Abstract
Summary Background
Myelofibrosis is a chronic myeloproliferative neoplasm characterised by splenomegaly, cytopenias, bone marrow fibrosis, and debilitating symptoms including fatigue, weight loss, and bone pain. Mutations in Janus kinase-2 (JAK2) occur in approximately 50% of patients. The only approved JAK2 inhibitor for myelofibrosis is the dual JAK1 and JAK2 inhibitor, ruxolitinib. 58–71% of patients treated with ruxolitinib in clinical trials so far have not achieved the primary endpoint of 35% or more reduction in spleen volume from baseline assessed by MRI or CT. Furthermore, more than 50% of patients discontinue ruxolitinib treatment after 3–5 years. On the basis of this unmet need, we investigated the efficacy and safety of fedratinib, a JAK2-selective inhibitor, in patients with ruxolitinib-resistant or ruxolitinib-intolerant myelofibrosis.
Methods
This single-arm, open-label, non-randomised, phase 2, multicentre study, done at 31 sites in nine countries, enrolled adult patients with a current diagnosis of intermediate or high-risk primary myelofibrosis, post-polycythaemia vera myelofibrosis, or post-essential thrombocythaemia myelofibrosis, found to be ruxolitinib resistant or intolerant after at least 14 days of treatment. Other main inclusion criteria were palpable splenomegaly (≥5 cm below the left costal margin), Eastern Cooperative Oncology Group performance status of 2 or less, and life expectancy of 6 months or less. Patients received oral fedratinib at a starting dose of 400 mg once per day, for six consecutive 28-day cycles. The primary endpoint was spleen response (defined as the proportion of patients with a ≥35% reduction in spleen volume as determined by blinded CT and MRI at a central imaging laboratory). We did the primary analysis in the per-protocol population only (patients treated with fedratinib, for whom a baseline and at least one post-baseline spleen volume measurement was available) and the safety analysis in all patients receiving at least one dose of fedratinib. This trial was registered with ClinicalTrials.gov (http://ClinicalTrials.gov), number NCT01523171 (http://clinicaltrials.gov/ct2/results?term=NCT01523171).
Findings
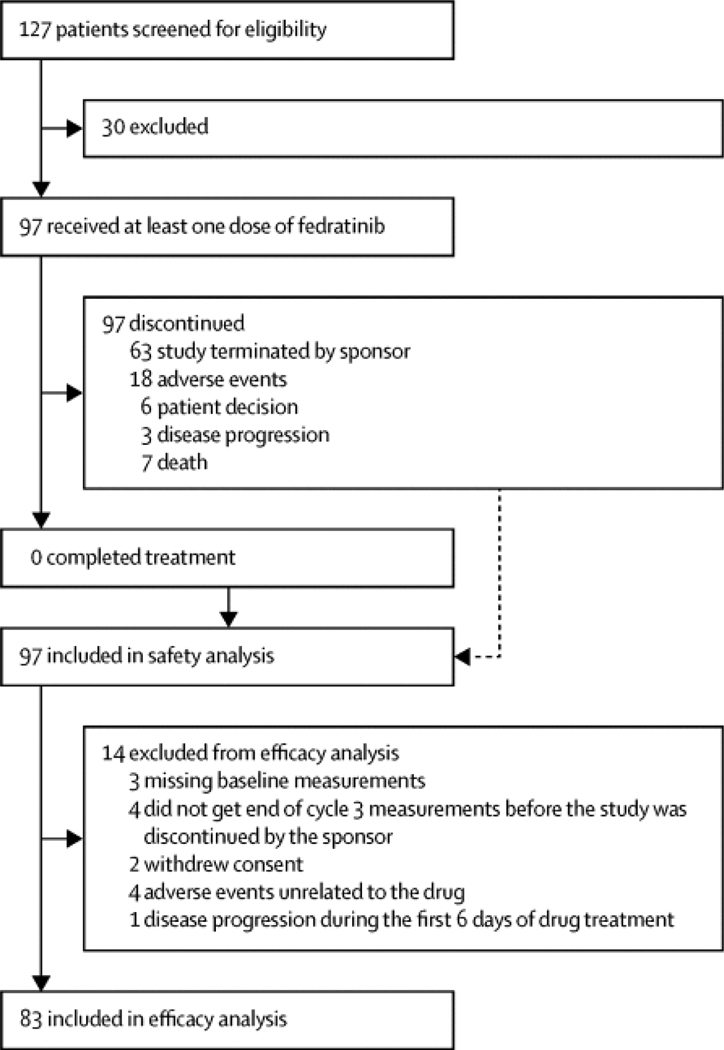
Between May 8, 2012, and Aug 29, 2013, 97 patients were enrolled and received at least one dose of fedratinib. Of 83 assessable patients, 46 (55%, 95% CI 44–66) achieved a spleen response. Common grade 3–4 adverse events included anaemia (37 [38%] of 97 patients) and thrombocytopenia (21 [22%] of 97), with 18 (19%) patients discontinuing due to adverse events. Seven (7%) patients died during the study, but none of the deaths was drug related. Suspected cases of Wernicke’s encephalopathy in other fedratinib trials led to study termination.
Interpretation
This phase 2 study met its primary endpoint, suggesting that patients with ruxolitinib-resistant or ruxolitinib-intolerant myelofibrosis might achieve significant clinical benefit with fedratinib, albeit at the cost of some potential toxicity, which requires further evaluation. Fedratinib development in this setting is currently being assessed.
Funding
Sanofi.
