <0.001 | ||
| Outcomes | ||||||
| Admitted to ICU | 16 | 3.3 | 3 (18.8) | 13 (81.3) | 0.081 | |
| High Flow oxygen requirement (FiO2 ≥0.36) | 350 | 71.0 | 60 (17.1) | 290 (82.7) | <0.001 | |
| ICU requirement (FiO2 ≥0.80) | 333 | 67.5 | 47 (14.1) | 286 (85.9) | <0.001 | |
Data expressed as mean ± standard deviation, median (interquartile range) or number (percentage). 1 SD: Standard deviation.
AST: aspartate transaminase; ALT: alanine aminotransferase; LDH: lactate dehydrogenase; ICU: intensive care unit; RT-PCT: real-time polymerase chain reaction; NLR: neutrophil-to-lymphocyte ratio.
HR: Hazard ratio; cHR: crude hazard ratio; aHR: adjusted hazard ratio; 95% CI: 95% confidence interval; NLR: neutrophil-to-lymphocyte ratio.
Treatment received by the study participants
Antibiotic therapy was administered to 98.8% (n=487) of the participants, including a combination of azithromycin + cephalosporins in 78.2% (n=381). Likewise, 65.7% (n=324) of the participants received corticosteroid treatment, with methylprednisolone being the preferred treatment in 64.8% (n=210), followed by dexamethasone with 27.2% (n=88). Additionally, 76.7% (n=378) of the sample received hydroxychloroquine, and 26.0% (n=128) received ivermectin, while 79.3% (n=391) were prescribed enoxaparin. Likewise, in the bivariate analysis, statistically significant differences were found between the types of corticosteroids received, having received enoxaparin, and mortality ( Table 2).
Table 2. Descriptive and bivariate analysis of the treatment received according to in-hospital death in the study sample.
| Variables | n | % | In-hospital death | ||
|---|---|---|---|---|---|
| Survivor | Non-survivor | P value | |||
| n=196
(39.8%) |
n=297
(60.2%) |
||||
| Received antibiotic therapy | 0.686 | ||||
| No | 6 | 1.2 | 3 (50.0) | 3 (50.0) | |
| Yes | 487 | 98.8 | 193 (39.6) | 294 (60.4) | |
| Types of antibiotic therapy | 0.919 | ||||
| Azithromycin+ Cephalosporins | 381 | 78.2 | 151 (39.6) | 230 (60.4) | |