Introduction
Myelofibrosis is a chronic myeloproliferative neoplasm that can occur de novo (primary myelofibrosis) or after transformation of polycythaemia vera (post-polycythaemia vera myelofibrosis) or of essential thrombocythaemia (post-essential thrombocythaemia myelofibrosis). Myelofibrosis is characterised by splenomegaly, cytopenias, and bone marrow fibrosis. Patients also have debilitating constitutional symptoms including fatigue, weight loss, early satiety, pruritus, night sweats, cough, pain due to splenomegaly, and pain in the bones. 1 Activating mutations in Janus kinase-2 (JAK2) and the thrombopoietin receptor (MPL) that result in deregulation of the JAK/STAT pathway are reported in most patients with myelofibrosis. 2 Patients without a JAK2 or MPL mutation frequently carry mutations in the calreticulin (CALR) gene, which also upregulates JAK signalling. 3
Ruxolitinib, a dual JAK1 and JAK2 inhibitor, was the first drug to be approved for the treatment of myelofibrosis. When compared with placebo in the COMFORT-I phase 3 study, 4 ruxolitinib reduced spleen volume by at least 35% at 24 weeks in 65 (42%) of 155 patients with intermediate-2 or high-risk myelofibrosis compared with placebo, albeit at the expense of increased anaemia and thrombocytopenia. In the COMFORT-II study, 5 41 (28%) of 146 patients given ruxolotinib achieved the same endpoint of a reduction in spleen volume by at least 35%. Long-term follow-up (median 149 weeks) analysis of the COMFORT-I trial showed a median 34% volume reduction in splenomegaly in patients receiving ruxolitinib compared with placebo. Only 77 (50%) of the 155 patients originally randomly assigned were still receiving ruxolitinib, and only 57 (51%) of 111 patients who crossed over from placebo were still taking ruxolitinib therapy. The proportions of patients randomly assigned to ruxolitinib who discontinued treatment were estimated to be 21% at year 1, 35% at year 2, and 51% at year 3, and were attributed to adverse events (19%), disease progression (23%), death (19%), and withdrawal of consent (15%). 6 For 78 (53%) of 146 patients in the COMFORT-II study 7 who were randomly assigned to ruxolitinib and achieved a 35% spleen volume reduction, median overall survival was not reached compared with 4·1 years on the best available therapy group, indicating a survival advantage for patients who responded to ruxolitinib therapy.
RESEARCH IN CONTEXT
Evidence before this study
We searched PubMed and meeting abstracts for articles published in English between June 1, 1950, and June 1, 2016, using the terms: “myelofibrosis”, “agnogenic myeloid metaplasia”, “myeloproliferative neoplasms”, “therapy”, and “treatment”. Myelofibrosisis is a myeloproliferative neoplasm, the characteristics of which include marked splenomegaly, extramedullary haemopoiesis, and debilitating symptoms. The only curative therapy is transplantation, but less than 10% of patients are transplant eligible. Ruxolitinib, a dual JAK1 and JAK2 inhibitor, is the only approved therapy for patients with myelofibrosis at present. Although ruxolitinib reduces splenomegaly and constitutional symptoms, a substantial proportion of patients either might not achieve the desired benefit or lose response over time (only 27% of patients remained on therapy after 5 years in the COMFORT-I trial); outcomes for patients who discontinue ruxolitinib in this situation are poor and such patients have a less optimum outcome from transplantation than patients who have a transplantation at the time of optimum response. Data from phase 2 and 3 studies of fedratinib in patients with myelofibrosis showed that fedratinib reduced splenomegaly and improved symptoms.
Added value of this study
To our knowledge JAKARTA-2 is the first study of fedratinib in patients with myelofibrosis who were either intolerant or resistant to ruxolitinib.
Implications of all the available evidence
Data from this study suggest that fedratinib therapy can provide meaningful results for patients who are resistant or intolerant to ruxolitinib.
Because at least 50% of patients with myelofibrosis do not maintain spleen responses with ruxolitinib or are intolerant of ruxolitinib long term, and because other JAK1 and JAK2 inhibitors have not proved to be more effective, a pressing unmet medical need exists for alternative therapies. 8 Ruxolitinib intolerance and resistance is associated with a substantially reduced life expectancy. In an analysis of 79 patients who stopped ruxolitinib, the median overall survival after discontinuation was 6 months, with an estimated overall survival of 34% at 1 year and 25% at 2 years. Notably, after a median follow-up of 10 months from stopping ruxolitinib, only 27 (34%) patients remained alive. 9 We aimed to assess the efficacy and safety of the JAK2 inhibitor fedratinib (SAR302503) in patients with myelofibrosis who were refractory or intolerant of ruxolitinib treatment.
Methods
Study design and participants
This was a single-arm, open-label, non-randomised, phase 2, multicentre study, done at 31 academic hospitals in Austria, Canada, France, Germany, Italy, the Netherlands, Spain, the UK, and the USA (appendix (sec1)). The study protocol was approved by independent ethics committees at each site and is provided in the appendix (sec1).
Eligible patients were aged 18 years or older, and had a current diagnosis of primary myelofibrosis, post-polycythaemia vera myelofibrosis, or post-essential thrombocythaemia myelofibrosis (according to the 2008 WHO classifications), 10 which was classified as intermediate-1, intermediate-2, or high-risk disease (according to the Dynamic International Prognostic Scoring System). 11 Patients with intermediate-1 disease had to have constitutional symptoms. For inclusion in this study, patients were required to have received ruxolitinib therapy for the treatment of primary myelofibrosis, post-polycythaemia vera myelofibrosis, or post-essential thrombocythaemia myelofibrosis for at least 14 days (unless the patient discontinued due to intolerance or allergy within 14 days). The other main inclusion criteria were palpable splenomegaly (≥5 cm below the left costal margin), Eastern Cooperative Oncology Group performance status of 2 or less, and life expectancy of 6 months or less. The main exclusion criteria were having received chemotherapy, including ruxolitinib, within 14 days before the start of the study (except hydroxyurea, which was permitted within 1 day of initiation of fedratinib), a history of other malignancies, and platelet count of less than 50 × 10 9 platelets per L. Eligible patients were identified and recruited at the study sites, and provided written informed consent before study initiation. The study was done according to the Declaration of Helsinki.
Procedures
Patients received oral fedratinib at a starting dose of 400 mg once per day, for six consecutive 28-day cycles. Dose adjustments of 100 mg/day were allowed to a minimum of 200 mg/day (due to toxicity) and a maximum of 600 mg/day (if the patient had not achieved a 50% reduction in spleen size by palpation and unacceptable toxicity had not been reported). Patients continuing to benefit after six cycles could remain on treatment until disease progression or unacceptable toxicity occurred. Patients discontinued study treatment in the event of unacceptable toxicity, disease progression (enlargement of spleen volume of ≥25% compared with baseline), splenectomy, relapse (according to modified International Working Group-Myeloproliferative Neoplasms Research and Treatment response criteria), 12 or voluntary withdrawal.
Spleen volume was assessed by blinded MRI or CT at a central imaging laboratory. Measurements were taken within 14 days before the first dose of fedratinib (baseline), at end of cycle 3 and end of cycle 6, at the end of every six cycles thereafter for 2 years, and at treatment discontinuation. Palpable spleen size was recorded at baseline and at every treatment cycle until cycle 6. Subsequent measurements were taken every three cycles, at the end of treatment visit, and at the 30-day follow-up visit. We assessed symptoms using a modified myelofibrosis symptom assessment form electronic diary 13 for 7 days before the start of fedratinib treatment (cycle 1, day 1), and daily for the first six cycles. We assessed six symptoms: abdominal discomfort, bone or muscle pain, early satiety, pruritus, pain under the ribs on the left side, and night sweats. A total symptom score was calculated daily as the sum of the scores for all six symptoms. We also calculated a weekly mean score for each symptom and a total symptom score if five of seven daily assessments per week were available for each patient. We analysed the weekly scores.
We assessed safety as the incidence of treatment-emergent adverse events, graded for severity using the National Cancer Institute Common Terminology Criteria for Adverse Events (version 4.03), and changes in clinical laboratory parameters and vital signs, relative to baseline.
On Nov 14, 2013 (2·5 months after completion of accrual), following the report of seven suspected cases of Wernicke’s encephalopathy among 877 patients given fedratinib in eight clinical trials (NCT01420770 (http://clinicaltrials.gov/ct2/results?term=NCT01420770), NCT01420783 (http://clinicaltrials.gov/ct2/results?term=NCT01420783), NCT00631462 (http://clinicaltrials.gov/ct2/results?term=NCT00631462), NCT00724334 (http://clinicaltrials.gov/ct2/results?term=NCT00724334), NCT01836705 (http://clinicaltrials.gov/ct2/results?term=NCT01836705), NCT0158562 (http://clinicaltrials.gov/ct2/results?term=NCT0158562), NCT01692366, NCT01437787 (http://clinicaltrials.gov/ct2/results?term=NCT01437787); including the present study), these trials were terminated and patients receiving fedratinib at that time were discontinued from ongoing fedratinib treatment. Because thiamine deficiency has been associated with the development of Wernicke’s encephalopathy, 14 all patients, including those who had previously discontinued fedratinib therapy, were required to initiate thiamine supplementation and have a safety follow-up for 90 days (protocol amendment 4, Nov 27, 2013). As a result, some patients did not reach end of cycle 6, and only the primary endpoint (spleen response at end of cycle 6), spleen response at end of cycle 3, proportion with a symptom response, and palpable spleen size at end of cycle 6 were analysed. In patients who did not reach end of cycle 6, we assessed the primary endpoint according to the last observation carried forward. Additional follow-up safety assessments were also included.
Outcomes
The primary endpoint was spleen response (the proportion of patients with a ≥35% reduction in spleen volume from baseline) at end of cycle 6 (24 weeks), assessed centrally. Secondary endpoints included symptom response (the proportion of patients with a 50% or more reduction in total symptom score from baseline to end of cycle 6), proportion of patients with a 50% or more reduction in palpable spleen length from baseline to end of cycle 6, spleen response at end of cycle 3 (12 weeks), percentage change in spleen volume from baseline to end of cycle 3 and end of cycle 6, and safety. Exploratory outcomes included pharmacokinetic analysis, duration of spleen response, JAK2 Val617Phe allele burden, and subgroup analyses of spleen and symptom responses of patients resistant or intolerant to ruxolitinib.
Statistical analysis
Assuming 25% of patients achieved the primary endpoint (a ≥35% reduction in spleen volume from baseline), 70 evaluable patients would provide at least 90% power at a one-sided 2·5% α level to test the null hypothesis of at least 10% of patients achieving the primary endpoint. Results from the COMFORT-1 study 4 showed that approximately 60% of patients receiving ruxolitinib did not respond to treatment.
Therefore, 42 patients (60% of 70 evaluable patients) would provide 80% power to test for at least 10% of patients achieving the primary endpoint among the subgroup of patients who did not reach the primary endpoint of splenic response during the ruxolitinib studies.