| Azithromycin | 45 | 9.3 | 20 (44.4) | 25 (55.6) | |
| Cephalosporins | 26 | 5.3 | 10 (38.5) | 16 (61.5) | |
| Azithromycin + Carbapenems | 23 | 4.7 | 7 (30.4) | 16 (69.6) | |
| Carbapenems | 6 | 1.2 | 3 (50.0) | 3 (50.0) | |
| Azithromycin + others | 4 | 0.8 | 1 (25.0) | 3 (75.0) | |
| Others | 2 | 0.4 | 1 (50.0) | 1 (50.0) | |
| Received corticosteroids | 0.971 | ||||
| No | 169 | 34.3 | 67 (39.6) | 102 (60.4) | |
| Yes | 324 | 65.7 | 129 (39.8) | 195 (60.2) | |
| Type of corticosteroids | <0.001 | ||||
| Methylprednisolone | 210 | 64.8 | 72 (34.3) | 138 (65.7) | |
| Dexamethasone | 88 | 27.2 | 48 (54.6) | 40 (45.4) | |
| Hydrocortisone | 21 | 6.5 | 4 (19.1) | 17 (80.9) | |
| Prednisone | 5 | 1.5 | 5 (100.0) | 0 (0.0) | |
| Received hydroxychloroquine | 0.554 | ||||
| No | 115 | 23.3 | 43 (37.4) | 72 (62.6) | |
| Yes | 378 | 76.7 | 153 (40.5) | 225 (59.5) | |
| Received ivermectin | 0.283 | ||||
| No | 365 | 74.0 | 140 (38.4) | 225 (61.6) | |
| Yes | 128 | 26.0 | 56 (43.8) | 72 (56.2) | |
| Received enoxaparin | <0.001 | ||||
| No | 102 | 20.7 | 55 (53.9) | 47 (46.1) | |
| Yes | 391 | 79.3 | 141 (36.1) | 250 (63.9) | |
| Received Lopinavir/Ritonavir | 0.283 | ||||
| No | 367 | 74.4 | 151 (41.1) | 216 (58.9) | |
| Yes | 126 | 25.6 | 45 (35.7) | 81 (64.3) | |
Survival estimated using Kaplan-Meier curves
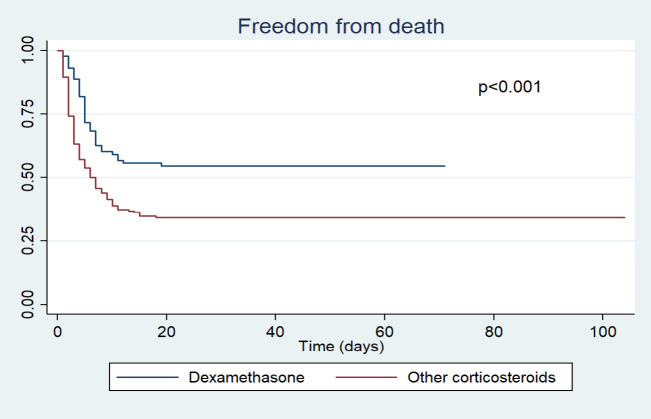
A better survival curve was found in participants admitted to hospital with a higher oxygen saturation (≥92% vs. 91-86% vs. 85-80% vs. <80%), which was statistically significant (p<0.001) ( Figure 1). Likewise, the survival curve was better in younger patients (<50 years vs. 50-59 vs. ≥60) and in those who received dexamethasone (dexamethasone vs. others) during hospitalization ( Figures 2 and 3). These differences were statistically significant (p<0.001).
Figure 1. Survival analysis by oxygen saturation level at hospital admission.
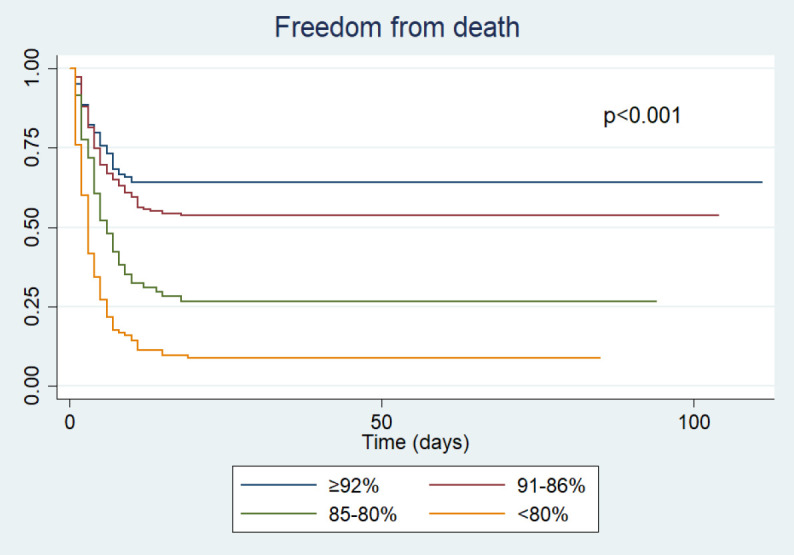
Figure 2. Survival analysis by age groups of the participants.
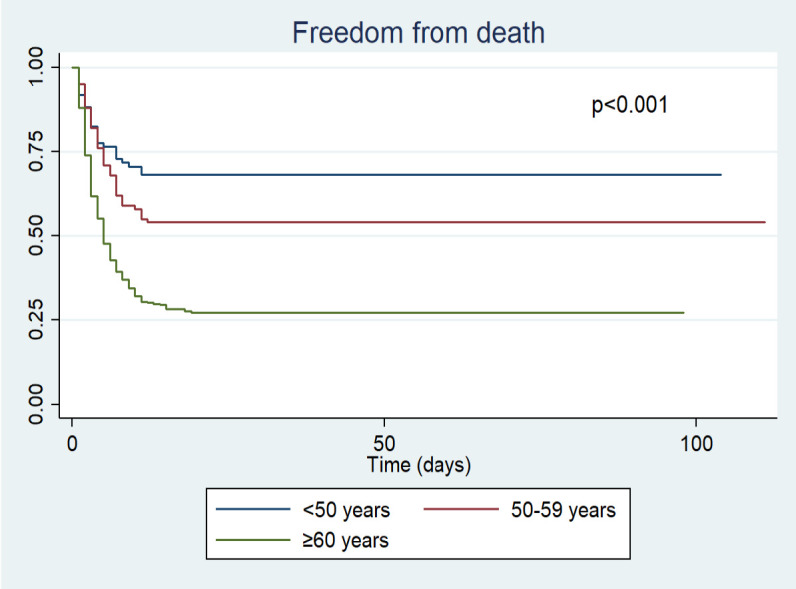
Figure 3. Survival analysis by group of corticosteroids received.
Risk factors for mortality in hospitalized COVID-19 patients in the study sample
In the crude Cox regression analysis, it was found that age greater than or equal to 60 years (crude hazard ratio [cHR]=2.52; 95% CI: 1.93-3.28), having two or more comorbidities (cHR=1.59; 95% CI: 1.19-2.12), oxygen saturation between 85-80% (cHR=2.70; 95% CI: 1.81-4.04) or less than 80% (cHR=5.25; 95% CI: 3.69-7.46), as well as the intermediate (cHR=2.16; 95% CI: 1.52-3.07) and high NLR tertile (cHR=3.40; 95% CI: 2.42-4.78) were associated with a higher risk of mortality in hospitalized COVID-19 patients in Peru. In the adjusted regression analysis, age greater than or equal to 60 years (adjusted hazard ratio [aHR]=1.57; 95% CI: 1.14-2.15), having two or more comorbidities (aHR=1.53; 95% CI: 1.10-2.14), oxygen saturation between 85-80% (aHR=2.52; 95% CI: 1.58-4.02), less than 80% (aHR=4.59; 95% CI: 3.01-7.00), as well as being in the intermediate (aHR=1.65; 95% CI: 1.15-2.39) and higher tertile NLR (aHR=2.18; 95% CI: 1.51-3.15) remained associated with a higher risk of mortality ( Table 3).
Table 3. Cox regression analysis to evaluate risk factors of mortality in the study sample.
| Variables | Crude model | Adjusted model | ||
|---|---|---|---|---|
| cHR (95% CI) | P value | aHR (95% CI) | P value | |
| Age | ||||
| ≥60 years | 2.52 (1.93-3.28) | <0.001 | 1.57 (1.14-2.15) | 0.006 |
| Sex | ||||
| Female | Reference | Reference | ||
| Male | 0.94 (0.73-1.21) | 0.645 | 1.05 (0.77-1.42) | 0.756 |
| Comorbidities | ||||
| 0 | Reference | Reference | ||
| 1 | 0.92 (0.70-1.21) | 0.559 | 0.92 (0.67-1.26) | 0.602 |
| 2 or more | 1.59 (1.19-2.12) | 0.002 | 1.53 (1.10-2.14) | 0.011 |
| Oxygen saturation | ||||
| ≥92% | Reference | Reference | ||
| 91-86% | 1.35 (0.93-1.97) | 0.116 | 1.18 (0.75-1.84) | 0.474 |
| 85-80% | 2.70 (1.81-4.04) | <0.001 | 2.52 (1.58-4.02) | <0.001 |
| <80% | 5.25 (3.69-7.46) | <0.001 | 4.59 (3.01-7.00) | <0.001 |
| NLR tertiles | ||||
| Low tertile | Reference | Reference | ||
| Intermediate tertile | 2.16 (1.52-3.07) | <0.001 | 1.65 (1.15-2.39) | 0.007 |
| High tertile | 3.40 (2.42-4.78) | <0.001 | 2.18 (1.51-3.15) | <0.001 |
Discussion
Main results
This study included 493 patients hospitalized in three hospitals in Peru. We found that approximately six out of 10 patients died during follow-up, and while about seven out of 10 required admission to the ICU, only 3.3% were actually admitted to this unit. In addition, it was found that being an older adult, having an oxygen saturation level of 85% or less at admission, and having a high NLR value were associated with a higher risk of mortality. In addition, approximately eight out of 10 hospitalized patients received hydroxychloroquine and nine out of 10 were prescribed azithromycin.