We did the primary efficacy analysis in the per-protocol population only as a result of the early termination (patients treated with fedratinib, for whom a baseline and at least one post-baseline spleen volume measurement was available). Due to the early termination of the study 35 of 83 patients had an end of cycle 3, but no end of cycle 6, measurement. To describe drug effect, we used the last observation carried forward method to impute the missing end of cycle 6 data with the end of cycle 3 measurement, except for patients who discontinued before end of cycle 6 due to disease progression. We did a post-hoc subgroup analysis of all efficacy endpoints according to investigator’s assessment of ruxolitinib failure (resistant or intolerant). For the primary endpoint analysis, ruxolitinib-resistant patients were further subdivided into those without a response (absence of a response or stable disease with ruxolitinib treatment), those with disease progression (an increase in spleen size during ruxolitinib treatment), or those with a loss of response (at any time during ruxolitinib treatment) as reported by the investigator. Patients classified as ruxolitinib intolerant had discontinued ruxolitinib therapy due to unacceptable toxicity (haematological [eg, thrombocytopenia, anaemia] or non-haematological in nature) during therapy. No formal statistical analyses were planned to compare efficacy endpoints between ruxolitinib-resistant and ruxolitinib-intolerant patients. We analysed the change in palpable spleen size in the intention-to-treat population (all enrolled patients). The symptom analysis population consisted of patients with a baseline and at least one post-baseline evaluable assessment of total symptom score. The safety population comprised all patients receiving at least one dose of fedratinib. We summarised data using descriptive statistics including the number of observations (n), mean, median, and IQR. Data were prepared using SAS version 9.4. A data monitoring committee oversaw the study. This trial was registered with ClinicalTrials.gov (http://ClinicalTrials.gov), number NCT01523171 (http://clinicaltrials.gov/ct2/results?term=NCT01523171).
Role of the funding source
Sanofi was involved in study design, data collection, data analysis, and data interpretation. CNH and RAM prepared the first draft of the manuscript with assistance from a medical writer funded by Sanofi. All authors had access to any data requested, reviewed and approved the manuscript, and vouched for the accuracy and completeness of the data. The corresponding author and medical writer had full access to all of the data in the study and had final responsibility for the decision to submit for publication.
Results
Between May 8, 2012, and Aug 29, 2013, a total of 97 patients were enrolled and all received at least one dose of fedratinib 400 mg (intention-to-treat population; figure 1 (fig1)). Median follow-up was 6·0 months (IQR 3·9–8·9). 14 patients were excluded from the efficacy analysis, including seven patients who were either missing baseline measurements (n=3) or did not get end of cycle 3 measurements before the study was discontinued by the sponsor (n=4), and seven patients who withdrew before the end of cycle 3 measurement due to withdrawn consent (n=2), adverse events unrelated to the drug (n=4), or disease progression during the first 6 days of drug treatment (n=1).
Figure 1.
Trial profile
The median age was 67·0 years (IQR 62·0–72·0) and 53 (55%) of 97 patients were male (table 1 (tbl1)). More than half of patients had primary myelofibrosis, and almost half had intermediate-2 disease. Overall, 61 (63%) of 97 patients had a JAK2 mutation. A third of patients had a platelet count of fewer than 100 × 10 9 platelets per L. Among the 83 patients included in the per-protocol population, 55 (66%) were classified as ruxolitinib resistant, and 27 (33%) were classified as ruxolitinib intolerant (table 2 (tbl2)). One additional patient had discontinued ruxolitinib treatment for other reasons (not definable), and was not classified. Most patients (69 [71%] of 97) had received ruxolitinib at an initial total daily dose of 30 mg or 40 mg, and the median duration of ruxolitinib exposure was 10·25 months (IQR 5·75–14·75; table 3 (tbl3)). Additionally, more than 40% of patients had achieved a reduction in spleen size of 50% or more during ruxolitinib therapy (table 3 (tbl3)).
Table 1.
Baseline characteristics
| Patients (n=97) | |
|---|---|
| Age (years) | 67·0 (62·0–72·0) |
| Sex | |
| Male | 53 (55%) |
| Female | 44 (45%) |
| Disease type | |
| Primary myelofibrosis | 53 (55%) |
| Post-polycythaemia vera myelofibrosis | 25 (26%) |
| Post-essential thrombocythaemia myelofibrosis | 19 (20%) |
| Risk status | |
| Intermediate-1 | 16 (16%) |
| Intermediate-2 | 47 (48%) |
| High-risk | 34 (35%) |
| Time since diagnosis (years) | 4·08 (2·93–5·23) |
| JAK2 mutational profile | |
| Wild type | 29 (30%) |
| Mutant | 61 (63%) |
| Missing | 7 (7%) |
| Platelet count | |
| <50 × 10 9 /L | 1 (1%) |
| 50 × 10 9 /L to <100 × 10 9 /L | 32 (33%) |
| ≥100 × 10 9 /L | 64 (66%) |
Data are median (IQR) or n (%). Data are from the intention-to-treat population. JAK2=Janus kinase-2.
Table 2.
Reasons for ruxolitinib failure assessed by investigator’s assessment
| Patients (n=83) | |
|---|---|
| Ruxolitinib resistance | |
| Patients (n=83) | |
| Total | 55 (66%) |
| Insufficient response | 19 (23%) |
| Disease progression | 13 (16%) |
| Loss of response | 23 (28%) |
| Ruxolitinib intolerance | |
| Total | 27 (33%) |
| Haematological toxicity | 20 (24%) |
| Thrombocytopenia | 11 (13%) |
| Anaemia | 6 (7%) |
| Other | 3 (4%) |
| Non-haematological toxicity | 7 (8%) |
| Other | |
| Insufficient efficacy | 1 (1%) |
Data are n (%).
Table 3.