It was found that about six in 10 hospitalized patients died, which is a high frequency compared to the 28.3%, 21.7% and 14.6% reported in patients hospitalized for COVID-19 from Wuhan, 6 New York 13 and Madrid, 14 respectively. Likewise, the mortality found in this study was higher than the 39.6% and 38% described in studies carried out in Brazil 15 and Mexico, 16 respectively. This could be due to patients having received hospital care on average 7.9 days after the onset of symptoms, with a subsequently higher risk of severe disease and mortality. Likewise, it was of note that 73.8% of the patients arrived at the hospital with an oxygen saturation lower than 92%, hypoxia being a risk factor for mortality. 17 On the other hand, despite 67.5% of the patients requiring admission to the ICU, only 3.3% were actually admitted, which could explain the high mortality and the shorter hospital stay in the group that died. In the present study, approximately 25% of the deaths occurred within the first 24 hours of hospitalization, suggesting that the population arrived late to medical care and adequate early monitoring of symptoms, which could avoid complications, was not carried out. 18
In this study, older adults were found to have a higher risk of mortality. This situation is consistent with what has been described in previous studies, 6, 19 and could be explained by greater dysregulation of immune function and immunosenescence in older adults, as well as a higher prevalence of comorbidities. 20 Certain comorbidities, such as hypertension, diabetes mellitus and chronic kidney disease, are treated with angiotensin converting enzyme inhibitors and angiotensin II receptor blockers, increasing the risk of severe disease and mortality. 19 However, the role of the immune system in the pathophysiology of COVID-19 in this age group is still under study.
Furthermore, elevated NLR values were found to be associated with an increased risk of mortality. Inflammation plays a relevant role in the pathophysiology of COVID-19 and allows establishing the prognosis of patients. Within the response of the innate immune system to a respiratory infection, there is a proliferation of neutrophils at the alveolar level, which could generate collateral damage and cytotoxicity. Furthermore, the release of anti-inflammatory cytokines could lead to lymphocyte apoptosis, producing lymphocytopenia. 21, 22 In this way, an elevated NLR has been described as an indicator of severe inflammation progression, which could lead to complications such as sepsis, multi-organ failure, and acute respiratory distress syndrome. 23 In previous studies, it has been described as a prognostic marker for COVID-19, and its usefulness is highlighted due to its low cost, easy implementation and practicality. 24
Hypoxemia was found to be a risk factor for mortality in the study sample, which is similar to results in previous studies. 25, 26 Approximately four out of 10 hospitalized patients arrived at the hospital with oxygen saturation lower than 86%, indicating that these patients arrived late at the hospital and correlates with the mean time of disease onset that exceeded seven days. Thus, the high proportion of patients with hypoxemia could be associated with the high mortality in the sample. Likewise, patients who arrive at the hospital with a higher degree of hypoxia require more intensive care, oxygen support, access to the ICU and mechanical ventilation, 17 which in Peru is limited 27 and could explain the high incidence of mortality. It should be noted that hypoxia has been associated with inflammation, which with the proliferation and elevation of cytokine levels, increases the already established lung damage and worsens the prognosis. 28
We found an association between having two or more comorbidities and a higher risk of mortality. The main comorbidities in the population were hypertension, diabetes mellitus and obesity, which have been described as predictors of severity and worse prognosis in previous studies. 29– 31 The pro-inflammatory role of obesity has been mentioned, which induces diabetes mellitus and oxidative stress, affecting cardiovascular function. 32 Likewise, a greater abdominal circumference increases breathing difficulty, which can restrict ventilation by decreasing the excursion of the diaphragm. 33 It should be noted that diabetes mellitus and obesity alter immune response to viral infections. 33
Nearly all of the participants received antibiotic therapy, the main drug being azithromycin. However, less than 7% of patients hospitalized for COVID-19 were reported as having bacterial coinfection. 34 Likewise, the use of azithromycin in conjunction with hydroxychloroquine gained relevance based on initial favorable reports in March 2020, 35 both drugs being approved for use in Peru as of April 2020. 36 However, later, the use of these drugs was rejected internationally, due to their null positive effect 37, 38 and the increased risk of mortality. 39 On the other hand, another drug frequently used was ivermectin, which began to gain relevance within the scheme of the Ministry of Health of Peru (MINSA) due to an in vitro study published during the study period. 40 However, this drug was consolidated in Peru over the following months after promoting its use through self-medication 41 and even subdermal application. 42 The use of these medical therapies based on studies with biases or design flaws 35, 43 could have caused people to have false security and to have not gone to the hospital on the appearance of symptoms, thereby leading to disease progression and an increased risk of death or the development of severe illness. On the other hand, corticosteroids were administered in two out of three people, with a predominance of methylprednisolone, while dexamethasone, which was reported to reduce mortality in hospitalized COVID-19 patients in June, 44 was not the most widely used.
The health system in Peru is fragmented and is currently overwhelmed. 45 Although progress has been made in universal insurance for the population, 46 the designated health budget is 2.3% of the annual gross domestic product. 47 Moreover, despite the low budget assigned, it is not fully executed annually, 47 which could further explain the shortcomings of the health system. In Peru, there were approximately 0.2 ICU beds per 100,000 inhabitants before the pandemic, 27 which, in addition to the deficit of oxygen and hospital beds, could explain the high mortality that led Peru to occupy the first place in mortality per 100,000 inhabitants for several months. 48 Likewise, the infodemic and high prevalence of self-medication are two latent problems in the Peruvian population. 11, 49 Both of these behaviors could increase the mortality of COVID-19 by providing false security and by people not attending health services in a timely manner, leading to a worse prognosis. The same occurs with the use of corticosteroids in the early and mild stages of the disease. 50 On the other hand, the high rate of job informality and poverty in Peru has also aggravated the situation and could explain the poor adherence to quarantine and the failure to implement community mitigation strategies. 51 This was reflected in a high mortality rate, especially in vulnerable groups. 52
This study has limitations: 1) The population included in the study were patients insured by social security, which is made up of salaried workers and their families, which may not be representative of the entire country due to its socioeconomic characteristics; 2) There was a high percentage of missing values in certain relevant laboratory markers, which limited their evaluation in the multivariate model. However, relevant and useful markers described in the literature were included; 3) There are laboratory markers of immune response such as cytokines that could not be measured in the present analysis. Despite these limitations, this study represents one of the first reports from Peru, 12, 53 a country that became the global epicenter of the pandemic and leaves many lessons to be dealt with in the future to improve the failures of the health system and management of evidence-based disease.
Conclusions
The risk factors found are consistent with what has been described in the literature and allow the identification of vulnerable groups in whom monitoring and early identification of symptoms should be prioritized. Likewise, the findings of our study describe what happened during the first stage of the pandemic in Peru, highlighting the late arrival to receiving medical attention, as well as the lack of ICU beds, leading to a high incidence of mortality. In addition, the number of ICU beds, hospital beds and access to oxygen in the population should be improved in order to reduce mortality. Finally, evidence-based treatment schemes must be implemented to combat the infodemic and self-medication in the population of Peru.
Acknowledgments
We would like to thank the staff of the Hospital Almanzor Aguinaga Asenjo, Luis Heysen Incháustegui and the EsSalud Chepén Hospital Clinic for the logistical support provided. In addition, we would like to thank the Universidad Científica del Sur, for the financial support in the payment of the article processing charge.
Funding Statement
The author(s) declared that no grants were involved in supporting this work.
[version 1; peer review: 2 approved]
Data availability
Underlying data
Figshare: Database including information of COVID-19 patients from Peru. DOI: https://doi.org/10.6084/m9.figshare.14170955.v1. 54
This project contains the following underlying data:
-
-
.xls file containing the information of COVID-19 patients from Peru.
Data are available under the terms of the Creative Commons Zero "No rights reserved" data waiver (CC BY 4.0 Public domain dedication).