Summary of previous ruxolitinib treatment
| Patients (n=97) | |
|---|---|
| Initial daily ruxolitinib dose (mg) | |
| ≤25 | 26 (27%) |
| 30 | 39 (40%) |
| 40 | 30 (31%) |
| 50 | 2 (2%) |
| Cumulative dose administered (mg) | 9040 (5075–13 005) |
| Duration of exposure (months) | 10·25 (5·75–14·75) |
| Reduction in palpable spleen size at best response | |
| Ruxolitinib-resistant (n=53) | |
| ≥50% | 23/53 (43%) |
| <50% | 30/53 (57%) |
| Ruxolitinib-intolerant (n=23) | |
| ≥50% | 10/23 (43%) |
| <50% | 13/23 (57%) |
Data are median (IQR), n (%), or n/N (%). Data are from the per-protocol population.
Patients received a median of six cycles of fedratinib therapy (IQR 3·9–8·9). Overall, 38 (39%) of 97 patients had at least one dose reduction; 13 (13%) patients had two dose reductions and four (4%) had more than two dose reductions. The most common reasons for dose reductions were gastrointestinal disorders (16 patients), anaemia (eight patients), and thrombocytopenia (six patients). All 97 patients discontinued study treatment: 63 (65%) patients due to the early termination of the study by the sponsor, 18 (19%) due to adverse events, six (6%) due to disease progression, three (3%) because of patient decision, and seven (7%) for other reasons (figure 1 (fig1)).
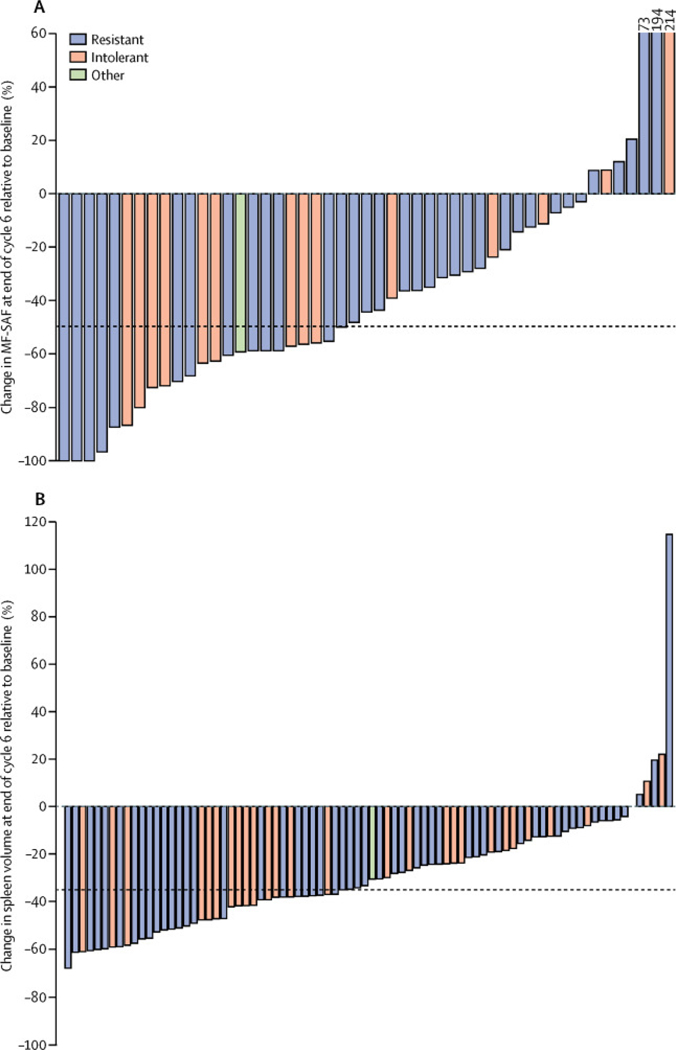
In the evaluable patients, 46 (55%, 95% CI 44–66) of 83 patients achieved a spleen response (≥35% reduction in spleen volume from baseline) at end of cycle 6 (table 4 (tbl4)). 29 (53%) of 55 patients resistant to ruxolitinib and 17 (63%) of 27 patients intolerant of ruxolitinib achieved spleen responses at end of cycle 6. 19 (61%) of 31 patients with baseline platelet counts of 50–100 × 10 9 platelets per L and 27 (52%) of 52 patients with baseline platelet counts of more than 100 × 10 9 platelets per L achieved a spleen response. At end of cycle 6, the median percentage change in spleen volume from baseline was −34·0% (95% CI −35·6 to 24·4; figure 2 (fig2)). In the intention-to-treat population, 33 (34%) of 97 patients at end of cycle 3 and 30 (31%) of 97 patients at end of cycle 6 showed a 50% or greater reduction in spleen size by palpation. An ad-hoc analysis suggested that both duration of ruxolitinib therapy and baseline spleen size (smaller or larger than 10 cm) did not have major effects on the proportion of patients achieving a spleen response (data not shown).
Table 4.
Spleen response
| EOC3 | EOC6 | |
|---|---|---|
| All (n=83)* (tbl4fn1) | 39 (47%) | 46 (55%) |
| Response by reason for ruxolitinib treatment failure | ||
| Ruxolitinib-resistant (n=55) | 25 (45%) | 29 (53%) |
| Insufficient response (n=19) | 8 (42%) | 10 (53%) |
| Disease progression (n=13) | 5 (38%) | 5 (38%) |
| Loss of response (n=23) | 12 (52%) | 14 (61%) |
| Ruxolitinib-intolerant (n=27) | 14 (52%) | 17 (63%) |
Data are n (%). Spleen response was defined as a 35% or more reduction in spleen volume from baseline. EOC=end of cycle.
One patient discontinued due to other reasons (not definable), and was therefore not classified as resistant or intolerant.
Figure 2.