References
- 1. Li X, Wang W, Zhao X, et al. : Transmission dynamics and evolutionary history of 2019-nCoV. J Med Virol. 2020;92(5):501–11. 10.1002/jmv.25701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Organization WH, organization Wh: Coronavirus disease (COVID-2019) situation reports. 2020.
- 3. Meneses-Navarro S, Freyermuth-Enciso MG, Pelcastre-Villafuerte BE, et al. : The challenges facing indigenous communities in Latin America as they confront the COVID-19 pandemic. Int J Equity Health. 2020;19:1–3. 10.1186/s12939-020-01178-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Gandhi RT, Lynch JB, del Rio C: Mild or moderate COVID-19. N Engl J Med. 2020. 10.1056/NEJMcp2009249 [DOI] [PubMed] [Google Scholar]
- 5. Yang AP, Liu J, Tao W, et al. : The diagnostic and predictive role of NLR, d-NLR and PLR in COVID-19 patients. Int Immunopharmacol. 2020:106504. 10.1016/j.intimp.2020.106504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Zhou F, Yu T, Du R, et al. : Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020. 10.1016/S0140-6736(20)30566-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Zheng Z, Peng F, Xu B, et al. : Risk factors of critical & mortal COVID-19 cases: A systematic literature review and meta-analysis. J Infect. 2020. 10.1016/j.jinf.2020.04.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Munayco C, Chowell G, Tariq A, et al. : Risk of death by age and gender from CoVID-19 in Peru, March-May, 2020. Aging (Albany NY). 2020;12(14):13869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Fraser B: COVID-19 strains remote regions of Peru. Lancet. 2020;395(10238):1684. 10.1016/S0140-6736(20)31236-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Dyer O: Covid-19 hot spots appear across Latin America. BMJ. 2020. 10.1136/bmj.m2182 [DOI] [PubMed] [Google Scholar]
- 11. Alvarez-Risco A, Mejia CR, Delgado-Zegarra J, et al. : The Peru Approach against the COVID-19 Infodemic: Insights and Strategies. Am J Trop Med Hyg. 2020:tpmd200536. 10.4269/ajtmh.20-0536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Mejía F, Medina C, Cornejo E, et al. : Oxygen saturation as a predictor of mortality in hospitalized adult patients with COVID-19 in a public hospital in Lima, Peru. PloS One. 2020;15(12):e0244171. 10.1371/journal.pone.0244171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Mikami T, Miyashita H, Yamada T, et al. : Risk factors for mortality in patients with COVID-19 in New York City. J Gen Intern Med. 2020:1–10. 10.1007/s11606-020-05983-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Giesen C, Diez-Izquierdo L, Saa-Requejo CM, et al. : Epidemiological characteristics of the COVID-19 outbreak in a secondary hospital in Spain. Am J Infect Control. 2020. 10.1016/j.ajic.2020.07.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Soares RCM, Mattos LR, Raposo LM: Risk factors for hospitalization and mortality due to COVID-19 in Espírito Santo State, Brazil. Am J Trop Med Hyg. 2020;103(3):1184–90. 10.4269/ajtmh.20-0483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Cortés-Tellés A, López-Romero S, Mancilla-Ceballos R, et al. : Risk factors for mortality among hospitalized patients with COVID-19. Tuberculosis and Respiratory Diseases. An overview in Mexican population. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kashani KB, editor Hypoxia in COVID-19: Sign of Severity or Cause for Poor Outcomes. Mayo Clin Proc. 2020; Mayo Foundation for Medical Education and Research. 10.1016/j.mayocp.2020.04.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Teo J: Early detection of silent hypoxia in Covid-19 pneumonia using smartphone pulse oximetry. J Med Syst. 2020;44(8):1–2. 10.1007/s10916-020-01587-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Shahid Z, Kalayanamitra R, McClafferty B, et al. : COVID-19 and older adults: what we know. J Am Geriatr Soc. 2020;68(5):926–9. 