Change in total symptom score (A) and spleen volume (B) from baseline to end of cycle 6, according to reason for ruxolitinib discontinuation
MF-SAF=modified myelofibrosis symptom assessment form.
90 patients were evaluable for symptom response, of whom 23 (26%) achieved a 50% or greater reduction in total symptom score from baseline to end of cycle 6 (figure 2 (fig2)). 13 (21%) of 61 patients resistant to ruxolitinib and nine (32%) of 28 patients intolerant of ruxolitinib achieved a symptom response. 12 (39%) of 31 patients with baseline platelet counts of 50–100 × 10 9 /L and 11 (19%) of 59 with baseline platelet counts of more than 100 × 10 9 /L achieved a symptom response. At the end of cycle 3, 29 (32%) of 90 patients achieved a symptom response, of whom 19 (31%) of 61 were ruxolitinib resistant and ten (36%) of 28 were ruxolitinib intolerant. Exploratory outcomes including pharmacokinetic analysis, duration of spleen response, and JAK2 Val617Phe allele burden were not available due to premature termination of the study, and will not be reported.
All 97 patients in the safety population had at least one adverse event. The most common haematological adverse events (laboratory assessment) were anaemia and thrombocytopenia (table 5 (tbl5)). The most common non-haematological adverse events were gastrointestinal symptoms, including diarrhoea, nausea, and vomiting. Grade 3–4 gastrointestinal toxicity was only seen for diarrhoea. The second most common class of non-haematological adverse events was infections or infestations; most cases were grade 1–2, and only urinary tract infection was reported in more than 10% of patients.
Table 5.
Adverse events
| Grade 1–2 | Grade 3–4 | Grade 5 | |
|---|---|---|---|
| Haematological adverse events * (tbl5fn1) (n=97) | |||
| Anaemia | 10 (10%) | 37 (38%) | 0 |
| Thrombocytopenia | 5 (5%) | 21 (22%) | 0 |
| Lymphopenia | 1 (1%) | 3 (3%) | 0 |
| Non-haematological adverse events (n=97) | |||
| Diarrhoea | 56 (58%) | 4 (4%) | 0 |
| Nausea | 54 (56%) | 0 | 0 |
| Vomiting | 40 (41%) | 0 | 0 |
| Constipation | 19 (20%) | 1 (1%) | 0 |
| Pruritus | 16 (16%) | 0 | 0 |
| Fatigue | 13 (13%) | 2 (2%) | 0 |
| Headache | 12 (12%) | 1 (1%) | 0 |
| Cough | 13 (13%) | 0 | 0 |
| Urinary tract infection | 12 (12%) | 0 | 0 |
| Dyspnoea | 11 (11%) | 1 (1%) | 0 |
| Dizziness | 11 (11%) | 0 | 0 |
| Abdominal pain | 7 (7%) | 2 (2%) | 0 |
| Alanine aminotransferase increased | 3 (3%) | 3 (3%) | 0 |
| Pneumonia | 3 (3%) | 2 (2%) | 1 (1%) |
| Hyperlipasaemia | 1 (1%) | 3 (3%) | 0 |
| Hyperuricaemia | 2 (2%) | 2 (2%) | 0 |
| Dehydration | 1 (1%) | 2 (2%) | 0 |
| Tumour lysis syndrome | 0 | 2 (2%) | 0 |
| Cardiac failure | 1 (1%) | 2 (2%) | 0 |
| Amylase increased | 1 (1%) | 2 (2%) | 0 |
| Blood bilirubin increased | 0 | 2 (2%) | 0 |
| Cardiac failure | 1 (1%) | 2 (2%) | 0 |
| Respiratory failure | 0 | 0 | 1 (1%) |
| Splenic rupture | 0 | 0 | 1 (1%) |
Data are n (%). Shown are any grade event occurring in more than 10% of patients, grade 3–4 events occurring in more than one patient, and all deaths (excluding four deaths due to disease progression).
Laboratory measurements.
A total of 33 (34%) of 97 patients had a serious adverse event, the most common being cardiac disorders (five [5%]), pneumonia (four [4%]), and pleural effusion (three [3%]). Seven (7%) patients died during the study, but none of the deaths was deemed to be related to fedratinib. Overall, 18 patients (19%) discontinued treatment due to adverse events, the most common being grade 3–4 thrombocytopenia (two patients). One additional patient had disease transformation to acute myeloid leukaemia. This event was deemed an adverse event, but the reason for discontinuation was recorded as disease progression. Grade 3 encephalopathy was reported in one male patient aged 62 years at 42 weeks (cycle 11) and treatment was discontinued. The patient fully recovered 1 week later. This case was assessed by an independent expert safety panel and deemed to be hepatic encephalopathy and not Wernicke’s encephalopathy.
At study discontinuation (protocol amendment 4), 63 patients were on active fedratinib treatment and 23 others were in safety follow-up. Thiamine supplementation and safety follow-up (planned duration 90 days) was initiated in 81 patients, although none showed evidence of thiamine deficiency and five patients refused thiamine supplementation. Mean thiamine exposure was 15·5 weeks. No encephalopathy or cardiac failure was reported during this extended safety follow-up period.