10.1111/jgs.16472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Pence BD: Severe COVID-19 and aging: are monocytes the key? GeroScience. 2020;42(4):1051–61. 10.1007/s11357-020-00213-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Rodriguez-Morales AJ, Cardona-Ospina JA, Gutiérrez-Ocampo E, et al. : Clinical, laboratory and imaging features of COVID-19: A systematic review and meta-analysis. Travel Med Infect Dis. 2020;101623. 10.1016/j.tmaid.2020.101623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wang D, Hu B, Hu C, et al. : Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus–infected pneumonia in Wuhan, China. JAMA. 2020;323(11):1061–9. 10.1001/jama.2020.1585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Liu Y, Du X, Chen J, et al. : Neutrophil-to-lymphocyte ratio as an independent risk factor for mortality in hospitalized patients with COVID-19. J Infect. 2020. 10.1016/j.jinf.2020.04.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kerboua KE: NLR: A Cost-effective Nomogram to Guide Therapeutic Interventions in COVID-19. Immunol Invest. 2020:1–9. 10.1080/08820139.2020.1773850 [DOI] [PubMed] [Google Scholar]
- 25. Xie J, Covassin N, Fan Z, et al., editors. Association between hypoxemia and mortality in patients with COVID-19. Mayo Clin Proc. 2020: Elsevier. 10.1016/j.mayocp.2020.04.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Petrilli CM, Jones SA, Yang J, et al. : Factors associated with hospital admission and critical illness among 5279 people with coronavirus disease 2019 in New York City: prospective cohort study. BMJ. 2020;369. 10.1136/bmj.m1966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Almeida F: Exploring the impact of COVID-19 on the sustainability of health critical care systems in South America. Int J Health Policy Manag. 2020. 10.34172/ijhpm.2020.116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Cavezzi A, Troiani E, Corrao S: COVID-19: hemoglobin, iron, and hypoxia beyond inflammation. A narrative review. Clin Pract. 2020;10(2). 10.4081/cp.2020.1271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Pranata R, Lim MA, Huang I, et al. : Hypertension is associated with increased mortality and severity of disease in COVID-19 pneumonia: a systematic review, meta-analysis and meta-regression. J Renin Angiotensin Aldosterone Syst. 2020;21(2). 10.1177/1470320320926899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Yang J, Tian C, Chen Y, et al. : Obesity aggravates COVID-19: an updated systematic review and meta-analysis. J Med Virol. 2020. 10.1002/jmv.26677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Huang I, Lim MA, Pranata R: Diabetes mellitus is associated with increased mortality and severity of disease in COVID-19 pneumonia–a systematic review, meta-analysis, and meta-regression. Diabetes Metab Syndr. 2020. 10.1016/j.dsx.2020.04.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Collaborators GO: Health effects of overweight and obesity in 195 countries over 25 years. N Engl J Med. 2017;377(1):13–27. 10.1056/NEJMoa1614362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kass DA, Duggal P, Cingolani O: Obesity could shift severe COVID-19 disease to younger ages. Lancet (London, England). 2020. 10.1016/S0140-6736(20)31024-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Lansbury L, Lim B, Baskaran V, et al. : Co-infections in people with COVID-19: a systematic review and meta-analysis. J Infect. 2020. 10.1016/j.jinf.2020.05.046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Gautret P, Lagier J-C, Parola P, et al. : Hydroxychloroquine and azithromycin as a treatment of COVID-19: results of an open-label non-randomized clinical trial. Int J Antimicrob Agents. 2020;105949. 10.1016/j.ijantimicag.2020.105949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Ministerio de Salud del Perú: N 193-2020-MINSA. Documento Técnico: Prevención, Diagnóstico y Tratamiento de personas afectadas por COVID-19 en el Perú. 2020.