Discussion
To our knowledge this is the first reported study of a selective JAK2 inhibitor therapy in patients with myelofibrosis who had previously received ruxolitinib. Overall, the results show that fedratinib was effective in reducing splenomegaly and symptom burden in patients who had previously discontinued ruxolitinib therapy due to either intolerance or resistance, as determined by the study investigators. Most patients achieved a reduction in spleen volume, with more than half (55%) of patients reaching the primary endpoint at end of cycle 6 of a 35% or greater reduction in spleen volume from baseline. Notably, the proportion of patients achieving a 35% reduction in spleen volume was greater than that reported in the COMFORT-I study 4 comparing ruxolitinib with placebo, in which 65 (42%) of 155 patients achieved a 35% or greater reduction in spleen volume, or the COMFORT-II study, 5 in which 41 (28%) of 146 patients given ruxolitinib achieved a 35% or greater reduction in spleen volume. The proportion of patients achieving splenic response in the present study depended on the reason for ruxolitinib failure, with patients who had disease progression during ruxolitinib treatment seeming to respond least well to fedratinib. Although the primary endpoint was met, early termination of the trial precluded assessment of the duration of spleen response. Most patients achieved an improvement in symptoms with fedratinib treatment, as measured using the modified myelofibrosis symptom assessment form, with around a quarter reaching the prespecified cutoff of 50% or greater improvement in total symptom score. Some differences were seen in both spleen and symptom responses according to the baseline platelet count; however, we suggest these subgroups are too small to draw meaningful inference with regard to these aspects of response.
The definitions and assessments of ruxolitinib resistance and intolerance were not prespecified in the study protocol, and therefore, these classifications were made by the investigators. So far, no consensus exists on the definition of ruxolitinib resistance. 15 16 This is a limitation of our study, as is the absence of a control group. At the time of study conception and still at the time of writing, no criteria have been universally agreed for ruxolitinib resistance or intolerance. We acknowledge that if different criteria (when they become available) were used, the study results might have been different.
A 2017 study 17 of the dual JAK1 and JAK2 inhibitor, momelotinib, enrolled patients who did not respond to ruxolitinib therapy. The prespecified definition of resistance in that study was the requirement for red blood cell transfusion during therapy, or dose reductions of ruxolitinib to less 20 mg twice per day, due to grade 3 or higher thrombocytopenia, anaemia, or haemorrhage. Although this approach might limit investigator bias, the definition is focused on ruxolitinib intolerance, and therefore can exclude other patient subgroups deemed to have stopped ruxolitinib due to insufficient efficacy, such as the ruxolitinib-resistant patients included in the present study. Additionally, seven patients who were included in our study had a non-haematological toxicity during ruxolitinib therapy and would probably have been excluded from the momelitinib trial on the basis of the eligibility criteria. This difference in eligibility criteria between trials highlights the need for a consensus definition of ruxolitinib resistance, so that the results of different trials might be more readily compared.
The safety profile of fedratinib was in line with that of previous fedratinib studies. 18 19 Gastrointestinal toxicities were the most common adverse events reported, but most of these events were grade 1–2. A single case of encephalopathy was reported, which was subsequently determined by an independent expert safety panel to be related to hepatic encephalopathy and inconsistent with Wernicke’s encephalopathy. The event resolved within 1 week after discontinuation of fedratinib treatment. As a result of seven potential cases of Wernicke’s encephalopathy in other fedratinib trials, the clinical programme was placed on clinical hold in the USA but not in Europe. 18 Because Wernicke’s encephalopathy is preventable with thiamine supplementation in patients with low thiamine levels, this study suggests that patients with primary or secondary myelofibrosis who are intolerant or resistant to ruxolitinib or other JAK1 and JAK2 inhibitors might achieve significant clinical benefit after treatment with fedratinib, a selective JAK2 inhibitor, even though our study did not have a control group, which is a limiting factor. The importance of the responses to fedratinib in this study are further underscored by data suggesting that patients who develop progressive disease, even after initial response to ruxolitinib, might fare badly with other salvage therapies such as transplantation 20 and have a poor overall survival after stopping ruxolitinib, 9 which is currently the only approved JAK inhibitor for myelofibrosis therapy.
Supplementary Material
Acknowledgments
The study was sponsored by Sanofi. The authors received writing assistance from Amy-Leigh Johnson of Adelphi Communications, funded by Sanofi.
Footnotes
Declaration of interests
CNH reports grants from Novartis and Shire; personal fees from Novartis, Sanofi, CTI, Baxalta, Gilead, and Shire; and other fees from Novartis, Sanofi, and CTI. AMV reports grants and personal fees from Novartis. J-JK reports personal fees and non-financial support from Novartis and Shire, and grants from AOP Orphan. RVT reports participation in advisory boards and speaker bureaus for Incyte and advisory meetings for Gilead Biosciences, and started employment at Eli Lilly and Company on Jan 19, 2015. EJ reports personal fees and non-financial support from Novartis. EW reports clinical trial grants from Sanofi-Aventis during the conduct of this study. HCS reports personal fees from Sanofi and Novartis. SZ reports grants for research and advisory board participation from Novartis. RAM reports grants from Sanofi, Incyte, Gilead, and CTI. RTS reports advisory board participation in AOP Orphan, PharmaEssentia, Incyte, and Gilead BioScience. MT reports research funds from Incyte, CTI Biopharma, NS Pharma, Gilead, and Sanofi; and advisory board participation for Sanofi, Gilead, CTI Biopharma, and Incyte. JL and ZS are employees of Sanofi. NS, PZ, and FP declare no competing interests.
Supplementary Material
Supplementary appendix (/ui/service/content/url?section=static%2fimage&eid=1-s2.0-S2352302617300881&path=23523026%2FS2352302617X00070%2FS2352302617300881%2Fmmc1.pdf)
References
- 1.Cervantes F: How I treat myelofibrosis. Blood 2014; 124: pp. 2635–2642. View In Article Cross Ref ( 10.1182/blood-2014-07-575373) [DOI] [PubMed] [Google Scholar]
- 2.Reiter A, Invernizzi R, Cross NC, Cazzola M: Molecular basis of myelodysplastic/myeloproliferative neoplasms. Haematologica 2009; 94: pp. 1634–1638. View In Article Cross Ref ( 10.3324/haematol.2009.014001) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nangalia J, Massie CE, Baxter EJ, et al. : Somatic CALR mutations in myeloproliferative neoplasms with nonmutated JAK2. N Engl J Med 2013; 369: pp. 2391–2405. View In Article [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Verstovsek S, Mesa RA, Gotlib J, et al. : A double-blind, placebo-controlled trial of ruxolitinib for myelofibrosis. N Engl J Med 2012; 366: pp. 799–807. View In Article Cross Ref ( 10.1056/NEJMoa1110557) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Harrison C, Kiladjian JJ, Al-Ali HK, et al. : JAK inhibition with ruxolitinib versus best available therapy for myelofibrosis. N Engl J Med 2012; 366: pp. 787–798. View In Article [DOI] [PubMed] [Google Scholar]
- 6.Verstovsek S, Mesa RA, Gotlib J, et al. : Efficacy, safety, and survival with ruxolitinib in patients with myelofibrosis: results of a median 3-year follow-up of COMFORT-I. Haematologica 2015; 100: pp. 479–488. View In Article Cross Ref ( 10.3324/haematol.2014.115840) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Harrison CN, Vannucchi AM, Kiladjian JJ, et al. : Leukemia 2016; 30: pp. 1701–1707. View In Article Cross Ref ( 10.1038/leu.2016.148) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stein BL, Cervantes F, Giles F, Harrison CN, Verstovsek S: Novel therapies for myelofibrosis. Leuk Lymphoma 2015; 56: pp. 2768–2778. View In Article Cross Ref ( 10.3109/10428194.2015.1037762) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kantarjian HM, Garcia-Manero G, Quintas-Cardama A, et al. : Outcome of patients (pts) with myelofibrosis (MF) after ruxolutinib (rux) therapy. Blood 2013; 122: pp. 1584. View In Article Cross Ref ( 10.1182/blood.v122.21.1584.1584) [DOI] [Google Scholar]
- 10.Vardiman JW, Thiele J, Arber DA, et al. : The 2008 revision of the World Health Organization (WHO) classification of myeloid neoplasms and acute leukemia: rationale and important changes. Blood 2009; 114: pp. 937–951. View In Article Cross Ref ( 10.1182/blood-2009-03-209262) [DOI] [PubMed] [Google Scholar]
- 11.Cervantes F, Pereira A: Prognostication in primary myelofibrosis. Curr Hematol Malig Rep 2012; 7: pp. 43–49. View In Article Cross Ref ( 10.1007/s11899-011-0102-1) [DOI] [PubMed] [Google Scholar]
- 12.Tefferi A, Cervantes F, Mesa R, et al. : Revised response criteria for myelofibrosis: International Working Group-Myeloproliferative Neoplasms Research and Treatment (IWG-MRT) & European LeukemiaNet (ELN) consensus report. Blood 2013; 122: pp. 1395–1398. View In Article Cross Ref ( 10.1182/blood-2013-03-488098) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mesa RA, Kantarjian H, Tefferi A, et al. : Evaluating the serial use of the myelofibrosis symptom assessment form for measuring symptomatic improvement: performance in 87 myelofibrosis patients on a JAK1 and JAK2 inhibitor (INCB018424) clinical trial. Cancer 2011; 117: pp. 4869–4877. View In Article Cross Ref ( 10.1002/cncr.26129) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schenker S, Henderson GI, Hoyumpa AM, McCandless DW: Hepatic and Wernicke’s encephalopathies: current concepts of pathogenesis. Am J Clin Nutr 1980; 33: pp. 2719–2726. View In Article Cross Ref ( 10.1093/ajcn/33.12.2719) [DOI] [PubMed] [Google Scholar]
- 15.Pardanani A, Tefferi A: Definition and management of ruxolitinib treatment failure in myelofibrosis. Blood Cancer J 2014; 4: pp. e268. View In Article Cross Ref ( 10.1038/bcj.2014.84) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Reilly JT, McMullin MF, Beer PA, et al. : Use of JAK inhibitors in the management of myelofibrosis: a revision of the British Committee for Standards in Haematology Guidelines for Investigation and Management of Myelofibrosis 2012. Br J Haematol 2014; 167: pp. 418–420. View In Article Cross Ref ( 10.1111/bjh.12985) [DOI] [PubMed] [Google Scholar]
- 17.Gupta V, Mesa RA, Deininger MW, et al. : A phase 1/2, open-label study evaluating twice-daily administration of momelotinib in myelofibrosis. Haematologica 2017; 102: pp. 94–102. View In Article Cross Ref ( 10.3324/haematol.2016.148924) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pardanani A, Harrison C, Cortes JE, et al. : Safety and efficacy of fedratinib in patients with primary or secondary myelofibrosis: a randomized clinical trial. JAMA Oncol 2015; 1: pp. 643–651. View In Article Cross Ref ( 10.1001/jamaoncol.2015.1590) [DOI] [PubMed] [Google Scholar]
- 19.Pardanani A, Tefferi A, Jamieson C, et al. : A phase 2 randomized dose-ranging study of the JAK2-selective inhibitor fedratinib (SAR302503) in patients with myelofibrosis. Blood Cancer J 2015; 5: pp. e335. View In Article Cross Ref ( 10.1038/bcj.2015.63) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shanavas M, Popat U, Michaelis LC, et al. : Outcomes of allogeneic hematopoietic cell transplantation in patients with myelofibrosis with prior exposure to Janus kinase 1/2 inhibitors. Biol Blood Marrow Transplant 2016; 22: pp. 432–440. View In Article Cross Ref ( 10.1016/j.bbmt.2015.10.005) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.