- 37. Consortium WST: Repurposed antiviral drugs for COVID-19—interim WHO SOLIDARITY trial results. N Engl J Med. 2020. 10.1056/NEJMoa2023184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Hernandez AV, Roman YM, Pasupuleti V, et al. : Hydroxychloroquine or chloroquine for treatment or prophylaxis of COVID-19: a living systematic review. Ann Intern Med. 2020. 10.7326/M20-2496 [DOI] [PubMed] [Google Scholar]
- 39. Soto-Becerra P, Culquichicón C, Hurtado-Roca Y, et al. : Real-world effectiveness of hydroxychloroquine, azithromycin, and ivermectin among hospitalized COVID-19 patients: results of a target trial emulation using observational data from a nationwide healthcare system in Peru. Azithromycin, and Ivermectin Among Hospitalized COVID-19 Patients: Results of a Target Trial Emulation Using Observational Data from a Nationwide Healthcare System in Peru. 2020. [Google Scholar]
- 40. Caly L, Druce JD, Catton MG, et al. : The FDA-approved drug ivermectin inhibits the replication of SARS-CoV-2 in vitro. Antiviral Res. 2020;104787. 10.1016/j.antiviral.2020.104787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Zavala-Flores E, Salcedo-Matienzo J: Medicación prehospitalaria en pacientes hospitalizados por COVID-19 en un hospital público de Lima-Perú. Acta Médica Peruana. 2020;37(3):393–5. 10.35663/amp.2020.373.1277 [DOI] [Google Scholar]
- 42. Ramal-Asayag C, Espinoza-Venegas LA, Celis-Salinas JC, et al. : Úlcera dérmica por ivermectina subcutánea en el tratamiento de COVID-19. Rev Soc Peru Med Interna. 2020:88-. 10.36393/spmi.v33i2.526 [DOI]
- 43. Podder CS, Chowdhury N, Sina MI, et al. : Outcome of ivermectin treated mild to moderate COVID-19 cases: a single-centre, open-label, randomised controlled study. IMC J Med Sci. 2020;14(002). 10.1101/2021.01.05.21249310 [DOI] [Google Scholar]
- 44. Group RC: Dexamethasone in hospitalized patients with Covid-19—preliminary report. N Engl J Med. 2020. 10.1056/NEJMoa2021436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Atun R, De Andrade LOM, Almeida G, et al. : Health-system reform and universal health coverage in Latin America. Lancet. 2015;385(9974):1230–47. 10.1016/S0140-6736(14)61646-9 [DOI] [PubMed] [Google Scholar]
- 46. Mezones-Holguín E, Amaya E, Bellido-Boza L, et al. : Cobertura de aseguramiento en salud: el caso peruano desde la Ley de Aseguramiento Universal. Rev Peru Med Exp Salud Publica. 2019;36:196–206. 10.17843/rpmesp.2019.362.3998 [DOI] [PubMed] [Google Scholar]
- 47. García E: Comex: Perú gasta en salud por debajo del promedio en América Latina. Diario Gestión. 2019. Reference Source [Google Scholar]
- 48. Solari LM: Peru–The Role of the National Government in Combatting the COVID-19 Pandemic. Good Public Governance in a Global Pandemic. 465. [Google Scholar]
- 49. Urrunaga-Pastor D, Benites-Zapata VA, Mezones-Holguín E: Factors associated with self-medication in users of drugstores and pharmacies in Peru: an analysis of the National Survey on User Satisfaction of Health Services, ENSUSALUD 2015. F1000Res. 2019;8. 10.12688/f1000research.17578.2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Pasin L, Navalesi P, Zangrillo A, et al. : Corticosteroids for Patients With Coronavirus Disease 2019 (COVID-19) With Different Disease Severity: A Meta-Analysis of Randomized Clinical Trials. J Cardiothorac Vasc Anesth. 2020. 10.1053/j.jvca.2020.11.057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Analytica O: COVID-19 will have wide-ranging impacts in Peru. Emerald Expert Briefings. (oxan-db).
- 52. Aguirre-Amaya K, Palomares-Custodio M, Quispe-Vicuña C, et al. : COVID-19 Mortality in Peruvian Older Adults: A Chronicle of a Health Crisis Foretold? J Frailty Aging. 2020:1–2. 10.14283/jfa.2020.66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Benites-Goñi H, Vargas-Carrillo E, Peña-Monge E, et al. : Clinical characteristics, management and mortality of patients hospitalized with COVID-19 in a reference hospital in Lima, Peru. Clinical characteristics, management and mortality of patients hospitalized with COVID-19 in a reference hospital in Lima, Peru. 2020.
- 54. Urrunaga-Pastor D: Database_v1. figshare. Dataset. 2021. 10.6084/m9.figshare.14170955.v1 [DOI]